Supplemental Digital Content is Available in the Text.
Visual allodynia, the burden of hypersensitivity to light and patterns, is enhanced in migraine patients, particularly in those with migraine with aura and chronic migraine.
Keywords: Migraine, Visual sensitivity, Allodynia, Cortical excitability
Abstract
Enhanced sensitivity to light (photophobia) and patterns is common in migraine and can be regarded as visual allodynia. We aimed to develop and validate a questionnaire to easily quantify sensitivity to light and patterns in large populations, and to assess and compare visual allodynia across different migraine subtypes and states. We developed the Leiden Visual Sensitivity Scale (L-VISS), a 9-item scale (score range 0-36 points), based on literature and patient interviews, and examined its construct validity. Furthermore, we assessed ictal and interictal visual sensitivity in episodic migraine with (n = 67) and without (n = 66) aura and chronic migraine with (n = 20) and without (n = 19) aura, and in healthy controls (n = 86). Differences between migraine subtypes and states were tested using a linear mixed model with 3 fixed factors (episodic/chronic, with/without aura, and ictal/interictal). Test–retest reliability and construct validity of L-VISS were good. Leiden Visual Sensitivity Scale scores correlated in the expected direction with light discomfort (Kendall's τ = −0.25) and pattern glare tests (τ = 0.35). Known-group comparisons confirmed its construct validity. Within migraine subtypes, L-VISS scores were higher in migraine with aura versus without aura and in chronic versus episodic migraine. The linear mixed model showed all factors affected the outcome (P < 0.001). The L-VISS is an easy-to-use scale to quantify and monitor the burden of bothersome visual sensitivity to light and patterns in large populations. There are remarkable ictal and interictal differences in visual allodynia across migraine subtypes, possibly reflecting dynamic differences in cortical excitability.
1. Introduction
Migraine is a common, multifactorial brain disorder characterized by recurring disabling attacks of headache and associated features (migraine without aura) and, in one-third of patients, neurological aura features (migraine with aura).21 Visual symptoms are common; most auras are visual38 and up to 90% of patients report photophobia during attacks.4 In-between attacks, 60% of migraineurs experience at least some enhanced sensitivity to light32 and many notice abnormal sensitivity to visual patterns40 or visual hallucinations,26 suggesting permanent patient burden caused by disturbed visual processing.
In concordance with “tactile allodynia,” ie, the painful response to nonpainful stimuli, this increased visual sensitivity has been termed “visual allodynia.”27 Tactile allodynia is a common phenomenon among migraineurs,9 in particular, those with chronic migraine1 or migraine with aura.25 Notably, in patients with chronic migraine,11 migraine with aura,7,16 and preictal photophobia,31 neurophysiological and neuroimaging evidence supporting ictal and interictal hyperexcitability of the visual migraine cortex5,16,17 is accumulating.20 Enhanced visual sensitivity might thus reflect visual cortex hyperexcitability,2 which in turn might predispose to cortical spreading depolarization, the likely mechanism for aura.20 These studies, however, were all using complex methods and, accordingly, could only investigate limited numbers of patients and migraine subtypes.
Quantifying sensitivity to light15 and visual patterns23 using questionnaires might be a promising noninvasive method to compare visual allodynia as a proxy for visual cortex excitability across large groups of patients with different migraine subtypes outside and during attacks. Existing questionnaires, however, measure only light or indirect pattern sensitivity and are mostly dichotomous.6,12,14 Moreover, studies applying these questionnaires were small and were focusing on only a single migraine subtype and state, precluding direct comparison between migraine subtypes and states.
We aimed to compare visual allodynia between large study populations across a spectrum of migraine subtypes both during and outside attacks. Therefore, we developed and validated an easy-to-use, self-report instrument to quantify visual sensitivity to light and patterns on a near-continuous linear scale (the “Leiden Visual Sensitivity Scale” [L-VISS]). Subsequently, we applied L-VISS to measure ictal and interictal visual allodynia in 4 large and clinically well-defined migraine subgroups with episodic or chronic migraine with or without aura.
2. Methods
This study consisted of 3 parts: (1) development of the L-VISS questionnaire; (2) validation of L-VISS as a reproducible and reliable easy-to-use self-report instrument to assess visual sensitivity; and (3) assessing and comparing ictal and interictal visual sensitivity using L-VISS in 4 migraine subgroups.
2.1. Participants
Subjects aged 18 to 65 with sufficient command of Dutch to fully understand the questionnaire were recruited from (1) the headache clinics of Leiden University Medical Centre and Tergooi Hospital; and (2) the Leiden University Migraine Neuro-Analysis database,35 which includes prescreened nonheadache controls and people with episodic or chronic migraine, willing to participate in studies on migraine.
Exclusion criteria for all participants were psychiatric or neurological disorder (except migraine for participants with migraine); use of chronic medication (other than oral contraceptives), including migraine prophylactics, in the 4 weeks preceding the measurements (except for participants with chronic migraine); and history of malignancy. Diagnosis was confirmed before participation by telephone interview for participants with episodic or chronic migraine (ie, headache on ≥15 days/month of which at least 8 fulfill migraine criteria) according to the International Classification of Headache Disorders (ICHD)-III-beta criteria.21 They were to have at least one attack per month in the 6 months before the measurement day. Controls and their first-degree relatives could not have migraine nor could they have any other form of headache on more than 1 day per month. The study was approved by the Medical Ethics Committee of Leiden University Medical Center, and all participants provided written informed consent before inclusion.
2.2. Development of Leiden Visual Sensitivity Scale and data collection
Items for the self-report scale to quantify sensitivity for light and patterns were based on the migraine literature13,19 and structured in-depth interviews with patients with migraine. After several revisions, using the feedback of patients with migraine from think-aloud interview sessions, we selected the items. For all items, we used a 5-point Likert-type response scale, to measure the degree rather than presence of visual sensitivity, and 5-point scales yield the best data quality.37 Per item, these 5 options were provided: “not at all” (0 points), “slightly” (1 point), “moderately” (2 points), “severely” (3 points), and “very severely” (4 points). Outcome of the scale (L-VISS score) is defined as the sum of the responses to all 9 questions (range 0-36 points). Participants were instructed to complete the questionnaire (a web-based or identical printed version) based on their experiences during the last month. Controls completed the questionnaire once. Participants with migraine completed the questionnaire twice, once while focusing on the interictal state and once while focusing on the ictal state. Patients with chronic migraine could opt-out for the interictal part of the questionnaire if they felt unable to identify an interictal state.
2.3. Measuring pattern glare and light discomfort
As part of the validation process of L-VISS, pattern glare and light sensitivity were measured in subgroups of controls and participants with episodic migraine in-between attacks, ie, at least 3 days after the last attack and at least 3 days before the next attack. Those who got an attack within 3 days after the measurement day were excluded. Measurements took place on the same day on which the participants completed L-VISS for the first time: first the pattern glare test and then, after an interval of at least 5 minutes, the light discomfort test. Participants with chronic migraine were not included in these experiments because they were not expected to be free of migraine for 6 consecutive days. We considered ictal state tests too burdensome for patients.
2.3.1. Pattern glare test
This test is used to measure pattern glare in response to printed patterns.18 Participants are presented with 3 black-and-white horizontally striped patterns with a different spatial frequency (pattern 1: 0.6 cycles per degree [cpd], pattern 2: 4.0 cpd, pattern 3: 12 cpd). Participants were seated in a lighted room at 70-cm distance of the pattern and instructed to binocularly focus for 5 seconds on the fixation dot in the middle of the pattern. Three variants of visual distortion were rated: color, motion (bending of lines and shimmer/flicker), and shapes (blurring of lines, fading, and shadowy shapes). After each measurement, patients were asked whether they suffered from afterimages. Test result was the pattern glare score, defined as the number of the reported visual distortions summed over the 3 patterns (0-9 points; modified from Ref. 42).
2.3.2. Light discomfort test
Individual discomfort to light was quantified using a custom-made setup comparable with other studies.43 All tests were performed in the same room with minimal background lighting. Participants were seated facing a 1000-W halogen lamp (QLT-1000; Falcon Eyes Ltd, Hong Kong, China) with their head positioned on a headrest. Heat-reducing and light-diffusing glass was mounted between the lamp and the headrest. A light intensity sequence was programmed through custom-written software, increasing from 1.6 loglux to 4.4 loglux in 2-second steps of 0.1 loglux with 2-second rest between each step. Light intensity was kept stable by automatic adjustments every 20 ms based on feedback from a luxmeter attached to the headrest above both eyes (SLD-70 BG2A Photodiode; Advanced Photonix, Inc, Ann Arbor, MI). Participants were instructed to indicate when the light intensity became uncomfortable; the test was stopped at that moment. The light discomfort test was repeated 3 times, with intervals of at least 3 minutes between each measurement to avoid habituation to the light stimulus. After each measurement, patients were asked whether they suffered from afterimages. Test outcome was the median light discomfort threshold of 3 subsequent tests.
2.4. Data analysis and statistics
2.4.1. Validity of standardized measurements
To validate our setup for the pattern glare and light discomfort tests, we compared our results with previous findings using these tests in migraine.42,43 Pattern glare scores and light discomfort threshold were compared between participants with episodic migraine and controls using independent-samples t tests. Presence of afterimages was compared between groups using Fisher exact test (light discomfort threshold test) and Pearson χ2 test (pattern glare test).
2.4.2. Internal consistency and test–retest reproducibility
Internal consistency was assessed in participants with episodic migraine (interictal state score) and controls, using Cronbach α coefficient (α ≥ 0.70 considered acceptable), interitem correlation (recommended 0.15-0.50), and item-total correlation (criterion value ≥0.30).34 Test–retest reproducibility was assessed using 1-way intraclass correlation coefficients (ICCs) for the sum score of the L-VISS and the individual items: ICC ≥0.41 acceptable; ICC ≥0.61 good; ICC ≥0.81 excellent.41 For this purpose, both subgroups completed the questionnaire a second time 2 to 3 weeks later.
2.4.3. Comparisons between sensory and behavioral testing
Leiden Visual Sensitivity Scale scores were correlated with pattern glare and light discomfort tests, 2 established measures of pattern and light sensitivity (see above). We hypothesized that L-VISS scores would correlate positively with pattern glare scores (ie, increased visual discomfort correlates with more visual sensitivity) and negatively with light discomfort threshold (ie, increased visual sensitivity correlates with lower light discomfort threshold). Correlations were assessed using Kendall's τ because the L-VISS scores in the validation subgroup (controls and participants with episodic migraine) were skewed to the left and our data set contains ties between scores. Correlations below 0.30 were considered poor, between 0.30 and 0.60 moderate, and above 0.60 good.24 Using independent-samples t tests, we assessed whether L-VISS scores were higher in those who had afterimages after the pattern glare and light discomfort tests compared with those who did not have afterimages.
Two known-group comparisons, ie, comparisons with expected outcome based on information from literature, were conducted. Photophobia is reported by 90% of migraineurs during attacks vs 60% outside attacks4,32 and by only less than 5% of controls.29,32 We hypothesized that L-VISS scores (1) within participants with migraine are higher during compared with outside attacks (paired-samples t test); and (2) in participants with migraine are higher compared with those in controls (independent-samples t test).
2.4.4. Comparison across migraine subtypes
Visual sensitivity was assessed in controls and 4 migraine subtypes outside and during attacks: (1) episodic migraine without aura; (2) episodic migraine with aura; (3) chronic migraine without aura; and (4) chronic migraine with aura. A linear mixed model was fitted on the L-VISS scores. The repeated-measures factor was set to compare the interictal vs ictal scores. Three fixed factors were included (1) diagnosis: episodic vs chronic migraine; (2) aura status: migraine with vs without aura; and (3) attack status: in-between or during the attack. Sex was included as covariate. The 2-way interactions between these factors, and between factors and covariates were also tested.
Baseline subject characteristics and L-VISS scores are reported as mean and SD. Independent t tests, χ2 tests, and Fisher exact test were used for comparison of baseline characteristics when appropriate. For all analyses, P values were considered significant when lower than 0.05. All data analyses were performed with IBM SPSS (version 22.0; Armonk, NY).
3. Results
3.1. Study population
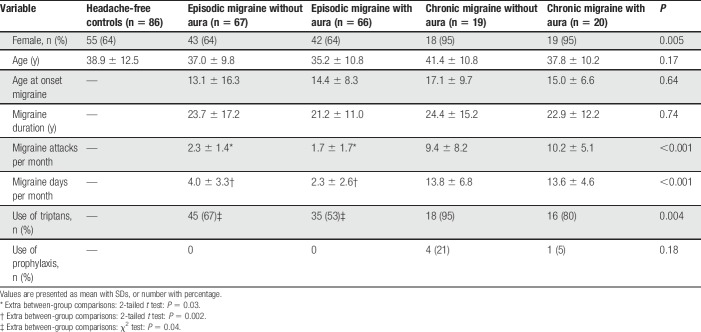
We included in total 258 participants: 133 with episodic migraine (n = 66 with and n = 67 without aura), 39 with chronic migraine (n = 20 with and n = 19 without aura; 19 participants (9 with, 10 without aura) had medication overuse according to ICHD-III-beta criteria21), and 86 age- and sex-balanced nonheadache controls (Table 1). As expected, participants with chronic migraine report more migraine days and attacks per month and higher triptan use compared with those with episodic migraine. Participants with chronic migraine reported sufficient days per month without headache to complete the questionnaire based on interictal days (chronic migraine without aura: 8.0 ± 6.0 headache-free days per month, chronic migraine with aura: 9.3 ± 5.5 days). Chronic migraine groups included more female participants than episodic migraine groups and controls. Triptan users' rate and monthly migraine attack and migraine day frequency were higher in participants with episodic migraine without aura compared to those with aura. Otherwise baseline characteristics of the control and migraine subgroups were similar.
Table 1.
Baseline characteristics of controls and migraine subgroups.
A sample of 146 participants (control: n = 46, episodic migraine without aura: n = 56; episodic migraine with aura: n = 44) completed the pattern glare test and 64 participants (control: n = 20; episodic migraine without aura: n = 23; episodic migraine with aura: n = 21) the light discomfort test. Five of these participants were subsequently excluded from the analysis; 4 (without aura: n = 3) developed a migraine attack within 3 days after the test; and 1 (without aura) had started using prophylactic medication in the period between the telephone interview and the measurement day. Two (without aura: n = 1; with aura: n = 1) participants were excluded from the test–retest analysis because they had completed the retest questionnaire after more than 3 weeks.
3.2. Development of Leiden Visual Sensitivity Scale
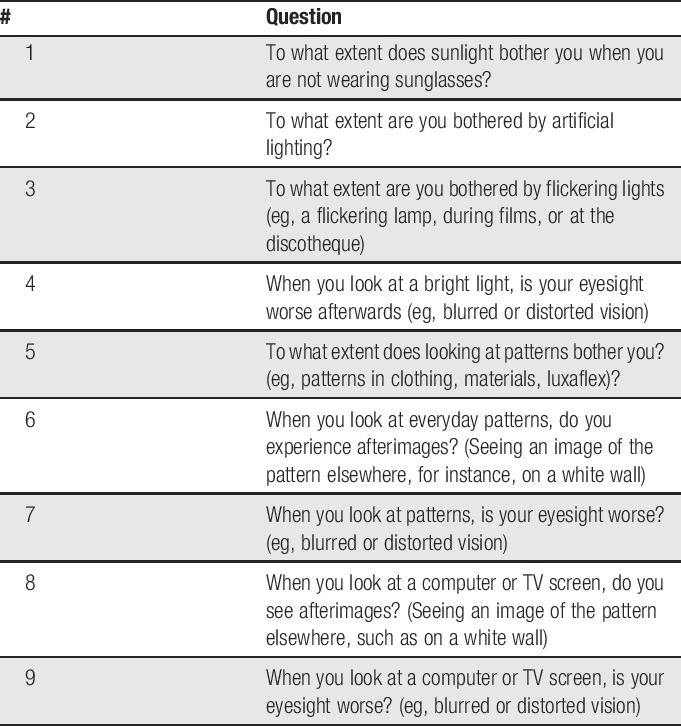
We identified 9 items from the literature13,19 and semistructured interviews with 10 patients with migraine based on their daily life experiences with visual allodynia. Think aloud interviews with 4 controls and 4 patients with episodic migraine did not reveal any missed aspects of visual allodynia, indicating completeness of the questions. These interviews also confirmed content validity (relevance and comprehensiveness) and acceptability of the questions (see Table 2 for an English translation and e-table 1, supplemental digital content, for the original questions in Dutch; available at http://links.lww.com/PAIN/A630).
Table 2.
English translation of the Leiden Visual Sensitivity Scale.
3.3. Standardized measurement of pattern glare and light discomfort
Differences between participants with episodic migraine and nonheadache controls on pattern glare test and light discomfort tests were in line with earlier reports,42,43 confirming the utility of these methods for validation purposes. Participants with migraine experienced more induced illusions when looking at patterns (pattern glare score: 4.9 ± 2.0 vs 3.2 ± 2.3; P < 0.001; Fig. 1A) and had a lower light discomfort threshold (mean threshold: 2.64 ± 0.5 vs 2.98 ± 0.5 loglux; P = 0.02; Fig. 1B).
Figure 1.
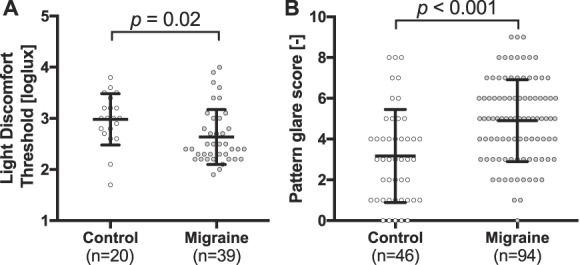
Light discomfort threshold and pattern glare score illustrate visual allodynia in episodic migraine. (A) Light discomfort threshold is decreased in patients with episodic migraine (n = 39, mean ± SD: 2.64 ± 0.5 loglux) compared with healthy controls (n = 20; 2.98 ± 0.5, P = 0.02). (B) Pattern glare score is enhanced in patients with episodic migraine (n = 94; 3.2 ± 2.3) when compared with controls (n = 46; 4.9 ± 2.0, P < 0.001).
Of the participants with episodic migraine, 9/39 (23%) reported nonpersistent afterimages after the light discomfort threshold test compared with 0/20 of nonheadache controls (P = 0.022). For the pattern glare test, the occurrence of afterimages depended on the cpd and was only significant for the 4.0 cpd pattern (Pearson χ2 test, reported as control vs episodic migraine: 0.6 cpd: 26/46 vs 71/100, P = 0.085; 4.0 cpd: 28/47 vs 83/101, P = 0.003; 12 cpd: 32/47 vs 79/101, P = 0.185).
3.4. Internal consistency and test–retest reproducibility
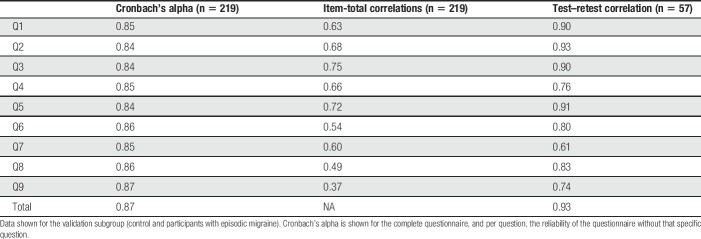
Results of the reliability analysis are presented in Table 3 (see e-table 2, supplemental digital content, which contains results per group, available at http://links.lww.com/PAIN/A630). Internal consistency of the L-VISS was excellent: Cronbach's alpha ranged from 0.73 (controls) to 0.83 (migraine); item-total correlations were above 0.30 except for one question in the control but not migraine group (Q6, correlation 0.23); interitem correlations ranged from 0.15 to 0.61 over all participants, except for one correlation of 0.08 between Q6 and Q8. Test–retest reliability was good to excellent: ICC of L-VISS scores ranged from 0.78 (controls) to 0.93 (migraine). No floor (2.6% reported lowest score) or ceiling (1.5% reported highest score) effects were present in the responses.
Table 3.
Reliability analysis assessed by internal consistency and test–retest reliability.
3.5. Comparisons between sensory and behavioral testing
Construct validity of light- and pattern-related questions was demonstrated by confirming that the pattern glare score and light discomfort threshold correlated with the L-VISS scores in the expected directions (pattern glare score: Kendall's τ = 0.35, P < 0.001; light discomfort threshold: τ = −0.25, P = 0.01; Figs. 2A and B). Presence of afterimages was also associated with a higher L-VISS score for the 4.0 cpd pattern (9.1 ± 5.9 vs 5.9 ± 5.1; P = 0.003), but not for the 0.6 cpd (9.0 ± 5.8 vs 7.1 ± 5.8; P = 0.054) and 12.0 cpd patterns (8.4 ± 5.7 vs 7.8 ± 6.2; P = 0.58) or light discomfort threshold (8.4 ± 6.2 vs 9.1 ± 6.1, P = 0.75). Construct validity was also established by confirming the pretest hypotheses that L-VISS scores are higher: (1) in 133 interictal participants with episodic migraine with or without aura (9.9 ± 5.7) than in 86 controls (3.6 ± 2.8; P < 0.001); and (2) within 133 participants with episodic migraine during (19.7 ± 7.2) compared with outside attacks (9.9 ± 5.7; P < 0.001) (Fig. 2C).
Figure 2.
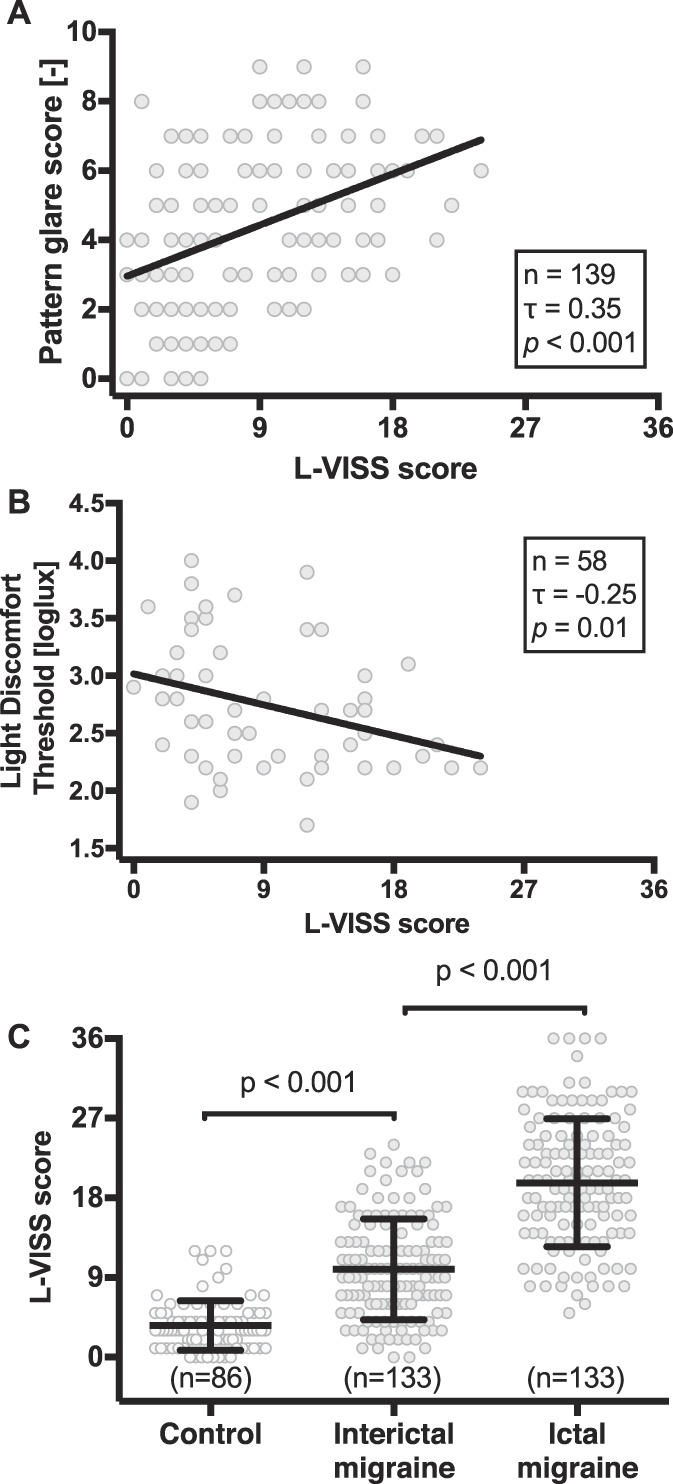
Construct validity was demonstrated by correlations of L-VISS score and standardized measures, and known-group comparisons. (A) Correlation of L-VISS score with pattern glare score (n = 139, of which episodic migraine n = 99; Kendall's τ = 0.35, P < 0.001). (B) Correlation of L-VISS score with light discomfort threshold (n = 58, of which episodic migraine n = 38; Kendall's τ = −0.25, P = 0.01). (C) Known-group comparisons show interictal L-VISS scores are higher in patients with episodic migraine (9.9 ± 5.7) compared with controls (3.6 ± 2.8; P < 0.001), and ictal L-VISS scores (19.7 ± 7.2) are increased compared with interictal scores (P < 0.001). L-VISS, Leiden Visual Sensitivity Scale.
3.6. Comparison across migraine subtypes and states
Mean L-VISS scores were lowest in controls (n = 86; 3.6 ± 2.8; Fig. 3) and highest in participants with chronic migraine with aura during attacks (n = 20; 25.8 ± 7.9). In between these extremes, L-VISS scores outside attacks were 8.5 ± 5.7 for episodic migraine without aura (n = 67), 11.3 ± 5.4 for episodic migraine with aura (n = 66), 10.9 ± 6.2 for chronic migraine without aura (n = 19), and 17.8 ± 6.9 for chronic migraine with aura (n = 20). During attacks, L-VISS scores were 18.3 ± 7.8 for episodic migraine without aura (n = 67), 21.2 ± 6.3 for episodic migraine with aura (n = 66), and 23.0 ± 8.0 for chronic migraine without aura (n = 19). Diagnosis, aura status, and attack status all influenced outcome (P < 0.001 for each factor). There were no significant 2-way interactions between these factors (all P > 0.11). Sex (P = 0.77) nor its interactions with 3 factors did affect outcome (all P > 0.12). Thus, L-VISS scores were higher: (1) in chronic vs episodic migraine, both for migraine with and without aura as well as during and outside attacks; (2) in migraine with aura vs without aura, both in episodic and chronic migraine as well as during and outside attacks; and (3) during vs outside attacks, both in episodic and chronic migraine as well as in migraine with and without aura.
Figure 3.
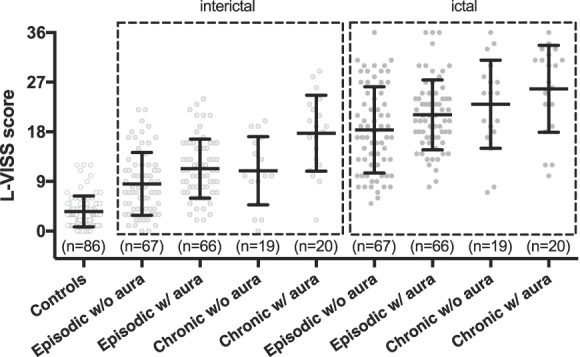
Individual L-VISS scores and mean per subgroup demonstrate effect of aura and chronic migraine on visual sensitivity. Participants with migraine reported interictal (light gray) and ictal (dark gray) scores. Subgroup scores are presented as mean and SD. Healthy controls (mean L-VISS score 3.6), and participants with episodic migraine without (interictal: 8.5/ictal: 18.3) and with aura (11.3/21.2) and participants with chronic migraine without (10.9/23.0) and with aura (17.8/25.8) were compared in-between and during attacks. Diagnosis, aura status, and attack status all affected the outcome (P < 0.001 per factor). L-VISS, Leiden Visual Sensitivity Scale.
Migraine attack frequency was weakly correlated with the L-VISS scores outside (r = 0.263; P = 0.001) and during attacks (r = 0.241; P = 0.002); aura frequency, however, was not correlated (r = −0.073; P = 0.570). The use of prophylactic medication in chronic migraine did not affect the L-VISS score in-between (P = 0.52) or during attacks (P = 0.16).
4. Discussion
We developed, validated, and applied L-VISS, an easy-to-use, 9-item, self-report questionnaire to rapidly and reliably quantify sensitivity to light and patterns on a near-continuous linear scale in large study populations. Leiden Visual Sensitivity Scale scores were higher in migraineurs: (1) with aura vs without aura; (2) with chronic vs episodic migraine; (3) during vs outside attacks; and (4) vs nonheadache controls, for all 4 migraine subtypes and both during and outside attacks. These findings reveal a fluctuating burden of visual allodynia, in particular, in patients with chronic migraine or migraine with aura, both outside and even more during attacks, and are well in line with, but do not prove, the hypothesis that the migraine brain is hyperexcitable.20
To the best of our knowledge, L-VISS is the first instrument to quantify visual sensitivity to light and patterns on a single, near-continuous, linear scale, enabling direct comparisons across multiple groups. Other instruments all use dichotomous or qualitative scales.6,12,14 Items included in the questionnaire were selected based on interviews with migraine patients and their feedback on the relevance and acceptability of these items. Validity was established over a broad range of tests. Internal consistency and test–retest reliability were both good to excellent, and there were no floor or ceiling effects. In an experimental setting, L-VISS scores increased with increasing light discomfort and pattern glare as measured with standard established psychophysical and behavioral tests,42,43 indeed suggesting that changes in L-VISS scores reflect changes in both phenomena.24 In known-group comparisons, L-VISS scores were higher in interictal migraineurs compared with controls and, within migraineurs, during compared with outside attacks. Construct validity was also confirmed by the finding that participants with afterimages to pattern glare reported higher L-VISS scores.
Various pathophysiological processes have been proposed to underlie photophobia and probably other forms of visual sensitivity, including enhanced excitability of the visual cortex.33 Leiden Visual Sensitivity Scale cannot differentiate between these different mechanisms. However, results from neurophysiological and neuroimaging studies, measuring cerebral excitability more directly,15 support the view, albeit indirectly, that differences in L-VISS scores might reflect differences in visual cortex excitability. Cortical excitability profiles across migraine subgroups and states in these studies7,11,15–17 were remarkably similar to the intersubgroup differences we found for L-VISS scores. Interictal excitability was higher in migraine with aura vs migraine without aura7,15,16 and in chronic vs episodic migraine.11 During attacks, visual sensitivity scores were increased even further, probably reflecting the symptom photophobia that might be caused by ictal increase of visual cortical excitability.7,17 Moreover, self-reported photophobia correlated well with visual cortex excitability as measured with positron emission tomography31 and blood-oxygen-level dependent activation after visual stimulation.15,23
Tactile allodynia and photophobia have both been linked to elevated levels of calcitonin gene–related peptide (CGRP),36 an important neurotransmitter in migraine pathophysiology.20 Moreover, the CGRP receptor–antagonist telcagepant has been shown to improve photophobia.22 Speculatively, increased visual sensitivity in chronic vs episodic migraine might thus reflect chronic central sensitization similar to what has been proposed for tactile allodynia.3,30 As CGRP plasma levels were higher in people with chronic migraine, in particular, in those with chronic migraine with aura,10 increased visual sensitivity might potentially reflect increased CGRP activity. Also, in triptan therapy, it was shown that treatment is more effective in patients with migraine with signs of tactile allodynia when triptans are administered before establishment of allodynic symptoms.8 The analgesic action of triptans seems to be specifically effective before central sensitization increases during the migraine attack.28 The level of visual allodynia as measured using the L-VISS might thus potentially prove a simple predictive test for migraine prophylactic efficacy of CGRP-blocking therapies39 and possibly be helpful in selecting candidates for early initiation of triptan treatment.
Recall and selection bias might have influenced our results, but we deem the risk and potential impact limited. Risk of recall bias, eg, by focusing while responding to L-VISS questions on the most recent days or on days with the most extreme visual hypersensitivity rather than on the whole month, or for chronic migraine by focusing not only on headache-free days but also tension-type headache days, cannot be excluded but is unlikely to explain differences between migraine subgroups. Participants with migraine with visual aura might perhaps have been focused more on visual symptoms. Selection bias, eg, because subjects with abnormal visual sensitivity were more likely to participate in this study than those without abnormal visual sensitivity, is also unlikely to have had a major effect. Most (76%) controls and participants with episodic migraine were in fact participating in studies which were unrelated to visual sensitivity and to which completing the L-VISS was added.
Leiden Visual Sensitivity Scale is a well-validated and inexpensive, easy-to-use, self-report instrument to reliably quantify and monitor visual allodynia in large study populations. Visual allodynia contributes to the burden of migraine, not only during but also outside migraine days. Our findings add to the clinical evidence that suggests hyperexcitability of the visual cortex is related to visual symptoms in patients with migraine, particularly in migraine with aura and in chronic migraine, and is increased during migraine attacks.
Conflict of interest statement
M.D. Ferrari reports grants and consultancy or industry support from Medtronic and independent support from the Netherlands Organization for Scientific Research (NWO), NIH, European Community, and the Dutch Heart Foundation. The remaining authors have no conflict of interest to declare.
This work was supported by grants of the Netherlands Organization for Scientific Research (NWO) (Dutch national science prize, Spinoza 2009 Award); and European Community (within the European Union's Seventh Framework program [“EUROHEADPAIN,” Grant agreement no. 602633]).
Supplementary Material
Acknowledgements
The authors thank Dr A.J. Shepherd for her advice concerning the pattern glare testing, C. de Wijs for technical development of the light discomfort test, and Ms J. Sykes for the translation of the Dutch L-VISS questionnaire to English for this publication.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/A630.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
M.J.L. Perenboom, A.H. Zamanipoor Najafabadi contributed equally to this work as first authors. J.A. Carpay, M.D. Ferrari contributed equally to this work as last authors.
References
- [1].Ashkenazi A, Sholtzow M, Shaw JW, Burstein R, Young WB. Identifying cutaneous allodynia in chronic migraine using a practical clinical method. Cephalalgia 2007;27:111–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Aurora SK, Wilkinson F. The brain is hyperexcitable in migraine. Cephalalgia 2007;27:1442–53. [DOI] [PubMed] [Google Scholar]
- [3].Bernstein C, Burstein R. Sensitization of the trigeminovascular pathway: perspective and implications to migraine pathophysiology. J Clin Neurol 2012;8:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bigal ME, Liberman JN, Lipton RB. Age-dependent prevalence and clinical features of migraine. Neurology 2006;67:246–51. [DOI] [PubMed] [Google Scholar]
- [5].Boulloche N, Denuelle M, Payoux P, Fabre N, Trotter Y, Géraud G. Photophobia in migraine: an interictal PET study of cortical hyperexcitability and its modulation by pain. J Neurol Neurosurg Psychiatry 2010;81:978–84. [DOI] [PubMed] [Google Scholar]
- [6].Braithwaite JJ, Marchant R, Takahashi C, Dewe H, Watson DG. The Cortical Hyperexcitability Index (CHi): a new measure for quantifying correlates of visually driven cortical hyperexcitability. Cogn Neuropsychiatry 2015;20:330–48. [DOI] [PubMed] [Google Scholar]
- [7].Brighina F, Bolognini N, Cosentino G, MacCora S, Paladino P, Baschi R, Vallar G, Fierro B. Visual cortex hyperexcitability in migraine in response to sound-induced flash illusions. Neurology 2015;84:2057–61. [DOI] [PubMed] [Google Scholar]
- [8].Burstein R, Collins B, Jakubowski M. Defeating migraine pain with triptans: a race against the development of cutaneous allodynia. Ann Neurol 2004;55:19–26. [DOI] [PubMed] [Google Scholar]
- [9].Burstein R, Yarnitsky D, Goor-Aryeh I, Ransil BJ, Bajwa ZH. An association between migraine and cutaneous allodynia. Ann Neurol 2000;47:614–24. [PubMed] [Google Scholar]
- [10].Cernuda-Morollón E, Larrosa D, Ramón C, Vega J, Martínez-Camblor P, Pascual J. Interictal increase of CGRP levels in peripheral blood as a biomarker for chronic migraine. Neurology 2013;81:1191–6. [DOI] [PubMed] [Google Scholar]
- [11].Chen WT, Wang SJ, Fuh JL, Lin CP, Ko YC, Lin YY. Persistent ictal-like visual cortical excitability in chronic migraine. PAIN 2011;152:254–8. [DOI] [PubMed] [Google Scholar]
- [12].Choi JY, Oh K, Kim BJ, Chung CS, Koh SB, Park KW. Usefulness of a photophobia questionnaire in patients with migraine. Cephalalgia 2009;29:953–9. [DOI] [PubMed] [Google Scholar]
- [13].Chronicle EP, Wilkins AJ. Colour and visual discomfort in migraineurs. Lancet 1991;338:890. [DOI] [PubMed] [Google Scholar]
- [14].Conlon EG, Lovegrove WJ, Chekaluk E, Pattison PE. Measuring visual discomfort. Vis Cogn 1999;6:637–63. [Google Scholar]
- [15].Cucchiara B, Datta R, Aguirre GK, Idoko KE, Detre J. Measurement of visual sensitivity in migraine: validation of two scales and correlation with visual cortex activation. Cephalalgia 2015;35:585–92. [DOI] [PubMed] [Google Scholar]
- [16].Datta R, Aguirre GK, Hu S, Detre JA, Cucchiara B. Interictal cortical hyperresponsiveness in migraine is directly related to the presence of aura. Cephalalgia 2013;33:365–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Denuelle M, Boulloche N, Payoux P, Fabre N, Trotter Y, Géraud G. A PET study of photophobia during spontaneous migraine attacks. Neurology 2011;76:213–8. [DOI] [PubMed] [Google Scholar]
- [18].Evans BJW, Stevenson SJ. The Pattern Glare Test: a review and determination of normative values. Ophthalmic Physiol Opt 2008;28:295–309. [DOI] [PubMed] [Google Scholar]
- [19].Evans RW, Digre KB. Expert opinion light sensitivity in migraineurs. Headache 2003;43:917–20. [DOI] [PubMed] [Google Scholar]
- [20].Ferrari MD, Klever RR, Terwindt GM, Ayata C, van den Maagdenberg AMJM. Migraine pathophysiology: lessons from mouse models and human genetics. Lancet Neurol 2015;14:65–80. [DOI] [PubMed] [Google Scholar]
- [21].Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013;53:137–46. [DOI] [PubMed] [Google Scholar]
- [22].Ho TW, Ferrari MD, Dodick DW, Galet V, Kost J, Fan X, Leibensperger H, Froman S, Assaid C. Efficacy and tolerability of MK-0974 (telcagepant), a new oral antagonist of calcitonin gene-related peptide receptor, compared with zolmitriptan for acute migraine: a randomised, placebo-controlled, parallel-treatment trial. Lancet 2008;372:2115–23. [DOI] [PubMed] [Google Scholar]
- [23].Huang J, Zong X, Wilkins A, Jenkins B, Bozoki A, Cao Y. fMRI evidence that precision ophthalmic tints reduce cortical hyperactivation in migraine. Cephalalgia 2011;31:925–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Innes E, Straker L. Validity of work-related assessments. Work 1999;13:125–52. [PubMed] [Google Scholar]
- [25].Jakubowski M, Silberstein S, Ashkenazi A, Burstein R. Can allodynic migraine patients be identified interictally using a questionnaire? Neurology 2005;65:1419–22. [DOI] [PubMed] [Google Scholar]
- [26].Jürgens TP, Schulte LH, May A. Migraine trait symptoms in migraine with and without aura. Neurology 2014;82:1416–24. [DOI] [PubMed] [Google Scholar]
- [27].Kowacs P, Utiumi M, Piovesan E. The visual system in migraine: from the bench side to the office. Headache 2015;55:84–98. [DOI] [PubMed] [Google Scholar]
- [28].Levy D, Jakubowski M, Burstein R. Disruption of communication between peripheral and central trigeminovascular neurons mediates the antimigraine action of 5HT 1B/1D receptor agonists. Proc Natl Acad Sci U S A 2004;101:4274–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lipton RB, Liberman JN, Kolodner KB, Bigal ME, Dowson A, Stewart WF. Migraine headache disability and health-related quality-of-life: a population-based case-control study from England. Cephalalgia 2003;23:441–50. [DOI] [PubMed] [Google Scholar]
- [30].Louter MA, Bosker JE, van Oosterhout WPJ, van Zwet EW, Zitman FG, Ferrari MD, Terwindt GM. Cutaneous allodynia as a predictor of migraine chronification. Brain 2013;136:3489–96. [DOI] [PubMed] [Google Scholar]
- [31].Maniyar FH, Sprenger T, Schankin C, Goadsby PJ. Photic hypersensitivity in the premonitory phase of migraine—a positron emission tomography study. Eur J Neurol 2014;21:1178–83. [DOI] [PubMed] [Google Scholar]
- [32].Mulleners WM, Aurora SK, Chronicle EP, Stewart R, Gopal S, Koehler PJ. Self-reported photophobic symptoms in migraineurs and controls are reliable and predict diagnostic category accurately. Headache 2000;41:31–9. [DOI] [PubMed] [Google Scholar]
- [33].Noseda R, Burstein R. Migraine pathophysiology: anatomy of the trigeminovascular pathway and associated neurological symptoms, cortical spreading depression, sensitization, and modulation of pain. PAIN 2013;154:S44–53. [DOI] [PubMed] [Google Scholar]
- [34].Nunnally JC, Bernstein IH. The theory of measurement error. New York: McGraw-Hill, 1994. [Google Scholar]
- [35].van Oosterhout WPJ, Weller CM, Stam AH, Bakels F, Stijnen T, Ferrari MD, Terwindt GM. Validation of the web-based LUMINA questionnaire for recruiting large cohorts of migraineurs. Cephalalgia 2011;31:1359–67. [DOI] [PubMed] [Google Scholar]
- [36].Recober A, Kuburas A, Zhang Z, Wemmie JA, Anderson MG, Russo AF. Role of calcitonin gene-related peptide in light-aversive behavior: implications for migraine. J Neurosci 2009;29:8798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Revilla MA, Saris WE, Krosnick JA. Choosing the number of categories in agree-disagree scales. Sociol Methods Res 2014;43:73–97. [Google Scholar]
- [38].Russell MB, Olesen J. A nosographic analysis of the migraine aura in a general population. Brain 1996;119:355–61. [DOI] [PubMed] [Google Scholar]
- [39].Russo AF. Calcitonin gene-related peptide (CGRP): a new target for migraine. Annu Rev Pharmacol Toxicol 2015;55:533–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Shepherd A. Increased visual after-effects following pattern adaptation in migraine: a lack of intracortical excitation? Brain 2001;124:2310–18. [DOI] [PubMed] [Google Scholar]
- [41].Shrout PE. Measurement reliability and agreement in psychiatry. Stat Methods Med Res 1998;7:301–17. [DOI] [PubMed] [Google Scholar]
- [42].Tibber MS, Guedes A, Shepherd AJ. Orientation discrimination and contrast detection thresholds in migraine for cardinal and oblique angles. Invest Ophthalmol Vis Sci 2006;47:5599–604. [DOI] [PubMed] [Google Scholar]
- [43].Vanagaite J, Pareja JA, Storen O, White LR, Sanc T, Stovner LJ, Støren O, White LR, Sand T, Stovner LJ. Light-induced discomfort and pain in migraine. Cephalalgia 1997;17:733–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.