Abstract
Retrospective studies have suggested that capecitabine combined with temozolomide (CAPTEM) is effective for treating patients with advanced neuroendocrine neoplasms (NENs); however, the efficacy and safety of this regimen needs to be verified by high-quality evidence or results of randomized controlled trials.
We carried out a meta-analysis to evaluate the safety and effectiveness of a CAPTEM protocol for patients with advanced NENs. Systematic electronic literature searches were conducted using PubMed, EMBASE, and the Cochrane Library, and among meeting abstracts of the American Society of Clinical Oncology, European Society for Medical Oncology, European Neuroendocrine Tumor Society, and North American Neuroendocrine Tumor Society, up to June 30, 2017. We selected studies describing CAPTEM regimens for treating advanced NENs and reported on tumor response and/or toxicities according to clear World Health Organization (WHO) grading of patients. Three reviewers independently and repeatedly identified studies, extracted data, and assessed the quality of the literature. A single-proportion meta-analysis was applied to included articles.
Fifteen studies with a total of 384 individuals were included. Medium overall survival in most studies was more than 12 months, whereas medium progression-free survival was similar or slightly higher than that in studies using other treatment regimes. Disease control rate of CAPTEM administration for patients with NENs was 72.89% (95% confidence interval, 64.04–81.73%; I2 = 82.4%; P < .01). WHO grade 3 to 4 toxicities, such as thrombocytopenia (3.36%), neutropenia (0.69%), lymphopenia (0.65%), anemia (0.59%), mucositis (0.57%), fatigue (0.54%), diarrhea (0.49%), nausea (0.39%), and transaminase elevation (0.13%) were reported in the trials included.
CAPTEM is effective and relatively safe for treating patients with advanced NENs.
Keywords: capecitabine, disease control rate, neuroendocrine neoplasms, temozolomide, toxicities, World Health Organization grade
1. Introduction
Neuroendocrine neoplasms (NENs) are a rare group of tumors whose incidence has been significantly increasing every year.[1] NENs are highly heterogeneous tumors, and their classification criteria and prognoses vary according to their presence in different organs.[2] One study of 35,618 patients reported that 20.60% of patients were found to already have distant metastases upon initial diagnosis.[1] In another study, median survival was only 19 months for patients with metastatic disease, 33 months for G1/G2 patients, and 5 months for G3 patients.[2] Comprehensive treatment should be applied in patients with advanced NENs. For patients with unresectable disease who are asymptomatic, and have a low tumor burden and stable disease, observation with marker assessment and abdominal/pelvic multiphasic computed tomography or magnetic resonance imaging scans every 3 to 12 months as clinically indicated should be considered until clinically significant disease progression occurs. For patients with symptomatic, severe tumor burden, or severe tumor progression, lanreotide or octreotide should be administered.[3,4] Several studies suggest that molecularly targeted therapies such as everolimus, sunitinib, or cytotoxic chemotherapy should be administered to patients with tumors that continue to progress.[5–10] The alkylating agents streptozocin and temozolomide appear to have the most antitumor activity in NENs, especially in pancreatic NENs. Many studies have suggested that capecitabine combined with temozolomide (CAPTEM) is effective in patients with advanced NENs, resulting in high objective response rates and considerably low toxicity[11–13]; however, the efficacy and safety of this regimen needs to be verified by high-quality evidence or results of randomized controlled trials. Therefore, in this article, we have evaluated the safety and efficacy of temozolomide combined with CAPTEM in patients with advanced NENs in a single-proportion meta-analysis.
2. Methods
2.1. Selection criteria
The following criteria were used to identify eligible studies: studies describing temozolomide and capecitabine as a combination treatment for advanced NENs; studies reporting tumor response outcome measures and/or toxicities, and studies clearly reporting World Health Organization (WHO) grading of patients. The following exclusion criteria were applied: case reports, editorials, commentaries, meta-analyses, review articles, and animal studies; experimental studies, and non-English language articles.
2.2. Literature search
A literature search for articles was performed using 3 major electronic databases (PubMed, EMBASE, and the Cochrane Library). The terms were combined with the Cochrane MEDLINE filter for studies. The search strategy for MEDLINE is available in the published protocol CRD42017071033. The search terms were adapted for use with other bibliographic databases in combination with database-specific filters for controlled trials, where these were available. Articles written in English were included. Meeting abstracts, including those of the American Society of Clinical Oncology, European Society for Medical Oncology, European Neuroendocrine Tumor Society, and North American Neuroendocrine Tumor Society were also checked. The search strategy was as follows: (“temozolomide” OR “methazolastone” OR “M and B-39831” OR “NSC-362856” OR “Temodal” OR “TMZ-Bioshuttle” OR “CCRG-81045” OR “Temodar”) AND (“Capecitabine” OR “N(4)-pentyloxycarbonyl-5’-deoxy-5-fluorocytidine” OR “Xeloda”) AND (“Neuroendocrine Tumors” OR “Carcinoma, Neuroendocrine” OR “Carcinoid Tumor” OR “Neuroendocrine Tumor” OR “Tumor, Neuroendocrine” OR “Tumors, Neuroendocrine” OR “Neuroendocrine Carcinoma” OR “Neuroendocrine Carcinomas” OR “Carcinoid Tumors” OR “Tumor, Carcinoid” OR “Tumors, Carcinoid” OR “Carcinoid” OR “Carcinoid, Goblet Cell” OR “Carcinoids, Goblet Cell” OR “Goblet Cell Carcinoid” OR “Argentaffinoma” OR “Argentaffinomas”). We retained any article that described the effect of CAPTEM in the treatment of advanced NENs. Review articles, opinion pieces, and articles published after June 2017 were excluded. All searched articles were reviewed by 3 authors, and disagreements were resolved via discussion.
2.3. Literature quality evaluation
We applied the Newcastle-Ottawa Quality Assessment Scale for Cohort Studies, which accommodated the studies included in this meta-analysis.[14] This methodology comprised 9 evaluation criteria, which included the following: research design (patients whose diagnoses were clearly defined, cohorts with >95% of patients having advanced NENs, and follow-up times of >1.5 years); comparability [records of patients’ ages and sexes, Eastern Cooperative Oncology Group performance status (ECOG ps), and tumor staging]; and assessment of outcomes [median progression-free survival (mPFS), disease control rate (DCR), and rate of patients who had grade 3–4 toxicities].
2.4. Data extraction
Data were extracted from studies independently by 3 reviewers, and included the following: author; publication year; study design; patients’ ages and sexes, ECOG ps; primary tumor location; WHO histology grade; DCR; rate of all grade 3 to 4 toxicities; mPFS; and median overall survival (mOS).
2.5. Statistical methods
Meta-analysis was performed for the study's main focuses to calculate proportions and rates with corresponding 95% confidence intervals (CIs) using RGui software version 3.4.1 (R Foundation for Statistical Computing, Vienna, Austria). Peters test and funnel plot were carried out to detect publication bias in the meta-analysis.[15] To evaluate heterogeneity in the outcomes across the included studies, Higgins’ I2 and Q test were used. A Q test P value of <.05 or an I2 value of >50% indicated substantial heterogeneity. When there was little or no substantial heterogeneity between tests, we used a fixed effect model; otherwise, a random effect model was used. Once significant heterogeneity was established, meta-regression, sensitivity analysis, and subgroup analysis were applied to determine the source of the heterogeneity.
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
3. Results
3.1. A rapid systematic review and meta-analysis
This systematic review was conducted in accordance with the Meta-analysis of Observational Studies in Epidemiology guidelines. A total of 657 articles were identified in the 3 major electronic databases using the aforementioned search strategy. We excluded 257 duplicated articles, 1 system review, 26 review articles, 22 non-English language articles, and 17 case reports. A total of 300 articles with irrelevant content were excluded after review of the title and abstract. Four articles were excluded for duplicated data; 12 were excluded for not having clear histologic classifications, and another 3 were excluded for lacking clear descriptions of tumor response and toxicities. Finally, 15 articles composed of 384 participants[10,11,13,16–27] (Fig. 1) were retained. Of the 15 studies, 1 was a single-arm phase II trial and 14 were retrospective studies; moreover, a majority of articles had a score of 7∗ or more in the Wells way literature quality evaluation[14] (Table 1). The general characteristics of the literature and the baseline data are reported in Table 2.
Figure 1.
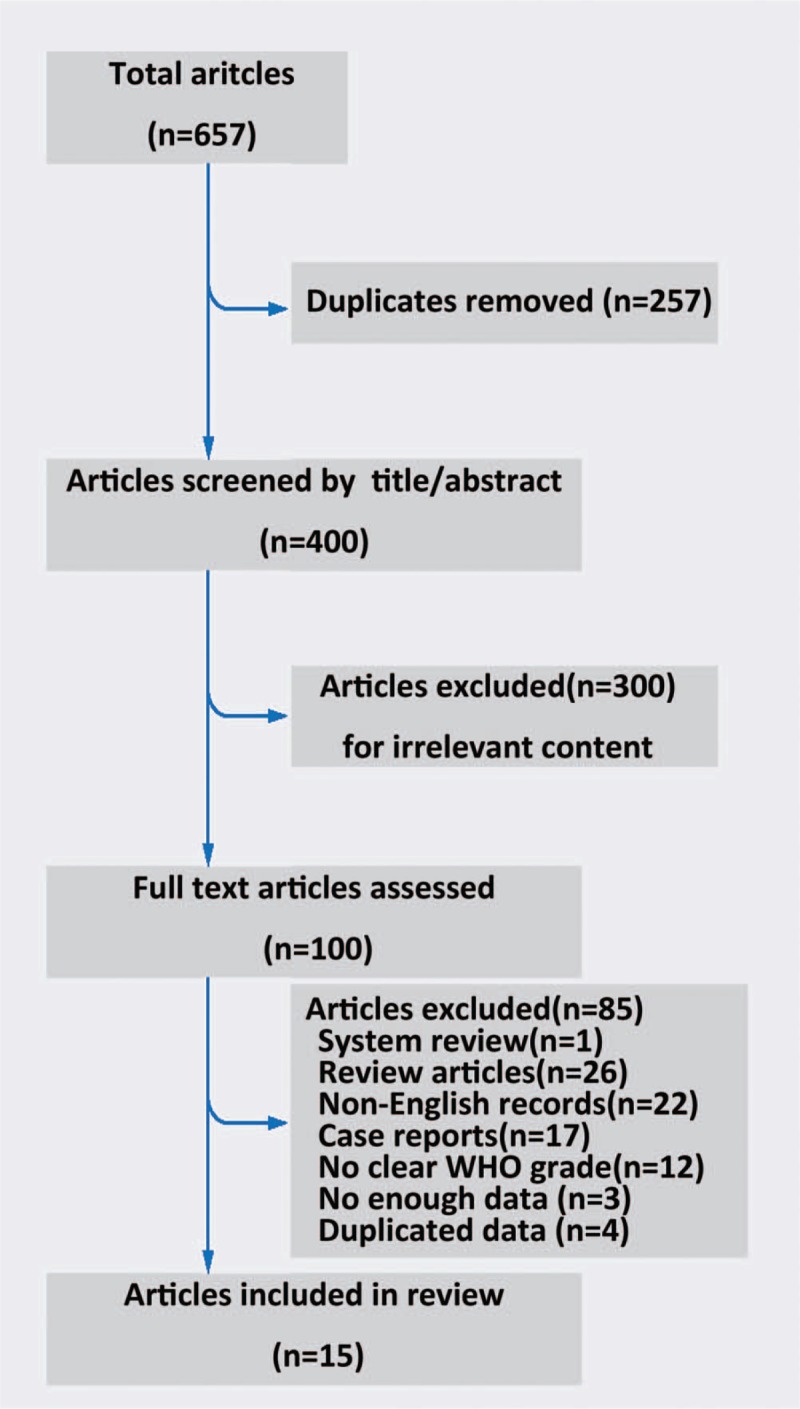
Literature search flowchart.
Table 1.
Literature quality evaluation.
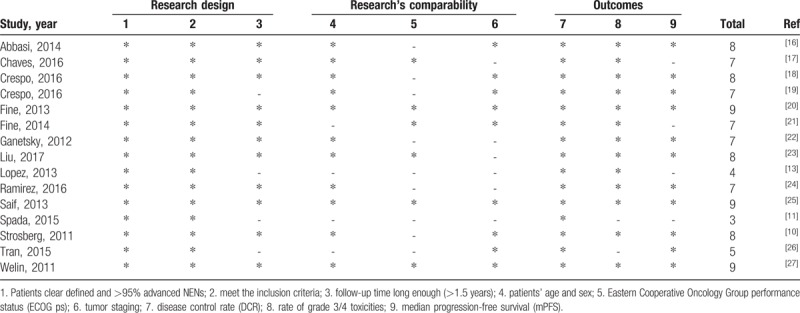
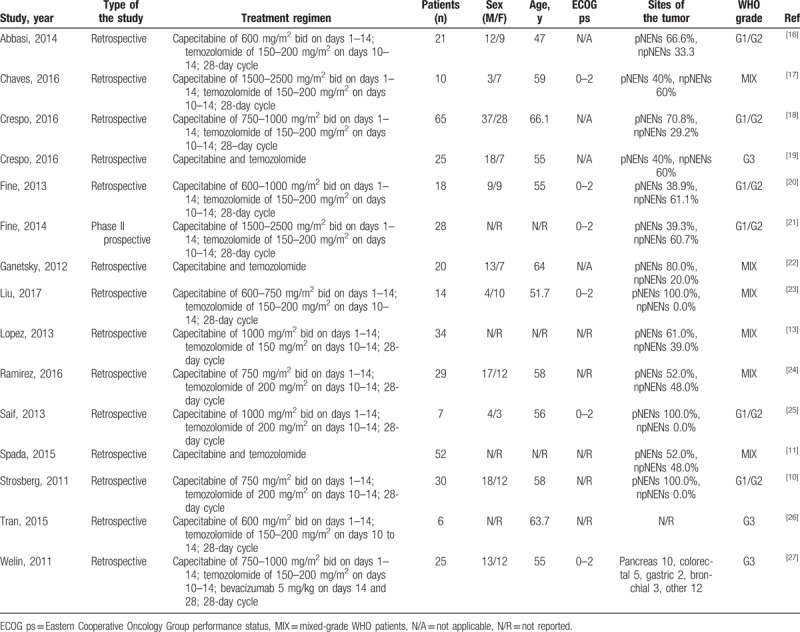
Table 2.
Characteristics of trials and patients.
3.2. Patients’ characteristics
The number of patients from the included studies ranged from 6 to 65, and the average age of patients ranged from 47 to 66 years. Six trials reported ECOG ps, which ranged from 0 to 2. All patients had diagnosed NENs.
3.3. Treatment regimens
A CAPTEM regimen was administered in all studies until either disease progression or unacceptable toxicity levels were reached (Fig. 2). The differences in dosing of CAPTEM are shown in Table 2.
Figure 2.
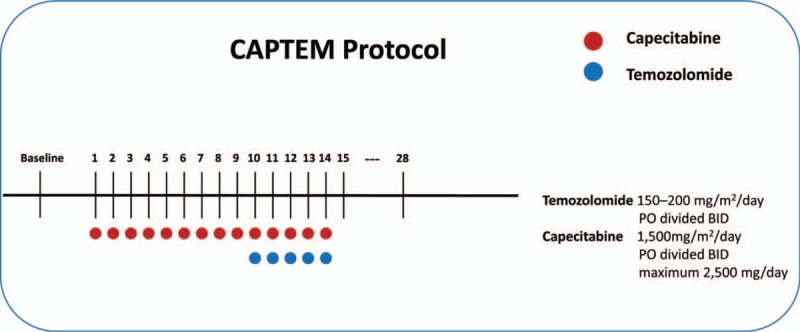
Treatment regimens of the capecitabine and temozolomide (CAPTEM) protocol.
3.4. Progression-free survival and overall survival
mPFS was reported in 12 of the included studies, ranging from 3.4 to 18 months, as shown in Table 3. The mPFS ranged between 3.4 and 6 months in studies of patients with WHO-graded G3 NENs, 12 to 18 months in studies reporting patients with G1/G2 NENs, and 8.9 to 16.4 months in studies with mixed-grade WHO patients.
Table 3.
Summary of tumor responses to CAPTEM.
mOS was reported in 7 out of 15 studies and ranged from 8 to 83 months (Table 3).
3.5. Tumor response
All studies assessed tumor response using Response Evaluation Criteria in Solid Tumors.[28] The DCR was reported in all studies and ranged from 44.00% to 96.67%. The meta-analysis of DCR showed that the DCR of CAPTEM for patients with NENs was 72.89% (95% CI, 64.04%–81.73%; I2 = 82.4%; P < .01) in a random effect model, and sensitivity analysis showed that no study directly affected the results of this meta-analysis (Fig. 3A, B). A single covariate meta-regression analysis showed that patients’ WHO grade (I2_res = 36.28%; P < .001) could account for the heterogeneity among the outcomes and, consequently, affect the results. According to subgroup analysis, DCR was 91.07% (95% CI, 85.19%–96.96%; I2 = 45.4%; P = .10) in studies that reported patients with G1/G2 NENs, 65.83% (95% CI, 58.46%–73.20%; I2 = 2.2%; P = .40) in studies that included multiple grades of NENs, and 56.91% (95% CI, 36.44%–77.39%; I2 = 56.2%; P = .10) in G3 exclusive studies (Fig. 3C). All results shown in a funnel plot were symmetric, and the P value of the Peters test was .1074 (t = −1.7294, df = 13, P = .107), which suggests no significant publication bias (Fig. 3D). Details of different response subtypes in each study are reported in Table 3.
Figure 3.

Measurements of disease control rates (DCRs) of capecitabine and temozolomide (CAPTEM) regimens to treat advanced neuroendocrine neoplasms (NENs). A, Forest plot; (B) sensitivity analysis; (C) subgroup analysis, and (D) funnel plot of single-proportion meta-analysis. CI = confidence interval.
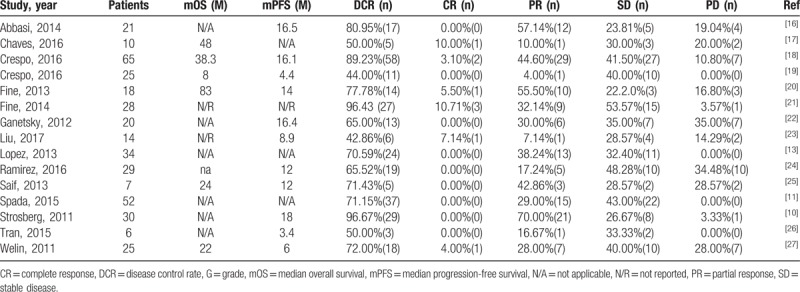
3.6. Toxicities
In the safety analysis, which included 13 studies, Higgins’ I2 and Q test results indicated no heterogeneity in the meta-analysis for toxicities (Fig. 4, Table 4). The most frequently observed severe toxicities (grades 3–4) were blood and lymphatic system disorders: thrombocytopenia (3.36%; 95% CI, 1.16%–5.55%), neutropenia (0.69%; 95% CI, 0–2.29%), lymphopenia (0.65%; 95% CI, 0–2.08%), and anemia (0.59%; 95% CI, 0–2.10%). Moreover, mucositis (0.57%; 95% CI, 0–2.02%), fatigue (0.54%; 95% CI, 0–1.93%), diarrhea (0.49%, 95% CI, 0–1.88%), and nausea (0.39%; 95% CI, 0–1.72%) were reported in the trials included. Only 1 in 336 patients had grade 3 transaminase elevation among all studies in this meta-analysis (Fig. 5).
Figure 4.
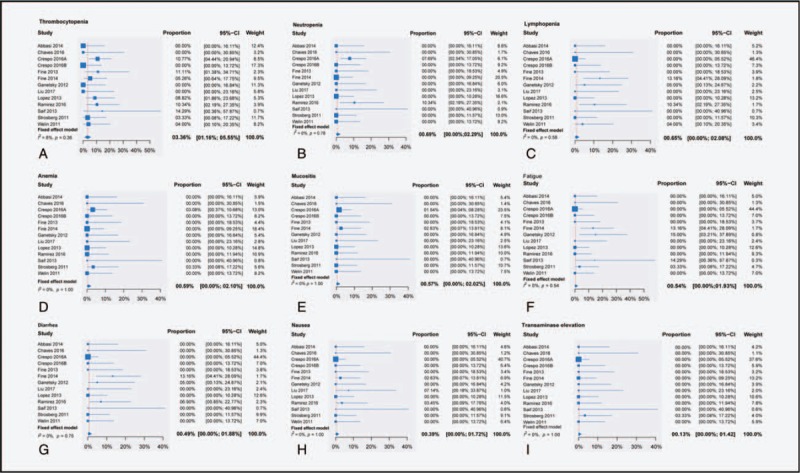
Single-proportion meta-analysis for all reported drug-related adverse events (grades 3–4). CI = confidence interval.
Table 4.
Grade 3 to 4 toxicities of CAPTEM.
Figure 5.
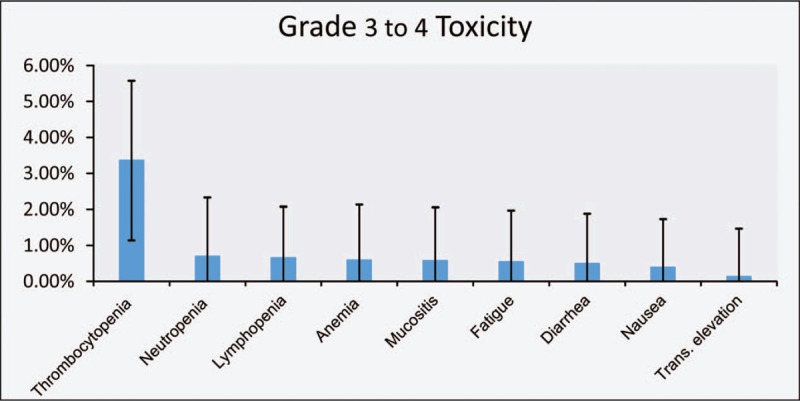
Rate of all reported adverse events after treatment with capecitabine and temozolomide (CAPTEM) for advanced neuroendocrine neoplasms (NENs). Trans. = transaminase. The vertical error bars represent the 95% confidence intervals for the rate of all reported adverse events from CAPTEM treatment in advanced neuroendocrine neoplasms (NENs).
4. Discussion
Recently, a number of randomized controlled trials have provided more treatment options for patients with advanced NENs.[3–6,29,30] However, due to the few number of known predictive factors for the treatment of advanced NENs, the management of these neoplasms have primarily relied on clinical and pathological factors, as well as each patient's specific circumstances. It has been suggested that somatostatin analogs such as octreotide or lanreotide should be considered for patients with symptomatic, clinically significant tumor burdens or severe disease progression.[3,4] With regard to disease progression, administration of everolimus, sunitinib, or cytotoxic chemotherapy has been confirmed as optimal.[5,6,29,30] The order in which the drugs are administered should consider both efficacy and toxicity, and maintenance therapy should avoid the toxicity of long-term use of chemotherapy or molecule-targeted drugs.
According to Yao et al,[5,30] for patients with advanced, low-grade, or intermediate-grade pancreatic neuroendocrine tumor (pNETs), the mPFS of the everolimus group was 11.0 months, which was significantly higher than the 4.6 months in the placebo group, whereas the mOS was 44.0 months in the everolimus group and 37.7 months in the placebo group. Similarly, a phase-3 trial conducted by Raymond et al[6] showed that, for advanced NENs, the mPFS of the sunitinib group was 11.4 months as compared to that of the placebo group, 5.5 months. However, in another study of advanced pNETs, mPFS in the sunitinib group was 12.6 versus 5.8 months in the placebo group, and the mOS of the sunitinib group was 38.6 versus 29.1 months in the placebo group.[29] Caplin et al[3] suggested that, for patients with ki-67 <10% with metastatic enteropancreatic neuroendocrine tumors treated with long-acting lanreotide and placebo, an mPFS for the lanreotide group was not reached versus the 18 months for the placebo group.
Although a number of treatment regimens have been associated with antitumor activity in these types of neoplasms, there has been no general consensus or guidance on the optimal cytotoxic chemotherapy regimen for patients with advanced NENs. One study revealed that capecitabine was safe and well tolerated by patients with advanced NENs and effective in patients with non-pNETs.[31] Meanwhile, another study suggested that temozolomide had an antitumor effect in advanced NENs when administered as a monotherapy, and its toxicity was acceptable.[32] Strosberg et al[10] showed that the CAPTEM elicited a high and durable response rate in metastatic NENs. There have been no high-grade evidence or results of an ongoing clinical trial (NCT01824875) regarding the safety and efficacy of CAPTEM.
Although a system review authored by Abdel-Rahman and Fouad[33] indicated that temozolomide-based combination therapy could be an effective and tolerable treatment option for advanced low-intermediate grade NENs, a quantitative result has not yet been clarified.
To the best of our knowledge, our study is the first meta-analysis to evaluate the efficacy and safety of CAPTEM in treating advanced NEN patients. Our protocol has been registered and published in the International Prospective Register of Systematic Reviews (PROSPERO), with the registration number of CRD42017071033. The primary endpoints were DCR and rate of patients who had grade 3 to 4 toxicities; other endpoints were mPFS and mOS. Altogether, 15 studies with a total of 384 patients with advanced NENs were included in this meta-analysis. Although there was moderate heterogeneity found in the meta-analysis of the included studies, the CAPTEM regimen was found to be an effective and safe regimen for treating patients with advanced NENs.
One previous study suggested that the mOS of patients with NETs with distant metastases was only 12 months.[2] Moreover, only 1 in 12 studies reported that the mOS was <12 months, perhaps because of the relatively large proportion of patients with Ki-67 >50%. The mPFS of most of the included studies was similar or slightly higher than that in studies using other treatment regimens (including temozolomide monotherapy).[7–9,31,32] These results suggest that CAPTEM regimens may prolong the survival of patients with advanced NENs and, therefore, deserve further attention.
All the included studies reported DCR, and this meta-analysis showed that DCR of a CAPTEM protocol for advanced NENs was greater than the DCRs reported in studies administering other agents or temozolomide monotherapy (Fig. 6).[7–9,32] Our results, as shown by the sensitivity analyses, indicate that no single study had a direct effect on the outcome. To explore the source of heterogeneity, a single covariate metaregression analysis was performed by restricted maximum likelihood methodology according to WHO grading, and the results indicate that WHO grading was associated with heterogeneity, even as high as 87.92%. Next, we divided the 15 included studies into 3 subgroups (G1/G2 group, G3 group, and mixed-grade group) by WHO grading. Subgroup analysis indicates that a CAPTEM regimen could be an effective treatment option for advanced low-intermediate grade NENs. Results also indicate low heterogeneity in that the P value of the Q test was >.05 in every group. Meanwhile, although all the studies used a CAPTEM protocol, the differences in drug dosing and cycles also led to heterogeneity (Table 1).
Figure 6.

Comparison of disease control rates (DCRs) of 4 chemotherapy treatment regimens for treating advanced neuroendocrine neoplasms (NENs). CAPTEM = capecitabine and temozolomide, TMZ = temozolomide.
Similarly to other cytotoxic chemotherapies, the CAPTEM regimen is known to be associated with toxicities such as myelosuppression, hepatotoxicity, and gastrointestinal toxicities. Our meta-analysis showed that the more frequently observed severe toxicities were thrombocytopenia, neutropenia, lymphopenia, and anemia. The most severe chemotherapy side effects reported were high levels of lymphocytes and neutropenia, which leave patients susceptible to opportunistic infections. Moreover, these have been reported as the primary cause of discontinuation and/or reduction in dose in many studies and as resulting in routine prescription of antibiotics for patients receiving CAPTEM therapy.
A number of studies have shown that O6-methylguanine-DNA methyltransferase (MGMT) is a predictor of the efficacy of temozolomide in treating patients with advanced NENs. However, the mechanism behind the association between MGMT and temozolomide is unclear.[34–36] A lack of MGMT deficiency in patients with NENs, as shown by immunohistochemistry, has been demonstrated in 24% to 51% of cases, whereas MGMT deficiency in cases of gastrointestinal NENs has not yet been reported.[35] Further studies and clinical trials such as NCT01525082 are required to demonstrate the relationship between MGMT and temozolomide.
The results of this meta-analysis might have been affected by the small sample sizes and the low evidence level of the studies included. In addition, absence of a control group might have led to indirect comparisons with other cytotoxic chemotherapies.
In summary, the meta-analysis of available data suggests that CAPTEM is effective and relatively safe for patients with advanced NENs. However, the efficacy and safety of CAPTEM protocols should be further confirmed in future prospective studies.
Acknowledgments
The authors thank Dr Yan, Yongjia for the assistance with the meta-analysis. The authors also thank M.M. Zhang, Guojing for the valuable discussion. The authors would like to thank Editage (www.editage.com) for English language editing.
Author contributions
Yaoheng Lu created this idea and Li Lu designed the research. Ji Wang, Zhicheng Zhao, Wenhao LV, and Yaoheng Lu searched the literature using major electronic databases and then selected studies and data. Yaoheng Lu drafted this article. Weihua Fu and Weidong Li made important changes to this article. All authors have seen the manuscript and approved the submission.
Conceptualization: Yaoheng Lu, Li Lu.
Data curation: Yaoheng Lu, Zhicheng Zhao, Ji Wang, Wenhao Lv.
Formal analysis: Yaoheng Lu.
Software: Yaoheng Lu.
Visualization: Yaoheng Lu.
Writing – original draft: Yaoheng Lu.
Writing – review and editing: Weihua Fu, Weidong Li.
Weidong Li orcid: 0000-0001-8516-0107.
Footnotes
Abbreviations: CAPTEM = capecitabine and temozolomide, CI = confidence interval, DCR = disease control rate, ECOG ps = Eastern Cooperative Oncology Group performance status, MGMT = O6-methylguanine-DNA methyltransferase, mOS = median overall survival, mPFS = median progression-free survival, NEN = neuroendocrine neoplasm, pNET = pancreatic neuroendocrine tumor, TMZ = temozolomide, WHO = World Health Organization.
Systematic Review Registration: PROSPERO CRD42017071033.
ORCiD ID 0000-0001-8516-0107
The authors have no funding and conflicts of interest to disclose.
Ethical approval: This article does not contain any studies with human participants or animals performed by any of the authors.
The authors of this work have nothing to disclose.
References
- [1].Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063–72. [DOI] [PubMed] [Google Scholar]
- [2].Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol 2017;3:1335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Caplin ME, Pavel M, Cwikla JB, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med 2014;371:224–33. [DOI] [PubMed] [Google Scholar]
- [4].Rinke A, Müller HH, Schadebrittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. Neuroendocrinology 2009;104:4656–63. [DOI] [PubMed] [Google Scholar]
- [5].Yao JC, Pavel M, Lombard-Bohas C, et al. Everolimus for the treatment of advanced pancreatic neuroendocrine tumors: overall survival and circulating biomarkers from the randomized, phase III RADIANT-3 study. J Clin Oncol 2016;34:3906–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 2011;364:501. [DOI] [PubMed] [Google Scholar]
- [7].Kouvaraki MA, Ajani JA, Hoff P, et al. Fluorouracil, doxorubicin, and streptozocin in the treatment of patients with locally advanced and metastatic pancreatic endocrine carcinomas. J Clin Oncol 2004;22:4762–71. [DOI] [PubMed] [Google Scholar]
- [8].Moertel CG, Lefkopoulo M, Lipsitz S, et al. Streptozocin-doxorubicin, streptozocin-fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med 1992;326:519–23. [DOI] [PubMed] [Google Scholar]
- [9].Sun W, Lipsitz S, Catalano P, et al. Eastern Cooperative Oncology Group. Phase II/III study of doxorubicin with fluorouracil compared with streptozocin with fluorouracil or dacarbazine in the treatment of advanced carcinoid tumors: Eastern Cooperative Oncology Group Study E1281. J Clin Oncol 2005;23:4897–904. [DOI] [PubMed] [Google Scholar]
- [10].Strosberg JR, Fine RL, Choi J, et al. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer 2011;117:268–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Spada F, Antonuzzo L, Marconcini R, et al. Chemotherapy with capecitabine plus temozolomide (CAP-TEM) in patients with advanced neuroendocrine neoplasms (NENs): an Italian multicenter retrospective analysis. Neuroendocrinology 2015;102:121. [Google Scholar]
- [12].Papaxoinis G, McCallum L, Nasralla M, et al. Efficacy of the combination of capecitabine and temozolomide in patients with advanced pulmonary carcinoid tumours. Neuroendocrinology 2016;103:71. [DOI] [PubMed] [Google Scholar]
- [13].Lopez Lopez C, Jimenez Fonseca P, Crespo Herrero G, et al. Temozolomide plus capecitabine as salvage treatment for patients with advanced neuroendocrine tumors (NETs) in the community setting. Eur J Cancer 2013;49:S626. [Google Scholar]
- [14].Wells GA, Shea BJ, O’Connell D, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of non-randomized studies in meta-analysis. Appl Eng Agric 2014;18:727–34. [Google Scholar]
- [15].Peters JL, Sutton AJ, Jones DR, et al. Comparison of two methods to detect publication bias in meta-analysis. JAMA 2006;295:676–80. [DOI] [PubMed] [Google Scholar]
- [16].Abbasi S, Kashashna A, Albaba H. Efficacy of capecitabine and temozolomide combination in well-differentiated neuroendocrine tumors: Jordan experience. Pancreas 2014;43:1303–5. [DOI] [PubMed] [Google Scholar]
- [17].Chaves A, Garcia A, Ribeiro J, et al. Capecitabine and temozolomide (CAPTEM) in patients with advanced neuroendocrine tumors: the experience of a Portuguese cancer center. Ann Oncol 2016;27:ii53. [Google Scholar]
- [18].Crespo G, Jiménez-Fonseca P, Custodio A, et al. Capecitabine and temozolomide in grade 1/2 neuroendocrine tumors: a Spanish multicenter experience. Future Oncol 2016;13:615–24. [DOI] [PubMed] [Google Scholar]
- [19].Crespo GH, Lopez C, Jimenez-Fonseca P, et al. Capecitabine-temozolomide in G3 neuroendocrine neoplasms. Neuroendocrinology 2016;103:66.25661647 [Google Scholar]
- [20].Fine RL, Gulati AP, Krantz BA, et al. Capecitabine and temozolomide (CAPTEM) for metastatic, well-differentiated neuroendocrine cancers: the Pancreas Center at Columbia University experience. Cancer Chemother Pharmacol 2013;71:663–70. [DOI] [PubMed] [Google Scholar]
- [21].Fine RL, Gulati AP, Tsushima D, et al. Prospective phase II study of capecitabine and temozolomide (CAPTEM) for progressive, moderately, and well-differentiated metastatic neuroendocrine tumors. J Clin Oncol 2014;32:179. [Google Scholar]
- [22].Ganetsky A, Adel NG, Do KG, et al. The efficacy of capecitabine and temozolomide for the treatment of metastatic neuroendocrine tumors: Memorial Sloan-Kettering Cancer Center experience. J Clin Oncol 2012;30:363. [Google Scholar]
- [23].Liu Q, Zhang P, Luo J, et al. Clinical observation of capecitabine and temozolomide in the treatment of advanced pancreatic neuroendocrine tumors. Chin J Clin Oncol 2017;44:228–32. [Google Scholar]
- [24].Ramirez RA, Beyer DT, Chauhan A, et al. The role of capecitabine/temozolomide in metastatic neuroendocrine tumors. Oncologist 2016;21:671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Saif MW, Kaley K, Brennan M, et al. A retrospective study of capecitabine/temozolomide (CAPTEM) regimen in the treatment of metastatic pancreatic neuroendocrine tumors (pNETs) after failing previous therapy. J Pancreas 2013;14:498. [DOI] [PubMed] [Google Scholar]
- [26].Tran Q, Bigby K. Single institution experience with capecitabine and temozolomide (Captem) in heavily pre-treated patients with metastatic well and poorly differentiated neuroendocrine malignancies. Asia Pac J Clin Oncol 2015;11:67. [Google Scholar]
- [27].Welin S, Sorbye H, Sebjornsen S, et al. Clinical effect of temozolomide-based chemotherapy in poorly differentiated endocrine carcinoma after progression on first-line chemotherapy. Cancer 2011;117:4617–22. [DOI] [PubMed] [Google Scholar]
- [28].Eisenhauer EA, Verweij J, Eisenhauer EA, et al. 11 New response evaluation criteria in solid tumors: RECIST guideline version 1.1. Eur J Cancer Suppl 2009;7:5. [DOI] [PubMed] [Google Scholar]
- [29].Faivre S, Niccoli P, Castellano D, et al. Sunitinib in pancreatic neuroendocrine tumors: updated progression-free survival and final overall survival from a phase III randomized study. Ann Oncol 2016;28:339–43. [DOI] [PubMed] [Google Scholar]
- [30].Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 2011;364:514–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Medley L, Morel AN, Farrugia D, et al. Phase II study of single agent capecitabine in the treatment of metastatic non-pancreatic neuroendocrine tumours. Br J Cancer 2011;104:1067–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ekeblad S, Sundin A, Janson ET, et al. Temozolomide as monotherapy is effective in treatment of advanced malignant neuroendocrine tumors. Clin Cancer Res 2007;13:2986–91. [DOI] [PubMed] [Google Scholar]
- [33].Abdel-Rahman O, Fouad M. Temozolomide-based combination for advanced neuroendocrine neoplasms: a systematic review of the literature. Future Oncol 2015;11:1275–90. [DOI] [PubMed] [Google Scholar]
- [34].Cros J, Hentic O, Rebours V, et al. MGMT expression predicts response to temozolomide in pancreatic neuroendocrine tumors. Endocr Relat Cancer 2016;23:625–33. [DOI] [PubMed] [Google Scholar]
- [35].Koumarianou A, Kaltsas G, Kulke MH, et al. Temozolomide in advanced neuroendocrine neoplasms: pharmacological and clinical aspects. Neuroendocrinology 2015;101:274–88. [DOI] [PubMed] [Google Scholar]
- [36].Pavel ME, Sers C. Women in cancer thematic review: systemic therapies in neuroendocrine tumors and novel approaches toward personalized medicine. Endocr Relat Cancer 2016;23:T135–54. [DOI] [PubMed] [Google Scholar]


