Abstract
Background:
Low HIV testing uptake prevents identification of adolescents living with HIV and linkage to care and treatment. We implemented an innovative service package at health care facilities to improve HIV testing uptake and linkage to care among adolescents aged 10–19 years in Western Kenya.
Methods:
This quasi-experimental study used preintervention and postintervention data at 139 health care facilities (hospitals, health centers, and dispensaries). The package included health worker capacity building, program performance monitoring tools, adolescent-focused HIV risk screening tool, and adolescent-friendly hours.
The study population was divided into early (10–14 years) and late (15–19 years) age cohorts. Implementation began in July 2016, with preintervention data collected during January–March 2016 and postintervention data collected during January–March 2017. Descriptive statistics were used to analyze the numbers of adolescents tested for HIV, testing HIV-positive, and linked to care services. Preintervention and postintervention demographic and testing data were compared using the Poisson mean test. χ2 testing was used to compare the linkage to care rates.
Results:
During the preintervention period, 25,520 adolescents were tested, 198 testing HIV-positive (0.8%) compared with 77,644 adolescents tested with 534 testing HIV-positive (0.7%) during the postintervention period (both P-values <0.001). The proportion of HIV-positive adolescents linked to care increased from 61.6% to 94.0% (P < 0.001). The increase in linkage to care was observed among both age cohorts and within each facility type (both P-values <0.001).
Conclusions:
The adolescent-focused case finding intervention package led to a significant increase in both HIV testing uptake and linkage to care services among adolescents in Western Kenya.
Key Words: adolescents, HIV, case finding
INTRODUCTION
Globally, there were 2.1 million adolescents aged 10–19 years living with HIV, with 260,000 new infections occurring within this age group in 2016.1 Poor access to health services, including HIV-related testing that represents a critical entry point into the HIV care and treatment cascade, hampers efforts to control the HIV epidemic among adolescents and youth.1–4
HIV testing rates among adolescents remain low in the middle- and low-income countries most affected by HIV epidemic.1,5–7 Recent data indicate that only 13% of adolescent girls and 9% of adolescent boys aged 15–19 years are tested for HIV in sub-Saharan Africa.1 In addition, poor linkage of adolescents who test positive for HIV is a major barrier to engagement in care and treatment services.5 Limited evidence exists on effective interventions to support adolescent linkage from diagnosis to initiation, retention, and treatment adherence.6 Group or individual counseling and education, financial incentives, expanded clinic accessibility, and provision of adolescent tailored services have shown promise as interventions.6 Most HIV testing and linkage to care and treatment interventions, however, have been developed for adult populations and are not tailored to the needs of the adolescents and youth.7
One of the key strategies for HIV case identification is health care facility (HCF)-based provider-initiated HIV testing and counseling (PITC). PITC has been extensively studied in adults and is often implemented for adolescents with the assumption that it will be equally efficient.6,8,9 To date, however, data on the efficacy of PITC for case finding among adolescents aged 10–19 years are limited.4,11–13 Factors associated with improved HIV testing uptake among adolescents and youth include availability of integrated testing at HCF, training of staff to offer comprehensive, adolescent-friendly health services, availability of flexible HCF hours, proximity of HCFs to the target populations, and increased number of testing sites.6,14–16 Furthermore, high HIV identification rates among children and adults has been associated with PITC at inpatient wards, tuberculosis (TB) clinics, and within maternal and child health (MCH) clinics.13 Due to the limited data available, it is not clear whether the same association applies to the adolescent population.
Most adult-oriented HIV programs lack disaggregated data on HIV testing among adolescents aged 10–19 years.11 Most published data refer to youth aged 15–24 years or to children aged 10 years and younger, whereas data on adolescents aged 10–19 years are limited.1,11 Practical, easy-to-use, and simplified data collection and reporting tools are urgently needed to inform programmatic targets, measure performance, and improve programming around HIV testing and linkage to care among adolescents in Africa.
To address the HIV testing and reporting gaps among adolescents, we developed an innovative adolescent service package to improve HIV testing uptake and linkage to care and treatment services among adolescents aged 10–19 years. It was implemented in 139 HCFs across 7 counties of Western Kenya, where HIV prevalence among adolescents aged 10–19 years is 10.4%.17,18 The service package intervention comprised capacity building for new and existing HCF staff, new program tools for monitoring performance, adolescent-focused HIV risk screening tool, and adolescent-friendly HCF hours, which included expanded evening and weekend hours. Implementation of the service package began in July 2016. In this study, we evaluated the effect of the new intervention service package on the uptake of HIV testing and linkage to care among adolescents aged 10–19 years in Western Kenya.
METHODS
Study Design
This was a preintervention versus postintervention study, which used program data from 139 HCFs in Western Kenya. Preintervention data were collected in May 2016 for the period of January to March 2016. Implementation of the package began in July 2016. Postintervention data were collected on a monthly basis for the period January to March 2017. Data on the numbers of adolescents tested for HIV, identified as HIV-positive, and linked to care and treatment services were collected. Linkage to care data were collected by entry point, including outpatient department (OPD) with general ambulatory services, inpatient wards, and specialized TB, MCH, and nutrition clinics.
Subjects and Setting
The study was conducted in Western Kenya, where HIV prevalence among adolescents is 10.4%.17,18 The University of Nairobi/Kenyatta National Hospital Ethical Review Committee reviewed and approved the study protocol (P345/04/2016). The study used aggregate programmatic data from the participating facilities. No patient-level data were abstracted; therefore, no consent procedure was required.
Study participants were all adolescents aged 10–19 years who were tested for HIV at 139 facilities in 7 counties of Western Kenya during 2 periods: January to March 2016 and January to March 2017. The HCFs included 38 dispensaries (graded as low-level HCFs), 57 health centers (graded as mid-level HCFs), and 44 hospitals (graded as high-level HCFs). Within these facilities, the diverse entry points (ie, OPD, inpatient wards, and TB, MCH, and nutrition clinics) were identified and adolescents tested for HIV at these entry points were tracked and linked to care. In addition, the study collected programmatic data from selected HCF-led adolescent activities such as integrated outreach, index case testing, and the adolescent-focused football league tournaments as part of the program supporting HIV testing and linkage to care activities.
Intervention Service Package
A comprehensive package of services designed to improve uptake of HIV testing and linkage to care specifically for adolescents was developed and implemented across all 139 HCFs (Fig. 1). First, capacity building was provided to HCF providers, including training by nationally approved trainers on the new national HIV testing algorithm and training by program staff on the new program-specific tools including an adolescent-focused HIV risk screening tool with follow-up individual-level mentorship and supervision. Services were also decentralized by introducing this training to HCF staff at additional entry points in dispensaries. In some high-volume HCFs (those with >2000 outpatient clients a month and multiple service delivery points), additional HIV testing service providers including linkage officers and triage staff were allocated.
Figure 1.
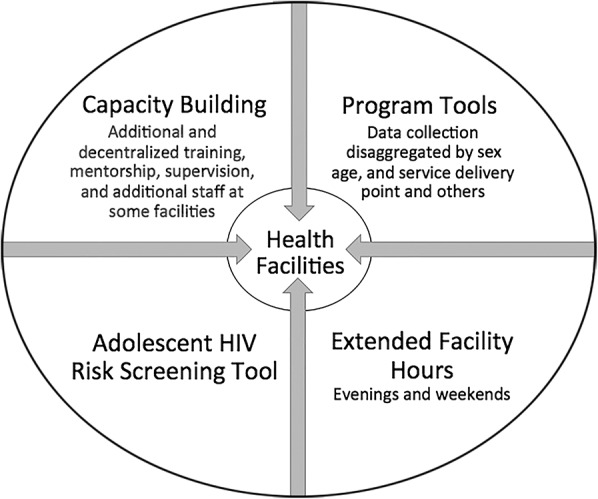
Illustration of the comprehensive intervention package of services implemented at all 139 sites. The capacity building element for HCF providers included training by nationally approved trainers on the new national HIV testing algorithm, and training by program staff on the new program-specific tools, including an adolescent-focused HIV risk screening tool, as well as individual-level follow-up mentorship and supervision. Second, newly developed program-specific tools, which collected data stratified by sex, age, and entry point, were introduced at HIV testing service points in the HCFs. Third, a newly developed, adolescent-focused HIV screening tool designed to identify eligibility for HIV testing, including assessment of HIV risk, among adolescents aged 10–19 years was implemented. Finally, extended evening (5–7 pm) and/or weekend hours (Saturdays and Sundays) were implemented at most HCFs to accommodate adolescents. In some high-volume HCFs (those with >2000 outpatient clients per month and multiple entry points), additional HIV testing service providers were allocated including linkage officers and triage staff.
Second, the newly developed program-specific tools, which collected data stratified by sex, age, and entry point, were introduced at HIV testing service points in the study HCFs. The tools captured staff work load at HCFs and numbers of adolescents eligible for testing by entry point. Tools for index client contact testing and follow-up were also designed and implemented. Third, a newly developed, adolescent-focused HIV screening tool designed to identify eligibility for HIV testing, including assessment of HIV risk, among adolescents aged 10–19 years was implemented. This tool was designed to be used by triage assistants at entry points and at the community events. When adolescents were determined to be eligible for HIV testing, they were escorted to the PITC room or space where HIV testing was conducted. The testing area was located at the same entry point. In some sites, adolescents were escorted to the voluntary counseling and testing room. Finally, extended evening (5–7 pm) and/or weekend hours (Saturdays and Sundays) were implemented at most HCFs to accommodate adolescents.
Data Collection
Preintervention data were collected using a study-specific tool administered by trained program staff at all 139 HCFs. Postintervention data were collected in the same HCFs by trained program staff using identical study-specific tool. Postintervention data were collected monthly in aggregate and sent to the study team. Key study variables included the number of adolescents tested for HIV, stratified by sex (males and females), age (10–14 and 15–19 years), and facility type (hospital, health center, and dispensary), and the number of adolescents testing HIV-positive. For those testing HIV-positive, linkage to care data were collected from both the testing register and the HIV care clinic registers. Linkage to care and treatment was defined as completing at least one HIV care visit and having a unique HIV care number issued. This number was documented in the HIV testing register, the HIV care clinic register, and ART register, which are routinely issued to the patients during their first HIV care visit to the HCFs (the same mechanism was used during the preintervention and postintervention periods).
Data Analysis
Data were entered monthly into an electronic password-protected database, where it was reviewed and validated before being exported to STATA (version 13) for analysis. Comparison of data between the preintervention and postintervention periods was done using the Poisson mean test. Comparisons were made overall and by sex, age group (10–14 years and 15–19 years), and facility type. Proportions of newly diagnosed adolescents who were successfully linked to care and treatment services were compared using the χ2 test, overall and by sex, age group (10–14 years and 15–19 years), and facility type.
RESULTS
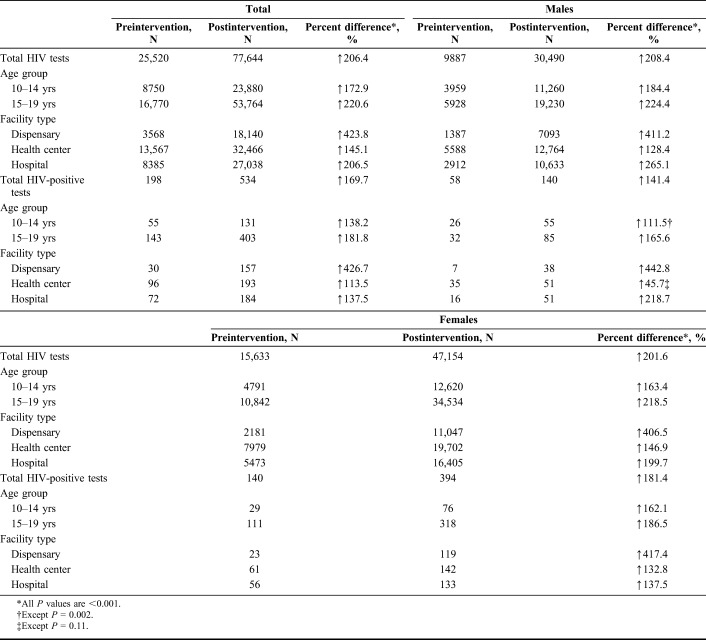
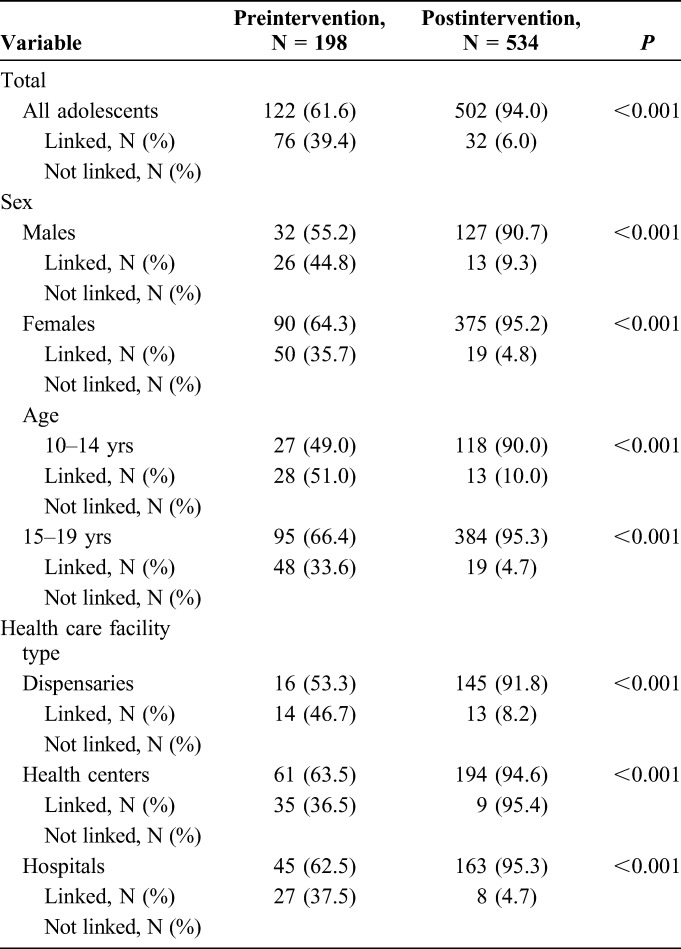
During the preintervention period (January–March 2016), a total of 25,520 adolescents aged 10–19 years were tested at the 139 HCFs (Table 1). During the postintervention period (January–March 2017), a total of 77,644 clients aged 10–19 years were tested at the same HCFs (P < 0.001). Significant increases were observed in the uptake of testing for both males and females, for each age group, and for each facility type in the postintervention compared with the preintervention periods (P-values <0.001, respectively). More females were tested for HIV compared with males; however, this disparity was more visible among older adolescents aged 15–19 years (Table 1).
TABLE 1.
Total and Sex-Based HIV Testing Among Adolescents in Western Kenya During Preintervention (January–March 2016) and Postintervention (January–March 2017) Periods
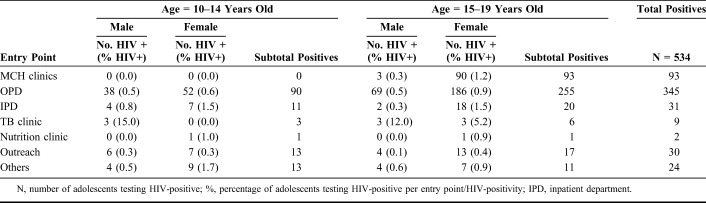
Data on HIV testing by entry point were not available for the preintervention period. For the postintervention period, the largest number of tests were conducted in OPD (67.6%), followed by the HCF-led outreach (14.4%) and MCH clinic (9.9%). The second largest number of females was diagnosed in MCH settings after OPD (Table 2).
Table 2.
HIV-Positivity Among Adolescents Aged 10–14 and 15–19 Years by Entry Point During the Preintervention (January–March 2016) and Postintervention (January–March 2017) Periods
The HIV-positivity rate was similar between the preintervention and the postintervention periods (0.77% versus 0.69%, respectively; P = 0.378). Given the large increase in numbers of youth tested, the total number of newly identified HIV-positive adolescents increased from 198 in the preintervention period to 534 in the postintervention period (P-values <0.001). This increase was significant across both age groups and sexes (P < 0.001, respectively). HIV-positivity was highest in TB clinics followed by MCH and inpatient clinics. The HIV-positivity was also relatively high in nutrition clinics especially among younger adolescents (Table 2). Selected HCFs led HIV testing outreach within nearby communities, although this contributed only a small number of those tested, and HIV-positivity was minimal in these settings (Table 2 and Fig. 2).
Figure 2.
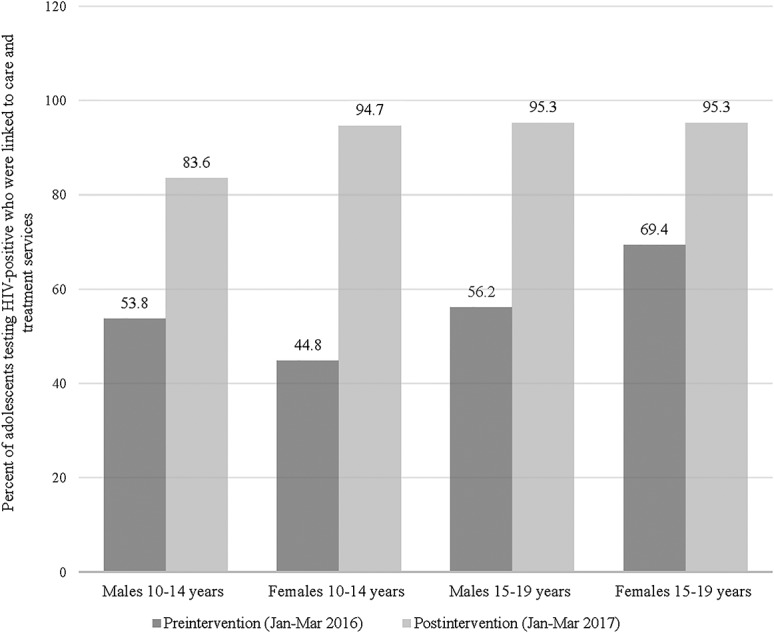
Change in proportion of adolescents testing HIV-positive linked to care and treatment services by age and sex during the preintervention (January–March 2016) and postintervention (January–March 2017) periods in Western Kenya. Linkage to care and treatment is a key challenge in adolescent HIV case finding. The comprehensive intervention package aimed to improve the rate of linkage to care for adolescents testing HIV-positive in the postintervention period. The linkage to care rate was assessed by age cohort (10–14 years and 15–19 years) and sex. The proportion of adolescents testing HIV-positive and linked to care improved from 61.6% in the preintervention period to 94% in the postintervention period.
The proportion of adolescents who tested HIV-positive and were linked to care and treatment services increased from 61.6% in the preintervention period to 94.0% in the postintervention period (P < 0.001) (Table 3). This increase was seen for both sexes, both age groups, and in all HCF types (all P-values <0.001). Comparison of the proportion of HIV-positive adolescents linked to care and treatment services during preimplementation and postimplementation periods showed a significant increase in linkage to care rates among different age and sex cohorts (all P-values <0.001) (Table 3).
TABLE 3.
Linkage to Care Among Adolescents Testing HIV-Positive During the Preintervention (January–March 2016) and Postintervention (January–March 2017) Periods
DISCUSSION
Implementation of the adolescent service package intervention in Western Kenya improved new HIV case identification and linkage to care and treatment services among adolescents aged 10–19 years. The package addressed several factors associated with increased testing such as staff training, monitoring of program performance, and provision of adolescent-friendly HCF hours. Moreover, the package included the adolescent screening tool to evaluate those eligible for HIV testing.4–6,11–16
This study did not set out to measure individual elements of the package but rather the effect of the combination package. Although implementation of separate parts of this package independently may have shown effectiveness in increasing HIV testing uptake and linkage to care and treatment services,6,15,16 we believe that the combination of all 4 interventions within the service package had additive effect and resulted in more positive outcomes than if each element of the package was implemented alone. The HIV testing and linkage to care and treatment rates in our study before the intervention implementation were comparable with those reported previously. In a meta-analysis of HIV testing and linkage among adolescents in sub-Saharan Africa, the uptake of the facility-based HIV testing was high at 73% and linkage to care for facility PITC was at 55% among young people (<25 years) including adolescents comprising 38% of facility PITC populations.19
Although the number of adolescents tested in both age categories increased significantly, the HIV-positivity rates did not change significantly in our study, suggesting stable adolescent HIV incidence rates in the Western Kenya. The HCFs within the reach of our intervention package had baseline community-based mobilization going on before the intervention, and there was no significant change in the scope or level of that mobilization throughout the implementation period. Because the package of intervention did not include modification of community-based mobilization, we do not believe that community-based mobilization significantly contributed to the increase in numbers of adolescents accessing HIV testing in our study. The implementation of our service package intervention led to increases in HIV testing uptake and the absolute number of newly identified HIV-positive adolescents, and improved the proportion linked to care and treatment services across sexes, age groups, and different facility types. Interestingly, for the first 12 months of implementation, the largest number of new HIV-positive tests by service delivery points has remained within OPD for both males and females (data not shown). For females, the second largest entry point was MCH settings, which is not surprising because most females are tested for HIV when attending MCH clinics for pregnancy and breastfeeding-related services.
Consistent with other studies, the disparity in HIV testing uptake among females and males was more visible among older adolescents aged 15–19 years, compared with the younger cohort of those aged 10–14 years. In many instances, females aged 15–19 years visit HCFs for antenatal and postnatal care.20–24 Higher HIV testing among 15–19-year-old females is also associated with their attendance at HCFs for perinatal or reproductive health issues in the recent past.25,26 By contrast, men aged 15–24 years are more likely to seek care from informal health centers than formal HCFs or to consult their peers.27–29 Our findings of higher HIV testing uptake among adolescent females emphasize the need for more integrated and effective ways of reaching adolescent males with HIV testing services.
In accordance with published data, we report the highest rates of HIV-positivity among the adolescents at inpatient wards and MCH settings. In our study, however, the HIV-positivity rate within MCH settings among older adolescent males (15–19 years) was almost 3 times that of older adolescent females (15–19 years). We speculate that adolescent males 15–19 years of age may be newly identified sexual partners of females seeking MCH-related care. These men may have been invited for partner testing, referred for management of sexually transmitted infections, or tested for HIV as part of integrated services for sexually transmitted infections. MCH settings could become a more focused entry point to reach older female and male adolescents for HIV case identification and linkage to care and treatment services, particularly where active youth-friendly partner notification programs exist.
In the postintervention period, most adolescents testing HIV-positive were identified within the OPD setting. In addition, although our study found an overall lower HIV-positivity in OPD, this setting still remains an important entry point to identify HIV-positive adolescents. Furthermore, we observed an unexpectedly high HIV-positivity rate among adolescents aged 10–14 years within nutrition clinics, suggesting that these adolescents may be perinatally infected, slow progressors. They likely have stunted growth as a result of the untreated HIV infection and thus, were referred for nutrition assessment and support. Nutrition clinics have historically been focused on reaching children younger than 5 years, and HIV testing and counseling rates there are high among children, with reported HIV-positivity rates around 14%–21%.30–32 Our study suggests that a stronger focus on nutrition clinics may be needed to optimize HIV case identification among adolescents, particularly for those younger than 15 years.
Moreover, we report numbers of overall adolescents tested and those testing HIV-positive to be significantly higher within dispensaries as compared to health centers and hospitals. Dispensaries generally are closer to clients and tend to serve the bulk of public health needs. Because the dispensaries are usually the first point of contact for many within the community, the uptake of testing and linkage to care services was significantly higher once they became the entry points for these services. The fewer number of staff at dispensary level of care makes capacity building quick, thereby allowing for rapid uptake and utilization of the tools introduced and facilitating buy in. This suggests the higher reach of testing at lower-level facilities such as the dispensaries, which is where most clients seek care, and provides insight into the decentralization strategy for case finding specifically for hard-to-reach adolescents. In our study, the selected HCF-led outreach testing and other community-based HIV outreach testing activities contributed a minimal number of those tested, and the HIV-positivity was also minimal in these settings.
Our study had several limitations. First, we used programmatic data, which relies on existing health systems, and no patient-level data were obtained. We also were not able to access the total number of adolescents accessing HCFs for health care needs, which could serve as a stronger denominator to the estimates of testing uptake and efficacy of the screening tool. Next, we were unable to collect HIV testing or linkage to care data by entry point during the preintervention. These data would have been helpful to compare with the postintervention data and to identify entry point bottlenecks in linkage to care services. However, in noting this limitation early in the study, we were able to develop program-specific tools that could provide data on entry points in the postintervention period. We also applied age disaggregation to the ongoing data collection that strengthened the data analysis.
CONCLUSIONS
Implementation of the combined adolescent service package, including capacity building, program monitoring tools, adolescent HIV risk screening tool, and adolescent-friendly HCF hours, was effective in optimizing case identification and linkage to care and treatment services for HIV-positive adolescents aged 10–19 years in Western Kenya. Decentralization to lower-level facilities, such as dispensaries, was helpful to improving identification and linkage rates in hard-to-reach communities. Testing in OPD settings continues to identify highest numbers of HIV-positive adolescents. Our findings reinforce the need to improve HIV case identification among adolescent males and suggest that targeting nutrition clinics to reach younger adolescent males and MCH clinics to reach older adolescent males (including male partners to female clients through community-based or self-testing) may hold promise.
Based on out study findings, we conclude that the efforts to maximize HCF-based case identification among adolescent populations in Kenya need to focus on OPD settings. In addition, new case identification among youth should also be strengthened in inpatient departments and particularly for females and males in MCH settings, whereas nutrition clinics could gain significance as a main entry point for younger adolescents. Disaggregated data collection and analysis are critically important in setting targets and evaluating the best strategies to increase HIV case finding among adolescents. Further research is needed to identify the best ways to reach adolescents, especially adolescent males, with HIV services.
ACKNOWLEDGMENTS
The authors thank the ELMA Kenya program team for their cooperation and support in the development of this manuscript.
Footnotes
Supported by ELMA program; Grant number: 15- F0027.
The authors have no funding or conflicts of interest to disclose.
REFERENCES
- 1.United Nations Child Fund. For Every Child, End AIDS—Seventh Stocktaking Report, 2016 [Internet]. New York, NY: UNICEF; 2016. Available at: https://data.unicef.org/wp-content/uploads/2016/12/HIV-and-AIDS-2016-Seventh-Stocktaking-Report.pdf. Accessed September 22, 2017. [Google Scholar]
- 2.World Health Organization, Joint United Nations Programme on HIV and AIDS. WHO, UNAIDS Statement on HIV Testing Services: New Opportunities and Ongoing Challenges [Internet]. Geneva, Switzerland: UNAIDS; 2017. Available at: http://www.unaids.org/sites/default/files/media_asset/2017_WHO-UNAIDS_statement_HIV-testing-services_en.pdf. Accessed October 2, 2017. [Google Scholar]
- 3.Gorogodo B, Anyachebelu E, Mabaleng Z, et al. HIV Testing as a critical entry point for a range of services: results from new start sites in four sub-districts in South Africa. Presented at the International AIDS Conference, Durban, South Africa; 2016. Available at: http://www.psi.org/wp-content/uploads/2016/09/Blessing-Gorogodo-Linking-MMC.pdf. Accessed October 2, 2017. [Google Scholar]
- 4.Kurth AE, Lally MA, Choko AT, et al. HIV testing and linkage to services for youth. J Int AIDS Soc. 2015;18(2 suppl 1):19433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. HIV and Adolescents: Guidance for HIV Testing and Counselling and Care for Adolescents Living with. Geneva, Switzerland: WHO; 2013. Available at: http://apps.who.int/iris/bitstream/10665/94334/1/9789241506168_eng.pdf?ua=1. Accessed October 2, 2017. [Google Scholar]
- 6.MacPherson P, Munthali C, Ferguson J, et al. Service delivery interventions to improve adolescents' linkage, retention and adherence to antiretroviral therapy and HIV care. Trop Med Int Health. 2015;20:1015–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong VJ, Murray KR, Phleps BR, et al. Adolescents, young people, and the 90-90-90 goals: to call to improve HIV testing and linkage to treatment. AIDS. 2017;31(suppl 3):S191–S194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson KS, Beima-Sofie KM, Moraa H, et al. At our age, we would like to do things the way we want: a qualitative study of adolescent HIV testing services in Kenya. AIDS. 2017;31(suppl 3):S213–S220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sam-Agudu NA, Folayan MO, Ezeanolue EE. Seeking wider access to HIV testing for adolescents in sub-Saharan Africa. Pediatr Res. 2016;79:838–845. [DOI] [PubMed] [Google Scholar]
- 10.Ruria EC, Masaba R, Kose J, et al. Optimizing linkage to care and initiation and retention on treatment of adolescents with newly diagnosed HIV infection. AIDS. 2017;31(suppl 3):S253–S260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Idele P, Gillespie A, Porth T, et al. Epidemiology of HIV and AIDS among adolescents: current status, inequalities, and data gaps. J Acquir Imune Defic Syndr. 2014;66(suppl 2):S144–S153. [DOI] [PubMed] [Google Scholar]
- 12.Kapogiannis BG, Legins KE, Chandan U, et al. Evidence-based programming for adolescent HIV prevention and care: operational research to inform best practices. J Acquir Immun Defic Syndr. 2014;66(suppl 2):S228–S235. [DOI] [PubMed] [Google Scholar]
- 13.Govindasamy D, Ferrand RA, Wilmore SMS, et al. Uptake and yield of HIV testing and counselling among children and adolescents in sub-Saharan Africa: a systematic review. J Int AIDS Soc. 2015;18:20182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mkandawire P. Assessing factors associated with HIV testing among adolescents in Malawi. Glob Public Health. 2017;12:927–940. [DOI] [PubMed] [Google Scholar]
- 15.Mavedzenge DN, Luecke E, Ross DA. Effective approaches or programming to reduce adolescent vulnerability to HIV infection, HIV risk, and HIV-related morbidity and mortality: a systematic review of systematic reviews. J Acquir Immune Defic Syndr. 2014;66(suppl 2):S154–S169. [DOI] [PubMed] [Google Scholar]
- 16.Buzi RS, Madanay FL, Smith PB. Integrating routine HIV testing into family planning clinics that treat adolescents and young adults. Public Health Rep. 2016;131(suppl 1):L130–L138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kenya Ministry of Health and Kenya National AIDS Control Council. Kenya AIDS Response Progress Report, 2016. http://nacc.or.ke/wp-content/uploads/2016/11/Kenya-AIDS-Progress-Report_web.pdf. Accessed September 22, 2017. [Google Scholar]
- 18.Kenya National AIDS Control Council. Kenya Fast-track Plan to End HIV and AIDS Among Adolescents and Young People. Nairobi, Kenya; National AIDS Control Council; 2015. Available at: http://www.ilo.org/wcmsp5/groups/public/–-ed_protect/–-protrav/–-ilo_aids/documents/legaldocument/wcms_532691.pdf. Accessed September 22, 2017. [Google Scholar]
- 19.Sharma M, Ying R, Tarr G, et al. Systematic review and meta-analysis of community and facility-based HIV testing to address linkage to care gaps in sub-Saharan Africa. Nature. 2015;528:S77–S85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ouma J, Asweta Co. Availability of antenatal care content in Kenya: analysis of KDHS 2008/9. Am J Public Health Res. 2017;5:115–123. [Google Scholar]
- 21.Obare F, Kwaak A, Adieri B, et al. HIV-positive adolescents in Kenya: access to sexual and reproductive health services. Bulletin 393, KIT Development Policy and Practice. Available at: http://www.bibalex.org/Search4Dev/files/375056/213610.pdf. Accessed October 2, 2017.
- 22.Kabiru CW, Luke N, Izugbara CO, et al. The correlates of HIV testing and impacts on sexual behavior: evidence from a life history of young people in Kisumu, Kenya. BMC Public Health. 2010;10:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Staveteig S, Wang S, Head SK, et al. Demographic Patterns of HIV Testing Uptake in Sub-Saharan Africa. DHS Comparative Reports No. 30. Calverton, Maryland: ICF International; 2013. Available at: https://dhsprogram.com/pubs/pdf/CR30/CR30.pdf. Accessed October 2, 2017. [Google Scholar]
- 24.Asaolu IO, Gunn JK, Center KE, et al. Predictors of HIV testing among youth in sub-Saharan Africa: a cross-sectional study. PLoS One. 2016;11:e0164052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahande M, Phimemon RN, Ramadhani HO. Factors associated with changes in uptake of HIV testing among young women (15-24) in Tanzania from 2003 to 2012. Infect Dis Poverty. 2012;5:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takarinda KC, Madyira LK, Mhangara M, et al. Factors associated with ever being HIV-tested in Zimbabwe: an extended analysis of the Zimbabwe Demographic and Health Survey (2010-2011). PLoS One. 2016;11:e0147828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar R, Prinja S, Lakshmi PV. Health care seeking behavior of adolescents: comparative study of two service delivery models. Indian J Pediatr. 2008;75:895–899. [DOI] [PubMed] [Google Scholar]
- 28.Mmari KN, OseniO Fatusi AO. STI treatment-seeking behaviors among youth in Nigeria: are there gender differences? Int Perspect Sex Reprod Health. 2010;36:72–79. [DOI] [PubMed] [Google Scholar]
- 29.Newton-Levinson A, Leichliter JS, Chandra-Mouli V. Help and care seeking for sexually transmitted infection among youth in low-and middle-income countries. Sex Transm Dis. 2017;44:319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kankasa C, Carter RJ, Briggs N, et al. Routine offering of HIV testing to hospitalized pediatric patients at university teaching hospital, Lusaka, Zambia: acceptability and feasibility. J Acquir Immune Defic Syndr. 2009;51:202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Midiani D, Saka E, Bhairavabhotia R, et al. Integrating pediatric HIV diagnosis with relevant health programs for optimal pediatric HIV uptake. IAS 2017 [Abstract]. Available at: http://programme.ias2017.org/Abstract/Abstract/4407. Accessed on October 24, 2017.
- 32.Thurstans S, Kerac M, Maleta K, et al. HIV prevalence in severely malnourished children admitted to nutrition rehabilitation units in Malawi: geographical and seasonal variations a cross-sectional study. BMC Pediatr. 2008;8:22. [DOI] [PMC free article] [PubMed] [Google Scholar]