Abstract
Background:
Recent evidence has shown that nicotinamide treatment may have an impact on phosphorus metabolism in hemodialysis patients. Nevertheless, the treatment remains controversial. This study aimed to precisely estimate the efficacy and safety of nicotinamide on phosphorus, calcium and iPTH in hemodialysis patients.
Methods:
We searched numerous information sources regarding randomized controlled trials (RCTs) of nicotinamide treatment in hemodialysis patients, including PubMed, EMBASE, and the Cochrane Library.
Results:
Nine relevant studies (n = 428) were included in the meta-analysis. Meta-analysis showed that levels of serum phosphorus (SMD −1.06; 95% CI, −1.27 to −0.85, P < .001), parathyroid hormone (SMD −1.09; 95% CI, −1.49 to −0.70, P < .001), and calcium–phosphorus (SMD −0.65; 95% CI, −0.97 to −0.34, P < .001) in the nicotinamide group were significantly lower than those of the control group. There was no significant difference in the levels of serum calcium (SMD 0.08; 95% CI, −0.15 to 0.30, P = .51) between the groups. The meta-analysis showed that the nicotinamide group had a significantly higher risk of adverse events (OR 3.99; 95% CI, 1.94–8.23, P < .001) than did the control group, especially for thrombocytopenia (OR 49.00; 95% CI, 2.68–897.36, P = .009). However, no serious adverse reactions were observed. There was no significant difference in the incidence of withdrawal (OR 3.51; 95% CI, 0.49–25.00, P = .21) between the groups.
Conclusion:
Evidence to date clearly indicates that nicotinamide is safe and effective for improving phosphorus metabolism in hemodialysis patients. However, nicotinamide probably causes thrombocytopenia. Further large-sample size, high-quality RCTs are needed.
Keywords: hemodialysis patients, nicotinamide, phosphorus metabolism
1. Introduction
Hyperphosphatemia is a common complication of end-stage renal disease (ESRD) and is an independent risk factor for increased mortality in dialysis patients.[1] A study found that long-term hyperphosphatemia can aggravate renal osteodystrophy, vascular calcification, and other cardiovascular diseases in ESRD patients. When the phosphorus level increased 0.323 mmol L−1, the risk of death increased by 18%.[2] Hyperphosphatemia seriously affects quality of life and survival rates in dialysis patients. Proper control of phosphorus has long been a dilemma for nephrologists. Only relying on dietary control and dialytic clearance to maintain phosphorus balance is often unsuccessful in achieving satisfactory results; therefore, there is a need to find effective oral phosphorus-lowering drugs.
Phosphorus-binding agents are the primary medications for the treatment of hyperphosphatemia. However, the representative calcium–phosphorus binding agents including calcium carbonate often cause hypercalcemia, the cause of increased risk of cardiovascular calcification and death in dialysis patients. Therefore, the Kidney Disease Outcome Quality Initiative (K/DOQI) recommends that dialysis patients with hypercalcemia use noncalcium phosphorus binding agents such as phosphorus-lowering drugs.[3] Aluminum-based phosphorus binders can cause the accumulation of aluminum in vivo.
Sevelamer and lanthanum carbonate have been marketed for phosphorus reduction in recent years. Sevelamer has been associated with reduction in LDL cholesterol as well. However, they can cause gastrointestinal adverse reactions and the accumulation of lanthanum in the body, respectively. In addition, high price greatly limits their application. There is an urgent need to find effective, safe, and affordable phosphate-lowering drugs for hemodialysis patients. In recent years, it has been found that the traditional lipid-lowering drug nicotinic acid reduces phosphorus while treating hyperlipidemia.[4–6] However, the representative calcium-based phosphorus binding agents such as calcium carbonate often cause hypercalcemia, further contributing to increased risk of cardiovascular calcification and death in dialysis patients. In this study, we conducted a comprehensive meta-analysis of nicotinamide to further explore its efficacy and safety for treatment of hyperphosphatemia in patients on dialysis.
1.1. Search strategy
Two researchers (YZ and PZ) performed a comprehensive literature search and 8 relevant studies were obtained that conformed to all eligibility criteria. To ascertain all relevant RCTs regardless of publication status, we searched the electronic databases PubMed, EMBASE, Cochrane Library until February 6, 2018. The following keywords were used: “niacinamide amide,” “nicotinic acid,” “nicotinamide,” “niacinamide amide,” “phosphorus,” “phosphate,” “dialysis.” Reference lists from the identified studies were incorporated to enrich the study.
1.2. Selection criteria
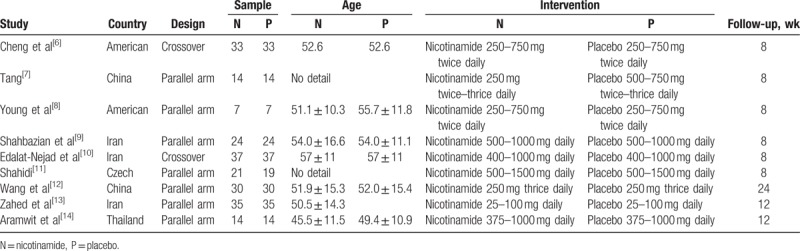
Two authors (YZ and TM) independently carried out the primary review to search for trials that met inclusion criteria. Any discrepancy was resolved by discussion and consensus (Fig. 1). The following criteria were used: the study design was an RCT; the study focused on patients undergoing regular hemodialysis or peritoneal dialysis treatment, with serum phosphorus >1.5 mmol/L; the experimental group received nicotinamide treatment, and the control group received placebo treatment, treatment time >1 month; and one of the following outcomes must have been included: serum calcium, phosphorus, calcium X phosphorus products, iPTH levels, and observed and recorded adverse reactions. Main characteristics of the included studies are listed in Table 1.
Figure 1.
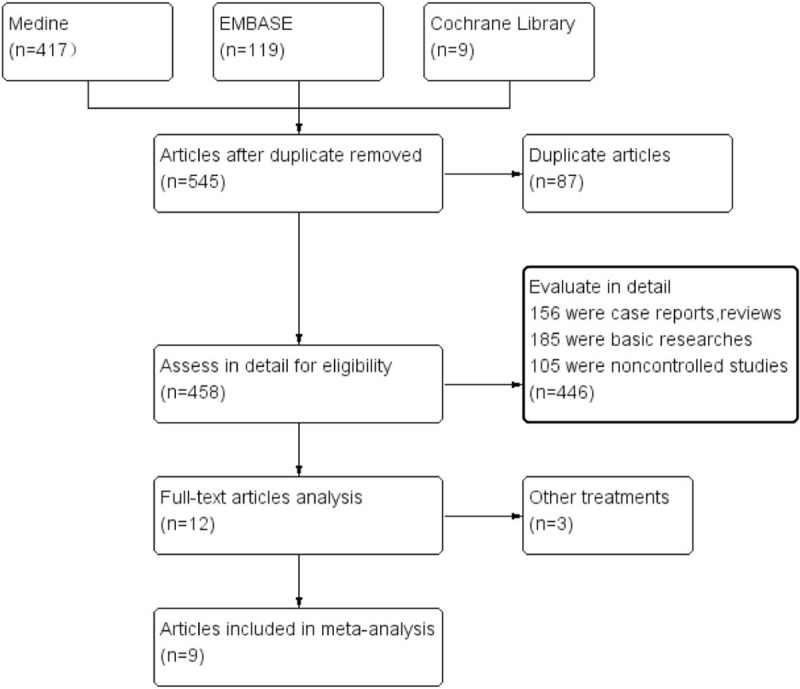
Flow chart of included studies in the meta-analysis.
Table 1.
Main characteristics of the included studies.
1.3. Risk of bias assessment
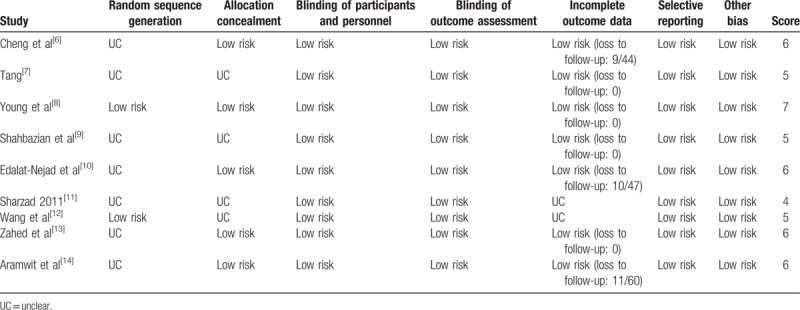
The quality of all trials was evaluated independently by 2 authors (YZ and TM) according to the Cochrane quality criteria (Table 2). Any disagreement between the authors (YZ and PZ) was settled by discussion with a third author (TM). Ethical approval is not required because this protocol will not involve any subject directly.
Table 2.
Risk of bias in published randomized control trials.
1.4. Statistical analysis
Stata 12.0 (StataCorp LP, College Station, TX) was used to perform statistical analyses. The risk ratio (RR) with its 95% confidence interval (CI) was used for the effect size of dichotomous data. Standard mean differences (SMDs) with their 95% CIs were computed for continuous data. The weighted fixed-effect model was used if there was no significant heterogeneity. Otherwise, the random-effects model was used. Heterogeneity was analyzed statistically by I2 and χ2 statistics. The critical value for homogeneity was a P-value less than .05. Sensitivity analysis was assessed by omitting each study in turn to evaluate the consistency and quality of the results through STATA 12.0. If there was significant heterogeneity, publication bias was assessed by Begg's plots and Egger's test.
Heterogeneity was categorized as follows: low when the I2 statistic was 0% to 25%; medium at 25% to 50%; high at 50% to 75%; and powerful at 75% to 100%.[15]P values were determined using the χ2 test; results were considered statistically significant at P < .05 for all included studies.
2. Results
2.1. Study selection
We identified 545 articles in the initial retrieval. Of these, 87 duplicate articles were excluded after carefully examining the titles and abstracts. When evaluated in detail, 446 articles were excluded because 156 were case reports or reviews, 185 were basic research and 105 were noncontrolled studies. The remaining 11 articles were reviewed for further selection. An additional 3 articles were excluded for insufficient data. Finally, 9 studies (n = 428) were included in this meta-analysis, as listed in Table 1. Our search strategy is described in the flow diagram (Fig. 1).
Main characteristics of the RCTs (country, design, sample size, age, intervention, and flow-up) included are described in Table 1.
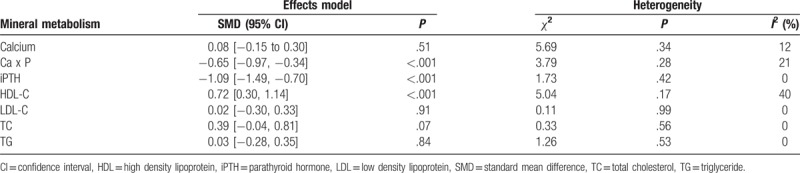
2.2. Effects on mineral metabolism parameters
Serum phosphorus value in the nicotinamide group was significantly lower (SMD −1.06; 95% CI, −1.27 to −0.85, P < .001, I2 = 79%, Fig. 2) than that in the control group. No significant difference in serum calcium level was observed between the 2 groups (SMD 0.08; 95% CI, −0.15 to 0.30, P = .51, I2 = 12%, Table 3). Ca x P level in nicotinamide group was also significantly lower than that in the control group (SMD −0.65; 95% CI, −0.97 to −0.34, P < .001, I2 = 21%, Table 3). The iPTH level in nicotinamide group was significantly lower than that in the control group (SMD −1.09; 95% CI, −1.49 to −0.70, P < .001, I2 = 0%, Table 3).
Figure 2.
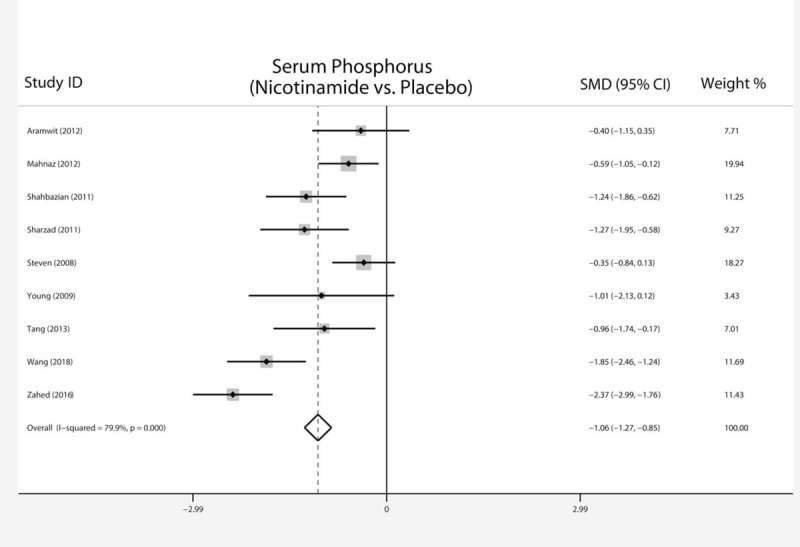
Forest plot of serum phosphorus of nicotinamide versus placebo in hemodialysis patients.
Table 3.
Calcium phosphorus and lipid metabolism of nicotinamide versus placebo in hemodialysis patients.
2.3. Serum lipid profile
The treatment group had significant higher HDL (SMD 0.72; 95% CI, 0.30–1.14, P < .001, I2 = 40%, Table 3) than did the control group. However, there was no significant difference between the 2 groups in terms of LDL (SMD 0.02, 95% CI, −0.30 to 0.33, P = .91, I2 = 0%, Table 3), TC (SMD, 0.39, 95% CI, −0.04 to 0.81, P = .07, I2 = 0%, Table 3), or TG (SMD 0.03; 95% CI, −0.28 to 0.35, P = .84, I2 = 0%, Table 3).
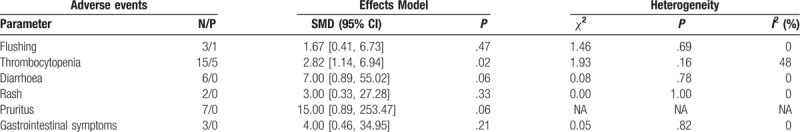
2.4. Adverse events
All of the studies reported adverse events during treatment. No significant difference was found in adverse events between the nicotinamide group and the control group in terms of diarrhea (RR 7.00; 95% CI, 0.89–55.02, P = .06, I2 = 0%, Table 4), pruritus (RR 15.00; 95% CI, 0.89–253.47, P = .06, Table 4), rash (RR 3.00; 95% CI, 0.33–27.28, P = .33, I2 = 0%, Table 4), gastrointestinal symptoms (RR 4.00; 95% CI, 0.46–34.95, P = .21, Table 4), or flushing (RR 1.67; 95% CI, 0.41–6.73, P = .47, I2 = 0%, Table 4). However, the nicotinamide group had a higher risk of thrombocytopenia (RR 2.82, 95% CI, 1.14–6.94, P = .02, I2 = 48%, Table 4). Overall, the adverse events were mild or tolerable.
Table 4.
Adverse events of nicotinamide versus placebo in haemodialysis patients.
2.5. Sensitivity analysis and publication bias
There was significant heterogeneity in serum phosphorus (I2 = 79%). Sensitivity analysis was conducted to ascertain the influence of each individual study by omitting individual studies. No individual study significantly affected the heterogeneity, suggesting that our statistical result was robust. No significant publication bias was found in the results of Begg's plots (P = .902) and Egger's test (P = .449) for phosphorus.
3. Discussion
The incidence of hyperphosphatemia in patients with end-stage renal disease is as high as 81% to 90%; it is an independent risk factor for death in dialysis patients.[16] The current primary treatment of hyperphosphatemia includes limitation of phosphorus in diet, the use of phosphorus-binding agents and blood purification treatment. Dietary phosphorus limitation requires foods with low phosphorus/protein ratios and low phosphorus uptake, with daily phosphorus intakes limited to 800 to 1000 mg; however, long-term low-phosphorus diets may increase the risk of adverse reactions in dialysis.[17]
Niacinamide, also called nicotinamide, is the amide of nicotinic acid (vitamin B3/niacin). It has been known as a drug that regulates lipid metabolism for more than 40 years. In 1998, Shimoda et al[18] used peneconide nicotinate in the treatment of low HDL-C with dialysis and found incidentally that the drug lowered serum phosphorus. Since then, the phosphorus-lowering effects of nicotinic acid and its derivatives have been confirmed in a series of clinical studies.[4,16] However, the efficacy and safety of this drug for dialysis patients still lack an adequate evidence-basis.
Debiec and Lorenc[19] found that there were 2 kinds of sodium–phosphate cotransporter in humans, type-2a sodium-dependent phosphate cotransporters (NaPi-2a) located in renal tubules, and type-2b sodium-dependent phosphate cotransporters (NaPi-2b) located in the small intestine; these mediate phosphate reabsorption in proximal tubules and intestine, respectively. Nicotinamide,[15] a known inhibitor of NaPi-2b (intestine) and NaPi-2a (kidney), has been used to reduce blood phosphate levels in CKD patients. Katai et al[20] found that nicotinamide not only inhibited chronic renal failure rat intestinal sodium/phosphorus transport activity but also had an inhibitory effect on intestinal phosphorus uptake. Eto et al[21] showed that nicotinamide significantly inhibited intestinal phosphate uptake and prevented the increase of serum phosphorus concentration in adenine-induced chronic renal failure model in rats; this effect was directly induced by nicotinamide in small intestine brush border membrane Na Pi-2b expression down-regulation. Similarly, a study[21] found that niceritrol, a prodrug of nicotinamide, increased the excretion of rat fecal phosphate. Nicotinamide also caused hypophosphatemia in normal mice.
The current meta-analysis included 9 RCTs and assessed the efficacy and safety of nicotinamide on phosphorus, calcium, iPTH in hemodialysis patients. As far as we know, no previous studies have specifically analyzed the role of nicotinamide in hemodialysis patients. Our present study found that nicotinamide was effective for the treatment in renal dialysis patients and could also decrease the Ca x P and iPTH without affecting serum calcium levels.
Our meta-analysis also showed that the nicotinamide group had significantly higher HDL levels than did the placebo group. There were no significant differences between the 2 groups in terms of levels of TC, TG, or LDL.
Overall, no significant difference was found in adverse events between the 2 groups. There was no significant difference in the incidence of withdrawal between patients in the nicotinamide and control groups. Most of the side-effects of nicotinamide were tolerable. However, we found that, compared with the placebo group, the nicotinamide group had a significantly higher risk of thrombocytopenia. Shahbazian et al's study[9] showed that thrombocytopenia occurred in 25% (6 of 25) of patients after nicotinamide treatment, and 2 among them had platelet counts < 100,000 cells/mm3, but recovered to 20,000 cells/mm3 at 20 days after treatment withdrawal.
The adverse reactions of nicotinamide drugs include hot flushes, burning and painful lips, diarrhea, hyperglycemia, and gastrointestinal symptoms. These may be associated with the release of prostaglandins caused by vasodilation.[7] Recent studies have found that the combination of type 1 selective D2 prostaglandin receptor inhibitors or the use of nicotinamide sustained release formulations can reduce the incidence of adverse reactions.
According to some reports, patients receiving nicotinamide treatment not only had lower serum phosphorus but also had reduced alkaline phosphatase levels,[22] pruritus,[23] plasma triglycerides, LDL and VLDL, as well as increased HDL levels.[5,6,9] This is also consistent with our results.
Several limitations of our meta-analysis should be considered. First, the information in several studies was incomplete. In particular, most studies lacked data on the serum lipid profile of patients after treatment. Second, this meta-analysis did not include a particularly large number of RCTs. The quality of several trials was not high, follow-up times were different, and the treatment doses were variable, ranging from 25 mg daily to 1500 mg/day. Furthermore, various therapy doses and follow-up times could have affected our conclusions. Third, significant heterogeneity existed in the results of serum phosphorus, and although no significant publication bias was found by Egger's test and Begg's plots, the validity of publication bias is limited. Finally, there appears to be a lack of published studies with negative outcomes. The risk of publication bias against studies with negative results is also a limitation.
4. Conclusions
Our meta-analysis demonstrated that nicotinamide treatment is effective for reducing blood phosphorus and does not increase hypercalcemia, thereby significantly reducing the calcium and phosphorus products in renal dialysis patients. Simultaneously, nicotinamide effectively raises blood HDL-C levels, which is a beneficial effect for reducing the risk of cardiovascular complications in patients undergoing hemodialysis. Combined with minor adverse reactions, high safety and low cost, nicotinamide provides a new option for the treatment of hyperphosphatemia in hemodialysis patients. For future research, the safety and efficacy of nicotinamide in the treatment of hyperphosphatemia in patients on maintenance dialysis needs to be further confirmed by more large-scale, high-quality, long-term follow-up clinical studies.
Acknowledgment
The authors are grateful to Bo Wang and Heng Zhang for valuable help with the literature search.
Author contributions
Conceptualization: Yong Zhang, Pan Zhang.
Data curation: Yong Zhang, Pan Zhang.
Formal analysis: Yong Zhang, Tean Ma, Pan Zhang.
Funding acquisition: Tean Ma, Pan Zhang.
Investigation: Yong Zhang, Tean Ma, Pan Zhang.
Methodology: Yong Zhang, Pan Zhang.
Project administration: Yong Zhang, Tean Ma, Pan Zhang.
Resources: Yong Zhang, Tean Ma, Pan Zhang.
Software: Yong Zhang, Tean Ma, Pan Zhang.
Supervision: Yong Zhang, Pan Zhang.
Validation: Yong Zhang, Tean Ma, Pan Zhang.
Visualization: Yong Zhang, Pan Zhang.
Writing – original draft: Yong Zhang, Tean Ma, Pan Zhang.
Writing – review & editing: Yong Zhang, Pan Zhang.
Footnotes
Abbreviations: CI = confidence interval, CKD = chronic kidney disease, ESRD = end-stage renal disease, HDL = high density lipoprotein, iPTH = parathyroid hormone, K/DOQI = Kidney Disease Outcome Quality Initiative, LDL = low density lipoprotein, NaPi-2a = type-2a sodium-dependent phosphate cotransporters, NaPi-2b = type-2b sodium-dependent phosphate cotransporters, RCT = randomized controlled trial, RR = risk ratio, SMD = standard mean difference, TC = total cholesterol, TG = triglyceride.
Ethics and dissemination: There are no ethical considerations associated with this review. Ethical approval is not required because this protocol will not involve any subject directly.
The authors have no conflicts of interest to disclose.
References
- [1].Tentori F, Blayney MJ, Albert JM, et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2008;52:519–30. [DOI] [PubMed] [Google Scholar]
- [2].Palmer SC, Hayen A, Macaskill P, et al. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. JAMA 2011;305:1119–27. [DOI] [PubMed] [Google Scholar]
- [3].National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003; 424 suppl 3:S1–S201 [PubMed] [Google Scholar]
- [4].Sampathkumar K, Selvam M, Sooraj YS, et al. Extended release nicotinic acid—a novel oral agent for phosphate control. Int Urol Nephrol 2006;38:171–4. [DOI] [PubMed] [Google Scholar]
- [5].Takahashi Y, Tanaka A, Nakamura T, et al. Nicotinamide suppresses hyperphosphatemia in hemodialysis patients. Kidney Int 2004;65:1099–104. [DOI] [PubMed] [Google Scholar]
- [6].Cheng SC, Young DO, Huang Y, et al. A randomized, double-blind, placebo-controlled trial of niacinamide for reduction of phosphorus in hemodialysis patients. Clin J Am Soc Nephrol 2008;3:1131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tang JL. Therapeutic observation of niacinamide for hemodialysis hyperphosphatemia. Clin J Postgrad Med 2013;13:44–6. [Google Scholar]
- [8].Young DO, Cheng SC, Delmez JA, et al. The effect of oral niacinamide on plasma phosphorus levels in peritoneal dialysis patients. Perit Dial Int 2009;29:562–7. [PubMed] [Google Scholar]
- [9].Shahbazian H, Zafar Mohtashami A, Ghorbani A, et al. Oral nicotinamide reduces serum phosphorus, increases HDL, and induces thrombocytopenia in hemodialysis patients: a double-blind randomized clinical trial. Nefrologia 2011;31:58–65. [DOI] [PubMed] [Google Scholar]
- [10].Edalat-Nejad M, Zameni F, Talaiei A. The effect of niacinamide on serum phosphorus levels in dialysis patients. Indian J Nephrol 2012;22:174–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Shahidi S, Sajjadieh S, Gholamrezaei A. Niacinamideamide for reduction of phosphorus in hemodialysis and peritoneal dialysis patients. A randomized, double-blind, placebo-controlled trial. Era-Edta Congress 2011;48:84–5. [Google Scholar]
- [12].Wang LH, Wang LL, Gao ZY, et al. Effect of nicotinamide on hyperphosphatemia in patients with maintenance hemodialysis. Chin Pract Med 2018;13:112–3. [Google Scholar]
- [13].Zahed NS, Zamanifar N, Nikbakht H. Effect of low dose nicotinic acid on hyperphosphatemia in patients with end stage renal disease. Indian J Nephrol 2016;26:239–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Aramwit P, Srisawadwong R, Supasyndh O. Effectiveness and safety of extended-release nicotinic acid for reducing serum phosphorus in hemodialysis patients. J Nephrol 2012;25:354–62. [DOI] [PubMed] [Google Scholar]
- [15].Kempson SA, Colon-Otero G, Ou SY, et al. Possible role of nicotinamide adenine dinucleotide as an intracellular regulator of renal transport of phosphate in the rat. J Clin Invest 1981;67:1347–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Maccubbin D, Tipping D, Kuznetsova O, et al. Hypophosphatemic effect of niacinamide in patients without renal failure: a randomized trial. Clin J Am Soc Nephrol 2010;5:582–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Uribarri J, Calvo MS. Hidden sources of phosphorus in the typical American diet: does it matter in nephrology? Semin Dial 2003;16:186–8. [DOI] [PubMed] [Google Scholar]
- [18].Shimoda K, Akiba T, Matsushima T, et al. [Niceritrol decreases serum phosphate levels in chronic hemodialysis patients]. Nihon Jinzo Gakkai shi 1998;40:1–7. [PubMed] [Google Scholar]
- [19].Debiec H, Lorenc R. Identification of Na+,Pi-binding protein in kidney and intestinal brush-border membranes. Biochem J 1988;255:185–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Katai K, Tanaka H, Tatsumi S, et al. Nicotinamide inhibits sodium-dependent phosphate cotransport activity in rat small intestine. Nephrol Dial Transplant 1999;14:1195–201. [DOI] [PubMed] [Google Scholar]
- [21].Eto N, Miyata Y, Ohno H, et al. Nicotinamide prevents the development of hyperphosphataemia by suppressing intestinal sodium-dependent phosphate transporter in rats with adenine-induced renal failure. Nephrol Dial Transplant 2005;20:1378–84. [DOI] [PubMed] [Google Scholar]
- [22].Vasantha J, Soundararajan P, Vanitharani N, et al. Safety and efficacy of nicotinamide in the management of hyperphosphatemia in patients on hemodialysis. Indian J Nephrol 2011;21:245–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Omidian M, Khazanee A, Yaghoobi R, et al. Therapeutic effect of oral nicotinamide on refractory uremic pruritus: a randomized, double-blind study. Saudi J Kidney Dis Transpl 2013;24:995–9. [DOI] [PubMed] [Google Scholar]


