Abstract
Objectives
The aim of this study was to identify anatomical indication ranges for different lateral wall cochlear implant electrodes to support surgeons in the preoperative preparation.
Methods
272 patients who were implanted with a FLEX20, FLEX24, FLEX28, or a custom-made device (CMD) were included in this study. The cochlear duct length (CDL) and basal cochlear diameter (length A) were measured within preoperative imaging data. The parameter A was then employed to additionally compute CDL estimates using literature approaches. Moreover, the inserted electrode length (IEL) and insertion angle (IA) were measured in postoperative CT data. By combining the preoperative measurements with the IA data, the covered cochlea length (CCL) and relative cochlear coverage (CC) were determined for each cochlea.
Results
The measurements of the CDL show comparable results to previous studies. While CDL measurements and estimations cover similar ranges overall, severe deviations occur in individual cases. The electrode specific IEL and CCL are fairly consistent and increase with longer electrodes, but relatively wide ranges of electrode specific CC values were found due to the additional dependence on the respective CDL. Using the correlation of IEL and CCL across electrode arrays, CDL ranges for selected arrays were developed (FLEX24: 31.3–34.4, FLEX28: 36.2–40.1, FLEXSoft: 40.6–44.9).
Conclusions
Our analysis shows that electrode specific CC varies due to the CDL variation. Preoperative measurement of the CDL allows for an individualized implant length selection yielding optimized stimulation and a reduced risk of intraoperative trauma. The CDL, as derived from preoperative CT imaging studies, can help the implant surgeon select the appropriate electrode array to maximize the patient’s outcomes.
Introduction
Cochlear implantation is a technology for patients with total, severe or frequency specific hearing loss which can restore the patient’s ability to understand speech [1,2]. The cochlear implant (CI) works by directly stimulating the auditory nerve. This is accomplished by inserting a cochlear implant electrode array into the patient’s cochlea. An electric field stimulus is then applied by a number of contacts distributed along the electrode array, targeting the spiral ganglion cells and auditory nerve fibers.
For cochlear implantation, various types of electrode arrays from different manufacturers are available. These electrode arrays differ in size, length, number of electrode contacts and material characteristics [3]. Prior to surgery, a decision must be made by the patient and physician on which electrode to implant. To do so, multiple factors must be taken into account including the residual hearing and medical history of the patient, which may include otosclerosis, patient preference as well as the length and shape of the cochlea. The cochlear length is known to have large variations [4–22]. Previous studies have shown that for patients who only hear with their CI, improved outcomes after CI surgery can be expected with longer electrode arrays and accordingly deeper insertion angles [23–25]. Other studies have shown that the insertion angle not only depends on the electrode array type but also on the length of the cochlea [26,27]. Furthermore, dysplasia and other syndromes exist which have an effect on cochlear geometry, especially regarding the length, shape and number of turns (e.g. Mondini dysplasia) [28].
Different methods are available for the evaluation of the cochlea duct length (CDL) and the depth of insertion within clinical imaging data of cochlea without malformation: one option is to manually trace the contour of the cochlea or electrode array and subsequently use spline interpolation to determine the corresponding length [15,29]. Other methods, which are based on mathematical correlations, use the basal diameter A (within a logarithmic equation) to estimate the cochlea or array length [30–32]. The benefit of the latter is that these types of estimates do not require special software tools but can be employed using common DICOM viewers. However, if these estimations are used for patient specific considerations on which CI array to use, the corresponding CDL values must be accurate and reliable. In order to address both the impact of cochlear length variations onto cochlear implant surgery as well as the reliability of popular literature approaches to assess this variability, the proposed study was conducted.
Based on a large dataset of imaging data of CI patients, evaluations were performed on the distribution of CDL values, the correlation of electrode array length and the length of the cochlea covered by the respective array and suitability of specific CI arrays for certain ranges of CDL values. Following up on the study of Rivas et al. [33] who addressed the impact of A-value assessment deviations onto the electrode choice, it was further evaluated to which extent inaccuracies of the A-value method itself [30] would have led to a different choice of CI array than the respective contour tracings.
Materials and methods
Ethics statement
The ethics committee of the Hannover Medical School, Germany, approved this retrospective study. Due to the retrospective design, no written information was given to the patients of the study group. All patient data were anonymized and de-identified prior the retrospective analysis.
Subjects
At the Hannover Medical School, pre- and postoperative imaging of all CI patients is obtained by either Cone Beam CT (CBCT) or standard CT scans. We performed a retrospective study of 272 preoperative imaging datasets of patients who were implanted with a MED-EL FLEX20, FLEX24 or FLEX28 electrode between 2006 and 2017. Postoperative scans were available in 259 of these cases. Furthermore, patients who received custom-made devices (CMD) of 16mm length as well as 16 mm partial insertions of FLEX24 and 20 mm insertions with the FLEX28 were evaluated. Partial insertions were performed in an attempt to preserve residual hearing which were all considered CMD for the purpose of data analysis. Within the overall study group, most patients were implanted with a MED-EL FLEX28 (165 patients), 46 patients with a FLEX24, 52 patients with a FLEX20 and 12 patients with a MED-EL Flex CMD (see Fig 1). In our practice, pre- and postoperative imaging are a routine part of clinical care analyses.
Fig 1. Study case overview.
Overview of implanted electrode arrays which were included into this study. Note that the CMD group consists of FLEX16 electrode arrays and partial insertions of FLEX24 and FLEX28 electrodes).
Imaging data analysis
All datasets were evaluated using the DICOM-Viewer OsiriX MD (version 2.5.1 64bit, Pixmeo SARL, Switzerland). The corresponding analysis included the following steps:
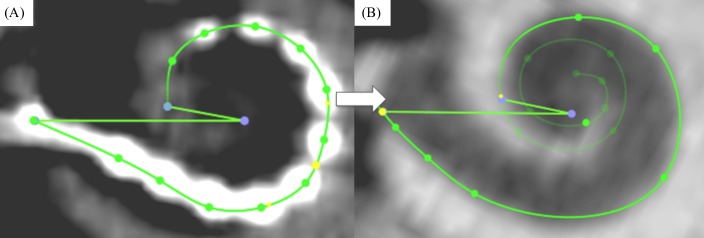
Tracings of the cochlear lateral wall from the center of the round window to the apex yield the corresponding CDL (see Figs 2 and 3)[15]. This is an automated feature within OsiriX MD. Performing one of these measurements takes approximately 2 minutes. An example is given in the supplementary material of this manuscript (see S1 Video).
Measurements of the basal turn diameter A as well as the cochlear angle (CA) (see Fig 4).
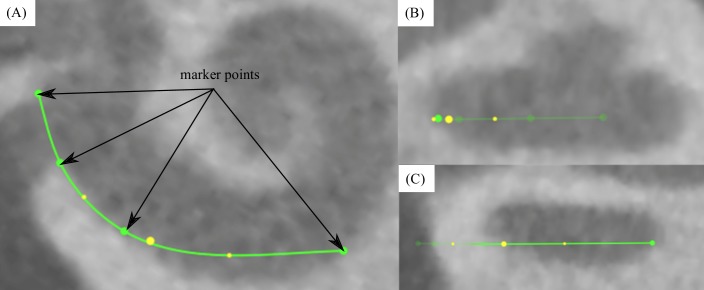
Measurement of the insertion angle (IA), defined as the angle from the center of the round window to the most apical contact (see Fig 5).
Tracings of the cochlear lateral wall in the preoperative scan (in order to avoid inaccuracies due to artifacts of the implanted array, see Fig 5) from the center of the round window to the insertion angle, yielding the covered cochlea length (CCL).
Measurement of the inserted electrode length (IEL) by placing marker points in the centers of all 12 electrode contacts as well as the entrance point of the electrode array in the round window (see Fig 5).
Computation of the individual cochlear coverage (CC) in percent by dividing the corresponding CCL by the respective CDL.
Fig 2. Spline measurement with OsiriX MD.
Visualization of lateral wall tracing in OsiriX MD in top (A) and side views (B, C).
Fig 3. Complete segmentation of the lateral wall.
Visualization of a completed lateral wall tracing in OsiriX MD (A, B) with the additional display of the enrolled path (C) for which the length is computed automatically.
Fig 4. Measurement of global cochlea dimensions.
Visualization of measurements of A (defined as the distance from the round window through the modiolus to the opposite wall of the cochlea) and B (maximal distance orthogonal to A).
Fig 5. Inclusion of postoperative imaging analysis.
(A) Postoperatively, marker points were placed in the center of the round window and along the inserted electrode array in the middle of the respective contact artefacts, and the corresponding insertion angle was measured. (B) The latter value was then used within the corresponding preoperative imaging data to determine the length along the lateral wall up to the insertion angle.
Measurement data analysis
After measurement data was acquired according to the methodology stated above, data analysis regarding the CDL and its estimation was performed in the following manner:
Estimation of the cochlea length using the estimation method of Escudé et al. [30] by using A and the average CA value of 900 deg (or 2.5 turns):
Comparison of the measured CDL values derived by the lateral wall tracings and the ones estimated using the above equation.
Results regarding the coverage of the cochlea with specific electrode arrays, corresponding anatomical indication ranges for each electrode array and clinically relevant evaluation errors were derived as follows:
Correlation of IEL and CCL in order to derive the relation between the length of the inserted electrode and the covered length of the cochlea.
Determining the CDL indication ranges for the different electrode arrays was based on (a) the previous correlation and (b) the manufacturer’s recommendation of 80% cochlear coverage (CC) [34].
Evaluating the accuracy of Escude’s method in terms of estimated CDL values suggesting the same CI array indication as the corresponding lateral wall measurements.
Statistical analysis
The data was statistically analyzed using IBM SPSS Statistics (Version 24.0.0.0). To test whether a normal distribution exists we used the Kolmogorov-Smirnov test.
Results
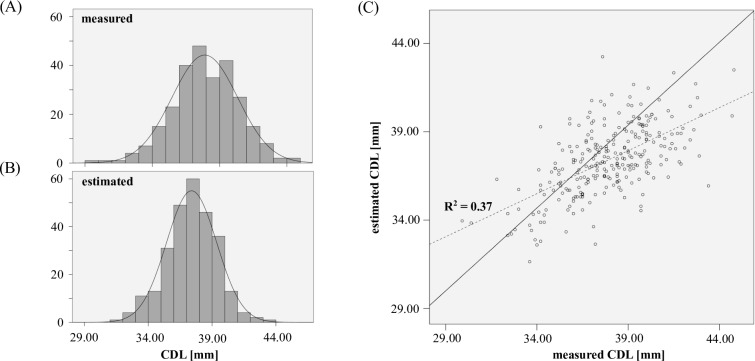
All measurement data can be found in S1 Table. Fig 6A and 6B show histograms of both measured and estimated CDLs of the 272 cochleae: In both cases a normal distribution was found with a mean length of 37.9mm and standard deviations of 2.4mm and 2.3mm respectively. The actual deviations of measurements and estimations are shown in Fig 6C: while the general trend of estimations and measurements is in agreement (R2 = 0.37), the mean absolute deviation was found to be at 1.4mm +/-1.1mm with a maximal deviation of 6.8mm.
Fig 6. CDL distribution.
Histograms of (A) measured and (B) estimated CDL values. (C) comparison of individual CDL measurements and estimations.
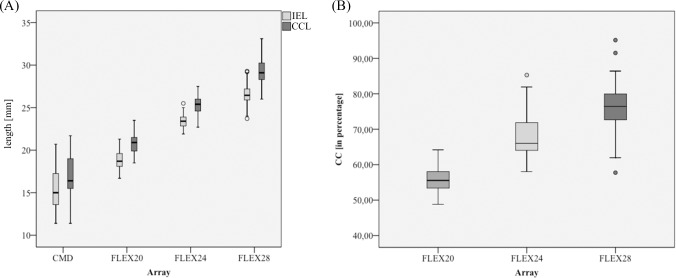
In order to evaluate the clinical relevance of these deviations, the postoperative measurement data was included into the analysis. Note that no tip fold over could be observed in any of the reviewed cases. Fig 7A shows the derived IEL and CCL values in a boxplot, grouped by the respective electrode array type. Means and the standard deviations of the IEL for the different arrays are 15.4mm +/-2.7mm for the CMD group, 18.7mm +/- 1.1 mm for the FLEX20, 23.5mm +/- 0.9 mm for the FLEX24 and 26.6mm +/- 1.1mm for the FLEX28. Mean values and standard deviations of the CCL are slightly larger (about 2 mm on average) than the respective IEL values with 20.8mm +/- 1.1mm for the FLEX20, 25.2mm +/- 1.2mm for the FLEX24 and 29.2mm +/-1.4mm for the FLEX28. The mean CCL of the CMD group was found to be 17mm +/- 2.9mm. Taking the preoperatively measured CDL into account, the individual cochlear coverage (CC) of the lateral wall can be calculated as the ratio of CCL and CDL, which is shown in Fig 7B. The achieved mean CC is 56% +/-3.5% for the FLEX20, 67.9% +/-6.1% for the FLEX24 and 76.4% +/-5.3% for the FLEX28. For the CMD devices a coverage of 46% +/- 7.6% was achieved. The CC variation for the longest electrode (FLEX28) ranged between 57.8% and 95.2% while it ranged from 58–85.3% for the FLEX24 and from 32.6–43.2% for the FLEX20.
Fig 7. Distribution of electrode specific CI outcomes.
(A) IEL and CCL distribution as well as (B) CC ranges for the different electrode array groups.
In order to correlate IEL and CCL, a scatter plot of the two is given in Fig 8A. Linear regression of these data points yielded the following correlation function:
Fig 8. Anatomical indication ranges.
(A) Depiction of the CCL range computed for a fully inserted (+/-5%) FLEX28 array, (B) visualization of the corresponding CDL indication ranges for FLEX24, FLEX28 and FLEXSoft electrode arrays.
The red area within the graph indicates (based on the example of a FLEX28 electrode array) the CCL range which can be expected for a successfully implanted array, if successful insertion is assumed to lie within +/- 5% of the array length that is supposed to be implanted (i.e. 28mm for a FLEX28 electrode array), the correlation function above can be employed to derive the corresponding CCL range. Table 1 shows the corresponding IEL and CCL ranges for different MED-EL electrode arrays suitable for patients without residual hearing. As mentioned before, the manufacturer recommends 80% CC (Mistrík & Jolly, 2016). Thus, translating the CCL to a clinical indicated range can be accomplished by dividing the derived CCL ranges by 0.8. The corresponding indicated ranges for the different arrays are listed in Table 1 and depicted in Fig 8B. Note that the indicated range for the FLEX28 array matches the peak of the derived CDL distribution.
Table 1. Indication range for the FLEX electrodes.
| Array | FLEX 24 | FLEX 28 | FLEX Soft |
|---|---|---|---|
| Array-length in mm | 24 | 28 | 31,5 |
| CCL in mm | 26.3 (25–27.5) |
30.5 (29–32) | 34.2 (32.5–35.9) |
| Recommended CDL (lateral wall) in mm | 32.9 | 38.2 | 42.7 |
| Indicated CDL (lateral wall) ranges (mm) | 31.3–34.4 | 36.2–40.1 | 40.6–44.9 |
| Recommended organ of Corti length (mm) | 26.5 | 30.5 | 34 |
| Indicated organ of Corti range (mm) | 25.3–27.7 | 29.1–32 | 32.4–35.7 |
Several publications refer to the CDL as the length of the organ of Corti (OC) and not the lateral wall. That is why the data of Hardy and Lee [5,31,35] was used to project the lateral wall indication ranges onto the organ of Corti (see Fig 9A). Linearly relating the CDL mean values +/- one standard deviation for lateral wall (37.9 mm +/- 2.4 mm) and organ of Corti (31.5 mm +/-2.3 mm) yielded the following equation:
Fig 9. Organ of Corti length.
(A) Histogram of the organ of Corti (OC) derived by Hardy and Lee (red) and MHH data (black), (B) Histogram of calculated OC length for MHH data with electrode lengths for FLEX24, FLEX28 and FLEXSoft (+/- 5%).
This equation was used to translate the lateral wall indication ranges into the ones for organ of Corti, which are displayed in Fig 9B and stated within the last two rows of Table 1.
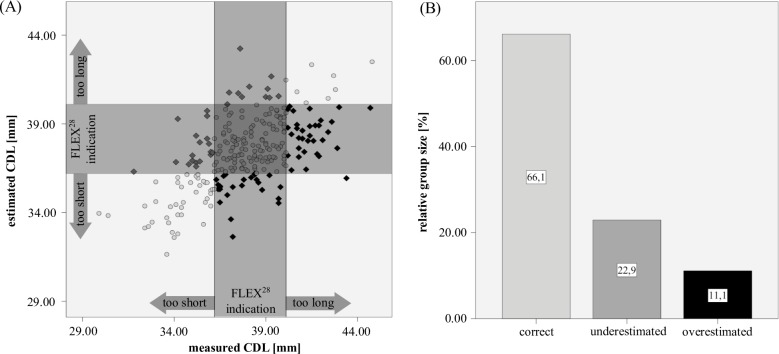
Finally, we evaluated if CDL estimations using the A value and CA [30] could be used instead of actual measurements to derive an accurate anatomical indication for a specific array. Note that only the length of the cochlea and not the length of the implanted array were taken into account for this analysis. Using the FLEX28 implanted group we then analyzed to what extent the CDL estimation method would predict cochlear anatomies as too short, applicable or too long for this array. Within Fig 10A, the x-axis represents the measured CDL whereas the y-axis shows the corresponding estimations. The shaded areas within this graph represent the FLEX28 indication ranges for measured and estimated CDL values respectively (3rd column of Table 1). Correct identification, i.e. a match of measured and estimated indication (highlighted in the table), of short cochleae could be achieved in 67.8%, of long cochleae in 17.1% and of matching cochleae in 77.2% of the cases (see Table 2 and Fig 10B).
Fig 10. CDL identification of FLEX28 candidates.
(A) the comparison of measurement and estimation-based identifications of FLEX28 candidates resulted in (B) correct identifications in 66% of the analyzed anatomies using Escudé’s method.
Table 2. Correct identification of cochlea length.
| Reference small | Reference match | Reference large | Correct Identification | |
|---|---|---|---|---|
| Estimation large | 0 | 11 | 7 | 7 |
| Estimation match | 19 | 132 | 33 | 132 |
| Estimation small | 40 | 28 | 1 | 40 |
| total number | 59 | 171 | 41 | 271 |
| correct identification in percentage | 67.8 | 77.2 | 17.1 | 66.1 |
Discussion
In our study, we analyzed the anatomy of the cochlea in 272 clinical imaging datasets. The postoperative location of different lateral wall electrode arrays could be assessed in 259 of these 272 cases. The derived variability of the cochlea length is in agreement with previous studies [4,5,15–17] and highlights again the importance of considering the patient specific anatomy in the field of cochlear implantation (see Fig 6A).
The projection of the wide portfolio of available electrode arrays [3] onto this range of cochlear length values then allows for optimal coverage of the intracochlear neural structures: achieving 80% CC with lateral wall electrodes in case of an average or long cochlea, for instance, would currently only be achievable with MED-EL devices who also offer electrode array lengths of more than 26mm. However, it was not yet clearly proven if electrode arrays stimulate the neural fiber endings at the organ of Corti or the spiral ganglion cells directly, i.e. what cochlear coverage achieves the best possible speech perception. Nevertheless, recent publications show superior speech understanding for the FLEX28 array (which typically achieves insertion angles of 540–720 degrees) in comparison to shorter electrode arrays [24]. The derived indication ranges yield a possible explanation for this finding, i.e. the sufficient coverage of neural structures for most cochleae with the FLEX28 array.
The comparison of measured and estimated [30] CDLs showed normal distributions in either case with no significant differences, but quite severe deviations were found for individual cases (see Fig 6C) with deviations of up to 6.8mm. Hence, the CDL estimation method described by Escudé et al. [30] may be applicable for retrospective analysis but is not suitable for clinical use where accuracy matters for each and every individual case. This was further highlighted in Fig 10B where the estimation deviations were evaluated in terms of anatomical indications for specific CI electrode array lengths: the results show that in total, correct indications could only be derived in 66% of the analyzed cochleae. Especially concerning are the 11% of the cochleae whose CDL values were overestimated: in a clinical setting, overestimations could result in implantations of electrode arrays too long for a specific cochlea such that the array cannot be fully inserted and/or possibly cause intracochlear damage. Underestimations, on the other hand, were found to occur in 22% of the cases and could entail insufficient coverage of the cochlear neurons and hence result in poor speech understanding, since studies show that longer electrodes result in a better speech understanding for electric stimulation only [23,24].
The IEL and corresponding CCL values for the analyzed electrode arrays are shown in Fig 7A. CCL values are larger than the according IEL values for each electrode array due to the latter being located in closer proximity to the modiolus than the lateral wall and thus representing a shorter distance. The projection of the array from the modiolus out onto the lateral wall hence creates a larger spiral profile and an accordingly larger CCL value. The group of CMDs shows the highest deviation regarding the IEL to CCL correlation. This fact is owed to the CMD group containing FLEX24 and FLEX28 arrays which were only partially inserted (covering only the individual frequency region with severe to profound hearing loss) as well as custom made devices of 16mm length.
While CC increases for longer electrodes overall (see Fig 7B), it can be seen that the CC distributions overlap especially regarding the FLEX24 and FLEX28 arrays. This can be explained by the fact that the CC is not only dependent on the length of the electrode array but also on the length of the cochlea [27]. In some cases, a FLEX24 in a short cochlea may therefore cover a larger fraction of the cochlea than a FLEX28 in a large cochlea. It should further be noted that the smallest CC values can be found within the FLEX20 group because this electrode was developed to not cover the full frequency range of the cochlea in order for patients to benefit from acoustic stimulation in the low frequencies using an EAS system.
Variations in CC values generated with the same array can also be seen in Fig 8A. Although the overall cochlea length does not directly influence CCL values, it can be assumed that the same kind of array will be situated differently in a small cochlea than in a large one. Variations in cochlear anatomy are therefore likely to also influence the length of the cochlea covered by an electrode array.
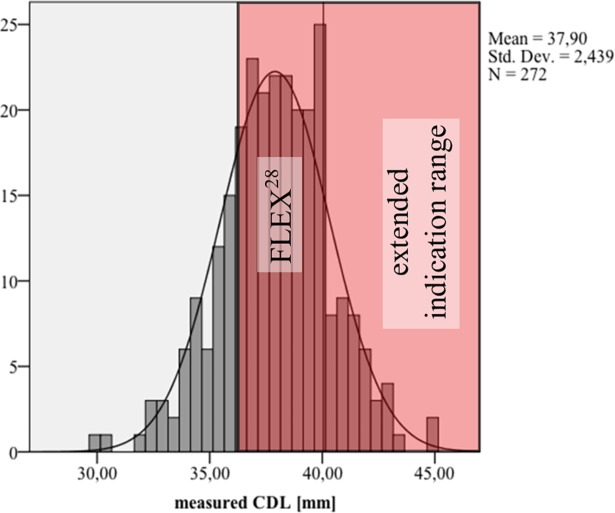
Regarding the anatomical indication ranges it was found based on the measured CDL that most cochleae are covered by the FLEX28 with a target CDL range of 36.2mm to 40.1mm (11+132+28 of the 271 cases corresponding to 63% of the analyzed cases). Without knowing the anatomy, a FLEX28 electrode also seems to be the best choice since it could be implanted in 76.8% (see Fig 11) of the cases with a reduced risk of harming any intracochlear structures.
Fig 11. Extended indication range for MED-EL FLEX28.
Structure preservation indication range for the FLEX28 array which could have been fully inserted in 76% of the investigated cases.
Conclusion
Following previous investigations, we showed that due to the variation of cochlear size, preoperative cochlea length assessment should be included into the routine preoperative care. Furthermore, individual implant selection based on the patient specific cochlea length is feasible and likely to improve implantation outcomes. However, length estimations exclusively based on mathematical correlations may result in false recommendations and could potentially injure the cochlea and lead to poorer outcomes. Furthermore, the presented results demonstrate that most cochleae are sufficiently covered by a FLEX28 electrode array while the FLEX24 and FLEXSoft should be employed for short and long cochleae respectively.
Supporting information
Preoperative measurement of a cochlear duct length (CDL) in a Cone Beam CT scan (CBCT).
(MOV)
(XLSX)
Acknowledgments
We are thankful to Daniel Schurzig, Ph.D., MED-EL, for his scientific support in this study.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by the German ministry for research and education (BMBF) under FKZ 13GW0160B "my-CI" and MED-EL Deutschland GmbH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. MedEl Deutschland GmbH paid for scientific congress charge, traveling and hotel costs for Max Eike Timm and Thomas Lenarz. No other author received specific funding for this work.
References
- 1.Clark G. Cochlear Implants: Fundamental and Applications. Spinger Verlag; 2003. [Google Scholar]
- 2.Rubinstein JT. How cochlear implants encode speech.:444–8. [DOI] [PubMed] [Google Scholar]
- 3.Dhanasingh A, Jolly C. An overview of cochlear implant electrode array designs. Hear Res [Internet]. 2017;356:93–103. Available from: 10.1016/j.heares.2017.10.005 [DOI] [PubMed] [Google Scholar]
- 4.Van Der Marel KS, Briaire JJ, Wolterbeek R, Snel-Bongers J, Verbist BM, Frijns JHM. Diversity in cochlear morphology and its influence on cochlear implant electrode position. Ear Hear. 2014;35(1):9–20. [DOI] [PubMed] [Google Scholar]
- 5.Hardy M. The length of the organ of Corti in man. Am J Anat [Internet]. 1938. January;62(2):291–311. Available from: http://doi.wiley.com/10.1002/aja.1000620204 [Google Scholar]
- 6.Erixon E, Högstorp H, Wadin K, Rask-Andersen H. Variational Anatomy of the Human Cochlea. Otol Neurotol [Internet]. 2009;30(1):14–22. Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00129492-200901000-00003 10.1097/MAO.0b013e31818a08e8 [DOI] [PubMed] [Google Scholar]
- 7.Ketten DR, Skinner MW, Wang G, Vannier MW, Gates GA, Neely JG. In vivo measures of cochlear length and insertion depth of nucleus cochlear implant electrode arrays. Ann Otol Rhinol Laryngol Suppl [Internet]. 1998. November;175:1–16. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9826942 [PubMed] [Google Scholar]
- 8.Lee J, Nadol JB, Eddington DK. Depth of electrode insertion and postoperative performance in humans with cochlear implants: A histopathologic study. Audiol Neurotol. 2010;15(5):323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meng ÃJ, Li ÃS, Zhang ÃF, Li Q, Qin ÃZ. Cochlear Size and Shape Variability and Implications in Cochlear Implantation Surgery. 2016;1307–13. [DOI] [PubMed] [Google Scholar]
- 10.Miller JD. Sex differences in the length of the organ of Corti in humans. J Acoust Soc Am [Internet]. 2007. April;121(4):EL151–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17471760 [DOI] [PubMed] [Google Scholar]
- 11.Verbist BM, Ferrarini L, Briaire JJ, Zarowski A, Admiraal-behloul F, Olofsen H, et al. Anatomic Considerations of Cochlear Morphology and Its Implications for Insertion Trauma in Cochlear Implant Surgery. 2009; [DOI] [PubMed] [Google Scholar]
- 12.Walby AP. Scala tympani measurement. Ann Otol Rhinol Laryngol [Internet]. 94(4 Pt 1):393–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/3896104 [PubMed] [Google Scholar]
- 13.Wright A, Davis A, Bredberg G, Ülehlová L, Spencer H, Davis A, et al. Hair Cell Distributions in the Normal Human Cochlea: A Report of a European Working Group a i r Cell Distributions in the Normal Human Cochlea. 2009;6489. [Google Scholar]
- 14.Wysocki J. Dimensions of the human vestibular and tympanic scalae. Hear Res. 1999;135(1–2):39–46. [DOI] [PubMed] [Google Scholar]
- 15.Würfel W, Lanfermann H, Lenarz T, Majdani O. Cochlear length determination using Cone Beam Computed Tomography in a clinical setting. Hear Res [Internet]. 2014. October;316:65–72. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0378595514001312 10.1016/j.heares.2014.07.013 [DOI] [PubMed] [Google Scholar]
- 16.Ulehlová L, Voldrich L, Janisch R. Correlative study of sensory cell density and cochlear length in humans. Hear Res [Internet]. 1987;28(2–3):149–51. Available from: http://www.ncbi.nlm.nih.gov/pubmed/3654386 [DOI] [PubMed] [Google Scholar]
- 17.Pelliccia P, Venail F, Bonafé A, Makeieff M, Iannetti G, Bartolomeo M, et al. Cochlea size variability and implications in clinical practice Variabilità delle dimensioni cocleari e sue implicazioni nella pratica clinica. ACTA Otorhinolaryngol Ital. 2014;34:42–9. [PMC free article] [PubMed] [Google Scholar]
- 18.Avci E, Nauwelaers T, Lenarz T, Hamacher V, Kral A. Variations in Microanatomy of the Human Cochlea. 2014;3261:3245–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adunka O, Unkelbach MH, Mack MG, Radeloff A, Gstoettner W. Predicting basal cochlear length for electric-acoustic stimulation. Arch Otolaryngol—Head Neck Surg. 2005;131(6):488–92. 10.1001/archotol.131.6.488 [DOI] [PubMed] [Google Scholar]
- 20.Biedron S, Prescher A, Ilgner J, Westhofen M. The Internal Dimensions of the Cochlear Scalae With Special Reference to Cochlear Electrode Insertion Trauma. 2010;731–7. [DOI] [PubMed] [Google Scholar]
- 21.Bredberg G, Teti A, Zambonin Zallone A, Lundevall E, Iurato S. Ultrastructural evaluation of the microslicing method for the study of temporal bone pathology. Acta Otolaryngol Suppl [Internet]. 1987;436:7–14. Available from: http://www.ncbi.nlm.nih.gov/pubmed/3314328 [DOI] [PubMed] [Google Scholar]
- 22.Cakir A, Labadie RF, Zuniga MG, Dawant BM, Noble JH. Evaluation of Rigid Cochlear Models for Measuring Cochlear Implant Electrode Position. Otol Neurotol [Internet]. 2016. December;37(10):1560–4. Available from: https://insights.ovid.com/crossref?an=00129492-201612000-00016 10.1097/MAO.0000000000001245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Connell BP, Hunter JB, Haynes DS, Holder JT, Dedmon MM, Noble JH, et al. Insertion depth impacts speech perception and hearing preservation for lateral wall electrodes. Laryngoscope [Internet]. 2017;1–6. Available from: http://doi.wiley.com/10.1002/lary.26467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Büchner A, Illg A, Majdani O, Lenarz T. Investigation of the effect of cochlear implant electrode length on speech comprehension in quiet and noise compared with the results with users of electro-acoustic-stimulation, a retrospective analysis. PLoS One. 2017;12(5):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Connell BP, Cakir A, Hunter JB, Francis DO, Noble JH, Labadie RF, et al. Electrode Location and Angular Insertion Depth Are Predictors of Audiologic Outcomes in Cochlear Implantation. Otol Neurotol [Internet]. 2016. September;37(8):1016–23. Available from: https://insights.ovid.com/crossref?an=00129492-201609000-00004 10.1097/MAO.0000000000001125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Seta D, Nguyen Y, Bonnard D, Ferrary E, Godey B, Bakhos D, et al. The Role of Electrode Placement in Bilateral Simultaneously Cochlear-Implanted Adult Patients. Otolaryngol Neck Surg [Internet]. 2016. September 22;155(3):485–93. Available from: http://journals.sagepub.com/doi/10.1177/0194599816645774 [DOI] [PubMed] [Google Scholar]
- 27.Franke-Trieger A, Jolly C, Darbinjan A, Zahnert T, Mürbe D. Insertion Depth Angles of Cochlear Implant Arrays With Varying Length. Otol Neurotol. 2014; [DOI] [PubMed] [Google Scholar]
- 28.Liu Y-K, Qi C-L, Tang J, Jiang M-L, Du L, Li Z-H, et al. The diagnostic value of measurement of cochlear length and height in temporal bone CT multiplanar reconstruction of inner ear malformation. Acta Otolaryngol [Internet]. 2017. February;137(2):119–26. Available from: https://www.tandfonline.com/doi/full/10.1080/00016489.2016.1221132 [DOI] [PubMed] [Google Scholar]
- 29.Lexow GJ, Schurzig D, Gellrich N-C, Lenarz T, Majdani O, Rau TS. Visualization, measurement and modelling of the cochlea using rotating midmodiolar slice planes. Int J Comput Assist Radiol Surg [Internet]. 2016. October 19;11(10):1855–69. Available from: http://link.springer.com/10.1007/s11548-016-1374-7 [DOI] [PubMed] [Google Scholar]
- 30.Escudé B, James C, Deguine O, Cochard N, Eter E, Fraysse B. The size of the cochlea and predictions of insertion depth angles for cochlear implant electrodes. Audiol Neurotol. 2006;11(SUPPL. 1):27–33. [DOI] [PubMed] [Google Scholar]
- 31.Alexiades G, Dhanasingh A, Jolly C. Method to estimate the complete and two-turn cochlear duct length. Otol Neurotol. 2015;36(5):904–7. 10.1097/MAO.0000000000000620 [DOI] [PubMed] [Google Scholar]
- 32.Koch RW, Ladak HM, Elfarnawany M, Agrawal SK. Measuring Cochlear Duct Length—A historical analysis of methods and results. J Otolaryngol—Head Neck Surg. 2017;46(1):1–11. 10.1186/s40463-016-0180-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rivas A, Cakir A, Hunter JB, Labadie RF, Zuniga MG, Wanna GB, et al. Automatic Cochlear Duct Length Estimation for Selection of Cochlear Implant Electrode Arrays. Otol Neurotol [Internet]. 2017;38(3):339–46. Available from: http://insights.ovid.com/crossref?an=00129492-201703000-00005 10.1097/MAO.0000000000001329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mistrík P, Jolly C. Optimal electrode length to match patient specific cochlear anatomy. Eur Ann Otorhinolaryngol Head Neck Dis. 2016;133:S68–71. 10.1016/j.anorl.2016.05.001 [DOI] [PubMed] [Google Scholar]
- 35.Lee J, Nadol JB Jr., Eddington DK. Depth of Electrode Insertion and Postoperative Performance in Humans with Cochlear Implants: A Histopathologic Study. Audiol Neurotol [Internet]. 2010;15(5):323–31. Available from: https://www.karger.com/Article/FullText/289571 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Preoperative measurement of a cochlear duct length (CDL) in a Cone Beam CT scan (CBCT).
(MOV)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.