Abstract
Ultrasonographic microcalcification is highly related to papillary thyroid cancer (PTC) and pathologic psammoma body is a poor prognostic factor. However, it is little known about whether the microcalcifications seen on ultrasonography are consistent with the pathologic psammoma bodies. We evaluated the relationship between ultrasonographic (US) calcification types and pathologic calcification features, and the consistency between observed pathologic and US calcifications.
US calcifications were classified into microcalcification (MC) and nonmicrocalcification (non-MC) types, and pathologic calcifications were classified into 3 types: psammoma bodies, stromal calcifications, and ossifications.
Among the 411 nodules that were reviewed by a pathologist, 38.9% (n = 160) had any type of US calcification. The larger the size of pathologic calcification, the more calcification was present in US (psammoma 46.1% < stromal 53.7% < ossification 73.3%). Psammoma bodies occurred in all US MC type. Ossification nodules occurred in nearly all (92.3%) non-MC type. The stromal-only nodules were 36.8% MC-type and 63.2% non-MC type. MC-type had a significantly higher odds ratio than non-MC type for predicting psammoma bodies according to the logistic regression.
The presence of MC in ultrasonography was consistent with the presence of psammoma bodies. This study suggests that US identification of MC may be a useful prognostic indicator of PTC aggressiveness.
Keywords: calcification, papillary thyroid cancer, pathology, psammoma body, ultrasonography
1. Introduction
Ultrasonographic (US) calcification is highly related to papillary thyroid cancer (PTC) diagnosis, and microcalcification is highly associated with PTC.[1–3] It has been reported that 19.8%–32.1% of thyroid nodules have some type of calcification.[4,5] Cancer risks have been reported to be as high as 59.2% when a thyroid nodule exhibits calcification.[1] In a previous study at our center, we classified the patterns of calcification into 5 types: microcalcification (fine stippling psammoma body), annular-like peripheral calcification, crescent-like peripheral calcification, intranodular coarse calcification, and calcified spot.[6] A previous study reported that calcifications were detected in 40.2% (524/1,305) of patients with PTC and microcalcifications were observed in 42.9% of calcifications (225/524). We hypothesize that since microcalcifications are more often reported than other calcification patterns in PTC, the clinical course for thyroid cancers with microcalcifications could be more aggressive than that of thyroid cancers without microcalcifications. Previous studies have revealed that the presence of pathologic psammoma bodies is a poor prognostic factor.[7–9] Intuitively, pathologic psammoma bodies are likely to present as microcalcifications on ultrasound images.
Several studies have suggested that psammoma bodies and US microcalcifications are poor prognostic factors. However, no studies have evaluated whether US calcifications synchronize with pathologic calcifications. Therefore, we preoperatively evaluated the presence and types of US calcifications as prognostic factors, to determine the relationship between US calcification and other pathologic factors. We also investigated the consistency between pathologic and US calcifications.
2. Materials and methods
2.1. Subjects
This study is a cross-sectional chart review. We retrospectively reviewed a total of 1297 thyroid nodules from 901 patients who were diagnosed with PTC. All patients underwent thyroidectomies between January 2008 and July 2011 at the Kosin University Gospel Hospital. Among these patients, 411 nodules were reviewed for pathologic features. The flow diagram is presented in Figure 1. We reviewed clinical information, US findings, and final pathology reports. This study was approved by the institutional review board (IRB) of the Kosin University Gospel Hospital (approval number: 11–87). We obtained informed consent from all participants.
Figure 1.
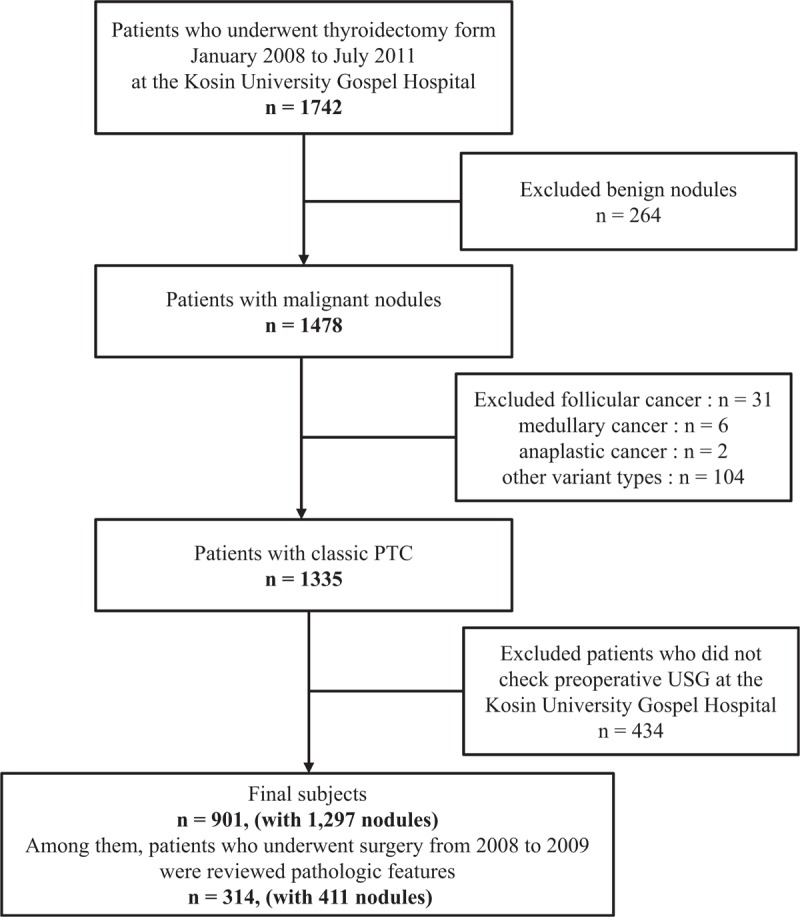
The flow diagram of this study.
2.2. Ultrasonographic calcification assessment
Before surgery, a thyroid US was performed for all nodules by 2 radiologists with real-time scanners (Philips Healthcare IU 22, Bothell, WA) equipped with a 5- to 12-MHz linear array transducers. All the US images were reviewed by one thyroid endocrinologist. The size and number of thyroid nodules and the presence and pattern of calcifications were recorded for each patient.
In this study, we classified US calcifications into only 2 types: microcalcification (MC) and nonmicrocalcification (non-MC). MC was defined as multiple punctate bright echoes (smaller than 2 mm) with or without acoustic shadowing. Non-MC calcification was defined as annular-type calcification, crescent-type calcification, intranodular calcification, and calcified spots (Fig. 2), according to a previously established classification system.[6]
Figure 2.
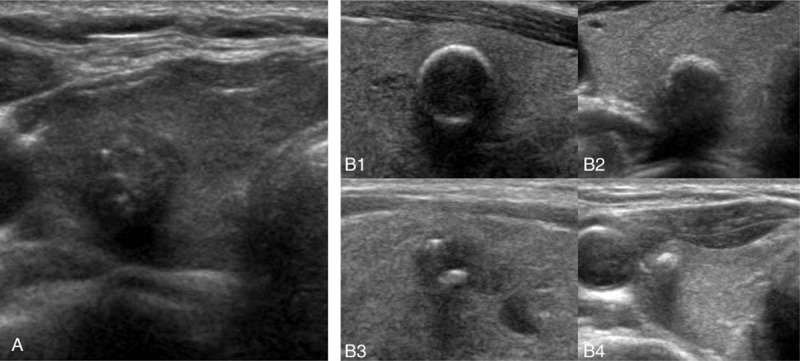
Transverse ultrasound image of thyroid nodules with calcifications. (A) Microcalcification, (B) nonmicrocalcification; (B-1) annular-type, (B-2) crescent-type, (B-3) intranodular, (B-4) calcified spot.
2.3. Pathologic calcification assessment
One pathologist retrospectively reviewed the pathologic calcifications of 411 nodules. Pathologic calcifications were divided into 3 types: psammoma bodies, stromal calcifications, and ossifications (Fig. 3). Psammoma bodies were defined as spherical calcified foci with concentric laminations. Ossification was considered positive only when both bone matrix and osteocytes were identifiable. Stromal calcifications were defined as calcifications that were not psammoma bodies or ossifications. We morphologically classified calcifications as irregular, linear, or mass type (Fig. 4).
Figure 3.
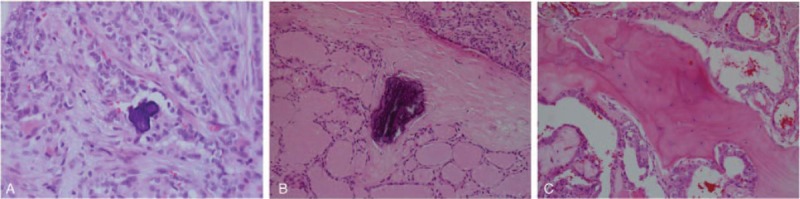
Classification of pathologic calcification. (A) Psammoma body, (B) stromal calcification, (C) ossification types.
Figure 4.
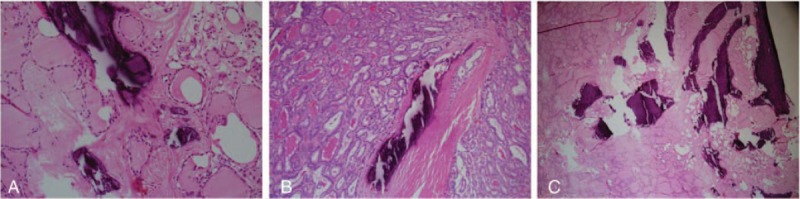
Morphology of pathologic calcification. (A) Irregular type: scattered with any type of calcification in the nodule. (B) Linear type: linear or curvilinear shapes larger than 0.1 cm. (C) Mass type: consisted of almost-calcification and few cells.
2.4. Statistical analysis
We analyzed the data in terms of frequency. Chi-square and Fisher's exact tests were performed for categorical variables. To evaluate the association between US and pathologic calcifications, logistic regression was used. The P values < .05 were considered statistically significant. SPSS, version 19.0 (SPSS Inc., Chicago, IL) was used for statistical analyses.
3. Results
3.1. Patient characteristics according to the presence of US calcification
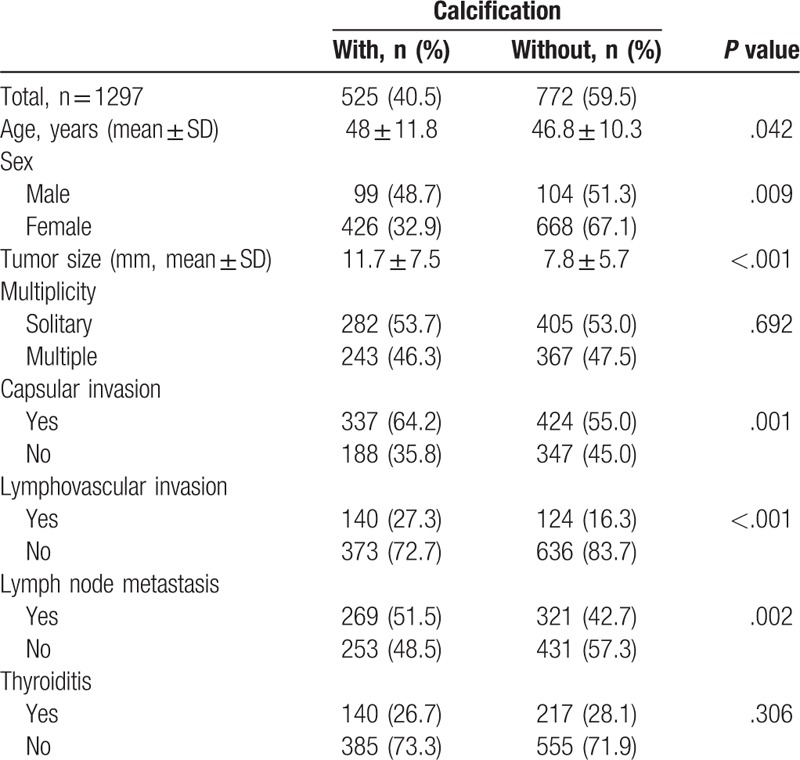
Clinicopathological characteristics according to the presence and absence of calcification are presented in Table 1. Among a total of 1297 PTC nodules, 40.5% (525 of 1297) had any type of calcification. The group of patients with calcifications was older than those without calcifications (48.0 ± 11.8 vs 46.8 ± 10.3 years, P < .001). In the male group, a larger number of PTC nodules had calcification (48.7 vs 32.9%, P = .009). Tumor size was significantly larger in the group with calcification than in the group without calcification (11.7 ± 7.5 vs 7.8 ± 5.7, P < .001). There were no significant differences in multiplicity or thyroiditis between the 2 groups. Capsular invasion, lymphovascular invasion, and lymph node metastasis were significantly higher in the group with calcification than the group without calcification (64.2 vs 55.0%, P = .001, 27.3 vs 16.3%, P < .001, 51.5 vs 48.5%, P = .002).
Table 1.
Clinicopathologic characteristics of patients with papillary thyroid carcinoma with and without calcification.
3.2. Patient characteristics according to US calcification type
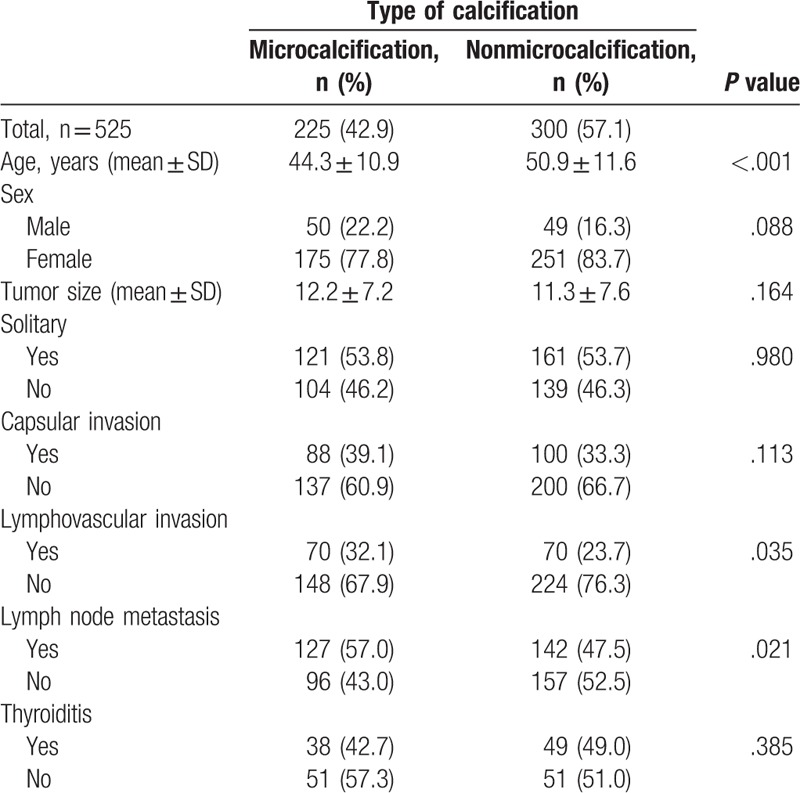
Clinicopathological characteristics according to the calcification type are shown in Table 2. Patients with MC type were younger than those with non-MC type (44.3 ± 10.9 vs 50.9 ± 11.6 years, P < .001). Tumor size was larger in the MC type than in the non-MC type, group, although this difference was not statistically significant. There were no significant differences in gender, solidarity, or thyroiditis between the 2 types. Capsular invasion was more frequent in MC type (39.1 vs 33.3%, P = .113), but this observation was not statistically significant. PTC with MC-type calcification showed higher incidences of lymphovascular invasion (32.1 vs 23.7%, P = .035) and lymph node metastasis (57.0 vs 47.5%, P = .021) than non-MC type.
Table 2.
Clinicopathologic characteristics of patients according to papillary thyroid carcinoma calcification type.
3.3. Morphology of pathologic calcification
Almost all psammoma bodies (98.7%) showed an irregular shape. On the contrary, ossification-type calcifications presented linear (46.7%) and irregular (46.7%) morphologies. Stromal-type calcifications showed irregular (87.5%) and linear (12.5) morphologies. The mass shape morphology was present only in ossification-type calcifications (Fig. 5).
Figure 5.
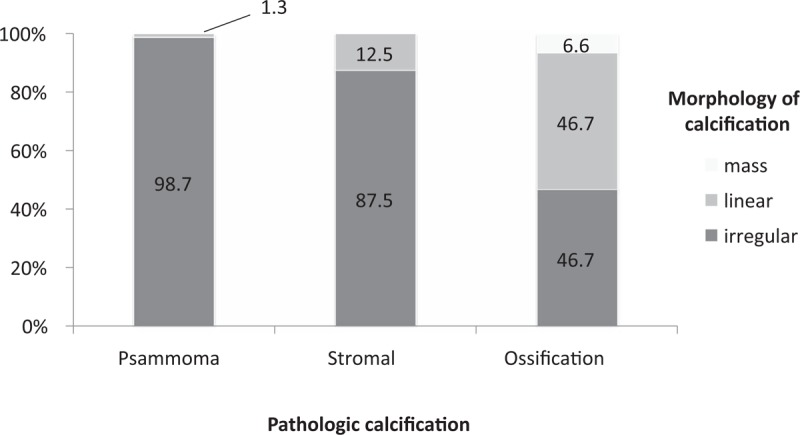
Morphology of calcification according to the pathologic calcification classifications.
3.4. Consistency of US and pathologic calcifications
Among the 411 nodules that were reviewed by a pathologist, 38.9% (n = 160) had any type of US calcification. The nodules with US calcification had significantly higher pathologic calcification than those without US calcification (71.6% vs 38.2%, P < .001). Figure 6 shows the presence of US calcification according to pathologic calcification type. The larger the size of pathologic calcification, the more calcification was present in US (psammoma 46.1% < stromal 53.7% < ossification 73.3%).
Figure 6.
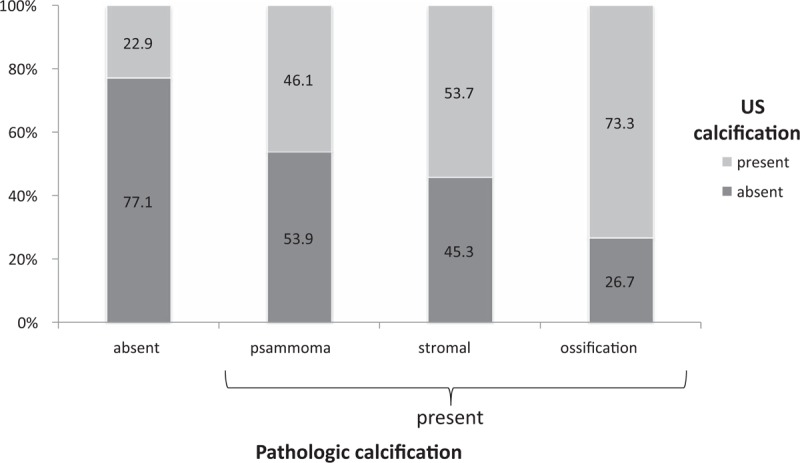
The existence of the ultrasonographic calcification according to pathologic calcification types.
The consistency of US and pathologic calcifications among only the nodules that had both US and pathologic calcifications are shown in Figure 7. Psammoma bodies occurred in all US MC type. Ossification nodules occurred in nearly all (92.3%) non-MC type. The stromal-only nodules were 36.8% MC-type and 63.2% non-MC type.
Figure 7.
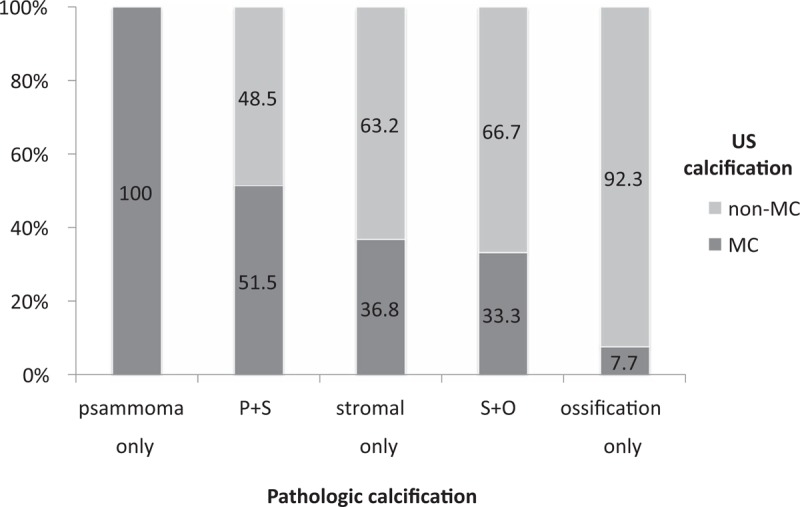
The consistency of US calcification and pathologic calcification among only nodules that had both ultrasonographic and pathologic calcification.
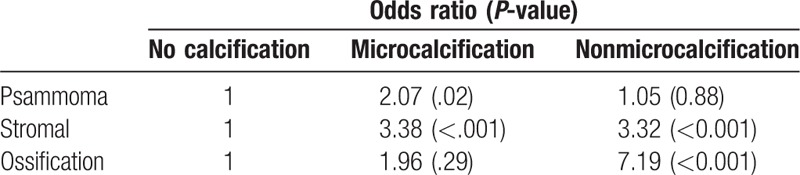
Table 3 shows the association between US calcification and pathologic calcification by logistic regression. MC type had a significantly higher odds ratio than non-MC type for predicting psammoma bodies. On the other hand, non-MC type had a significantly higher odds ratio for predicting ossification.
Table 3.
Association between ultrasonographic calcification and pathologic calcification patterns.
4. Discussion
The prevalence of thyroid cancer has been increasing worldwide since the 1990s,[10–12] and among all countries, Korea has had the most rapid increase in thyroid cancer incidence. From 1997 to 2011, the annual percent change in thyroid cancer was 25.1% in men and 23.7% in women.[13,14] Papillary thyroid cancer is a nonaggressive cancer with very low cancer-related mortality.[15,16] However, locoregional recurrences and distant metastases occur in up to 30% and 20% of patients, respectively, during long-term follow-up after initial treatment.[17,18] Locoregional recurrences and distant metastases have significant relationships with long-term mortality.[18–20] Therefore, the preoperative selection of high-risk patients is very important for making decisions about treatment strategies.
In this study, we evaluated postoperative pathologic prognostic factors according to preoperative US calcification assessments. Tumor size, capsular invasion, lymphovascular invasion, and lymph node metastasis are known predictors of aggressive PTC.[21,22] This study showed that patients with any type of calcification were older and had larger tumors than patients without calcification. Calcification could be part of the aging process in patients with thyroid cancer,[23] which suggests that older patients will show increased tumor sizes and more calcifications and could explain why tumors with calcifications have more aggressive features.
Interestingly, our study showed that tumors with MC were larger than those without MC, although patients with MC-type tumors were younger. In this study, MC-type tumors had worse pathologic features compared to non-MC-type tumors, and this is the most important finding of our study. We anticipate that MC-type tumors, which can be seen with ultrasonography, will have different pathogenesis and more aggressive PTC patterns. Previous studies have revealed that the presence of pathologic psammoma bodies is a poor prognostic factor.[7–9] Therefore, US MC may be related to pathologic psammoma bodies. However, no study has investigated the association between US MC and pathologic psammoma bodies. Therefore, we hypothesize that the MC type, with a poor US prognosis, may be pathologically related to psammoma bodies.
In this study, we showed that pathologic calcification is consistent with US MC type. We classified pathologic calcification into 3 types: psammoma body, stromal calcification, and ossification, and evaluated the relationships between these pathologic types and 2 US types of calcification. Nodules with any types of pathologic calcification had higher incidences of US calcification, although pathologic calcifications were not exactly coincident with US calcifications (Fig. 6). The nodules with psammoma bodies showed US MC-type more frequently than non-MC type (Fig. 7). This does not completely explain why the MC type had aggressive PTC patterns, but does partially suggest some feature. Additional studies are necessary to further address this observation.
The most important limitation of this study is that the study population could be potentially unrepresentative, because this is a retrospective single-center study that included only patients who were treated with surgery. This is a simple cross-sectional study, so we could not show mortality rates. At the time of data collection, only 12 patients had recurrences, of whom 6 had calcifications, and of these 6, 3 were MC-type and 3 were non-MC type. Therefore, we could not demonstrate that MC-type calcification and psammoma bodies were independent factors after adjusting for other factors through statistical methods. Although this is a potential weakness, our study still has several strengths. To our knowledge, this is the first study that shows the relationship between preoperative US calcification and pathologic calcification. This study also included a large number of patients with PTC and clearly showed differences between prognostic factors.
In conclusion, MC-type PTC had a higher incidence of capsular invasion, lymphovascular invasion, and lymph node metastasis compared to non-MC-type, although patients with MC were younger than those without. And pathologic calcification types partially coincided with US calcification types. MC-type PTC had more psammoma bodies and less ossification than non-MC type. Our results suggest that US MC-type calcification may be a useful prognostic indicator of aggressive PTC features. Therefore, US MC-type calcification could be a useful indicator for preoperative decision-making. Different pathophysiologic mechanisms may exist according to US calcification types, and further studies are needed to assess calcification type-associated pathogeneses.
Author contributions
Conceptualization: Bu Kyung Kim, Young Sik Choi, Young Ok Kim.
Data curation: Bu Kyung Kim, So Young Oak, Young Ok Kim.
Investigation: Bu Kyung Kim, Eun Mi Lee, So Young Oak, Su Kyoung Kwon, Young Ok Kim.
Methodology: Bu Kyung Kim, Eun Mi Lee, Young Sik Choi, Young Ok Kim.
Resources: Jeong Hoon Kim, Young Ok Kim.
Supervision: Jeong Hoon Kim, Su Kyoung Kwon, Young Sik Choi, Young Ok Kim.
Validation: Young Ok Kim.
Writing – original draft: Bu Kyung Kim.
Writing – review & editing: Bu Kyung Kim, Young Ok Kim.
Bu Kyung Kim orcid: 0000-0001-7845-4377
Footnotes
Abbreviations: MC = microcalcification, non-MC = nonmicrocalcification, PTC = papillary thyroid carcinoma, US = ultrasonographic.
Presented at the Annual meeting of the American Thyroid Association (ATA) 2014 and the International Thyroid Congress Program (ITC) 2015.
This work was supported in part by a grant from the Kosin University College of Medicine.
The authors have no conflicts of interest to disclose.
References
- [1].Khoo ML, Asa SL, Witterick IJ, et al. Thyroid calcification and its association with thyroid carcinoma. Head Neck 2002;24:651–5. [DOI] [PubMed] [Google Scholar]
- [2].Kakkos SK, Scopa CD, Chalmoukis AK, et al. Relative risk of cancer in sonographically detected thyroid nodules with calcifications. J Clin Ultrasound 2000;28:347–52. [DOI] [PubMed] [Google Scholar]
- [3].Koike E, Noguchi S, Yamashita H, et al. Ultrasonographic characteristics of thyroid nodules: prediction of malignancy. Arch Surg 2001;136:334–7. [DOI] [PubMed] [Google Scholar]
- [4].Lu Z, Mu Y, Zhu H, et al. Clinical value of using ultrasound to assess calcification patterns in thyroid nodules. World J Surg 2011;35:122–7. [DOI] [PubMed] [Google Scholar]
- [5].Chen G, Zhu XQ, Zou X, et al. Retrospective analysis of thyroid nodules by clinical and pathological characteristics, and ultrasonographically detected calcification correlated to thyroid carcinoma in South China. Eur Surg Res 2009;42:137–42. [DOI] [PubMed] [Google Scholar]
- [6].Kim BK, Choi YS, Kwon HJ, et al. Relationship between patterns of calcification in thyroid nodules and histopathologic findings. Endocr J 2013;60:155–60. [DOI] [PubMed] [Google Scholar]
- [7].Bai Y, Zhou G, Nakamura M, et al. Survival impact of psammoma body, stromal calcification, and bone formation in papillary thyroid carcinoma. Mod Pathol 2009;22:887–94. [DOI] [PubMed] [Google Scholar]
- [8].Das DK, Mallik MK, Haji BE, et al. Psammoma body and its precursors in papillary thyroid carcinoma: a study by fine-needle aspiration cytology. Diagn Cytopathol 2004;31:380–6. [DOI] [PubMed] [Google Scholar]
- [9].Cai YF, Wang QX, Ni CJ, et al. The clinical relevance of psammoma body and Hashimoto thyroiditis in papillary thyroid carcinoma: a large case-control study. Medicine (Baltimore) 2015;94:e1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ito Y, Nikiforov YE, Schlumberger M, et al. Increasing incidence of thyroid cancer: controversies explored. Nat Rev Endocrinol 2013;9:178–84. [DOI] [PubMed] [Google Scholar]
- [11].Chen AY, Jemal A, Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer 2009;115:3801–7. [DOI] [PubMed] [Google Scholar]
- [12].Kilfoy BA, Zheng T, Holford TR, et al. International patterns and trends in thyroid cancer incidence, 1973–2002. Cancer Causes Control 2009;20:525–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Colonna M, Uhry Z, Guizard AV, et al. Recent trends in incidence, geographical distribution, and survival of papillary thyroid cancer in France. Cancer Epidemiol 2015;39:511–8. [DOI] [PubMed] [Google Scholar]
- [14].Jung KW, Won YJ, Kong HJ, et al. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat 2013;45:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Oh CM, Jung KW, Won YJ, et al. Age-period-cohort analysis of thyroid cancer incidence in Korea. Cancer Res Treat 2015;47:362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Grebe SK, Hay ID. Thyroid cancer nodal metastases: biologic significance and therapeutic considerations. Surg Oncol Clin N Am 1996;5:43–63. [PubMed] [Google Scholar]
- [17].Kim WB. A closer look at papillary thyroid carcinoma. Endocrinol Metab (Seoul) 2015;30:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med 1994;97:418–28. [DOI] [PubMed] [Google Scholar]
- [19].Tubiana M, Schlumberger M, Rougier P, et al. Long-term results and prognostic factors in patients with differentiated thyroid carcinoma. Cancer 1985;55:794–804. [DOI] [PubMed] [Google Scholar]
- [20].Palme CE, Waseem Z, Raza SN, et al. Management and outcome of recurrent well-differentiated thyroid carcinoma. Arch Otolaryngol Head Neck Surg 2004;130:819–24. [DOI] [PubMed] [Google Scholar]
- [21].Mercante G, Frasoldati A, Pedroni C, et al. Prognostic factors affecting neck lymph node recurrence and distant metastasis in papillary microcarcinoma of the thyroid: results of a study in 445 patients. Thyroid 2009;19:707–16. [DOI] [PubMed] [Google Scholar]
- [22].Baek SK, Jung KY, Kang SM, et al. Clinical risk factors associated with cervical lymph node recurrence in papillary thyroid carcinoma. Thyroid 2010;20:147–52. [DOI] [PubMed] [Google Scholar]
- [23].Seifert G. Heterotopic (extraosseous) calcification (calcinosis). Etiology, pathogenesis and clinical importance. Pathologe 1997;18:430–8. [DOI] [PubMed] [Google Scholar]


