Abstract
We sought to establish whether continuous positive airway pressure (CPAP) for obstructive sleep apnoea (OSA) in people with type 2 diabetes and diabetic macular oedema (DMO) improved visual acuity.
We randomly assigned 131 eligible patients aged 30–85 years from 23 UK centres with significant DMO causing visual impairment (LogMAR letters identified ≥39 and ≤78, score 0.92–0.14) plus severe OSA on screening to either usual ophthalmology care (n=67) or usual ophthalmology care plus CPAP (n=64) for 12 months.
Mean age of participants was 64 years, 73% male, mean body mass index 35.0 kg·m−2. Mean 4% oxygen desaturation index was 36 events·h−1. There was no significant difference in the visual acuity at 12 months between the CPAP group and the control group (mean LogMAR 0.33 (95% CI 0.29–0.37) versus 0.31 (95% CI 0.27–0.35); p=0.39), and no significant correlation between change in LogMAR and average CPAP use. The median±sd (range) daily CPAP use was 3.33±2.25 (0–7.93) h at 3 months, 3.19±2.54 (0–8.07) h at 6 months and 3.21±2.70 (0–7.98) h at 12 months.
CPAP therapy for OSA did not improve visual acuity in people with type 2 diabetes and DMO compared with usual care alone over 12 months.
Short abstract
UK ROSA RCT shows 1 year of CPAP for OSA in people with type 2 diabetes and macular oedema does not improve visual acuity http://ow.ly/bw8H30lrAX8
Introduction
By 2030, diabetes mellitus is expected to affect 8% of the world's adult population [1]. The risk of developing diabetic retinopathy and maculopathy correlates with diabetes duration [2]. Targeted treatment of glycaemia, hypertension and dyslipidaemia is recommended to reduce the risk of diabetic retinopathy [3–5]. When diabetic retinopathy and maculopathy are present, ophthalmic treatment options include retinal laser photocoagulation to prevent visual loss [6] and intravitreal anti-vascular endothelial growth factor (VEGF) [7] or intraocular corticosteroid to improve visual acuity [8].
Obstructive sleep apnoea (OSA) occurs during sleep with recurrent upper airway obstruction, causing apnoea, hypoxia and subsequent arousal with pulse and blood pressure increases [9]. This sleep fragmentation can cause excessive sleepiness, but may be asymptomatic. OSA is commonly associated with obesity and is increasing in worldwide prevalence alongside type 2 diabetes [10].
OSA is particularly prevalent in adults with type 2 diabetes; rates vary from 23% to 87% [11, 12]. People with diabetic retinopathy and maculopathy have a high OSA prevalence, with 34–54% of people with diabetic macular oedema (DMO) having OSA on screening sleep studies [13, 14]. Guidelines recommend screening for OSA in people with type 2 diabetes; many remain undiagnosed [15].
Treatment of significant OSA includes weight loss and continuous positive airway pressure (CPAP), i.e. a positive air pressure applied via a mask covering the nose and/or mouth to splint the pharynx, and thus prevent obstruction and consequent intermittent hypoxia [16]. CPAP improves daytime sleepiness and quality of life in those with moderate to severe OSA and is widely used. There are well-documented improvements in blood pressure with CPAP [17]. Randomised controlled trials (RCTs) of CPAP have not shown overall effects on diabetes control or insulin resistance [18–20], nor a benefit in cardiovascular risk [21, 22].
Hypoxia, oxidative stress and inflammation are proposed mechanisms in the development of diabetic retinopathy [23]. Serum rhodopsin mRNA changes occur in people with OSA [24] and the retinal nerve fibre layer thickness is significantly reduced, correlating with OSA severity [25]. Changes can be seen in the retinal vasculature, with OSA patients having increased retinal venular dilation compared with controls [26]. People with OSA and type 2 diabetes have significantly higher grades of retinopathy and prevalence of maculopathy than those without OSA [27]. A meta-analysis showed OSA to be significantly associated with increased risk of diabetic retinopathy [28]; recently, OSA was identified as an independent predictor for retinopathy progression [29]. One uncontrolled study using CPAP in people with OSA and DMO showed benefits in visual acuity in high CPAP compliers [30].
We hypothesised that treatment of OSA with CPAP would improve DMO and thus visual acuity. We performed an RCT of CPAP in people with diabetic retinopathy and impaired vision due to DMO and concurrent OSA.
Methods
Study design and oversight
The multicentre Retinopathy and concurrent Obstructive Sleep Apnoea (ROSA) trial was a 12-month follow-up RCT conducted between 2012 and 2017 (ISRCTN number 95411896). The study had ethical approval (REC 12/NE/0234); all participants gave written informed consent. There were 23 UK recruiting ophthalmology centres; the Newcastle Regional Sleep Service was the coordinating centre.
Patients and procedures
Details of an initial patient identification phase have been previously published [14]. Patients with severe OSA (4% oxygen desaturation index ≥20 events·h−1 or apnoea–hypopnoea index ≥30 events·h−1) were contacted by the coordinating centre. Eligible patients were those with best corrected visual acuity (BCVA) ≥39 and ≤78 letters in at least one eye (using the Early Treatment Diabetic Retinopathy Study (ETDRS) protocol at a testing distance of 4 m), central macular oedema in the visually impaired eye(s) and willing to have CPAP. Exclusion criteria were previous CPAP for OSA, significant cataract affecting vision, disability precluding informed consent or protocol adherence, excessive sleepiness in any driver (based on raised Epworth Sleepiness Scale (ESS) score) and respiratory failure. Subjects with the latter two exclusion criteria were referred for urgent OSA management.
Randomisation and interventions
Patients were randomised (1:1) to CPAP or control by a central telephone service using computer-derived treatment allocation (Sealed Envelope; www.sealedenvelope.com) with minimisation for office blood pressure, OSA severity, glycated haemoglobin (HbA1c) and visual acuity severity. All patients had usual best-practice clinical care for DMO during the trial, with ocular interventions as clinically indicated, determined by their ophthalmologist. They were all provided with written information on optimising sleep hygiene and benefits of weight loss to OSA by the research team. In addition to usual care, patients randomised to CPAP (S9 Autoset; ResMed, Didcot, UK) were instructed by staff who routinely initiate CPAP. Humidification and interface choices were made individually. Patients were given written and audiovisual information on CPAP plus telephone support: the coordinating centre contacted all patients after 2, 7 and 30 days to help manage any CPAP difficulties. Patients could telephone for help and additional visits at the local recruiting centre were arranged as required.
Study measurements
The primary end-point of the trial was BCVA of the study eye (LogMAR with refraction, 4 m ETDRS) at 12 months. Assessments were performed at baseline, and 3, 6 and 12 months. Ophthalmological measurements were completed by trained individuals blind to the patient's study group. BCVA testing was performed with ETDRS charts after an assessment for lens status and refraction [31]. Visual acuity was recorded as number of letters identified and equivalent LogMAR value. Central 1 mm macular thickness was measured through optical coherence tomography (Spectralis (Heidelberg Engineering, Franklin, MA, USA), Topcon 2000 (Topcon, Tokyo, Japan) or Cirrus (Carl Zeiss Meditec, Dublin, CA, USA), according to centre). Digital retinal photography was used to take one fovea- and one disc-centred image. The same equipment was used by each site for all patients. Retinal photographs were graded by two independent graders using UK diabetic eye screening grading definitions (version 1.3) [32]. If grades conflicted, an arbitration review was completed. During the baseline and 12-month assessment, grading of any cataract was completed using the Lens Opacities Classification System version II protocol [33]. The total number of all ocular interventions in 12 months was recorded (anti-VEGF therapy, intravitreal corticosteroids, and focal and grid photocoagulation).
Office blood pressure, weight, height, and neck and waist circumference were measured, plus oxygen saturation via a pulse oximeter. Nonfasting cholesterol, high-density lipoprotein (HDL), triglycerides and HbA1c were checked at each visit, plus thyroid function at baseline only. Self-assessed health status measurements were taken at each visit: Short Form-12 (SF-12) [34], 25-item Visual Function Questionnaire (VFQ-25) [35], short Calgary Sleep Apnea Quality of Life Index (Short SAQLI) [36] plus the ESS [37].
Statistical analysis
We calculated 90 patients randomised 1:1 would provide 80% power to detect 0.1 difference in LogMAR with 5% significance, based on pilot data showing the mean±sd difference in LogMAR at 6 months between compliers with CPAP and noncompliers was 0.1±0.17 [30]. We allowed extra recruitment for an estimated 5% dropout and 15% nonadherence rate based on previous CPAP studies. To enable exploratory subset analysis, an increase in numbers of 30% was planned. Recruitment took longer than anticipated; 131 completed follow-up by trial completion. All analyses were on an intention-to-treat (ITT) basis. All participants with at least one post-randomisation assessment of the primary outcome were included in the primary analysis. The primary outcome of LogMAR at 12 months was analysed using a mixed-effects model, accounting for repeated measures over time. The model includes LogMAR score as a response variable. A two-sided p-value <0.05 was considered significant. Retinopathy, maculopathy and photocoagulation at 12 months were analysed by comparing group proportions using the Chi-squared test. We further investigated whether adherence to CPAP therapy influenced the primary outcome in different ways. We defined “high” and “low” compliers based around the mean total hours of CPAP used per night at 6 and 12 months, to give similar sized numbers in each group (thus high compliers ≥2 h·night−1 and low compliers <2 h·night−1). In addition, the patients were divided into quartiles (Q1–Q4) based on median daily usage of CPAP and with a comparison of the change in LogMAR at 12 months adjusting for the baseline LogMAR between Q2, Q3 and Q4 versus the reference group Q1. Analyses were undertaken using Stata version 14.2 (StataCorp, College Station, TX, USA) and validated in SAS version 9.4 (SAS Institute, Cary, NC, USA) by independent clinical trial statisticians.
Results
Study participants
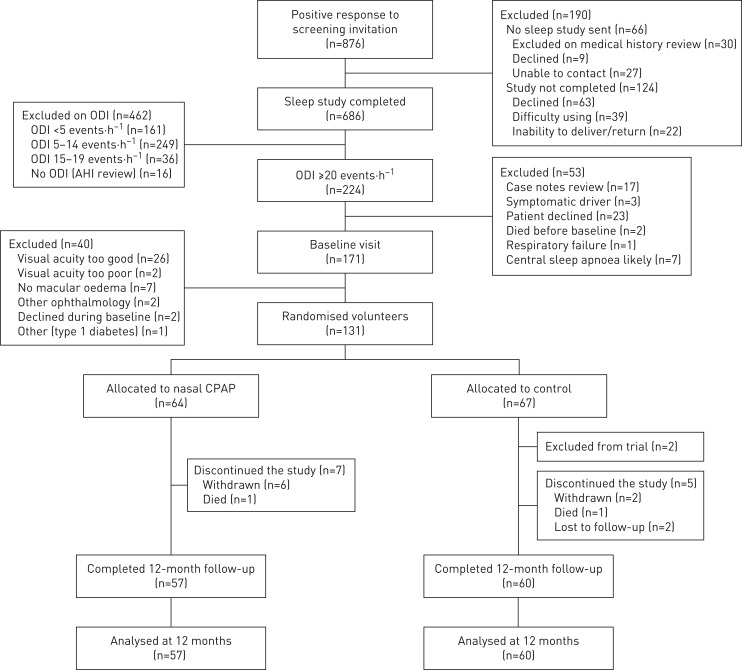
There were 224 patients who met the initial eligibility criteria with severe OSA (figure 1). At baseline visit, 131 participants were eligible to be randomised. Two patients randomised to control requested CPAP during the study, due to worsening sleepiness. Some patients randomised to CPAP had zero adherence. The baseline characteristics of the participants are shown in table 1. Based on ethnic group classification: 92% White European, 5% Asian, 3% Black and 1% Egyptian. The distribution of minimisation factors for randomisation was well balanced between the two groups.
FIGURE 1.
Screening, randomisation and follow-up analyses. ODI: 4% oxygen desaturation index; AHI: apnoea–hypopnoea index; CPAP: continuous positive airway pressure.
TABLE 1.
Baseline characteristics of the study participants
| CPAP group | Control group | |
| Subjects | 64 | 67 |
| Sex | ||
| Male | 43 (67.2) | 52 (80.0) |
| Female | 21 (32.8) | 13 (20.0) |
| Age years | 64.88±10.44 (41.66–83.42) | 64.19±9.13 (39.03–81.10) |
| BMI kg·m−2 | 34.78±8.74 (20.3–82.7) | 35.23±6.32 (22.0–50.5) |
| Neck circumference cm | 43.16±4.09 (34.5–60.0) | 44.81±4.07 (36.0–54.0) |
| Waist circumference cm | 115.15±11.99 (93–149.5) | 118.59±17.21 (67–151) |
| Duration of diabetes years | 15.91±8.73 (1–37) | 15.63±9.47 (1–51) |
| ESS score | 9.1±5.9 (0–22) | 9.0±5.8 (0–24) |
| AHI events·h−1 | 32.9±16.9 (0–82) | 33.7±18.67 (0–107) |
| Daytime oxygen saturation on air at rest % | 96.4±1.9 (89–100) | 96.5±1.6 (91–99) |
| ODI events·h−1 | 36.5±17.9 (20–99) | 36.3±15.6 (20–84) |
| Short SAQLI score | 3.0±1.4 (1.1–5.7) | 3.1±1.4 (1.0–6.3) |
| SF-12 total score | ||
| Physical health composite score | 38.6±6.5 (24.2–53.2) | 38.7±6.4 (25.3–49.7) |
| Mental health composite score | 50.2±8.2 (29.9–67.8) | 50.4±7.6 (29.4–62.1) |
| VFQ-25 score | 70.0±19.9 (28.0–98.2) | 75.3±19.4 (18.9–98.5) |
| Total cholesterol mmol·L−1 | 4.27±1.09 (2.4–7.1) | 4.09±1.08 (2.2–7.4) |
| HDL mmol·L−1 | 1.18±0.36 (0.6–2.3) | 1.20±0.36 (0.6–2.03) |
| Triglycerides mmol·L−1 | 2.08±1.08 (0.7–5.5) | 2.08±1.41 (0.6–7.3) |
| TSH mU·L−1 | 2.5±1.5 (0.7–7.5) | 2.0±1.2 (0.4–6.6) |
| Thyroxine pmol·L−1 | 14.8±2.3 (10–19) | 14.7±2.2 (9.3–19.2) |
| HbA1c mmol·mol−1 | 66.9±17.6 (40–127) | 66.0±23.3 (13.4–163) |
| LogMAR | 0.36±0.21 (−0.08–1.08) | 0.35±0.22 (−0.18–0.9) |
| CMT µm | 364.3±107.9 (173–742) | 346.3±102.6 (119–671) |
| Retinopathy grade¶ | ||
| 1 | 36 (59) | 33 (54) |
| 2 | 18 (30) | 21 (34) |
| 3 | 7 (11) | 7 (12) |
| Maculopathy grade# | ||
| 0 | 17 (28) | 28 (46) |
| 1 | 44 (72) | 33 (54) |
| Photocoagulation grade# | ||
| 0 | 31 (51) | 27 (44) |
| 1 | 30 (49) | 34 (56) |
Data are presented as n (%) or mean±sd (range). CPAP: continuous positive airway pressure; BMI: body mass index; ESS: Epworth Sleepiness Scale; AHI: apnoea–hypopnoea index; ODI: 4% oxygen desaturation index; SAQLI: Calgary Sleep Apnea Quality of Life Index; SF-12: Short Form-12; VFQ-25: 25-item Visual Function Questionnaire; HDL: high-density lipoprotein; TSH: thyroid stimulating hormone; HbA1c: glycated haemoglobin; CMT: central macular thickness. #: n=61 CPAP, n=61 control.
LogMAR
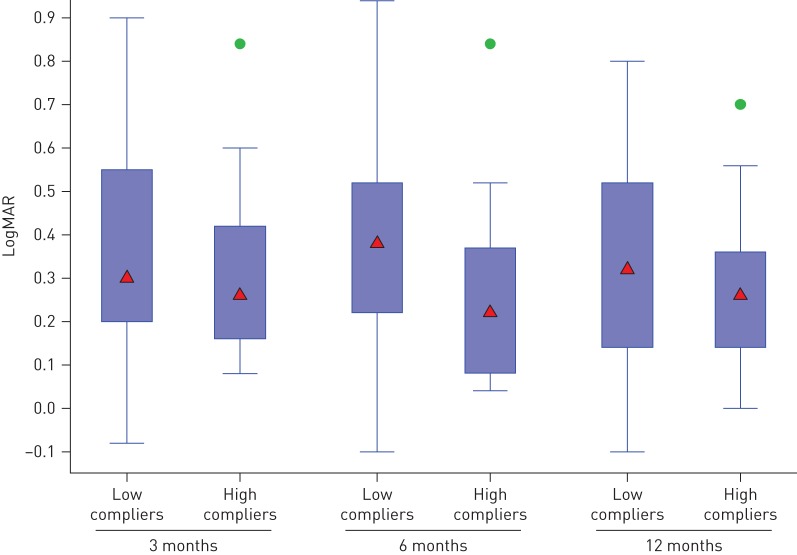
There were no significant differences in LogMAR at 3, 6 or 12 months between the patients in the CPAP group and the control group, after adjusting for the minimisation factors (table 2 and figure 2). The mean±sd change in LogMAR at 12 months was CPAP −0.02±0.15 and control −0.04±0.17. After adjustment for baseline central macular thickness (CMT), this result remained not statistically significant. There was no significant correlation between the change in LogMAR and average CPAP use at 3, 6 or 12 months (r= −0.15; p=0.31). The change in LogMAR from baseline to 6 months or from 6 to 12 months was not statistically significant in either the low and high CPAP compliers group, with the paired t-test (figure 3). Further division of the CPAP group into quartiles based on median daily usage showed no significant change in LogMAR at 12 months in any group or any suggestion of a dose–response relationship (mean LogMAR Q1 0.34 (95% CI 0.26–0.42); Q2 0.31 (95% CI 0.23–0.39), p=0.63; Q3 0.37 (95% CI 0.28–0.45), p=0.64; Q4 0.29, 95% (CI 0.21–0.37), p=0.41).
TABLE 2.
Results of LogMAR at 3, 6 and 12 months
| CPAP group | Control group | Mean difference (95% CI) | p-value# | |||
| n | LogMAR | n | LogMAR | |||
| 3 months | 59 | 0.34 (0.31–0.38) | 60 | 0.30 (0.27–0.34) | 0.04 (−0.01–0.09) | 0.113 |
| 6 months | 60 | 0.33 (0.30–0.37) | 60 | 0.30 (0.26–0.33) | 0.03 (−0.02–0.09) | 0.217 |
| 12 months | 57 | 0.33 (0.29–0.37) | 60 | 0.31 (0.27–0.35) | 0.03 (−0.03–0.08) | 0.390 |
Data are presented as mean (95% CI), unless otherwise stated. CPAP: continuous positive airway pressure. #: calculated from linear mixed model adjusting for baseline LogMAR and minimisation factors.
FIGURE 2.
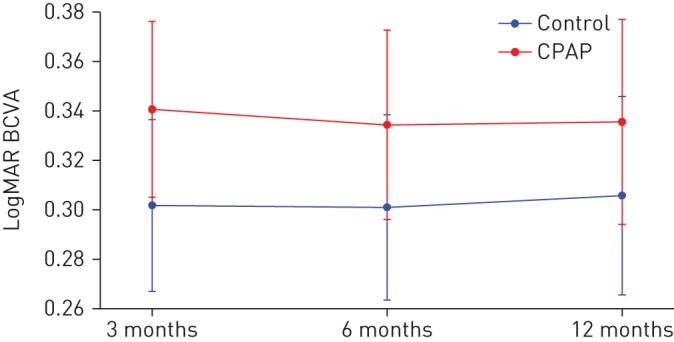
Difference in LogMAR best corrected visual acuity (BCVA) at 3, 6 and 12 months. CPAP: continuous positive airway pressure. Data are presented as mean (95% CI).
FIGURE 3.
LogMAR after continuous positive airway pressure therapy in high versus low compliers. Data are presented as box-and-whisker plots showing median (triangles), interquartile range (boxes) and minimum–maximum range (whiskers) with outliers (circles).
Central macular thickness
There was a significant difference in CMT at 3 months, with the CPAP group having increased CMT implying worsening oedema compared with the control group: CPAP mean 339.4 (95% CI 319.5–359.3) µm versus control 312.6 (95% CI 296.1–329.2) µm; p=0.045. At 6 and 12 months there was no significant difference between the two groups: CPAP 12-month mean 320.4 (95% CI 298.2–342.6) µm versus control 309.2 (95% CI 286.5–331.9) µm; p=0.53.
Ocular interventions
There was no statistically significant difference between the mean number of ocular interventions over 12 months in the CPAP group (5.2 (95% CI 3.6–6.8)) and the control group (3.4 (95% CI 2.3–4.6)); p=0.08.
Progression of diabetic retinopathy
The changes in proportions of retinopathy, maculopathy and photocoagulation from baseline to 12 months were not statistically significant in either group (table 3).
TABLE 3.
Progression of diabetic retinopathy, maculopathy and photocoagulation at 12 months
| CPAP group | Control group | p-value# | |
| Subjects | 53 | 54 | |
| Retinopathy grade | |||
| 1 | 32 (60) | 31 (57) | 0.825 |
| 2 | 13 (25) | 16 (30) | |
| 3 | 8 (15) | 7 (13) | |
| Maculopathy grade | |||
| 0 | 22 (42) | 25 (46) | 0.618 |
| 1 | 31 (58) | 29 (54) | |
| Photocoagulation grade | |||
| 0 | 22 (42) | 20 (37) | 0.636 |
| 1 | 31 (58) | 34 (63) |
Data are presented as n or n (%), unless otherwise stated. CPAP: continuous positive airway pressure. #: Chi-squared test.
Self-assessed health status
There was no significant difference between the groups in VFQ-25 or SF-12 scores at 3, 6 or 12 months. The Short SAQLI score showed a statistically significant difference at 3 months between the groups, implying symptomatic benefit from CPAP (mean CPAP 2.3 (95% CI 2.1–2.5) versus control 2.8 (95% CI 2.6–2.9); p=0.003), but no significant difference between groups at 6 or 12 months.
Epworth Sleepiness Scale
There was no significant difference in the ESS score between groups at 3, 6 or 12 months (table 4). High CPAP compliers showed a statistically significant decrease of ESS score at 3 months (p=0.02), but no significant decrease at 6 and 12 months.
TABLE 4.
Epworth Sleepiness Scale (ESS) score
| CPAP group | Control group | Mean difference (95% CI) | p-value# | |||
| n | ESS score | n | ESS score | |||
| 3 months | 59 | 7.7 (6.8–8.7) | 60 | 8.0 (7.2–8.9) | −0.3 (−1.6–1.0) | 0.633 |
| 6 months | 59 | 6.7 (5.7–7.7) | 60 | 7.6 (6.7–8.5) | −0.9 (−2.2–0.5) | 0.210 |
| 12 months | 57 | 7.2 (6.1–8.3) | 60 | 7.6 (6.7–8.5) | −0.4 (−1.9–1.1) | 0.587 |
Data are presented as mean (95% CI), unless otherwise stated. CPAP: continuous positive airway pressure. #: calculated from linear mixed model adjusting for the baseline ESS score and minimisation factors.
Continuous positive airway pressure
The CPAP download data from the last study period is shown in table 5. The proportion of nights with ≥4 h using CPAP data shows 19% of people used CPAP for 60–100% of nights at 3 months, 27% at 6 months and 22% at 12 months.
TABLE 5.
Continuous positive airway pressure usage at 3, 6 and 12 months
| 3 months | 6 months | 12 months | ||||
| n | Mean±sd (range) | n | Mean±sd (range) | n | Mean±sd (range) | |
| Pressure 95th centile cmH2O | 57 | 10.9±3.6 (4–19.3) | 48 | 11.6±3.6 (4.6–19.2) | 42 | 11.5±3.6 (4.0–18.2) |
| AHI events·h−1 | 57 | 1.9±2.7 (0–10) | 48 | 1.9±2.5 (0–11.8) | 42 | 1.7±2.3 (0–8.4) |
| Obstructive events·h−1 | 57 | 0.78±1.44 (0–8.3) | 48 | 0.65±1.11 (0–6.2) | 42 | 0.54±0.79 (0–3.1) |
| Central events·h−1 | 57 | 0.84±1.66 (0–8.5) | 48 | 0.75 91.34 (0–6.2) | 42 | 0.70±1.37 (0–6.5) |
| Median daily usageh | 57 | 3.33±2.25 (0–7.93) | 57 | 3.19±2.54 (0–8.07) | 57 | 3.21±2.70 (0–7.98) |
| Average daily usage h | 57 | 2.35±2.09 (0–7.48) | 57 | 2.16±2.30 (0–6.9) | 57 | 1.78±2.18 (0–6.53) |
AHI: apnoea–hypopnoea index.
Blood tests
There were no statistically significant changes in HbA1c, cholesterol, HDL and triglycerides between the treatment groups at any of the time-points.
Discussion
This multicentre UK RCT of CPAP in patients with OSA and impaired vision due to DMO and type 2 diabetes has shown no benefit to visual acuity from CPAP compared with standard ophthalmological care over a 12-month period. There was no statistically or clinically significant improvement with CPAP in the variables of vision, DMO or retinal photography at any time-point, nor change in other measures. There have been no other RCTs conducted in this area. This is therefore novel data, useful for clinical practice.
OSA was found in 75% of people studied in the total cohort. Those people with symptomatic OSA with daytime sleepiness should be referred for CPAP to improve these symptoms [16]. There has been increasing interest in whether CPAP can be used to treat conditions causally associated with OSA, such as hypertension, insulin resistance, type 2 diabetes and cardiovascular disease [18, 21, 22, 38]. This is particularly relevant to those people with asymptomatic OSA, who do not require CPAP for daytime sleepiness, but in whom it might mitigate risk from another condition. The numbers of people with these comorbid conditions are enormous, so robust evidence to guide therapeutic decisions is essential.
There was no additional benefit of CPAP for severe OSA for any ocular measure. The CPAP group had deterioration in CMT at 3 months that just reached statistical significance; it did not persist at 6 and 12 months. This was not associated with any significant difference in visual acuity between the two groups. This difference is not clinically significant; typically this would require an increase in CMT of ≥10% from baseline and a decrease in visual acuity of ≥5 letters [39]. The CPAP group had a nonsignificant higher average number of ocular interventions than the control group at 12 months; perhaps these interventions occurred because of the CMT increase and they were effective at correcting ongoing CMT differences between groups. It is not clear why the CPAP group would have increased CMT at 3 months compared with the control group; changes in ocular perfusion with CPAP are potentially plausible, causing an effect on ocular vascular permeability, but unlikely. No changes in CMT were found in the only other study of CPAP on DMO [30]. That smaller, uncontrolled study gave 32 people with DMO and OSA CPAP for 6 months. Post-randomisation division into high and low compliers showed those who used CPAP well (n=13, mean ≥2.5 h·night−1) had significant improvements in visual acuity compared with those who were less compliant (n=15), with an adjusted treatment effect on visual acuity of high adherence versus low adherence of 0.11 (95% CI 0.21– −0.002; p=0.047), equivalent to a one-line improvement on the LogMAR chart. There was no significant improvement in DMO or retinal photographs, but interest in the potential benefit of CPAP to visual acuity led to this RCT.
In this trial, patients randomised to CPAP received additional support from sleep teams to optimise their adherence with this therapy. Despite this, the adherence to CPAP was lower than that seen in other trials of CPAP in individuals with OSA also found by screening specific nonsleep clinic populations: mean adherence in the SAVE study of people with coronary or cerebrovascular disease was 3.3 h·night−1 over several years and it was 3.5 h·night−1 in a study of CPAP versus oxygen in OSA found in patients with cardiovascular disease or known cardiovascular risk factors [22, 40]. The SAVE trial gave pre-randomisation subtherapeutic CPAP to ensure participants were adherent for an average of 3 h·night−1; this excluded 324 people (10%), which no doubt improved trial CPAP adherence rates by excluding those who could not tolerate it at the outset. We felt a “real-world” trial including all patients would be more applicable to clinical practice. All these studies prove that high adherence to CPAP in patients who have not presented to the sleep clinic with symptoms is difficult to achieve. It is known that CPAP use varies in patients in clinical practice. Possibly the patients in this study with type 2 diabetes, DMO and other medical problems, but without sleepiness, found the additional burden of CPAP too much. The level of CPAP adherence may have been insufficient to affect visual outcomes, but there was no suggestion of any correlation of changes in visual acuity with CPAP use nor any improvement seen in high compared with low CPAP users. A per protocol analysis was conducted for the primary outcome of LogMAR BCVA at 12 months, excluding the patients in the control arm who received CPAP (n=2) and those in the CPAP group who never used CPAP (n=5): it showed no difference to the results found in the ITT analysis (p=0.38). It therefore seems that although CPAP effectively treated OSA, confirmed by CPAP download data, it did not improve visual outcomes. CPAP may be unable to reverse established ocular damage in people who have DMO. Alternatively, best ophthalmic care (anti-VEGF, intravitreal corticosteroids and photocoagulation) may stabilise and improve vision, leaving no additional role for CPAP to reduce DMO. Whether CPAP could be used as a potential tool to prevent diabetic retinopathy, rather than reversing it, or delaying its progression, remains an area of interest and future studies are needed to ascertain whether CPAP has a valid role in this arena, particularly given the poor adherence in this group of patients.
In this study, daytime sleepiness and quality of life, measured by the ESS and the Short SAQLI, respectively, significantly improved in those with high CPAP compliance at 3 months. It is surprising this was not sustained throughout the trial and that there was no significant improvement in the SF-12 between groups. Both have been shown to improve in previous RCTs of CPAP [41]. The lower median CPAP adherence than other studies of people with symptomatic OSA may be the reason or the lack of therapeutic benefit may have tempered their use of CPAP. The participants in this study were screened for OSA, but had never sought previous help for OSA symptoms. Although the mean baseline ESS score was within the normal range, other studies have shown that improvements in sleepiness occur regardless of the baseline ESS score [21, 42]. While the prevalence of OSA was high in this cohort, these were not people who had presented with sleep symptoms and therefore may have different treatment responses to those people with symptomatic OSA. At the end of the trial, 63% of patients randomised to CPAP opted to continue, suggesting they had some form of symptomatic benefit from this.
An observational prospective longitudinal study assessed 230 patients from diabetes clinics at two UK centres with assessments of diabetic retinopathy via photography and OSA via home sleep studies [29]. Sight-threatening diabetic retinopathy prevalence was higher in patients with OSA than those without OSA (42.9% versus 24.1%; p=0.004). After a median follow-up of 43 months, patients with OSA were more likely to develop pre-proliferative/proliferative diabetic retinopathy (18.4% versus 6.1%; p=0.02). OSA remained an independent predictor of progression after adjustment for confounders (OR 5.2 (95% CI 1.2–23.0); p=0.03). All patients with moderate or severe OSA at baseline were referred for CPAP. High CPAP adherence significantly lowered progression to advanced diabetic retinopathy and maculopathy (n=15). While that study showed potential benefits from CPAP, it was in a small subgroup of nonrandomised patients. The authors emphasised the need for an RCT of CPAP.
In conclusion, this is the only RCT to evaluate the effect of CPAP for OSA on visual acuity in people with macular oedema and type 2 diabetes. CPAP should continue to be given to patients with symptomatic OSA, but there is no evidence from this study to support its use as an alternative therapy for DMO when standard ophthalmic therapy is already being given.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-01177-2018_Supplement (5.2KB, pdf)
Acknowledgements
We are grateful to the UK Local Comprehensive Research network who facilitated involvement of the UK sites in this trial, plus all the patients who took part in this research. Thanks to the Trial Steering Committee: Mary Morrell (Chair; Imperial College London, London, UK), John Stradling (NIHR Oxford Biomedical Research Centre, Oxford, UK), Sonya Craig (University Hospital Aintree, Liverpool, UK), Rajen Gupta (Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK), Emma Hedley (Oxford Respiratory Trials Unit, Oxford, UK), Ly-Mee Yu (Centre for Statistics in Medicine, University of Oxford, Oxford, UK) and Chris Rogers (Sleep Apnoea Trust Association, Chinnor, UK); and to the independent data monitoring committee: Anthony De Soyza (Chair; Newcastle University, Newcastle upon Tyne, UK), John Gibson (Newcastle University, Newcastle upon Tyne, UK), David Steel (Sunderland Eye Infirmary, Sunderland, UK) and Louise Linsell (Centre for Statistics in Medicine, University of Oxford, Oxford, UK). Thanks to the Oxford Respiratory Trials Unit (Oxford, UK) management team: Najib Rahman, Emma Hedley, Jack Quaddy, Gyathri Kagithala, Assunta Sabia, Molly Glaze, Maria Bantouna and Charlotte Carter, and to Gavin Reilly for statistical input.
Footnotes
This article has supplementary material available from erj.ersjournals.com.
This study has been registered at www.isrctn.com with identification number 95411896. Individual de-identified participant data will be shared with participants. This data will include all the data collected in this trial. Additional, related documents have been made available (study protocol, statistical analysis plan).
ROSA Principal Investigators: Rajen Gupta (Royal Victoria Infirmary, Newcastle upon Tyne, UK), Maged Habib (Sunderland Eye Infirmary, Sunderland, UK), Martin McKibbin (St James's University Hospital, Leeds, UK), Faruque Ghanchi (Bradford Royal Infirmary, Bradford, UK), Abosede Cole (Bristol Eye Hospital, Bristol, UK), Patrick Richardson (Royal Derby Hospital, Derby, UK), Sahar Al-Husainy (Heartlands Hospital, Birmingham, UK), Andy Morris (The Royal Bournemouth Hospital, Bournemouth, UK), Imran Rahman (Blackpool Victoria Hospital, Blackpool, UK), Sajjad Haider and Saju Thomas (University Hospital of North Durham, Durham and Darlington Memorial Hospital, Darlington, UK), Sridhar Manvikar (The James Cook University Hospital, Middlesbrough, UK), Yvonne D'Souza (Manchester Royal Eye Hospital, Manchester, UK), Nonavinakere P. Manjunatha (Hospital of St Cross, Rugby and University Hospital Coventry, Coventry, UK), Christina Rennie (University Hospital Southampton, Southhampton, UK), Anju Kadyan (Royal Shrewsbury Hospital, Shrewsbury, UK), Naren Dhingra (Pinderfields Hospital, Wakefield, UK), Justin Pepperell (Musgrove Park Hospital, Taunton, UK), Andrew Brown (University Hospital of North Staffordshire, Stoke-on-Trent, UK), Neil Ward (Derriford Hospital, Plymouth, UK), Giuliana Silvestri (Royal Victoria Hospital, Belfast, UK), Rehna Khan (Huddersfield Royal Infirmary, Huddersfield, UK), Tim Jackson (King's College Hospital, London, UK) and Chris Brand (Royal Hallamshire Hospital, Sheffield, UK).
Author contributions: Conception and design: S.D. West and J.R. Stradling. Recruitment and data collection: B. Prudon, J. Hughes and R. Gupta. Analysis: S.B. Mohammed and S. Gerry. Data interpretation: S.D. West, J.R. Stradling and R. Gupta. Drafting the manuscript for important intellectual content: S.D. West. All authors have approved the final manuscript.
Conflict of interest: S.D. West has nothing to disclose.
Conflict of interest: B. Prudon reports grants (paid to institution) and supply of equipment for the project from ResMed UK, during the conduct of the study.
Conflict of interest: J. Hughes has nothing to disclose.
Conflict of interest: R. Gupta reports travel support from Bayer and Novartis, and personal fees from Bayer, outside the submitted work.
Conflict of interest: S.B. Mohammed has nothing to disclose.
Conflict of interest: S. Gerry has nothing to disclose.
Conflict of interest: J.R. Stradling reports grants from ResMed Foundation, during the conduct of the study; personal fees for consultancy work from ResMed UK and Bayer Germany, outside the submitted work.
Support statement: This study was funded with a grant from The ResMed Foundation. ResMed UK also provided financial support for clinical research staff, the Apnealinks, CPAP equipment and to ROSA study days for research site team members. The UK Local Comprehensive Research network facilitated involvement of the UK sites in this trial. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010; 87: 4–14. [DOI] [PubMed] [Google Scholar]
- 2.Yau JW, Rogers SL, Kawasaki R, et al. . Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012; 35: 556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837–853. [PubMed] [Google Scholar]
- 4.Klein R, Knudtson MD, Lee KE, et al. . The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXII. The twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology 2008; 115: 1859–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ucgun NI, Yildirim Z, Kilic N, et al. . The importance of serum lipids in exudative diabetic macular edema in type 2 diabetic patients. Ann NY Acad Sci 2007; 1100: 213–217. [DOI] [PubMed] [Google Scholar]
- 6.The Diabetic Retinopathy Study Research Group. Preliminary report on effects of photocoagulation therapy. Am J Ophthalmol 1976; 81: 383–396. [DOI] [PubMed] [Google Scholar]
- 7.Heier JS, Korobelnik JF, Brown DM, et al. . Intravitreal aflibercept for diabetic macular edema: 148-week results from the VISTA and VIVID studies. Ophthalmology 2016; 123: 2376–2385. [DOI] [PubMed] [Google Scholar]
- 8.Boyer DS, Yoon YH, Belfort R Jr, et al. . Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology 2014; 121: 1904–1914. [DOI] [PubMed] [Google Scholar]
- 9.Dempsey JA, Veasey SC, Morgan BJ, et al. . Pathophysiology of sleep apnea. Physiol Rev 2010; 90: 47–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heinzer R, Vat S, Marques-Vidal P, et al. . Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med 2015; 3: 310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.West SD, Nicoll DJ, Stradling JR. Prevalence of obstructive sleep apnoea in men with type 2 diabetes. Thorax 2006; 61: 945–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foster GD, Sanders MH, Millman R, et al. . Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes Care 2009; 32: 1017–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mason RH, West SD, Kiire CA, et al. . High prevalence of sleep disordered breathing in patients with diabetic macular edema. Retina 2012; 32: 1791–1798. [DOI] [PubMed] [Google Scholar]
- 14.Prudon B, Hughes J, West S. A novel postal-based approach to diagnosing obstructive sleep apnoea in a high-risk population. Sleep Med 2017; 33: 1–5. [DOI] [PubMed] [Google Scholar]
- 15.Shaw JE, Punjabi NM, Wilding JP, et al. . Sleep-disordered breathing and type 2 diabetes: a report from the International Diabetes Federation Taskforce on Epidemiology and Prevention. Diabetes Res Clin Pract 2008; 81: 2–12. [DOI] [PubMed] [Google Scholar]
- 16.Giles TL, Lasserson TJ, Smith BH, et al. . Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev 2006; 1: CD001106. [DOI] [PubMed] [Google Scholar]
- 17.Bratton DJ, Gaisl T, Wons AM, et al. . CPAP vs mandibular advancement devices and blood pressure in patients with obstructive sleep apnea: a systematic review and meta-analysis. JAMA 2015; 314: 2280–2293. [DOI] [PubMed] [Google Scholar]
- 18.West SD, Nicoll DJ, Wallace TM, et al. . Effect of CPAP on insulin resistance and HbA1c in men with obstructive sleep apnoea and type 2 diabetes. Thorax 2007; 62: 969–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lam JC, Lam B, Yao TJ, et al. . A randomised controlled trial of nasal continuous positive airway pressure on insulin sensitivity in obstructive sleep apnoea. Eur Respir J 2010; 35: 138–145. [DOI] [PubMed] [Google Scholar]
- 20.Shaw JE, Punjabi NM, Naughton MT, et al. . The effect of treatment of obstructive sleep apnea on glycemic control in type 2 diabetes. Am J Respir Crit Care Med 2016; 194: 486–492. [DOI] [PubMed] [Google Scholar]
- 21.Craig SE, Kohler M, Nicoll D, et al. . Continuous positive airway pressure improves sleepiness but not calculated vascular risk in patients with minimally symptomatic obstructive sleep apnoea: the MOSAIC randomised controlled trial. Thorax 2012; 67: 1090–1096. [DOI] [PubMed] [Google Scholar]
- 22.McEvoy RD, Antic NA, Heeley E, et al. . CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med 2016; 375: 919–931. [DOI] [PubMed] [Google Scholar]
- 23.Arden GB, Sivaprasad S. Hypoxia and oxidative stress in the causation of diabetic retinopathy. Curr Diabetes Rev 2011; 7: 291–304. [DOI] [PubMed] [Google Scholar]
- 24.Wong A, Merritt S, Butt AN, et al. . Effect of hypoxia on circulating levels of retina-specific messenger RNA in type 2 diabetes mellitus. Ann NY Acad Sci 2008; 1137: 243–252. [DOI] [PubMed] [Google Scholar]
- 25.Kargi SH, Altin R, Koksal M, et al. . Retinal nerve fibre layer measurements are reduced in patients with obstructive sleep apnoea syndrome. Eye 2005; 19: 575–579. [DOI] [PubMed] [Google Scholar]
- 26.Shankar A, Peppard PE, Young T, et al. . Sleep-disordered breathing and retinal microvascular diameter. Atherosclerosis 2013; 226: 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.West SD, Groves DC, Lipinski HJ, et al. . The prevalence of retinopathy in men with Type 2 diabetes and obstructive sleep apnoea. Diabet Med 2010; 27: 423–430. [DOI] [PubMed] [Google Scholar]
- 28.Zhu Z, Zhang F, Liu Y, et al. . Relationship of obstructive sleep apnoea with diabetic retinopathy: a meta-analysis. Biomed Res Int 2017; 2017: 4737064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altaf QA, Dodson P, Ali A, et al. . Obstructive sleep apnea and retinopathy in patients with type 2 diabetes. a longitudinal study. Am J Respir Crit Care Med 2017; 196: 892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mason RH, Kiire CA, Groves DC, et al. . Visual improvement following continuous positive airway pressure therapy in diabetic subjects with clinically significant macular oedema and obstructive sleep apnoea: proof of principle study. Respiration 2012; 84: 275–282. [DOI] [PubMed] [Google Scholar]
- 31.Early Treatment Diabetic Retinopathy Study Research Group. Early Treatment of Diabetic Retinopathy Study (ETDRS): Manual of Operations. Washington, National Technical Information Service, 1985. [Google Scholar]
- 32.NHS Screening Service. Diabetic Eye Screening Revised Grading Definitions Version 1.3 2012. http://bmec.swbh.nhs.uk/wp-content/uploads/2013/03/Diabetic-Screening-Service-Revised-Grading-Definitions-November-2012.pdf Date last accessed: August 17, 2018.
- 33.Chylack LT Jr, Leske MC, McCarthy D, et al. . Lens opacities classification system II (LOCS II). Arch Ophthalmol 1989; 107: 991–997. [DOI] [PubMed] [Google Scholar]
- 34.Jenkinson C, Layte R. Development and testing of the UK SF-12. J Health Serv Res Policy 1997; 2: 14–18. [DOI] [PubMed] [Google Scholar]
- 35.Mangione CM, Lee PP, Gutierrez PR, et al. . Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol 2001; 119: 1050–1058. [DOI] [PubMed] [Google Scholar]
- 36.Flemons WW, Reimer MA. Measurement properties of the Calgary Sleep Apnea Quality of Life Index. Am J Respir Crit Care Med 2002; 165: 159–164. [DOI] [PubMed] [Google Scholar]
- 37.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991; 14: 540–545. [DOI] [PubMed] [Google Scholar]
- 38.Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, et al. . Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet 2002; 359: 204–210. [DOI] [PubMed] [Google Scholar]
- 39.Wells JA, Glassman AR, Ayala AR, et al. . Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med 2015; 372: 1193–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gottlieb DJ, Punjabi NM, Mehra R, et al. . CPAP versus oxygen in obstructive sleep apnea. N Engl J Med 2014; 370: 2276–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jenkinson C, Davies RJ, Mullins R, et al. . Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trial. Lancet 1999; 353: 2100–2105. [DOI] [PubMed] [Google Scholar]
- 42.Bratton DJ, Gaisl T, Schlatzer C, et al. . Comparison of the effects of continuous positive airway pressure and mandibular advancement devices on sleepiness in patients with obstructive sleep apnoea: a network meta-analysis. Lancet Respir Med 2015; 3: 869–878. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-01177-2018_Supplement (5.2KB, pdf)