Abstract
Background
Associations between well-being, resilience to trauma and the volume of grey-matter regions involved in affective processing (e.g., threat/reward circuits) are largely unexplored, as are the roles of shared genetic and environmental factors derived from multivariate twin modelling.
Methods
This study presents, to our knowledge, the first exploration of well-being and volumes of grey-matter regions involved in affective processing using a region-of-interest, voxel-based approach in 263 healthy adult twins (60% monozygotic pairs, 61% females, mean age 39.69 yr). To examine patterns for resilience (i.e., positive adaptation following adversity), we evaluated associations between the same brain regions and well-being in a trauma-exposed subgroup.
Results
We found a correlated effect between increased well-being and reduced grey-matter volume of the pontine nuclei. This association was strongest for individuals with higher resilience to trauma. Multivariate twin modelling suggested that the common variance between the pons volume and well-being scores was due to environmental factors.
Limitations
We used a cross-sectional sample; results need to be replicated longitudinally and in a larger sample.
Conclusion
Associations with altered grey matter of the pontine nuclei suggest that basic sensory processes, such as arousal, startle, memory consolidation and/or emotional conditioning, may have a role in well-being and resilience.
Introduction
Mental health is more than the absence of mental illness; mental health and illness share only one-quarter common variance.1–4 Whereas mental illness is usually measured using clinical symptom checklists or severity scales, mental health is measured using indices of mental well-being that assess levels of positive affect and life satisfaction (subjective well-being), as well as other adaptive attributes, including mastery, autonomy and goal-striving (psychological well-being). 5 Well-being is therefore a state that is measurable at different time points, and can vary from languishing to flourishing levels. It is also something slightly different from resilience, which is the process of adaptive recovery (that is, returning to an optimal mental state) following adversity or trauma exposure.6 In resilience, the presence of the environmental trauma is a necessary condition. To date, although neural circuits potentially involved in well-being and resilience have been proposed,7,8 they remain largely untested. It is important that we understand how brain circuits may vary for well-being and resilience, given that they are different constructs, differently affected by environmental (and genetic) factors.
Fundamental to mental health are the processes of adaptive emotional and cognitive functioning. These adaptive processes rely on at least 3 core brain circuits subserving the evaluative processing of rewarding cues or positive affect (e.g., the striatum, orbitofrontal cortex); potential threat or negative affect (e.g., amygdala, hippocampus, insula, brainstem, medial frontal cortex); and attention/cognitive control (e.g., dorsolateral prefrontal cortex, anterior cingulate, inferior parietal lobule).9 The structural integrity of these circuits may play a role in modulating well-being and resilience from stress.7,8 Alterations in the structure of these circuits may lead to stress-mediated disorders (such as posttraumatic stress disorder and major depression) by way of an accentuated threat-over-reward network, or to pleasure-seeking disorders (such as dependent drug use and compulsive disorders) by way of an accentuated reward network coupled with a lack of inhibitory control.7,8,10–13 It is important to understand how the structure of these circuits involved in affective disorders relates to levels of well-being and resilience, separate from illness symptoms, in order to understand the mechanisms to target for health-promotion strategies.
Few studies have investigated the associations between the structural integrity of regions in the affective circuits and resilience or well-being. For resilience, the few available studies have compared brain structure in trauma-exposed controls to clinical groups, identifying variations in hippocampal volume when resilience is defined as an absence of psychopathology given trauma exposure.14,15 However, we do not believe that the definition of resilience as the absence of psychopathology alone is sufficient, because the “healthy” population varies considerably in levels of well-being, even in the absence of psychopathology. For instance, we have shown that only 23% of healthy individuals (as defined by an absence of psychopathology on the Depression, Anxiety, Stress Scale [DASS-42])16 rated within optimal flourishing levels of well-being on the COMPAS-W5 well-being scale, with another 67% scoring at moderate levels, and a further 10% at languishing levels (and therefore at higher risk for developing illness symptomatology).1 No study to date has examined the brain-structure correlates of resilience as defined by increased positive adaptation (e.g., a flourishing well-being score) in the presence of trauma exposure, rather than the absence of psychopathology alone. This different definition is crucial and needs to be incorporated into resilience research, given these known relationships between mental health and illness and their small phenotypic overlap.1–4
Similarly for well-being, only a small number of studies have examined associations with brain structure in healthy samples. All studies have focused on samples of adolescents or young adults, and have used either a measure of subjective well-being (i.e., positive affect and life satisfaction) or psychological well-being (e.g., life purpose, mastery, personal growth), but not both.17–21 Considering associations using composite indices of well-being that measure both subjective and psychological well-being may be useful, because evidence suggests that both constructs are strongly associated with up to 74% shared variance.22,23 However, previous findings generally suggest a dichotomous pattern of association, varying by the well-being construct. Greater subjective well-being has been associated with greater volume of the bilateral hippocampus and right parahippocampus, and lower volume of regions typically involved in the affective circuit of threat: the prefrontal cortex (left ventromedial and left rostrolateral portions), dorsal anterior cingulate, left precuneus, right caudate and left amygdala.17–19 In contrast, increased psychological well-being has been associated with increased volume of regions typically involved in the affective circuit of reward: cortical (orbitofrontal and right insula), left rostral anterior cingulate and basal ganglia nuclei (left accumbens, bilateral caudate and left pallidum).20,21 Given that no study has compared both measures of well-being, it is unclear whether these effects were a property of the different well-being constructs or were sample-specific. Notably, in the study demonstrating negative associations between subjective well-being and the left precuneus, the effect was shown to be partially mediated by levels of self-esteem17 — an aspect of psychological well-being — suggesting that both subjective and psychological well-being may be contributing to shared associations.
In the present study, we examined whether the grey matter of regions defining affective circuits were associated with levels of well-being and resilience to trauma in a large sample of 263 healthy adult twins. We hypothesized that levels of well-being and resilience to trauma would be associated with grey-matter volume in regions of the brain implicated in emotional functioning and attention/cognitive control, with opposite patterns apparent for mental illness risk symptoms. We used the COMPAS-W well-being scale5 to measure subjective and psychological well-being. We used the same scale to define levels of resilience in a subsample of participants who reported exposure to significant childhood trauma to determine whether similar brain regions were associated with both well-being and resilience. We used the Depression, Anxiety, Stress Scale (DASS-42)16 to measure mental illness risk symptoms. We also hypothesized that relationships between measures of well-being and grey-matter volume would reflect contributions from shared variance due to genetics and environment, assessed using multivariate twin modelling of monozygotic and dizygotic twin pairs. This second hypothesis drew on our previous work showing that both genetics and environment contribute to variance in well-being at 48% heritability.5
Methods
Participants
Healthy same-sex monozygotic and dizygotic twins from the TWIN-E study conducted at the University of Sydney, Australia, 24 participated in this study. The study received approval from the Human Research Ethics Committees of the University of Sydney (03–2009/11430) and Flinders University (FCREC#08/09). Prior to enrolment, all participants provided written informed consent after receiving a written description of the study.
The present study included 263 twins who completed the MRI testing phase. The mean age of the sample was 39.69 ± 12.91 (range 18–62) years. The sample included 160 women with a mean education duration of 14.59 ± 2.86 years. It included 159 monozygotic twins (91 women, mean age 40.79 ± 12.28 yr) and 98 dizygotic twins (67 women, mean age 37.96 ± 13.68 yr), and had birth-order distributions of 132 twin 1 (81 women, mean age 39.82 ± 12.86 yr) and 131 twin 2 (79 women, mean age 39.55 ± 13.01 yr). Eligible participants were same-sex, healthy, adult twin pairs, with English as their primary language and European ancestry. For further details of inclusion/exclusion criteria, see Appendix 1, available at jpn.ca/170125-a1.
Measures
The methodology used in the present study24 has been validated in healthy and clinical populations.25–28 The self-report measures were assessed using the WebQ online test battery.24 We measured well-being using the 26-item COMPAS-W scale of well-being5; depression and anxiety mood symptoms using the DASS-4216; and early-life stress (trauma) using the 19-item Early Life Stress Questionnaire, which assesses the occurrence of specific early-life stressors up to age 18 years that have been shown to have a psychological impact in childhood, including abuse, neglect, family conflict, illness/death and natural disasters.29 To examine resilience, we measured well-being scores using the COMPAS-W scale of well-being in a trauma-exposed subgroup.
We acquired MRI data on a 3.0 T GE Signa HDx scanner (GE Healthcare) using an 8-channel head coil. We acquired a T1-weighted, high-resolution SPGR scan with the following parameters: 180 slices, 1 mm cubic voxels, 256 × 256 matrix (repetition time 8.3 ms, echo time 3.2 ms, inversion time 500 ms, flip angle 11°). The 9 regions of interest were grey-matter regions that underpin the affective circuits of reward, threat and attention/cognitive control: amygdala/hippocampus, anterior cingulate, basal ganglia (including the caudate, putamen, pallidum, substantia nigra), brainstem (including the midbrain and pons), thalamus, inferior parietal gyrus, insula, medial frontal gyrus and orbital frontal gyrus. These were determined a priori and defined by standardized masks using the Automated Anatomic Labelling toolbox.30 For further details of measures, see Appendix 1.
Statistical analysis
To examine the association between well-being and grey-matter volume, we estimated multiple linear regression models of total COMPAS-W well-being scores predicting grey-matter volume for each region of interest in SPM8 using voxel-based morphometry, with age and sex as covariates. Output was in the form of statistical parametric maps based on a cluster-level, false-discovery-rate–corrected threshold of p < 0.05. We extracted averages of significant clusters to use in quantitative analyses. We repeated this analysis with total DASS-42 depression/anxiety scores to examine whether any of the brain regions identified for well-being were also associated with depression/anxiety scores. For resilience, we repeated the above well-being analysis in the trauma-exposed subgroup, whereby participants exposed to trauma yet reporting a flourishing well-being score were considered to be more resilient. Each of the voxel-based morphometry analyses in SPM8 were conducted in twin 1 and then verified across both twins using linear mixed models in SPSS using the extracted brain cluster variables.
We conducted genetic and environment twin analyses for each significant cluster region (average extracted cluster converted to a log-transformed score) using OpenMx version 1.731 on R version 2.13.2.32 We undertook univariate genetic modelling using the classic twin design33 to estimate the genetic and environmental contributions to variance for each cluster region. For these models, A refers to additive genetic effects, D to nonadditive genetic (or dominance) effects, C to common environment and E to nonshared (unique) environment. Full ACE and ADE models were fitted to each variable using maximum likelihood estimation, and model fit was assessed by dropping parameters. Multivariate genetic models were then fitted to total COMPAS-W well-being and DASS-42 scores and the specific brain cluster region to assess shared and unique genetic and environmental variance between the variables. We ran a saturated model including scores for well-being, total DASS-42 and brain cluster region to confirm the nature of the mean and variances between twin pairs and across monozygotic and dizygotic twins. We included age, sex and education as covariates on the means. We tested and compared the saturated model with means and variances constrained across twin pairs and zygosity for comparative fit to a correlated factors model, which examined the genetic and environmental contribution to the correlation or covariance between variables.33 We examined the role of shared versus unique genetic and environment factors between well-being, DASS-42 and brain cluster region by testing the A and E components for each variable and correlations between all components. We then tested the significance of the genetic and environmental correlations between variables by systematically setting each correlation to zero, with a significant difference between models indicating a significant correlation. We compared the models for best fit using the χ2 difference test of the −2 log-likelihood statistic, where significance was indicated when the critical value of p = 0.05 was exceeded. We also used a higher degree of freedom and lower Aikake information criterion (AIC) value as indicators of the better overall model.
Results
Demographic characteristics
We found no significant differences between monozygotic and dizygotic twins, or between twin 1 and 2 groups for age, sex or education. The groups also did not differ in mean well-being scores, depression/anxiety scores, or average total number of early-life stress events (Table 1). We found no significant differences in age (t = −1.002, p = 0.318) or sex (t = 0.853, p = 0.395) with well-being scores using a linear mixed model (controlling for birth order and zygosity). We also found no sex difference for depression/anxiety scores (t = −1.360, p = 0.176) using a similar linear mixed model, but we did find a significant effect for age, whereby an increase in age was associated with reduced symptom scores (t = −2.224, p = 0.028).
Table 1.
| Characteristic | MZ (n = 159) | DZ (n = 98) | Twin 1 (n = 132) | Twin 2 (n = 131) |
|---|---|---|---|---|
| Mental well-being | 100.0 ± 10.2 | 99.5 ± 10.1 | 100.5 ± 9.9 | 99.3 ± 10.7 |
| Depression/anxiety symptoms | 10.4 ± 10.8 | 11.7 ± 10.7 | 10.4 ± 10.6 | 11.1 ± 10.7 |
| Early-life stress events | 1.7 ± 1.7 | 1.4 ± 1.4 | 1.6 ± 1.7 | 1.6 ± 1.6 |
| Premature birth or other birth complications | 47% | 44% | 45% | 47% |
| Adoption | 0% | 0% | 0% | 0% |
| Major surgery or repeated hospitalization | 11% | 15% | 12% | 14% |
| Life-threatening illness or injury | 10% | 8% | 11% | 8% |
| Sustained bullying | 17% | 15% | 13% | 20% |
| Physical abuse | 5% | 3% | 5% | 4% |
| Sexual abuse | 5% | 6% | 7% | 4% |
| Emotional abuse | 9% | 10% | 10% | 8% |
| Poverty or neglect | 2% | 0% | 1% | 2% |
| Natural disaster | 4% | 0% | 2% | 4% |
| House destroyed by fire or other means | 1% | 0% | 0% | 1% |
| Witness of warfare | 0% | 0% | 0% | 0% |
| Parents divorced or separated | 10% | 5% | 9% | 8% |
| Long period of separation from immediate family | 7% | 4% | 7% | 5% |
| Sustained family conflict | 12% | 13% | 14% | 11% |
| Death of immediate family | 3% | 3% | 4% | 3% |
| Life-threatening illness in immediate family | 6% | 7% | 8% | 7% |
| Witness domestic violence in family | 12% | 5% | 8% | 10% |
| Witness or experience some other traumatic event | 6% | 5% | 6% | 5% |
DZ = dizygotic; MZ = monozygotic.
Results presented as mean ± standard deviation, or as frequency (%).
No significant differences between groups for well-being, depression and anxiety scores, or average early-life events.
Apart from birth complications, the top 5 early-life stress events reported were sustained bullying (16%), sustained family conflict (13%), major surgery or repeated hospitalization (13%), emotional abuse (10%) and life-threatening illness or injury (9%). Using a linear mixed model, we examined categorical group differences in individuals with and without early-life stress exposure across twin pairs (controlling for age, sex and zygosity) and found no significant differences in scores for well-being (t = 0.280, p = 0.780) or depression/anxiety (t = −0.769, p = 0.443). Partial correlation analyses identified that a continuous increase in number of early-life stress events was associated with an increase in depression/anxiety scores, controlling for age, sex, zygosity and family relatedness (r = 0.248, p < 0.001), with no associations with well-being scores (r = 0.036, p = 0.57). However, an increase in well-being scores was correlated with a reduction in depression/anxiety scores (r = −0.406, p < 0.001).
Grey-matter volume associations with well-being
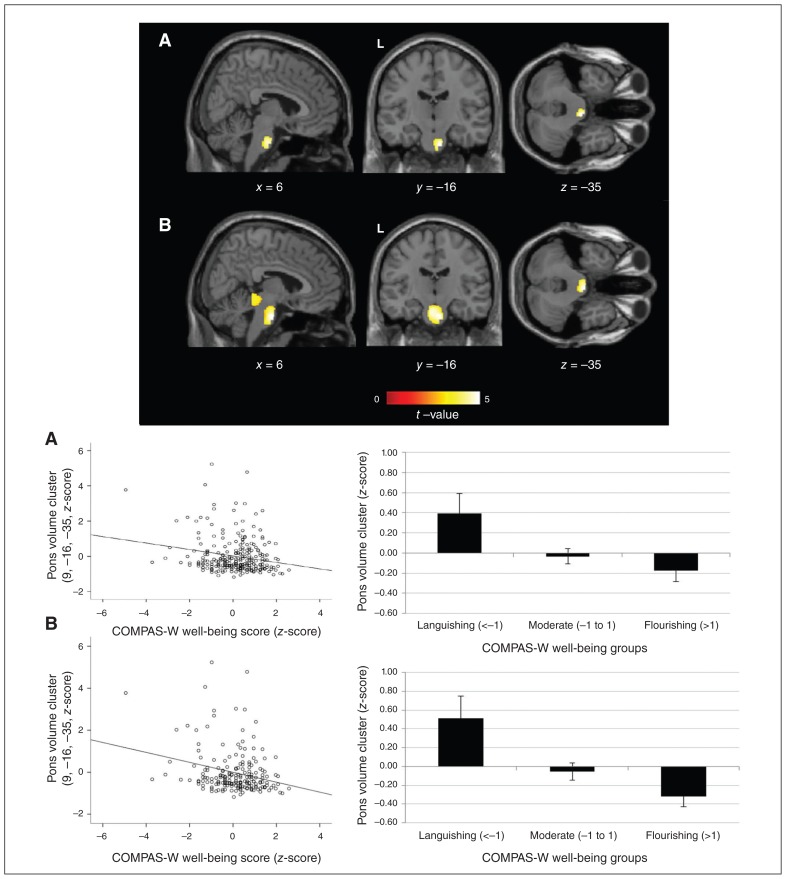
We used voxel-based morphometry to examine associations between well-being and grey-matter volume for the selected regions of interest in twin 1, controlling for the effects of age and sex. We found a significant negative correlation between well-being and the pons (Table 2 and Fig. 1a), the locus of which is near the fourth ventricle of the pontine nuclei and fibres. While we observed no significant relationships at the FDR-corrected threshold in other regions for well-being, we did observe some effects at the uncorrected threshold (p < 0.005) in the inferior parietal gyrus, the medial prefrontal cortex (mPFC) and ventromedial prefrontal cortex (vmPFC; see Appendix 1). We also found a marginal positive correlation between the pons and DASS-42 depression/anxiety scores, whereby an increase in depression/anxiety scores was associated with an increase in pons volume (t = 3.86, k = 3471, p = 0.058). No other associations were significant at the corrected threshold for depression/anxiety scores, other than some effects for the anterior cingulate, dorsomedial prefrontal cortex and caudate at uncorrected thresholds (see Appendix 1). An exploratory whole-brain analysis revealed no further significant effects.
Table 2.
Grey-matter volume regions of interest, associations with well-being and resilience*
| Effect/region | Side | Coordinates, MNI | Cluster size, mm3 | t | pFDR | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| x | y | z | |||||
| Well-being: negative correlation/pons (brainstem) | Right | 9 | −16 | −35 | 4244 | 4.66 | 0.039† |
| Resilience: negative correlation/pons (brainstem) | Right | 6 | −13 | −33 | 5120 | 4.70 | 0.013† |
| Depression/anxiety: positive correlation/pons (brainstem) | Left | −2 | −16 | −23 | 3471 | 3.86 | 0.058 |
FDR = false discovery rate; MNI = Montreal Neurological Institute; VBM = voxel-based morphometry.
Analyses conducted in twin 1, controlling for age and sex. Verification of results in twin 2 was conducted using linear mixed models for significant extracted clusters in SPSS (p < 0.05, corrected). The well-being and depression/anxiety VBM analysis was conducted in all twin 1 participants (n = 132). For the resilience VBM analysis, associations between grey-matter volume and well-being scores were evaluated in the early-life stress group (n = 97). Trend effects for other regions of interest at uncorrected levels are provided in Appendix 1.
Result is significant at pFDR < 0.05 (cluster-level).
Fig. 1.
Voxel-based morphometry image and graphs of group means for significant grey-matter volume effects in the pons for (A) well-being and (B) resilience (levels of well-being given trauma exposure). Individuals with higher levels of well-being and resilience showed significant grey-matter reductions in the pons. Coordinates and statistics for these effects are shown in Table 2.
The grey-matter volume effects demonstrated in twin 1 identified for well-being were confirmed across both twins in SPSS using a linear mixed model of the extracted pons cluster. The effect was significant for the whole sample in terms of well-being (t = −2.581, p = 0.010).
Grey-matter volume associations with resilience
We examined associations with well-being in the trauma-exposed group to test for resilience, and we found that reductions in the same region (pons) were significantly associated with elevated well-being scores, and the size/effect of the cluster was also larger (Table 2 and Fig. 1b). Again, we found no further significant relationships for the other regions at the corrected level, but several effects were apparent at the uncorrected threshold for the hippocampus, anterior cingulate, vmPFC, insula and inferior parietal gyrus (see Appendix 1). An exploratory whole-brain analysis revealed no further significant effects.
The grey-matter volume effects demonstrated in twin 1 identified for resilience were confirmed across both twins in SPSS using a linear mixed model of the extracted pons cluster. The effect was significant in the trauma-exposed subgroup in terms of resilience (t = −3.165, p = 0.002), and not significant in the nontrauma subgroup (t = 0.102, p = 0.919). The effect remained significant in the trauma-exposed subgroup even when excluding premature birth as a traumatic event (t = −2.301, p = 0.023).
Twin modelling
We conducted a univariate heritability analysis on the extracted pons cluster region. Intra-class correlation coefficients for this cluster were 0.247 (p = 0.013) for monozygotic twin pairs and −0.029 (p = 0.574) for dizygotic twin pairs, suggesting that an ADE rather than an ACE model was most appropriate to test (because dizygotic correlation was less than half of the monozygotic correlation). Univariate modelling for the pons cluster suggested an E model was the best fit (AE v. E: −2LL243 = −222.20, AIC = −708.20, p = 0.060) with parameter estimates of A (additive genetics or “heritability”: 20% [95% confidence interval (CI) 0–0.39]) and E (unique environment: 80% [95% CI 0.61–1.0]), suggesting that at the univariate level, unique environment contributed significantly to volumetric variance of this pons cluster region. We ran similar univariate models for well-being and depression/anxiety scores, and in both cases, an AE model was the best fit: well-being (ACE v. AE: A 37% [95% CI 0.18–0.54]; E 63% [95% CI 0.46–0.82]; −2LL242 = 703.38; AIC = 219.38; p > 0.99) and depression/anxiety scores (ACE v. AE: A 30% [95% CI 0.11–0.47]; E 70% [95% CI 0.53–0.87]; −2LL242 = 251.80; AIC = −232.20; p = 0.73).
We conducted a multivariate twin model to examine whether any of the environmental variance was shared between the pons volume cluster and well-being. We also included total depression/anxiety scores in the model due to their phenotypic association with well-being and trend level associations with the pons. We tested an AE model to account for both genetic and environmental variance between pairs of variables. Initial modelling of the saturated model indicated that the means and variances between twin pairs within and between dizygotic and monozygotic twins could be equated. The correlated factors model was not significantly different from the saturated model, with means and variances equated between twin pairs and zygosity, indicating that it provided a comparable model of the data (Table 3).
Table 3.
Key model fit comparisons
| Model | −2LL | df | AIC | Δ−2LL | Δdf | p |
|---|---|---|---|---|---|---|
| Saturated with equal means and variances | 672.25 | 705 | −737.75 | — | — | — |
| Correlated factors AE model (all A and E correlations fitted) | 688.71 | 726 | −763.29 | 16.46 | 21 | 0.74 |
| Correlated factors AE model (A correlation specified between well-being and depression/anxiety, and E correlations specified between well-being and pons, and between depression/anxiety and well-being)* | 692.13 | 729 | −765.87 | 3.42 | 3 | 0.33 |
−2LL = −2 log-likelihood; A = additive genetics; AIC = Aikake information criterion; E = unique environment.
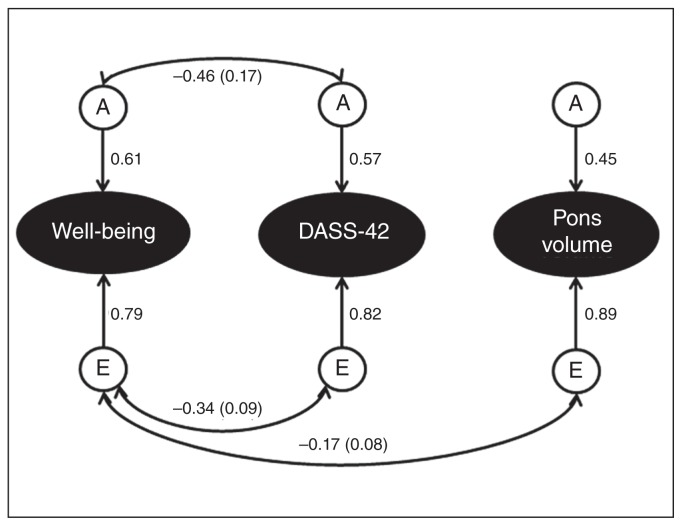
Setting the additive genetic correlation between well-being and depression/anxiety scores to zero did not result in significant deterioration of the model fit, indicating no significant genetic correlation between well-being and depression/anxiety. However, because the correlation was moderate in size (r = −0.45) and at trend-level significance (p = 0.066), we decided to keep the correlation in the model. Setting the unique environment correlation between well-being and depression/anxiety scores to zero resulted in significant deterioration in the model fit, indicating a significant correlation (r = −0.34, p = 0.0002). Setting the additive genetic correlation between well-being and the pons, and between depression/anxiety scores and the pons, to zero did not significantly reduce model fit and reduced the AIC value, suggesting these correlations were not significant. Setting the unique environment correlation between well-being and pons volume to zero led to significant reductions in model fit, indicating a significant correlation (r = −0.17, p = 0.02), while setting the unique environment correlation between depression/anxiety scores and pons volume to zero did not significantly reduce the model fit, indicating no significant correlation. The most parsimonious model for the data was therefore a correlated factors model with correlations between additive genetic factors contributing to variance in scores on well-being and depression/anxiety scores, and correlations between unique environment factors contributing to variance in well-being and depression/anxiety scores, and well-being scores and pons volume (Fig. 2).
Fig. 2.
The final correlated factors model for well-being, DASS-42 total scores and pons volume in twin pairs with corresponding standardized path estimates. We fitted an AE correlated factors model to the 3 variables across both twin pairs. The ellipses indicate the observed variables and the circles indicate unobserved latent factors (either A or E). Single-headed arrows indicate the effect of 1 latent factor on an observed variable, and double-headed arrows indicate correlations or covariances between latent factors. Results suggest significant negative correlations between A and E factors, contributing to variance in well-being and DASS-42 scores. Additive genetics contributing to DASS-42 and well-being scores did not correlate with those contributing to pons volume. There was a significant negative correlation between E factors contributing to well-being scores and pons volume. A = additive genetics; DASS-42 = Depression, Anxiety, Stress Scale; E = unique environment.
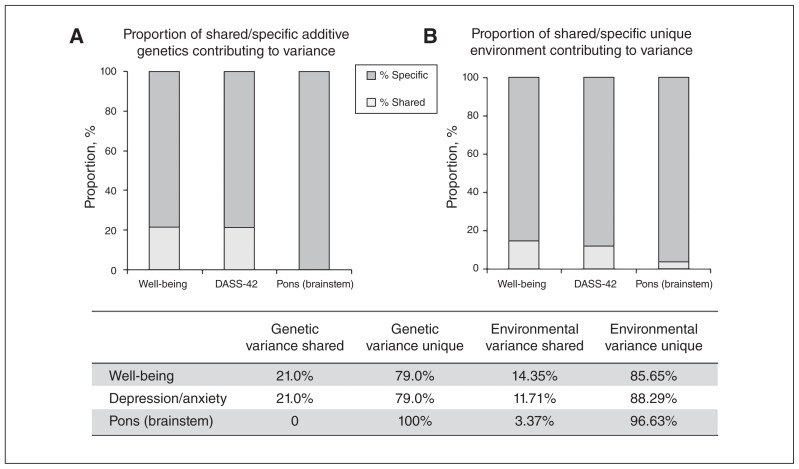
Additive genetics (heritability) contributed 37.14% to variance in well-being scores, 31.62% to variance in depression/anxiety scores and 19.93% to variance in pontine volume. The remaining variance was attributable to environmental factors unique to the individual. We found a significant negative correlation between well-being and depression/anxiety scores (phenotypic r = −0.38), with 40.68% of this covariance attributable to genetic factors and 59.32% to environmental factors. We found a small significant negative correlation between well-being and pons volume (phenotypic r = −0.12), which was wholly attributable to environmental factors. The specific proportion of genetics and environment that was shared versus unique between the variables is presented in Fig. 3.
Fig. 3.
Proportion of variance attributable to specific and shared (A) additive genetics and (B) unique environment sources for the measures of well-being, Depression, Anxiety, Stress Scale (DASS-42) score and brainstem (pons) volume.
Discussion
To our knowledge, this was the first study to establish the association between grey matter of affective brain regions that underpin reward, threat and attention/cognitive control with composite well-being and resilience in healthy adult twins. We found a significant negative correlation between our composite index of well-being (using the COMPAS-W well-being scale) and a specific reduction in the volume of the pontine nuclei located within the brainstem. Moreover, this same region was negatively correlated with resilience in the trauma-exposed subgroup, but with a larger effect (and cluster) size evident than when considering associations with levels of well-being across the whole sample. DASS-42 depression/anxiety scores also showed a positive association with the same region, albeit not at the corrected threshold level. To our knowledge, this is also the first study to examine shared genetic or environmental variance between well-being, depression/anxiety scores and brain volume using multivariate twin modelling. Twin modelling of well-being, depression/anxiety scores and pons volume suggested that unique environment contributed to the most variance for each variable, with heritability (genetic) estimates of 37%, 30% and 20%, respectively. Multivariate modelling of shared variance between the variables identified that the proportion of unique environment variance in pons volume shared with well-being was 3.4%. In contrast, the proportion of genetic variance in well-being shared with depression/anxiety was 20.4%.
Our data identified the pons as a region that is structurally associated with composite well-being and resilience; in particular, elevations in well-being and resilience were associated with reductions in this brain region. The direction of this effect with composite well-being was consistent with previous studies demonstrating negative associations between measures of subjective well-being and regions of the prefrontal cortex (ventromedial and rostrolateral), dorsal anterior cingulate, precuneus, caudate and amygdala.17–19 In the present study, we also tested these regions, and while we found a significant effect specific to the pons, we also found a converging trend effect for the mPFC with well-being at an uncorrected threshold. In contrast, studies of psychological well-being have previously reported a positive correlation with volume of the insula,20 orbitofrontal cortex, anterior cingulate and basal ganglia.21 In the present study, we found no significant positive associations between these brain regions and well-being. However, we did find trend positive associations (uncorrected) between well-being and vmPFC volume, and between resilience and volume of the hippocampus, anterior cingulate, vmPFC and insula. It is possible many of these effects did not reach significance due to variations in the size and characteristics of the present study compared to previous studies (i.e., smaller, older or less culturally diverse samples).18,20 In addition, many of the previous studies did not include the brainstem in their region-of-interest analysis, 17,19,21 so in future studies it will be important to consider all regions examined here.
Contrary to the above association studies, experimental evidence from an intervention study has shown that grey-matter changes in the brainstem (including the pons) are associated with improvements in psychological well-being.34,35 In this study, a mindfulness-based intervention was administered to 16 healthy adults over 8 weeks and compared with 17 healthy wait-list controls.34 The results suggested that treatment participants showed increases in several brain regions, including clusters in the cerebellum that extended into the brainstem and several pontine nuclei. In a later study of the same participants, the researchers showed that in a subset of the 14 healthy individuals receiving treatment, significant improvements in psychological well-being were evident over the 8-week period, and these changes were positively correlated with grey-matter volume in the brainstem, including the pontine tegmentum and its nuclei.35 Notably, the direction of this effect was positive, which contrasts with the negative correlations reported in the present study. However, the authors did say that these results were only speculative, given the extremely small sample size (14 participants). Moreover, changes were examined over an 8-week period, so whether these effects persisted beyond this period (or indeed, reversed) remains to be confirmed.
The pons locus identified in the present study is the pontine nucleus, which are individual nuclei that fill the basilar pons (the caudal part, as opposed to the dorsal pons) as apparent in Figure 1. These nuclei receive projections from the cortex and relay them to the cerebellum. The pons is involved in several core functions, including wakefulness and rapid-eye-movement (REM) sleep; blood pressure and startle response; basic emotional expression; and memory consolidation and learning.36–41 The pons contains various neurochemical neurons, including cholinergic neurons that promote wakefulness and REM sleep.36 It receives projections from the cholinergic pedunculopontine within the reticular activating system of the brainstem, which has been linked to REM sleep initiation and maintenance.36 There is increased cortical blood flow in the brainstem and limbic areas during REM sleep, which has been suggested to play a role in memory consolidation and storage during dream states.36 The pons is also involved in autonomic functions during potentially stressful states. During stress, the amygdala projects signals via the brainstem (and pons) that suppress parasympathetic cardiac control and redirect sympathetic outflow and subsequent blood pressure reactivity to ready the organism for appropriate behavioural response.37 The caudal pons in particular is the sensorimotor interface of the primary startle pathway. The protective startle response is elicited by sudden sensory stimuli via caudal pontine reticular nucleus projections to spinal, cranial and facial motor neurons, whereas activation of glutamate receptors mGluRIIIs in the pontine reticular nucleus strongly inhibits startle-mediating neurons.38 It is therefore conceivable that alterations to the pons could affect an individual’s basic arousal levels and autonomic sensory responses to potential threat, whereby individuals with higher levels of well-being and resilience would display more efficient and/or attenuated sensory responses to stress (i.e., an attenuated autonomic or startle response to potential threat). In contrast, individuals with lower levels of well-being and resilience would display less effective control and increased sympathetic responses to the same stimuli.
The pontine nuclei are also involved in basic memory function, such as procedural memory learning via relayed information to the cerebellum,39 and eyeblink conditioning — a form of classical conditioning — via direct projections from the mPFC to pontine nuclei, enabling the conditioned stimulus response by the cerebellum.40 Lesions in this area prevent the relay of conditioned stimulus information to the cerebellum, severely impairing the eyeblink learning response.41 In other studies, it has been suggested that the amygdala may facilitate the cerebellum-mediated motor memory system by modulating the emotional significance of stimuli via a sensory gating function.42 This study showed that the amygdala may gate input to the cerebellum via either direct projections to the pontine nucleus or via indirect pathways through thalamic regions (i.e., the medial geniculate body and the suprageniculate nucleus) or the periaqueductal grey, acting as an attention-like mechanism that increases input to the cerebellum for significant stimuli.42 Alterations to the pons structure found in the present study may highlight alterations to the emotional conditioning process for specific stimuli — particularly threatening stimuli — whereby individuals with reduced well-being and resilience have an increased propensity to condition emotional “significance” to irrelevant stimuli of perceived threat.
Our twin analyses suggested that the negative relationship between volume of the pons and well-being was driven by factors from the environment rather than genetics. It is quite possible that individuals with higher well-being, particularly those who are resilient, may be exposed to similar life experiences during childhood (positive and/or negative) that contribute to altered development of the pons (or vice versa: altered pons contributes to higher levels of well-being). One possible avenue is a shift of the synaptic pruning process for this region during development. Individuals with higher levels of well-being and resilience may experience a more effective pruning process of ineffective synapses for basic sensory processes and memory conditioning. Such negative associations between improved function and reduced brain volume are not unusual and are increasingly reported in healthy individuals.17–19 Increased synaptic pruning in some brain regions may in fact be quite adaptive, particularly when recovering from traumatic life experiences during childhood.
Several potential implications of these findings are worth noting. We found that individuals with higher levels of well-being and resilience showed reduced volume in the pontine nuclei, and that this association was driven by unique environment (rather than genetic) factors. The literature suggests that the pons is involved in several core sensory functions, including wakefulness, REM sleep and memory consolidation during dream states; the startle response to potential threat; and mediating emotional conditioning to specific stimuli. Therefore, one implication of these findings is the creation of avenues for new research studies. Such studies could aim to pinpoint the specific pathway of mechanism (e.g., via sleep or startle) of these effects, but also their direction (i.e., whether well-being influences pons volume or the other way around). This type of research can be conducted in human experimental laboratory studies or in longitudinal neuroimaging studies that evaluate changes in the brain to specific stimuli or simply over time. Parallel studies in animals could also be implemented to obtain a more precise localization of changes in the brainstem during development in the presence/absence of environmental stress. The outcomes of such studies would then have clinical implications in promoting well-being and resilience in the general population. They would inform the mechanism to aim for with intervention (e.g., by way of targeted psychological or pharmacological health promotion strategies) and in whom (i.e., those with lower well-being scores, or who show specific alterations to pons, or other regions). These implications are of course suggested in the absence of knowledge of how other neural networks may also be altered with well-being and resilience (e.g., white-matter tracts or functional connectivity), and therefore other possible pathways for change, which is a matter for future work.
Limitations
Several study limitations need to be noted. These results provide insight into the neural basis of well-being and resilience, but the causal directions of these relationships cannot be confirmed. It is equally possible that higher levels of well-being/resilience caused the reduction of the pons during development, or that reductions in the pons facilitated increases in well-being and resilience to trauma. Longitudinal studies evaluating changes in brain structure over time will help address these issues. Larger-sample studies should also be conducted to confirm whether effects can also be localized to other brain regions for which we found effects only at uncorrected levels. Segmentation and normalization of the brainstem grey matter can also be problematic in MRI studies,43 so the exact localization of the region identified here should be confirmed. Still, in the present study, image acquisition was tuned to cover the brainstem and pons, whereas other studies often cover this area of interest only partially, making this a notable strength of the present study.
Conclusion
The present study suggests that individuals with higher levels of well-being and resilience show reduced volume of the pons relative to individuals with lower levels of well-being and resilience, and that environmental factors contributed to these associations. Future studies could consider the functional MRI correlates for well-being and resilience to pinpoint whether these structural alterations affect connectivity to other regions, such as the amygdala and mPFC, in modulating emotional and cognitive functions.
Acknowledgements
This project was supported by an Australian Research Council (ARC) Linkage grant (LP0883621), with Brain Resource Ltd as industry partner. J. Gatt and M. Korgaonkar are supported by National Health & Medical Research Council (NHMRC) Career Development Fellowships (1062495 awarded to JMG and 1090148 to MSK). K. Routledge was supported by a NHMRC Postgraduate Public Health Scholarship (1055839). K. Grasby was supported by NHMRC grants (1103603 and 1103623). P. Schofield is supported by a NHMRC Program Grant (1037196). This research was facilitated through the Australian Twin Registry, a national research resource in part supported by a Centre of Research Excellence Grant from the National Health & Medical Research Council (NHMRC ID 1079102). S. Grieve acknowledges the support of the Parker Hughes bequest and the Heart Research Institute. The authors gratefully acknowledge Sicong Tu for helping create the MRI images in Figure 1, and George Paxinos for advice on localization of the brain region.
Footnotes
Competing interests: J. Gatt has previously received consultancy fees from Brain Resource Ltd unrelated to this study and is a stockholder in MAP Corp. Pte Ltd. A. Harris has consulted for Lundbeck Australia and received fees for lectures from Janssen-Cilag and Lundbeck Australia. He has been an investigator on industry-sponsored trials by Janssen-Cilag and Brain Resource Ltd. C.R. Clark holds a small quantum of stock and has received fees from Brain Resource Ltd for consultancies unrelated to this study. L. Williams has received fees for consultancies unrelated to this study and previously held stock options from Brain Resource Ltd. No other authors declare competing interests.
Contributors: J. Gatt., P. Schofield, C.R. Clark and L. Williams designed the study. J. Gatt, K. Burton, P. Schofield, C.R. Clark and L. Williams acquired the data, which J. Gatt, K. Burton, K. Routledge, K. Grasby analyzed. J. Gatt wrote the article, which all authors reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
References
- 1.Routledge KM, Burton KLO, Williams LM, et al. Shared versus distinct genetic contributions of mental wellbeing with depression and anxiety symptoms in healthy twins. Psychiatry Res. 2016;244:65–70. doi: 10.1016/j.psychres.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 2.Kendler KS, Myers JM, Keyes CL. The relationship between the genetic and environmental influences on common externalising psychopathology and mental wellbeing. Twin Res Hum Genet. 2011;14:516–23. doi: 10.1375/twin.14.6.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kendler KS, Myers JM, Maes HH, et al. The relationship between the genetic and environmental influences on common internalizing psychiatric disorders and mental well-being. Behav Genet. 2011;41:641–50. doi: 10.1007/s10519-011-9466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keyes CL. Mental illness and/or mental health? Investigating axioms of the complete state model of health. J Consult Clin Psychol. 2005;73:539–48. doi: 10.1037/0022-006X.73.3.539. [DOI] [PubMed] [Google Scholar]
- 5.Gatt JM, Burton KLO, Schofield PR, et al. The heritability of mental health and wellbeing defined using COMPAS-W, a new composite measure of wellbeing. Psychiatry Res. 2014;219:204–13. doi: 10.1016/j.psychres.2014.04.033. [DOI] [PubMed] [Google Scholar]
- 6.Alexander R, Gatt JM. Resilience. In: Miu AC, Homberg JR, Lesch K-P, editors. Genes, brain and emotions: from resilience to psychopathology. Oxford: Oxford University Press; in press. [Google Scholar]
- 7.Feder A, Nestler EJ, Charney DS. Psychobiology and molecular genetics of resilience. Nat Rev Neurosci. 2009;10:446–57. doi: 10.1038/nrn2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berridge KC, Kringelbach ML. Building a neuroscience of pleasure and well-being. Psychol Well Being. 2011;1:1–26. doi: 10.1186/2211-1522-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams LM. Precision psychiatry: a neural circuit taxonomy for depression and anxiety. Lancet Psychiatry. 2016;3:472–80. doi: 10.1016/S2215-0366(15)00579-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bryant RA, Kemp AH, Felmingham KL, et al. Enhanced amygdala and medial prefrontal activation during nonconscious processing of fear in posttraumatic stress disorder: an fMRI study. Hum Brain Mapp. 2008;29:517–23. doi: 10.1002/hbm.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris JS, Frith CD, Perrett DI, et al. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383:812–5. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- 12.Williams LM, Palmer D, Liddell BJ, et al. The “when” and “where” of perceiving signals of threat versus non-threat. Neuroimage. 2006;31:458–67. doi: 10.1016/j.neuroimage.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Williams LM, Phillips ML, Brammer MJ, et al. Arousal dissociates amygdala and hippocampal fear responses: evidence from simultaneous fMRI and skin conductance recording. Neuroimage. 2001;14:1070–9. doi: 10.1006/nimg.2001.0904. [DOI] [PubMed] [Google Scholar]
- 14.Chan SW, Harmer CJ, Norbury R, et al. Hippocampal volume in vulnerability and resilience to depression. J Affect Disord. 2016;189:199–202. doi: 10.1016/j.jad.2015.09.021. [DOI] [PubMed] [Google Scholar]
- 15.Yehuda R, Golier JA, Tischler L, et al. Hippocampal volume in aging combat veterans with and without post-traumatic stress disorder: relation to risk and resilience factors. J Psychiatr Res. 2007;41:435–45. doi: 10.1016/j.jpsychires.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Lovibond SH, Lovibond PF. Manual for the Depression Anxiety Stress Scales. Sydney: Psychology Foundation; 1995. [Google Scholar]
- 17.Kong F, Ding K, Yang Z, et al. Examining gray matter structures associated with individual differences in global life satisfaction in a large sample of young adults. Soc Cogn Affect Neurosci. 2015;10:952–60. doi: 10.1093/scan/nsu144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takeuchi H, Taki Y, Nouchi R, et al. Anatomical correlates of quality of life: evidence from voxel-based morphometry. Hum Brain Mapp. 2014;35:1834–46. doi: 10.1002/hbm.22294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dennison M, Whittle S, Yucel M, et al. Trait positive affect is associated with hippocampal volume and change in caudate volume across adolescence. Cogn Affect Behav Neurosci. 2015;15:80–94. doi: 10.3758/s13415-014-0319-2. [DOI] [PubMed] [Google Scholar]
- 20.Lewis GJ, Kanai R, Bates TC. Neural correlates of the ‘good life’: eudaimonic well-being is associated with insular cortex volume. Soc Cogn Affect Neurosci. 2014;9:615–8. doi: 10.1093/scan/nst032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dolcos S, Hu Y, Iordan AD, et al. Optimism and the brain: trait optimism mediates the protective role of the orbitofrontal cortex gray matter volume against anxiety. Soc Cogn Affect Neurosci. 2016;11:263–71. doi: 10.1093/scan/nsv106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keyes CLM, Shmotkin D, Ryff CD. Optimizing well-being: the empirical encounter of two traditions. J Pers Soc Psychol. 2002;82:1007–22. [PubMed] [Google Scholar]
- 23.Waterman AS. Two conceptions of happiness: contrasts of personal expressiveness (eudaimonia) and hedonic enjoyment. J Pers Soc Psychol. 1993;64:678–91. [Google Scholar]
- 24.Gatt JM, Korgaonkar MS, Schofield PR, et al. The TWIN-E project in emotional wellbeing: study protocol and preliminary heritability results across four MRI and DTI measures. Twin Res Hum Genet. 2012;15:419–41. doi: 10.1017/thg.2012.12. [DOI] [PubMed] [Google Scholar]
- 25.Williams LM, Simms E, Clark CR, et al. The test-retest reliability of a standardized neurocognitive and neurophysiological test battery: neuromarker. Int J Neurosci. 2005;115:1605–30. doi: 10.1080/00207450590958475. [DOI] [PubMed] [Google Scholar]
- 26.Paul RH, Gunstad J, Cooper N, et al. Cross-cultural assessment of neuropsychological performance and electrical brain function measures: additional validation of an international brain database. Int J Neurosci. 2007;117:549–68. doi: 10.1080/00207450600773665. [DOI] [PubMed] [Google Scholar]
- 27.Paul RH, Lawrence J, Williams LM, et al. Preliminary validity of “integneuro”: a new computerized battery of neurocognitive tests. Int J Neurosci. 2005;115:1549–67. doi: 10.1080/00207450590957890. [DOI] [PubMed] [Google Scholar]
- 28.Silverstein SM, Berten S, Olson P, et al. Development and validation of a world-wide-web-based neurocognitive assessment battery: WebNeuro. Behav Res Methods. 2007;39:940–9. doi: 10.3758/bf03192989. [DOI] [PubMed] [Google Scholar]
- 29.Sanders B, Becker-Lausen E. The measurement of psychological maltreatment: early data on the Child Abuse and Trauma Scale. Child Abuse Negl. 1995;19:315–23. doi: 10.1016/s0145-2134(94)00131-6. [DOI] [PubMed] [Google Scholar]
- 30.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–31. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 31.Neale MC, Boker SM, Xie G, et al. Mx: statistical modeling. Richmond (VA): Virginia Commonwealth University, Department of Psychiatry, Viginia Institute for Psychiatric and Behavioral Genetics; 2003. [Google Scholar]
- 32.R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. [Google Scholar]
- 33.Neale MC, Maes HHM. Methodology for genetic studies of twins and families. Netherlands: Kluwer Academic Publishers B.V.; 1998. [Google Scholar]
- 34.Hölzel BK, Carmody J, Vangel M, et al. Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Res Neuroimaging. 2011;191:36–43. doi: 10.1016/j.pscychresns.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singleton O, Hölzel BK, Vangel M, et al. Change in brainstem gray matter concentration following a mindfulness-based intervention is correlated with improvement in psychological well-being. Front Hum Neurosci. 2014;8:33. doi: 10.3389/fnhum.2014.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garcia-Rill E, Charlesworth A, Heister D, et al. The developmental decrease in REM sleep: the role of transmitters and electrical coupling. Sleep. 2008;31:673–90. doi: 10.1093/sleep/31.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gianaros PJ, Sheu LK, Matthews KA, et al. Individual differences in stressor-evoked blood pressure reactivity vary with activation, volume, and functional connectivity of the amygdala. J Neurosci. 2008;28:990–9. doi: 10.1523/JNEUROSCI.3606-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmid S, Brown T, Simons-Weidenmaier N, et al. Group III metabotropic glutamate receptors inhibit startle-mediating giant neurons in the caudal pontine reticular nucleus but do not mediate synaptic depression/short-term habituation of startle. J Neurosci. 2010;30:10422–30. doi: 10.1523/JNEUROSCI.0024-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirai Y, Morishima M, Karube F, et al. Specialized cortical subnetworks differentially connect frontal cortex to parahippocampal areas. J Neurosci. 2012;32:1898–913. doi: 10.1523/JNEUROSCI.2810-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siegel JJ, Kalmbach B, Chitwood RA, et al. Persistent activity in a cortical-to-subcortical circuit: bridging the temporal gap in trace eyelid conditioning. J Neurophysiol. 2012;107:50–64. doi: 10.1152/jn.00689.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Campolattaro MM, Kashef A, Lee I, et al. Neuronal correlates of cross-modal transfer in the cerebellum and pontine nuclei. J Neurosci. 2011;31:4051–62. doi: 10.1523/JNEUROSCI.4142-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farley SJ, Radley JJ, Freeman JH. Amygdala modulation of cerebellar learning. J Neurosci. 2016;36:2190–201. doi: 10.1523/JNEUROSCI.3361-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beissner F, Deichmann R, Baudrexel S. fMRI of the brainstem using dual-echo EPI. Neuroimage. 2011;55:1593–9. doi: 10.1016/j.neuroimage.2011.01.042. [DOI] [PubMed] [Google Scholar]