Abstract
Objective
Necrotizing enterocolitis (NEC) is the most common surgical emergency in preterm infants, and pathogenesis associates with changes in the fecal microbiome. As fecal samples incompletely represent microbial communities in intestinal mucosa, we sought to determine the NEC tissue-specific microbiome and assess its contribution to pathogenesis.
Design
We amplified and sequenced the V1-V3 hypervariable region of the bacterial 16S rRNA gene extracted from intestinal tissue and corresponding fecal samples from 12 surgical patients with NEC and 14 surgical patients without NEC. Low quality and non-bacterial sequences were removed, and taxonomic assignment was made with the Ribosomal Database Project. Operational taxonomic units were clustered at 97%. We tested for differences between NEC and non-NEC samples in microbiome alpha- and beta-diversity and differential abundance of specific taxa between NEC and non-NEC samples. Additional analyses were performed to assess the contribution of other demographic and environmental confounding factors on the infant tissue and fecal microbiome.
Results
The fecal and tissue microbial communities were different. NEC was associated with a distinct microbiome, which was characterized by low diversity, higher abundances of Staphylococcus and Clostridium_sensu_stricto, and lower abundances of Actinomyces and Corynebacterium. Infant age and vancomycin exposure correlated with shifts in the tissue microbiome.
Conclusion
The observed low diversity in NEC tissues suggests that NEC is associated with a bacterial bloom and a distinct mucosal bacterial community. The exact bacterial species that constitute the bloom varied by infant and were strongly influenced by age and exposure to vancomycin.
Introduction
Necrotizing enterocolitis (NEC) is a common and frequently fatal intestinal complication in premature infants [1,2]. Experiments in germ-free animals and toll-like receptor targeted knock out mice strongly suggest a bacterial antigen is critical for the initiation of intestinal inflammation and NEC development [3–6]. Bacterial DNA is present in larger quantities in acute human NEC specimens compared to samples collected after NEC has clinically resolved [7]. A number of different gram-positive and gram-negative bacteria as well as viruses have been associated with NEC [8]. Indeed, microbial community studies using 16S rRNA gene sequencing of the fecal microbiome demonstrate a reduction in microbial community diversity with a shift towards potentially pathogenic subgroups [9–12].
We previously detected significant differences in the microbiome between surgical tissue and parallel collected fecal samples in preterm infants without NEC [13]. We hypothesized the existence of a specific microbial profile at the site of injury in the small intestinal mucosa of premature infants with NEC that has not been previously recognized in fecal microbiome studies. Hence, we sought to interrogate differences in the tissue-level and fecal microbiomes in infants with and without NEC to determine bacterial communities at the site of injury and their representation in feces. As intestinal tissue cannot ethically be collected from healthy infants, we included infants with intestinal diseases other than NEC in this study for comparison. We detected a statistically significant increase in the abundance of Staphylococcus and Clostridium_sensu_stricto in NEC compared to non-NEC tissue samples when controlling for age and history of antibiotic exposure.
Materials and methods
Ethics statement
This study was approved by the Vanderbilt University Institutional Review Board (protocol number 090161). All infants hospitalized at the Monroe Carell Jr. Children’s Hospital at Vanderbilt were eligible for the study if they underwent intestinal resection at <180 days of age. We obtained written informed consent from parents, the next of kin, caretakers, or guardians on behalf of the minors/children enrolled in the study to permit collection of tissue and metadata from the medical records including gestational age, birth weight, race, sex, mode of delivery, maternal or fetal indications for delivery, antibiotic exposure, enteral feeding regimens, diagnoses and type of surgical resection.
Sample collection
Tissue collected at the time of surgery was gently rinsed with sterile saline solution, and immediately cryopreserved in sterile containers [13]. Fecal material was collected by either taking the patient’s first post-operative stool or by scraping surgical tissue; samples were immediately cryopreserved (Table 1). The clinical and intraoperative diagnosis of NEC was confirmed by a pediatric pathologist after histologic examination of the resected specimen and by review of the operative and surgical pathology reports.
Table 1. Clinical characteristics of patient samples.
| Sample ID | Indication for intestinal resection | Gestational age (wks) | Birth weight (g) | Sex | Age at surgery (d) | Tissue type | Mode of delivery | Feeding | Preoperative antibiotics (d)* | Antibiotic type† | Sample types included in analysis |
|---|---|---|---|---|---|---|---|---|---|---|---|
| N4 | NEC | 26 | 780 | F | 6 | Ileum | Vaginal | NPO | 2 | A, G, V, MZ | Fecal, tissue |
| N6 | NEC | 29 | 1,630 | M | 46 | Colon | Vaginal | NPO | 22 | V, G, P | Fecal, tissue |
| N9 | NEC | 28 | 850 | M | 38 | Jejunum | C-section | EBM | 2 | A, G | Fecal, tissue |
| N20 | NEC | 33 | 1,740 | M | 5 | Colon | Vaginal | Formula | 5 | A, G | Tissue |
| N27 | NEC | 25 | 440 | F | 27 | Ileum | C-section | EBM | 1 | V, G, M | Fecal‡, tissue |
| N33 | NEC | 30 | 1,583 | F | 7 | Ileum | C-section | Formula | 6 | A, G, V, C, MZ | Fecal, tissue |
| N34 | NEC | 30 | 1,550 | M | 9 | Jejunum | Vaginal | Formula | 3 | A, G, V, MZ | Fecal, tissue |
| N37 | NEC | 25 | 650 | F | 8 | Ileum | C-section | EBM | 3 | A, G, V | Fecal, tissue |
| N39 | NEC | 33 | 2,101 | F | 8 | Ileum | Vaginal | Formula | 6 | A, G, MZ | Fecal, tissue |
| N324 | NEC | 30 | 1,420 | F | 11 | Ileum | C-section | Formula | 1 | A, G, V, P | Tissue |
| C22 | Spontaneous perforation | 25 | 850 | M | 6 | Ileum | Vaginal | NPO | 6 | A, G, MZ | Fecal, tissue |
| C23 | Spontaneous perforation | 24 | 650 | F | 1 | Ileum | Vaginal | NPO | 1 | A, G, MZ, M | Fecal |
| C18 | Congenital volvulus | 31 | 1,400 | F | 1 | Ileum | C-section | NPO | 1 | A, G | Fecal, tissue |
| C28 | Congenital volvulus | 32 | 2,590 | F | 0 | Ileum | C-section | NPO | 0 | A, G, MZ | Fecal, tissue |
| C17 | Mesenteric ischemia | 26 | 700 | M | 6 | Jejunum | C-section | EBM | 6 | A, G | Fecal, tissue |
| C5 | Intestinal atresia | 35 | 2,605 | M | 3 | Ileum | Vaginal | NPO | 2 | A, G | Fecal‡, tissue |
| C8 | Intestinal atresia | 34 | 2,015 | F | 5 | Jejunum | Vaginal | NPO | 5 | A, G | Fecal, tissue |
| C24 | Stricture removal | 28 | 1,268 | M | 57 | Jejunum | Vaginal | Formula | 17 | A, G, CL, V | Fecal, tissue |
| C26 | Stricture removal | 29 | 1,664 | M | 43 | Ileum | C-section | NPO | 18 | A, G, CL, V | Fecal, tissue |
| C19 | Stricture removal | 39 | 3,454 | F | 132 | Colon | Vaginal | NPO | 131 | A, G, C, CP, CT, M, V | Fecal, tissue |
| C16 | Hirschsprung’s disease | 36 | 2,793 | M | 4 | Colon | Vaginal | NPO | 0 | None | Tissue |
| C14 | Re-anastomosis | 27 | 830 | F | 60 | Ileum | Vaginal | Formula | 14 | A, G, C, MZ, V | Fecal, tissue |
| C15 | Re-anastomosis | 26 | 790 | F | 65 | Ileum | C-section | Formula | 12 | A, G, MZ, P, V | Fecal, tissue |
| C21 | Re-anastomosis | 32 | 1,660 | M | 57 | Ileum | Vaginal | Formula | 24 | A, G, M, V, T, CL | Fecal, tissue |
* 0, less than 24 hours
† A, ampicillin; G, gentamicin; V, vancomycin; C, cefotaxime; CL, clindamycin; CP, cefepime; CT, ceftriaxone; M, meropenem; P piperacillin-tazobactam; MZ, metronidazole; T, tobramycin
‡ Feces adherent to collected mucosa; all other fecal samples collected at patient’s first post-operative stool
NEC, necrotizing enterocolitis; NPO, nil per mouth; EBM, expressed breast milk; DBM, donor breast milk
DNA extraction and amplification of 16S rRNA gene
We extracted DNA from fresh NEC and non-NEC surgical tissue and corresponding fecal samples as previously described [13]. Briefly, we extracted DNA from 15–25 mg of intestinal tissue and 180–200 mg of feces and amplified the V1-V3 hypervariable region of bacterial 16S rRNA with previously validated primers: 5F (5’-TGGAGAGTTTGATCCTGGCTCAG-3’) and 532R (5’-TACCGCGGCTGCTGGCAC-3’) [14]. PCR was conducted as described [13] and barcoded amplicons were gel purified (Qiagen), quantified, and pooled prior to sequencing on a 454 FLX Titanium sequencer. Sequencing negative controls—template-free sterile water, processed with the same DNA extraction and PCR amplification kits as the real samples—were sequenced on the same run [15].
Pyrosequencing and data analysis
Sequences generated from the pyrosequencing of barcoded 16S rRNA gene PCR amplicons were analysed using mothur (http://mothur.org) [16] by following the 454 SOP as of 13 March 2017. Sequences were aligned to the SILVA database release 123 [17] and taxonomically classified with the Ribosomal Database Project (RDP) classifier 11 [18]. Chimeric sequences as detected by UCHIME were removed [19]. OTUs were clustered at 97% similarity. Prior to statistical analysis, samples with <400 reads were discarded (N = 3).
Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) was used to predict metagenomic and functional composition of the samples from 16S rRNA sequences [20]. Prior to PICRUSt analyses, closed reference OTUs were picked against the GreenGenes database 13_5 [21] using uclust [22] in QIIME 1.9.1 [23]; taxonomy assignments were made using the RDP Classifier 2.2 [24]. Functions of genes were assigned using the KEGG Orthology database [25].
Statistical analysis was performed in R using MGSAT (https://bitbucket.org/andreyto/mgsat), which wraps a number of R packages, including vegan [26] to perform alpha- and beta- diversity analyses and DESeq2 [27], GeneSelector [28], and stabsel [29] for testing taxonomic associations with metadata. When testing taxonomic associations with metadata, we report the q-values computed with the Benjamini & Hochberg false discovery rate method to adjust for multiple comparisons [30].
For diversity and richness estimates, full count matrices as produced by the mothur annotation were used [31]. To compare microbial alpha diversity estimates between groupings, we estimated Shannon-Wiener (H') and Simpson’s diversity indices; to compare microbial richness estimates, we estimated observed OTUs and calculated S. chao1 estimates. Counts were rarefied to the lowest library size of all the samples (number of reads per sample = 445), and then abundance-based and incidence-based alpha diversity indices and richness estimates were computed. This was repeated multiple times (n = 400), and the results were averaged. Incidence-based estimates were computed on pools of observations split by the relevant metadata attribute, and in each repetition, observations were also stratified to balance the number of observations at each level of the metadata attribute. Inverted Simpson and Shannon diversity indices were converted into corresponding Hill numbers [32]. Linear models were fit to test for associations between abundance-based richness and diversity estimates and metadata attributes.
We applied the PermANOVA (permutation-based analysis of variance) [33] test of statistical significance (as implemented in the Adonis function of the R vegan package) [34] on the association between the abundance profile dissimilarities and the metadata variables. We used the Bray-Curtis dissimilarity index [35] and 4000 permutations. The counts were normalized to simple proportions within each observation.
When differential abundance analysis was performed, in order to remove the likely non-informative features and to reduce the associated penalty from the multiple testing correction applied after univariate tests, we used unbiased metadata-independent filtering at each taxonomy level by eliminating all taxa that were detected with a mean proportional abundance of less than 0.0005. The absolute counts from the removed features were aggregated into a category “other,” which was taken into an account when computing simple proportions during data normalization, but were otherwise discarded. When testing taxonomic associations with metadata, for each feature, we also obtained, from the same test done on the full dataset, the p-value computed using the test implementation from R exactRankTests package [36], the q-value computed with the Benjamini & Hochberg false discovery rate method in the package function p.adjust [37], and several types of the effect size such as common language effect size and rank biserial correlation [38]. To evaluate the influence of confounders, models were built in DESeq2 with pre-selected covariates added in.
Stabsel is a stability selection approach implemented in the R package stabs [29]. This feature selection method implements a stability selection procedure described in [39] with the improved error bounds described in [40]. Elastic net (from R package glmnet [41]) was used as the base feature selection method that was wrapped by the stability protocol. For groupings with two factor levels, a binomial family model was built with the grouping as a response and the matrix of the abundance values as predictors. The mixing parameter α of glmnet was selected based on a 15-fold cross-validation minimizing deviance on the full dataset. The predictors were first normalized to simple proportions within each multivariate observation, transformed with the inverse hyperbolic sign , and then standardized to zero means and unit variances. With its multivariate base feature selection method, this protocol can potentially detect those correlated groups of biologically relevant features that will be missed by the univariate methods. The ranking of taxa and their probability of being selected into the model were reported, as well as the probability cutoff corresponding to the per-family error rate (PFER) that is controlled by this method. Our PFER cutoff was set to 0.05, and the target number of features selected by the base classifier was set to where p is the total number of features (39). In our experience with omics datasets, the PFER control in this method is fairly conservative, and we typically look at the ranking of features as opposed to only concentrating on features that pass the PFER cutoff.
Data deposition
All sequences reported in this paper have been deposited into the NCBI sequence short read archive (accession no. SRR7993700-SRR7993745).
Results
Demographic and antimicrobial exposure characteristics were similar between NEC and non-NEC infants
We collected and analyzed fresh surgical tissue and corresponding fecal samples from 10 patients with NEC and 14 patients without NEC; in total, 44 samples were analyzed (fecal N = 21; tissue N = 23) (Table 1). Surgical samples included patients with spontaneous intestinal perforations, ileal and jejunal atresias, midgut volvulus, and mesenteric ischemic bowel injuries. Mean gestational age, birth weight and postnatal age were 29 weeks (range 25–33 weeks), 1,274 grams (range 440–2,101 grams), and 17 days (range 5–46 days) for NEC infants and 30 weeks (range 24–39 weeks), 1,662 grams (range 650–3,454 grams), and 31 days (0–132 days) for non-NEC patients, respectively (all t-tests p>0.05). Female infants represented 60% and 50% of the study population in the NEC and non-NEC groups, respectively. Except for two colon samples among the non-NEC group and two colon samples within the NEC group, all analyzed tissues were from the ileum or jejunum. For the non-NEC group, one fecal sample (C5) was adherent to the mucosa when collected, for the NEC group there was one (N27). All but one infant from the non-NEC group had perinatal antibiotic exposure. Mean number of antibiotic exposure days prior to surgery were less in the NEC group (5 days, range 1–22) compared to the non-NEC group (17 days, range 0–131) but means were not statistically different (t-test with Welch’s correction, p = 0.180). Both the NEC and non-NEC groups contained infants receiving breast milk, infant formula, or no enteral nutrition prior to sample collection. Of non-NEC infants, 36% were delivered via C-section compared to 50% of infants in the NEC group.
Microbial diversity was reduced in NEC samples compared to non-NEC samples
After quality filtering and removal of chimeras and non-bacterial sequences, barcoded 16S rRNA amplicons generated a total of 59,778 sequences for fecal and 72,791 sequences for tissue samples. The mean (range) number of fecal sample sequences was 4,719 (697–15,319) for NEC and 2,510 (589–6,530) for non-NEC subjects, and the mean (range) number of tissue sample sequences was 2,322 (445–6,066) sequences for NEC and 2,799 (634–7,906) for non-NEC subjects.
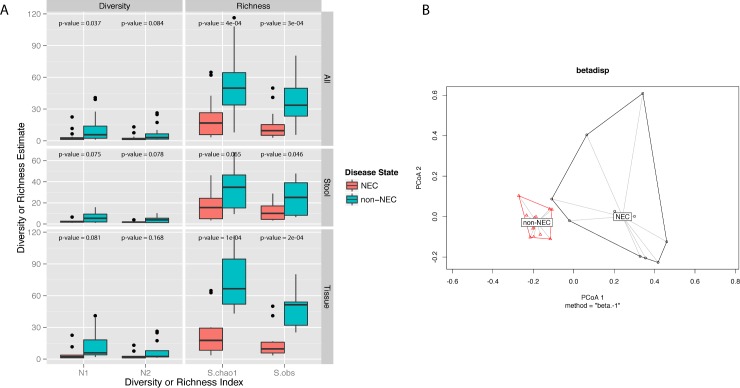
Prior to estimating microbial alpha-diversity or richness, samples were rarefied to the lowest library size of all the samples (445 reads per sample). When testing for a non-zero coefficient of a normal linear model that used NEC/non-NEC group membership as predictor of richness, microbial richness and diversity were lower in NEC samples compared to non-NEC samples (Fig 1A). In tissue samples, when comparing microbial richness or diversity in tissue from NEC and non-NEC subjects, p-values for all tested richness estimates were <0.05 and there was a trend towards lower alpha diversity estimates in tissue from infants with NEC compared to those without NEC (p-values: N1 = 0.081, N2 = 0.168) (Fig 1A). Both microbial richness (observed OTU counts (S.obs) p-value = 0.046, S.Chao1 p-value = 0.065) and alpha diversity (N1 p-value = 0.075, N2 p-value = 0.078) were at or near significantly lower in stool from infants with NEC compared to those without (Fig 1A).
Fig 1.
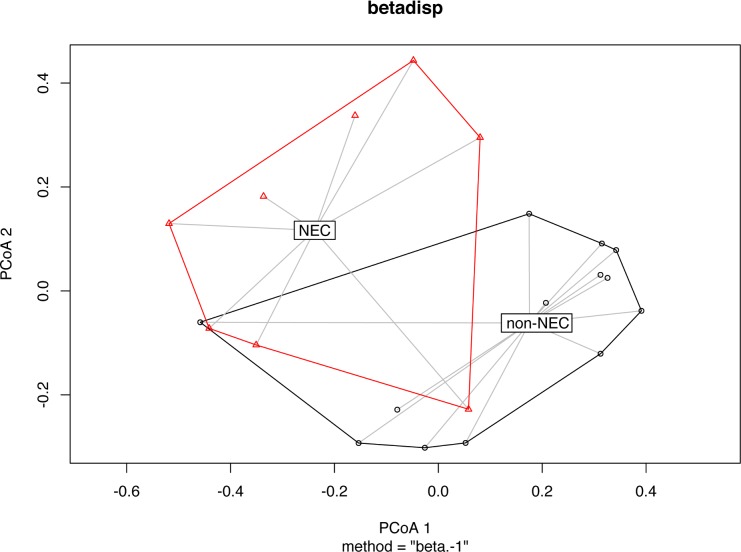
A) Boxplots of tissue microbial diversity and richness in infants with and without necrotizing enterocolitis (NEC) at the operational taxonomic unit (OTU) level for all samples, stool alone, and tissue alone. After rarefaction to the lowest library size of all the samples (445 reads per sample), α diversity and richness estimates were calculated per each sample. This process was repeated 400 times and results were averaged. The Shannon and inverse Simpson indices were calculated to estimate abundance-based OTU diversity, while the Chao1 estimator and observed taxa counts were calculated to estimate abundance-based OTU richness. Displayed p-values were obtained after testing for a non-zero coefficient of a normal linear model that used NEC/non-NEC group membership as predictor of richness or diversity. All tested richness and diversity indices for both tissue and stool samples were at or near significantly lower in NEC compared to non-NEC samples. B) Principal coordinates analysis (PCoA) plot of tissue samples, labelled by NEC status. Bray-Curtis dissimilarities between samples were calculated at the genus level after normalizing read counts to simple proportions and after rarefaction to the lowest library size (445 reads per sample). The centroids between the NEC and non-NEC samples were significantly dissimilar (Adonis PerMANOVA p-value = 0.0002).
NEC and non-NEC samples exhibited distinct microbial profiles
Prior to estimating beta diversity, samples were rarefied to the lowest library size (445 reads/sample). Principal Component Analysis (PCoA) using pairwise Bray-Curtis dissimilarities demonstrates distinct microbial genus composition of tissue samples from NEC versus non-NEC patients (Adonis test p-value = 0.0003) (Fig 1B). The microbial communities isolated from NEC and non-NEC fecal samples were also significantly dissimilar (Fig 2, Bray-Curtis dissimilarities calculated at the genus level, Adonis test p-value = 0.003). In contrast to the more uniform pattern in non-NEC tissues, microbial composition in NEC tissue clustered in separate coordinates indicating discrete colonization types.
Fig 2. Principal coordinates analysis (PCoA) plots of stool samples, labelled by necrotizing enterocolitis (NEC) status.
Bray-Curtis dissimilarities between samples were calculated at the genus level after normalizing read counts to simple proportions. The centroids between the NEC and non-NEC samples were significantly dissimilar (Adonis PerMANOVA p-value = 0.003).
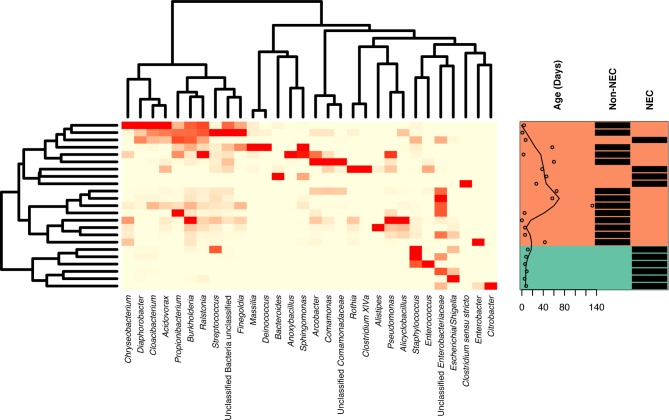
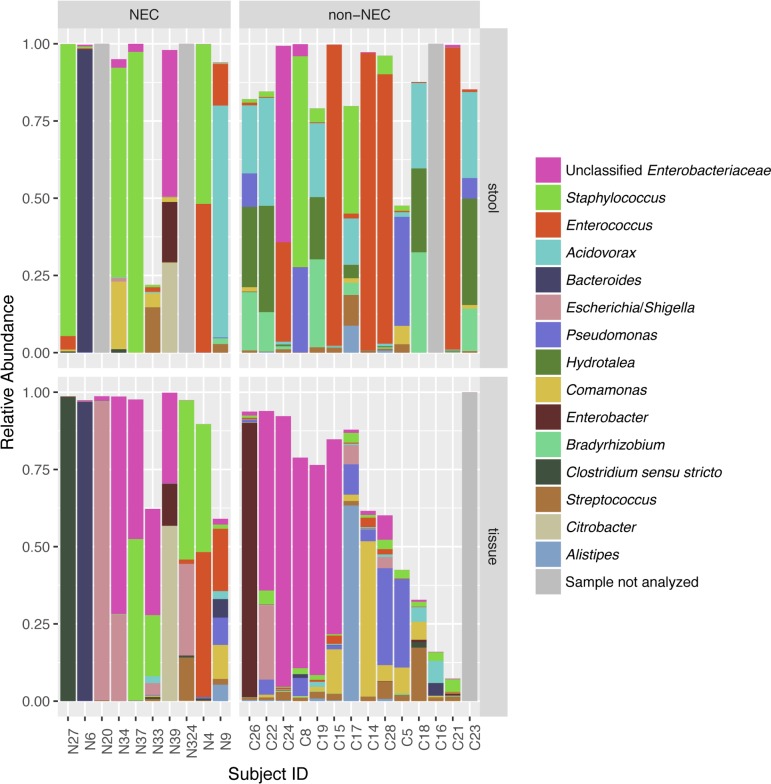
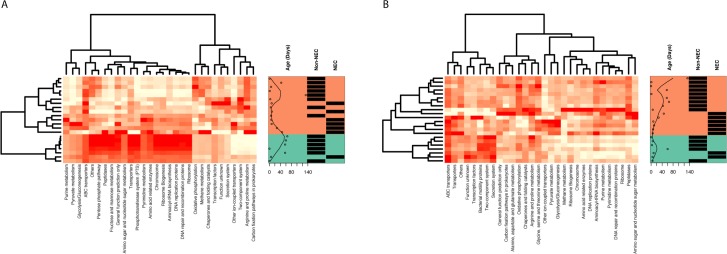
Specific taxa associated with the differential microbial profiles of NEC and non-NEC samples. Fig 3 shows a heatmap of the top 30 most abundant genera found in tissue samples across the bottom, with sample clustering on the left and each individual sample marked on the right with both infant age in days at time of collection and whether the sample was from an infant with or without NEC. NEC and non-NEC samples generally formed two distinct clusters. Bacterial genus level assignments for tissue and fecal samples comparing NEC with non-NEC patients are depicted in Fig 4; NEC tissue samples were more likely to be dominated by a single genus (Fig 1A), including Staphylococcus, Clostridium, Escherichia, or Bacteroides than non-NEC samples. Stool and tissue communities were significantly dissimilar (Bray-Curtis dissimilarities Adonis test p-value = 0.0005), with tissue and stool communities from the same infant sharing little overlap (Fig 4).
Fig 3. Heatmap of microbial abundance profiles of infant gut tissue at the genus level.
The 30 most abundant genera are shown. Infant age, in days, and necrotizing enterocolitis (NEC) status are labelled for each sample. Clustering due to NEC status can be observed.
Fig 4. Stacked bar graph showing genus level taxonomic composition for each individual sample, expressed as a proportion of reads.
Infant sample ID is on the x-axis. The top 15 genera with the highest average relative abundance are shown. Samples are stratified by necrotizing enterocolitis (NEC) status, and whether sample was tissue or stool. Tissue and stool samples from the same infant had dissimilar microbial profiles.
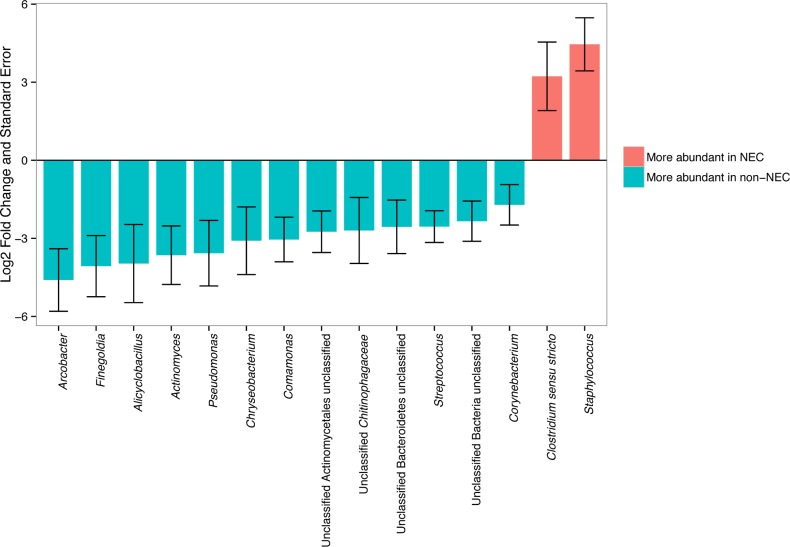
When tissue samples were analyzed alone, 15 taxa at the genus level had differential abundances in NEC compared to non-NEC samples with DESeq2 test q-values < 0.1 (Fig 5). Staphylococcus was ranked first in the DESeq2 model as most significantly different between NEC and non-NEC samples. Clostridium_sensu_stricto was near significantly more abundant in NEC tissue compared to non-NEC tissue (Table 2). Both groups were the only two genera identified as being significantly or near significantly more abundant in NEC compared to non-NEC samples. Clostridium_sensu_stricto was significantly more abundant in NEC than non-NEC tissues when the GeneSelector test using Wilcoxon test rankings was applied (q-value = 0.021). Clostridium_sensu_stricto abundance being higher in NEC infants appears to be due mostly to a single infant, N27, who had nearly 100% Clostridium_sensu_stricto abundance; the relative abundance of this genus was low for the remainder of samples (Fig 4).
Fig 5. Comparison of the abundance of tissue bacterial genera between infants with and without necrotizing enterocolitis (NEC).
Only bacterial genera that were significantly different between groups after adjusting for multiple comparisons using the DESeq2 package (see text for details) are indicated by an asterisk. Log2 fold change and log2 fold change standard error of tissue bacterial genera according to NEC status as calculated with the DESeq2 analysis. A log2 fold change of >0 (pink bars) indicates that abundance was detected to be higher during NEC, while a log2 fold change <0 (blue bars) indicates that abundance was detected to be higher in infants without NEC.
Table 2. Difference in the abundance of selected tissue and fecal genera or OTUs in infants for a number of tested comparisons for taxa that were significant with the DESeq2 test.
| Taxon | Sample type | Groups compared | Base mean* | Log2 fold change† | q-value‡ |
|---|---|---|---|---|---|
| Staphylococcus | tissue | NEC/non-NEC | 177.975 | 4.455 | 0.0006 |
| Clostridium sensu stricto | tissue | NEC/non-NEC | 1.552 | 3.226 | 0.0519 |
| Staphylococcus | fecal | NEC/non-NEC | 317.6 | 4.696 | 0.0002 |
| Staphyloocccus.OTU0004 | fecal | NEC/non-NEC | 230.90 | 5.32 | 6.26e-04 |
| Staphyloocccus.OTU0004 | tissue | NEC/non-NEC | 148.7 | 4.703 | 0.001 |
| Actinomyces | tissue | NEC/non-NEC | 1.550 | -3.647 | 0.008 |
| Corynebacterium | tissue | NEC/non-NEC | 1.960 | -1.711 | 0.087 |
| Staphylococcus | tissue | Male/female | 78.701 | -3.699 | 0.012 |
| Actinomyces | fecal | Age (days) | 7.725 | 0.0871 | 0.004 |
| Staphylococcus | tissue | Vancomycin (yes/no) | 78.701 | 3.758 | 0.015 |
* Base means are calculated in the DESeq2 package for each taxon after normalizing read counts for each sample to account for differences in sequencing depth.
† Log2 fold changes are calculated by the DESeq2 package and indicate the magnitude of the difference in abundance for each comparison. For categorical tests, positive values indicate that DESeq2 estimated the taxon was more abundant in the first tested group while negative values indicate that DESeq2 estimated the taxon was more abundant in the second group. When age in days was used as the group to test, a positive value indicates that DESeq2 found that taxon increased in abundance with each age day.
‡ Reported q-values are the result of a Wald test with the Benjamini and Hochberg correction for multiple comparisons.
NEC, necrotizing enterocolit
When fecal samples were analyzed alone, while Clostridium_sensu_stricto did not differ in abundance between NEC and non-NEC samples, Staphylococcus as a genus was more abundant during NEC (Table 2), consistent with findings from a recent study describing fecal microbiome samples from NEC patients [42]. A single Staphylococcus OTU, identified as OTU0004, was dominated in NEC fecal samples (Table 2) compared to non-NEC samples. This same Staphylococcus OTU0004 was also found to be significantly more abundant in tissue samples in infants with NEC (Table 2) compared to those without NEC. Due to the limited read lengths obtained, this OTU could not confidently be classified below the genus level.
NEC-associated changes in the microbiome were stronger than the influence of other measured potential confounders
Although infants in the NEC and non-NEC groups were similar demographically and had similar environmental exposures in aggregate (all p-values >0.05), we conducted additional analyses to assess the effect of potential gut microbiome confounders.
Mode of delivery did not have a significant correlation with infant microbiome richness, alpha or beta diversity, or abundance of specific taxa in either stool or tissue samples. Infant sex did not have a significant correlation with infant microbiome richness or alpha or beta diversity in either tissue or fecal samples; however, tissue from males had lower abundance of Staphylococcus (Table 2). Age of the infant at time of sampling was found to correlate with trends in the gut microbiome: overall, the microbial communities significantly differed between age groups (pairwise Bray-Curtis dissimilarities were calculated between each sample and infants were quartered into age groups of as even size as possible and the PermANOVA Adonis test was performed on these groupings; p-value = 0.011). We observed an association between microbial richness, diversity and infant age and this was strongly correlated with NEC status (Fig 2).
Prior to sampling, all but one infant had been exposed to antibiotics (Table 1). Of infants who had received antibiotics, all had received at least two different antibiotics and at least one broad-spectrum antibiotic. Half of all infants in this study (12/24) were treated with vancomycin. Staphylococcus abundance was higher in tissue taken from infants who had received vancomycin (Table 2). In contrast, Staphylococcus was not significantly more abundant in stool taken from infants with vancomycin exposure. Vancomycin exposure was not significantly associated with differential abundance of any other taxa or with alpha diversity or richness in either fecal or tissue samples.
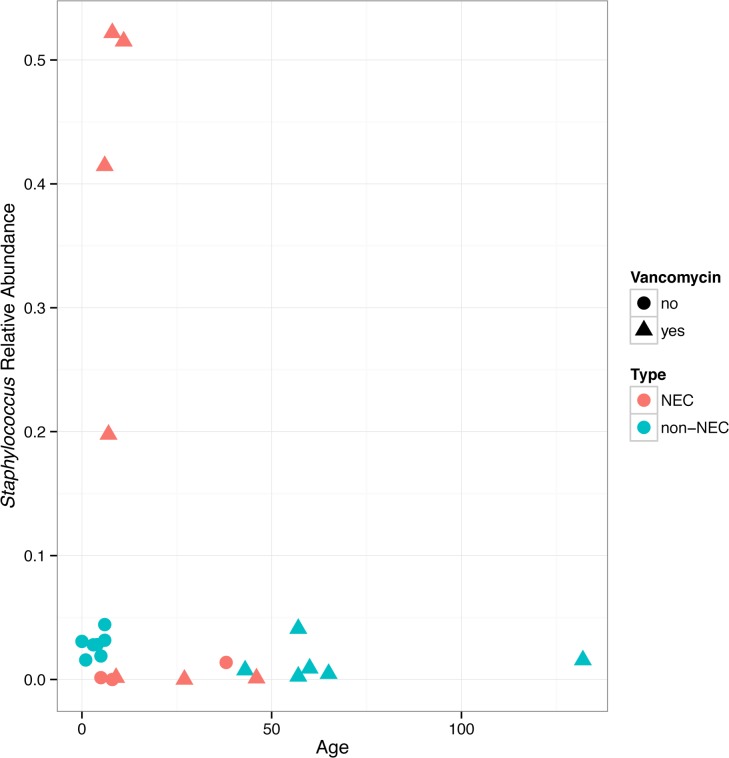
To further assess the influence of confounding variables on the effect of NEC on the microbiome, we built models in DESeq2 to explicitly account for a priori selected covariates that may affect the gut microbiome (delivery mode, infant sex, infant age, diet, tissue type, exposure to vancomycin). Regardless of covariates added, the DESeq2 calculated log2 fold change in Staphylococcus abundance between infants with and without NEC directionality did not change (i.e., Staphylococcus abundance was always higher in NEC infants). Infant age and exposure to vancomycin had the strongest effect on the association between NEC on Staphylococcus abundance: young infants with NEC who had been exposed to vancomycin generally had high Staphylococcus tissue abundance (Fig 6A).
Fig 6. Age of the infant in days at time of sample collection is plotted on the x-axis and Staphylococcus relative abundance is plotted on the y-axis.
Tissue Staphylococcus abundance is highest in infants with early necrotizing enterocolitis (NEC) resection, who received vancomycin.
For ethical reasons, intestinal tissue cannot be collected from healthy infants; therefore, all the non-NEC infants in this study were in the hospital for ailments unrelated to NEC. There were two disparate groups of non-NEC infants recruited for this study: those who were very young (<1 week old) and those who were older (40+ days). To assess the impact of these two disparate non-NEC groups, we repeated our main analyses, after separating out the samples into three groups: 1) NEC 2) “young” non-NEC and 3) “old” non-NEC. A PCoA plot constructed using pairwise Bray-Curtis dissimilarities at the genus level revealed that the two non-NEC groups clustered together, separately from samples from infants with NEC (S1 Fig). Additionally, microbial richness was lowest in infants who had NEC and was similar in the two non-NEC groups, regardless of infant age (S2 Fig). Staphylococcus abundance was highest in the NEC group, followed by the youngest non-NEC group, and lowest in the older non-NEC group suggesting intestinal Staphylococcus colonization at early age.
PICRUSt analyses reveals different functional profiles in NEC compared to non-NEC samples
Overall, the predicted functional profile in NEC and non-NEC samples were generally distinct in stool (Fig 7A) and tissue samples (Fig 7B). Processes and pathways related to signatures of infectious diseases, i.e., bacterial toxins (base mean = 661.6, log2 fold change = 1.049, q-value = 2.045e-05) and Staphylococcus aureus infection (base mean = 831.7, log2 fold change = 0.724, q-value = 1.209e-02) were enriched in NEC tissue samples compared to non-NEC tissues.
Fig 7. Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) was used to predict metagenomic and functional composition of the samples from 716S rRNA sequences.
Heatmaps of normalized counts of microbial function pathways detected with the PICRUSt pipeline for infant A) stool and B) tissue are shown. Infant age, in days, and necrotizing enterocolitis (NEC) status are labelled for each sample. Both sample types display clustering associated with NEC status.
Discussion
Only a few studies have interrogated the tissue-level intestinal microbiome in NEC, despite the relative proximal location of intestinal injury and previous reports on the existence of a site-specific intestinal microbiome [13,43,44]. Here, we report a tissue-specific overrepresentation of Firmicutes, specifically Staphylococcus sp. and Clostridium sp. in NEC. We are aware of only two other reports on the NEC tissue-level microbiome in humans: a study from Denmark performed a retrospective analysis of formalin-fixed and paraffin-embedded tissue specimens using fluorescent in situ hybridization with bacterial rRNA-targeting oligonucleotide probes [45]. They detected Proteobacteria (49.0%), Firmicutes (30.4%), Actinobacteria (17.1%) and Bacteroidetes (3.6%) in tissue samples. More recently Brower-Sinning et al. applied 16S rRNA technology to compare the microbiome of 16 cryopreserved NEC samples and 10 controls [46]. Except for a higher bacterial load in NEC tissues, no statistically significant distinction was found between the composition of NEC and non-NEC microbial communities. The different results in our study may be explained by the fact that in the work by Brower-Sinning et al. all but one control patient were former NEC patients. In contrast, we included samples from infants with no history of NEC.
While we observed that the infant gut microbiome was significantly dissimilar in infants with NEC compared to those without NEC, we conducted a number of analyses to test the influence of potential confounders. After adding multiple covariates to the model in DESeq2 suggests that a combination of variables is likely to influence the infant tissue microbiome, for example age, vancomycin exposure, and NEC were found to correlate with Staphylococcus abundance. We observed that very young infants with NEC who had been exposed to vancomycin were most likely to have high Staphylococcus abundance in their gut tissue. We do not know how to explain this unexpected finding except by the fact that vancomycin does not penetrate tissue very well. Delivery by C-section has been associated with colonization of the neonate with Staphylococcus [47]. Therefore, we were surprised by our finding that mode of delivery did not correlate with specific taxa in our dataset. However, three out of four samples with high abundance of Staphylococcus were from C-section-delivered infants indicating that our sample size may have been insufficient to detect a statistical significance.
One unique aspect of our study is the direct comparison between tissue and fecal samples. This allows for an additional level of quality control as each patient is his/her own control and results between fecal and tissue samples were distinct in both non-NEC and NEC patients. Consistent with previous studies in preterm infants [9,48,49], we confirmed the dominant phyla as Proteobacteria and Firmicutes, with a smaller contribution (<20%) from Bacteroidetes and Actinobacteria. Several fecal microbiome studies reported a bloom of γ-Proteobacteria with a concomitant decrease in Firmicutes in NEC patients [10,12]. This shift in microbial communities in NEC patients appears to start 1–2 weeks prior to diagnosis and has been associated with metabolic changes [9]. While our data do not replicate this shift in Proteobacteria in fecal samples, possibly as we measured the gut microbiome during rather than prior to NEC diagnosis, we confirmed the previously reported reduced microbial diversity and loss of Actinobacteria in NEC patients, especially patients with severe (surgical) disease [10,50].
Given numerous previous reports on the dominance of Proteobacteria in NEC [9,10], we were surprised to find the high prevalence of Firmicutes and specifically Staphylococcus sp. in NEC tissue. However, different forms of dysbiosis have been reported in NEC [11,48] including recently an association between Clostridium and Staphylococcus with NEC in European preterm infants [42]. Importantly, NEC dysbiosis with Firmicutes including Staphylococcus has been associated with earlier disease and higher mortality [48]. Our study included only infants with surgical NEC, the group of patients with highest mortality [51]. When comparing NEC patients with heavy versus light Staphylococcus abundance, NEC patients with high abundance required surgical resection significantly earlier. Staphylococcus is the major colonizing organism of the infant gut shortly after birth [49,52,53]. In preterm neonates, culture-based studies detected Staphylococcus in 50% of meconium and 100% of fecal samples from the first week post-partum [54]. Staphylococcus sp. are frequently cultured from meconium and have been associated with increased risk for NEC [8,55].
Our study has limitations. While we collected tissue and fecal samples prospectively, technical and ethical limitations do not allow for tissue sampling prior to surgical resection. Therefore, we cannot perform time series experiments to evaluate the dynamic microbiome changes in NEC tissue. Similarly, since it is currently not possible to sample intestinal tissue from normal infants, we lack a healthy control cohort in which to characterize the standard infant tissue microbiome. While we attempted to match for important variables such as mode of delivery, antibiotic exposure and type of feeding, given the nature of this human study that explores both tissue and stool of a surgical emergency in a very vulnerable population, we were not able to control for all possible microbiome confounders. In addition, the lack of shotgun metagenomic sequencing prohibits further classification of the bacteria, especially those of important genera e.g., Staphylococcus and Clostridium identified in this study. However, based on the recent findings by Rozé et al, we speculate that the majority of Staphylococcus and Clostridium species would be S. aureus and C. neonatale [42]. Future studies implementing whole genome sequencing will be necessary to address strain identification and implications for derangements in metabolic function associated with the distinct microbial community structure we detected. An additional future aim could be to measure Staphylococcus-specific endotoxin production in stool samples, especially as our PICRUSt data suggests there was an increase in bacterial toxin pathways in NEC compared to non-NEC tissue samples.
Conclusion
To the best of our knowledge, we define here for the first time corresponding fecal and tissue-level microbial communities comparing NEC patients with patients without a history of NEC and confirm age and antimicrobial exposure as defining factors.
Supporting information
Bray-Curtis dissimilarities between samples were calculated at the OTU level after normalizing read counts to simple proportions. NEC and non-NEC samples are observed to cluster separately, while both the young and old non-NEC samples clustered together.
(TIF)
Two age disparate groups of non-necrotizing enterocolitis (NEC) infants were included in the analysis; however, from this figure it can be observed that NEC/non-NEC status had a much stronger effect on microbial richness than infant age.
(TIF)
Data Availability
All sequences reported in this paper have been deposited into the NCBI sequence short read archive (accession numbers SRR7993700-SRR7993745).
Funding Statement
This work was supported by the American Academy of Pediatrics Marshall Klaus Perinatal Research Award (to J.R.K.) and the Eunice Kennedy Shriver National Institute Of Child Health & Human Development (NICHD) [T32HD068256 (to J.R.K.), K08HD061607 (to J.H.W.)]; the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) [K08DK090146 (to D.J.M.)], the National Institute of Health (NIH) Division of Loan Repayment National Institute of Diabetes and Digestive and Kidney Diseases Award (to J.R.K.), the National Science Foundation [DEB-1046149] and The Vanderbilt Microbiome Initiative (to S.R.B.), and U19AI095227 and P30 AI110527, the National Institute of Allergy and Infectious Diseases (NIAID) (to S.R.D.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD, NIDDK, NIAID or the National Institutes of Health (NIH). This project was also funded by the Vanderbilt Digestive Disease Research Center [P30DK058404], Vanderbilt Diabetes Center [P30DK20593], and the Vanderbilt CTSA Grant UL1 RR024975-01 from the National Center for Research Resources (NCRR/NIH). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gordon P, Christensen R, Weitkamp JH, Maheshwari A. Mapping the New World of Necrotizing Enterocolitis (NEC): Review and Opinion. EJ Neonatol Res.2(4):145–72. [PMC free article] [PubMed] [Google Scholar]
- 2.Weitkamp JH. More than a gut feeling: predicting surgical necrotising enterocolitis. Gut. 2014;63(8):1205–6. 10.1136/gutjnl-2013-305928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Musemeche CA, Kosloske AM, Bartow SA, Umland ET. Comparative effects of ischemia, bacteria, and substrate on the pathogenesis of intestinal necrosis. Journal of pediatric surgery. 1986;21(6):536–8. [DOI] [PubMed] [Google Scholar]
- 4.Rozenfeld RA, Liu X, DePlaen I, Hsueh W. Role of gut flora on intestinal group II phospholipase A2 activity and intestinal injury in shock. American journal of physiology Gastrointestinal and liver physiology. 2001;281(4):G957–63. 10.1152/ajpgi.2001.281.4.G957 [DOI] [PubMed] [Google Scholar]
- 5.Jilling T, Simon D, Lu J, Meng FJ, Li D, Schy R, et al. The Roles of Bacteria and TLR4 in Rat and Murine Models of Necrotizing Enterocolitis1. J Immunol. 2006;177(5):3273–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leaphart CL, Cavallo J, Gribar SC, Cetin S, Li J, Branca MF, et al. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol. 2007;179(7):4808–20. [DOI] [PubMed] [Google Scholar]
- 7.Bucher BT, McDuffie LA, Shaikh N, Tarr PI, Warner BB, Hamvas A, et al. Bacterial DNA Content in the Intestinal Wall from Infants with Necrotizing Enterocolitis. Journal of pediatric surgery. 2011;46(6):1029–33. 10.1016/j.jpedsurg.2011.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coggins SA, Wynn JL, Weitkamp JH. Infectious causes of necrotizing enterocolitis. Clinics in perinatology. 2015;42(1):133–54, ix. 10.1016/j.clp.2014.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Claud EC, Keegan KP, Brulc JM, Lu L, Bartels D, Glass E, et al. Bacterial community structure and functional contributions to emergence of health or necrotizing enterocolitis in preterm infants. Microbiome. 2013;1(1):20 10.1186/2049-2618-1-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mai V, Young CM, Ukhanova M, Wang X, Sun Y, Casella G, et al. Fecal microbiota in premature infants prior to necrotizing enterocolitis. PloS one. 2011;6(6):e20647 10.1371/journal.pone.0020647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sim K, Shaw AG, Randell P, Cox MJ, McClure ZE, Li MS, et al. Dysbiosis anticipating necrotizing enterocolitis in very premature infants. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2015;60(3):389–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Hoenig JD, Malin KJ, Qamar S, Petrof EO, Sun J, et al. 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J. 2009;3(8):944–54. 10.1038/ismej.2009.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romano-Keeler J, Moore DJ, Wang C, Brucker RM, Fonnesbeck C, Slaughter JC, et al. Early life establishment of site-specific microbial communities in the gut. Gut microbes. 2014;5(2):192–201. 10.4161/gmic.28442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang YW, Ellis NM, Hopkins MK, Smith DH, Dodge DE, Persing DH. Comparison of phenotypic and genotypic techniques for identification of unusual aerobic pathogenic gram-negative bacilli. Journal of clinical microbiology. 1998;36(12):3674–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salter SJ, Cox MJ, Turek EM, Calus ST, Cookson WO, Moffatt MF, et al. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC biology. 2014;12:87 10.1186/s12915-014-0087-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schloss PD, Gevers D, Westcott SL. Reducing the effects of PCR amplification and sequencing artifacts on 16S rRNA-based studies. PloS one. 2011;6(12):e27310 10.1371/journal.pone.0027310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pruesse E, Quast C, Knittel K, Fuchs BM, Ludwig W, Peplies J, et al. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic acids research. 2007;35(21):7188–96. 10.1093/nar/gkm864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cole JR, Wang Q, Cardenas E, Fish J, Chai B, Farris RJ, et al. The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic acids research. 2009;37(Database issue):D141–5. 10.1093/nar/gkn879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 272011. p. 2194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langille MG, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nature biotechnology. 2013;31(9):814–21. 10.1038/nbt.2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Applied and environmental microbiology. 2006;72(7):5069–72. 10.1128/AEM.03006-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26(19):2460–1. 10.1093/bioinformatics/btq461 [DOI] [PubMed] [Google Scholar]
- 23.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 7 United States2010. p. 335–6. 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Applied and environmental microbiology. 2007;73(16):5261–7. 10.1128/AEM.00062-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanehisa M, Institute for Chemical Research KU, Uji, Kyoto 611–0011, Japan, Sato Y, Healthcare Solutions Department FKSL, Hakata-ku, Fukuoka 812–0007, Japan, Kawashima M, Healthcare Solutions Department FKSL, Hakata-ku, Fukuoka 812–0007, Japan, et al. KEGG as a reference resource for gene and protein annotation. Nucleic acids research. 2017;44(D1). [DOI] [PMC free article] [PubMed]
- 26.Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O'Hara RB, et al. vegan: Community Ecology Package. R package version 2.0–10. 2014.
- 27.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology. 2014;15(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boulesteix AL, Slawski M. Stability and aggregation of ranked gene lists. Briefings in bioinformatics. 2009;10(5):556–68. 10.1093/bib/bbp034 [DOI] [PubMed] [Google Scholar]
- 29.Hofner B, Hothorn T. stabs: Stability Selection with Error Control 2014 [updated 2014. Available from: http://cran.r-project.org/package=stabs.
- 30.Shilts MH, Rosas-Salazar C, Tovchigrechko A, Larkin EK, Torralba M, Akopov A, et al. Minimally invasive sampling method identifies differences in taxonomic richness of nasal microbiomes in young infants associated with mode of delivery. Microbial ecology. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Applied and environmental microbiology. 2009;75(23):7537–41. 10.1128/AEM.01541-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill MO. Diversity and evenness: a unifying notation and its consequences. Ecology. 1973;54(2):427–32. [Google Scholar]
- 33.Anderson MJ. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001;26(1):32–46. [Google Scholar]
- 34.Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O'Hara RB, et al. vegan: Community Ecology Package 2014 [updated 2014. Available from: http://cran.r-project.org/package=vegan.
- 35.Bray JR, Curtis JT. An ordination of the upland forest communities of southern Wisconsin. Ecol Monogr. 1957;27(4):325–49. [Google Scholar]
- 36.Hothorn T, Hornik K. exactRankTests: Exact Distributions for Rank and Permutation Tests. 2013. [Google Scholar]
- 37.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57(1):289–300. [Google Scholar]
- 38.Grissom RJ, Kim JJ. Effect Sizes for Research: Univariate and Multivariate Applications, Second Edition London: Routledge; 2012. 2012/04/23/. 453 p. [Google Scholar]
- 39.Meinshausen N, Bühlmann P. Stability selection. J R Stat Soc Series B Stat Methodol. 2010;72(4):417–73. [Google Scholar]
- 40.Shah RD, Samworth RJ. Variable selection with error control: another look at stability selection. J R Stat Soc Series B Stat Methodol. 2013;75(1):55–80. [Google Scholar]
- 41.Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33(1):1–22. [PMC free article] [PubMed] [Google Scholar]
- 42.Roze JC, Ancel PY, Lepage P, Martin-Marchand L, Al Nabhani Z, Delannoy J, et al. Nutritional strategies and gut microbiota composition as risk factors for necrotizing enterocolitis in very-preterm infants. Am J Clin Nutr. 2017;106(3):821–30. 10.3945/ajcn.117.152967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, et al. Diversity of the human intestinal microbial flora. Science. 2005;308(5728):1635–8. 10.1126/science.1110591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gevers D, Kugathasan S, Denson LA, Vazquez-Baeza Y, Van Treuren W, Ren B, et al. The treatment-naive microbiome in new-onset Crohn's disease. Cell Host Microbe. 2014;15(3):382–92. 10.1016/j.chom.2014.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith B, Bodé S, Petersen BL, Jensen TK, Pipper C, Kloppenborg J, et al. Community analysis of bacteria colonizing intestinal tissue of neonates with necrotizing enterocolitis. BMC Microbiol. 112011. p. 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brower-Sinning R, Zhong D, Good M, Firek B, Baker R, Sodhi CP, et al. Mucosa-associated bacterial diversity in necrotizing enterocolitis. PloS one. 2014;9(9):e105046 10.1371/journal.pone.0105046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107(26):11971–5. 10.1073/pnas.1002601107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morrow AL, Lagomarcino AJ, Schibler KR, Taft DH, Yu Z, Wang B, et al. Early microbial and metabolomic signatures predict later onset of necrotizing enterocolitis in preterm infants. Microbiome. 2013;1(1):13 10.1186/2049-2618-1-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, et al. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci U S A. 2011;108 Suppl 1:4578–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McMurtry VE, Gupta RW, Tran L, Blanchard EEt, Penn D, Taylor CM, et al. Bacterial diversity and Clostridia abundance decrease with increasing severity of necrotizing enterocolitis. Microbiome. 2015;3:11 10.1186/s40168-015-0075-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guthrie SO, Gordon PV, Thomas V, Thorp JA, Peabody J, Clark RH. Necrotizing enterocolitis among neonates in the United States. Journal of perinatology: official journal of the California Perinatal Association. 2003;23(4):278–85. [DOI] [PubMed] [Google Scholar]
- 52.Jacquot A, Neveu D, Aujoulat F, Mercier G, Marchandin H, Jumas-Bilak E, et al. Dynamics and clinical evolution of bacterial gut microflora in extremely premature patients. J Pediatr. 2011;158(3):390–6. 10.1016/j.jpeds.2010.09.007 [DOI] [PubMed] [Google Scholar]
- 53.Subramanian S, Huq S, Yatsunenko T, Haque R, Mahfuz M, Alam MA, et al. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature. 2014;510(7505):417–21. 10.1038/nature13421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moles L, Gomez M, Heilig H, Bustos G, Fuentes S, de Vos W, et al. Bacterial diversity in meconium of preterm neonates and evolution of their fecal microbiota during the first month of life. PloS one. 2013;8(6):e66986 10.1371/journal.pone.0066986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stewart CJ, Marrs EC, Magorrian S, Nelson A, Lanyon C, Perry JD, et al. The preterm gut microbiota: changes associated with necrotizing enterocolitis and infection. Acta paediatrica (Oslo, Norway: 1992). 2012;101(11):1121–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bray-Curtis dissimilarities between samples were calculated at the OTU level after normalizing read counts to simple proportions. NEC and non-NEC samples are observed to cluster separately, while both the young and old non-NEC samples clustered together.
(TIF)
Two age disparate groups of non-necrotizing enterocolitis (NEC) infants were included in the analysis; however, from this figure it can be observed that NEC/non-NEC status had a much stronger effect on microbial richness than infant age.
(TIF)
Data Availability Statement
All sequences reported in this paper have been deposited into the NCBI sequence short read archive (accession numbers SRR7993700-SRR7993745).