Abstract
Hearing loss affects 30 million people in the United States, and a subset of these patients have normal low-frequency hearing and ski-sloped high-frequency hearing loss. For these patients, hearing aids alone may not provide adequate benefit. Cochlear implantation alone has been utilized to improve speech perception. The addition of high-frequency electric hearing to low-frequency acoustic hearing in these patients is beneficial. Technical improvements have allowed preservation of low-frequency hearing in cochlear implant recipients, allowing for electric and acoustic stimulation in the same ear with significant improvements in speech perception, sound localization, music appreciation, and quality of life.
Keywords: cochlear implantation, electric and acoustic stimulation, hearing loss, hearing preservation cochlear implantation
Learning Outcomes: As a result of this activity, the participant will be able to (1) describe at least three technical and medical developments that have led to improvements in low-frequency hearing preservation during cochlear implantation; (2) summarize the benefits of EAS listening devices over hearing aids or cochlear implants alone; (3) summarize the long-term evidence on rates of hearing preservation after cochlear implantation; (4) summarize current challenges in programming EAS devices for individual patient use.
Electric and Acoustic Stimulation
Hearing loss affects nearly 15% of the U.S. population, or roughly 28 million people. 1 For cases of mild-to-moderate hearing loss, hearing aids (HAs) are sufficient to amplify sound for improved speech perception compared with listening in an unaided condition. With severe-to-profound hearing loss, however, HAs may not provide improvements in speech perception that allow for communication. 2 Cochlear implants (CIs) offer improved speech perception for patients with severe-to-profound sensorineural hearing loss and limited speech perception abilities with HAs. Despite this advance, speech perception in certain conditions, such as in noise, has been variable.
A subset of patients exists with normal-to-moderate low-frequency hearing sensitivity and down-sloping, or ski-sloped severe-to-profound high-frequency hearing loss. This configuration is often observed with presbycusis, familial hearing loss, and noise-induced hearing loss ( Fig. 1 ). These patients have poor speech perception since consonants are typically recognized in the high frequencies 3 and therefore are not effectively presented via amplification with HAs. Until recently, these patients were not considered candidates for cochlear implantation due to normal-to-moderate low-frequency hearing and the risk of disrupting residual hearing with a full-length electrode array. Historically, cochlear implantation disrupted residual hearing in the implanted ear. However, in the mid-1990s, methods to preserve low-frequency hearing during cochlear implantation were developed, either through reduced insertion depths or shorter electrodes, resulting in the first animal and human data demonstrating hearing preservation was possible. 4 5 6 Cochlear implantation candidacy criteria have subsequently been relaxed in an effort to effectively treat patients with normal-to-moderate low-frequency hearing who do not gain sufficient benefit from HAs. Cochlear implantation in this patient population with preservation of hearing has led to improved speech perception in quiet and noise due to the combination of acoustic and electric stimulation, known as electric and acoustic stimulation (EAS). The acoustic component provides low-frequency cues that are beneficial for complex listening tasks, such as speech perception in noise, sound localization, and music/melody perception. The CI component provides stimulation of mid-to-high frequency information for improved representation of speech sounds in this region, providing speech perception improvements over HAs alone.
Figure 1.
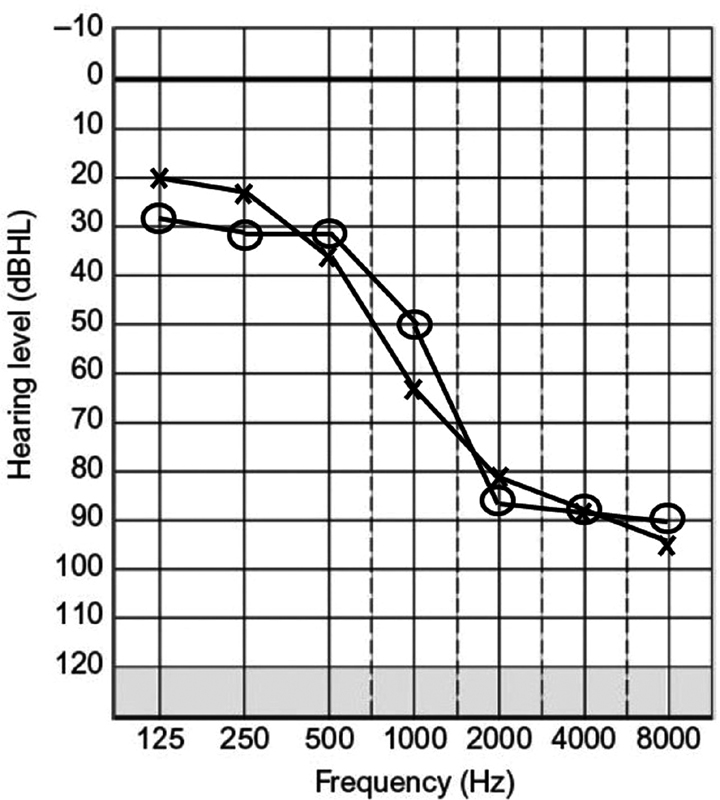
Example of “ski-sloped” hearing loss, or preservation of low-frequency hearing with severe-to-profound high-frequency hearing loss.
Hearing Preservation in Cochlear Implantation: Surgical Techniques, Adjunctive Therapies, and Long-Term Hearing Preservation Expectations
A variety of technical advances have enabled preservation of low-frequency hearing. These advances include progressive changes in electrode array designs, evolving soft surgical approaches and insertion techniques, and neuroprotective drug utilization.
Among the first developments that allowed hearing preservation was alteration of the CI electrode array. The first electrode arrays utilized to preserve hearing were either standard electrodes that were inserted short of normal insertion depths, or dramatically shorter electrode arrays. More recently, shallow insertion with a shorter array in the basal region of the cochlea has allowed for preservation of functional cochlear structures toward the mid and apical region of the cochlea and thus preserved low-frequency hearing sensitivity. 4 7 8 9 10 These shorter electrode arrays are specifically designed to minimize damage to intracochlear structures and preserve low-frequency residual hearing while allowing the high-frequency components of speech and other sounds to be coded electrically. 5 11 12 13 14 Additionally, this new generation of electrode arrays are typically more flexible, have smaller diameters than their predecessors, and follow the lateral wall of the cochlea, and each change has resulted in improved hearing preservation. 7 13 14
Overall, there is generally no consensus on optimal electrode array length for hearing preservation and postactivation speech perception. 15 Santa Maria et al 16 analyzed several studies and found that no specific electrode array design demonstrated a clear advantage in hearing preservation. Hearing preservation has been demonstrated in studies with electrode lengths ranging from 6 to 31 mm. 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 Despite this, strong arguments have been made for intermediate length electrode arrays in this spectrum. Intracochlear trauma increases with depth of insertion, resulting from progressive increases in force during insertion beyond 20 mm. 33 34 Thus, 20 mm may be a key target, as this distance represents a frequency match of near 1 kHz, sufficient to enable EAS. Additionally, electrode arrays shorter than this have been reported to have poorer speech performance with electric stimulation. 35 Some conceptual advantages also exist with the use of longer electrode arrays—for example, the ability to reprogram the device to present the full speech frequency spectrum should residual low-frequency hearing be lost or progress over time. 22 23 29 However, some studies have shown differing effects, with deeper insertion of electrode arrays resulting in no improvement in speech perception 36 or resulting in poorer speech perception outcomes with a perimodiolar electrode array. 37 Taking into consideration these results, the HEARRING group has recommended a flexible approach to electrode array selection for children, which could be broadly applied to adults as well based on extended indications for EAS devices ( Fig. 2 ). They recommend a shorter electrode array length (e.g., 24 mm) for patients with robust low-frequency hearing, a slightly longer electrode array for those with mild-to-moderate low-frequency hearing loss (e.g., 28 mm), and a long electrode array for patients with moderate or worse hearing loss and/or risk factors for hearing loss. 38
Figure 2.
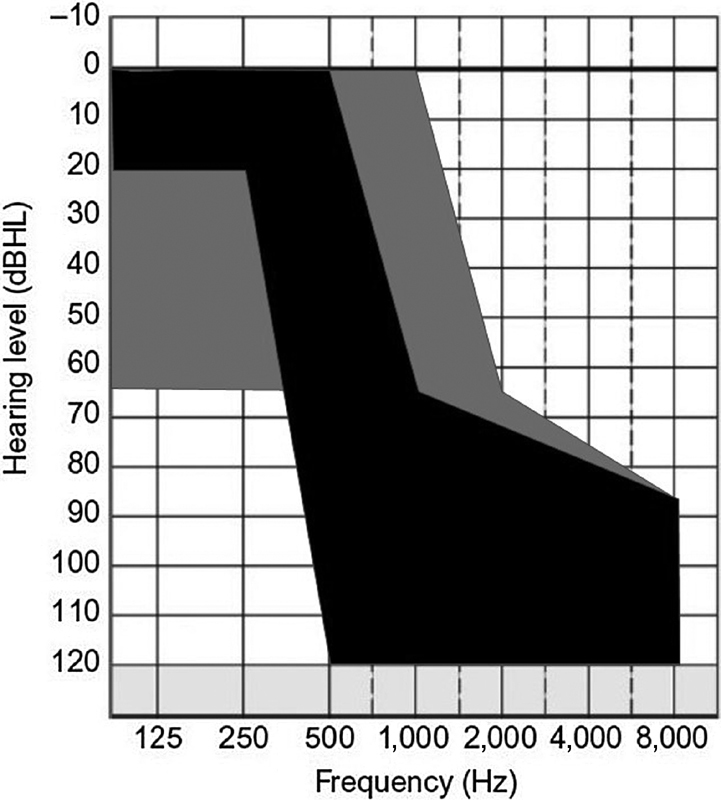
Audiogram parameters demonstrating basic and expanded indications for hearing preservation cochlear implantation and electric and acoustic stimulation. Gray indicates the expanded indications and black indicates the classic indications.
Assessing cochlear length when choosing an electrode array has been studied for some time, but recent debate on appropriate electrode array length for EAS has renewed interest in these measures. Recent studies have demonstrated that electrode array lengths around 28 to 30 mm are likely suitable for full cochlear coverage. 39 Angular insertion depth (AID) measurements are another method for determining insertion depth as opposed to electrode array length alone. AID measures are likely more accurate measures of cochlear coverage, as they account for variance in both linear insertion depth and length of the cochlear duct. Furthermore, it has been recently demonstrated that increasing AID is directly correlated with improved speech perception outcomes from the CI component, but ultimately poorer hearing preservation rates, even with newer electrodes designed for hearing preservation. 40 These findings support the tradeoff between attempts to improve speech perception with the CI component, and hearing preservation for the acoustic component.
Another area of debate has been the approach to electrode array insertion, via either a cochleostomy or round window insertion. The first insertions were via the round window but with large, rigid electrode arrays, significant insertion trauma resulted, and thus cochleostomies became more popular. However, round window insertion techniques have again become more widespread due to a more favorable trajectory along the lateral wall of the cochlea, with AIDs correlating directly with hearing preservation with the new generation of flexible electrodes. 31 41
A variety of studies have assessed cochleostomy versus round window insertion in both animals and humans. Several conceptual arguments have been made for round window insertion. Round window insertion typically avoids acoustic trauma, bone dust and blood entry into the cochlea, perilymph loss, and long-term inflammatory and ossifying changes. 42 These concepts have been supported by animal studies with histopathologic results. 42 43 Long-term retrospective studies also have supported round window approaches. 44 However, a systematic review by Havenith et al found that no clear benefit could be found for either approach, largely due to variances in definitions, methodology, and the lack of randomized controlled studies in the clinical setting. 45 More recent studies have also suggested hearing preservation with either approach. 16 46 47 Additionally, after cochleostomy or round window opening, demonstrable effects have been shown with the speed of insertion of the electrode array, with less trauma incurred with slower speeds. 48
However, even with meticulous surgical technique and optimal electrode array choice and placement, accessing the cochlea and inserting a foreign body induces significant inflammatory and cellular responses. A large body of research has sought pharmacologic agents to reduce these responses in an effort to minimize damage to cochlear structures and to preserve hearing long-term. The most widely researched group of agents is the corticosteroids. Corticosteroids have a myriad of effects on the auditory system, 47 and have shown the greatest benefit to date for hearing preservation after cochlear implantation.
A variety of methods for administering corticosteroids have been conceived, including intravenous, intratympanic, topical at the time of cochlear implantation, intracochlear, and via the implant itself. There is considerable variation in how much steroid should be used pre-, intra-, and postoperatively, but the consensus is that steroids positively impact hearing preservation when compared with no steroids regardless of treatment method. 49 50 In a meta-analysis, intraoperative topical administration along with postoperative steroid administration was beneficial, but preoperative intratympanic administration had no effect. 16 In contrast, Rajan et al showed that preoperative as well as intraoperative transtympanic administration of steroids improved hearing preservation. 51 The mechanism of hearing preservation, however, is unclear as it has been shown that topical application of steroids leads to exposure for only about an hour and the drug effects may only last for 24 hours. 52 Additionally, some of the effects are not seen at frequencies below 2,000 Hz, 16 suggesting that steroids may not reach the apex of the cochlea where hearing preservation is desired. Thus, other methods have been considered to allow more directed and long-term access to the cochlea by steroids. A variety of studies have shown that hearing loss induced by implant insertion trauma could be reduced with drug-eluting materials placed during implantation, catheters for infusion, or drug-eluting devices themselves. 53 54 55 56 57 58 59 60 61 The efficacy and feasibility of such a drug-eluting system also has been demonstrated in humans. 62 Regardless of the method, corticosteroids reduce inflammatory processes caused by electrode insertion resulting in better preservation of cochlear structures, 63 and stabilization of hearing levels. 51
Other methods for reducing inflammatory and oxidative stress during cochlear implantation have been considered. A variety of antioxidants decrease oxidative stress and can prevent hearing loss. 64 N-acetyl cysteine is one agent that has been applied in a guinea pig model with evidence of both short-term and long-term effects on hearing preservation. 65
Different pharmacologic targets have been considered in protecting cochlear structures against trauma. One of the targets that has shown efficacy is the c-Jun N-terminal kinase and its signaling pathway. A DJNK inhibitor (DJNKI-1) was applied in a guinea pig model and reduced immediate and long-term auditory dysfunction. 66 67 Studies have investigated pharmacologic agents that might promote long-term survival of spiral ganglion neurons (SGNs) in an attempt to preserve hearing long-term. Hair cells and the organ of Corti secrete neurotrophins which support SGNs, and loss of these neurotrophins, as happens when hair cells and the organ of Corti are damaged, can lead to degeneration. Direct application of these neurotrophins to the cochlea can rescue this degenerative process 68 and decrease ABR thresholds in response to injury. 69 Such neurotrophins have been coated onto CI electrode arrays and tested in animal models, demonstrating increased survival of SGNs. 70
Another agent commonly used during CI surgery, hyaluronic acid, has been utilized due to its utility as a lubricant and sealant. It is thought to act as a sealant for perilymph, to reduce friction during electrode insertion, and prevent entry of debris generated during implantation into the cochlea. A meta-analysis by Santa Maria et al demonstrated no benefit from this material. 16 There is some suggestion of cytotoxicity from its use with high concentrations and intracochlear administration, 71 but at lower concentrations no adverse effects have been seen when incorporated into soft insertion techniques. 72
The ultimate goal of the advances described earlier is the ability to preserve all residual hearing in all patients undergoing cochlear implantation. Postoperative hearing preservation has been reported in a high number of EAS recipients, but many experience some change in residual hearing thresholds. Defining the extent of threshold changes in the long term is made difficult due to the use of a variety of different electrode arrays, surgical techniques, and definitions of hearing preservation. Gantz et al demonstrated that placement of an electrode array in the cochlea alone did result in a hearing decrement, but this stabilized over time. Their results suggested that changes in residual hearing thresholds stabilize and that long-term utility of an EAS device is feasible. 73 These hearing changes are likely the result of a two-component process that results in neuronal damage. The initial trauma is caused by insertion of the electrode array with resultant mechanical damage. Later-onset damage is likely due to chronic inflammatory changes in response to the foreign body within the cochlea as well as degeneration of neural structures.
Given the recency of EAS as a technological development, it has taken some time to accumulate subject numbers to ascertain both short-term and long-term hearing preservation results. Several recent studies have delineated long-term results with hearing preservation after cochlear implantation, allowing expectations to be set. One of the early studies using the Hybrid 10 electrode (Cochlear Corporation) demonstrated that 2 of 87 patients had complete loss of low-frequency hearing at 1 month, and at 2 year follow-up an additional 6 patients had progressed, for a preservation rate of 90.8%. 74
Results from the European trial of the longer L24 Hybrid electrode (Cochlear Corporation) demonstrated preservation of hearing in 89% of patients at 1 month, with a decrease to 74% at 1 year. 75 Similar results were found in another study contrasting hearing preservation with the Nucleus Freedom CI422 electrode versus the L24 Hybrid electrode (Cochlear Corporation) with results favoring the L24 Hybrid. 76
An additional study retrospectively analyzed a variety of implanted electrodes (Cochlear Slim Straight, MED-EL Standard, Medium, Flex20, and Flex24) and demonstrated similar short-term results with 79% of patients with complete or partial hearing preservation immediately postoperatively with 8 cases of complete loss, and 67% of patients having complete or partial hearing preservation at 1 year with an additional 7 completely losing residual hearing. In the long-term follow-up (mean of 4.3-year postoperative), the researchers found that 50% of patients had complete or partial preservation of hearing suggesting gradual decrement over time. Ultimately 21% of patients completely lost all low-frequency hearing. 77 As expected, they found that hearing preservation was dependent on electrode array design and surgical approach. They found that patients who lost all low-frequency hearing performed more poorly and they also suggested that these patients be considered for reimplantation with a full-length electrode given prior evidence of the utility of this approach. 78
A longitudinal study by Gantz et al demonstrated that 83 to 92% of their subjects undergoing cochlear implantation with the S8, S12, and L24 electrodes (Cochlear Corporation) maintained low-frequency hearing. They noted an initial decrement in hearing postoperatively and a second period of decrement at 3 to 6 months postimplantation, but stabilization after that period. 79 They found that long-term hearing stabilized with a decline per year consistent with previous studies around 1 dB per year. 77 80 More recent updated results with the L24 electrode array alone demonstrated similar preservation rates at 5 years, with 94% preserving some degree of low-frequency hearing but only 72% using the EAS component of the system. 81 Similarly, Pillsbury et al demonstrated hearing preservation using the MED-EL EAS system, reporting that 79% of patients experienced less than 30 dB change in low-frequency hearing, but they reported a much higher continued use of the EAS system at 97% of patients at 1 year follow-up. 82 These findings were in line with previous studies of a similar EAS system in a smaller number of subjects. 83 However, other multicenter studies have shown a less robust preservation rate with hearing preservation electrode arrays, with 66% of patients demonstrating functional low-frequency hearing preservation at 6 months and 54% in another. 84 85 Several of the patients who did not retain low-frequency hearing chose to have their device replaced with a standard electrode array. 84
Reports of even longer-term follow-up have, expectedly, had small subject numbers aside from the report from Gantz et al described earlier. 79 Mertens et al demonstrated some degree of hearing preservation in 81% of subjects out to 10 years of follow-up 86 and Moteki et al reported on 19 patients with 89.4% retaining hearing sufficient to allow EAS use. 87
Thus, despite a myriad of electrode arrays, surgical approaches, and definitions of hearing preservation, it can generally be concluded that with newer hearing preservation techniques and electrode arrays, low-frequency hearing can be preserved long term in the majority of patients allowing for long-term EAS use. Worth noting, however, is that within each study, a significant proportion of patients completely lost all low-frequency hearing, up to 20% of all patients in one study. This possibility requires upfront counseling before surgery. Additionally, some studies exclude patients shown to have poor long-term outcomes, such as those with older than 65 years and with greater than 30 years of prior deafness and this also should be taken into consideration with the reported hearing preservation rates. 82
Benefits of Low-Frequency Hearing Preservation and Utilization in Electric and Acoustic Stimulation
The successes described earlier with low-frequency hearing preservation have enabled the fitting of EAS devices. The combination of acoustic and electric stimulation has resulted in improved speech perception in quiet and noise for patients, which exceeds the speech perception abilities with either stimulation modality alone. 5 8 11 12 13 73 74 83 88 89 90 91 92 93 Benefits have also been reported for song/instrument perception and localization of sound targets in a noisy background. 94 95 96 97 These gains are likely derived from the addition of acoustic low-frequency cues that are not effectively represented by CI signal coding strategies.
Several studies have demonstrated the importance of low-frequency acoustic information in EAS devices. 5 8 11 12 13 73 74 83 88 89 90 91 92 93 To further support the utility of EAS devices, it has been demonstrated that when low frequency or complex sounds are presented to places in the cochlea that are coded for higher frequencies, pitch perception and identification of low-frequency information is diminished or disappears. 23 This strongly suggests that providing electrical or acoustic stimulation to apical regions to carry this low-frequency information would improve performance with music and tonality as perception of these components is reliant on this low-frequency information. When the temporal code and place code conflict, sound localization is primarily coded by the temporal fine structure which is carried in low frequencies. Conversely, word perception is driven by the signal envelope which is carried in higher frequencies. This is clearly demonstrated in EAS users: EAS provides both correct place and time coding in the low frequencies via acoustic stimulation which allows for improvements in speech perception outcomes via utilization of the temporal fine structure. The benefit of adding this acoustic information, even when minimal, to electric stimulation has been demonstrated in bimodal listeners, who listen with a HA in the ear contralateral to the CI (CI + HA). 98 99 Listening in a bimodal condition results in greater performance than when listening with the CI alone. 96 98 However, the bimodal condition may be inferior to the EAS condition as each ear will be processing sound cues in entirely different methods, potentially distorting perception. 100
Previous studies have demonstrated that both interaural level and time differences (ILD and ITD) allow listeners to locate sound sources spatially as well as improve speech perception in complex listening environments such as the simulated cocktail party. 101 102 103 Zhang et al 104 demonstrated improved speech perception in background noise with provision of acoustic input for the bimodal condition relative to a CI alone. ILDs are predominant in higher frequencies above 1,500 Hz and ITDs are predominant in frequencies below 1,000 Hz, 105 both of which may be accessible to EAS users. The better performance of the EAS recipients in challenging test conditions also could be explained by the improved transmission of fundamental frequency cues in the lower-frequency region of acoustic hearing, which likely helps group auditory targets. 106
Unilateral EAS recipients listening with a HA in the contralateral ear (EAS + HA) demonstrate improved speech perception in spatially separated noise and localization tasks likely due to the presence of bilateral acoustic low-frequency stimulation. The benefit of listening with EAS + HA as compared with EAS alone is often not realized when speech perception materials are presented in quiet. 107 108 Dillon et al 108 reported a significant improvement with EAS + HA over EAS alone on a speech perception task where the target and 4-talker masker were co-located. Similarly, Gifford et al 109 demonstrated an improvement in speech perception abilities of CI subjects with binaural low-frequency hearing as compared with a bimodal listening configuration when presented with target and masker that were either co-located or spatially separated. With localization, subjects listening with EAS + HA perform similarly when listening with bilateral HAs. However, localization is poorer in a bimodal condition than when bilateral acoustic information is provided, which suggests again a binaural benefit from the acoustic component alone. 97
Interestingly, the utility of EAS in speakers of tonal languages, such as Mandarin Chinese, where use of CIs alone results in perception of only about half of presented tones, is likely to be quite high. Intonation cues appear to be derived from low-frequency information, and as such are often excluded with electric stimulation alone. 110 Amplification of frequencies around the fundamental frequency allowed better tone identification and strategies have been undertaken to improve access to these signals which are located in the lower frequencies for tonal listeners. 111
While retaining the capacity to improve speech perception, sound localization, and music perception through hearing preservation as well as offering different CI signal coding strategies due to greater cochlear coverage, there are suggestions of additional benefits with the use of longer electrode arrays. Some authors suggest that the 24-mm range may be ideal to offer better coverage of the cochlea tonotopically, and also good residual hearing preservation. The ideal length will likely vary in each individual based on anatomy, cochlear duct length, and parameters of hearing loss, which can be assessed by AID. 41 Furthermore, the refinement of atraumatic insertion techniques as well as softer, more flexible, lateral cochlear wall electrodes will likely enhance hearing preservation rates. 31
Electric and Acoustic Stimulation Device Programming Considerations
While the benefits of combining acoustic and electric information in an ipsilateral condition are known, there are fewer studies investigating the optimization of EAS device programming. One parameter that has been studied is the crossover frequency between acoustic and electric stimulation. This allows for the sound signal presented by the processor to be mapped to either acoustic or electric stimulation, or even both. CIs of conventional CI recipients are programmed where the full-speech spectrum is represented electrically. In cases of EAS, better speech perception outcomes have been obtained when the electrical stimulation was limited to the mid and high-frequency information. 112 113 114 115 Overlapping acoustic and electrical stimulation, or those with a gap between the two, yielded poorer performance than the “meet” program where there was minimal overlap. 91 92 112 115 116 Typically, determining the crossover frequency has relied on the unaided audiogram for the implanted ear. The audiologist reviews where the patient's hearing exceeds that of where benefit can be achieved with the acoustic component. However, the definition for this cutoff has been variable, with some suggesting assigning the frequency to where residual hearing exceeds 65 up to 80 dB HL. 10 13 15 74 88 112 117 More recent analyses of the fitting methods of EAS recipients have demonstrated improved outcomes when greater spectral overlap was provided by assigning the crossover to a lower frequency or providing a higher low-pass frequency cutoff. 117 Recent work by Gifford and colleagues has demonstrated that setting the low-frequency CI cutoff to where the audiogram is reaches 70 dB hearing loss. Additionally, utilizing full CI bandwidth was not the ideal condition, but setting a boundary at 313 or 438 Hz in the bilateral EAS condition provided the greatest benefits, particularly in noisy environments. Again, competing hypotheses may suggest why these cutoffs are effective, either with better place mapping from the CI component or better transmission of low-frequency harmonics. 118 Additional strategies have utilized more personalized calculations, such as frequency-to-place map calculations 92 and individually optimized frequency measurements 115 119 to improve encoding of both the acoustic and electrical signals. In any case, researchers may be underestimating the benefits of EAS due to limited knowledge on the programming of these combined devices.
In addition to changing how sounds are presented via the processor, researchers have demonstrated that there is cortical plasticity that occurs with continued implant use. McDermott and Varsavsky 120 and Reiss et al 121 both showed that pitch interpretation through a CI changes with duration of use in a predictable manner. Providing a higher percentage of cochlear coverage by extending electrodes into the apical region reduces the degree of required cortical remapping and may lead to a faster rate of learning with the implant, though nearly all implant users require several months for adaptation to the new device. There also appear to be limitations to this cortical plasticity and remapping with only a range of tolerance for remapping. 122 Buchman and colleagues 35 investigated the influence of electrode array insertion depth on CI performance, reporting a rapid improvement in speech perception with longer electrode arrays. As discussed in previous sections, this improvement in speech perception with longer electrode arrays may come at the tradeoff of loss of hearing with deeper insertion depths. Lastly, new electric signal coding strategies that incorporate temporal fine structure such as fundamental frequency in these lower frequencies 123 124 provided significantly improved speech perception as well as music appreciation with these new strategies only after 1 year of use.
Future Directions to Improve Speech Perception Outcomes
Over time, it is anticipated that the technological advances described herein will expand the indications for cochlear implantation into patients with greater amounts of residual hearing. Bilateral EAS use, taking advantage of residual hearing bilaterally, is currently considered the most advanced listening condition, though some studies suggest that unilateral EAS coupled with a contralateral HA, providing binaural acoustic hearing, may be best for speech perception in nearly all conditions without requiring additional surgery or the risk of damage to the hearing in the contralateral ear. A great deal of research remains in defining optimized programming of the acoustic and electric components for maximum benefit.
Many factors that are responsible for preservation of hearing in cochlear implantation beyond preventing physical and inflammatory damage to cochlear structures remain a mystery. The maintenance of existing hearing is critical for EAS, and research has focused on numerous factors that could protect hair cells and SGNs after hearing loss and during cochlear implantation including neurotrophic factors, anti-inflammatory steroids, antiapoptotic agents, or a combination of these. The means of locally delivering these agents continues to be researched and advanced, in particular the challenge of providing longer-term exposure of these agents to deeper cochlear structures to allow for their preservation.
In addition to pharmacological techniques, much research has focused on soft insertion techniques as well as the use of shorter electrode arrays to allow avoidance of damage to the low-frequency regions located in the apex of the cochlea. As noted earlier, flexible long straight electrode arrays have become available which allow deeper insertion into the cochlea while avoiding trauma due to the flexible nature of the electrodes as well as their smaller cross-section. These electrode arrays have the potential to maintain low-frequency hearing as well as additional benefits of improved tonotopic stimulation.
Attempts have been made to assess for possible cochlear damage and hearing loss intraoperatively during electrode insertion. Round window electrocochleography has been shown to be highly indicative of preimplant cochlear functional status, 125 126 127 and can be predictive of speech perception outcome in adults. 127 It is highly sensitive to electrophysiologic changes during electrode array insertion and may allow for the future prediction of hearing loss, or adjustment of insertion technique intraoperatively to prevent hearing loss intraoperatively and immediately postoperatively. 128
Conclusion
The image of the CI candidate has evolved from those with profound hearing loss to those with normal to moderate levels of low-frequency hearing thanks to innovations in surgical procedures and electrode array design. When postoperative hearing preservation is achieved, EAS recipients experience significant improvements in speech perception in noise, sound localization, and quality of life. Even music appreciation is maintained. Further gains in these areas may be realized with new research into the programming of combined devices.
References
- 1.Hoffman H J, Dobie R A, Losonczy K G, Themann C L, Flamme G A. Declining prevalence of hearing loss in US adults aged 20 to 69 years. JAMA Otolaryngol Head Neck Surg. 2017;143(03):274–285. doi: 10.1001/jamaoto.2016.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ching T Y, Dillon H, Byrne D. Speech recognition of hearing-impaired listeners: predictions from audibility and the limited role of high-frequency amplification. J Acoust Soc Am. 1998;103(02):1128–1140. doi: 10.1121/1.421224. [DOI] [PubMed] [Google Scholar]
- 3.Turner C W. Hearing loss and the limits of amplification. Audiol Neurootol. 2006;11 01:2–5. doi: 10.1159/000095606. [DOI] [PubMed] [Google Scholar]
- 4.von Ilberg C, Kiefer J, Tillein J et al. Electric-acoustic stimulation of the auditory system. New technology for severe hearing loss. ORL J Otorhinolaryngol Relat Spec. 1999;61(06):334–340. doi: 10.1159/000027695. [DOI] [PubMed] [Google Scholar]
- 5.Gantz B J, Turner C W. Combining acoustic and electrical hearing. Laryngoscope. 2003;113(10):1726–1730. doi: 10.1097/00005537-200310000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Hodges A V, Schloffman J, Balkany T. Conservation of residual hearing with cochlear implantation. Am J Otol. 1997;18(02):179–183. [PubMed] [Google Scholar]
- 7.Skarzyński H, Lorens A, Piotrowska A. A new method of partial deafness treatment. Med Sci Monit. 2003;9(04):CS20–CS24. [PubMed] [Google Scholar]
- 8.Gstoettner W, Kiefer J, Baumgartner W-D, Pok S, Peters S, Adunka O. Hearing preservation in cochlear implantation for electric acoustic stimulation. Acta Otolaryngol. 2004;124(04):348–352. doi: 10.1080/00016480410016432. [DOI] [PubMed] [Google Scholar]
- 9.Kiefer J, Gstoettner W, Baumgartner W et al. Conservation of low-frequency hearing in cochlear implantation. Acta Otolaryngol. 2004;124(03):272–280. doi: 10.1080/00016480310000755a. [DOI] [PubMed] [Google Scholar]
- 10.James C, Albegger K, Battmer R et al. Preservation of residual hearing with cochlear implantation: how and why. Acta Otolaryngol. 2005;125(05):481–491. doi: 10.1080/00016480510026197. [DOI] [PubMed] [Google Scholar]
- 11.Gantz B J, Turner C. Combining acoustic and electrical speech processing: Iowa/Nucleus hybrid implant. Acta Otolaryngol. 2004;124(04):344–347. doi: 10.1080/00016480410016423. [DOI] [PubMed] [Google Scholar]
- 12.Lenarz T, Stover T, Buechner A et al. Temporal bone results and hearing preservation with a new straight electrode. Audiol Neurootol. 2006;11 01:34–41. doi: 10.1159/000095612. [DOI] [PubMed] [Google Scholar]
- 13.Lenarz T. Electro-acoustic stimulation of the cochlea. Editorial. Audiol Neurootol. 2009;14 01:1. doi: 10.1159/000206488. [DOI] [PubMed] [Google Scholar]
- 14.Woodson E A, Reiss L AJ, Turner C W, Gfeller K, Gantz B J. The Hybrid cochlear implant: a review. Adv Otorhinolaryngol. 2010;67:125–134. doi: 10.1159/000262604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Abel K M, Dunn C C, Sladen D P et al. Hearing preservation among patients undergoing cochlear implantation. Otol Neurotol. 2015;36(03):416–421. doi: 10.1097/MAO.0000000000000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santa Maria P L, Gluth M B, Yuan Y, Atlas M D, Blevins N H. Hearing preservation surgery for cochlear implantation: a meta-analysis. Otol Neurotol. 2014;35(10):e256–e269. doi: 10.1097/MAO.0000000000000561. [DOI] [PubMed] [Google Scholar]
- 17.Skarzynski H, Lorens A. Electric acoustic stimulation in children. Adv Otorhinolaryngol. 2010;67:135–143. doi: 10.1159/000262605. [DOI] [PubMed] [Google Scholar]
- 18.Bruce I A, Felton M, Lockley M et al. Hearing preservation cochlear implantation in adolescents. Otol Neurotol. 2014;35(09):1552–1559. doi: 10.1097/MAO.0000000000000542. [DOI] [PubMed] [Google Scholar]
- 19.Skarzyński H, Lorens A, D'Haese P et al. Preservation of residual hearing in children and post-lingually deafened adults after cochlear implantation: an initial study. ORL J Otorhinolaryngol Relat Spec. 2002;64(04):247–253. doi: 10.1159/000064134. [DOI] [PubMed] [Google Scholar]
- 20.Skarzyński H, Lorens A, Piotrowska A, Podskarbi-Fayette R. Results of partial deafness cochlear implantation using various electrode designs. Audiol Neurootol. 2009;14(01) 01:39–45. doi: 10.1159/000206494. [DOI] [PubMed] [Google Scholar]
- 21.Baumgartner W D, Jappel A, Morera C et al. Outcomes in adults implanted with the FLEXsoft electrode. Acta Otolaryngol. 2007;127(06):579–586. doi: 10.1080/00016480600987784. [DOI] [PubMed] [Google Scholar]
- 22.Helbig S, Baumann U, Hey C, Helbig M. Hearing preservation after complete cochlear coverage in cochlear implantation with the free-fitting FLEXSOFT electrode carrier. Otol Neurotol. 2011;32(06):973–979. doi: 10.1097/MAO.0b013e31822558c4. [DOI] [PubMed] [Google Scholar]
- 23.Hochmair I, Hochmair E, Nopp P, Waller M, Jolly C. Deep electrode insertion and sound coding in cochlear implants. Hear Res. 2015;322:14–23. doi: 10.1016/j.heares.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Mick P, Amoodi H, Shipp D et al. Hearing preservation with full insertion of the FLEXsoft electrode. Otol Neurotol. 2014;35(01):e40–e44. doi: 10.1097/MAO.0b013e318291c66d. [DOI] [PubMed] [Google Scholar]
- 25.Prentiss S, Sykes K, Staecker H. Partial deafness cochlear implantation at the University of Kansas: techniques and outcomes. J Am Acad Audiol. 2010;21(03):197–203. doi: 10.3766/jaaa.21.3.8. [DOI] [PubMed] [Google Scholar]
- 26.Rodgers B, Prentiss S, Staecker H. Expanding cochlear implantation to patients with residual mid and high frequency hearing. Otorinolaringol. 2012;68:183–190. [Google Scholar]
- 27.Tamir S, Ferrary E, Borel S, Sterkers O, Bozorg Grayeli A. Hearing preservation after cochlear implantation using deeply inserted flex atraumatic electrode arrays. Audiol Neurootol. 2012;17(05):331–337. doi: 10.1159/000339894. [DOI] [PubMed] [Google Scholar]
- 28.Usami S, Moteki H, Suzuki N et al. Achievement of hearing preservation in the presence of an electrode covering the residual hearing region. Acta Otolaryngol. 2011;131(04):405–412. doi: 10.3109/00016489.2010.539266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bruce I A, Bates J E, Melling C, Mawman D, Green K M. Hearing preservation via a cochleostomy approach and deep insertion of a standard length cochlear implant electrode. Otol Neurotol. 2011;32(09):1444–1447. doi: 10.1097/MAO.0b013e3182355824. [DOI] [PubMed] [Google Scholar]
- 30.Nordfalk K F, Rasmussen K, Bunne M, Jablonski G E. Deep round window insertion versus standard approach in cochlear implant surgery. Eur Arch Otorhinolaryngol. 2016;273(01):43–50. doi: 10.1007/s00405-014-3451-2. [DOI] [PubMed] [Google Scholar]
- 31.Skarzynski H, Lorens A, Zgoda M, Piotrowska A, Skarzynski P H, Szkielkowska A. Atraumatic round window deep insertion of cochlear electrodes. Acta Otolaryngol. 2011;131(07):740–749. doi: 10.3109/00016489.2011.557780. [DOI] [PubMed] [Google Scholar]
- 32.Sun C H, Hsu C J, Chen P R, Wu H P. Residual hearing preservation after cochlear implantation via round window or cochleostomy approach. Laryngoscope. 2015;125(07):1715–1719. doi: 10.1002/lary.25122. [DOI] [PubMed] [Google Scholar]
- 33.Adunka O, Kiefer J. Impact of electrode insertion depth on intracochlear trauma. Otolaryngol Head Neck Surg. 2006;135(03):374–382. doi: 10.1016/j.otohns.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 34.Adunka O, Gstoettner W, Hambek M, Unkelbach M H, Radeloff A, Kiefer J. Preservation of basal inner ear structures in cochlear implantation. ORL J Otorhinolaryngol Relat Spec. 2004;66(06):306–312. doi: 10.1159/000081887. [DOI] [PubMed] [Google Scholar]
- 35.Buchman C A, Dillon M T, King E R, Adunka M C, Adunka O F, Pillsbury H C. Influence of cochlear implant insertion depth on performance: a prospective randomized trial. Otol Neurotol. 2014;35(10):1773–1779. doi: 10.1097/MAO.0000000000000541. [DOI] [PubMed] [Google Scholar]
- 36.van der Marel K S, Briaire J J, Verbist B M, Muurling T J, Frijns J H. The influence of cochlear implant electrode position on performance. Audiol Neurootol. 2015;20(03):202–211. doi: 10.1159/000377616. [DOI] [PubMed] [Google Scholar]
- 37.Holden L K, Finley C C, Firszt J B et al. Factors affecting open-set word recognition in adults with cochlear implants. Ear Hear. 2013;34(03):342–360. doi: 10.1097/AUD.0b013e3182741aa7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rajan G, Tavora-Vieira D, Baumgartner W D et al. Hearing preservation cochlear implantation in children: the HEARRING group consensus and practice guide. Cochlear Implants Int. 2018;19(01):1–13. doi: 10.1080/14670100.2017.1379933. [DOI] [PubMed] [Google Scholar]
- 39.Johnston J D, Scoffings D, Chung M et al. Computed tomography estimation of cochlear duct length can predict full insertion in cochlear implantation. Otol Neurotol. 2016;37(03):223–228. doi: 10.1097/MAO.0000000000000955. [DOI] [PubMed] [Google Scholar]
- 40.O'Connell B P, Hunter J B, Haynes D S et al. Insertion depth impacts speech perception and hearing preservation for lateral wall electrodes. Laryngoscope. 2017;127(10):2352–2357. doi: 10.1002/lary.26467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Connell B P, Cakir A, Hunter J B et al. Electrode location and angular insertion depth are predictors of audiologic outcomes in cochlear implantation. Otol Neurotol. 2016;37(08):1016–1023. doi: 10.1097/MAO.0000000000001125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adunka O, Unkelbach M H, Mack M, Hambek M, Gstoettner W, Kiefer J. Cochlear implantation via the round window membrane minimizes trauma to cochlear structures: a histologically controlled insertion study. Acta Otolaryngol. 2004;124(07):807–812. doi: 10.1080/00016480410018179. [DOI] [PubMed] [Google Scholar]
- 43.Burghard A, Lenarz T, Kral A, Paasche G. Insertion site and sealing technique affect residual hearing and tissue formation after cochlear implantation. Hear Res. 2014;312:21–27. doi: 10.1016/j.heares.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 44.Skarzynski H. Long-term results of partial deafness treatment. Cochlear Implants Int. 2014;15 01:S21–S23. doi: 10.1179/1467010014Z.000000000170. [DOI] [PubMed] [Google Scholar]
- 45.Havenith S, Lammers M J, Tange R A et al. Hearing preservation surgery: cochleostomy or round window approach? A systematic review. Otol Neurotol. 2013;34(04):667–674. doi: 10.1097/MAO.0b013e318288643e. [DOI] [PubMed] [Google Scholar]
- 46.Eshraghi A A, Ahmed J, Krysiak E et al. Clinical, surgical, and electrical factors impacting residual hearing in cochlear implant surgery. Acta Otolaryngol. 2017;137(04):384–388. doi: 10.1080/00016489.2016.1256499. [DOI] [PubMed] [Google Scholar]
- 47.Wanna G B, O'Connell B P, Francis D O et al. Predictive factors for short- and long-term hearing preservation in cochlear implantation with conventional-length electrodes. Laryngoscope. 2018;128(02):482–489. doi: 10.1002/lary.26714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rajan G P, Kontorinis G, Kuthubutheen J. The effects of insertion speed on inner ear function during cochlear implantation: a comparison study. Audiol Neurootol. 2013;18(01):17–22. doi: 10.1159/000342821. [DOI] [PubMed] [Google Scholar]
- 49.Carlson M L, Driscoll C L, Gifford R H et al. Implications of minimizing trauma during conventional cochlear implantation. Otol Neurotol. 2011;32(06):962–968. doi: 10.1097/MAO.0b013e3182204526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Causon A, Verschuur C, Newman T A. A retrospective analysis of the contribution of reported factors in cochlear implantation on hearing preservation outcomes. Otol Neurotol. 2015;36(07):1137–1145. doi: 10.1097/MAO.0000000000000753. [DOI] [PubMed] [Google Scholar]
- 51.Rajan G P, Kuthubutheen J, Hedne N, Krishnaswamy J. The role of preoperative, intratympanic glucocorticoids for hearing preservation in cochlear implantation: a prospective clinical study. Laryngoscope. 2012;122(01):190–195. doi: 10.1002/lary.22142. [DOI] [PubMed] [Google Scholar]
- 52.Hargunani C A, Kempton J B, DeGagne J M, Trune D R. Intratympanic injection of dexamethasone: time course of inner ear distribution and conversion to its active form. Otol Neurotol. 2006;27(04):564–569. doi: 10.1097/01.mao.0000194814.07674.4f. [DOI] [PubMed] [Google Scholar]
- 53.Vivero R J, Joseph D E, Angeli S et al. Dexamethasone base conserves hearing from electrode trauma-induced hearing loss. Laryngoscope. 2008;118(11):2028–2035. doi: 10.1097/MLG.0b013e31818173ec. [DOI] [PubMed] [Google Scholar]
- 54.Jolly C, Garnham C, Mirzadeh H et al. Electrode features for hearing preservation and drug delivery strategies. Adv Otorhinolaryngol. 2010;67:28–42. doi: 10.1159/000262594. [DOI] [PubMed] [Google Scholar]
- 55.Liu Y, Jolly C, Braun S et al. Effects of a dexamethasone-releasing implant on cochleae: a functional, morphological and pharmacokinetic study. Hear Res. 2015;327:89–101. doi: 10.1016/j.heares.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 56.Liu Y, Jolly C, Braun S et al. In vitro and in vivo pharmacokinetic study of a dexamethasone-releasing silicone for cochlear implants. Eur Arch Otorhinolaryngol. 2016;273(07):1745–1753. doi: 10.1007/s00405-015-3760-0. [DOI] [PubMed] [Google Scholar]
- 57.Douchement D, Terranti A, Lamblin J et al. Dexamethasone eluting electrodes for cochlear implantation: effect on residual hearing. Cochlear Implants Int. 2015;16(04):195–200. doi: 10.1179/1754762813Y.0000000053. [DOI] [PubMed] [Google Scholar]
- 58.Farhadi M, Jalessi M, Salehian P et al. Dexamethasone eluting cochlear implant: histological study in animal model. Cochlear Implants Int. 2013;14(01):45–50. doi: 10.1179/1754762811Y.0000000024. [DOI] [PubMed] [Google Scholar]
- 59.Dolgin E. Sound medicine. Nat Med. 2012;18(05):642–645. doi: 10.1038/nm0512-642. [DOI] [PubMed] [Google Scholar]
- 60.Bas E, Bohorquez J, Goncalves S et al. Electrode array-eluted dexamethasone protects against electrode insertion trauma induced hearing and hair cell losses, damage to neural elements, increases in impedance and fibrosis: a dose response study. Hear Res. 2016;337:12–24. doi: 10.1016/j.heares.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 61.Wilk M, Hessler R, Mugridge K et al. Impedance changes and fibrous tissue growth after cochlear implantation are correlated and can be reduced using a dexamethasone eluting electrode. PLoS One. 2016;11(02):e0147552. doi: 10.1371/journal.pone.0147552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Plontke S K, Götze G, Rahne T, Liebau A. Intracochlear drug delivery in combination with cochlear implants: current aspects. HNO. 2017;65 01:19–28. doi: 10.1007/s00106-016-0285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van De Water T R, Abi Hachem R N, Dinh C T et al. Conservation of hearing and protection of auditory hair cells against trauma-induced losses by local dexamethasone therapy: molecular and genetic mechanisms. Cochlear Implants Int. 2010;11 01:42–55. doi: 10.1179/146701010X12671178390834. [DOI] [PubMed] [Google Scholar]
- 64.Kannan K, Jain S K. Oxidative stress and apoptosis. Pathophysiology. 2000;7(03):153–163. doi: 10.1016/s0928-4680(00)00053-5. [DOI] [PubMed] [Google Scholar]
- 65.Eastwood H, Pinder D, James Det al. Permanent and transient effects of locally delivered n-acetyl cysteine in a guinea pig model of cochlear implantation Hear Res 2010259(1-2):24–30. [DOI] [PubMed] [Google Scholar]
- 66.Eshraghi A A. Prevention of cochlear implant electrode damage. Curr Opin Otolaryngol Head Neck Surg. 2006;14(05):323–328. doi: 10.1097/01.moo.0000244189.74431.df. [DOI] [PubMed] [Google Scholar]
- 67.Eshraghi A A, Wang J, Adil Eet al. Blocking c-Jun-N-terminal kinase signaling can prevent hearing loss induced by both electrode insertion trauma and neomycin ototoxicity Hear Res 2007226(1-2):168–177. [DOI] [PubMed] [Google Scholar]
- 68.Wise A K, Fallon J B, Neil A J et al. Combining cell-based therapies and neural prostheses to promote neural survival. Neurotherapeutics. 2011;8(04):774–787. doi: 10.1007/s13311-011-0070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kanzaki S, Stöver T, Kawamoto K et al. Glial cell line-derived neurotrophic factor and chronic electrical stimulation prevent VIII cranial nerve degeneration following denervation. J Comp Neurol. 2002;454(03):350–360. doi: 10.1002/cne.10480. [DOI] [PubMed] [Google Scholar]
- 70.Richardson R T, Wise A K, Thompson B C et al. Polypyrrole-coated electrodes for the delivery of charge and neurotrophins to cochlear neurons. Biomaterials. 2009;30(13):2614–2624. doi: 10.1016/j.biomaterials.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roland J T, Jr, Magardino T M, Go J T, Hillman D E. Effects of glycerin, hyaluronic acid, and hydroxypropyl methylcellulose on the spiral ganglion of the guinea pig cochlea. Ann Otol Rhinol Laryngol Suppl. 1995;166:64–68. [PubMed] [Google Scholar]
- 72.Khater A, El-Anwar M W. Methods of hearing preservation during cochlear implantation. Int Arch Otorhinolaryngol. 2017;21(03):297–301. doi: 10.1055/s-0036-1585094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gantz B J, Dunn C, Oleson J, Hansen M, Parkinson A, Turner C. Multicenter clinical trial of the Nucleus Hybrid S8 cochlear implant: final outcomes. Laryngoscope. 2016;126(04):962–973. doi: 10.1002/lary.25572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gantz B J, Hansen M R, Turner C W, Oleson J J, Reiss L A, Parkinson A J. Hybrid 10 clinical trial: preliminary results. Audiol Neurootol. 2009;14 01:32–38. doi: 10.1159/000206493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lenarz T, James C, Cuda D et al. European multi-centre study of the Nucleus Hybrid L24 cochlear implant. Int J Audiol. 2013;52(12):838–848. doi: 10.3109/14992027.2013.802032. [DOI] [PubMed] [Google Scholar]
- 76.Jurawitz M C, Büchner A, Harpel T et al. Hearing preservation outcomes with different cochlear implant electrodes: Nucleus® Hybrid™-L24 and Nucleus Freedom™ CI422. Audiol Neurootol. 2014;19(05):293–309. doi: 10.1159/000360601. [DOI] [PubMed] [Google Scholar]
- 77.Helbig S, Adel Y, Rader T, Stöver T, Baumann U. Long-term hearing preservation outcomes after cochlear implantation for electric-acoustic stimulation. Otol Neurotol. 2016;37(09):e353–e359. doi: 10.1097/MAO.0000000000001066. [DOI] [PubMed] [Google Scholar]
- 78.Fitzgerald M B, Sagi E, Jackson M et al. Reimplantation of hybrid cochlear implant users with a full-length electrode after loss of residual hearing. Otol Neurotol. 2008;29(02):168–173. doi: 10.1097/mao.0b013e31815c4875. [DOI] [PubMed] [Google Scholar]
- 79.Gantz B J, Dunn C C, Oleson J, Hansen M R. Acoustic plus electric speech processing: long-term results. Laryngoscope. 2018;128(02):473–481. doi: 10.1002/lary.26669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yao W N, Turner C W, Gantz B J. Stability of low-frequency residual hearing in patients who are candidates for combined acoustic plus electric hearing. J Speech Lang Hear Res. 2006;49(05):1085–1090. doi: 10.1044/1092-4388(2006/077). [DOI] [PubMed] [Google Scholar]
- 81.Roland J T, Jr, Gantz B J, Waltzman S B, Parkinson A J.Long-term outcomes of cochlear implantation in patients with high-frequency hearing lossLaryngoscope 2018; Epub ahead of print [DOI] [PMC free article] [PubMed]
- 82.Pillsbury H C, III, Dillon M T, Buchman C A et al. Multicenter US clinical trial with an electric-acoustic stimulation (EAS) system in adults: final outcomes. Otol Neurotol. 2018;39(03):299–305. doi: 10.1097/MAO.0000000000001691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Helbig S, Van de Heyning P, Kiefer J et al. Combined electric acoustic stimulation with the PULSARCI(100) implant system using the FLEX(EAS) electrode array. Acta Otolaryngol. 2011;131(06):585–595. doi: 10.3109/00016489.2010.544327. [DOI] [PubMed] [Google Scholar]
- 84.Roland J T, Jr, Gantz B J, Waltzman S B, Parkinson A J; Multicenter Clinical Trial Group.United States multicenter clinical trial of the cochlear nucleus hybrid implant system Laryngoscope 201612601175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mady L J, Sukato D C, Fruit J et al. Hearing preservation: does electrode choice matter? Otolaryngol Head Neck Surg. 2017;157(05):837–847. doi: 10.1177/0194599817707167. [DOI] [PubMed] [Google Scholar]
- 86.Mertens G, Punte A K, Cochet E, De Bodt M, Van de Heyning P. Long-term follow-up of hearing preservation in electric-acoustic stimulation patients. Otol Neurotol. 2014;35(10):1765–1772. doi: 10.1097/MAO.0000000000000538. [DOI] [PubMed] [Google Scholar]
- 87.Moteki H, Nishio S Y, Miyagawa M, Tsukada K, Iwasaki S, Usami S I. Long-term results of hearing preservation cochlear implant surgery in patients with residual low frequency hearing. Acta Otolaryngol. 2017;137(05):516–521. doi: 10.1080/00016489.2016.1252061. [DOI] [PubMed] [Google Scholar]
- 88.Gstoettner W K, van de Heyning P, O'Connor A F et al. Electric acoustic stimulation of the auditory system: results of a multi-centre investigation. Acta Otolaryngol. 2008;128(09):968–975. doi: 10.1080/00016480701805471. [DOI] [PubMed] [Google Scholar]
- 89.Turner C W, Gantz B J, Vidal C, Behrens A, Henry B A. Speech recognition in noise for cochlear implant listeners: benefits of residual acoustic hearing. J Acoust Soc Am. 2004;115(04):1729–1735. doi: 10.1121/1.1687425. [DOI] [PubMed] [Google Scholar]
- 90.Turner C W, Reiss L AJ, Gantz B J.Combined acoustic and electric hearing: preserving residual acoustic hearing Hear Res 2008242(1-2):164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gantz B J, Turner C, Gfeller K E, Lowder M W. Preservation of hearing in cochlear implant surgery: advantages of combined electrical and acoustical speech processing. Laryngoscope. 2005;115(05):796–802. doi: 10.1097/01.MLG.0000157695.07536.D2. [DOI] [PubMed] [Google Scholar]
- 92.Kiefer J, Pok M, Adunka O et al. Combined electric and acoustic stimulation of the auditory system: results of a clinical study. Audiol Neurootol. 2005;10(03):134–144. doi: 10.1159/000084023. [DOI] [PubMed] [Google Scholar]
- 93.Adunka O F, Dillon M T, Adunka M C, King E R, Pillsbury H C, Buchman C A. Hearing preservation and speech perception outcomes with electric-acoustic stimulation after 12 months of listening experience. Laryngoscope. 2013;123(10):2509–2515. doi: 10.1002/lary.23741. [DOI] [PubMed] [Google Scholar]
- 94.Gfeller K E, Olszewski C, Turner C, Gantz B, Oleson J. Music perception with cochlear implants and residual hearing. Audiol Neurootol. 2006;11 01:12–15. doi: 10.1159/000095608. [DOI] [PubMed] [Google Scholar]
- 95.Gfeller K, Turner C, Oleson J et al. Accuracy of cochlear implant recipients on pitch perception, melody recognition, and speech reception in noise. Ear Hear. 2007;28(03):412–423. doi: 10.1097/AUD.0b013e3180479318. [DOI] [PubMed] [Google Scholar]
- 96.Dorman M F, Gifford R H. Combining acoustic and electric stimulation in the service of speech recognition. Int J Audiol. 2010;49(12):912–919. doi: 10.3109/14992027.2010.509113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dunn C C, Perreau A, Gantz B, Tyler R S. Benefits of localization and speech perception with multiple noise sources in listeners with a short-electrode cochlear implant. J Am Acad Audiol. 2010;21(01):44–51. doi: 10.3766/jaaa.21.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ching T Y, Incerti P, Hill M. Binaural benefits for adults who use hearing aids and cochlear implants in opposite ears. Ear Hear. 2004;25(01):9–21. doi: 10.1097/01.AUD.0000111261.84611.C8. [DOI] [PubMed] [Google Scholar]
- 99.Kong Y Y, Stickney G S, Zeng F G.Speech and melody recognition in binaurally combined acoustic and electric hearing J Acoust Soc Am 2005117(3, Pt 1):1351–1361. [DOI] [PubMed] [Google Scholar]
- 100.van Hoesel R J.Contrasting benefits from contralateral implants and hearing aids in cochlear implant users Hear Res 2012288(1-2):100–113. [DOI] [PubMed] [Google Scholar]
- 101.Cherry C. Some experiments on the recognition of speech, with one and two ears. J Acoust Soc Am. 1953;26:554–559. [Google Scholar]
- 102.Yost W A, Dye R H, Jr, Sheft S. A simulated “cocktail party” with up to three sound sources. Percept Psychophys. 1996;58(07):1026–1036. doi: 10.3758/bf03206830. [DOI] [PubMed] [Google Scholar]
- 103.Hirsh I. The influence of interaural phase on interaural summation and inhibition. J Acoust Soc Am. 1948;20(04):536–544. [Google Scholar]
- 104.Zhang T, Dorman M F, Spahr A J. Information from the voice fundamental frequency (F0) region accounts for the majority of the benefit when acoustic stimulation is added to electric stimulation. Ear Hear. 2010;31(01):63–69. doi: 10.1097/aud.0b013e3181b7190c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Loiselle L H, Dorman M F, Yost W A, Cook S J, Gifford R H. Using ILD or ITD cues for sound source localization and speech understanding in a complex listening environment by listeners with bilateral and with hearing-preservation cochlear implants. J Speech Lang Hear Res. 2016;59(04):810–818. doi: 10.1044/2015_JSLHR-H-14-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rader T, Fastl H, Baumann U. Speech perception with combined electric-acoustic stimulation and bilateral cochlear implants in a multisource noise field. Ear Hear. 2013;34(03):324–332. doi: 10.1097/AUD.0b013e318272f189. [DOI] [PubMed] [Google Scholar]
- 107.Incerti P V, Ching T Y, Cowan R. A systematic review of electric-acoustic stimulation: device fitting ranges, outcomes, and clinical fitting practices. Trends Amplif. 2013;17(01):3–26. doi: 10.1177/1084713813480857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dillon M T, Buss E, Adunka O F, Buchman C A, Pillsbury H C. Influence of test condition on speech perception with electric-acoustic stimulation. Am J Audiol. 2015;24(04):520–528. doi: 10.1044/2015_AJA-15-0022. [DOI] [PubMed] [Google Scholar]
- 109.Gifford R H, Dorman M F, Skarzynski H et al. Cochlear implantation with hearing preservation yields significant benefit for speech recognition in complex listening environments. Ear Hear. 2013;34(04):413–425. doi: 10.1097/AUD.0b013e31827e8163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wei C G, Cao K, Zeng F G.Mandarin tone recognition in cochlear-implant subjects Hear Res 2004197(1-2):87–95. [DOI] [PubMed] [Google Scholar]
- 111.Luo X, Chang Y P, Lin C Y, Chang R Y. Contribution of bimodal hearing to lexical tone normalization in Mandarin-speaking cochlear implant users. Hear Res. 2014;312:1–8. doi: 10.1016/j.heares.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Karsten S A, Turner C W, Brown C J, Jeon E K, Abbas P J, Gantz B J. Optimizing the combination of acoustic and electric hearing in the implanted ear. Ear Hear. 2013;34(02):142–150. doi: 10.1097/AUD.0b013e318269ce87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nopp P, Polak M. From electric acoustic stimulation to improved sound coding in cochlear implants. Adv Otorhinolaryngol. 2010;67:88–95. doi: 10.1159/000262600. [DOI] [PubMed] [Google Scholar]
- 114.Simpson A, McDermott H J, Dowell R C, Sucher C, Briggs R J. Comparison of two frequency-to-electrode maps for acoustic-electric stimulation. Int J Audiol. 2009;48(02):63–73. doi: 10.1080/14992020802452184. [DOI] [PubMed] [Google Scholar]
- 115.Vermeire K, Anderson I, Flynn M, Van de Heyning P. The influence of different speech processor and hearing aid settings on speech perception outcomes in electric acoustic stimulation patients. Ear Hear. 2008;29(01):76–86. doi: 10.1097/AUD.0b013e31815d6326. [DOI] [PubMed] [Google Scholar]
- 116.Büchner A, Illg A, Majdani O, Lenarz T. Investigation of the effect of cochlear implant electrode length on speech comprehension in quiet and noise compared with the results with users of electro-acoustic-stimulation, a retrospective analysis. PLoS One. 2017;12(05):e0174900. doi: 10.1371/journal.pone.0174900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rader T, Adel Y, Fastl H, Baumann U. Speech perception with combined electric-acoustic stimulation: a simulation and model comparison. Ear Hear. 2015;36(06):e314–e325. doi: 10.1097/AUD.0000000000000178. [DOI] [PubMed] [Google Scholar]
- 118.Gifford R H, Davis T J, Sunderhaus L W et al. Combined electric and acoustic stimulation with hearing preservation: effect of cochlear implant low-frequency cutoff on speech understanding and perceived listening difficulty. Ear Hear. 2017;38(05):539–553. doi: 10.1097/AUD.0000000000000418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Baumann U, Rader T, Helbig S, Bahmer A. Pitch matching psychometrics in electric acoustic stimulation. Ear Hear. 2011;32(05):656–662. doi: 10.1097/AUD.0b013e31821a4800. [DOI] [PubMed] [Google Scholar]
- 120.McDermott H, Varsavsky A. Better fitting of cochlear implants: modeling loudness for acoustic and electric stimuli. J Neural Eng. 2009;6(06):65007. doi: 10.1088/1741-2560/6/6/065007. [DOI] [PubMed] [Google Scholar]
- 121.Reiss L A, Turner C W, Karsten S A, Gantz B J. Plasticity in human pitch perception induced by tonotopically mismatched electro-acoustic stimulation. Neuroscience. 2014;256(256):43–52. doi: 10.1016/j.neuroscience.2013.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Reiss L A, Ito R A, Eggleston J L, Wozny D R. Abnormal binaural spectral integration in cochlear implant users. J Assoc Res Otolaryngol. 2014;15(02):235–248. doi: 10.1007/s10162-013-0434-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vermeire K, Punte A K, Van de Heyning P. Better speech recognition in noise with the fine structure processing coding strategy. ORL J Otorhinolaryngol Relat Spec. 2010;72(06):305–311. doi: 10.1159/000319748. [DOI] [PubMed] [Google Scholar]
- 124.Müller J, Brill S, Hagen R et al. Clinical trial results with the MED-EL fine structure processing coding strategy in experienced cochlear implant users. ORL J Otorhinolaryngol Relat Spec. 2012;74(04):185–198. doi: 10.1159/000337089. [DOI] [PubMed] [Google Scholar]
- 125.Choudhury B, Fitzpatrick D C, Buchman C A et al. Intraoperative round window recordings to acoustic stimuli from cochlear implant patients. Otol Neurotol. 2012;33(09):1507–1515. doi: 10.1097/MAO.0b013e31826dbc80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Radeloff A, Shehata-Dieler W, Scherzed A et al. Intraoperative monitoring using cochlear microphonics in cochlear implant patients with residual hearing. Otol Neurotol. 2012;33(03):348–354. doi: 10.1097/MAO.0b013e318248ea86. [DOI] [PubMed] [Google Scholar]
- 127.McClellan J H, Formeister E J, Merwin W H, III et al. Round window electrocochleography and speech perception outcomes in adult cochlear implant subjects: comparison with audiometric and biographical information. Otol Neurotol. 2014;35(09):e245–e252. doi: 10.1097/MAO.0000000000000557. [DOI] [PubMed] [Google Scholar]
- 128.Adunka O F, Giardina C K, Formeister E J, Choudhury B, Buchman C A, Fitzpatrick D C. Round window electrocochleography before and after cochlear implant electrode insertion. Laryngoscope. 2016;126(05):1193–1200. doi: 10.1002/lary.25602. [DOI] [PMC free article] [PubMed] [Google Scholar]


