Abstract
Contrast-enhanced ultrasound (CEUS) is highly accurate in depicting the vascularity of liver nodules. The aim of this study was to verify the characteristics of CEUS in distinguishing small (≤3 cm) hepatocellular carcinoma (HCC) from intrahepatic cholangiocarcinoma (ICC).
A total of 65 patients with a liver nodule (HCC, n = 58; ICC, n = 7) smaller than 3 cm who underwent liver CEUS and pathologic confirmation were retrospectively reviewed. CEUS findings were compared with histopathologic and clinical data.
Arterial-phase hyperenhancement and portal-delayed-phase wash-out on CEUS were observed in 77.6% (45/58) of HCCs and 85.7% (6/7) of ICCs. Time of arterial-phase hyperenhancement (11 seconds [6–20] vs 16 seconds [14–19], P = .008), time of portal-delayed-phase wash-out (65 seconds (15–260) vs 35 secconds (27–54), P = .002), and time interval between arterial-phase hyperenhancement and portal-delayed-phase wash-out (50 seconds [5–249] vs 19 seconds [13–35], P < .001) on CEUS were significantly different between HCCs and ICCs showing arterial-phase hyperenhancement and portal-delayed-phase wash-out. The sensitivity, specificity, positive predictive value, and negative predictive value of time interval more than 25 seconds between arterial-phase hyperenhancement and portal-delayed-phase wash-out on CEUS for the differentiation of HCCs and ICCs were 91.1%, 83.3%, 97.6%, and 55.6%, respectively.
The time interval between arterial-phase hyperenhancement and portal-delayed-phase wash-out on CEUS was the most sensitive indicator in distinguishing small HCC from ICC showing arterial-phase hyperenhancement and portal-delayed-phase wash-out.
Keywords: contrast-enhanced ultrasound, hepatocellular carcinoma, intrahepatic cholangiocarcinoma
1. Introduction
Primary liver cancer carries a very poor prognosis; it is the second leading cause of cancer death worldwide.[1] Among primary liver cancers, hepatocellular carcinoma (HCC) accounts for 80% of cases, and intrahepatic cholangiocarcinoma (ICC) accounts for approximately 15% of cases.[2] Because the treatment and prognosis of HCC and ICC are somewhat different, accurate differentiation is necessary, and early diagnosis is needed to improve the prognosis. Although the gold standard method of differential diagnosis for HCC and ICC is pathologic confirmation, the majority of HCCs and some ICCs occur in patients with liver cirrhosis (LC), making it difficult to perform biopsies due to the risk of bleeding and/or the presence of ascites.
Typical imaging features such as arterial-phase hyperenhancement and portal-delayed-phase wash-out in dynamic computed tomography (CT) or magnetic resonance imaging (MRI) are highly specific for the diagnosis of HCC. However, in some small-sized, well-differentiated HCCs, the typical pattern is not observed because arterial tumor vessels have not sufficiently developed, making diagnosis difficult.[3,4]
Recently, contrast-enhanced ultrasound (CEUS) using a microbubble contrast agent allowing a continuous view of the enhancement pattern has made it possible to evaluate the vascular characteristics of tumors in real-time and has been shown to be useful for differentiation of focal hepatic lesions.[5–8] A previous study demonstrated that CEUS is a valuable imaging modality for the characterization of dysplastic nodules and HCCs in cirrhotic patients with small liver nodules showing atypical or noncoincidental typical vascular patterns on dynamic CT and MRI.[9] However, the role of CEUS in the diagnosis of HCCs is still controversial due to the perceived possibility of false-positive diagnosis of HCC in patients with ICC, as the latter can also display arterial-phase hyperenhancement and portal-delayed-phase wash-out on CEUS.[10–12] For this reason, recent recommendations from the American Association for the Study of Liver Diseases (AASLD) have excluded CEUS in the diagnostic techniques for HCC.[13]
The aim of this study was to verify the characteristics of CEUS in distinguishing small (≤3 cm) HCCs from ICCs.
2. Materials and methods
2.1. Patients
A total of 66 patients with small (≤3 cm) liver nodules and pathologically confirmed HCC or ICC who underwent liver CEUS from August 2012 to May 2016 were retrospectively reviewed. One case of combined HCC and ICC was excluded. Finally, 65 patients with pathologically confirmed HCC or ICC who underwent liver CEUS were enrolled. CEUS findings were compared with histopathologic and clinical data. LC was diagnosed via a liver biopsy or based on clinical findings such as a radiologic coarse liver echotexture with nodularity, the presence of features of portal hypertension (e.g., ascites, splenomegaly, and varices), and thrombocytopenia (<100,000/mm3).[14] The study protocol was approved by the Institutional Review Board of Gachon University Gil Medical Center (IRB no: GCIRB2018-167).
2.2. Histology of liver nodules
Ultrasound-guided percutaneous biopsy (n = 59) or surgical resection (n = 6) was performed for histologic evaluation. Tru-cut biopsy needles (ACE-CUT biopsy needle, TSK) were used for sonography-guided percutaneous biopsy, and at least 2 biopsies were obtained from each patient. Of the 65 nodules, 58 nodules were pathologically confirmed as HCCs, and 7 nodules were confirmed as ICCs.
2.3. Contrast-enhanced ultrasound
All CEUS was performed within 1 month before percutaneous biopsy or surgery. After a 2.4-mL SonoVue (Bracco, Milano, Italy) contrast agent bolus with a low mechanical index (0.12) was administered in the left antecubital vein, CEUS was performed using a convex transducer (4 MHz, LOGIQ E9, GE Healthcare, Milwaukee, WI). CEUS was performed by the same physician with over 20 years of experience of sonography. CEUS findings were recorded and evaluated in 3 stages: an arterial phase (10–20 seconds), a portal venous phase (30–45 seconds), and a delayed phase (>120 seconds). In addition, the time at arterial-phase hyperenhancement, the time at portal-delayed-phase wash-out, and the time interval between arterial-phase hyperenhancement and portal-delayed-phase wash-out after contrast agent injection were measured (Fig. 1).
Figure 1.
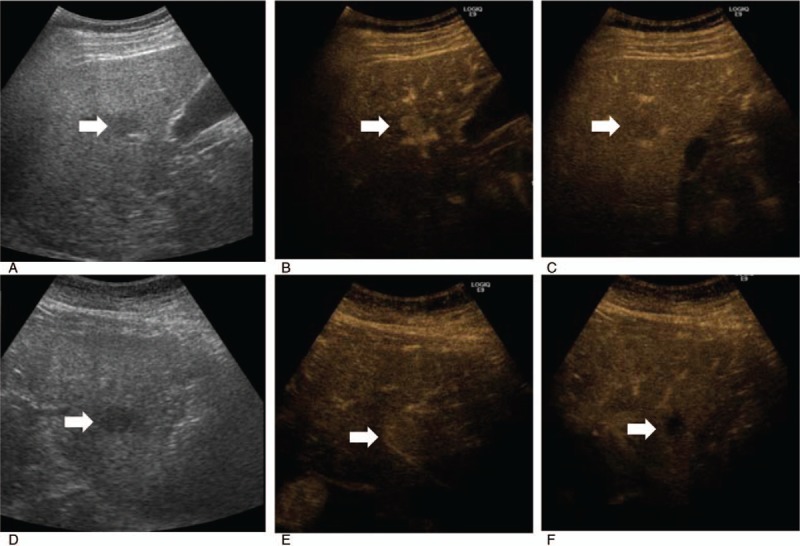
Hepatocellular carcinoma in a 59-year-old male with hepatitis-B-virus-related cirrhosis. (A) Baseline ultrasound image showing a 2.0-cm hypoechoic nodule (arrow). Dynamic contrast-enhanced ultrasound image showing (B) a nodule with homogenous hyperenhancement during the arterial phase (arrow) (13 seconds after contrast administration), and (C) a nodule with wash-out during the portal-delayed phase (arrow) (71 seconds after contrast administration). Intrahepatic cholangiocarcinoma in a 66-year-old female with hepatitis-B-virus-related cirrhosis. (D) Baseline ultrasound image showing a 2.5-cm hypoechoic nodule (arrow). Dynamic contrast-enhanced ultrasound image showing (E) a 2.5-cm nodule with homogenous hyperenhancement (arrow) during the arterial phase (16 seconds after contrast administration), and (F) a nodule with wash-out (arrow) during the portal venous phase (30 seconds after contrast administration).
2.4. Magnetic resonance imaging
The MRIs were obtained using a 3-T unit (Verio; Siemens Medical Solutions, Erlangen, Germany) or a 1.5-T unit (Avanto; Siemens Medical Solutions). The MRI protocol consisted of a breath-hold fat-saturated T2-weighted fast spin-echo or turbo spin-echo sequence, a breath-hold T1-weighted dual-echo (in-phase and opposed-phase) sequence, precontrast and 3-dimensional fat-saturated T1-weighted dynamic contrast-enhanced sequences, and free-breathing diffusion-weighted imaging (DWI), using a single-shot echo-planar imaging sequence, and 20 minutes delayed hepatobiliary phase. For the contrast-enhanced dynamic MRIs, Gd-EOB-DTPA (Primovist, Eovist; Bayer-Schering, Berlin, Germany) was administered at 0.025 mmol/kg of body weight at 2 mL/s. After contrast injection, the hepatic arterial, portal-venous, and transitional phase images were acquired at 40, 60, and 120 seconds, respectively. DWI with simultaneous respiratory triggering was performed during the period prior to the 20 minutes delayed imaging. For each patient, the repetition time was matched to the length of the respiratory cycle; every patient had b-values of 0, 400, and 1000 s/mm2. The apparent diffusion coefficient (ADC) values of the HCC and ICC were measured on an ADC map, and the slice's location was identical to that of the selected image on the DWI and hepatobiliary-phase images. The ADC values were automatically calculated by a computer program included in the GE workstation software. The MRIs were retrospectively analyzed by 2 radiologists who were unaware of the pathologic results.
2.5. Image analysis
The CEUS images were retrospectively and separately analyzed by 2 physicians (YSK and SKS) with 21 and 4 years’ experience of sonography who were unaware of the clinical data of the patients, pathologic results, and the findings of CT or MRI. The hyper-, iso- or hypoenhancement of the target lesion in the arterial, portal venous, and delayed phase was evaluated, and the times were recorded when the target lesion began to show hyperenhanement or wash-out. The κ-value representing interobserver agreements in arterial-phase heperenhancement and portal-delayed-phase wash-out were 0.82 and 0.64, respectively. The MRIs were retrospectively analyzed by 2 radiologists with over 6 years’ experience in abdominal disease diagnosis who were unaware of the clinical data of the patients and the pathologic results.
2.6. Statistical analysis
Interobserver agreement for the evaluation of arterial-phase hyperenhancement or portal-delayed-phase wash-out on CEUS was evaluated using κ statistics, with a κ-value of 0 to 0.20 indicating slight agreement, 0.21 to 0.40 fair agreement, 0.41 to 0.60 moderate agreement, 0.61 to 0.80 substantial agreement, and 0.80 to 1.00 almost perfect agreement.[15] Quantitative data are expressed as medians (ranges). The Mann–Whitney U test was used to test the differences of CEUS between HCCs and ICCs, such as the considerably time variations of arterial hyperenhancement and portal-delayed wash-out.
The logistic regression analysis was applied to identify the related factors in distinguishing HCCs from ICCs. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and the area under the curve (AUC) were used to determine diagnostic usefulness of CEUS for differentiation between HCC and ICC. Statistical significance was accepted for P-values <.05, and the statistical analysis was performed using the SPSS ver. 12.0 software package (SPSS Inc, Chicago, IL).
3. Results
3.1. Baseline characteristics of total patents
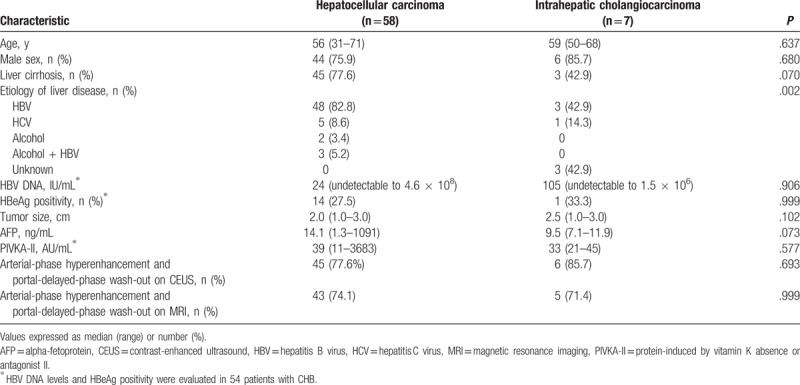
The mean age of the 65 patients was 58 ± 9 years, and 76.9% (n = 50) of the patients were males. Of the 58 patients with HCC, 77.6% (n = 45) had LC, 82.8% (n = 48) were infected with hepatitis B virus (HBV), and 8.6% (n = 5) were infected with hepatitis C virus (HCV). Of the 7 patients with ICC, 42.9% (n = 3) had LC, 42.9% (n = 3) were infected with HBV, and 14.3% (n = 1) were infected with HCV. There were no significant differences among these indicators, including median of HBV DNA levels (24 [undetectable to 4.6 × 108] IU/mL vs 105 [undetectable to 1.5 × 106] IU/mL, P = .906) and HBeAg(+) rate (27.5% vs 33.3%, P = .999) between patients with chronic hepatitis B (CHB)-related HCC and ICC at the time of malignancy confirmed diagnosis (Table 1).
Table 1.
Baseline characteristics of total patients.
The median tumor size (HCC 2.0 cm [1.0–3.0] vs ICC 2.5 cm [1.0–3.0]), alpha-fetoprotein (AFP) (HCC 14.1 ng/mL [1.3–1091] vs HCC 9.5 ng/mL [7.1–11.9]), and protein induced by vitamin K absence or antagonist-II (PIVKA-II) (HCC 39 AU/mL [11–3683] vs ICC 33 AU/mL [21–45]) were not significantly different. The typical enhancement pattern of HCC (arterial-phase hyperenhancement and portal-delayed-phase wash-out) on CEUS was observed in 77.6% (45/58) of the patients with HCC and in 85.7% (6/7) of the patients with ICC. Arterial-phase hyperenhancement and portal-delayed-phase wash-out on CEUS did not show any significant difference between the 2 tumors (Table 1).
Likewise, arterial-phase hyperenhancement and portal-delayed-phase wash-out on MRI also did not show any significant difference between the 2 tumors (Table 1). High signal on T2-weighted image (87.9% vs 85.7%, P = .999) and DWI (93.1% vs 100%, P = .999), and low signal on hepatobiliary phase (98.3% vs 100%, P = .999) were shown in most of HCC and ICC, and there were no significant differences between HCC and ICC. The mean ADC values between the HCCs (1.21 ± 0.22 × 10−3 mm2) and ICCs (1.07 ± 0.24 × 10−3 mm2) were not significant different (P = .141) (data not shown).
3.2. Baseline characteristics of patents with small (≤3 cm) HCC and ICC showing arterial-phase hyperenhancement and portal-delayed-phase wash-out on CEUS
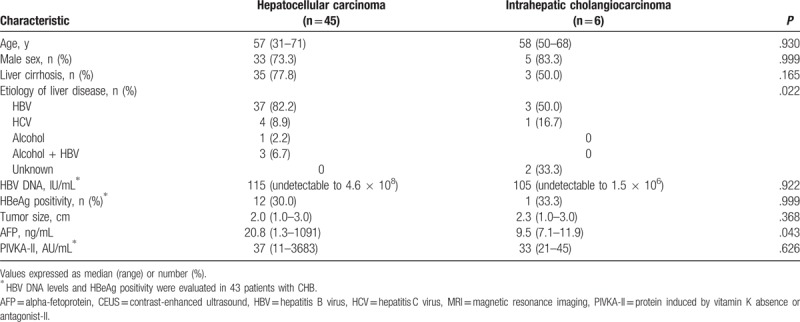
The mean age of the 51 patients with small (≤3 cm) HCC and ICC showing arterial-phase hyperenhancement and portal-delayed-phase wash-out on CEUS was 57 ± 9 years, and 74.5% (n = 38) of the patients were male. Of the 45 patients with HCC showing arterial-phase hyperenhancement and portal-delayed-phase wash-out on CEUS, 77.8% (n = 35) had LC, 88.9% (n = 40) were infected with HBV, and 8.9% (n = 4) were infected with HCV. Of the 6 patients with ICC showing arterial-phase hyperenhancement and portal-delayed-phase wash-out on CEUS, 50.0% (n = 3) had LC, 50.0% (n = 3) were infected with HBV, and 16.6% (n = 1) were infected with HCV. There were no significant differences in median HBV DNA levels (115 [undetectable to 4.6 × 108] vs 105 [undetectable to 1.5 × 106], P = .922) and HBeAg positivity (30.0% vs 33.3%, P = .999) between patients with CHB with HCC and patients with CHB with ICC at the time of pathologic diagnosis for cancers. The median AFP levels were significantly different between patients with HCC and patients with ICC. However, the median tumor size (HCC 2.0 cm [1.0–3.0] vs ICC 2.3 cm [1.0–3.0]) and PIVKA-II (HCC 37 AU/mL [11–3683] vs ICC 33 AU/mL [21–45]) were not significantly different (Table 2).
Table 2.
Baseline characteristics of the patients with small (≤3 cm) hepatocellular carcinoma and intrahepatic cholangiocarcinoma showing arterial-phase hyperenhancement and portal-delayed-phase wash-out on CEUS.
3.3. Differences of CEUS findings between small (≤3 cm) HCC and ICC showing arterial-phase hyperenhancement and portal-delayed-phase wash-out on CEUS
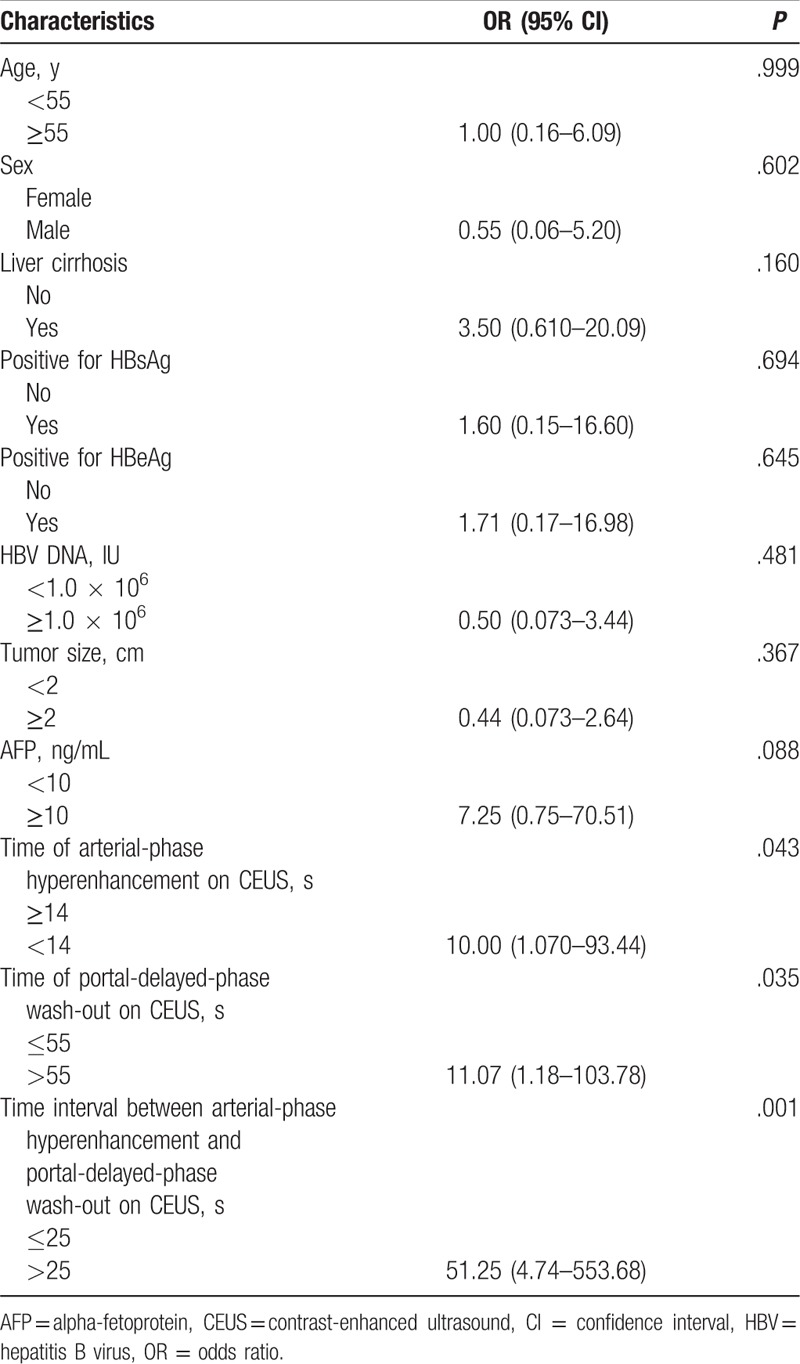
In the 6 cases of ICC showing arterial-phase enhancement and portal-delayed-phase wash-out, all displayed a homogenous enhancement pattern rather than a rim-enhancement pattern, which did not help to differentiate between HCC and ICC. However, the median time at arterial-phase hyperenhancement after contrast injection between HCC (11 seconds, range 6–20) and ICC (16 seconds, range 14–19) was significantly different (P = .008). The median time at portal-delayed-phase wash-out after contrast injection between HCC (65 seconds, range 15–260) and ICC (35 seconds, range 27–54) was significantly different (P = .002). In addition, the time interval between arterial-phase hyperenhancement and portal-delayed-phase wash-out was 50 seconds (range 5–249) in HCC and 19 seconds (range 13–35) in ICC (P < .001) (Fig. 2). In univariate analysis, the time <14 seconds at arterial-phase hyperenhancement, the time >55 seconds at portal-delayed-phase wash-out after contrast injection, and the time interval >25 seconds between arterial-phase hyperenhancement and portal-delayed-phase wash-out on CEUS were significant factors in differentiating HCCs from ICCs (Table 3).
Figure 2.
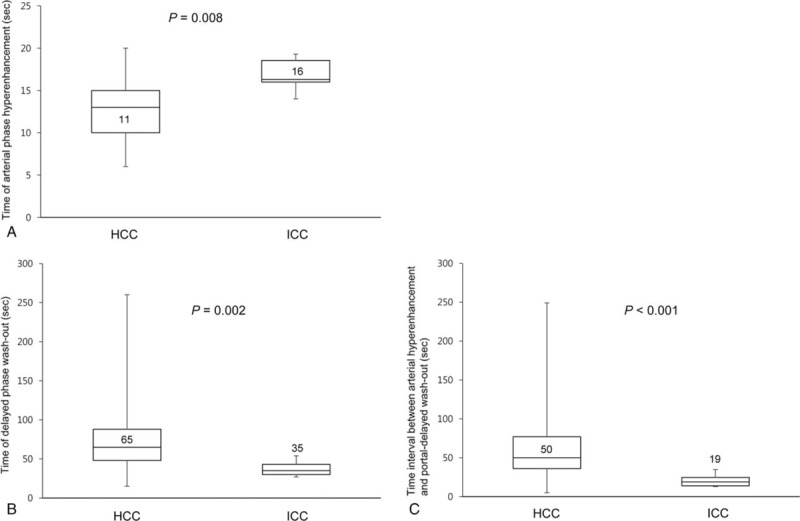
Differences in considerably time variations of arterial hyperenhancement and portal-delayed wash-out on contrast-enhanced ultrasound: (A) time of arterial-phase hyperenhancement after contrast injection (seconds), (B) time of portal-delayed-phase wash-out after contrast injection (seconds), and (C) time interval between arterial-phase hyperenhancement and portal-delayed-phase wash-out (seconds) between small (≤3 cm) hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC) showing arterial-phase hyperenhancement and portal-delayed-phase wash-out.
Table 3.
Univariate analysis for the characteristics in differentiating of small (≤3 cm) hepatocellular carcinomas from intrahepatic cholangiocarcinomas showing arterial-phase hyperenhancement and portal-delayed-phase wash-out on CEUS.
3.4. Sensitivity and specificity of CEUS findings for differentiation between HCC and ICC
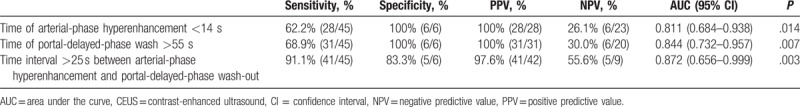
The sensitivity, specificity, PPV, and NPV of the time at arterial-phase hyperenhancement <14 seconds after contrast injection to distinguish HCC from ICC were 62.2%, 100%, 100%, and 26.1%, respectively. The sensitivity, specificity, PPV, and NPV of the time at portal-delayed-phase wash-out more than 55 seconds after contrast injection to distinguish HCC from ICC were 68.9%, 100%, 100%, and 30.0%, respectively. The sensitivity, specificity, PPV, and NPV of the time interval between arterial-phase hyperenhancement and portal-delayed-phase wash-out more than 25 seconds after contrast injection to distinguish HCC from ICC were 91.1%, 83.3%, 97.6%, and 55.6%, respectively. The time at arterial-phase hyperenhancement <14 seconds or the time at portal-delayed-phase wash-out >55 seconds after contrast injection was most specific feature of distinguishing HCC from ICC. The time interval >25 seconds between arterial-phase hyperenhancement and portal-delayed-phase wash-out after contrast injection was the most sensitive feature of distinguishing HCC from ICC (Table 4).
Table 4.
Sensitivity and specificity of CEUS findings for the differentiation of small (≤3 cm) hepatocellular carinomas from intrahepatic cholangiocarcinomas showing arterial-phase hyperenhancement and portal-delayed-phase wash-out.
4. Discussion
Most patients with HCC have an established underlying chronic liver disease and/or cirrhosis with major risk factors for developing HCC, including chronic infection with HBV, hepatitis C virus, alcoholic liver disease, nonalcoholic fatty liver disease, and aflatoxin-contaminated food. Treatment outcomes in cases of HCC are affected by multiple factors including liver function, performance status of the patient, and tumor stage. The treatment of HCC can be divided into curative treatments (liver transplantation, resection, and local treatment such as radiofrequency ablation and ethanol injection) and palliative treatments including transarterial chemoembolization and sorafenib.[16]
The etiology of ICC that arises from peripheral bile ducts within the liver parenchyma distal to the second-order bile ducts is not well known. Surgical resection is the only treatment that can improve the survival rate, and palliative treatments such as radiation therapy and chemotherapy are known to have low therapeutic efficacy.[17] Therefore, accurate differentiation between HCC and ICC is important because the treatment and prognosis of the 2 tumors are quite different. Hepatic tumors with increased AFP in high-risk patients with HCC can be considered as HCC rather than ICC. However, increased AFP is uncommon in patients with small HCCs. In addition, both HCC and ICC may occur in patients with LC.[18] In our study, 77.6% of the patients with HCC suffered from LC, while 42.9% of the patients with ICC also suffered from LC. Therefore, it is more difficult to differentiate between small HCCs and ICC in patients with LC.
Although radiologic features that can distinguish ICC from HCC such as rim-enhancement, delayed-phase enhancement, distal biliary dilatation, and capsular retraction have been suggested,[19] these were not helpful in differentiating between small HCCs and ICC on CEUS. In the present study, 6 of 7 patients with ICCs showed no delayed-phase hyperenhancement but rather arterial-phase hyperenhancement. In all patients with ICCs, homogeneous enhancement and not rim-enhancement was present, and distal biliary dilatation was not observed. Capsular retraction was observed in only 1 patients. On the contrary, rim-enhancement pattern on CEUS was present in most of the patients with ICCs larger than 4 cm in our data (data not shown). Although delayed phase hyperenhancement was not observed in all of the patients with ICC, the time at hyperenhancement after contrast injection in the patients with ICC (median 16 seconds, range 14–19) was significantly delayed compared to that of the patients with HCC (median 11 seconds, range 6–20).
Because of the characteristic blood flow changes such as unpaired arterial blood flow increases and portal flow decrease, a typical enhancement pattern of arterial-phase hyperenhancement followed by portal or delayed “wash-out” on dynamic contrast-enhanced imaging was shown in HCC, which allows the diagnosis of HCC without histologic examination.[13,20] However, this typical enhancement pattern of HCC can be observed in ICC. In our study, arterial-phase hyperenhancement and portal-delayed-phase wash-out on CEUS was shown in 77.6% of HCCs and 85.7% of ICC, respectively. Similar results were also obtained in the enhancement pattern on MRI.
Recently, various imaging techniques such as DWI and ADC sequences on MRI compared with CT which has the disadvantage of radiation exposure and is limited to observation of enhancement pattern, have been proposed to predict liver diseases including HCC.[21–26] DWI is known to detect Brownian motion of water protons, thus reflecting the biologic character of tissue and the ADC is used to quantify the Brownian motion. The areas of malignant tissues can be observed with high signal intensity on DWI and a decrease in the ADC is expected with increased intracellular tissue caused by either cell swelling or increased cellular density.[27] However, our results did not show any significant differences of ADC values between HCCs and ICCs.
Although there are several shortcomings including the difficulty of observation in deep portion on ultrasound, ultrasound has the advantages of being safe, easily repeatable, and relatively low cost.[28,29] Especially, CEUS has the advantage of observing the enhancement pattern in real-time compared with CT and MRI. Our study demonstrated that the significant differences of CEUS findings between HCCs and ICCs were the time of arterial-phase hyperenhancement, the time of portal-delayed-phase wash-out, and the time interval between arterial-phase hyperenhancement and portal-delayed-phase wash-out on CEUS among the tumors with arterial-phase hyperenhancement and portal-delayed-phase wash-out. In patients with ICC, the time at portal-delayed-phase wash-out after contrast injection was 35 seconds (27–54 seconds), whereas the time at portal-delayed-phase wash-out after contrast injection was 65 seconds (15–260 seconds) in patients with HCC. Recently, Liu et al[30] demonstrated that the time at portal-delayed-phase wash-out after contrast injection between ICC (27.5 seconds) and HCC (70.1 seconds) was significantly different. Additionally, the time interval between arterial-phase hyperenhancement and portal-delayed-phase wash-out was significantly different between ICC and HCC cases, and this was the most sensitive feature involving CEUS for differentiation between ICC and HCC in the present study. In comparison with HCC, portal-delayed-phase wash-out occurred quickly after arterial-phase hyperenhancement in ICC. On the contrary, in 4 of 45 cases of HCC, portal-delayed-phase wash-out after arterial-phase hyperenhancement occurred in a short time. However, arterial-phase hyperenhancement was within 10 seconds after contrast injection in all 4 cases, and this allowed us to differentiate HCC from ICC. In our study, the characteristics such as the time at arterial-phase hyperenhancement <14 seconds or the time at portal-delayed-phase wash-out >55 seconds after contrast injection were the specific features of distinguishing HCC from ICC.
Consequently, our results showed that the timing of enhancement or wash-out observed in CEUS, which could not be observed in MRI, might be useful for distinguishing HCC from ICC. Although MRI is known to be an objective and excellent modality in detecting soft tissue tumors,[31] the timing of MRI scan is predetermined and the contrast agents used in MRI leak out into the interstitium of tissues. However, CEUS can observe the enhancement pattern in real time, especially the very early or late enhancement patterns of tumors that are difficult to identify with MRI. In addition, unique intravascular properties of the microbubbles used in CEUS make it possible to more clearly depict tumor vascularity.[8]
Our study has several limitations. First, the number of cases of ICC <3 cm was much lower than that of HCC. Because the prevalence of ICC is lower than that of HCC in Korea and proper screening tests are not performed for ICC, few cases of ICC <3 cm are diagnosed. Second, as the nature of retrospective design, there might have been selection bias in the present study. Although there are limitations, this study is meaningful in regard to identifying the features of CEUS that can distinguish HCC from ICC in which rim-enhancement, delayed enhancement, distal biliary dilatation, or capsular retraction are not observed.
In conclusion, characteristics of CEUS such as the time at arterial-phase hyperenhancement, the time at portal-delayed-phase wash-out, and the time interval between arterial-phase hyperenhancement and portal-delayed-phase wash-out on CEUS can be useful for differentiating between HCC and ICC <3 cm. Further large-scale studies are needed to confirm the present results.
Author contributions
Conceptualization: Duck Joo Choi, Yun Soo Kim.
Data curation: Seung Kak Shin, Yun Soo Kim.
Investigation: Seung Kak Shin, Yun Soo Kim, Oh Sang Kwon.
Resources: Ju Hyun Kim, Oh Sang Kwon.
Supervision: Duck Joo Choi, Ju Hyun Kim.
Visualization: Yun Soo Kim.
Writing – original draft: Seung Kak Shin.
Footnotes
Abbreviations: AASLD = American Association for the Study of Liver Disease, ADC = apparent diffusion coefficient, AFP = alpha-fetoprotein, AUC = area under the curve, CEUS = contrast-enhanced ultrasound, CHB = chronic hepatitis B, CT = computed tomography, HBV = hepatitis B virus, HCC = hepatocellular carcinoma, HCV = hepatitis C virus, ICC = intrahepatic cholangiocarcinoma, LC = liver cirrhosis, MRI = magnetic resonance imaging, NPV = negative predictive value, PIVKA-II = protein induced by vitamin K absence or antagonist-II, PPV = positive predictive value.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- [2].Walther Z, Jain D. Molecular pathology of hepatic neoplasms: classification and clinical significance. Patholog Res Int 2011;2011:403929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sugimachi K, Tanaka S, Terashi T, et al. The mechanisms of angiogenesis in hepatocellular carcinoma: angiogenic switch during tumor progression. Surgery 2002;131:S135–41. [DOI] [PubMed] [Google Scholar]
- [4].Nicolau C, Catala V, Vilana R, et al. Evaluation of hepatocellular carcinoma using SonoVue, a second generation ultrasound contrast agent: correlation with cellular differentiation. Eur Radiol 2004;14:1092–9. [DOI] [PubMed] [Google Scholar]
- [5].Leen E, Angerson WJ, Yarmenitis S, et al. Multi-centre clinical study evaluating the efficacy of SonoVue (BR1), a new ultrasound contrast agent in Doppler investigation of focal hepatic lesions. Eur J Radiol 2002;41:200–6. [DOI] [PubMed] [Google Scholar]
- [6].Bolondi L, Gaiani S, Celli N, et al. Characterization of small nodules in cirrhosis by assessment of vascularity: the problem of hypovascular hepatocellular carcinoma. Hepatology 2005;42:27–34. [DOI] [PubMed] [Google Scholar]
- [7].Sugimoto K, Moriyasu F, Shiraishi J, et al. Assessment of arterial hypervascularity of hepatocellular carcinoma: comparison of contrast-enhanced US and gadoxetate disodium-enhanced MR imaging. Eur Radiol 2012;22:1205–13. [DOI] [PubMed] [Google Scholar]
- [8].Kim TK, Noh SY, Wilson SR, et al. Contrast-enhanced ultrasound (CEUS) liver imaging reporting and data system (LI-RADS) 2017 - a review of important differences compared to the CT/MRI system. Clin Mol Hepatol 2017;23:280–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Shin SK, Kim YS, Choi SJ, et al. Contrast-enhanced ultrasound for the differentiation of small atypical hepatocellular carcinomas from dysplastic nodules in cirrhosis. Digest Liver Dis 2015;47:775–82. [DOI] [PubMed] [Google Scholar]
- [10].Vilana R, Forner A, Bianchi L, et al. Intrahepatic peripheral cholangiocarcinoma in cirrhosis patients may display a vascular pattern similar to hepatocellular carcinoma on contrast-enhanced ultrasound. Hepatology 2010;51:2020–9. [DOI] [PubMed] [Google Scholar]
- [11].Xu HX, Lu MD, Liu GJ, et al. Imaging of peripheral cholangiocarcinoma with low-mechanical index contrast-enhanced sonography and SonoVue: initial experience. J Ultrasound Med 2006;25:23–33. [DOI] [PubMed] [Google Scholar]
- [12].Chen LD, Xu HX, Xie XY, et al. Intrahepatic cholangiocarcinoma and hepatocellular carcinoma: differential diagnosis with contrast-enhanced ultrasound. Eur Radiol 2010;20:743–53. [DOI] [PubMed] [Google Scholar]
- [13].Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lee HS, Kim JK, Cheong JY, et al. Prediction of compensated liver cirrhosis by ultrasonography and routine blood tests in patients with chronic viral hepatitis. Korean J Hepatol 2010;16:369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–74. [PubMed] [Google Scholar]
- [16].El-Serag HB. Hepatocellular carcinoma. N Engl J Med 2011;365:1118–27. [DOI] [PubMed] [Google Scholar]
- [17].Ryu KH, Lee KT, Lee JK, et al. Prognostic factors in resectable peripheral cholangiocarcinoma. Kor J Gastroenterol 2000;36:686–94. [Google Scholar]
- [18].Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology 2011;54:173–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chung YE, Kim MJ, Park YN, et al. Varying appearances of cholangiocarcinoma: radiologic-pathologic correlation. Radiographics 2009;29:683–700. [DOI] [PubMed] [Google Scholar]
- [20].Yoon JH, Park JW, Lee JM. Noninvasive diagnosis of hepatocellular carcinoma: elaboration on Korean Liver Cancer Study Group-National Cancer Center Korea Practice Guidelines compared with other guidelines and remaining issues. Korean J Radiol 2016;17:7–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Razek AA, Massoud SM, Azziz MR, et al. Prediction of esophageal varices in cirrhotic patients with apparent diffusion coefficient of the spleen. Abdom Imaging 2015;40:1465–9. [DOI] [PubMed] [Google Scholar]
- [22].Besheer T, Razek AAKA, El Bendary M, et al. Does steatosis affect the performance of diffusion-weighted MRI values for fibrosis evaluation in patients with chronic hepatitis C genotype 4? Turk J Gastroenterol 2017;28:283–8. [DOI] [PubMed] [Google Scholar]
- [23].Razek AAKA, Khashaba M, Abdalla A, et al. Apparent diffusion coefficient value of hepatic fibrosis and inflammation in children with chronic hepatitis. Radiol Med 2014;119:903–9. [DOI] [PubMed] [Google Scholar]
- [24].Razek AA, Abdalla A, Omran E, et al. Diagnosis and quantification of hepatic fibrosis in children with diffusion weighted MR imaging. Eur J Radiol 2011;78:129–34. [DOI] [PubMed] [Google Scholar]
- [25].Heo SH, Jeong YY, Shin SS, et al. Apparent diffusion coefficient value of diffusion-weighted imaging for hepatocellular carcinoma: correlation with the histologic differentiation and the expression of vascular endothelial growth factor. Korean J Radiol 2010;11:295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Razek AA, Tawfik AM, Elsorogy LG, et al. Perfusion CT of head and neck cancer. Eur J Radiol 2014;83:537–44. [DOI] [PubMed] [Google Scholar]
- [27].Abdel Razek AA, Gaballa G, Denewer A, et al. Diffusion weighted MR imaging of the breast. Acad Radiol 2010;17:382–6. [DOI] [PubMed] [Google Scholar]
- [28].Razek AA, Al Mahdy Al Belasy F, Ahmed WM, et al. Assessment of articular disc displacement of temporomandibular joint with ultrasound. J Ultrasound 2015;18:159–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Razek AA, El-Basyouni SR. Ultrasound of knee osteoarthritis: interobserver agreement and correlation with Western Ontario and McMaster Universities Osteoarthritis. Clin Rheumatol 2016;35:997–1001. [DOI] [PubMed] [Google Scholar]
- [30].Liu GJ, Wang W, Lu MD, et al. Contrast-enhanced ultrasound for the characterization of hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Liver Cancer 2015;4:241–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Abdel Razek AA, Castillo M. Imaging appearance of primary bony tumors and pseudo-tumors of the spine. J Neuroradiol 2010;37:37–50. [DOI] [PubMed] [Google Scholar]


