Abstract
Background:
Digestive system cancers are recognized as associated with high morbidity and mortality. It is generally accepted that N-myc downstream-regulated gene 1 (NDRG1) is aberrantly overexpressed or downregulated in digestive system cancers, and its prognostic value remains controversial. Accordingly, we herein conducted a meta-analysis to explore whether NDRG1 expression is correlated with overall survival (OS) and clinicopathological characteristics of patients with digestive system cancers.
Methods:
We systematically searched PubMed, EMBASE, and Web of Science for eligible studies up to June 6, 2017. In all, 19 publications with 21 studies, were included.
Results:
The pooled results showed that low NDRG1 expression was significantly associated with worse OS in colorectal cancer (pooled HR = 1.67, 95% CI: 1.22–2.28, P < .001) and pancreatic cancer (pooled HR = 1.87, 95% CI: 1–3.5, P < .0001). Moreover, the relationships between low NDRG1 expression and higher OS ratio of patients with liver cancer (pooled HR = 0.44, 95% CI: 0.32–0.62, P = .009) and gallbladder cancer (pooled HR = 0.56, 95% CI: 0.23–1.38, P = .01) were observed. Nevertheless, no significant association was observed between low NDRG1 expression and OS in gastric cancer (pooled HR = 0.81, 95% CI: 0.45–1.43, P = .46) or esophageal cancer (pooled HR = 0.76, 95% CI: 0.26–2.24, P = .62).
Conclusion:
The prognostic significance of NDRG1 expression varies according to cancer type in patients with DSCs. Considering that several limitations existed in this meta-analysis, more studies are required to further assess the prognostic value of NDRG1 expression in patients with DSCs and relevant mechanisms.
Keywords: digestive system cancers, meta-analysis, N-myc downstream-regulated gene 1 (NDRG1), prognostic
1. Introduction
Digestive system cancers (DSCs) are one of the most deadly threats to humans due to the high morbidity and mortality rates.[1] Despite the identification of many biomarkers related to DSCs, it still be difficult to predict the prognoses of patients with digestive system malignancies, which depend on distant metastasis, lymph node invasion, and local recurrence.[2] Moreover, patients may experience diverse clinical outcomes and even they have similar patterns of lymph node metastases, TNM stage, and tumor differentiation.[3] Therefore, it is imperative to identify new credible prognostic markers to predict patient prognosis and o devise better therapies for patients with DSCs.
It is common knowledge that the NDRG1 protein is mainly expressed in epithelial cells in humans,[4] while some specific tissues including muscle, connective tissue, blood vessels, and most of the nervous system show without expression of NDRG1.[5] Concerning its subcellular localization, NDRG1 can be found in the nucleus, plasma membrane and cytoplasm depending on the cell type.[5] NDRG1 protein was initially identified as a predominantly cytoplasmic protein,[6] it has been shown to be involved in various biological functions including cell growth, differentiation, embryogenesis, development, lipid biosynthesis, myelination, stress and immunity responses.[7] Meanwhile, NDRG1 was also primarily known as a suppressor of metastasis, and demonstrated to suppress angiogenesis, cell proliferation and invasion processes in multiple cancers, including prostate cancer, pancreatic cancer, and colorectal cancer.[7–9] Inversely, some studies have reported that NDRG1 was overexpressed in various cancers such as hepatocellular carcinoma, gastric cancer, cervical cancer, renal cancer and squamous cell carcinoma, which indicates its tumorigenic effects.[7,10,11] Moreover, elevated NDRG1 expression was reported to promote proliferation and invasion in vitro, as well as tumor growth and angiogenesis in vivo in liver cancer and gastric cancer.[12–14] In addition to the effect of NDRG1 in tumorigenesis, many researchers have also investigated that NDRG1 might serve as a prognostic marker in cancer patients.[15–20] Specifically, numerous studies of DSCs had the limitations concerning tumor type, disparities in tumor stage and experimental schemes, and the prognostic role of NDRG1 in DSCs was inconsistent.[13–22] For instance, some previous studies showed that decreased NDRG1 expression was associated with better overall survival,[9,21,23,24] while others indicated that increased level of NDRG1 expression was correlated with poorer overall survival.[10,14,25,26] Even though the molecular functions of NDRG1 and its potential as a molecular target for cancer therapy have already been reviewed comprehensively,[10] its precise prognostic role in patients with DSCs has not been assessed in a systematic review with a meta-analysis, and as a result, its role is still controversial.
Therefore, we conducted this meta-analysis and systematic review to assess the influence of decreased NDRG1 expression on overall and disease-free survival, as well as the association between decreased NDRG1 expression and clinicopathological factors of patients with DSCs.
2. Materials and methods
2.1. Ethics and dissemination
Ethical approval and informed consent are not required, as the study will be a literature review and will not involve direct contact with patients or alterations to patient care.
2.2. Literature search strategy and study selection
To identify all the studies that assessed the association between NDRG1 expression and survival outcome of patients with digestive system cancers, 2 reviewers performed a comprehensive literature search in the following databases: PubMed, EMBASE, and Web of Science. The last search was updated on June 6, 2017. The publication language was restricted to English. Key words used were (“NDRG1” or “N-myc downstream regulated gene 1”), (“cancer” or “tumor” or “malignancy” or “carcinoma”), and (“prognosis or prognostic”).
All the studies were included if they met the following inclusion criteria: the studies investigated the association between NDRG1 and overall survival (OS) of patients with digestive system cancers; relevant clinicopathologic characteristics were presented; tumor tissues from patients with digestive system cancers were used for the determination of NDRG1 expression; patients were grouped into high and low expression arms according to the NDRG1 expression level; sufficient information and data were available to calculate hazard ratios (HRs) with 95% confidence intervals (CIs).
The exclusion criteria for this study were as follows: studies that were published as reviews, abstracts, case reports, letters, or comments as well as duplicate studies; studies in which human cell lines or animals were used; studies that failed to provide the HRs with 95% confidence intervals or K-M survival curves used to calculate overall survival and disease-free survival.
2.3. Data extraction and quality assessment
All the candidate publications was extracted from each selected study independently by 2 independent investigator. The third review investigator was responsible for reconciling disagreements when the results were controversial. The following information was extracted: the first author's name, publication year, country, tumor type, number of patients, tumor stage, clinical and pathological features, cut-off value, OS, and DFS. If the results of both the univariate and multivariate analyses were provided in the studies, only the latter one was extracted due to its higher accuracy since multivariate analyses account for confounding factors. Three aspects including the selection of participants, comparability, and ascertainment of the outcome were assessed. A study with a score ≥6 was considered as high-quality study after the selected publication were evaluated with the Newcastle-Ottawa Scale (NOS) ranging from 0 (minimum) to 9 (maximum).
2.4. Statistical analysis
The meta-analysis was performed using Stata SE12.0 (StataCorp, College Station, TX). HRs and 95% confidence intervals (95% CIs) were used to assess the prognostic value of NDRG1, and ORs (odds ratios) with 95% CIs were used to evaluate the association between NDRG1 expression and clinicopathological features of digestive system cancers. The sensitivity analysis was performed to assess the validity and reliability of the pooled overall survival in patients with a specific type of DSC. Chi-square-based Q tests and I2 statistics were applied to evaluate study heterogeneity, with I2 > 50% and P < .05 indicating statistical heterogeneity. If no severe statistical heterogeneity was detected, a fixed-effects model was used to assess the pooled HRs; otherwise, a random-effects model was used.
3. Results
3.1. Study selection and study characteristics
A total of 173 articles were identified in PubMed, EMBASE, and Web of Science. Twenty duplicated articles were excluded. The remaining abstracts and full-texts of the references were meticulously reviewed, 19 publications, which included 21 studies, were finally determined to be eligible for the present pooled analysis of the prognostic value of NDRG1 in digestive system cancers (DSCs).[8–10,13–16,19–30] The inclusion of all publications was based on the selection criteria mentioned above and the detailed selection process is shown in Fig. 1.
Figure 1.
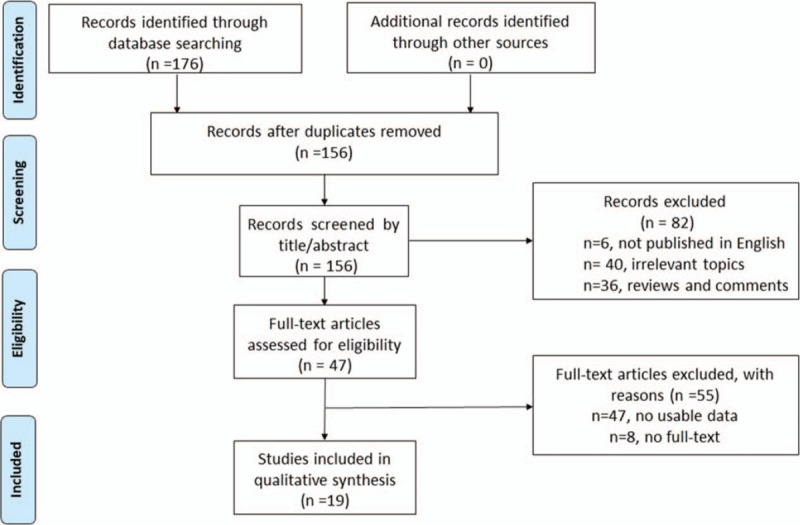
Flow diagram of study selection process.
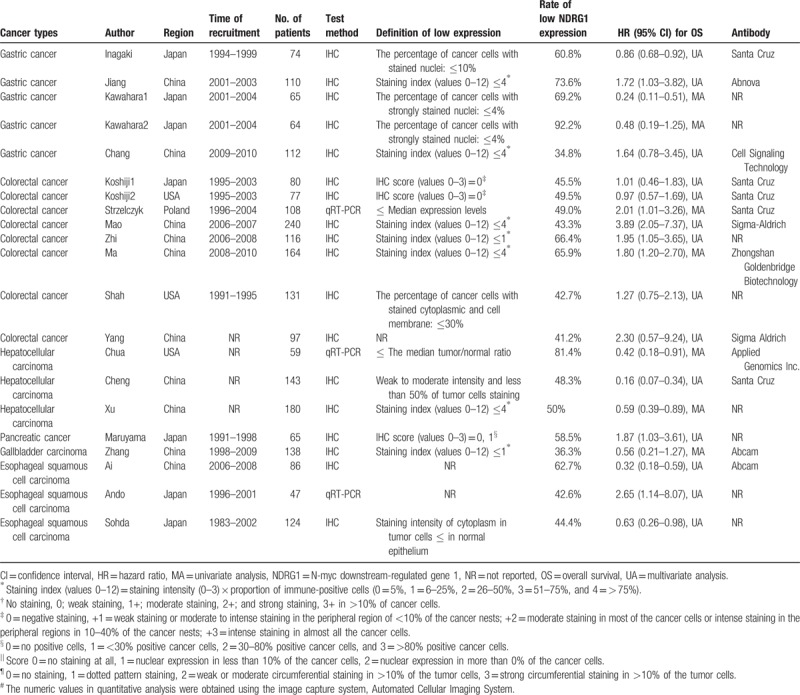
The basic characteristics of the included studies are summarized in Table 1. Twenty-one studies with 2280 patients were totally included in the current meta-analysis, the sample size of which ranged from 47 to 240. Specifically, Kawahara et al and Koshiji et al conducted 2 studies of each, therefore, we marked them as Kawahara 1 and Kawahara 2[14]; Koshiji 1 and Koshiji 2.[22] All the included studies were published in English and the recruitment time of patients ranged from 1993 to 2010. There are 5 studies focused on gastric cancer,[13,14,16,23] 8 studies focused on colorectal cancer,[8,9,22,24,28–30] 3 studies focused on hepatocellular carcinoma (HCC),[10,25,26] 3 studies involved esophageal squamous cell carcinoma (ESCC),[15,19,21] 1 study involved pancreatic cancer[27] and 1 study involved gallbladder cancer,[20] among the 21 studies. Ten studies were performed in China, 7 studies were performed in Japan, and 4 studies were performed in the USA and Poland, regarding the population of the cases. The majority of studies on NDRG1 expression used IHC to detect NDRG1 protein, while 3 studies used qRT-PCR (Table 1). In addition, 18 studies reported cut-off values of NDRG1 expression, however, they were not consistent (Table 1). The sources of antibody included Sigma Aldrich, Santa Cruz, Abcam (UK), Abnova (China), Cell Signaling Technology and Zhongshan Goldenbridge Biotechnology (China) (Table 1).
Table 1.
Baseline characteristics of studies included in the meta-analysis.
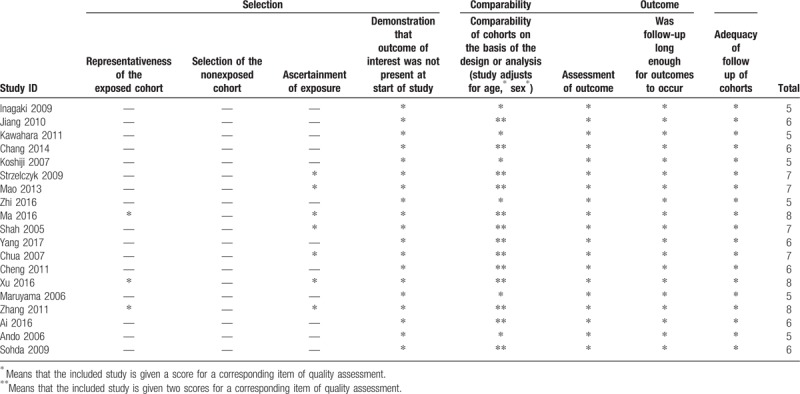
The study quality score was assessed using the modification of the Newcastle-Ottawa scale, in which the scores ranged from 5 to 7, which indicated that the quality of the included studies was moderate to high (Table 2).
Table 2.
The Newcastle-Ottawa Scale (NOS) quality assessment of the enrolled studies.
3.2. Low NDRG1 expression and OS in digestive system cancers
The pooled result revealed that low NDRG1 expression was significantly associated with worse overall survival (OS) of patients with colorectal cancer (pooled HR = 1.67, 95% CI: 1.22–2.28, P < .001) (Fig. 2 and Table 3) and those with pancreatic cancer (pooled HR = 1.87, 95% CI: 1–3.5, P < .0001) (Table 3). Furthermore, the associations between low NDRG1 expression and better OS of patients with liver cancer (pooled HR = 0.36, 95% CI: 0.16–0.78, P = .01) (Fig. 3 and Table 3) or gallbladder cancer (pooled HR = 0.56, 95% CI: 0.23–1.38, P = .01) were observed (Table 3). However, no significant association was found between low NDRG1 expression and OS in gastric cancer (pooled HR = 0.81, 95% CI: 0.45–1.43, P = .46) (Fig. 4 and Table 3) and esophageal cancer (pooled HR = 0.76, 95% CI: 0.26–2.24, P = .62) (Fig. 5 and Table 3).
Figure 2.
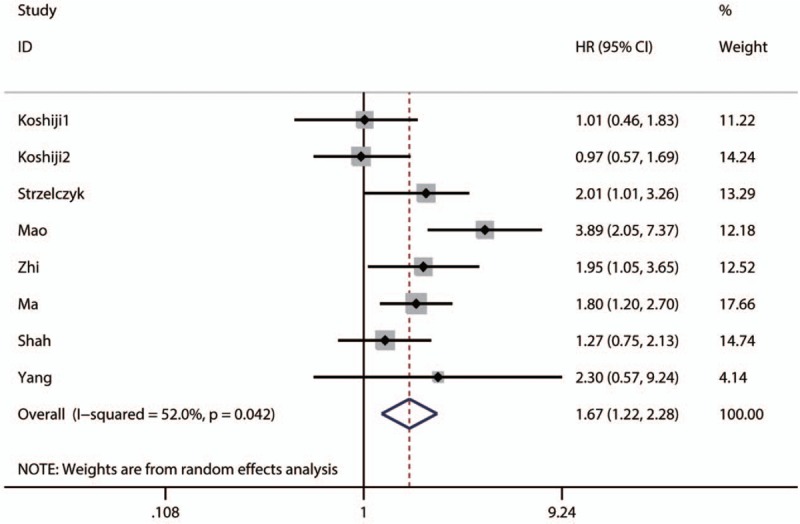
Forest plot of pooled HR for the association between low NDRG1 expression and OS of patients with colorectal cancer.
Table 3.
Results of pooled hazard ratios of overall survival of patients with low NDRG11 expression level.

Figure 3.
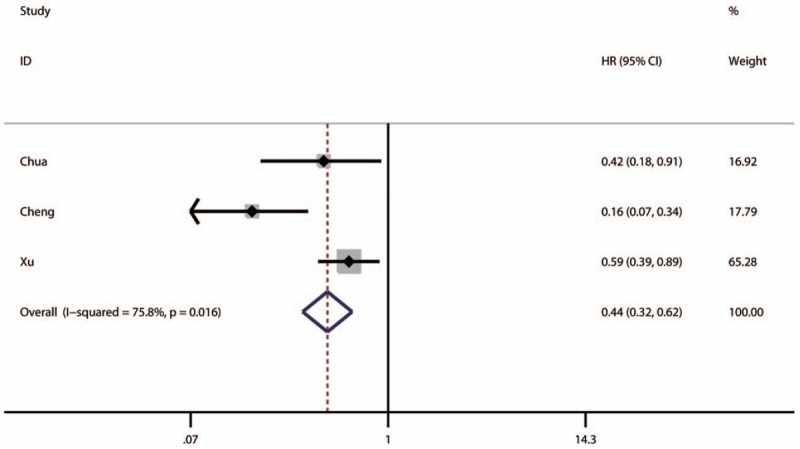
Forest plot of pooled HR for the association between low NDRG1 expression and OS of patients with liver cancer.
Figure 4.
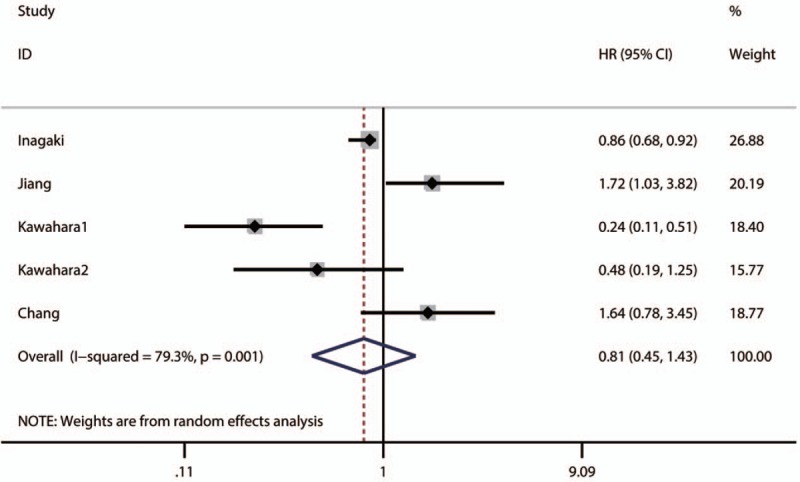
Forest plot of pooled HR for the association between low NDRG1 expression and OS of patients with gastric cancer.
Figure 5.
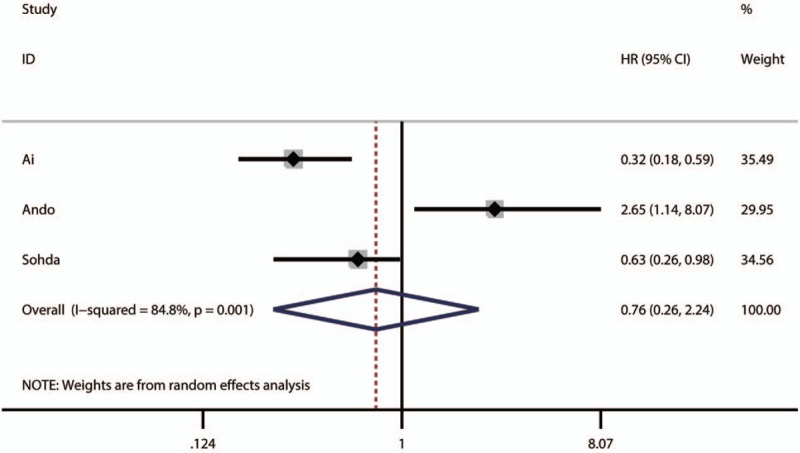
Forest plot of pooled HR for the association between low NDRG1 expression and OS of patients with gastric cancer.
3.3. Low NDRG1 expression and clinicopathological factors in gastric cancer
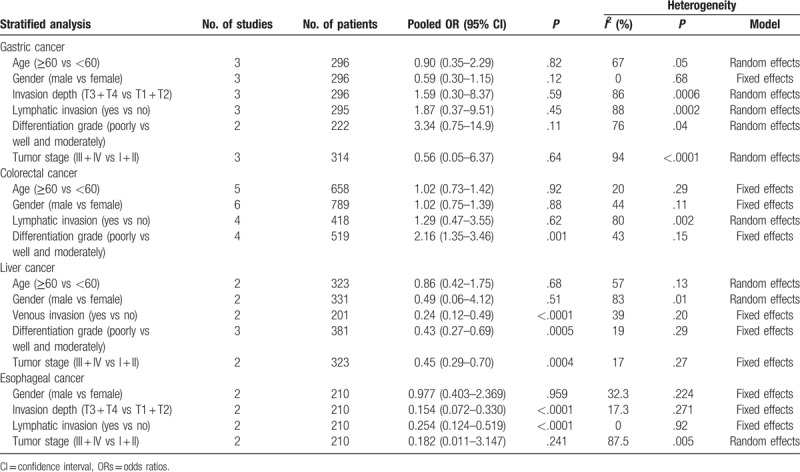
Four studies reported the relationship between low NDRG1 expression and clinicopathological factors in gastric cancer,[9,19,20,25] including 3 studies that investigated tumor invasion depth, lymphatic invasion, TNM stage, age, and gender, while 2 studies that involved tumor differentiation and grade (Table 4). Except for gender (I2 = 0, P = .68), significant heterogeneity was observed in age (I2 = 67, P = .05), tumor invasion depth (I2 = 86, P = .0006), lymphatic invasion (I2 = 88, P = .0002), differentiation grade (I2 = 76, P = .004), and tumor stage (I2 = 94, P < .0001). Therefore, the random-effects model was employed for variables with the exception of gender, while the fixed-effects model was applied for gender (Table 4). Nevertheless, the pooled analysis showed no significance in the association between low NDRG1 expression and age (OR = 0.90, 95% CI: 0.35–2.29, P = .82), gender (OR = 0.59, 95% CI: 0.30–1.15, P = .12), invasion depth (OR = 1.59, 95% CI: 0.30–8.37, P = .59), lymphatic invasion (OR = 1.87, 95% CI: 0.37–9.5, P = .45), or tumor differentiation grade (OR = 3.34, 95% CI: 0.75–14.9, P = .11) (Table 4).
Table 4.
Results of meta-analysis of high NDRG1 expression level and clinicopathological features in gastric cancer, colorectal cancer, and hepatocellular carcinoma.
3.4. Low NDRG1 expression and clinicopathological factors in colorectal cancer
A total of 6 studies described the association between NDRG1 expression and clinicopathological factors in colorectal cancer,[9,10,16,22,24,28,30] including age, gender, tumor differentiation grade, and lymphatic invasion (Table 4). No significant heterogeneity was found between low NDRG1 expression and age (I2 = 32, P = .22), gender (I2 = 49, P = .12), or tumor differentiation grade (I2 = 43, P = .15); therefore, a fixed-effects model was applied for analysis. Even so, significant heterogeneity was observed in studies that reported lymphatic invasion (I2 = 80, P = .007); therefore, a random-effects model was applied (Table 4). The pooled analysis revealed no significant relationship between decreased NDRG1 expression and age (OR = 1.02, 95% CI: 0.73–1.42, P = .92), gender (OR = 1.02, 95% CI: 0.75–1.39, P = .88), or lymphatic invasion (OR = 1.29, 95% CI: 0.47–3.55, P = .62), while low NDRG1 expression was obviously related to poor tumor differentiation grade (OR = 2.16, 95% CI: 1.35–3.46, P = .001) (Table 4).
3.5. NDRG1 expression and clinicopathological factors in hepatocellular carcinoma
Three studies reported the relationship of NDRG1 expression and clinicopathological factors in hepatocellular carcinoma.[10,14,26] Age, gender, venous invasion, and tumor stage were all reported in 2 studies, while tumor differentiation grade was described in 3 studies (Table 4).
No significant heterogeneity was detected in the studies regarding venous invasion (I2 = 39, P = .20), differentiation grade (I2 = 19, P = .29), or tumor stage (I2 = 17, P = .27); therefore, a fixed-effects model was applied. Nevertheless, significant heterogeneity was detected in studies that involved age (I2 = 57, P = .13) and gender (I2 = 83, P = .01); therefore, a random-effects model was used. The pooled results showed that low NDRG1 expression was significantly correlated with venous invasion (OR = 0.24, 95% CI: 0.12–0.49, P < .0001), tumor differentiation grade (OR = 0.43, 95% CI: 0.27–0.69, P = .0005), and tumor stage (OR = 0.45, 95% CI: 0.29–0.70, P < .0004). However, no significant relationship was found between low NDRG1 expression and age (OR = 0.86, 95% CI: 0.42–1.75, P = .13) or gender (OR = 0.49, 95% CI: 0.06–4.12, P = .51).
3.6. Sensitivity analysis
The sensitivity analyses were performed to evaluate the stability of the pooled results for OS in gastric cancer (Fig. 6A), colorectal cancer (Fig. 6B), ESCC (Fig. 6C), and HCC (Fig. 6D). The results of the sensitivity analyses showed that the pooled HRs for OS did not change substantially, which indicates that the conclusions from our meta-analysis were relatively reliable.
Figure 6.
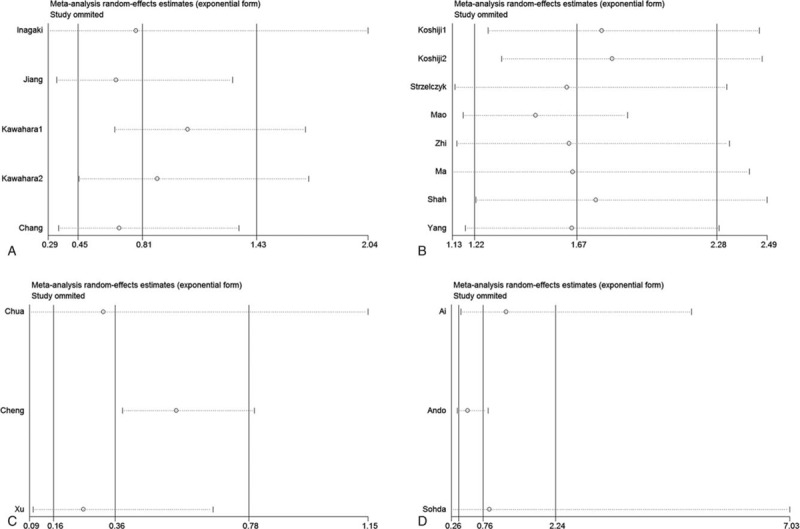
The sensitivity analyses for OS in gastric cancer (A), colorectal cancer (B), ESCC (C) and liver cancer (D).
4. Discussion
It has been reported that the effect of NDRG1 in the carcinogenesis of digestive system cancers (DSCs) is conflicting[7,10]; therefore, its prognostic value in patients with DSCs is also inconsistent and remains unknown.[8–10,13,14,16,19–27,29] Despite that a previous study comprehensively reviewed the molecular functions of NDRG1 and its potential as a molecular target for cancer therapy, the impact of low NDRG1 expression on the prognosis of patients with DSCs has not been fully explored. Hence, we herein combined 19 publications that included 21 studies and 2280 patients to perform the first meta-analysis that has evaluated the association of NDRG1 with overall survival (OS) of patients with DSCs.[8–10,13–16,19–30] The purpose was to provide a comprehensive and relatively reliable conclusion. In addition, we also explored the relationship between low NDRG1 expression and clinicopathological features of DSCs.
The pooled results of the meta-analysis revealed that low NDRG1 expression was significantly associated with worse overall survival (OS) in colorectal cancer (pooled HR = 1.67, 95% CI: 1.22–2.28, P < .001) and pancreatic cancer (pooled HR = 1.87, 95% CI: 1–3.5, P < .0001). However, associations between better OS and low NDRG1 expression in liver cancer (pooled HR = 0.36, 95% CI: 0.17–0.78, P = .01) and gallbladder cancer (pooled HR = 0.56, 95% CI: 0.23–1.38, P = .01) were also observed. However, the results showed that low NDRG1 expression was not related to OS in gastric cancer (pooled HR = 0.81, 95% CI: 0.45–1.43, P = .46) and esophageal cancer (pooled HR = 0.76, 95% CI: 0.26–2.24, P = .62). Moreover, sensitivity analyses demonstrated that the pooled HRs evaluating the prognostic value of decreased NDRG1 expression in gastric cancer, colorectal cancer, esophageal cancer, and liver cancer were not significantly altered, which indicates that the pooled results were robust. Considering the above findings, we hypothesized that the prognostic value of decreased NDRG1 expression varies according to cancer type in patients with DSCs.
The finding that decreased expression of NDRG1 differently associated with patients’ OS in differently DSCs made sense to us due to several possible mechanisms. First, NDRG1 expression levels throughout the digestive system are different. It is expressed in esophagus, gastric, small intestine, colon, and rectum but it has been reported no expression found in liver.[12,26] For those tissues with high expression of NDRG1, it may play important roles in keeping the cells in their normal status. In those tissues where NDRG1 is weakly expressed or no expression, changes of NDRG1 expression may make a relatively dismal effect on cells. Second, it has been reported that NDRG1 is involved in the regulation of various cellular functions,[31] thus it is a target protein and/or mediator protein for multiple signaling pathways. Those signaling pathways play different roles during carcinogenesis in different cancers. If the signaling pathway that NDRG1 regulates or involves is pivotal for the carcinogenesis, then it is very possible for NDGR1 to be associated with the OS for the patients and vice versa. More studies are needed to investigate the functions of NDRG1 in different cancers to fulfill the requirement of precisely explaining the prognostic value of NDRG1.
In addition, we also investigated the relationship of NDRG1 expression and clinicopathological characteristics to further validate the pooled results of the association between OS and NDRG1 expression. We assessed the associations between low NDRG1 expression and clinicopathological characteristics in gastric cancer, colorectal cancer, hepatocellular carcinoma (HCC), and esophageal squamous cell carcinoma (ESCC), considering that the biology, pathology, clinical courses, and treatments vary enormously among different types of DSCs. However, low expression of NDRG1 was not evaluated in pancreatic cancer and gallbladder carcinoma due to limited data on the clinicopathological features. The results showed no correlation between NDRG1 expression and the clinicopathological characteristics of gastric cancer, which is inconsistent with the pooled results for OS in gastric cancer. Without any doubt, this result should also be interpreted with caution. First, the prognostic value and association of NDRG1 with the clinicopathological features of gastric cancer might vary with the subcellular localization of NDRG1 expression and subtypes of gastric cancer. Nonetheless, only the study by Kawahara et al[14] specifically explored the impacts of 2 factors on the prognostic value of NDRG1 in gastric cancer. Inversely, the in vitro studies verified that NDRG1 overexpression inhibited cell proliferation and invasiveness and induced G1 cell cycle arrest and early apoptosis in gastric cancer. This suggests that NDRG1 may act as a tumor suppressor gene, and its expression upregulation can cause favorable prognosis.[16,23] Similarly, decreased NDRG1 expression in colorectal cancer was significantly associated with poor tumor differentiation grade, suggesting that NDRG1 may also act as a tumor suppressor gene. Moreover, it was demonstrated that NDRG1 overexpression could inhibit the invasion, metastasis and epithelial–mesenchymal transition (EMT) of colorectal cancer via multiple pathways, including NF-κB and nuclear β-catenin signaling pathways.[8,9] Additionally, some studies found that N-myc downstream-regulated gene 1 could promote apoptosis in colorectal cancer cells by enhancing ubiquitination of Bcl-2[30] and upregulation of death receptor 4,[32] and meanwhile some recent researches have also verified that NDRG1 could play anti-tumor roles by inhibiting the ErbB signaling pathway through restraining the formations of epidermal growth factor receptor (EGFR)/human epidermal growth factor receptor 2 (HER2) and HER2/HER3 heterodimers, as well as promoting the degradation of EGFR.[33] Paradoxically, a recent study by Kim et al[34] demonstrated that downregulation of NDRG1 could cause the resensitization of radioresistant rectal cancer cells by causing more DNA double-strand breakages, indicating that NDRG1 act as a protumor factor in rectal cancer. In HCC, up to date nearly all relevant literatures suggested that NDRG1 acted as a tumorigenic element by promoting HCC cell migration, invasion, and growth.[25,35,36] Furthermore, several mechanisms have been investigated to explain the tumorigenic effects of NDRG1. For instance, it has been demonstrated that NDRG1 could trigger numerous oncogenic signaling pathways in cancer cells, including the AKT, EGF, ErbB, Wnt/β-catenin, MAPK, and Jak-STAT pathways.[25,37–39] A most recent study by Sevinsky et al[40] reported that high NDRG1 expression was associated with worse prognosis in breast cancer patients, and they demonstrated that NDRG1 promoted breast cancer aggressiveness by regulating the fate of lipids in cells. In addition, some evidence hold on that NDRG1 plays critical role in activating the stress-induced, prosurvival autophagic pathway in cancer cells.[41,42] More interestingly, recently Luo et al[43] demonstrated that NDRG1 can promote HCC progression by regulating tumor microenvironment. They found that forkhead box Q1 (FOXQ1)/NDRG1 axis in HCC cells could activate pSTAT6/C-C motif chemokine ligand 26 (CCL26) signaling, thus recruiting hepatic stellate cells (HSCs), the main cellular source of cancer associated fibroblast, which is a well-known microenvironment contributor for HCC progression.[43] Overall, the biological functions of NDRG1 in tumors may vary according to tumor type, which is consistent with the results of our meta-analysis. In future, more studies are required to uncover the concrete mechanisms for the anti- or protumor effects of NDRG1 in different tumors, so as to develop NDRG1 as a therapeutic target.
We aware that our study may have several significant limitations. First, it may have introduced publication bias since that only English publications were considered in this meta-analysis. Second, no pooling analysis could be used to synthetically assess the prognostic value of NDRG1 expression in these 2 cancer type due to that only a single study on gallbladder carcinoma and pancreatic cancer was identified through thorough literature search. Additionally, although the current meta-analysis focused on comprehensively assessing the prognostic value of NDRG1 expression in digestive system tumors, no eligible studies referred to biliary tumor. Therefore, more studies are warranted to further explore the prognostic value of NDRG1 expression in biliary tumor, gallbladder carcinoma, and pancreatic cancer. Third, there are many differences in tumor biology between right and left colon cancers. Moreover, it has been considered that the primary colorectal tumor location (right and left) is closely associated with response to chemotherapy and long-term prognosis in patients, especially for metastatic colorectal cancer.[44] Therefore, it will be more reasonable to analyze the prognostic value of NDRG1 in left and right colorectal cancer, respectively. However, all the included studies about colorectal cancer did not analyze the prognostic value of NDRG1 in left and right colorectal cancer, respectively, thus we cannot obtain relevant data to conduct the pooled analysis in this regard. Fourth, the definitions of overexpression of NDRG1 were inconsistent throughout various studies, which probably may partly account for the heterogeneity among the included studies. Fifth, despite that HRs with 95% CIs in most of the included studies were produced by a multivariate analysis, variables added into the Cox proportional hazard models varied from study to study. It may be one of sources of heterogeneity in this meta-analysis as well. Sixth, NDRG1 staining was heterogeneous and it can be detected in nuclei, cytoplasm and cell membrane. However, the majority of the included studies did not assess the prognostic value of NDRG1 expressed in nuclear, cytoplasm or membrane, respectively. This may also introduce bias into our meta-analysis in a degree, thus weakening the reliability of the pooled analysis. Hence, future studies are required to evaluate the prognostic value of nuclear, cytoplasmic, and membranous NDRG1 expression, respectively. Last but not least, only few eligible studies provided available data for synthetically assessing the associations of low NDRG1 expression with the clinicopathological parameters, which may reduce the reliability of those pooled results due to the limitation of small sample size.
In conclusion, the prognostic significance of NDRG1 expression varies according to cancer type in patients with DSCs. Low NDRG1 expression was significantly associated with worse OS in colorectal cancer and pancreatic cancer, while it was closely related to better OS in liver cancer and gallbladder cancer. However, in gastric cancer and esophageal cancer, no associations of NDRG1 expression with prognosis were found. Considering that several limitations existed, more studies are required to further assess the prognostic value of NDRG1 expression in patients with DSCs and relevant mechanism.
Author contributions
Data curation: Hai-Peng Liu.
Investigation: Kang Chen, Hai-Peng Liu.
Methodology: Hai-Peng Liu, Ze-Ping Huang.
Software: Ze-Ping Huang.
Supervision: Fu-Rong Wang, Xiao Chen.
Writing – original draft: Kang Chen.
Writing – review & editing: Xiao-hong Liu, Fu-Rong Wang, Xiao Chen.
Footnotes
Abbreviations: CI = confidence interval, DFS = disease-free survival, DSCs = digestive system cancers, ESCC = esophageal squamous cell carcinoma, HCC = hepatocellular carcinoma, HR = hazard ratio, NDRG1 = N-myc downstream-regulated gene 1, OS = overall survival.
The authors have no conflicts of interest to disclose.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- [2].Ng L, Poon RT, Pang R. Biomarkers for predicting future metastasis of human gastrointestinal tumors. Cell Mol Life Sci 2013;70:3631–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hari DM, Leung AM, Lee JH, et al. AJCC Cancer Staging Manual 7th edition criteria for colon cancer: do the complex modifications improve prognostic assessment? J Am Coll Surg 2013;217:181–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kurdistani SK, Arizti P, Reimer CL, et al. Inhibition of tumor cell growth by RTP/rit42 and its responsiveness to p53 and DNA damage. Cancer Res 1998;58:4439–44. [PubMed] [Google Scholar]
- [5].Lachat P, Shaw P, Gebhard S, et al. Expression of NDRG1, a differentiation-related gene, in human tissues. Histochem Cell Biol 2002;118:399–408. [DOI] [PubMed] [Google Scholar]
- [6].van Belzen N, Dinjens WN, Diesveld MP, et al. A novel gene which is up-regulated during colon epithelial cell differentiation and down-regulated in colorectal neoplasms. Lab Invest 1997;77:85–92. [PubMed] [Google Scholar]
- [7].Fang BA, Kovacevic Z, Park KC, et al. Molecular functions of the iron-regulated metastasis suppressor, NDRG1, and its potential as a molecular target for cancer therapy. Biochim Biophys Acta 2014;1845:1–9. [DOI] [PubMed] [Google Scholar]
- [8].Wangpu X, Yang X, Zhao J, et al. The metastasis suppressor, NDRG1, inhibits “stemness” of colorectal cancer via down-regulation of nuclear beta-catenin and CD44. Oncotarget 2015;6:33893–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ma J, Gao Q, Zeng S, et al. Knockdown of NDRG1 promote epithelial-mesenchymal transition of colorectal cancer via NF-kappaB signaling. J Surg Oncol 2016;114:520–7. [DOI] [PubMed] [Google Scholar]
- [10].Xu X, Liu Z, Wang J, et al. Global proteomic profiling in multistep hepatocarcinogenesis and identification of PARP1 as a novel molecular marker in hepatocellular carcinoma. Oncotarget 2016;7:13730–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wang Y, Zhou Y, Tao F, et al. N-myc downstream regulated gene 1 (NDRG1) promotes the stem-like properties of lung cancer cells through stabilized c-Myc. Cancer Lett 2017;401:53–62. [DOI] [PubMed] [Google Scholar]
- [12].Akiba J, Ogasawara S, Kawahara A, et al. N-myc downstream regulated gene 1 (NDRG1)/Cap43 enhances portal vein invasion and intrahepatic metastasis in human hepatocellular carcinoma. Oncol Rep 2008;20:1329–35. [PubMed] [Google Scholar]
- [13].Inagaki Y, Tang W, Xu HL, et al. Localization of N-myc downstream-regulated gene 1 in gastric cancer tissue. Dig Liver Dis 2009;41:96–103. [DOI] [PubMed] [Google Scholar]
- [14].Kawahara A, Akiba J, Hattori S, et al. Nuclear expression of N-myc downstream regulated gene 1/Ca (2+)-associated protein 43 is closely correlated with tumor angiogenesis and poor survival in patients with gastric cancer. Exp Ther Med 2011;2:471–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ai R, Sun Y, Guo Z, et al. NDRG1 overexpression promotes the progression of esophageal squamous cell carcinoma through modulating Wnt signaling pathway. Cancer Biol Ther 2016;17:943–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chang X, Xu X, Ma J, et al. NDRG1 expression is related to the progression and prognosis of gastric cancer patients through modulating proliferation, invasion and cell cycle of gastric cancer cells. Mol Biol Rep 2014;41:6215–23. [DOI] [PubMed] [Google Scholar]
- [17].Dos Santos M, da Cunha Mercante AM, Nunes FD, et al. Prognostic significance of NDRG1 expression in oral and oropharyngeal squamous cell carcinoma. Mol Biol Rep 2012;39:10157–65. [DOI] [PubMed] [Google Scholar]
- [18].Nagai MA, Gerhard R, Fregnani JH, et al. Prognostic value of NDRG1 and SPARC protein expression in breast cancer patients. Breast Cancer Res Treat 2011;126:1–4. [DOI] [PubMed] [Google Scholar]
- [19].Sohda M, Mochida Y, Kato H, et al. Overexpression of Cap43 is associated with malignant status of esophageal cancer. Anticancer Res 2009;29:965–70. [PubMed] [Google Scholar]
- [20].Zhang SB, Song SP, Li B, et al. Expression of N-myc downstream-regulated gene 1 in primary gallbladder carcinoma and its correlation with clinicopathological features and clinical outcome. Med Oncol 2012;29:1866–72. [DOI] [PubMed] [Google Scholar]
- [21].Ando T, Ishiguro H, Kimura M, et al. Decreased expression of NDRG1 is correlated with tumor progression and poor prognosis in patients with esophageal squamous cell carcinoma. Dis Esophagus 2006;19:454–8. [DOI] [PubMed] [Google Scholar]
- [22].Koshiji M, Kumamoto K, Morimura K, et al. Correlation of N-myc downstream-regulated gene 1 expression with clinical outcomes of colorectal cancer patients of different race/ethnicity. World J Gastroenterol 2007;13:2803–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jiang K, Shen Z, Ye Y, et al. A novel molecular marker for early detection and evaluating prognosis of gastric cancer: N-myc downstream regulated gene-1 (NDRG1). Scand J Gastroenterol 2010;45:898–908. [DOI] [PubMed] [Google Scholar]
- [24].Mao Z, Sun J, Feng B, et al. The metastasis suppressor, N-myc downregulated gene 1 (NDRG1), is a prognostic biomarker for human colorectal cancer. PLoS ONE 2013;8:e68206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cheng J, Xie HY, Xu X, et al. NDRG1 as a biomarker for metastasis, recurrence and of poor prognosis in hepatocellular carcinoma. Cancer Lett 2011;310:35–45. [DOI] [PubMed] [Google Scholar]
- [26].Chua MS, Sun H, Cheung ST, et al. Overexpression of NDRG1 is an indicator of poor prognosis in hepatocellular carcinoma. Mod Pathol 2007;20:76–83. [DOI] [PubMed] [Google Scholar]
- [27].Maruyama Y, Ono M, Kawahara A, et al. Tumor growth suppression in pancreatic cancer by a putative metastasis suppressor gene Cap43/NDRG1/Drg-1 through modulation of angiogenesis. Cancer Res 2006;66:6233–42. [DOI] [PubMed] [Google Scholar]
- [28].Shah MA, Kemeny N, Hummer A, et al. Drg1 expression in 131 colorectal liver metastases: correlation with clinical variables and patient outcomes. Clin Cancer Res 2005;11:3296–302. [DOI] [PubMed] [Google Scholar]
- [29].Strzelczyk B, Szulc A, Rzepko R, et al. Identification of high-risk stage II colorectal tumors by combined analysis of the NDRG1 gene expression and the depth of tumor invasion. Ann Surg Oncol 2009;16:1287–94. [DOI] [PubMed] [Google Scholar]
- [30].Yang X, Zhu F, Yu C, et al. N-myc downstream-regulated gene 1 promotes oxaliplatin-triggered apoptosis in colorectal cancer cells via enhancing the ubiquitination of Bcl-2. Oncotarget 2017;8:47709–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ellen TP, Ke Q, Zhang P, et al. NDRG1, a growth and cancer related gene: regulation of gene expression and function in normal and disease states. Carcinogenesis 2008;29:2–8. [DOI] [PubMed] [Google Scholar]
- [32].Zhang X, Feng B, Zhu F, et al. N-myc downstream-regulated gene 1 promotes apoptosis in colorectal cancer via up-regulating death receptor 4. Oncotarget 2017;8:82593–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Menezes SV, Sahni S, Kovacevic Z, et al. Interplay of the iron-regulated metastasis suppressor NDRG1 with epidermal growth factor receptor (EGFR) and oncogenic signaling. J Biol Chem 2017;292:12772–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kim SC, Shin YK, Kim YA, et al. Identification of genes inducing resistance to ionizing radiation in human rectal cancer cell lines: re-sensitization of radio-resistant rectal cancer cells through down regulating NDRG1. BMC Cancer 2018;18:594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lu WJ, Chua MS, So SK. Suppressing N-Myc downstream regulated gene 1 reactivates senescence signaling and inhibits tumor growth in hepatocellular carcinoma. Carcinogenesis 2014;35:915–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lu WJ, Chua MS, Wei W, et al. NDRG1 promotes growth of hepatocellular carcinoma cells by directly interacting with GSK-3beta and Nur77 to prevent beta-catenin degradation. Oncotarget 2015;6:29847–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yi X, Luk JM, Lee NP, et al. Association of mortalin (HSPA9) with liver cancer metastasis and prediction for early tumor recurrence. Mol Cell Proteomics 2008;7:315–25. [DOI] [PubMed] [Google Scholar]
- [38].Nakanishi K, Sakamoto M, Yamasaki S, et al. Akt phosphorylation is a risk factor for early disease recurrence and poor prognosis in hepatocellular carcinoma. Cancer 2005;103:307–12. [DOI] [PubMed] [Google Scholar]
- [39].Apte U, Zeng G, Muller P, et al. Activation of Wnt/beta-catenin pathway during hepatocyte growth factor-induced hepatomegaly in mice. Hepatology 2006;44:992–1002. [DOI] [PubMed] [Google Scholar]
- [40].Sevinsky CJ, Khan F, Kokabee L, et al. NDRG1 regulates neutral lipid metabolism in breast cancer cells. Breast Cancer Res 2018;20:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wang H, Li W, Xu J, et al. NDRG1 inhibition sensitizes osteosarcoma cells to combretastatin A-4 through targeting autophagy. Cell Death Dis 2017;8:e3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sahni S, Bae DH, Lane DJ, et al. The metastasis suppressor, N-myc downstream-regulated gene 1 (NDRG1), inhibits stress-induced autophagy in cancer cells. J Biol Chem 2014;289:9692–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Luo Q, Wang CQ, Yang LY, et al. FOXQ1/NDRG1 axis exacerbates hepatocellular carcinoma initiation via enhancing crosstalk between fibroblasts and tumor cells. Cancer Lett 2018;417:21–34. [DOI] [PubMed] [Google Scholar]
- [44].Gallois C, Pernot S, Zaanan A, et al. Colorectal cancer: why does side matter? Drugs 2018;78:789–98. [DOI] [PubMed] [Google Scholar]


