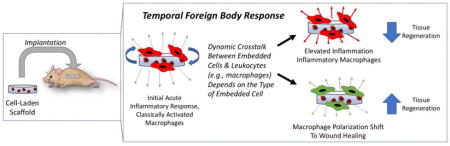
Abstract
Implantation of cell-laden scaffolds is a promising strategy for regenerating tissue that has been damaged due to injury or disease. However, the act of implantation initiates an acute inflammatory response. If the scaffold is non-biologic (i.e., a modified biologic scaffold or synthetic-based scaffold), inflammation will be prolonged through the foreign body response (FBR), which eventually forms a fibrous capsule and walls off the implant from the surrounding host tissue. This host response, from a cellular perspective, can create a harsh environment leading to long-lasting effects on the tissue engineering outcome. At the same time, cells embedded within the scaffold can respond to this environment and influence the interrogating immune cells (e.g., macrophages). This crosstalk, depending on the type of cell, can dramatically influence the host response. This review provides an overview of the FBR and highlights important and recent advancements in the host response to cell-laden scaffolds with a focus on the impact of the communication between immune cells and cells embedded within a scaffold. Understanding this complex interplay between the immune cells, notably macrophages, and the tissue engineering cells is a critically important component to a successful in vivo tissue engineering therapy.
Keywords: Macrophage, Foreign Body Response, Scaffold, Mesechymal Stem Cells
Graphical abstract
Introduction
Tissue engineering holds great promise for regenerating tissues and whole organs, which have been damaged due to injury or disease. When damage is severe or when endogenous cells are incapable of regeneration, a strategy that incorporates tissue-specific or stem cells into a scaffold will be important. One of the benefits of a scaffold-based strategy is the ability to design the scaffold with biochemical cues and degradation rates to enhance differentiation and tissue synthesis [1,2]. To this end, there have been significant advancements in scaffold research and development, spanning biologic and synthetic-based scaffolds, for tissue engineering.
Despite significant research, only a few implantable cell-laden scaffolds have reached clinical success. One of the challenges is that the surgical implantation of the scaffold creates an injury and initiates an acute inflammatory response. In addition, scaffolds made from non-biologic materials will be recognized as foreign and elicit a foreign body response (FBR), which is characterized by chronic inflammation and fibrosis [3,4]. The FBR has been well-documented to acellular non-biologic scaffolds, including chemically crosslinked decellularized extracellular matrix (ECM) [5], crosslinked collagen gels [6], poly(α-hydroxy ester) scaffolds [7], alginate hydrogels [8], poly(ethylene glycol) (PEG)-based hydrogels [9]. Since the FBR is the body’s normal response to an implanted non-biologic material, the FBR has often been used to classify a material as biocompatible [3]. However, the scaffolds that have been clinically successful, albeit only a few, have been largely limited to natural, unmodified, biologic materials (e.g. [10,11]). This raises the question as to whether the prolonged inflammatory response is limiting the advancement of cell-laden non-biologic scaffolds.
Although many implantable medical devices function despite the FBR, long-term exposure to this environment has led to unwanted corrosion or degradation of highly stable biomaterials [12]. For tissue engineering, where cells are embedded within a scaffold, cells are known to be highly sensitive to their environment. Thus, understanding the effects of the host response to the embedded cells within scaffolds is critically important, but has received little attention. This review presents an overview of the FBR and highlights recent advancements in the host response with a focus on the communication between immune cells and cells embedded within a scaffold (i.e., non-immune cells) using in vitro models and in in vivo implantation of cell-laden scaffolds.
The Foreign Body Response
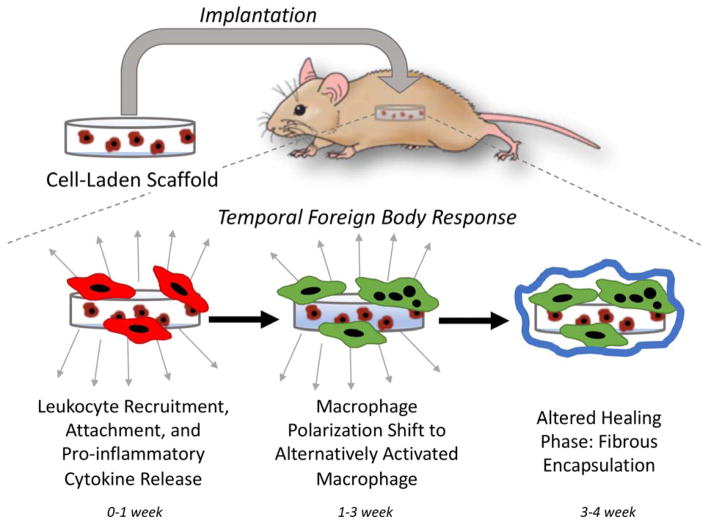
The FBR is a temporal response characterized by chronic inflammation and a dense, avascular fibrous capsule (Figure 1) [3,4]. As part of the initial acute inflammatory response, phagocytes are recruited to the site of implantation with neutrophils arriving first followed by long-lived macrophages. Neutrophils are thought to be short-lived in the FBR, however, recent evidence suggests that their presence may be longer than previously thought [13]. Phagocytes recognize the implant as foreign through surface adsorbed proteins, release pro-inflammatory cytokines, and when attempts to phagocytose the foreign material fail, chronic inflammation ensues. Macrophages, specifically, become frustrated and fuse into foreign body giant cells (FBGCs), a characteristic feature of the FBR. Although not well understood, polarization of the macrophage changes from inflammatory to an alternatively activated state and the FBR transitions into an altered healing phase. This phase leads to the formation of the fibrous capsule. In total, the FBR takes ~3–4 weeks after which the response stabilizes. Macrophages and FBGCs remain at the implant surface encased within the fibrous capsule for the lifetime of the implant, maintaining low grade chronic inflammation. Once the scaffold has degraded completely, the fibrous capsule eventually breaks down [14].
Figure 1.
The temporal host response upon implantation of a cell-laden scaffold. An acute inflammatory response initiates as part of the surgical implantation, which creates a wound. This initial response is accompanied by recruitment of leukocytes, notably inflammatory macrophages (shown in red), which release pro-inflammatory cytokines. If the scaffold is non-biologic, the host response evolves eventually shifting to an altered healing phase that is accompanied by a polarization shift in the macrophage and formation of multinucleated foreign body giant cells (shown in green) and the eventual walling off of the implant by a fibrous capsule. The resolution of the foreign body response is the presence of macrophages and FBGCs at the implant surface, maintaining low grade chronic inflammation, and the isolation of the implant from the host tissue.
Macrophages are considered the orchestrators of the FBR and as a result have received the most attention. Macrophages are complex cells due to their involvement in inflammation, regulation, and wound healing [15]. As a result, macrophages display a high degree of plasticity [15,16]. Initially, inflammatory macrophages derived from blood monocytes are recruited to the implantation site. These macrophages are characterized by NF-κB activation of pro-inflammatory cytokines (e.g., tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-1β (IL-1β)) [17]. This phenotype can be simulated in vitro by factors such as lipopolysaccharide (LPS) and/or interferon gamma (IFN-γ) [18]. The alternatively activated macrophage phenotype that emerges as the FBR transitions to the healing phase is less understood. Several macrophage subtypes have been defined within the alternatively activated phenotype and FBGCs fall within this subset [16]. In vitro FBGC formation has been recapitulated using interleukin-4 (IL-4) or interleukin-13 (IL-13) cytokines [19]. IL-4/IL-13 stimulated macrophages have been reported to secrete molecules such as interleukin-10 (IL-10) and IL-1 receptor antagonist (IL-1ra), which attenuate and self-regulate inflammation [20,21], and TGF-β, which is involved in normal and altered healing (i.e., fibrosis) [22]. Although little is known about the neutrophil, a recent review suggests their role in instigating the FBR [23]. This review, however, focuses on the macrophage.
From a cellular perspective, the FBR creates a harsh environment with chronic inflammation and isolation from the host tissue and vasculature. Although the FBR eventually regresses in response to a degradable implant, the FBR may have long-lasting effects. Thus, understanding the complex interplay between different macrophage phenotypes, the overall FBR, and the embedded cells is important to advancing tissue engineering in vivo.
The Crosstalk In Vitro: Macrophages and Cell-Laden Scaffolds
During the FBR, macrophages accumulate at the implant surface and depending on scaffold chemistry and architecture can migrate into the scaffold. As a result, macrophages can interact with embedded cells within a scaffold through paracrine or juxtacrine signaling. This signaling is two-way where molecules secreted by macrophages can signal to the embedded cells and vice versa. To tease out the signaling mechanisms involved in this dynamic crosstalk, in vitro models have been developed [24]. In this section, we highlight several examples of ‘one-way’ signals from macrophages to non-immune cells, ‘one-way’ signals from non-immune cells to macrophages, and finally the ‘two-way’ signals that create a highly dynamic and continuous crosstalk.
The Influence of Macrophage Paracrine Signaling on Non-Immune Cells
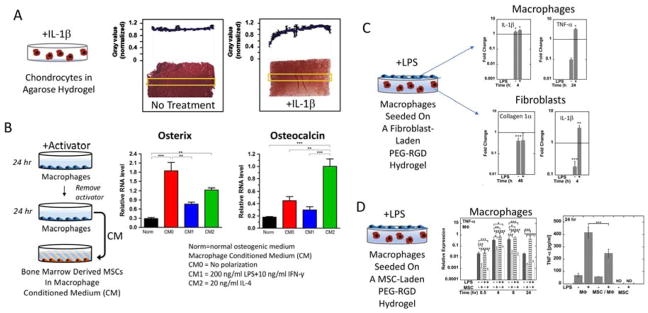
Prolonged exposure to pro-inflammatory cytokines, which are secreted by inflammatory macrophages under chronic inflammation, has been shown to negatively affect embedded cells within scaffolds. For example, osteogenic cells seeded onto nanofibrous scaffolds exhibited a marked reduction in mineralization with TNF-α [25]. When chondrocytes encapsulated in hydrogels were continuously exposed to IL-1α or IL-1β, cartilaginous matrix deposition and mechanical properties decreased (Figure 2A) [26]. Interestingly, some inflammatory molecules, such as TNF-α, have pleiotropic effects [27,28]. For example, low concentrations of TNF-α during osteogenic differentiation of mesenchymal stem cells (MSCs) enhanced alkaline phosphatase and mineralization, while high TNF-α concentration inhibited these osteogenic functions [29]. When macrophage conditioned medium was used, the findings were different (Figure 2B). Contrarily to the above mentioned studies, conditioned medium from LPS/IFN-γ stimulated (inflammatory) macrophages enhanced adipogenesis and conditioned medium from IL-4 stimulated (alternatively activated) macrophages enhanced osteogenesis [30]. This seemingly contradictory finding may be attributed to the secretion of anti-inflammatory molecules, which follow secretion of pro-inflammatory cytokines, as a means of self-regulation [31]. It is possible that the anti-inflammatory molecules in the conditioned medium may have been present at higher concentration and/or more stable than the pro-inflammatory cytokines. The complex milieu of paracrine signaling factors and their temporal profile within macrophage conditioned medium requires further study.
Figure 2.
In vitro assessment of the inflammatory environment (A), the one-way signals from macrophages to differentiating stem cells (B), and the two-way signals in a simulated FBR with macrophages at the surface of a cell-laden hydrogel (C, D). In (A), cartilage cells (i.e., chondrocytes) embedded in an agarose hydrogel and cultured continuously with interleukin-1β (IL-1β) led to reduce cartilage extracellular matrix deposition shown by the red staining for sulfated glycosaminoglycans. Reproduced with permission from [26]. In (B), macrophages were conditioned with different activators (i.e., no activator (CM0), lipopolysaccharide (LPS) + interferon gamma (IFN-γ) for a classically activated (inflammatory) macrophage (CM1), or interleukin-4 (IL-4) for an alternatively activated macrophage (CM2). After 24 hours, the medium with activator was removed, and fresh media applied without activator for 24 hours, to create the conditioned medium (CM). The CM was then supplemented with osteogenic factors and applied to bone marrow derived mesenchymal stem cells (MSCs) for seven days. Gene expression of two osteogenic genes, osterix and osteocalcin, are shown. Reproduced from [30]. In (C), macrophages were seeded on top of a fibroblast-laden poly(ethylene glycol) (PEG) hydrogel with RGD and cultured with or without LPS. The cell populations were separated after 24 hours and gene expression analyzed for the macrophage by IL-1β and tumor necrosis factor-α (TNF-α) and for the fibroblast for collagen 1α and IL-1β. The horizontal line represents the corresponding mono-culture. Reproduced with permission from [39]. In (D), macrophages were seeded on top of a MSC-laden poly(ethylene glycol) (PEG) hydrogel with RGD and cultured with or without LPS for 24 hours. Relative expression for the macrophage was assessed by TNF-α and TNF-α protein was measured in the medium. Reproduced with permission from [35].
The Influence of Non-Immune Cells on Macrophage Polarization
Several studies have investigated growth factors, which are important to tissue engineering, and shown an effect on macrophage polarization. For example, vascular endothelial growth factor (VEGF) induced migration of inflammatory macrophages and shifted their polarization to an alternatively activated polarization state [32]. Bone morphogenetic protein 2 (BMP2) downregulated inflammatory cytokines and enhanced angiogenic factors secreted by macrophages [33]. Immunomodulatory effects from stem cells has also been investigated. Under an inflammatory stimulant, stem cells secrete high levels of anti-inflammatory factors of which several have been identified and include prostaglandin E2 (PGE2), tumor necrosis factor-inducible gene 6 (TSG6), and IL-1ra [34]. Using MSC conditioned medium, LPS stimulated macrophages cultured on top of a PEG-RGD hydrogel secreted less TNF-α and this down-regulation was re-capitulated with exogenous PGE2 [35]. MSC conditioned medium was also shown to promote monocyte differentiation into an alternatively activated macrophage, but independent of PGE2 [36]. These studies suggest that PGE2 may be important in attenuating inflammation, but not in shifting macrophage polarization. While stem cells have been shown to have a positive effect on attenuating inflammatory macrophages, their immunomodulatory properties are highly dependent on the culture environment. For example, 3D cultures produce greater immunomodulatory effects than 2D cultures [37]. Scaffolds that promote a stem cell morphology in between round and fully stretched showed improved immunomodulatory effects [38]. Importantly, these studies demonstrate that non-immune cells, and specifically stem cells, and their secretome can have a significant impact on macrophage inflammatory responses and polarization states, but is dependent on the nature of the scaffold. Less is known in how tissue-specific (i.e., differentiated cells) influence macrophages. Since tissue engineering strategies often use differentiated cells, more research is needed to understand how the inflammatory environment influences differentiated cells and how their secretome in turn may affect macrophages.
Continuous Signaling Between Macrophages and Non-Immune Cells: Co-Cultures
The in vivo scenario is more complex where cells continually communicate, receiving signals from each other and responding in real time. To recapitulate this dynamic crosstalk in vitro, studies have used co-cultures such as a) transwell inserts or other forms that physically separate the two cell types [24], b) co-cultures that simulate the FBR where macrophages are seeded directly on top of scaffolds containing embedded cells [39], and c) directly mixing of cells to facilitate cell-cell contacts [24].
In co-cultures that capture paracrine-only signaling, the dynamic crosstalk can have a positive or negative impact on each cell type. For example, macrophage motility was higher in the presence of fibroblasts compared to mono-cultures [40]. For 2D cultures of calvarial osteoblasts, the presence of macrophages was necessary to induce mineralization when differentiation factors such as dexamethasone were absent [41]. When macrophages were seeded on top of a fibroblast-laden PEG-RGD hydrogel with LPS, pro-inflammatory gene expression levels were elevated in both cell types while expression of collagen genes in the fibroblast cells was reduced when compared to mono-cultures (Figure 2C) [39]. On the contrary when macrophages were seeded on top of a similar PEG hydrogel, but which contained embedded MSCs and stimulated with LPS, pro-inflammatory cytokine gene expression in macrophages was reduced when compared to macrophages seeded on acellular hydrogels (Figure 2D) [35]. Other studies have reported similar findings where macrophages cultured in the presence of MSCs led to higher IL-10, but lower TNF-α secretion [42]. Interestingly, in these co-cultures with MSCs and macrophages, PGE2 levels were low in the co-culture despite being high in the MSC mono-culture [35,42]. This observation points to a complex interplay that may have averted an inflammatory response. These in vitro studies demonstrate that the type of embedded cell (e.g., differentiated versus stem cell) has a significant impact on the crosstalk, elevating or dampening the inflammatory response.
A few studies have developed in vitro models to study juxtacrine signaling, where both paracrine and cell-cell contacts exist. In co-cultures with macrophages and fibroblasts, there were no significant differences between the effects of paracrine and juxtacrine signaling [43]. However, differences were noted between macrophages and adipocytes, where TNF-α levels were higher under paracrine-only signaling compared to juxtacrine signaling [44]. Preosteoblastic cells cultured in direct contact with macrophages had higher mineralization regardless of the polarization state of the macrophage (i.e., no, LPS, or IL-4 stimulation) compared to monocultures [45]. However, LPS-stimulated macrophages led to a significant decrease in osteocalcin expression in the preosteoblastic cells compared to monoculture [45]. When vascular smooth muscle cells were cultured directly with monocytes on a scaffold, their contractile phenotype was suppressed while migration enhanced when compared to monocultures; a finding that was partly mediated by IL-6 [46]. Although the mechanisms involved in juxtacrine signaling between macrophages and non-immune cells are not known, rapid communication due to close proximity and/or direct cell-cell contacts may play important roles.
Collectively, these in vitro studies using conditioned medium or co-cultures demonstrate that the cell-to-cell communication between macrophages and non-immune cells (e.g., the embedded cells) will have a significant impact on both cell types. Depending on the nature of the communication, the macrophage polarization state, and the type of non-immune cell (e.g., differentiated or stem-like), the communication can promote either an anti-inflammatory/pro-regenerative environment or elevate the inflammatory environment. Moreover, continuous crosstalk can lead to cooperative communication that can differ from the sum of the individual effects in mono-cultures [35,47].
The Host Response and Tissue Engineering In Vivo
The in vivo host response is complex encompassing immune and other non-immune cells in a highly coordinated temporal process. Therefore, in vivo studies are critical to the ‘complete picture.’ In this section, we highlight several studies to illustrate the importance of the crosstalk between the host and embedded cells in mediating the overall host response to implanted cell-laden scaffolds.
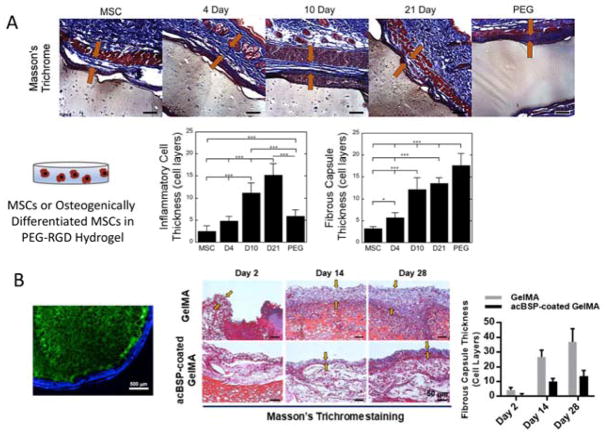
A number of studies have demonstrated that the scaffold chemistry (e.g., biologic versus non-biological [5]), scaffold architecture (e.g., porosity [48]), and incorporation of anti-inflammatory ECM molecules (e.g., chondroitin sulfate [49], netrin-1 [50]) can improve the host response by attenuating the overall inflammatory response. However, only a few studies have examined the effect of incorporating cells of autologous or syngeneic (i.e., cells from a genetically identical animal) origin within an implanted scaffold on the host response. These studies, although few, indicate that these cells will indeed have an impact on the host response. For example, when autologous cells were seeded onto a decellularized ECM biologic scaffold, which on its own promoted constructive healing, the host response instead consisted of classically activated macrophages and a fibrotic, scar-like healing response [51]. PEG hydrogels, which elicit a FBR on their own [9], were shown to exhibit an even more severe FBR when the hydrogel contained either syngeneic dermal fibroblasts [39] or syngeneic osteogenically differentiated MSCs (Figure 3A) [35]. In the former, the cellular content and ECM deposited by the dermal fibroblasts decreased over time, suggesting that the embedded cells were also negatively impacted. These studies point to the crosstalk between differentiated cells and host immune cells in elevating the inflammatory response. Several mechanisms may contribute to a more severe response including the embedded cells releasing their own inflammatory molecules, which is supported by in vitro co-culture studies [39], or cellular death, which releases endogenous intracellular molecules that act as danger associated molecular patterns. Similarly in accordance with in vitro studies, when the embedded cells were MSCs, the FBR to a cell-laden PEG hydrogel was attenuated, but was dependent on the differentiation stage of the MSC (Figure 3A) [35]. A recent study combined the need to develop scaffolds for tissue engineering and to modulate the immune response (Figure 3B) [52]. In this study, a polysaccharide coating, which induced a macrophage polarization state spanning inflammatory, pro-angiogenic, and pro-osteogenic, was applied to a MSC-laden crosslinked gelatin hydrogel. In vivo, the coating reduced the FBR, improved vascularization, and enhanced osteogenesis of the embedded MSCs. Collectively, these studies further support the in vitro studies indicating that the crosstalk between the embedded cells and interrogating immune cells has a significant impact on both the host response and the embedded cells’ ability to synthesize and deposit new tissue.
Figure 3.
In vivo assessment of cell-laden scaffolds. In (A), a PEG-RGD hydrogel with no cells (labeled as PEG) or with embedded MSCs at varying stages of osteogenic differentiation (i.e., MSCs or differentiated for 4, 10, or 21 days prior to encapsulation and implantation). Top row shows the host response after 28 days by Masson’s Trichrome and plots indicate quantitative assessment of the thickness of inflammatory cells at the surface of the hydrogel implant and the thickness of the resulting fibrous capsule. Reproduced with permission from [35]. In (B), a polysaccharide coating was applied to a MSC-laden gelatin crosslinked hydrogel. Far left image of scaffold (green) with coating (blue). Histological assessment of the fibrous capsule by Masson’ts trichrome staining and corresponding fibrous capsule thickness quantified. Reproduced with permission from [52].
Concluding Remarks
Tissue engineering strategies that involve the implantation of cell-laden scaffolds offers a promising approach to regenerating functional tissue in vivo. However, a successful strategy requires that the host responds with a minimal inflammatory response and promotes a normal healing response long-term. It is well-known that acute inflammation occurs as a result of the surgical procedure of implantation and that non-biologic scaffolds induce a FBR, prolonging inflammation. Recent evidence has emerged from in vitro and in vivo studies that implicate cells embedded within the scaffold (i.e., a cell-laden scaffold) as factors that contribute to the host response. Importantly these findings demonstrate that, depending on the type of cell within the scaffold, the inflammatory response can be elevated or dampened. The immunomodulatory effects of stem cells and their ability to not only dampen the inflammatory response, but also shift the healing from a pro-fibrotic to a pro-regenerative healing response is exciting for tissue engineering. There is however more research needed to identify the signaling factors, both good and bad, that are involved in the crosstalk between embedded cells and the host immune cells.
Highlights.
Implantation of cell-laden scaffolds for tissue engineering is promising
Non-biologic scaffolds elicit a foreign body response
The foreign body response is characterized by chronic inflammation
Crosstalk between embedded cells and immune cells affects the host response
Stem cells in a scaffold can attenuate inflammation of the host response
Acknowledgments
This work was supported by supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number 1R01AR069060. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors also acknowledge support from a Department of Education GAANN fellowship to LSS.
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
* of special interest
** of outstanding interest
- 1.Bryant SJ, Vernerey FJ. Programmable Hydrogels for Cell Encapsulation and Neo-Tissue Growth to Enable Personalized Tissue Engineering. Adv Healthc Mater. 2018:7. doi: 10.1002/adhm.201700605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tibbitt MW, Rodell CB, Burdick JA, Anseth KS. Progress in material design for biomedical applications. Proc Natl Acad Sci U S A. 2015;112:14444–51. doi: 10.1073/pnas.1516247112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ratner BD, Bryant SJ. Biomaterials: Where we have been and where we are going. Annu Rev Biomed Eng. 2004;6:41–75. doi: 10.1146/annurev.bioeng.6.040803.140027. [DOI] [PubMed] [Google Scholar]
- 4.Anderson JM. Biological responses to materials. Annu Rev Mater Res. 2001;31:81–110. [Google Scholar]
- 5.Badylak SF, Valentin JE, Ravindra AK, McCabe GP, Stewart-Akers AM. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng Part A. 2008;14:1835–42. doi: 10.1089/ten.tea.2007.0264. [DOI] [PubMed] [Google Scholar]
- 6.Delgado LM, Bayon Y, Pandit A, Zeugolis DI. To Cross-Link or Not to Cross-Link? Cross-Linking Associated Foreign Body Response of Collagen-Based Devices. Tissue Eng Part B Rev. 2015;21:298–313. doi: 10.1089/ten.teb.2014.0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pihlajamaki H, Salminen S, Laitinen O, Tynninen O, Bostman O. Tissue response to polyglycolide, polydioxanone, polylevolactide, and metallic pins in cancellous bone: An experimental study on rabbits. J Orthop Res. 2006;24:1597–606. doi: 10.1002/jor.20191. [DOI] [PubMed] [Google Scholar]
- 8.Liang Y, Liu WS, Han BQ, Yang CZ, Ma Q, Song FL, et al. An in situ formed biodegradable hydrogel for reconstruction of the corneal endothelium. Colloids Surf B-Biointerfaces. 2011;82:1–7. doi: 10.1016/j.colsurfb.2010.07043. [DOI] [PubMed] [Google Scholar]
- 9.Lynn AD, Blakney AK, Kyriakides TR, Bryant SJ. Temporal progression of the host response to implanted poly(ethylene glycol) based hydrogels. J Biomed Mater Res A. 2011;96A:621–631. doi: 10.1002/jbm.a.33015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qi M. Transplantation of Encapsulated Pancreatic Islets as a Treatment for Patients with Type 1 Diabetes Mellitus. Adv Med. 2014;2014:429710. doi: 10.1155/2014/429710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saris D, Price A, Widuchowski W, Bertrand-Marchand M, Caron J, Drogset JO, et al. Matrix-Applied Characterized Autologous Cultured Chondrocytes Versus Microfracture: Two-Year Follow-up of a Prospective Randomized Trial. Am J Sports Med. 2014;42:1384–94. doi: 10.1177/0363546514528093. [DOI] [PubMed] [Google Scholar]
- 12.Gibon E, Cordova LA, Lu L, Lin T-H, Yao Z, Hamadouche M, et al. The biological response to orthopedic implants for joint replacement. II: Polyethylene, ceramics, PMMA, and the foreign body reaction. J Biomed Mater Res Part B-Appl Biomater. 2017;105:1685–91. doi: 10.1002/jbm.b.33676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jhunjhunwala S, Aresta-DaSilva S, Tang K, Alvarez D, Webber MJ, Tang BC, et al. Neutrophil Responses to Sterile Implant Materials. PloS One. 2015;10:e0137550. doi: 10.1371/journal.pone.0137550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rockey DC, Bell PD, Hill JA. Fibrosis--a common pathway to organ injury and failure. N Engl J Med. 2015;372:1138–49. doi: 10.1056/NEJMra1300575. [DOI] [PubMed] [Google Scholar]
- 15.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *16.Klopfleisch R. Macrophage reaction against biomaterials in the mouse model – Phenotypes, functions and markers. Acta Biomater. 2016;43:3–13. doi: 10.1016/j.actbio.2016.07.003. Comprehesive review of macrophage polarization stimuli and the resulting phenotype and corresponding markers. [DOI] [PubMed] [Google Scholar]
- 17.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci-Landmark. 2008;13:453–61. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 18.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McNally AK, Anderson JM. Interleukin-4 Induces Foreign-Body Giant-Cells From Human Monocytes Macrophages - Differential Lymphokine Regulation Of Macrophage Fusion Leads To Morphological Variants Of Multinucleated Giant-Cells. Am J Pathol. 1995;147:1487–99. [PMC free article] [PubMed] [Google Scholar]
- 20.Moore KW, Malefyt RD, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 21.Arend WR. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev. 2002;13:323–40. doi: 10.1016/S1359-6101(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 22.Morikawa M, Derynck R, Miyazono K. TGF-beta and the TGF-beta Family: Context-Dependent Roles in Cell and Tissue Physiology. Cold Spring Harb Perspect Biol. 2016:8. doi: 10.1101/cshperspect.a021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Selders GS, Fetz AE, Radic MZ, Bowlin GL. An overview of the role of neutrophils in innate immunity, inflammation and host-biomaterial integration. Regen Biomater. 2017;4:55–68. doi: 10.1093/rb/rbw041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bogdanowicz DR, Lu HH. Studying cell-cell communication in co-culture. Biotechnol J. 2013;8:395–6. doi: 10.1002/biot.201300054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mountziaris PM, Tzouanas SN, Mikos AG. The interplay of bone-like extracellular matrix and TNF-alpha signaling on in vitro osteogenic differentiation of mesenchymal stem cells. J Biomed Mater Res A. 2012;100A:1097–106. doi: 10.1002/jbm.a.34058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lima EG, Tan AR, Tai T, Bian L, Ateshian GA, Cook JL, et al. Physiologic deformational loading does not counteract the catabolic effects of interleukin-1 in long-term culture of chondrocyte-seeded agarose constructs. J Biomech. 2008;41:3253–9. doi: 10.1016/j.jbiomech.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holtmann MH, Neurath MF. Differential TNF-signaling in chronic inflammatory disorders. Curr Mol Med. 2004;4:439–44. doi: 10.2174/1566524043360636. [DOI] [PubMed] [Google Scholar]
- 28.TRACEY K, CERAMI A. Tumor-Necrosis-Factor - A Pleiotropic Cytokine And Therapeutic Target. Annu Rev Med. 1994;45:491–503. doi: 10.1146/annurev.med.45.1.491. [DOI] [PubMed] [Google Scholar]
- 29.Qin Z, Fang Z, Zhao L, Chen J, Li Y, Liu G. High dose of TNF-alpha suppressed osteogenic differentiation of human dental pulp stem cells by activating the Wnt/beta-catenin signaling. J Mol Histol. 2015;46:409–20. doi: 10.1007/s10735-015-9630-7. [DOI] [PubMed] [Google Scholar]
- 30.He X-T, Li X, Yin Y, Wu R-X, Xu X-Y, Chen F-M. The effects of conditioned media generated by polarized macrophages on the cellular behaviours of bone marrow mesenchymal stem cells. J Cell Mol Med. 2018;22:1302–615. doi: 10.1111/jcmm.13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest. 2000;117:1162–72. doi: 10.1378/chest.117.4.1162. [DOI] [PubMed] [Google Scholar]
- 32.Wheeler KC, Jena MK, Pradhan BS, Nayak N, Das S, Hsu C-D, et al. VEGF may contribute to macrophage recruitment and M2 polarization in the decidua. PloS One. 2018;13:e0191040–e0191040. doi: 10.1371/journal.pone.0191040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei F, Zhou Y, Wang J, Liu C, Xiao Y. The Immunomodulatory Role of BMP-2 on Macrophages to Accelerate Osteogenesis. Tissue Eng Part A. 2017 doi: 10.1089/ten.TEA.2017.0232. [DOI] [PubMed] [Google Scholar]
- 34.Prockop DJ, Oh JY. Mesenchymal Stem/Stromal Cells (MSCs): Role as Guardians of Inflammation. Mol Ther. 2012;20:14–20. doi: 10.1038/mt.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swartzlander MD, Blakney AK, Amer LD, Hankenson KD, Kyriakides TR, Bryant SJ. Immunomodulation by mesenchymal stem cells combats the foreign body response to cell-laden synthetic hydrogels. Biomaterials. 2015;41:79–88. doi: 10.1016/j.biomaterials.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Magatti M, Vertua E, De Munari S, Caro M, Caruso M, Silini A, et al. Human amnion favours tissue repair by inducing the M1-to-M2 switch and enhancing M2 macrophage features. J Tissue Eng Regen Med. 2017;11:2895–911. doi: 10.1002/term.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Follin B, Juhl M, Cohen S, Perdersen AE, Kastrup J, Ekblond A. Increased Paracrine Immunomodulatory Potential of Mesenchymal Stromal Cells in Three-Dimensional Culture. Tissue Eng Part B-Rev. 2016;22:322–9. doi: 10.1089/ten.teb.2015.0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *38.Su N, Gao P-L, Wang K, Wang J-Y, Zhong Y, Luo Y. Fibrous scaffolds potentiate the paracrine function of mesenchymal stem cells: A new dimension in cell-material interaction. Biomaterials. 2017;141:74–85. doi: 10.1016/j.biomaterials.2017.06.028. Demonstrates that the immunomodulatory effects of MSCs can be controlled by the fibrous nature of the scaffold, which was attributed in part to the shape of the MSCs. [DOI] [PubMed] [Google Scholar]
- 39.Swartzlander MD, Lynn AD, Blakney AK, Kyriakides TR, Bryant SJ. Understanding the host response to cell-laden poly(ethylene glycol)-based hydrogels. Biomaterials. 2013;34:952–64. doi: 10.1016/j.biomaterials.2012.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou G, Loppnow H, Groth T. A macrophage/fibroblast co-culture system using a cell migration chamber to study inflammatory effects of biomaterials. Acta Biomater. 2015;26:54–63. doi: 10.1016/j.actbio.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 41.Chang MK, Raggatt L-J, Alexander KA, Kuliwaba JS, Fazzalari NL, Schroder K, et al. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J Immunol Baltim Md 1950. 2008;181:1232–44. doi: 10.4049/jimmunol.181.2.1232. [DOI] [PubMed] [Google Scholar]
- *42.Saldana L, Valles G, Bensiamar F, Jose Mancebo F, Garcia-Rey E, Vilaboa N. Paracrine interactions between mesenchymal stem cells and macrophages are regulated by 1,25-dihydroxyvitamin D3. Sci Rep. 2017;7:14618. doi: 10.1038/s41598-017-15217-8. Provides further evidence that cell-cell signaling between macrophages and mesenchymal stem cells in co-cultures has a significant effect on inflammatory responses and osteogenic potential of MSCs, but is dependent on the 3D nature of the culture. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holt DJ, Chamberlain LM, Grainger DW. Cell-cell signaling in co-cultures of macrophages and fibroblasts. Biomaterials. 2010;31:9382–94. doi: 10.1016/j.biomaterials.2010.07.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nitta CF, Orlando RA. Crosstalk between immune cells and adipocytes requires both paracrine factors and cell contact to modify cytokine secretion. PloS One. 2013;8:e77306. doi: 10.1371/journal.pone.0077306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *45.Loi F, Cordova LA, Zhang R, Pajarinen J, Lin T, Goodman SB, et al. The effects of immunomodulation by macrophage subsets on osteogenesis in vitro. Stem Cell Res Ther. 2016;7:15. doi: 10.1186/s13287-016-0276-5. Demonstrates that direct co-culture between macrophages and preosteoblastic cells has a greater effect on osteoblastic genes than the polarization state of the macrophage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Battiston KG, Ouyang B, Labow RS, Simmons CA, Santerre JP. Monocyte/macrophage cytokine activity regulates vascular smooth muscle cell function within a degradable polyurethane scaffold. Acta Biomater. 2014;10:1146–55. doi: 10.1016/j.actbio.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 47.Ballotta V, Smits AIPM, Driessen-Mol A, Bouten CVC, Baaijens FPT. Synergistic protein secretion by mesenchymal stromal cells seeded in 3D scaffolds and circulating leukocytes in physiological flow. Biomaterials. 2014;35:9100–13. doi: 10.1016/j.biomaterials.2014.07.042. [DOI] [PubMed] [Google Scholar]
- 48.Sussman EM, Halpin MC, Muster J, Moon RT, Ratner BD. Porous Implants Modulate Healing and Induce Shifts in Local Macrophage Polarization in the Foreign Body Reaction. Ann Biomed Eng. 2014;42:1508–16. doi: 10.1007/s10439-013-0933-0. [DOI] [PubMed] [Google Scholar]
- **49.Corradetti B, Taraballi F, Corbo C, Cabrera F, Pandolfi L, Minardi S, et al. Immune tuning scaffold for the local induction of a pro-regenerative environment. Sci Rep. 2017;7:17030. doi: 10.1038/s41598-017-16895-0. Demonstrates that grafting chondroitin sulfate onto a scaffold enhances healing in vivo in a subcutaneous implant model through the recruitment of anti-inflammatory macrophages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Y, Wan S, Liu G, Cai W, Huo D, Li G, et al. Netrin-1 Promotes Inflammation Resolution to Achieve Endothelialization of Small-Diameter Tissue Engineering Blood Vessels by Improving Endothelial Progenitor Cells Function In Situ. Adv Sci. 2017;4:1700278. doi: 10.1002/advs.201700278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown BN, Valentin JE, Stewart-Akers AM, McCabe GP, Badylak SF. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials. 2009;30:1482–91. doi: 10.1016/j.biomaterials.2008.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **52.Niu Y, Li Q, Xie R, Liu S, Wang R, Xing P, et al. Modulating the phenotype of host macrophages to enhance osteogenesis in MSC-laden hydrogels: Design of a glucomannan coating material. Biomaterials. 2017;139:39–55. doi: 10.1016/j.biomaterials.2017.05.042. Demonstrates that a polysaccharide coating can affect macrophage polarization to improve vascularization and enhance osteogenesis of MSCs that are delivered in a scaffold and implanted subcutaneously. [DOI] [PubMed] [Google Scholar]