Abstract
Background:
The phospholipid mediator platelet activating factor (PAF) activates an inflammatory response that includes arachidonic acid release and prostaglandin production in the eye, increasing vascular permeability and inflammation.
The purpose of this study is to investigate the action of LAU-0901, a novel PAF receptor antagonist, on experimental uveitis.
Methods:
Uveitis was induced in Lewis rats by lipopolysaccharide (LPS) treatment. LAU-0901 was then delivered systemically in different concentrations at plus 4 and 16 hours, or vehicle injected as controls. Additional animals were used for histological analyses of untreated, uveitis, and uveitis-plus-LAU-0901 retinas. Conventional histological and immunohistochemical methods were employed. A slit lamp and Spectral Domain-Ocular Coherence Tomography (SD-OCT) retinal imager was used for anterior segment photography and posterior pole OCT. Rats were euthanized 4 hours after the second LAU-0901 injection in this 24-hour model. Aqueous humor was collected and quantified, and also analyzed for tumor necrosis factor alpha (TNF-α).
Results:
Uveitic eyes demonstrated hypopyon formation, leukocyte infiltration, and an increase in aqueous protein and TNF-α levels. LAU-0901 treatment resulted in a dose-dependent reduction in inflammation, reflected by reduced total protein levels (up to a 64% reduction). Moreover, hypopyon was prevented, leukocytes were absent in vitreous and aqueous humor, and TNF-α levels were reduced by 91%.
Conclusions:
The PAF receptor antagonist LAU-0901 decreases ocular inflammation in a rat model of anterior uveitis in a dose-dependent manner, suggesting that use of this molecule may provide a means to attenuate inflammation onset and offer a future alternative or adjunctive treatment for ocular inflammation.
Keywords: Inflammation, LAU-0901, Lipopolysaccharide, Neuroprotection, Platelet activating factor, Uveitis
Introduction
Uveitis is an ocular inflammatory disease responsible for 10–20% of blindness1 with an estimated incidence of 1 in 200 people developing uveitis in the United States.2 Ocular inflammation from uveitis may cause cataract, glaucoma, persistent macular edema, and potential destruction of the retina and choroid. Permanent visual loss is usually due to retinal insults from longstanding cystoid macular edema or from retinal tissue damage and scarring caused by the release of arachidonic acid metabolites, cytokines, and oxidative processes.3
Many of the systemic therapies for uveitis continue to have significant side effects and are used off-label. Currently, the mainstay of local therapy is corticosteroid treatment, but this is a major cause of glaucoma and cataracts,4 limiting its usefulness for long-term administration. Systemic immunosuppression with anti-metabolites is sometimes necessary and may have few ocular side effects, but long-term use may increase susceptibility to infections, anemia, and neoplasm. The use of newer biologic agents, usually monoclonal antibodies targeted to a particular component of the inflammatory cascade, have been reported with some success. Most recently, the FDA approved the use of the anti-TNF agent Adalimumab (Humira) for the treatment of non-infectious posterior, intermediate, and pan-uveitis; however, this agent and other biologics also have systemic side effects and associated significant adverse events.5,6 Moreover, many patients require more than one agent for disease control and improvement in quality of life. Thus, there is a continued need for the development of effective treatment options for uveitis.
One potential target for therapeutic development is the phospholipid mediator, platelet activating factor (PAF). PAF acts through a G protein-coupled receptor, activating the inflammatory cascade, and has also been implicated in the inflammatory response in uveitis.7–9 PAF activates the release of arachidonic acid and the production of prostaglandins in the eye and increases vascular permeability, which contributes to cystoid macular edema, a common cause of vision loss in intermediate and posterior uveitis. In addition, PAF has been implicated in the development of chorioretinitis10,11 and chorioretinal neovascularization,12,13 which are devastating complications of uveitis.
LAU-0901 (Louisiana State University and Alcalá (Spain) Universities) is a potent PAF receptor antagonist (Patent # US 6566359 B1. Bazan NG, Sunkel C, Marcheselli V, Alvarez-Builla Gomez J. Assignee: LSUHSC. “2,4,6-Trimethyl-1,4-dihydro-pyridine-3,5-dicarboxylic acid esters as neuroprotective drugs.” 2003) that has been shown to protect photoreceptors from light-induced oxidative stress.14–16 It is highly neuroprotective in cerebral ischemia,17 suppresses inflammation and apoptosis in response to corneal injuries,16 and specifically suppresses cyclo-oxygenase-2 (COX-2) and nuclear factor kappa B (NF-κB), both important mediators of inflammation. Moreover, it represses the chemotaxis of inflammatory cells and inhibits inflammatory responses in retinal, corneal, and neuronal cells,15,16,18 further suggesting neuroprotective, as well as anti-inflammatory bioactivity. Here, we show the potent anti-inflammatory effects of the PAF receptor antagonist LAU-0901, using a well-established endotoxin-induced uveitis (EIU) rodent model: lipopolysaccharide (LPS)-induced uveitis.
Methods
Rats
All animal experiments conformed to the Association for Research in Vision and Ophthalmology (ARVO) statement for the use of animals in ophthalmic and vision research and were approved by the LSUHSC Institutional Animal Care and Use Committee. Male Lewis rats (150–175 g) were obtained from Charles River Laboratories (Wilmington, MA) and maintained in the LSUHSC animal colony on a 12 h:12 h light–dark cycle (0600 h on; 1800 h off) with an average in-cage luminance of 25 lux at bedding level. Animals were fed normal rat chow and supplied with water ad libitum. Eighty-five rats were used to assess the effects of LAU-0901 in control and lipopolysaccharide (LPS)-induced uveitis. Animals were anesthetized with ketamine (90 mg/kg) and xylazine (10 mg/kg) prior to hypopyon analysis and Spectral Domain Ocular Coherence Tomography (SD-OCT) imaging. All rats were sacrificed by CO2 asphyxiation.
Induction of Uveitis and Drug Treatment
Uveitis was induced by injecting 100 μl (200 μg) of LPS into the right hind paw.19 The LPS protocol was validated against a sham at 24 hours post-injection and significantly elevated protein levels in both eyes (data not shown); after validation, sham treatments were no longer administered. When LPS was used alone, these animals became the uveitis control. One μl/g of LAU-0901 was injected intraperitoneally (ip) at different concentrations (15 μg/μl, 30 μg/μl, 45 μg/μl, and 60 μg/μl) 4 hours after LPS (Sigma-Aldich Corp, St Louis, MO) injection, and injections were repeated 16 hours later. One set of control animals did not undergo LPS treatment; another set was injected ip with only the LAU-0901 vehicle (45% cyclodextran, Sigma-Aldrich Corp). In experiments that measured aqueous protein, leukocyte accumulation, and inflammatory marker distribution, rats were treated with 60 μg/μl of LAU-0901. Animals were euthanized 4 hours after the second LAU-0901 injection in this 24-hour model. These treatments were conducted in duplicate.
Ocular Imaging
Rats were anesthetized by ip injection of 100 mg/kg ketamine and 10 mg/kg xylazine (Vedco Inc., St. Joseph, MO) 24 hours after LPS injection, and the eyes were dilated with tropicamide (1%, Bausch & Lomb, Tampa, FL). Then they were examined by slit lamp (Model SL-07; Topcon, Inc., Tokyo, Japan), and images of the anterior segments were captured. Manifestations of inflammation were observed and evaluated. These manifestations included iris and conjunctival vasodilation, flare or cells in the anterior chamber, the formation of hypopyon, fibrin formation in the anterior chamber, and seclusion of the pupil. Vitreal infiltrates and retina were observed by Spectral Domain-Ocular Coherence Tomography (SD-OCT) using the Spectralis HRA+OCT imaging system (Heidelberg Engineering, Heidelberg, DE), and OCT images were archived for later evaluation.
Collection and Analysis of Aqueous Humor
Twenty-four hours after LPS injection, rats from each group were sacrificed and whole eyes removed, rinsed in phosphate-buffered saline (PBS; GIBCO, Grand Island, NY), blotted, and placed on parafilm. A cornea puncture was made with the tip of a #11 surgical blade and the aqueous humor collected from the wax surface with a 200 μm micropipette. Aqueous humor was preserved in 1.5 ml Eppendorf tubes at −20°C until analysis. Total protein concentration in the aqueous humor was used as an indicator of the presence and degree of anterior inflammation. The total protein concentration in each aqueous humor sample was measured using the Bio-Rad Protein Assay kit with an Eppendorf Biophotometer (model 6131, v1.26). Briefly, a 1:500 dilution of the aqueous humor was used. After centrifugation (14,000 rpm for 10 minutes at 4°C), 2 μl of the supernatant was added to 1 ml of 20% Coomassie Brilliant Blue G-250 dye and incubated at room temperature for 10 minutes. Absorbance was determined spectrophotometrically at 595 nm. Protein concentration was then calculated from a standard curve.
Histopathology and Immunohistochemistry
Conventional methods were employed to produce plastic sections for light microscopy. Briefly, following removal of eyes, corneas were slit and globes placed in fixative (2% paraformaldehyde and 2% glutaraldehyde in 0.1 M sodium cacodylate buffer) overnight at 4° C. Eyes were then hemi-sectioned through the optic nerve along the vertical meridian and corneas notched near the superior margin for embedding orientation. After three buffer rinses, tissue was post-fixed for 1 hour in sodium cacodylate-buffered 1% OsO4, and rinsed again. Dehydration occurred through an ethanol series to acetone, followed by embedding in an Epon/Araldite epoxy mixture. Sections (1 μm-thick) were obtained, stained with toluidine blue (1% toluidine blue in 1% sodium borate), viewed by light microscopy, and digitized for analysis.
Immunolocalization was performed on 20 μm-thick cryosections. A dot was placed at the superior edge of the cornea with a fine point permanent marker for embedding and orientation purposes. Eyes were treated as above using 4% fresh paraformaldehyde in PBS and cryoprotected in 15% sucrose for 8 hours and 30% sucrose for 24 hours. Eyes were oriented and embedded in Tissue-Tek OCT embedding compound just prior to sectioning. Sections were placed on glass microscope slides on a 32° C slide warmer for 30 minutes. Some sections were stained with hematoxylin and eosin, while others were prepared for immunolocalization by confocal microscopy. These sections were rinsed, post-fixed with 100% cold methanol, and permeabilized with 1% triton-X 100. After treatment with 95° C citric acid for epitope retrieval, and blocking for 1 hour in 2% donkey serum, primary antibody was placed on each section for 48 hours at 4° C. This was followed by incubation with secondary antibody and nuclear staining for 1 hour at room temperature. Sections were then washed, coverslipped, and imaged. Primary antibodies for localization of inflammation were COX-2 (Cell Signaling, 25 μg/ml) and vascular endothelial growth factor (VEGF) (Santa Cruz Biotechnology, 25 μg/ml). Secondary antibodies for fluorescence detection were AlexaFluor 488 (10 μg/ml) and AlexaFluor 633 (10 μg/ml) (Invitrogen, Carlsbad, CA). Nuclei were stained with Hoechst 33258 (10 μg/mL, H3569, Invitrogen). Sections were imaged with a Zeiss LSM-510 Meta laser confocal microscope with either a 20× air or a 40× oil-immersion objective (Zeiss Plan-NEOFLUAR 40×/1.3 Oil DIC). Visualization of fluorescent signal was obtained as follows (excitation; emission; pinhole Ø): Hoechst 33258 (405nm; 420–490nm; 1.27 Airy), AlexaFluor® 488 (488 nm; 505–550 nm; 1.00 Airy), AlexaFluor® 568 (543 nm; 560–615 nm; 0.94 Airy), AlexaFluor® 633 (633 nm; long pass 650 nm; 0.87 Airy).
Enzyme Linked Immunoassay (ELISA) of TNF alpha
TNF-α was quantified with the Quantikine Immunoassay kit (RTA00, Minneapolis, MN) according to the suggested protocol.
Statistical Analysis
The protein concentration of left and right eyes was measured after inducing uveitis. For LAU-0901 dosage comparisons, treatments were vehicle, 15, 30, 45, and 60 ug/uL LAU-0901. Treatments were administered to 10 animals each (n=50). A repeated measures within-subjects ANOVA design was used here (degrees of freedom = 4). The independent variable was protein concentration and the dependent variable was treatment factor. Post-hoc comparisons of treatments with the vehicle treatment were performed with Dunnet’s test. The mean effects with 95% family-wise confidence levels where * = p<0.05 and *** = p<0.001, demonstrating the protective effect of LAU-0901. All statistics were performed using R.20
For TNF-α quantification, treatments were vehicle alone, LPS-induced uvitis, uveitis-plus-LAU-0901 (60 ug/uL), and LAU-0901 alone. Treatments were administered to 4 animals each (n=16). Data (standard errors shown) were analyzed by one-way ANOVA using Origin software, and demonstrated a significant difference between uveitis and uveitis-plus-LAU-0901 treatments (p=0.003).
Results
LAU-0901 reduced uveitis-induced anterior chamber inflammation on clinical exam
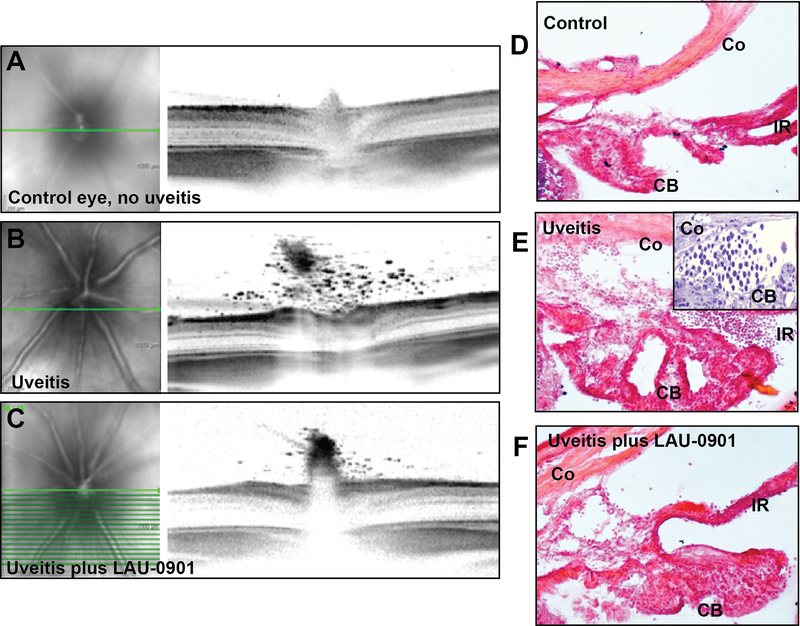
After inducing uveitis we first examined the rats using slit lamp biomicroscopy to evaluate anterior chamber inflammation. Typical findings seen in an anterior uveitis by other groups include dilated iris vessels and hypopyon formation.10,21 Slit lamp photography demonstrated significant anterior chamber cell with hypopyon formation and dilated iris vessels (Figure 1A, B) in the non-treated uveitis rats. Higher magnification slit lamp exam revealed inflammation of the ciliary body with a hypopyon or cellular aggregate behind the iris (Figure 1B), which is consistent findings in a recent study imaging the anterior segment in acute uveitis.22 In contrast to the robust inflammation viewed on exam, LAU-0901-treated rats showed no hypopyon (Figure 1C, D), indicating that the PAF receptor antagonist inhibited the accumulation of leukocytes associated with a severe inflammatory response of the anterior chamber.
Figure 1. LAU-0901 prevents hypopyon formation in the anterior segment.
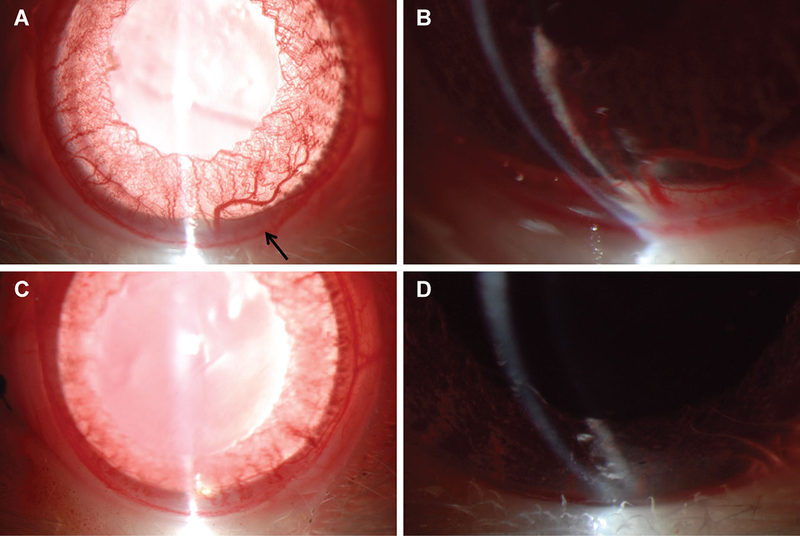
Slit lamp photography of the anterior segment revealed hypopyon formation (arrow), increased prominence of iris vessels, and reduced dilation in animals that received LPS treatment (A). Magnified image reveals the hypopyon in the anterior segment, as well as a similar aggregate in the ciliary sulcus behind the iris (B). However, when LPS treatment was accompanied by LAU-0901 administration, no hypopyon was observed and there was mimimal engorgement of iris vessels (C and D).
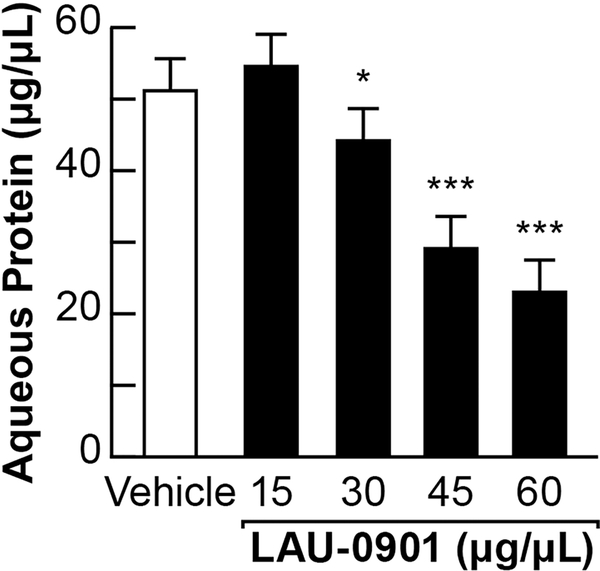
LAU-0901 decreased aqueous humor protein accumulation in EIU
To further characterize the effect of LAU-0901 on EIU in rats, we next conducted a dose-response experiment, measuring aqueous protein levels in response to LAU-0901. Intraperitoneal treatment of uveitic animals with escalating doses of LAU-0901 (15 μg/μl, 30 μg/μl, 45 μg/μl, and 60 μg/μl) resulted in a dose-dependent reduction (54 μg/ml, 43 μg/ml, 29 μg/ml, 19 μg/ml, respectively) of total aqueous protein levels (Figure 2). Treatment of uveitic animals with the LAU-0901 vehicle alone had no modulatory effect (50 μg/ml). In clinical practice, reduction of aqueous protein, also known as “flare”, is a sign of attenuation of uveitic inflammation. Therefore, LAU-0901 reduced the amount of protein in the aqueous humor in a dose dependent response.
Figure 2. LAU-0901 reduces uveitis-induced aqueous humor protein.
A concentration series of 15 μg/μl, 30 μg/μl, 45 μg/μl, and 60 μg/μl, delivered ip, of LAU-0901 administered at 4 and 20 hours post-endotoxin treatment, resulted in a dose-dependent reduction (54 μg/ml, 43 μg/ml, 29 μg/ml, 19 μg/ml, respectively) of total aqueous protein levels. A 64% reduction in aqueous protein with the 60 μg/μl dose occurred.
LAU-0901 decreased infiltration of inflammatory cells to the anterior chamber, iris, ciliary body, and vitreous in EIU
During the initial stages of the inflammatory reaction, the endothelium is activated, allowing for inflammatory cell diapedesis. This results in leukocyte accumulation within the eye at sites close to the endothelial cells. To define the effect of LAU-0901 on inflammatory cell infiltration, we compared control non-uveitic eyes and those with endotoxin-induced uveitis, with and without LAU-0901 treatment, using SD-OCT and conventional histology. SD-OCT images revealed the accumulation of numerous cells in the posterior vitreous of non-LAU-0901-treated rats with uveitis. The number of these cells was greatly reduced in animals treated with LAU-0901, and cells were non-existent in controls (Figure 3A-C). Fundus images associated with each SD-OCT scan delineate the precise location (bright green line) of the OCT image at the optic disk. Cryo-sections were taken through the periphery of the anterior chamber. We selected frozen sections to minimize the possible loss of free-floating cells within the aqueous humor as a result of extensive tissue processing through numerous fixation, dehydration, and embedding steps. These sections revealed the accumulation of cells within the anterior chamber angle and in the posterior segment between the iris and ciliary body. In addition, we conventionally prepared some tissue for hematoxylin and eosin-stained cryosections and toluidine blue-stained plastic sections, which required multiple prolonged steps through various liquids. Despite extensive processing, images of these sections demonstrated comparable accumulations of inflammatory cells in the iris and the ciliary body of LPS induced eyes, and a lack of these cells in the iris and ciliary body of both controls and LAU-0901-treated eyes (Figure 3D-F and inset). Therefore, LAU-0901 reduces inflammatory cell accumulation in the uveal tissue, the anterior chamber angle, and the posterior vitreous as compared to positive controls.
Figure 3. LAU-0901 greatly reduces neutrophil infiltration into uveitic eyes.
SD-OCT imaging of endotoxin-induced uveitis revealed extensive vitreal neutrophil infiltration at the optic nerve, and greatly reduced neutrophil presence in the LAU-0901-treated eyes. No neutrophil infiltration was present in control eyes. The green line on the fundus images at left denotes the position of the scan image to the right (A-C). Hematoxylin and eosine-stained cryo-sections demonstrated dense neutrophil accumulation associated with the ciliary body (arrows) in uveitic eyes, only slight presence in LAU-0901-treated uveitic eyes, and no accumulation in controls (D-F). Inset in E shows neutrophil accumulation within the angle in a 1 μm-thick plastic toluidine blue section. CB, ciliary body; Co, cornea; IR, iris.
LAU-0901 reduced levels of the inflammatory markers COX-2, VEGF, and TNF-α
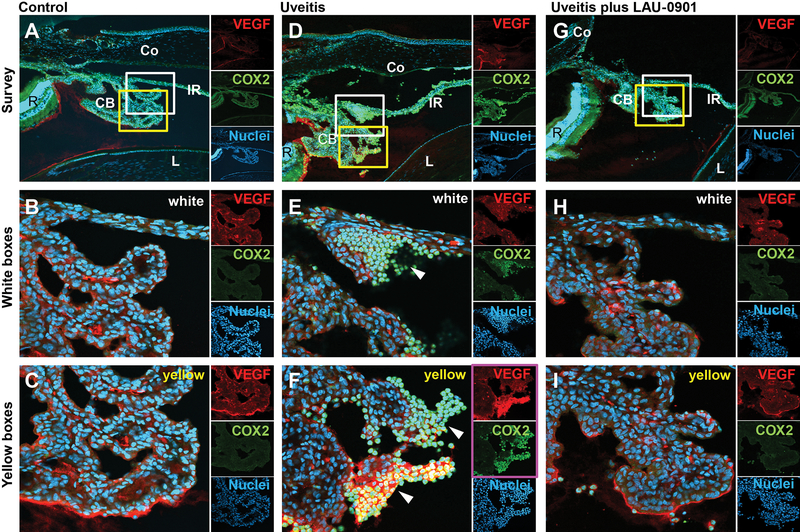
Finally, we wished to determine if LAU-0901 was effective at decreasing the level of certain inflammatory mediators known to be active in uveitis. Thus, we performed immunohistochemistry to define regions where inflammation-associated molecules accumulate in EIU and to monitor the effect of LAU-0901 treatment at these sites. VEGF and COX-2 were prominently abundant within the ciliary body and at the base of the iris in the uveitis eyes. Interestingly, both COX-2 and VEGF labeling were most prevalent along the inner edges of the ciliary processes. However, non-uveitis control eyes and uveitis + LAU-0901-treated eyes showed only uniform background levels of VEGF and no COX-2 labeling within these structures (Figure 4).
Figure 4. LAU-0901 prevents up-regulation of inflammatory markers in the ciliary bodies of uveitic eyes.
Immunolocalization of the inflammatory markers vascular endothelial growth factor (VEGF) and cyclo-oxygenase-2 (COX-2) (A-I) reveals COX-2 highly up-regulated near the base of the iris and the ciliary body, and VEGF up-regulation within the inner regions of the ciliary body in endotoxin-induced uveitic eyes (D-F) (also, white arrow heads in E and F). However, in the LAU-0901-treated animals, both the VEGF and COX-2 labels are reduced to background levels (G-I), similar to the control, untreated animals (A-C). In each of the three treatments, the white and yellow boxes in the top survey images (A, D, and G) are enlarged below (B, E, and H, white boxes; C, F, and I, yellow boxes). The small insets to the right of each image show discreet labeling for the two inflammatory markers (VEGF, red; COX-2, green). Nuclei are labeled blue with Hoechst. CB, ciliary body; Co, cornea; IR, iris; L, lens; R, retina.
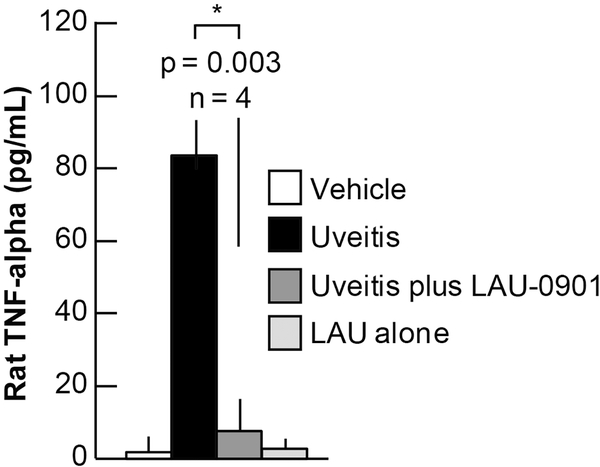
In uveitis, inflammatory mediators from the affected tissues and the infiltrating leukocytes become abundant within the aqueous humor as the disease progresses. One molecule, TNF-α, is quickly up-regulated. We used immunoquantification to determine if LAU-0901 reduced this early response cytokine by employing an ELISA immunoassay to quantify TNF-α within the aqueous humor. TNF-α levels in control non-uveitic, uveitic, uveitic + LAU-0901, and non-uveitic + LAU-0901 rats were measured (2 pg/ml, 83 pg/ml, 7 pg/ml, and 3 pg/ml, respectively). These results demonstrated a low concentration of TNF-α within the aqueous humor of control eyes, those with LAU-0901-treated uveitis, and control eyes with LAU-0901 treatment, as compared to a very high concentration of TNF-α in those eyes with uveitis (Figure 5). Thus LAU-0901 reduces uveitic inflammatory mediators in LPS-induced uveitis eyes as compared to positive controls.
Figure 5. Up-regulation of the inflammatory cytokine TNF-α is prevented by LAU-0901.
Analysis of TNF-α expression in aqueous humor by ELISA revealed about 82 pg/ml in uveitis-induced eyes, while uveitis plus LAU-0901 treatment showed about 8 pg/ml. Control and LAU-0901 alone showed about 2 and 4 pg/ml, respectively, indicating strong suppression (about 98%) of the TNF-α response by LAU-0901.
Discussion
Lipid mediators are potent regulators of inflammation,22–24 and recent advances have helped to further elucidate their roles in neuroprotection and modulation of the immune response.25 The small molecule PAF receptor antagonist LAU-0901 attenuated inflammation in a rat model of EIU and resulted in a clinical decrease in anterior chamber inflammation. SD-OCT imaging also demonstrated a reduction in vitreous cells. Histology of the angle demonstrated rapid recruitment of neutrophils in uveitic eyes and a reduction in this cellularity in LAU-0901-treated animals. Total protein levels, a measure of vascular permeability, were reduced by 64% in LAU-0901-treated versus control eyes. Furthermore, this effect was noted to occur in a dose-dependent fashion.
We have shown that a hallmark of this animal model of uveitis is the infiltration of neutrophils associated with the ciliary body and the optic nerve head, and subsequent hypopyon formation. As inflammation was initiated, we detected a robust increase in TNF-α, a cytokine associated with endothelial cell activation, the up-regulation of adhesion molecules (e.g., selectins), and vascular permeability. Circulating leukocytes interact with these adhesion molecules, ultimately undergoing extravasation from the post-capillary venules through chemoattraction, rolling and tight adhesion, and endothelial transmigration.26,27 We also found high immunolabeling of VEGF associated with the ciliary body. While VEGF is not usually deemed an inflammatory marker, it has been linked to adhesion and migration when associated with integrins and angiogenesis.27 It has been shown that under conditions of stress, PAF and arachidonic acid are released, which enhance prostaglandin synthesis by inducing COX-2 up-regulation.28 Therefore, in this model accumulation of TNF-α, VEGF, and COX-2 in control uveitic retinas indicates onset of inflammation and up-regulation of the events that lead to neutrophil accumulation near the optic nerve head and the ciliary body. In comparison to those control uveitis retinas, little to no evidence of neutrophils was observed in LAU-0901-treated rat eyes over a 24-hour period, which suggests that LAU-0901 acts rapidly in a manner in which the initial cytokine response is inhibited.
In addition to the accumulation of neutrophils, the inflammatory state leads to excessive departure from tissue homeostasis, with eventual apoptosis and neuronal cell death ultimately leading to tissue destruction and permanent damage. In uveitis, chorioretinal damage is one of the main causes of permanent visual loss. It is also known that PAF increases vascular permeability and is associated with choroidal and retinal neovascularization. Thus, inhibition may reduce ocular vascular leakage, which is of significant clinical impact, given the morbidity and risk of vision loss due to uveitis-induced cystoid macular edema and neovascularization. In addition to its anti-inflammatory effect, previous studies with LAU-0901 have shown it is a potent neuroprotector. LAU-0901 treatment of rats in experimental focal cerebral ischemia is highly neuroprotective, greatly reducing neuronal cell death,17,29 and LAU-0901 inhibition of PAF reduces the up-regulation of COX-2 and inhibits prostaglandin production, preventing corneal cell loss.28 Furthermore, in rodent models of light-induced photoreceptor damage, LAU-0901 preserves photoreceptors from oxidative stress-induced apoptotic cell death.15 Thus, LAU-0901 has potential to block many of the downstream destructive effects of the inflammatory state in uveitis.
In summary, we have shown LAU-0901 to be an efficient inhibitor of uveitis in an innate immune response rodent model by six methods: 1) Anterior segment examination of inflammation using slit lamp biomicroscopy; 2) Quantification of aqueous proteins; 3) SD-OCT imaging of neutrophils in the posterior vitreous; 4) Light microscopy of cryo- and plastic sections demonstrating neutrophils associated with the angle, the iris, and the ciliary body; 5) Immunolocalization by confocal microscopy of inflammatory markers associated with the angle, the iris, and the ciliary body; and 6) Quantification of the inflammatory marker TNF-α.
This study strongly highlights the potential of LAU-0901 in the attenuation of inflammation, as well as retinal damage, in posterior uveitis. It also represents PAF as a potential therapeutic target in the treatment of uveitis. To further elucidate the role of LAU-0901 in attenuating the adaptive immune response, we hope to test this agent in the experimental autoimmune uveitis (EAU) rat model. In addition, evaluation of ocular penetration, kinetics, drug efficacy, and toxicity need to be performed.
Acknowledgments
Funding
This work was supported by the Louisiana State University Health Sciences Center Translational Research Initiative (JRE) and National Institutes of Health grants EY005121 and GM103340 (NGB). It was also supported in part by the Louisiana Lions Eye Foundation, New Orleans, LA and by an unrestricted departmental grant from Research to Prevent Blindness, Inc., New York, NY.
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Suttorp-Schulten MS, Rothova A. The possible impact of uveitis on blindness: a literature review. Br J Ophthalmol. 1996;8:844–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gritz D Incidence and prevalence of uveitis in Northern California: The Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004;111:491–500. [DOI] [PubMed] [Google Scholar]
- 3.Rau NA, Wu GS. Free radical mediated photoreceptor damage in uveitis. Prog Retina Eye Res. 2000;19:41–68. [DOI] [PubMed] [Google Scholar]
- 4.Sen HN, Vitale S, Gangaputra SS, Nussenblatt RB, Liesegang TL, Levy-Clarke GA et al. Periocular corticosteroid injections in uveitis: Effects and complications. Ophthalmology. 2014;S0161–6420:00485–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imrie F, Dick A. Biologics in the treatment of uveitis. Curr Opin Ophthalmol. 2007;18:481–486. [DOI] [PubMed] [Google Scholar]
- 6.Jaffe GJ, Dick AD, Brézin AP, Nguyen QD, Thorne JE, Kestelyn P, et al. Adalimumab in Patients with Active Noninfectious Uveitis. N Engl J Med. 2016;375:932–943. [DOI] [PubMed] [Google Scholar]
- 7.Nitoda E, Moschos MM, Mavragani CP, Koutsilieris M. Ocular actions of platelet-activating factor: clinical implications. Expert Opin Ther Targets. 2012;16:1027–1039. [DOI] [PubMed] [Google Scholar]
- 8.Tsuji F, Shirasawa E. The role of platelet-activating factor in cell infiltration in endotoxin-induced uveitis in guinea pigs. Curr Eye Res. 1998;17:501–505. [DOI] [PubMed] [Google Scholar]
- 9.Garland RC, Sun D, Zandi S, Xie F, Faez S, Tayyari F et al. Noninvasive molecular imaging reveals role of PAF in leukocyte-endothelial interaction in LPS-induced ocular vascular injury. FASEB J. 2011;25:1284–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenbaum JT, Angell E, Wilson D, Broquet C, Boney RS, Braquet P. Intravitreally injected platelet activating factor induces retinitis in experimental animals. Curr Eye Res. 1999;18:342–348. [DOI] [PubMed] [Google Scholar]
- 11.De Kozak Y, Faure JP, Thillaye B, Ruchoux MM, Doly M, Droy-Lefaix MT et al. Ginkgo biloba extract (EGb 761) and a platelet-activating factor antagonist protect the retina in experimental autoimmune uveoretinitis. Ocul Immunol Inflamm. 1994;2:231–237. [DOI] [PubMed] [Google Scholar]
- 12.Bhosle VK, Rivera JC, Zhou TE, Omri S, Sanchez M, Hamel D et al. Nuclear localization of platelet-activating factor receptor controls retinal neovascularization. Cell Discov. 2016;2:16017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H, Yang Y, Takeda A, Yoshimura T, Oshima Y, Sonoda KH et al. A Novel Platelet-Activating Factor Receptor Antagonist Inhibits Choroidal Neovascularization and Subretinal Fibrosis. PLoS One. 2013;8:e68173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin N, Bazan HE, Braquet P, Bazan NG. Prolonged effects of a new platelet-activating factor antagonist on ocular vascular permeability in an endotoxin model of uveitis. Curr Eye Res. 1991;10:19–24. [DOI] [PubMed] [Google Scholar]
- 15.Cortina MS, Gordon WC, Lukiw WJ, Bazan NG. Oxidative stress-induced retinal damage up-regulates DNA polymerase gamma and 8-oxoguanine-DNA-glycosylase in photoreceptor synaptic mitochondria. Exp Eye Res. 2005;81:742–750. [DOI] [PubMed] [Google Scholar]
- 16.He J, Bazan NG, Bazan HE. Alkali-induced corneal stromal melting prevention by a novel platelet activating factor receptor antagonist. Arch Ophthalmol. 2006;124:70–78. [DOI] [PubMed] [Google Scholar]
- 17.Belayev L, Khoutorova L, Atkins K, Gordon WC, Alvarez-Builla J, Bazan NG. LAU-0901, a novel platelet-activating factor antagonist, is highly neuroprotective in cerebral ischemia. Exp Neurol. 2008;214:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bazan NG. Synaptic lipid signaling: significance of polyunsaturated fatty acids and platelet-activating factor. J Lipid Res. 2003;44:2221–2233. [DOI] [PubMed] [Google Scholar]
- 19.Couturier A, Bousquet E, Zhao M, Naud MC, Klein C, Jonet L et al. Anti-vascular endothelial growth factor acts on retinal microglia/macrophage activation in a rat model of ocular inflammation. Mol Vis. 2014;20:908–920. [PMC free article] [PubMed] [Google Scholar]
- 20.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: 2012; ISBN 3–900051-07–0, URL http://www.R-project.org/. [Google Scholar]
- 21.D’Alessandro LP, Forster DJ, Rao NA. Anterior uveitis and hypopyon. Trans Am Ophthalmol Soc. 1991;89:303–311. [PMC free article] [PubMed] [Google Scholar]
- 22.Pepple KL, Choi WJ, Wilson L, Van Gelder RN, Wang RK. Quantitative Assessment of Anterior Segment Inflammation in a Rat Model of Uveitis Using Spectral-Domain [DOI] [PMC free article] [PubMed]
- 23.Bazan NG, Halabi A, Ertel M, Petasis NA. Neuroinflammation In: Siegel G, Albers RW, Brady ST, Price DL, eds. Basic Neurochemistry: Molecular, Cellular and Medical Aspects, 8th edition. Burlington: Elsevier Academic Press; 2012 [Google Scholar]
- 24.Bazan NG, Calandria JM, Serhan CN. Rescue and repair during photoreceptor cell renewal mediated by docosahexaenoic acid-derived neuroprotectin D1. J Lipid Res. 2010;51:2018–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu H, Forrester JV, Liversidge J, Crane IJ Leukocyte trafficking in experimental autoimmune uveitis: breakdown of blood retinal barrier and upregulation of cellular adhesion molecules. Invest Ophthalmol Vis Sci. 2003;44:226–234. [DOI] [PubMed] [Google Scholar]
- 26.Xu H, Dawson R, Crane IJ, Liversidge J. Leukocyte diapedesis in vivo induces transient loss of tight junction protein at the blood retina barrier. Invest Ophthalmol Vis Sci. 2005;46:2487–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heil M, Clauss M, Suzuki K, Buschmann IR, Willuweit A, Fischer S, et al. Vascular endothelial growth factor (VEGF) stimulates monocyte migration through endothelial monolayers via increased integrin expression. Eur J Cell Biol. 2000;79:850–857. [DOI] [PubMed] [Google Scholar]
- 28.Bazan HE, Tao Y, DeCoster MA, Bazan NG. Platelet-activating factor induces cyclooxygenase-2 gene expression in corneal epithelium. Requirement of calcium in the signal transduction pathway. Invest Ophthalmol Vis Sci. 1997;38:2492–2501. [PubMed] [Google Scholar]
- 29.Belayev L, Khoutorova L, Atkins K, Cherqui A, Alvarez-Builla J, Bazan NG. LAU-0901, a novel platelet-activating factor-receptor antagonist, confers enduring neuroprotection in experimental focal cerebral ischemia in the rat. Brain Res. 2009;1253:184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]