Abstract
INTRODUCTION:
Adherence to aromatase inhibitor (AI) therapy is poor, often due to treatment-emergent side effects, including musculoskeletal symptoms, fatigue and insomnia. In this analysis, we examined sleep patterns and daytime function both objectively using actigraphy and subjectively using validated questionnaires in women initiating AI therapy.
METHODS:
Post-menopausal women with stage 0-III hormone-receptor positive breast cancer who were initiating AI therapy were eligible. Patients wore actigraphy devices for 10 consecutive days and completed questionnaires at baseline prior to initiation of AI and 3 months after AI therapy. Associations between baseline demographics and symptoms, changes in patient-reported outcomes (PROs) and actigraphy measures from baseline to three months on AI therapy and discontinuation of AI therapy were examined using sign tests, logistic regression models, Spearman correlation and linear mixed models.
RESULTS:
Forty two patients (86%) completed baseline assessments and 23 patients (47%) completed both baseline and 3 month assessments. Objectively measured daytime function as measured by total daytime activity decreased over 3 months after starting AI (232566 activity count (AC) v. 204205 AC, p=0.023), and the decrease was more evident in women with higher baseline physical function. Reduced daytime activity was correlated with increased fatigue (ρ=−0.49, p=0.017).
CONCLUSIONS:
Daytime function decreased after initiation of AI therapy and was moderately correlated with increased fatigue, although no association was identified with change in pain or sleep quality. Additional studies are required to understand why function is reduced, which could have implications for interventions to improve tolerance of and persistence with AI therapy.
Keywords: Breast cancer, adjuvant endocrine therapy, aromatase inhibitors, actigraphy, patient-reported outcomes, treatment discontinuation
MICROABSTRACT:
Treatment-emergent side effects often affect adherence to aromatase inhibitor (AI) therapy. In this study, we examined sleep patterns and daytime function both objectively using actigraphy and subjectively using validated questionnaires in women on AI therapy. Daytime function significantly decreased after 3 months on AI therapy and was correlated with increased fatigue. Larger studies are necessary to understand why daytime function is affected by AI therapy and to identify interventions to improve daytime function, which may be helpful in improving adherence to AI therapy.
INTRODUCTION
Aromatase inhibitor (AI) therapy is commonly used for adjuvant treatment of postmenopausal women with hormone receptor-positive breast cancer. New data have emerged favoring extended endocrine therapy beyond five years for women with early-stage breast cancer.1 However, despite these data up to 50% of women discontinue treatment prior to 5 years.2, 3 Poor adherence and persistence with endocrine therapy have been associated with worse breast cancer-related mortality.4–6
Treatment-emergent toxicity is a key reason for early treatment discontinuation. In the multicenter prospective randomized Exemestane and Letrozole Pharmacogenetics (ELPh) trial in postmenopausal women with early stage breast cancer, 32.4% of patients discontinued initial AI therapy within 2 years with a median time to treatment discontinuation of 6 months.7 Of the patient-reported reasons for discontinuation during treatment, AI-associated musculoskeletal syndrome (AIMSS) was the primary reason for treatment discontinuation, while fatigue and insomnia were the next leading causes.7 Women with poor sleep quality prior to AI initiation were more likely to discontinue therapy because of toxicity by one year compared to women with good sleep quality.8 It is unknown how or if AIMSS impact fatigue and insomnia, as well as daytime activity.
The majority of studies reporting the effect of AI therapy on sleep have relied on subjective assessment with patient-reported outcomes. Previously, objective assessment required polysomnography, which remains the gold standard for assessment of sleep, but this is burdensome and costly. More recently, studies have demonstrated that actigraphy, an objective measurement used to quantify sleep and circadian rhythms, has a high correlation with polysomnography. Over the last two decades, the use of actigraphy has gained popularity since it utilizes non-invasive methodology, is low cost, and provides accurate sleep measurements in an ambulatory or home setting.9, 10 While changes in sleep quality have been previously reported using objective and subjective assessments in breast cancer patients undergoing surgery, chemotherapy and radiation, there is a lack of published studies evaluating changes in daytime function and sleep disturbance using actigraphy and validated surveys in patients receiving treatment with AI therapy.
In this study, we examined changes in daytime function and sleep parameters in patients on AI therapy both objectively using actigraphy and subjectively using validated questionnaires. We hypothesized that symptoms from AI therapy would interfere with daytime function and sleep for a subset of treated patients. In addition, we hypothesized that patients with worsening daytime function and/or sleep disturbance over time are more likely to discontinue AI therapy.
METHODS
Study Participants
Participants were recruited from December 2013 through February 2016 at the University of Michigan Comprehensive Cancer Center. Post-menopausal women with stage 0-III hormone-receptor positive breast cancer who had completed all indicated surgery, radiation therapy, and chemotherapy and were initiating treatment with an AI were eligible for enrollment. Patients who previously received tamoxifen therapy were permitted to enroll. Those with sleep apnea requiring continuous positive airway pressure, restless leg syndrome requiring medication for treatment, prior treatment with an AI, a history of medical or arthritic diseases that would interfere with evaluation of pain or activity level, use of a wheelchair for ambulation most of the time, or those working second or third shifts with non-traditional sleep schedules were excluded from the study. The Institutional Review Board at the University of Michigan approved the clinical trial (clinicaltrials.gov NCT01983995), and patients were required to provide written informed consent prior to undergoing study-related procedures.
Study Procedures
Prior to initiation of AI therapy, enrolled patients wore the actigraphy device for 10 consecutive days and completed validated questionnaires, described below. Patients then initiated AI therapy with anastrozole 1 mg daily, exemestane 25 mg daily, or letrozole 2.5 mg daily; choice of AI medication was at the discretion of the treating provider. Three months after initiation of AI therapy, patients again completed 10 consecutive days of actigraphy and the same validated questionnaires.
Outcome Measures
Actigraphy
Daytime activity and sleep were assessed using the wrist-worn Actiwatch Spectrum Pro accelerometer (Philips Healthcare, Bend, OR). This device is an omnidirectional accelerometer that records acceleration changes at a frequency of 32 times per second and stores the data as “activity counts” per 30-second sampling period. Parameters used to evaluate daytime activity included total daytime activity (sum of all valid activity counts), average daytime activity per minute, daytime maximum activity (the highest valid activity count obtained), immobile time and variability in activity. Daytime activity parameters were measured as activity counts during awake hours, while immobile time was measured as minutes of presumed rest pre-defined by the actigraphy algorithm during daytime hours. Daytime maximum activity and variability in activity were used to characterize maximum activity performed and changes in activity during daytime hours, respectively.
Sleep quality was assessed using the following parameters: total sleep time, sleep time during night hours, wake after sleep onset, total activity at night and sleep efficiency. Total sleep time and wake after sleep onset (during time in bed) were determined by actigraphy sleep algorithm. A sleep diary was used in conjunction with actigraphy to verify sleep and wake times, as well as record pain levels and sleep aids. Total activity at night was defined as pre-defined movement captured by the accelerometer during night time hours, while sleep efficiency was defined as time asleep divided by time in bed.
Questionnaires
Each patient completed validated questionnaires at baseline and three months after initiation of AI therapy. The validated questionnaires used for the study include questionnaires assessing sleep quality [Pittsburgh Sleep Quality Index (PSQI)11 and Patient-Reported Outcomes Measurement Information System (PROMIS) Sleep Disturbance12], fatigue [PROMIS Fatigue13], physical function [PROMIS Physical Function14], symptoms due to endocrine therapy [Functional Assessment of Cancer Therapy-Endocrine Subscale (FACT-ES)15] and pain [Brief Pain Inventory (BPI)16, 17].
The PSQI measures sleep quality and disturbance retrospectively over the previous month. It consists of 19 items that produce a global sleep quality score and seven component scores: sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbance, use of sleep medications, and daytime dysfunction.11 Higher scores reflect worse sleep quality. Psychometric data suggests the PSQI is a valid and reliable instrument to use in women with breast cancer.18
The PROMIS Sleep Disturbance, PROMIS Fatigue and PROMIS Physical Function are 8-item, 7-item and 10-item questionnaires, respectively, developed by the NIH roadmap initiative PROMIS (www.NIHPROMIS.org) used to characterize sleep and physical function in those with or without sleep disorders. Raw scores are translated to a T-score, with a mean score of 50 and a 10 point standard deviation. Higher scores represent a greater amount of the quantity being measured (e.g., more sleep disturbance, better physical function).
The FACT-ES is a 47-item questionnaire used to measure the quality of life (QOL) as well as side effects of endocrine treatment in patients with breast cancer. It assesses 4 QOL domains (physical, emotional, social/family, and functional well-being) as well as 19 items that measure symptoms commonly related to endocrine therapy. Higher scores reflect better quality of life. It has been validated in women with breast cancer and has good test-retest reliability.15
The BPI is a 17-item patient self-rating scale that assesses sensory and reactive components of pain. For the sensory components, it addresses severity, location, chronicity, and degree of relief due to therapy. For the reactive components, it assesses depression, suffering, and perceived availability of relief.19 It has been validated in patients with both cancer and non-cancer pain.16, 17 Higher scores reflect more pain or pain interference.
Statistical Analysis
The primary objective of the study was to characterize changes in daytime function and sleep patterns through actigraphy and validated questionnaires in women on AI therapy. Significant changes from baseline to three months in actigraphy parameters and patient-reported symptoms were assessed using sign tests. Pairwise associations between baseline patient demographics, baseline patient symptoms, changes in patient symptoms, and changes in actigraphy were examined using Spearman correlation coefficients. Changes in measures were computed as the difference in the observed value at the month 3 and baseline visits. For actigraphy measures collected daily during the 10-day reporting period, the mean of each measure was used to summarize each visit.
The McNemar and sign tests were performed to evaluate whether there was a significant difference in the proportion of women taking sleep medications and the number of sleep medications used at baseline and after three months of AI therapy, respectively. Sleep medications were defined as any medication used to induce sleep, such as sedatives (i.e. benzodiazepines), hypnotic agents (i.e. zolpidem tartrate) or other sleep aids (i.e. melatonin, diphenhydramine-containing products, etc.).
A chronologic association between sleep and daytime activity was assessed using linear mixed models scaling and transforming the outcome variable if needed. In separate models, we evaluated sleep and daytime activity bidirectionally as both predictors and outcomes. Specifically, sleep efficiency and sleep time at night were the variables of interest to quantify sleep whereas total activity and average activity per minute were of interest to quantify daytime activity. All mixed models included a fixed visit effect and random subject effect and assessed the addition of the sleep or daytime activity predictor using a chi-square test of nested models. Logistic regression assessed the associations between binary outcomes, including discontinuation of therapy and use of prior chemotherapy, with baseline patient characteristics and changes in both actigraphy and patient-reported symptoms. Discontinuation of therapy was defined as discontinuation of AI for >14 days, including women who temporarily held AI therapy for at least 14 days prior to resuming AI.
The threshold for statistical significance was pre-specified as p-value <0.05 and no correction was made for multiple comparisons due to the small sample size and exploratory nature of the analysis.
RESULTS
Patient characteristics
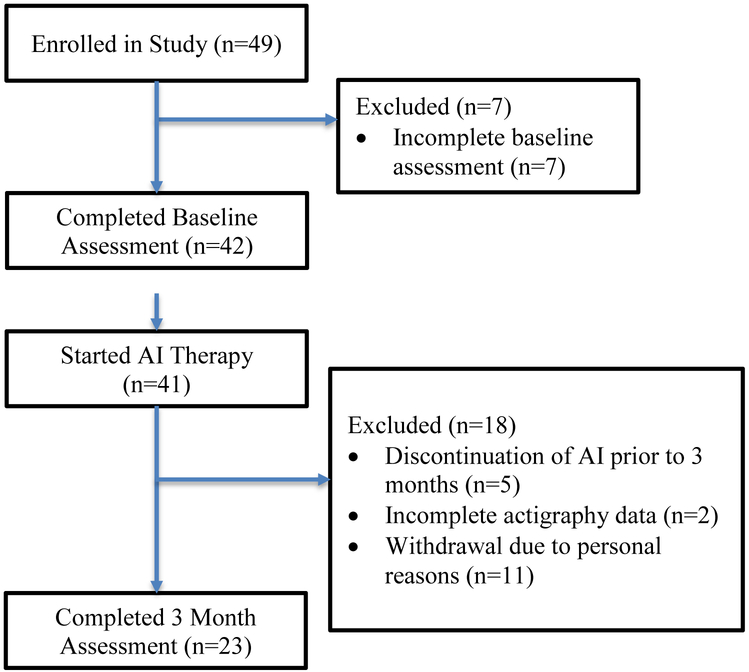
Forty-two of the 49 (86%) enrolled patients completed baseline assessments and twenty-three (47%) patients completed both baseline and 3 month assessments (Figure 1). Eighteen patients had incomplete 3 month assessments due to discontinuation of AI therapy prior to 3 months (N=5), incomplete actigraphy data (N=2) or withdrawal due to personal reasons (N=11). Participants were predominantly white (94%) with stage I disease (71%) (Table 1). In addition to undergoing surgical resection, fourteen patients received neoadjuvant or adjuvant chemotherapy (29%) and twenty-nine patients (59%) underwent adjuvant radiation therapy. Seven patients (14%) had previously received tamoxifen. All but two enrolled patients started treatment with anastrozole (96%).
Figure 1: Consort Diagram of Patient Enrollment.
AI: aromatase inhibitor.
Table 1:
Demographic information (N=49)
| Patient Characteristic | Number (%) |
|---|---|
| Mean age, years (range) | 63 (49–86) |
| Body mass index, mean (range) | 28.5 (19.5–42.2) |
| Ethnicity | |
| Hispanic/Latino | 3 (6.1) |
| Non-Hispanic/Latino | 41 (83.7) |
| Not Reported | 5 (10.2) |
| Race | |
| Asian | 2 (4.1) |
| Black or African American | 1 (2.0) |
| White | 46 (93.9) |
| Tumor Stage | |
| Stage I | 35 (71.4) |
| Stage II | 14 (28.6) |
| Receptor Status | |
| Estrogen Receptor Positive | 49 (100) |
| Progesterone Receptor Positive | 44 (89.8) |
| HER2 Receptor Positive | 6 (12.2) |
| Treatment | |
| Surgery and Endocrine Therapy | 35 (71.4) |
| Surgery, Chemotherapy and Endocrine Therapy | 14 (28.6) |
| Radiation Therapy | 29 (59.2) |
| Trastuzumab | 5 (10.2) |
| Adjuvant Endocrine Therapy | |
| History of Tamoxifen | 7 (14.3) |
| Anastrozole | 47 (95.9) |
| Letrozole | 2 (4.1) |
| Exemestane | 0 (0) |
Changes in actigraphy and patient-reported symptoms during AI therapy
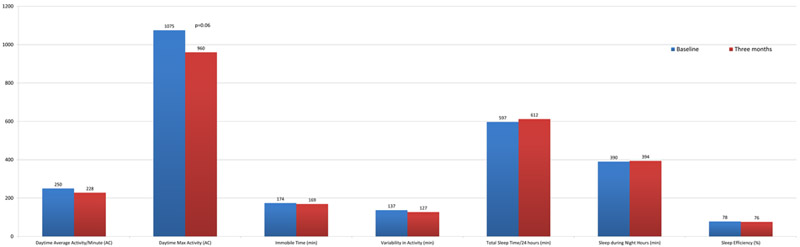
Daytime function as measured by total daytime activity exhibited a significant overall decrease between baseline and three months after starting AI therapy (232566 activity count (AC) v. 204205 AC, p=0.023; Table 2). Of the 24 patients with paired baseline and 3 month total AC, 5 patients (21%) had a modest 10–20% reduction in total daytime activity and 8 (33%) had a substantial >20% decrease in activity, whereas the activity level for 6 (25)% of patients remained relatively stable and 5 (21%) had an increased total activity level of at least 10%. There was also a trend towards reduced daytime maximum activity [1075 AC v. 960 AC, p=0.06; Figure 2] over three months. No statistically significant differences between visits were found in daytime average activity per minute, variability in activity, or immobile time. In addition, there were no statistically significant changes in any sleep parameters during the first 3 months of AI therapy.
Table 2: Change in actigraphy variables on aromatase inhibitor therapy over 3 months for the entire cohort.
AC: activity count; min: minutes
| Change in Measure |
Visit 1 Mean N=38–39 |
Visit 2 Mean N=27–28 |
p-value |
|---|---|---|---|
| Daytime Activity (AC) | 232565.8 | 204204.7 | 0.023 |
| Daytime Average Activity per Minute (AC) | 249. 6 | 227.7 | 0.15 |
| Daytime Maximum Activity (AC) | 1075.2 | 959.9 | 0.06 |
| Variability in Activity (AC) | 137.3 | 126.6 | 0.54 |
| Immobile Time (min) | 173.7 | 169.0 | 0.84 |
| Total Sleep Time (min) | 597.0 | 612.0 | 1.0 |
| Sleep Time during Night Hours (min) | 390.4 | 393.7 | 1.0 |
| Wake After Sleep Onset (min) | 71.0 | 76.5 | 0.42 |
| Sleep Efficiency (%) | 78.3 | 75.8 | 0.11 |
| Total Activity at Night (AC) | 5826.0 | 6460.8 | 0.23 |
Figure 2:
Changes in Actigraphy Measures on AI Therapy
During the first 3 months of AI therapy there was a statistically significant increase in mean worst pain (baseline 1.38, 3 month 2.17, p=0.035) and a trend towards an increase in mean average pain (baseline 0.87, 3 month 1.27, p=0.09; Table 3). There were no significant changes in any other patient-reported symptoms during the three month period. Sleep medications were used by nine women at baseline and twelve women after three months of AI therapy; this difference was not statistically significant.
Table 3: Change in patient-reported symptoms on aromatase inhibitor therapy over 3 months for the entire cohort.
PSQI: Pittsburgh Sleep Quality Index, PROMIS: Patient-Reported Outcomes Measurement Information System, FACT-ES: Functional Assessment of Cancer Therapy-Endocrine Subscale, BPI: Brief Pain Inventory
| Change in Measure |
Visit 1 Mean N=45–49 |
Visit 2 Mean N=30–31 |
p-value |
|---|---|---|---|
| PSQI | 5.6 | 5.9 | 0.68 |
| PROMIS Sleep Disturbance (T-score) | 46.7 | 45.5 | 0.18 |
| PROMIS Physical Function (T-score) | 50.9 | 49.7 | 0.38 |
| PROMIS Fatigue (T-score) | 49.7 | 47.9 | 0.71 |
| FACT-ES | 153.2 | 154.6 | 0.86 |
| BPI Average | 0.9 | 1.3 | 0.09 |
| BPI Worst | 1.4 | 2.2 | 0.035 |
| BPI Interference | 0.9 | 0.8 | 1.0 |
There was no temporal association between sleep and daytime activity. The prior night’s sleep quality (sleep efficiency and total sleep at night) was not found to affect activity the following day (p=0.66 and p=0.11, respectively). Similarly, activity on a given day (total activity during the day and average daytime activity per minute) was not found to affect that night’s sleep quality (p=0.31 and p=0.80, respectively).
Associations between baseline patient characteristics and changes in actigraphy or patient-reported symptoms
Patients with higher physical function at baseline experienced a larger decrease in daytime function after the first three months of AI therapy. Moderate-to-strong correlations between baseline physical function as measured by PROMIS physical function scale and total daytime activity (Spearman correlation coefficient ρ=−0.64, p=0.001), daytime average activity per minute (ρ=−0.70, p=0.0002), and variability in activity (ρ=−0.68, p=0.0003) were identified (Supplemental Table A). In addition, there was a moderately strong correlation between age and change in total sleep time from baseline to three months on AI therapy, with older patients experiencing a larger decrease in total sleep time (ρ=−0.43, p=0.039; Supplemental Table B). Furthermore, there was a moderate positive correlation between lower body mass index (BMI) and a greater decrease in daytime activity over 3 months on AI therapy (ρ=0.48, p=0.017). There were no associations between prior chemotherapy or other baseline patient-reported symptoms with changes in actigraphy (Supplemental Table C and data not shown).
Age was also associated with change in pain interference over time. Older patients experienced less pain interference from baseline to 3 months after initiation of AI (ρ=−0.40, p=0.039; Supplemental Table D). There were no correlations between baseline BMI and changes in patient-reported symptoms on AI therapy and no association between prior chemotherapy and changes in patient symptoms on AI therapy.
Associations between change in patient-reported symptoms and change in actigraphy
Patient-reported physical function assessed by PROMIS physical function was moderately to strongly positively correlated with changes in daytime function, as assessed with multiple parameters (Table 4). A larger reduction in patient-reported physical function correlated with a larger reduction in total daytime activity (ρ=0.61, p=0.0026), daytime average activity per minute (ρ=0.69, p=0.0004) and variability in activity (ρ=0.51, p=0.016). In addition, increase in physical function from baseline to three months was associated with a larger decrease in total sleep time (ρ=−0.63, p=0.0018) and immobile time during daytime hours (ρ=−0.56, p=0.006).
Table 4:
Association between change in patient-reported physical function and changes in actigraphy variables over 3 months
|
Change in Actigraphy Measure |
Spearman Correlation Coefficient |
P-Value |
|---|---|---|
| Daytime Activity (n=22) | 0.61 | 0.0026 |
| Daytime Avg. Activity per Minute (n=22) | 0.69 | 0.0004 |
| Daytime Maximum Activity (n=22) | 0.20 | 0.38 |
| Variability in Activity (n=22) | 0.51 | 0.016 |
| Immobile Time (n=22) | −0.56 | 0.006 |
| Total Sleep Time (n=22) | −0.63 | 0.0018 |
| Wake After Sleep Onset (n=21) | −0.04 | 0.86 |
| Sleep Efficiency (n=21) | 0.02 | 0.92 |
| Total Activity at Night (n=21) | −0.10 | 0.68 |
Patient-reported fatigue assessed by PROMIS fatigue was moderately negatively correlated with changes in daytime function and moderately positively correlated with change in total sleep time (Table 5). Women with more fatigue at three months compared to baseline had a larger decrease in daytime activity (ρ=−0.49, p=0.017) and daytime average activity per minute (ρ=−0.48, p=0.020) at three months after AI initiation. Patients with more fatigue at three months compared to baseline also had a larger increase in total sleep time at three months compared to baseline (ρ=0.38, p=0.08). Changes in patient-reported symptoms of sleep disturbance, sleep quality, symptoms from endocrine therapy, and pain were not associated with changes in actigraphy daytime or sleep variables.
Table 5:
Association between change in patient-reported fatigue and changes in actigraphy variables over 3 months
|
Change in Actigraphy Measure |
Spearman Correlation Coefficient |
P-Value |
|---|---|---|
| Daytime Activity (n=23) | −0.49 | 0.017 |
| Daytime Avg. Activity per Minute (n=23) | −0.48 | 0.020 |
| Daytime Maximum Activity (n=23) | −0.08 | 0.73 |
| Variability in Activity (n=23) | −0.35 | 0.010 |
| Immobile Time (n=23) | 0.30 | 0.16 |
| Total Sleep Time (n=23) | 0.38 | 0.08 |
| Wake After Sleep Onset (n=22) | −0.14 | 0.54 |
| Sleep Efficiency (n=22) | 0.19 | 0.39 |
| Total Activity at Night (n=22) | −0.19 | 0.39 |
Discontinuation of AI therapy
Thirteen of the forty nine (26.5%) enrolled patients discontinued AI therapy between the time of enrollment and November 2016. There were no associations between discontinuation of therapy and baseline patient characteristics, including age, BMI, baseline patient-reported symptoms and actigraphy variables. Similarly, there were no associations between discontinuation of AI therapy at any time and changes in any of these variables during the first 3 months of AI therapy.
DISCUSSION
In this analysis of the effects of AI therapy on daytime activity and sleep disturbance, we utilized both actigraphy measures and validated subjective questionnaires to thoroughly evaluate changes in both daytime activity and sleep quality in women on AI therapy. Our findings suggest that AI therapy can negatively affect patients’ daytime activity without a statistically significant change in sleep patterns. In addition, this decrease in physical activity does not appear to be related to persistent effects of their prior therapy. These results have implications for patient management and development of strategies to improve persistence with AI therapy.
As expected, pain modestly worsened after initiation of AI therapy. Although it is possible that an increase in pain may have contributed to a decrease in daytime activity, we were unable to find such an association in our cohort. Alternatively, other factors such as fatigue also likely contributed to decreased activity, as demonstrated by the strongly negative correlation between change in fatigue and daytime activity parameters during the 3 months following initiation of AI therapy. It is unknown whether patients experienced increased fatigue, which led them to reduce their activity levels, or whether the decreased activity resulted in more fatigue. It is also unclear if this is independent of pain or sleep disturbance, although patients experiencing fatigue may subsequently experience more pain because of inactivity. However, guidelines recommend physical activity as a key intervention for managing chronic fatigue in cancer patients.20 In addition, a randomized phase III trial demonstrated that moderate exercise can improve pain in women with AIMSS. Therefore, proactively encouraging patients to be more active could potentially lead to improved tolerance of therapy and increased persistence with therapy.21
While musculoskeletal symptoms have been clearly shown to be a primary cause of premature discontinuation of AI therapy, it is unknown what role other potentially modifiable symptoms such as sleep disturbance play. While we hypothesized that sleep quality would worsen on AI therapy, in this relatively small study we were unable to identify any associations between AI discontinuation and changes in either objective assessment of sleep quality or sleep disturbance with actigraphy or patient-reported sleep symptoms. This is in contrast to the findings of larger studies that relied on patient-reported symptoms.8, 22 We also examined the temporal relationship between daytime activity and sleep quality. However, we were unable to demonstrate an impact on the prior night’s sleep quality on the subsequent day’s activity, or an impact of the daytime activity on sleep quality the following night. Therefore, the magnitude of the effect of sleep disturbance on AI-associated symptoms and persistence with therapy remains poorly defined.
We closely examined the associations between actigraphy measures and patient-reported symptoms to determine whether actigraphy provided additional insight into daytime activity and sleep quality in women on AI therapy. Use of actigraphy failed to add substantially to the patient-reported assessment of activity since there was a high correlation between changes in PROMIS physical function and the objectively-assessed changes in daytime activity. In contrast, changes in the other subjective and objective sleep quality assessments were not as highly correlated. While we may be able to rely on patient-reported symptoms for routine evaluation of daytime function, it is difficult to determine the role of objective versus subjective measures for examination of sleep quality in women on AI therapy based on the findings in this study, in part because of the low variability in sleep measures across patients and time points using the different methodologies. In addition, there are specific instances where objective measurement of activity may be beneficial, such as examination of associations between physical activity levels and other symptoms.23
The strengths of this study include utilizing complementary subjective and objective measurement of daytime function and sleep quality, in addition to validated questionnaires to assess multiple other symptoms in women initiating AI therapy. However, the low variability in daytime activity and sleep variables and high study discontinuation rate due to factors other than toxicity limit the interpretation of the results. Accrual to this study was slow in part because of timing issues related to endocrine therapy initiation. Some patients had their treatment discussion with the oncologist prior to completion of radiotherapy and were not interested in returning to clinic to enroll in the study, and some patients did not want to wait an additional 10 days while they wore the actigraphy device prior to initiating AI therapy.
Additional research is needed to independently validate the findings in this study, and to determine why activity levels decrease in patients after they start AI therapy. In addition, it will be important to understand the impact of these changes in physical function and activity on adherence and persistence to AI therapy. If future studies confirm significantly reduced daytime activity in women on AI therapy, and in particular if the reduction in activity is associated with decreased tolerance of therapy, intervention for patients specifically to increase activity levels at the time of AI initiation will be warranted.
Supplementary Material
CLINICAL PRACTICE POINTS.
While multiple studies have outlined the early and late-onset side effects of aromatase inhibitor (AI) therapy, to our knowledge this is the first study to examine sleep patterns and daytime function objectively using actigraphy and subjectively using validated questionnaires in women starting AI therapy. Daytime function was significantly reduced after 3 months of AI therapy and was moderately correlated with increased fatigue. Changes in daytime function were also correlated with changes in subjectively measured physical function. Further investigation is necessary to confirm these results and identify interventions to improve daytime function for women on AI therapy.
Acknowledgements:
NLH was a Damon Runyon-Lilly Clinical Investigator supported (in part) by the Damon Runyon Cancer Research Foundation (grant number CI-53–10). Support for KAS was provided by the Cancer Center Biostatistics Training Grant 5T32-CA083654 (to J.G.M. Taylor).
Footnotes
Disclosures:
None of the authors have any relevant disclosures to report.
References:
- 1.Burstein HJ, Temin S, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology Clinical Practice Guideline focused update. J Clin Oncol 2014;32:2255–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28:4120–4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Partridge AH, LaFountain A, Mayer E, Taylor BS, Winer E, Asnis-Alibozek A. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol 2008;26:556–562. [DOI] [PubMed] [Google Scholar]
- 4.Hershman DL, Shao T, Kushi LH, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat 2011;126:529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barron TI, Cahir C, Sharp L, Bennett K. A nested case-control study of adjuvant hormonal therapy persistence and compliance, and early breast cancer recurrence in women with stage I-III breast cancer. Br J Cancer. 2013;109:1513–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chirgwin JH, Giobbie-Hurder A, Coates AS, et al. Treatment adherence and its impact on disease-free survival in the Breast International Group 1–98 trial of tamoxifen and letrozole, alone and in sequence. J Clin Oncol 2016;34:2452–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henry NL, Azzouz F, Desta Z, et al. Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. J Clin Oncol 2012;30:936–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kidwell KM, Harte SE, Hayes DF, et al. Patient-reported symptoms and discontinuation of adjuvant aromatase inhibitor therapy. Cancer. 2014;120:2403–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madsen MT, Huang C, Gogenur I. Actigraphy for measurements of sleep in relation to oncological treatment of patients with cancer: a systematic review. Sleep Med Rev. 2015;20:73–83. [DOI] [PubMed] [Google Scholar]
- 10.Sadeh A The role and validity of actigraphy in sleep medicine: an update. Sleep Med Rev 2011;15:259–267. [DOI] [PubMed] [Google Scholar]
- 11.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- 12.Yu L, Buysse DJ, Germain A, et al. Development of short forms from the PROMIS sleep disturbance and Sleep-Related Impairment item banks. Behav Sleep Med 2011;10:6–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cook KF, Jensen SE, Schalet BD, et al. PROMIS measures of pain, fatigue, negative affect, physical function, and social function demonstrated clinical validity across a range of chronic conditions. J Clin Epidemiol 2016;73:89–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen RE, Potosky AL, Reeve BB, et al. Validation of the PROMIS physical function measures in a diverse US population-based cohort of cancer patients. Qual Life Res 2015;24:2333–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fallowfield LJ, Leaity SK, Howell A, Benson S, Cella D. Assessment of quality of life in women undergoing hormonal therapy for breast cancer: validation of an endocrine symptom subscale for the FACT-B. Breast Cancer Res Treat 1999;55:189–199. [DOI] [PubMed] [Google Scholar]
- 16.Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J Pain. 2004;5:133–137. [DOI] [PubMed] [Google Scholar]
- 17.Tittle MB, McMillan SC, Hagan S. Validating the brief pain inventory for use with surgical patients with cancer. Oncol Nurs Forum. 2003;30:325–330. [DOI] [PubMed] [Google Scholar]
- 18.Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh Sleep Quality Index. J Psychosom Res. 1998;45:5–13. [DOI] [PubMed] [Google Scholar]
- 19.Daut RL, Cleeland CS, Flanery RC. Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain. 1983;17:197–210. [DOI] [PubMed] [Google Scholar]
- 20.Bower JE. Cancer-related fatigue--mechanisms, risk factors, and treatments. Nat Rev Clin Oncol 2014;11:597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McTiernan A, Irwin M, Vongruenigen V. Weight, physical activity, diet, and prognosis in breast and gynecologic cancers. J Clin Oncol 2010;28:4074–4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desai K, Mao JJ, Su I, et al. Prevalence and risk factors for insomnia among breast cancer patients on aromatase inhibitors. Support Care Cancer. 2013;21:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy SL, Smith DM, Clauw DJ, Alexander NB. The impact of momentary pain and fatigue on physical activity in women with osteoarthritis. Arthritis Rheum. 2008;59:849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.