Abstract
Background
Brain connectome research based on graph theoretical analysis shows that small-world topological properties play an important role in the structural and functional alterations observed in patients with psychiatric disorders. However, the reported global topological alterations in small-world properties are controversial, are not consistently conceptualized according to agreed-upon criteria, and are not critically examined for consistent alterations in patients with each major psychiatric disorder.
Methods
Based on a comprehensive PubMed search, we systematically reviewed studies using noninvasive neuroimaging data and graph theoretical approaches for 6 major psychiatric disorders: schizophrenia, major depressive disorder (MDD), attention-deficit/hyperactivity disorder (ADHD), bipolar disorder (BD), obsessive–compulsive disorder (OCD) and posttraumatic stress disorder (PTSD). Here, we describe the main patterns of altered small-world properties and then systematically review the evidence for these alterations in the structural and functional connectome in patients with these disorders.
Results
We selected 40 studies of schizophrenia, 33 studies of MDD, 5 studies of ADHD, 5 studies of BD, 7 studies of OCD and 5 studies of PTSD. The following 4 patterns of altered small-world properties are defined from the perspectives of segregation and integration: “regularization,” “randomization,” “stronger small-worldization” and “weaker small-worldization.” Although more differences than similarities are noted in patients with these disorders, a prominent trend is the structural regularization versus functional randomization in patients with schizophrenia.
Limitations
Differences in demographic and clinical characteristics, pre-processing steps and analytical methods can produce contradictory results, increasing the difficulty of integrating results across different studies.
Conclusion
Four psychoradiological patterns of altered small-world properties are proposed. The analysis of altered small-world properties may provide novel insights into the pathophysiological mechanisms underlying psychiatric disorders from a connectomic perspective. In future connectome studies, the global network measures of both segregation and integration should be calculated to fully evaluate altered small-world properties in patients with a particular disease.
Introduction
Recent advances in neuroscience methodology have under-pinned a large increase in human brain research studies. In particular, by taking advantage of noninvasive imaging approaches, the developing field of psychoradiology (https://radiopaedia.org/articles/psychoradiology) promises not only insights into abnormal brain function and underlying neuropsychopathologies, but also potential clinical utility in patients with mental illness, from diagnosis to planning and monitoring therapeutic interventions.1
Psychoradiological studies have revealed abnormal brain networks in patients with several major psychiatric disorders. 2–5 However, reported neuroimaging studies to date have sometimes neglected the interactions among multiple brain regions. Widespread and subtle pathology across the brain may be only imperfectly detected using a traditional regional or voxel-based analysis.6 In contrast, a large-scale network analysis enables the assessment of overall connectivity patterns among all regions of the brain. This approach has been stimulated by pioneering studies modelling the brain as a complex network in the so-called connectome.7 In these terms, the human brain network exhibits topological properties known as “small-worldness,” an optimal balance between the segregation and integration of information-processing procedures.8 We suggest that a clear definition of the main patterns of altered small-world properties might yield new insights into how these processes are perturbed in patients with pathological brain conditions, with potential benefits to diagnosis and therapy.
Graph theory provides a rigorous mathematical framework to analyze the topology of complex networks.9 Graph theoretical approaches have been widely applied to connectome studies to investigate the topological organization of large-scale functional and structural brain networks in healthy and disease states.10–13 Brain disorders clearly cause significant alterations in global network topology;14 however, connectome studies have often reported inconsistent quantitative accounts of these. Occasionally, similar alterations have been described in terms of different patterns of change. For example, 2 functional connectome studies in patients with schizophrenia reported a decreased clustering coefficient (Cp) and an increased path length (Lp) compared with healthy controls.15,16 However, 1 study interpreted this result as a shift toward more random organization,15 whereas the other study described the result as “weaker small-worldization” (i.e., a shift toward less marked small-world properties).16 Conversely, different topological alterations may be interpreted as showing the same pattern. For example, 2 functional studies of patients with schizophrenia reported a shift toward a more random organization;15,17 however, 1 study observed an increased Lp and decreased Cp,15 whereas the other study noted a decreased Lp (marginal group difference) and decreased Cp.17 Definitions of unified criteria for characterizing small-world properties in terms of global topological parameters are urgently needed.
Furthermore, functional and structural networks may show discrepancies in alterations in small-world properties in patients with the same disorder. For example, in patients with schizophrenia, increased Cp and Lp have been reported in the structural connectome,18 but decreased Cp and Lp in the resting-state functional connectome have also been reported. 15 Meanwhile, other studies on the structural and functional connectome have reported consistent alterations in small-world properties (e.g., decreased global efficiency and a maintained local efficiency)19,20 and increased Cp and Lp.18,21 Further studies are required to explore whether there are potential common trends in functional and structural networks.
Several reviews have recently summarized findings for both the functional and structural connectomes in patients with psychiatric disorders.2,22–25 However, to date, no study has reviewed the clinically altered small-world properties of the human brain connectome from the perspectives of information segregation and integration. In this review, we explore the main patterns of altered small-world properties using a graph theoretical analysis, and then summarize recent findings from studies of patients with several major psychiatric disorders.
First, we introduce the construction of the brain connectome, connectome measures, and network types (for additional details, please refer to the study by Liao and colleagues25).
Rationale for brain connectome construction
In graph theory, a brain network is defined as a graph G (N, K), with N and K representing the number of nodes and edges, respectively. Modern brain mapping techniques include structural magnetic resonance imaging (sMRI), functional MRI (fMRI), electroencephalography (EEG) and magnetic encephalography (MEG); in order to reduce the confounding effects of heterogeneous image acquisition techniques, we did not consider the latter 2 techniques.
Brain nodes are often defined based on anatomic landmarks, functional activation26–28 and regional connectivity profiles.29 Anatomic landmarks include the automated anatomic labelling (AAL) atlas, Harvard–Oxford atlas and Desikan– Killiany atlas; the AAL atlas has been frequently used,30 but different investigators have used different numbers of regions. Some researchers have used software tools, such as FreeSurfer, to parcellate the cortex into a given number of nodes. Most templates have constructed brain networks with fewer than 1000 nodes.31 Independent component analysis (ICA) is the most popular of the many data-driven analyses for delineating resting-state functional brain networks. The number of components identified using the ICA approach is typically less than 100 for a whole-brain analysis. 26 There is no gold standard for node parcellation. Researchers typically choose network nodes based on anatomic templates for convenience. The use of an anatomic atlas is known to introduce potential bias because of its inhomogeneity, and a reasonable approach in most neuroimaging studies is to use a single atlas for analysis.32 A recent functional study investigating the impact of parcellation choice on group differences found that patient-specific global network properties are robustly observed using different parcellation schemes, whereas graph metrics characterizing impairments of individual nodes vary considerably.33 Overall, the correct definition of nodes in connectome studies is still an open question that needs further technical investigation.
Once the nodes have been determined, the edges are defined. The edges of the brain functional connectome are constructed by calculating the Pearson, partial, or wavelet correlation for every possible pair of regional time series among the nodes. The Pearson correlation measures the interdependence between 2 time series. However, correlation may result from indirect associations with common sources. To minimize this problem, in the partial correlation approach the brain activity in one region is correlated with that in another region after the activity in all other regions is regressed out; this attempts to remove the effects of indirect paths34 and provides a better estimate of the true macroscopic functional connectome.35 However, this strategy has the opposite problem of losing real connections, which is likely to underestimate topological measures.36 The best recent evidence is that Pearson correlation is more valid and reliable.37
After construction, the edges of the brain structural connectome can be measured in 2 ways. The first, using structural data directly, is by computing the chosen (partial or Pearson) correlation coefficients of the morphological features of the brain (cortical thickness or grey-matter volume) among nodes. The second approach is by reconstructing diffusion MRI-traced white matter using either deterministic or probabilistic tractography, and the latter is more advanced. The tracking procedure used in deterministic tractography terminates when it reaches regions with fibre crossings,38 and this tends to reduce sensitivity. Probabilistic tractography overcomes this shortcoming, and offers a natural approach to modelling uncertainty by generating multiple curves originating from a seed point,39 providing a more comprehensive picture of connectivity. The probabilistic algorithm has better test–retest reliability and is more robust than deterministic tractography in areas of high uncertainty (notably fibre crossings).40,41 However, the lower computational cost of deterministic tractography makes it more often used in clinical applications. High-field imaging and the application of advanced diffusion reconstruction algorithms offer the potential to characterize more complex fibre patterns when edges are defined.
Once the nodes and edges are defined, a matrix representation is generated where each entry records the edge weight of corresponding node pairs denoted by the row and column index of the matrix. The reconstructed network is classified as the directed or undirected type, depending on whether the edges display directionality. Depending on whether the edges are assigned with different strengths, a binary network is obtained by thresholding the weights of the edges, where the edges are either 1 (connected) or 0 (unconnected); otherwise, the network is a weighted type.
Brain connectome measures
Once the brain connectome is constructed, the connectome measurement is calculated. In graph theory, a complex network is characterized by 2 types of measures, integration and segregation, which represent crucial information processing patterns of the brain and ensure efficient global communication and functional specialization.42
Specifically, topological integration refers to the efficiency of global information communication or the ability to integrate distributed information in the network, which is measured by the parameters Lp, λ and Eglob. The characteristic path length, Lp, is calculated by averaging the minimum number of connections that link any paired nodes in the network.43 The normalized characteristic path length, λ, is the normalized Lp, which is calculated for the mean Lp of 100 matched random networks that preserve the same number of nodes and edges as the real network. The global efficiency, Eglob, measures how efficiently information is exchanged at the global level.44
In contrast, segregation refers to the ability of densely interconnected groups of brain regions to perform specialized processing procedures and is measured by the parameters Cp, γ and Eloc. The network clustering coefficient, Cp, is calculated by averaging the Cp over all nodes in the network: Cp is equivalent to the fraction of the node’s neighbours that are also neighbours of each other.43 The normalized clustering coefficient, γ, is the normalized Cp, which is calculated for the mean Cp of 100 matched random networks that preserve the same number of nodes and edges as the real network. Local efficiency, Eloc, measures how efficiently information is exchanged at the local level.44 The level of clustering measured by Eloc expresses the level of local connectedness of a network, with high levels of clustering interpreted as high levels of local organization of the network.22
The small-world index, σ, is γ ÷ λ, which is > 1 when it fulfills the conditions of γ > 1 and λ ≈ 1.43 Graph measures are discussed in more detail elsewhere.42
Brain network types
Based on the perspectives of segregation and integration, networks are divided into 3 main types: regular, random and small-world networks. A regular network is characterized by a high Cp (the probability that neighbouring nodes are also interconnected with other neighbouring nodes) and a long Lp (the average distance from one node to any other node in the network, expressed as the number of links that must be travelled). A random network is characterized by a low Cp and a short Lp. A small-world network has a higher Cp but a similar Lp to a random network, has both high global and local information transformation capacities, and maintains an optimized balance between segregation and integration. Quantitatively, the small-world index, σ, should be > 1 for small-world networks, as measured by both γ > 1 and λ ≈ 1.
Although connectome studies have confirmed that the brain networks of patients and healthy controls all exhibit small-world topology (σ > 1, with γ > 1 and λ ≈1), significant group differences in global network measures have been reported, implying different types of alterations in small-world properties in patients with different disorders. However, to date, the conceptualization of these altered small-world properties has varied, with no agreed-upon definitive criteria.
Aims of this review
This review is designed to address the following questions. Can unified criteria be defined for the altered small-world properties? What are the consistently altered small-world properties in patients with specific psychiatric disorders? Are common alterations observed between functional and structural networks? Specifically, we first propose 4 defined patterns of altered small-world properties and then systematically review published connectome studies to identify the most consistently altered small-world properties in patients with 1 of 6 specific psychiatric disorders: schizophrenia, major depressive disorder (MDD), attention-deficit/hyperactivity disorder (ADHD), bipolar disorder (BD), obsessive–compulsive disorder (OCD) and posttraumatic stress disorder (PTSD). We treated structural and functional studies separately to assess their consistency and evaluate any discrepancies.
Methods
Study selection
We searched PubMed using the following search terms: [(schizophrenia) or (depression) or (attention-deficit/hyperactivity disorder or ADHD) or (bipolar disorder or BD) or (obsessive compulsive disorder or OCD) or (post-traumatic stress disorder or PTSD)] and (graph analysis or graph theory or small world or connectome). We screened 943 studies published before Dec. 1, 2016. We excluded studies that did not examine global properties (Cp, γ, Eloc, Lp, λ, Eglob and σ), studies that did not compare patients with one of the aforementioned psychiatric disorders with healthy controls and studies using EEG or MEG.
In addition, we manually cross-referenced the studies with the bibliographies of recent reviews in the field to ensure that no studies of significance were omitted from the review,2,23,45 after which 1 additional paper was included.46
Patterns of altered small-world properties
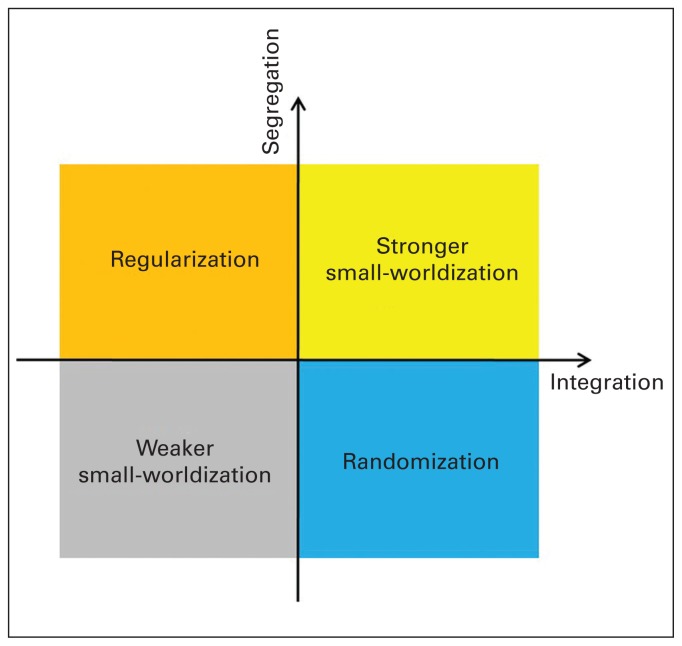
Based on the perspectives of segregation (Cp, γ and Eloc) and integration (Lp, λ and Eglob), we propose 4 patterns of altered small-world properties: “regularization,” in which the network transforms from a small-world network to a relatively regular network; “randomization,” in which the network transforms from a small-world network to a relatively random network; “stronger small-worldization,” in which the network transforms from a small-world network to a relatively stronger small-world network; and “weaker small-worldization,” in which the network transforms from a small-world network to a relatively weaker marked small-world network (Table 1 and Fig. 1).
Table 1.
Four patterns of alterations in small-world properties from the perspectives of segregation and integration
| Segregation | Integration | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Pattern | Cp | γ | Eloc | Lp | λ | Eglob | |
| Randomization | ↓ | ↓ | ↓ | and/or | ↓ | ↓ | ↑ |
| Regularization | ↑ | ↑ | ↑ | and/or | ↑ | ↑ | ↓ |
| Stronger small-worldization | ↑ | ↑ | ↑ | and | ↓ | ↓ | ↑ |
| Weaker small-worldization | ↓ | ↓ | ↓ | and | ↑ | ↑ | ↓ |
γ = normalized clustering coefficient; λ = normalized characteristic path length; Cp = clustering coefficient; Eglob = global efficiency; E = local efficiency; Lp = characteristic path length.
Fig. 1.
Representation of the 4 patterns of altered small-world properties in the brain connectome — regularization, randomization, stronger small-worldization and weaker small-worldization — based on the perspectives of segregation and integration. Regularization is defined as increased segregation and/or decreased integration. Randomization is defined as decreased segregation and/or increased integration. Stronger small-worldization is defined as increased segregation and increased integration. Weaker small-worldization is defined as decreased segregation and decreased integration. Integration
Regularization was defined where at least 1 altered measurement of segregation (increased Cp, γ or Eloc) and/or at least 1 altered measurement of integration (decreased Eglob or increased Lp or λ) were present. Randomization was defined where at least 1 altered measurement of segregation (decreased Cp, γ or Eloc) and/or at least 1 altered measurement of integration (increased Eglob or decreased Lp or λ) were present. Stronger small-worldization was defined where at least 1 altered measurement of segregation (increased Cp, γ or Eloc) and at least 1 altered measurement of integration (increased Eglob or decreased Lp or λ) were present. Weaker small-worldization was defined where at least 1 altered measurement of segregation (decreased Cp, γ or Eloc) and at least 1 altered measurement of integration (decreased Eglob or increased Lp or λ) were present.
Results
Methodological review of connectome studies
We included 95 papers reporting global properties: 40 studies of schizophrenia, 33 studies of MDD, 5 studies of ADHD, 5 studies of BD, 7 studies of OCD and 5 studies of PTSD. More details are presented in Appendix 1, Tables S1–S3, available at jpn.ca/170214-a1). Of the studies we included, 53 used AAL, 7 used the Desikan–Killiany atlas, 6 used the Harvard– Oxford atlas, 1 used the Destrieux atlas, 1 used a spatially unbiased infratentorial template, 1 used a voxelwise approach and 11 used FreeSurfer to parcellate the cortex into diverse numbers of nodes. The brain nodes in 14 papers were defined based on functional activation; 11 of these studies involved an ICA. The brain nodes in 1 paper were defined based on the regional connectivity profiles.
Twenty-nine, 11 and 6 papers used the Pearson, partial, and wavelet correlations of the regional time series, respectively, to construct the edges of the functional connectome. One paper estimated functional connectivity in the brain using the higher-order statistical dependence. One paper used the IMaGES algorithm to detect functional connections. In 1 paper, functional networks were built using the correlations among ICA time courses of brain nodes. For the edges of structural connectome of morphological features in the brain, 3 and 2 papers, respectively, computed the Pearson and partial correlations of cortical thickness; 3 papers calculated the Pearson correlations of grey-matter volume. For the structural connectome reconstructing diffusion MRI data, 30 and 7 papers, respectively, performed deterministic and probabilistic tractography. One paper used the generalized q-sampling imaging (GQI) to construct the edges.47
Global topological alterations in patients with schizophrenia
Forty-nine data sets from 22 functional and 18 structural connectome studies were included, of which 26 used fMRI, 20 used diffusion MRI and 1 used sMRI (Fig. 2 and Appendix 1, Table S1). Of the functional studies, 2 recruited subgroups of patients: 1 recruited patients with familial and sporadic schizophrenia and analyzed the data with and without global signal regression (GSR),48 and the other recruited patients with and without auditory verbal hallucinations (AVH).16 One study examined global properties during a task and at rest.15 Another study investigated the networks of the left and right hemispheres.49 One study explored both the structural and functional connectomes.16
Fig. 2.
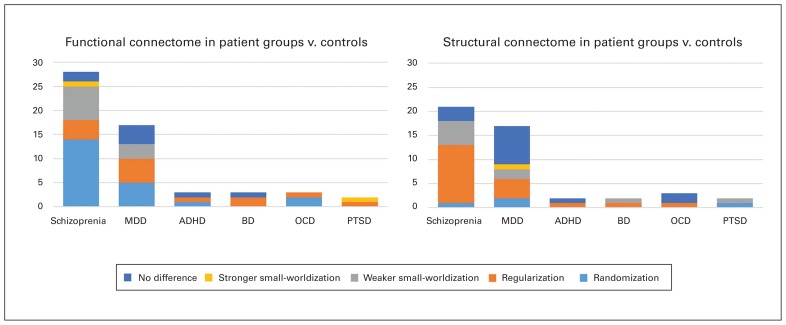
Reports describing either the randomization, regularization, weaker small-worldization, stronger small-worldization or no difference in functional and structural brain connectomes between patient groups and healthy controls. ADHD = attention-deficit/hyperactivity disorder; BD = bipolar disorder; MDD = major depressive disorder; OCD = obsessive–compulsive disorder; PTSD = posttraumatic stress disorder.
Functional connectome studies
Fourteen data sets from 12 studies showed a shift toward randomization,15,17,48–57 including 7 reports of decreased segregation, and 7 reports of decreased segregation and increased integration. Specifically, in the study by Ma and colleagues,15 patients with schizophrenia showed decreased segregation (decreased Cp) and increased integration (decreased Lp) at rest. Yu and colleagues49 reported decreased segregation (decreased γ) in the task-related network in the right hemisphere. Zhu and colleagues48 observed decreased segregation (decreased γ and Eloc) with GSR and decreased segregation (decreased γ and Eloc) and increased integration (decreased λ) without GSR in patients with familial schizophrenia as well as decreased segregation (decreased Eloc) in patients with sporadic schizophrenia without GSR.
Seven data sets from 6 studies showed a shift toward weaker small-worldization with decreased segregation and integration.15,16,49,58–60 Specifically, patients with schizophrenia showed decreased segregation (decreased Cp) and decreased integration (increased Lp) during the auditory oddball (AOD) task15 and decreased segregation (decreased γ) and decreased integration (decreased Eglob and increased Lp) in the task-related network in the left hemisphere.49 Patients with schizophrenia with and without AVH showed decreased segregation (decreased Cp and Eloc) and decreased integration (decreased Eglob and increased Lp).16
Four studies showed a shift toward regularization,20,21,61,62 including 3 reports of both increased segregation and decreased integration, and 1 report of decreased integration. One study showed a shift toward stronger small-worldization, with increased segregation (increased Cp) and increased integration (increased Eglob).63 Two studies, however, did not report any significant difference in global properties. 48,64
Structural connectome studies
Twelve studies showed a shift toward regularization,16,18,19,65–73 including 11 reports of decreased integration and 1 report of both decreased integration and increased segregation. Five studies showed a shift toward weaker small-worldization, with decreased segregation and integration.71,74–77 Specifically, of the 2 data sets in the study by van den Heuvel and colleagues, 71 1 data set showed a shift toward regularization with decreased integration (decreased Eglob) and the other independent data set showed a shift toward weaker small-worldization with decreased segregation (decreased Cp) and decreased integration (decreased Eglob) in patients with schizophrenia. One study showed a shift toward randomization with increased integration. 78 Three studies, however, did not report any significant difference in global properties.16,79,80
Summary of altered small-world properties
Our review included 818 patients with schizophrenia and 791 healthy controls with 28 data sets from 22 functional studies, as well as 945 patients with schizophrenia and 912 healthy controls with 21 data sets from 19 structural studies. In sMRI studies, the largest proportion (12 of 21 data sets) of altered small-world properties included regularization in 675 of 945 patients, but in fMRI studies, randomization was observed more frequently (14 of 28 data sets, 390 of 818 patients) (Fig. 2). Most patients with schizophrenia were currently taking medications; only 3 data sets included medication- naive and medication-washout patients, and in 5 data sets medication status was not available. For the data sets including currently medicated patients with schizophrenia, the most prominent trend was still structural regularization (10 of 16 data sets) rather than functional randomization (12 of 25 data sets).
Global topological alterations in patients with MDD
Thirty-five data sets from 16 functional and 17 structural connectome studies were included, of which 17 used fMRI, 12 used DTI and 5 used sMRI (Fig. 2 and Appendix 1, Table S2). One of the fMRI studies recruited patients with late-life depression (LLD) with and without comorbid amnestic mild cognitive impairment (aMCI).81 One functional study analyzed the data with and without GSR.82
Functional connectome studies
Five data sets from 4 studies showed a shift toward randomization, including 2 reports of increased integration,13,83 and 3 reports of decreased segregation.81,82 Specifically, Borchardt and colleagues82 observed decreased segregation (decreased Cp) in patients with MDD with and without GSR. One data set from the study by Li and colleagues81 showed decreased segregation (decreased Eloc) in patients with LLD. Five studies showed a shift toward regularization, including 3 reports of decreased integration characterized by increased Lp 84,85 or decreased Eglob and increased Lp,86 1 report of increased segregation (increased Eloc),87 and 1 report of increased segregation (increased Eloc) and decreased integration (decreased Eglob).88
Three studies showed a shift toward weaker small-worldization with decreased segregation and integration, including decreased Cp with decreased Eglob and increased Lp,89 and decreased Eloc with decreased Eglob and increased Lp.81,90 Four studies, however, reported no significant differences. 46,91–93 One study showed a decreased σ.94
Structural connectome studies
Four studies showed a shift toward regularization, including 1 report of increased segregation (higher transitivity used as an alternative to Cp),95 1 report of decreased integration ( decreased Eglob and increased Lp),96 2 reports of both increased segregation and decreased integration characterized by an increased γ with a decreased Eglob and increased λ,97 and increased Cp and Eloc with increased Lp.47
Two studies showed a shift toward randomization with decreased segregation characterized by a decreased γ and Eloc,98 as well as a decreased Cp.99 Two studies showed a shift toward weaker small-worldization with decreased segregation and decreased integration: 1 study showed a decreased γ and increased λ,100 and the other study showed a decreased Cp and Eloc along with a decreased Eglob.101 One study showed a shift toward stronger small-worldization with increased segregation (increased Eloc) and increased integration (increased Eglob and decreased Lp and λ).102 Eight studies did not report significant differences.103–110
Summary of altered small-world properties
Global topology was not significantly altered in 12 reports. Nine studies showed regularization, 7 showed randomization, 5 showed weaker small-worldization, and 1 showed stronger small-worldization. In 1 study, the global network alterations, which depend on a single measurement, σ, were difficult to classify. In 17 data sets patients with MDD were currently taking medicine, 15 data sets included medication-naive and medication-washout patients, and medication status was not available in 3 data sets. No prominent trends in altered small-world properties were observed either in the medicated patients or in the medication-free patients.
Global topological alterations in patients with ADHD
Only 5 studies, 3 using fMRI and 2 using DTI, were included (Fig. 2 and Appendix 1, Table S3). Of the fMRI studies, 1 study showed a shift toward regularization by increased segregation (increased Eloc) in patients with ADHD.111 One additional fMRI study reported decreased segregation (decreased Eloc) in the visual attention network of children with ADHD during a task, suggesting a shift toward randomization. 112 Another fMRI study reported no significant between-group differences in Cp, γ, Lp, or λ.113
The white-matter networks in boys with ADHD showed decreased integration (decreased Eglob and increased Lp and λ),114 showing a shift toward regularization. A white-matter study in adults with ADHD reported no significant between-group differences in Cp, γ, λ, or Eglob.115
Global topological alterations in patients with BD
Only 5 studies, 3 using fMRI and 2 DTI, were included (Fig. 2 and Appendix 1, Table S3). In the fMRI studies, patients with BD type I showed decreased integration ( increased Lp) during a task, indicating a shift toward regularization. 84 Two resting-state fMRI studies included patients with BD types I and II; 1 study suggested a shift toward regularization characterized by increased segregation (increased Cp) and decreased integration (decreased Eglob)116 and the other showed a lack of significant between-group differences in Cp, Lp, Eloc or Eglob.92
Two white-matter network studies included patients with BD type I; 1 study showed a shift toward regularization characterized by decreased integration (decreased Eglob)117 and the other study suggested a shift toward weaker small-worldization characterized by decreased segregation ( decreased Cp) and decreased integration (decreased Eglob and increased Lp).118
Global topological alterations in patients with OCD
Seven studies, 4 fMRI and 3 sMRI, were included (Fig. 2 and Appendix 1, Table S3). Of the 4 fMRI studies, 1 study found increased segregation (increased γ) of the top–down control network in patients with OCD, suggesting a shift toward regularization.12 Another 2 fMRI studies showed a shift toward randomization in patients with OCD; 1 study (focusing on treatment) reported decreased segregation (decreased γ and Eloc) at baseline,119 and the other study reported decreased segregation (decreased γ) and increased integration (decreased λ) in the orbitofrontal–striato–thalamic circuit.120 Decreased σ has been reported in children with OCD.121
Of the 2 white matter network studies, 1 study reported increased segregation (increased γ) and decreased integration (decreased Eglob and increased Lp and λ), indicating a shift toward regularization,122 while the other study reported no significant differences in λ or γ.123 One cortical thickness study showed no significant between-group differences in Eglob or Eloc.124
Global topological alterations in patients PTSD
Only 5 studies, 2 fMRI and 3 sMRI, were included (Fig. 2 and Appendix 1, Table S3). Of the 2 fMRI studies, 1 study reported increased segregation (increased Cp and Eloc) and increased integration (increased Eglob and decreased Lp) in adults with PTSD, showing a shift toward stronger small-worldization, 11 whereas the other study reported increased segregation (increased Cp and Eloc) and decreased integration (increased λ) in children with PTSD, suggesting a shift toward regularization.125
Of the 2 white matter studies, 1 study reported increased integration (decreased Lp and λ) in adults with PTSD, indicating a shift toward randomization,126 whereas the other study reported a shift toward weaker small-worldization in children with PTSD characterized by decreased segregation ( decreased Eloc) and decreased integration (decreased Eglob and increased Lp).127 In an additional structural network study of grey matter, decreased σ was reported in patients with PTSD.128
Discussion
Our systematic review highlights several important findings in brain connectome research in patients with psychiatric disorders. Based on the perspectives of information segregation and integration, we explicitly propose 4 patterns of altered small-world properties: regularization, randomization, stronger small-worldization and weaker small-worldization. According to these criteria, the most prominent trend in the psychiatric studies reviewed here is the regularization of the structural connectome with randomization of the functional connectome in patients with schizophrenia (Fig. 2). However, no consistent alterations were observed in patients with MDD, ADHD, BD, OCD or PTSD.
Brain connectome research based on graph theoretical analysis has proven that small-world topological properties are important in structural and functional alterations in the brains of patients with psychiatric disorders. However, the reported alterations of small-world topological properties in previous connectome studies are controversial and often contradictory (see the Introduction), which may be partially due to the lack of unified criteria for conceptualizing these alterations. Here, we have attempted to address this issue from the perspectives of segregation and integration to classify altered small-world properties into 4 patterns: regularization, randomization, stronger small-worldization and weaker small-worldization. In future studies we recommend comprehensive calculation of the global network measures of segregation (Cp, γ, and Eloc) and integration (Lp, λ, and Eglob). Additionally, the small-world index, σ, should be calculated to define the small-world network: σ is calculated as γ ÷ λ, which is > 1 for a small-world organization that fulfills only the conditions of γ > 1 and λ ≈ 1. An important technical point is that reporting σ > 1 is not sufficient to prove small-worldness. 94,121,128 A regular network with a large γ (e.g., γ = 3) and a long λ (e.g., λ = 2) is possible, which together yield σ > 1.129 In other words, regular networks may have σ > 1, and therefore, both γ > 1 and λ ≈ 1 are required to prove small-worldness.
We systematically reviewed all current reports examining altered small-world properties in both the structural and functional connectomes of patients with 1 of 6 major psychiatric disorders. The prominent trend is the structural regularization and functional randomization in patients with schizophrenia. In the structural connectome, large proportions of the reported connectomes were constructed using diffusion MRI, and most node and edge definitions used the AAL 90 template and deterministic tractography to construct weighted networks. In contrast, in the functional connectome, obviously consistent definitions for nodes and edges were not used. In addition, neural activity is shaped, but not defined, by the underlying anatomy,16 as relatively fixed structural organizations produce diverse functional network patterns.130 Nevertheless, structural connections constrain and shape the diverse patterns of functional connections, while the functional connection patterns reflect the architecture of structural connections.131
Patients with depression did not display consistent alterations in small-world properties. A substantial proportion of alterations preserved global organization, rather than the falling into 1 of the 4 patterns defined here. Where there were significant between-group differences in the global network measures, most studies on the structural connectome in patients with MDD seems to indicate a trend toward regularization. Our attempt to identify consistent altered small-world properties might be limited by the heterogeneity of the patient samples (e.g., age of the patients). An exploratory subgroup analysis of patients with MDD revealed that the structural connectome in patients with LLD95–97,99,104,105 seemed to show a shift toward regularization.95–97
To date, there is too little brain connectome research in patients with ADHD, BD, OCD and PTSD for us to infer consistent alterations in small-world properties. In patients with BD type I, the brain networks appear to show a trend toward regularization. Future research is needed to confirm this speculation.
Several potential factors likely contributed to the variable findings. The first is the preprocessing procedure. Global signal regression is controversial, possibly leading to false-positive results:132,133 only a few studies stated that they did not regress out the global signal, and in most studies we could not ascertain any information. Another potential influence is the choice of the correlation metric. Most functional connectome studies used the Pearson correlation coefficient to define network edges. According to a reported test–retest analysis, the reliability of global topological properties is modulated by the correlation metrics and the global signal, with the highest reliability observed for Pearson correlation-based brain networks without global signal removal.37 The second confounding factor is the brain parcellation schemes. Lord and colleagues33 showed that different parcellation schemes for global network properties are comparably consistent. However, they presented only a comparison between 2 atlases (AAL and Dosenbach) for the graph theoretical analysis, which might be not generalizable to many other commonly used atlases. Third, we cannot exclude the potential confounding effects of medications, although the trend toward structural regularization rather than functional randomization was still prominent in the data sets comprising medicated patients with schizophrenia. Several studies showed appearance of significant structural and functional alterations in the brain following the administration of antipsychotics. 3,134,135 Fourth, variability may result from the use of different diagnostic categories for patients with the same disorder. A recent study showed a greater stability for some patients with a first psychosis diagnosis, as assigned using ICD-10 criteria rather than DSM-IV criteria.136 Fifth, subtype heterogeneity may be a potential confounding factor. For example, 2 schizophrenia studies recruited subgroups of patients: 1 study recruited patients with familial and sporadic schizophrenia, and the other study recruited patients with and without AVH. Two DTI studies recruited patients with the most common paranoid subtype of schizophrenia: 1 study recruited a population considered a homogeneous genetic subtype of schizophrenia, namely, the 22q11.2 deletion syndrome (22q11.2DS). A detailed description of the subtypes analyzed in the remaining studies was not reported. We performed an exploratory analysis of subtypes of LLD and BD type I, which was provided by the original reports. Despite the significant variability among the different studies, 2 prominent trends of structural regularization rather than functional randomization emerged in patients with schizophrenia. Future studies will be facilitated by strategies overcoming these issues of methodological boundaries and patient heterogeneity.
Different alterations in the global property measures belonging to the same perspective were identified. For example, in the structural connectome, decreased γ and increased Eloc, both of which belong to the segregation perspective, were found in patients with MDD.102 The analysis of γ and Eloc revealed seemingly opposite results; γ decreased, whereas Eloc increased in this group of patients. Initially, the results appeared to be contradictory. Notably, γ is normalized relative to Cp of the 100 matched random networks that preserve the number of nodes, edges and degree distribution of the real network. The ambiguity was likely the result of the choice of a null model for normalization, which was similar to the finding of increased γ and decreased Cp in patients with focal epilepsy.137 The Eloc of each node was similar to its Cp. In this circumstance, we believe that the alterations in small-worldness should be evaluated using non-normalized global measurements.
Inconsistent alterations in several original studies were reported compared with the current criteria. A lower σ in the functional networks in the brains of patients with schizophrenia displays a shift toward randomization during an AOD task.15 However, according to the current criteria, decreased segregation (decreased Cp) and decreased integration (increased Lp) suggest a shift toward weaker small-worldization. In another AOD study, decreased segregation (decreased γ) in both the left and right hemisphere task-related networks in patients schizophrenia were reported to be similar to randomization. 49 However, according to the current criteria, decreased segregation (decreased γ) and decreased integration (decreased Eglob and increased Lp) indicated a shift toward weaker small-worldization in the task-related networks in the left hemisphere. In the structural connectome of children with PTSD, decreased integration (decreased Eglob and increased Lp) suggested a shift toward regularization.127 However, according to the current criteria, the decreased integration (decreased Eglob and increased Lp) and decreased segregation (decreased Eloc) showed a shift toward weaker small-worldization. Those inconsistencies might be attributable to the previous unclear definition of the altered small-world properties.
Limitations
This study has several limitations. First, we described the patterns of altered small-world properties from the perspectives of segregation and integration. However, 10 reports investigated only 1 perspective, including 9 reports from 8 studies involving only integration65,66,84,107,108,116,117,121 and 1 study involving only segregation.55 Based on the findings from those 9 studies, our hypothesis may be not accurate. For example, increased segregation alone without information on integration will appear as regularization, but stronger small-worldization may actually occur. Second, 3 studies94,121,128 reported only changes in σ, which is not sufficient to determine small-worldness alterations. Third, the differences in preprocessing steps and analytical methods (e.g., parcellation and correlation calculations)37,138–140 can produce inconsistent results, and thus the integration of results across different studies is difficult. Fourth, although 3 studies reported altered small-world properties in patients with schizophrenia and LLD,17,95,110 these differences did not reach statistical significance. Fifth, combining evidence from patients with different disease subtypes will increase the variance of the results. However, detailed subtype information was not available for most studies included. Sixth, most studies reported p values for between-group comparisons and did not show the values of the global properties. A better strategy is to report the magnitude of the effects and differences, because the p value may be a biased estimate. We were consequently unable to perform analyses of effect sizes and publication bias.
Conclusion
We propose 4 patterns of altered small-world properties — namely, regularization, randomization, stronger small-worldization and weaker small-worldization — and use these patterns to summarize the altered small-world properties in patients with 1 of 6 major psychiatric disorders. Although we observed more differences than similarities in patients with these disorders, a prominent trend was the regularization of the structural connectome with randomization of the functional connectome in patients with schizophrenia. An analysis of altered small-world properties may provide novel insights into the pathophysiological mechanisms underlying psychiatric disorders from a connectomic perspective. However, several important challenges for future research remain.
First, as described, mixed results for the brain networks have been reported. The heterogeneity of the patient samples may be a major reason for these discrepancies. Researchers will need to select more homogeneous samples by considering demographic variables in more detail. The accumulation of validated evidence from connectome studies will reveal biological markers that indicate specific phenotypes of psychiatric disorders. Second, the combination of multimodal imaging modalities in future studies will provide additional integrative information to map the patterns of the brain connectome. Third, most of these connectome changes were detected based on cross-sectional data and thus may be influenced by interparticipant variability and unbalanced cohort distributions. Investigations of longitudinal networks are needed. Fourth, caution should be exercised in choosing the most reliable methods for analyzing structural and functional connectomes. For resting- state fMRI studies, higher test–retest network reliabilities are obtained using functionally rather than structurally defined nodes and Pearson rather than partial correlations.37,140 Finally, the emergence of novel connectome analytical approaches (e.g., dynamic connectivity) and imaging protocols (e.g., multiband fMRI) should dramatically increase our knowledge of network dysfunction in patients with brain disorders. Additional imaging research on the connectome in patients with the major psychiatric disorders is urgently needed.
Acknowledgements
This study was supported by the National Natural Science Foundation (Grant Nos. 81501452, 81621003, 81761128023, 81220108013, 81227002 and 81030027), the Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT, grant IRT16R52) of China, the Changjiang Scholar Professorship Award (Award No. T2014190) of China, and the CMB Distinguished a Professorship Award (Award No. F510000/G16916411) administered by the Institute of International Education. D. Lei is supported by the Newton International Fellowship from the Royal Society, and X. Suo is supported by the Graduate Student’s Research and Innovation Fund of Sichuan University (No. 2018YJSY099).
Footnotes
Competing interests: None declared.
Contributors: X. Suo, D. Lei, H. Zhu and Q. Gong designed the study. X. Suo, D. Lei, L. Li, W. Li, S. Wang and G. Kemp acquired the data, which X. Suo, D. Lei, L. Li, W. Li, J. Dai, S. Wang, M. He and G. Kemp analyzed. X. Suo, D. Lei, L. Li and G. Kemp wrote the article, which all authors reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
References
- 1.Lui S, Zhou XJ, Sweeney JA, et al. Psychoradiology: the frontier of neuroimaging in psychiatry. Radiology. 2016;281:357–72. doi: 10.1148/radiol.2016152149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gong Q, He Y. Depression, neuroimaging and connectomics: a selective overview. Biol Psychiatry. 2015;77:223–35. doi: 10.1016/j.biopsych.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Gong Q, Lui S, Sweeney JA. A selective review of cerebral abnormalities in patients with first-episode schizophrenia before and after treatment. Am J Psychiatry. 2016;173:232–43. doi: 10.1176/appi.ajp.2015.15050641. [DOI] [PubMed] [Google Scholar]
- 4.Sarpal DK, Lencz T, Malhotra AK. In support of neuroimaging biomarkers of treatment response in first-episode schizophrenia. Am J Psychiatry. 2016;173:732–3. doi: 10.1176/appi.ajp.2016.16030320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gong Q. Response to Sarpal et al.: importance of neuroimaging biomarkers for treatment development and clinical practice. Am J Psychiatry. 2016;173:733–4. doi: 10.1176/appi.ajp.2016.16030320r. [DOI] [PubMed] [Google Scholar]
- 6.Li F, Lui S, Yao L, et al. Altered white matter connectivity within and between networks in antipsychotic-naive first-episode schizophrenia. Schizophr Bull. 2018;44:409–18. doi: 10.1093/schbul/sbx048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sporns O, Tononi G, Kotter R. The human connectome: a structural description of the human brain. PLOS Comput Biol. 2005;1:e42. doi: 10.1371/journal.pcbi.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sporns O. Network attributes for segregation and integration in the human brain. Curr Opin Neurobiol. 2013;23:162–71. doi: 10.1016/j.conb.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 9.Fornito A, Zalesky A, Breakspear M. Graph analysis of the human connectome: promise, progress, and pitfalls. Neuroimage. 2013;80:426–44. doi: 10.1016/j.neuroimage.2013.04.087. [DOI] [PubMed] [Google Scholar]
- 10.He Y, Chen ZJ, Evans AC. Small-world anatomical networks in the human brain revealed by cortical thickness from MRI. Cereb Cortex. 2007;17:2407–19. doi: 10.1093/cercor/bhl149. [DOI] [PubMed] [Google Scholar]
- 11.Lei D, Li K, Li L, et al. Disrupted functional brain connectome in patients with posttraumatic stress disorder. Radiology. 2015;276:818–27. doi: 10.1148/radiol.15141700. [DOI] [PubMed] [Google Scholar]
- 12.Zhang T, Wang J, Yang Y, et al. Abnormal small-world architecture of top-down control networks in obsessive-compulsive disorder. J Psychiatry Neurosci. 2011;36:23–31. doi: 10.1503/jpn.100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J, Wang J, Wu Q, et al. Disrupted brain connectivity networks in drug-naive, first-episode major depressive disorder. Biol Psychiatry. 2011;70:334–42. doi: 10.1016/j.biopsych.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 14.Aerts H, Fias W, Caeyenberghs K, et al. Brain networks under attack: robustness properties and the impact of lesions. Brain. 2016;139:3063–83. doi: 10.1093/brain/aww194. [DOI] [PubMed] [Google Scholar]
- 15.Ma S, Calhoun VD, Eichele T, et al. Modulations of functional connectivity in the healthy and schizophrenia groups during task and rest. Neuroimage. 2012;62:1694–704. doi: 10.1016/j.neuroimage.2012.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu J, Wang C, Liu F, et al. Alterations of functional and structural networks in schizophrenia patients with auditory verbal hallucinations. Front Hum Neurosci. 2016;10:114. doi: 10.3389/fnhum.2016.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He H, Sui J, Yu Q, et al. Altered small-world brain networks in schizophrenia patients during working memory performance. PLoS One. 2012;7:e38195. doi: 10.1371/journal.pone.0038195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Lin L, Lin CP, et al. Abnormal topological organization of structural brain networks in schizophrenia. Schizophr Res. 2012;141:109–18. doi: 10.1016/j.schres.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 19.Wang Q, Su TP, Zhou Y, et al. Anatomical insights into disrupted small-world networks in schizophrenia. Neuroimage. 2012;59:1085–93. doi: 10.1016/j.neuroimage.2011.09.035. [DOI] [PubMed] [Google Scholar]
- 20.Su TW, Hsu TW, Lin YC, et al. Schizophrenia symptoms and brain network efficiency: a resting-state fMRI study. Psychiatry Res. 2015;234:208–18. doi: 10.1016/j.pscychresns.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 21.Yu Q, Sui J, Rachakonda S, et al. Altered topological properties of functional network connectivity in schizophrenia during resting state: a small-world brain network study. PLoS One. 2011;6:e25423. doi: 10.1371/journal.pone.0025423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Filippi M, van den Heuvel MP, Fornito A, et al. Assessment of system dysfunction in the brain through MRI-based connectomics. Lancet Neurol. 2013;12:1189–99. doi: 10.1016/S1474-4422(13)70144-3. [DOI] [PubMed] [Google Scholar]
- 23.Fornito A, Zalesky A, Pantelis C, et al. Schizophrenia, neuroimaging and connectomics. Neuroimage. 2012;62:2296–314. doi: 10.1016/j.neuroimage.2011.12.090. [DOI] [PubMed] [Google Scholar]
- 24.Mears D, Pollard HB. Network science and the human brain: using graph theory to understand the brain and one of its hubs, the amygdala, in health and disease. J Neurosci Res. 2016;94:590–605. doi: 10.1002/jnr.23705. [DOI] [PubMed] [Google Scholar]
- 25.Liao X, Vasilakos AV, He Y. Small-world human brain networks: perspectives and challenges. Neurosci Biobehav Rev. 2017;77:286–300. doi: 10.1016/j.neubiorev.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 26.Shen X, Tokoglu F, Papademetris X, et al. Groupwise whole-brain parcellation from resting-state fMRI data for network node identification. Neuroimage. 2013;82:403–15. doi: 10.1016/j.neuroimage.2013.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Power JD, Cohen AL, Nelson SM, et al. Functional network organization of the human brain. Neuron. 2011;72:665–78. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dosenbach NU, Nardos B, Cohen AL, et al. Prediction of individual brain maturity using fMRI. Science. 2010;329:1358–61. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Craddock RC, James GA, Holtzheimer PE, III, et al. A whole brain fMRI atlas generated via spatially constrained spectral clustering. Hum Brain Mapp. 2012;33:1914–28. doi: 10.1002/hbm.21333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 31.Xia M, He Y. Functional connectomics from a “big data” perspective. Neuroimage. 2017;160:152–67. doi: 10.1016/j.neuroimage.2017.02.031. [DOI] [PubMed] [Google Scholar]
- 32.Park B, Ko JH, Lee JD, et al. Evaluation of node-inhomogeneity effects on the functional brain network properties using an anatomy- constrained hierarchical brain parcellation. PLoS One. 2013;8:e74935. doi: 10.1371/journal.pone.0074935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lord A, Ehrlich S, Borchardt V, et al. Brain parcellation choice affects disease-related topology differences increasingly from global to local network levels. Psychiatry Res Neuroimaging. 2016;249:12–9. doi: 10.1016/j.pscychresns.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Smith SM, Miller KL, Salimi-Khorshidi G, et al. Network modelling methods for FMRI. Neuroimage. 2011;54:875–91. doi: 10.1016/j.neuroimage.2010.08.063. [DOI] [PubMed] [Google Scholar]
- 35.Smith SM, Vidaurre D, Beckmann CF, et al. Functional connectomics from resting-state fMRI. Trends Cogn Sci. 2013;17:666–82. doi: 10.1016/j.tics.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zalesky A, Fornito A, Bullmore E. On the use of correlation as a measure of network connectivity. Neuroimage. 2012;60:2096–106. doi: 10.1016/j.neuroimage.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 37.Liang X, Wang J, Yan C, et al. Effects of different correlation metrics and preprocessing factors on small-world brain functional networks: a resting-state functional MRI study. PLoS One. 2012;7:e32766. doi: 10.1371/journal.pone.0032766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mori S, van Zijl PC. Fiber tracking: principles and strategies — a technical review. NMR Biomed. 2002;15:468–80. doi: 10.1002/nbm.781. [DOI] [PubMed] [Google Scholar]
- 39.Ratnarajah N, Simmons A, Davydov O, et al. A novel approach for improved tractography and quantitative analysis of probabilistic fibre tracking curves. Med Image Anal. 2012;16:227–38. doi: 10.1016/j.media.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 40.Behrens TE, Woolrich MW, Jenkinson M, et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50:1077–88. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- 41.Buchanan CR, Pernet CR, Gorgolewski KJ, et al. Test-retest reliability of structural brain networks from diffusion MRI. Neuroimage. 2014;86:231–43. doi: 10.1016/j.neuroimage.2013.09.054. [DOI] [PubMed] [Google Scholar]
- 42.Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52:1059–69. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 43.Watts DJ, Strogatz SH. Collective dynamics of ‘small-world’ networks. Nature. 1998;393:440–2. doi: 10.1038/30918. [DOI] [PubMed] [Google Scholar]
- 44.Latora V, Marchiori M. Efficient behavior of small-world networks. Phys Rev Lett. 2001;87:198701. doi: 10.1103/PhysRevLett.87.198701. [DOI] [PubMed] [Google Scholar]
- 45.Cao M, Shu N, Cao Q, et al. Imaging functional and structural brain connectomics in attention-deficit/hyperactivity disorder. Mol Neurobiol. 2014;50:1111–23. doi: 10.1007/s12035-014-8685-x. [DOI] [PubMed] [Google Scholar]
- 46.Lord A, Horn D, Breakspear M, et al. Changes in community structure of resting state functional connectivity in unipolar depression. PLoS One. 2012;7:e41282. doi: 10.1371/journal.pone.0041282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen VC, Shen CY, Liang SH, et al. Assessment of abnormal brain structures and networks in major depressive disorder using morphometric and connectome analyses. J Affect Disord. 2016;205:103–11. doi: 10.1016/j.jad.2016.06.066. [DOI] [PubMed] [Google Scholar]
- 48.Zhu J, Zhuo C, Liu F, et al. Distinct disruptions of resting-state functional brain networks in familial and sporadic schizophrenia. Sci Rep. 2016;6:23577. doi: 10.1038/srep23577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu Q, Sui J, Rachakonda S, et al. Altered small-world brain networks in temporal lobe in patients with schizophrenia performing an auditory oddball task. Front Syst Neurosci. 2011;5:7. doi: 10.3389/fnsys.2011.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alexander-Bloch AF, Gogtay N, Meunier D, et al. Disrupted modularity and local connectivity of brain functional networks in childhood-onset schizophrenia. Front Syst Neurosci. 2010;4:147. doi: 10.3389/fnsys.2010.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu F, Zhuo C, Yu C. Altered cerebral blood flow covariance network in schizophrenia. Front Neurosci. 2016;10:308. doi: 10.3389/fnins.2016.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lo CY, Su TW, Huang CC, et al. Randomization and resilience of brain functional networks as systems-level endophenotypes of schizophrenia. Proc Natl Acad Sci U S A. 2015;112:9123–8. doi: 10.1073/pnas.1502052112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lynall ME, Bassett DS, Kerwin R, et al. Functional connectivity and brain networks in schizophrenia. J Neurosci. 2010;30:9477–87. doi: 10.1523/JNEUROSCI.0333-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang L, Metzak PD, Honer WG, et al. Impaired efficiency of functional networks underlying episodic memory-for-context in schizophrenia. J Neurosci. 2010;30:13171–9. doi: 10.1523/JNEUROSCI.3514-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu Q, Plis SM, Erhardt EB, et al. Modular organization of functional network connectivity in healthy controls and patients with schizophrenia during the resting state. Front Syst Neurosci. 2012;5:103. doi: 10.3389/fnsys.2011.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alexander-Bloch AF, Vertes PE, Stidd R, et al. The anatomical distance of functional connections predicts brain network topology in health and schizophrenia. Cereb Cortex. 2013;23:127–38. doi: 10.1093/cercor/bhr388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tomasi D, Volkow ND. Mapping small-world properties through development in the human brain: disruption in schizophrenia. PLoS One. 2014;9:e96176. doi: 10.1371/journal.pone.0096176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu Y, Liang M, Zhou Y, et al. Disrupted small-world networks in schizophrenia. Brain. 2008;131:945–61. doi: 10.1093/brain/awn018. [DOI] [PubMed] [Google Scholar]
- 59.Sheffield JM, Repovs G, Harms MP, et al. Fronto-parietal and cingulo-opercular network integrity and cognition in health and schizophrenia. Neuropsychologia. 2015;73:82–93. doi: 10.1016/j.neuropsychologia.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu Q, Erhardt EB, Sui J, et al. Assessing dynamic brain graphs of time-varying connectivity in fMRI data: application to healthy controls and patients with schizophrenia. Neuroimage. 2015;107:345–55. doi: 10.1016/j.neuroimage.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hadley JA, Kraguljac NV, White DM, et al. Change in brain network topology as a function of treatment response in schizophrenia: a longitudinal resting-state fMRI study using graph theory. NPJ Schizophr. 2016;2:16014. doi: 10.1038/npjschz.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li X, Xia S, Bertisch HC, et al. Unique topology of language processing brain network: a systems-level biomarker of schizophrenia. Schizophr Res. 2012;141:128–36. doi: 10.1016/j.schres.2012.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu Q, Sui J, Kiehl KA, et al. State-related functional integration and functional segregation brain networks in schizophrenia. Schizophr Res. 2013;150:450–8. doi: 10.1016/j.schres.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fornito A, Yoon J, Zalesky A, et al. General and specific functional connectivity disturbances in first-episode schizophrenia during cognitive control performance. Biol Psychiatry. 2011;70:64–72. doi: 10.1016/j.biopsych.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen J, Yao Z, Qin J, et al. Abnormal inter- and intra-hemispheric integration in male paranoid schizophrenia: a graph-theoretical analysis. Shanghai Arch Psychiatry. 2015;27:158–66. doi: 10.11919/j.issn.1002-0829.215036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Griffa A, Baumann PS, Ferrari C, et al. Characterizing the connectome in schizophrenia with diffusion spectrum imaging. Hum Brain Mapp. 2015;36:354–66. doi: 10.1002/hbm.22633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim DJ, Kent JS, Bolbecker AR, et al. Disrupted modular architecture of cerebellum in schizophrenia: a graph theoretic analysis. Schizophr Bull. 2014;40:1216–26. doi: 10.1093/schbul/sbu059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ottet MC, Schaer M, Debbane M, et al. Graph theory reveals dysconnected hubs in 22q11DS and altered nodal efficiency in patients with hallucinations. Front Hum Neurosci. 2013;7:402. doi: 10.3389/fnhum.2013.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sun Y, Chen Y, Lee R, et al. Disruption of brain anatomical networks in schizophrenia: a longitudinal, diffusion tensor imaging based study. Schizophr Res. 2016;171:149–57. doi: 10.1016/j.schres.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 70.Sun Y, Chen Y, Collinson SL, et al. Reduced hemispheric asymmetry of brain anatomical networks is linked to schizophrenia: a connectome study. Cereb Cortex. 2017;27:602–15. doi: 10.1093/cercor/bhv255. [DOI] [PubMed] [Google Scholar]
- 71.van den Heuvel MP, Sporns O, Collin G, et al. Abnormal rich club organization and functional brain dynamics in schizophrenia. JAMA Psychiatry. 2013;70:783–92. doi: 10.1001/jamapsychiatry.2013.1328. [DOI] [PubMed] [Google Scholar]
- 72.Yeo RA, Ryman SG, van den Heuvel MP, et al. Graph metrics of structural brain networks in individuals with schizophrenia and healthy controls: group differences, relationships with intelligence, and genetics. J Int Neuropsychol Soc. 2016;22:240–9. doi: 10.1017/S1355617715000867. [DOI] [PubMed] [Google Scholar]
- 73.Zalesky A, Fornito A, Seal ML, et al. Disrupted axonal fiber connectivity in schizophrenia. Biol Psychiatry. 2011;69:80–9. doi: 10.1016/j.biopsych.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Collin G, Kahn RS, de Reus MA, et al. Impaired rich club connectivity in unaffected siblings of schizophrenia patients. Schizophr Bull. 2014;40:438–48. doi: 10.1093/schbul/sbt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sun Y, Lee R, Shen K, et al. Structural connectivity analysis reveals topological aberrations in patients with schizophrenia. Conf Proc IEEE Eng Med Biol Soc. 2013;2013:1386–9. doi: 10.1109/EMBC.2013.6609768. [DOI] [PubMed] [Google Scholar]
- 76.Yan H, Tian L, Wang Q, et al. Compromised small-world efficiency of structural brain networks in schizophrenic patients and their unaffected parents. Neurosci Bull. 2015;31:275–87. doi: 10.1007/s12264-014-1518-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang R, Wei Q, Kang Z, et al. Disrupted brain anatomical connectivity in medication-naive patients with first-episode schizophrenia. Brain Struct Funct. 2015;220:1145–59. doi: 10.1007/s00429-014-0706-z. [DOI] [PubMed] [Google Scholar]
- 78.Cabral J, Fernandes HM, Van Hartevelt TJ, et al. Structural connectivity in schizophrenia and its impact on the dynamics of spontaneous functional networks. Chaos. 2013;23:046111. doi: 10.1063/1.4851117. [DOI] [PubMed] [Google Scholar]
- 79.Hu M, Zong X, Zheng J, et al. Risperidone-induced topological alterations of anatomical brain network in first-episode drug-naive schizophrenia patients: a longitudinal diffusion tensor imaging study. Psychol Med. 2016;46:2549–60. doi: 10.1017/S0033291716001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van den Heuvel MP, Mandl RC, Stam CJ, et al. Aberrant frontal and temporal complex network structure in schizophrenia: a graph theoretical analysis. J Neurosci. 2010;30:15915–26. doi: 10.1523/JNEUROSCI.2874-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li W, Douglas Ward B, Liu X, et al. Disrupted small world topology and modular organisation of functional networks in late-life depression with and without amnestic mild cognitive impairment. J Neurol Neurosurg Psychiatry. 2015;86:1097–105. doi: 10.1136/jnnp-2014-309180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Borchardt V, Lord AR, Li M, et al. Preprocessing strategy influences graph-based exploration of altered functional networks in major depression. Hum Brain Mapp. 2016;37:1422–42. doi: 10.1002/hbm.23111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guo H, Cheng C, Cao X, et al. Resting-state functional connectivity abnormalities in first-onset unmedicated depression. Neural Regen Res. 2014;9:153–63. doi: 10.4103/1673-5374.125344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Manelis A, Almeida JR, Stiffler R, et al. Anticipation-related brain connectivity in bipolar and unipolar depression: a graph theory approach. Brain. 2016;139:2554–66. doi: 10.1093/brain/aww157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Borchardt V, Krause AL, Starck T, et al. Graph theory reveals hyper- functionality in visual cortices of seasonal affective disorder patients. World J Biol Psychiatry. 2015;16:123–34. doi: 10.3109/15622975.2014.966144. [DOI] [PubMed] [Google Scholar]
- 86.Meng C, Brandl F, Tahmasian M, et al. Aberrant topology of striatum’s connectivity is associated with the number of episodes in depression. Brain. 2014;137:598–609. doi: 10.1093/brain/awt290. [DOI] [PubMed] [Google Scholar]
- 87.Ye M, Yang T, Qing P, et al. Changes of functional brain networks in major depressive disorder: a graph theoretical analysis of resting- state fMRI. PLoS One. 2015;10:e0133775. doi: 10.1371/journal.pone.0133775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Park CH, Wang SM, Lee HK, et al. Affective state-dependent changes in the brain functional network in major depressive disorder. Soc Cogn Affect Neurosci. 2014;9:1404–12. doi: 10.1093/scan/nst126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Luo Q, Deng Z, Qin J, et al. Frequency dependant topological alterations of intrinsic functional connectome in major depressive disorder. Sci Rep. 2015;5:9710. doi: 10.1038/srep09710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang Z, Yuan Y, Bai F, et al. Altered topological patterns of brain networks in remitted late-onset depression: a resting-state fMRI study. J Clin Psychiatry. 2016;77:123–30. doi: 10.4088/JCP.14m09344. [DOI] [PubMed] [Google Scholar]
- 91.Bohr IJ, Kenny E, Blamire A, et al. Resting-state functional connectivity in late-life depression: higher global connectivity and more long distance connections. Front Psychiatry. 2013;3:116. doi: 10.3389/fpsyt.2012.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.He H, Yu Q, Du Y, et al. Resting-state functional network connectivity in prefrontal regions differs between unmedicated patients with bipolar and major depressive disorders. J Affect Disord. 2016;190:483–93. doi: 10.1016/j.jad.2015.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Peng D, Shi F, Shen T, et al. Altered brain network modules induce helplessness in major depressive disorder. J Affect Disord. 2014;168:21–9. doi: 10.1016/j.jad.2014.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jin C, Gao C, Chen C, et al. A preliminary study of the dysregulation of the resting networks in first-episode medication-naive adolescent depression. Neurosci Lett. 2011;503:105–9. doi: 10.1016/j.neulet.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 95.Mak E, Colloby SJ, Thomas A, et al. The segregated connectome of late-life depression: a combined cortical thickness and structural covariance analysis. Neurobiol Aging. 2016;48:212–21. doi: 10.1016/j.neurobiolaging.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bai F, Shu N, Yuan Y, et al. Topologically convergent and divergent structural connectivity patterns between patients with remitted geriatric depression and amnestic mild cognitive impairment. J Neurosci. 2012;32:4307–18. doi: 10.1523/JNEUROSCI.5061-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ajilore O, Lamar M, Leow A, et al. Graph theory analysis of cortical- subcortical networks in late-life depression. Am J Geriatr Psychiatry. 2014;22:195–206. doi: 10.1016/j.jagp.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang T, Wang K, Qu H, et al. Disorganized cortical thickness covariance network in major depressive disorder implicated by aberrant hubs in large-scale networks. Sci Rep. 2016;6:27964. doi: 10.1038/srep27964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li X, Steffens DC, Potter GG, et al. Decreased between-hemisphere connectivity strength and network efficiency in geriatric depression. Hum Brain Mapp. 2017;38:53–67. doi: 10.1002/hbm.23343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Singh MK, Kesler SR, Hadi Hosseini SM, et al. Anomalous gray matter structural networks in major depressive disorder. Biol Psychiatry. 2013;74:777–85. doi: 10.1016/j.biopsych.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen JH, Yao ZJ, Qin JL, et al. Aberrant global and regional topological organization of the fractional anisotropy-weighted brain structural networks in major depressive disorder. Chin Med J (Engl) 2016;129:679–89. doi: 10.4103/0366-6999.178002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Long Z, Duan X, Wang Y, et al. Disrupted structural connectivity network in treatment-naive depression. Prog Neuropsychopharmacol Biol Psychiatry. 2015;56:18–26. doi: 10.1016/j.pnpbp.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 103.Ajilore O, Lamar M, Kumar A. Association of brain network efficiency with aging, depression, and cognition. Am J Geriatr Psychiatry. 2014;22:102–10. doi: 10.1016/j.jagp.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Charlton RA, Leow A, GadElkarim J, et al. Brain connectivity in late-life depression and aging revealed by network analysis. Am J Geriatr Psychiatry. 2015;23:642–50. doi: 10.1016/j.jagp.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lim HK, Jung WS, Aizenstein HJ. Aberrant topographical organization in gray matter structural network in late life depression: a graph theoretical analysis. Int Psychogeriatr. 2013;25:1929–40. doi: 10.1017/S104161021300149X. [DOI] [PubMed] [Google Scholar]
- 106.Qin J, Wei M, Liu H, et al. Abnormal brain anatomical topological organization of the cognitive-emotional and the frontoparietal circuitry in major depressive disorder. Magn Reson Med. 2014;72:1397–407. doi: 10.1002/mrm.25036. [DOI] [PubMed] [Google Scholar]
- 107.Sacchet MD, Prasad G, Foland-Ross LC, et al. Elucidating brain connectivity networks in major depressive disorder using classification-based scoring. Proc IEEE Int Symp Biomed Imaging. 2014;2014:246–9. doi: 10.1109/ISBI.2014.6867855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sacchet MD, Prasad G, Foland-Ross LC, et al. Support vector machine classification of major depressive disorder using diffusion-weighted neuroimaging and graph theory. Front Psychiatry. 2015;6:21. doi: 10.3389/fpsyt.2015.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tymofiyeva O, Connolly CG, Ho TC, et al. DTI-based connectome analysis of adolescents with major depressive disorder reveals hypoconnectivity of the right caudate. J Affect Disord. 2017;207:18–25. doi: 10.1016/j.jad.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Korgaonkar MS, Fornito A, Williams LM, et al. Abnormal structural networks characterize major depressive disorder: a connectome analysis. Biol Psychiatry. 2014;76:567–74. doi: 10.1016/j.biopsych.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 111.Wang L, Zhu C, He Y, et al. Altered small-world brain functional networks in children with attention-deficit/hyperactivity disorder. Hum Brain Mapp. 2009;30:638–49. doi: 10.1002/hbm.20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xia S, Foxe JJ, Sroubek AE, et al. Topological organization of the “small-world” visual attention network in children with attention deficit/hyperactivity disorder (ADHD) Front Hum Neurosci. 2014;8:162. doi: 10.3389/fnhum.2014.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cocchi L, Bramati IE, Zalesky A, et al. Altered functional brain connectivity in a non-clinical sample of young adults with attention- deficit/hyperactivity disorder. J Neurosci. 2012;32:17753–61. doi: 10.1523/JNEUROSCI.3272-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cao Q, Shu N, An L, et al. Probabilistic diffusion tractography and graph theory analysis reveal abnormal white matter structural connectivity networks in drug-naive boys with attention deficit/hyperactivity disorder. J Neurosci. 2013;33:10676–87. doi: 10.1523/JNEUROSCI.4793-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sidlauskaite J, Caeyenberghs K, Sonuga-Barke E, et al. Whole-brain structural topology in adult attention-deficit/hyperactivity disorder: Preserved global - disturbed local network organization. Neuroimage Clin. 2015;9:506–12. doi: 10.1016/j.nicl.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Spielberg JM, Beall EB, Hulvershorn LA, et al. Resting state brain network disturbances related to hypomania and depression in medication- free bipolar disorder. Neuropsychopharmacology. 2016;41:3016–24. doi: 10.1038/npp.2016.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Collin G, van den Heuvel MP, Abramovic L, et al. Brain network analysis reveals affected connectome structure in bipolar I disorder. Hum Brain Mapp. 2016;37:122–34. doi: 10.1002/hbm.23017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Leow A, Ajilore O, Zhan L, et al. Impaired inter-hemispheric integration in bipolar disorder revealed with brain network analyses. Biol Psychiatry. 2013;73:183–93. doi: 10.1016/j.biopsych.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shin DJ, Jung WH, He Y, et al. The effects of pharmacological treatment on functional brain connectome in obsessive-compulsive disorder. Biol Psychiatry. 2014;75:606–14. doi: 10.1016/j.biopsych.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 120.Jung WH, Yucel M, Yun JY, et al. Altered functional network architecture in orbitofronto-striato-thalamic circuit of unmedicated patients with obsessive-compulsive disorder. Hum Brain Mapp. 2017;38:109–19. doi: 10.1002/hbm.23347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Armstrong CC, Moody TD, Feusner JD, et al. Graph-theoretical analysis of resting-state fMRI in pediatric obsessive-compulsive disorder. J Affect Disord. 2016;193:175–84. doi: 10.1016/j.jad.2015.12.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhong Z, Zhao TD, Luo J, et al. Abnormal topological organization in white matter structural networks revealed by diffusion tensor tractography in unmedicated patients with obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2014;51:39–50. doi: 10.1016/j.pnpbp.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 123.Reess TJ, Rus OG, Schmidt R, et al. Connectomics-based structural network alterations in obsessive-compulsive disorder. Transl Psychiatry. 2016;6:e882. doi: 10.1038/tp.2016.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kim SG, Jung WH, Kim SN, et al. Disparity between dorsal and ventral networks in patients with obsessive-compulsive disorder:evidence revealed by graph theoretical analysis based on cortical thickness from MRI. Front Hum Neurosci. 2013;7:302. doi: 10.3389/fnhum.2013.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Suo X, Lei D, Li K, et al. Disrupted brain network topology in pediatric posttraumatic stress disorder: a resting-state fMRI study. Hum Brain Mapp. 2015;36:3677–86. doi: 10.1002/hbm.22871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Long Z, Duan X, Xie B, et al. Altered brain structural connectivity in post-traumatic stress disorder: a diffusion tensor imaging tractography study. J Affect Disord. 2013;150:798–806. doi: 10.1016/j.jad.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 127.Suo X, Lei D, Chen F, et al. Anatomic insights into disrupted small-world networks in pediatric posttraumatic stress disorder. Radiology. 2017;282:826–34. doi: 10.1148/radiol.2016160907. [DOI] [PubMed] [Google Scholar]
- 128.Mueller SG, Ng P, Neylan T, et al. Evidence for disrupted gray matter structural connectivity in posttraumatic stress disorder. Psychiatry Res. 2015;234:194–201. doi: 10.1016/j.pscychresns.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 129.Bullmore ET, Bassett DS. Brain graphs: graphical models of the human brain connectome. Annu Rev Clin Psychol. 2011;7:113–40. doi: 10.1146/annurev-clinpsy-040510-143934. [DOI] [PubMed] [Google Scholar]
- 130.Park HJ, Friston K. Structural and functional brain networks: from connections to cognition. Science. 2013;342:1238411. doi: 10.1126/science.1238411. [DOI] [PubMed] [Google Scholar]
- 131.Wang Z, Dai Z, Gong G, et al. Understanding structural-functional relationships in the human brain: a large-scale network perspective. Neuroscientist. 2015;21:290–305. doi: 10.1177/1073858414537560. [DOI] [PubMed] [Google Scholar]
- 132.Fox MD, Zhang D, Snyder AZ, et al. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101:3270–83. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Murphy K, Birn RM, Handwerker DA, et al. The impact of global signal regression on resting state correlations: Are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Navari S, Dazzan P. Do antipsychotic drugs affect brain structure? A systematic and critical review of MRI findings. Psychol Med. 2009;39:1763–77. doi: 10.1017/S0033291709005315. [DOI] [PubMed] [Google Scholar]
- 135.Fusar-Poli P, Smieskova R, Kempton MJ, et al. Progressive brain changes in schizophrenia related to antipsychotic treatment? A meta-analysis of longitudinal MRI studies. Neurosci Biobehav Rev. 2013;37:1680–91. doi: 10.1016/j.neubiorev.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Salvatore P, Baldessarini RJ, Tohen M, et al. McLean-Harvard International First-Episode Project: two-year stability of ICD-10 diagnoses in 500 first-episode psychotic disorder patients. J Clin Psychiatry. 2011;72:183–93. doi: 10.4088/JCP.09m05311yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Výtvarová E, Marecek R, Fousek J, et al. Large-scale cortico-subcortical functional networks in focal epilepsies: the role of the basal ganglia. Neuroimage Clin. 2016;14:28–36. doi: 10.1016/j.nicl.2016.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang J, Wang L, Zang Y, et al. Parcellation-dependent small-world brain functional networks: a resting-state fMRI study. Hum Brain Mapp. 2009;30:1511–23. doi: 10.1002/hbm.20623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liao XH, Xia MR, Xu T, et al. Functional brain hubs and their test-retest reliability: a multiband resting-state functional MRI study. Neuroimage. 2013;83:969–82. doi: 10.1016/j.neuroimage.2013.07.058. [DOI] [PubMed] [Google Scholar]
- 140.Wang JH, Zuo XN, Gohel S, et al. Graph theoretical analysis of functional brain networks: test-retest evaluation on short- and long-term resting-state functional MRI data. PLoS One. 2011;6:e21976. doi: 10.1371/journal.pone.0021976. [DOI] [PMC free article] [PubMed] [Google Scholar]