Supplemental Digital Content is available in the text
Keywords: cardiovascular events, meta-analysis, regorafenib, solid tumors
Abstract
Background:
The present comparative meta-analysis was conducted to evaluate the cardiovascular events of regorafenib in patients with solid tumors.
Methods:
Eligible studies from MEDLINE, Google Scholar, Cochrane Library, Clinical key, EBSCO publishing and Ovid, which had reported cardiovascular adverse events potentially caused by regorafenib were absorbed. Data of clinical characteristics and cardiovascular events including hypertension, hemorrhage, thrombosis, and heart failure were extracted from selected literatures for the final analysis. Pooled analysis of cardiovascular adverse events was developed by relative risks (RRs) and corresponding 95% confidence intervals (CIs) with software STATA 13.0 and RevMan 5.3.
Results:
Thirty studies including 3813 patients were fit into analysis. The incidences of cardiovascular events of all-grade were: hypertension, 36.8% (95% CI, 29.8%–43.8%), hemorrhage, 8.6% (95% CI, 3.2%–14%), thrombosis, 1.4% (95% CI, 0.1%–2.8%), and heart failure, 2.9% (95% CI, 0.3%–5.6%). The incidences of cardiovascular events of high-grade were: hypertension, 9.9% (95% CI, 7.4%–12.4%), hemorrhage, 1.2% (95% CI, 0.3%–2.2%), thrombosis, 1.6% (95% CI, 0.2%–3.4%), and heart failure, 2.9% (95% CI, 0.3%–5.6%). The RRs and their 95% CIs of all-grade cardiovascular events among patients treated with regorafenib were: hypertension, 4.10 (95% CI, 3.07–5.46; P < .00001), hemorrhage, 2.71 (95% CI, 1.45–5.08; P = .002), thrombosis, 1.27 (95% CI, 0.49–3.27; P = .62), and heart failure, 0.79 (95% CI, 0.16–3.94; P = .77). The RRs and their 95% CIs of high-grade cardiovascular events among patients treated with regorafenib were: hypertension, 5.82 (95% CI, 3.46–9.78; P < .00001), hemorrhage, 0.90 (95% CI, 0.50–1.61; P = .72), thrombosis, 1.28 (95% CI, 0.48–3.41; P = .62), and heart failure, 1.15 (95% CI, 0.23–5.69; P = .86), respectively.
Conclusion:
The present meta-analysis has demonstrated that regorafenib is associated with an increasing risk of hypertension at all-grade and high-grade, as well as hemorrhage at all-grade. Adequate awareness of cardiovascular adverse events of regorafenib should be established for clinicians.
1. Introduction
Regorafenib, also known as Stivarga or BAY 73-4506, an oral small molecule multi-kinase inhibitor, has emerged as a targeted agent which inhibit the activity of various kinase, including fms-related tyrosine kinase 1 (FLT1, also known as vascular endothelial growth factor receptor 1,VEGFR1), kinase insert domain receptor (KDR, also known as VEGFR2), FLT4 (VEGFR3), platelet-derived growth factor α (PDGFR-α), PDGFR-β, KIT proto-oncogene receptor tyrosine kinase, fibroblast growth factor receptor 1 (FGFR1), FGFR2, TEK receptor tyrosine kinase, RAF-1, tyrosine-protein kinase TIE-2, v-RAF murine sarcoma viral oncogene homolog B (BRAF), BRAFV600E, stress-activated protein kinase 2 (SAPK2), and protein tyrosine kinase 5 (PTK5).[1,2] With the positive results of several related clinical trials, regorafenib has been approved by the United States Food and Drug Administration (FDA) for the therapy of metastatic colorectal carcinoma (mCRC),[3] advanced gastrointestinal stromal tumors (GIST),[4] and hepatocellular carcinoma (HCC).[5]
However, like other VEGFR tyrosine kinase inhibitors (TKIs), most of the randomized clinical trials on regorafenib were focus primarily on its anti-tumor efficacy, which might be more beneficial for its approval by administration, rather than cardiovascular events, which were reported as secondary outcomes.[3–8] That makes a precise definition of the complete spectrum of adverse events challenging. In addition, owing to the low incidence rate of cardiovascular events in one research, the specific types of events, incidence rate, relative risk to placebo, and potential influence on the management during the treatment of carcinoma still remain elusive.[9,10]
Thus, to provide some suggestion for clinicians, as well as onco-cardiology patients, the present systematic review and meta-analysis was performed to evaluate the incidence rate and relative risk (RR) of cardiovascular events induced by regorafenib in patients with solid carcinomas among available clinical trials.
2. Methods
2.1. Literature search
A literature review among databases including MEDLINE, Google Scholar, Cochrane Library, Clinical key, EBSCO publishing, and Ovidwas conducted up to November 2017 with the main key word regorafenib. The search was limited to clinical trials including perspective or prospective ones, which published in English. The present meta-analysis was conducted in compliance with the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions and was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.[11]
2.2. Inclusion and exclusion criteria
Inclusion criteria: Clinical trials related to regorafenib in patients with solid tumors; participants were suggested to receive regorafenib or placebo treatment in controlled studies or regorafenib alone in single-armed studies; adverse events at all-grade or high-grade of hypertension, hemorrhage, thrombosis, or heart failure were reported. Exclusion criteria: publications of review articles, case reports, correspondences, and basic researches were excluded; meeting abstracts without data of cardiovascular event reported; classification of adverse event did not meet the standard of National Cancer Institute Common Toxicity Criteria (NCI CTC).
2.3. Data extraction
Data extraction was performed by 2 independent investigator. The following information was extracted from each included literature: first author's name, year of publication, region, cancer sites, previous treatment lines, number of patients, median age, sex, European cooperative oncology group performance status (ECOG PS), median treatment duration, and the dose of regorafenib. In terms to cardiovascular events, adverse events including hypertension, hemorrhage, thrombosis, and heart failure at all-grade and high-grade were recorded according to NCI CTC versions. Any discrepancies between investigators were resolved by consensus.
2.4. Quality assessment of included studies
The quality of the included randomized clinical trials (RCTs) was evaluated with the criteria of Cochrane Collaboration's tool for assessing risk of bias of RCTs by the 2 reviewers. The following items were adopted for the assessment: random sequence generation, allocation concealment, binding of participants and personnel, binding of outcome assessments, incomplete outcome data, selective reporting and other bias, which were presented with figures, such as “risk of bias graph” and “risk of bias summary.”
2.5. Statistical analysis
The primary objective of the present study was to investigate the incidence rate, RR, and corresponding 95% confidence intervals (CIs) of cardiovascular events at all-grade and high-grade of regorafenib in patients with solid tumors. For the calculation of incidence rate, data of the number of patients, as well as the number of cardiovascular events at all-grade and high-grade, were extracted from all the selected literatures, including randomized, controlled trials, and single-armed researches. Non-comparative binary data were analyzed as follows. P = X/n; SE (P) = (P [1–P)/n)1/2 (P, incidence rate; X, events; n, total; SE, standard error). The formulas were appropriate for the circumstance of approximate normal distribution of sampling. If the data were unmatched to normal distribution, the formulas were adopted as follows, P = ln (X/[n–X]); SE (P) = (1/X + 1/[n–X])1/2; Pt = OR/(1 + OR); 95%CI lower limit, LL = LLOR/(1 + LLOR); 95%CI upper limit, UL = ULOR/(1 + ULOR). The incidences of cardiovascular events were performed with software STATA 13.0 (StataCorp LP, Texas). For the analysis of RR, data of number of patients and the ones of cardiovascular events at all-grade and high-grade were extracted from randomized, placebo-controlled trials only. Cochrane Q test and inconsistency statistic (I2) were applied for the heterogeneity evaluation among the included literatures. The calculations of RR were developed with software RevMan 5.3 (Cochrane Collaboration, USA). P-value >.1 and I2 < 50% were supposed to show no substantial heterogeneity existed. Random effect model or fixed effect one were applied for the analysis of data of heterogeneous or not for both incidence and RR. A P-value <.05 was considered statistically significant. Potential publication bias was detected with funnel plots in software RevMan 5.3.
3. Results
3.1. Search results
A total of 884 potential literatures has been initially searched in databases including MEDLINE, Google Scholar, Cochrane Library, Clinical key, EBSCO Publishing and Ovid. Five hundred twenty articles were removed because of duplications. Two hundred fourty-six researches were further excluded with the property of clinical trials in regard to regorafenib. With the inclusion criteria, 34 papers were adsorbed for the potentially final assessment. After full text carefully reviewed, 4 researches had been conclusively eliminated because of reasons including insufficient data of cardiovascular events (n = 2), confusing classification of events (n = 1), and therapeutic drugs rather than placebo or blank as control (n = 1). Accordingly, a total of 30 clinical trials related to regorafenib were considered eligible for the final analysis. Six studies were placebo-controlled prospective clinical trials, and the other 24 researches were single-armed trials. A flow diagram which detailed the selection of included studies was presented in Fig. 1.
Figure 1.
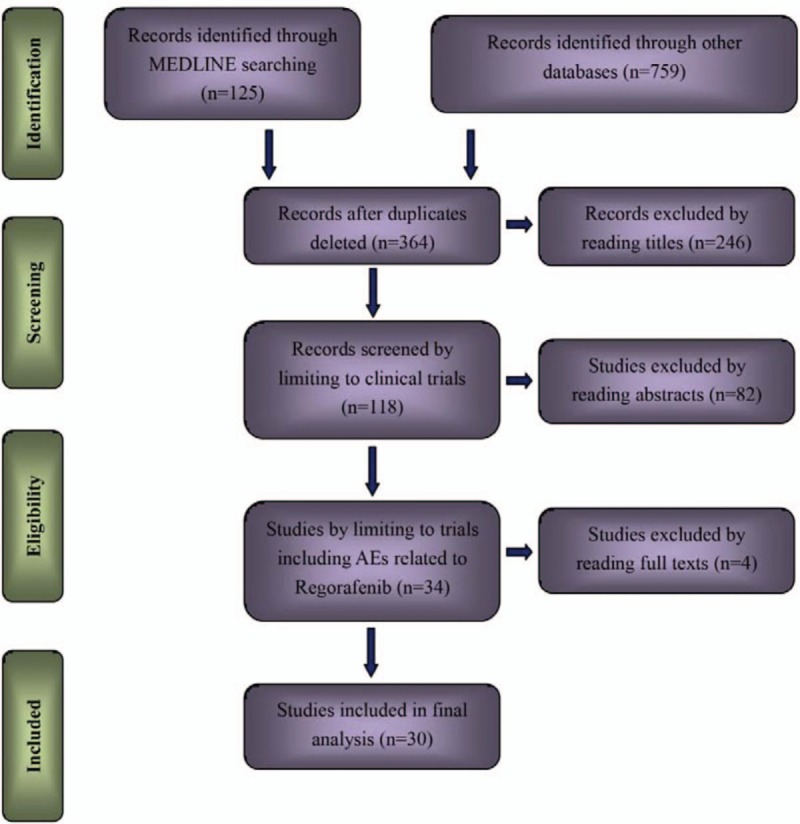
Study selection procedure with PRISMA flow diagram. PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
3.2. Quality assessment of the included studies
With the performance of quality evaluation within the criteria of Cochrane Collaboration's tool for assessing risk of bias of RCTs, we found that all the included RCTs satisfied the items including random sequence generation, allocation concealment, binding of participants and personnel, and binding of outcome assessments. However, cardiovascular events such as hypertension, hemorrhage, thrombosis, or heart failure were not entirely reported in selective literatures, results of which were presented in Figs. 2 and 3.
Figure 2.
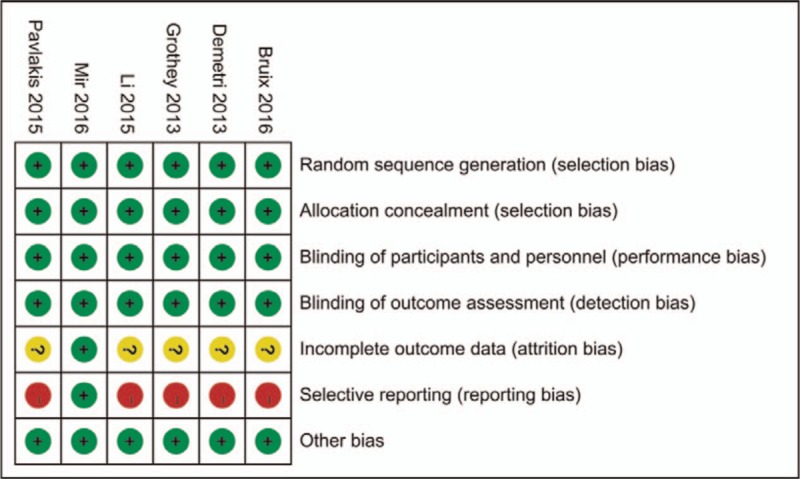
Risk of bias summary: review authors’ judgments about each risk of bias item for each included study.
Figure 3.
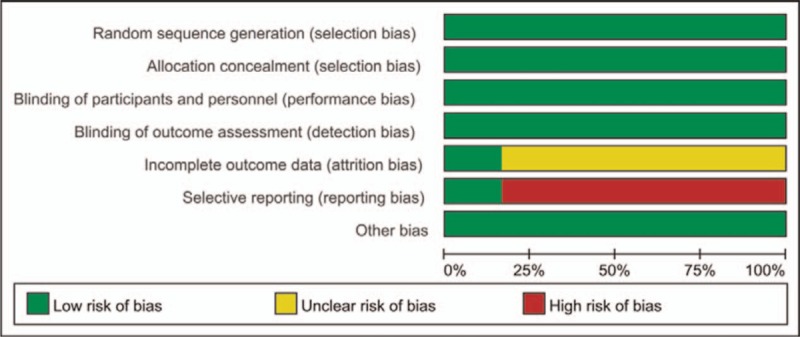
Risk of bias graph: review authors’ judgments about each risk of bias item presented as percentages among all included studies.
3.3. Population characteristics
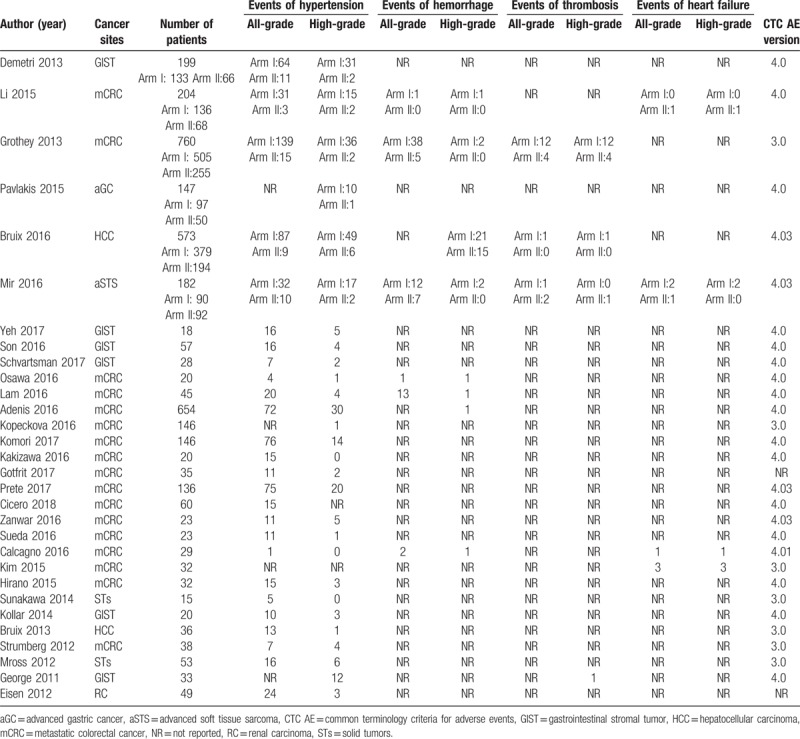
A total of 3813 patients were considered available in the present meta-analysis, solid tumors of which included GIST, mCRC, advanced gastric cancer (aGC), HCC, advanced soft tissue sarcoma (aSTS), and renal carcinoma (RC). The majority of patients have Eastern Cooperative Oncology Group performance status (ECOG PS) as 0, 1, and 2. The baseline clinic-pathological characteristics and the number of cardiovascular events of all-grade and high-grade in all included researches were presented in Tables 1 and 2.
Table 1.
Baseline characteristics of the researches included in present study.
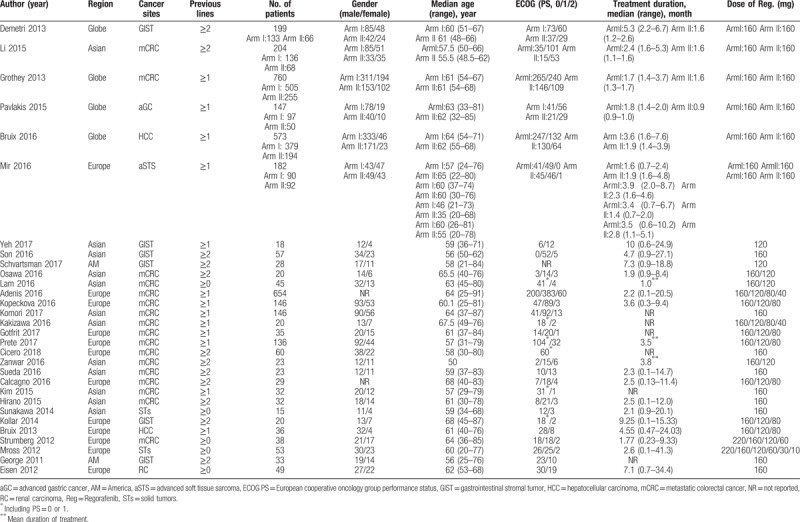
Table 2.
Number of events reported in included literatures.
3.4. Overall incidence of cardiovascular events
All the including researches were pooled analyzed for the overall incidence of cardiovascular events. The incidences of all-grade cardiovascular events were: hypertension, 36.8% (95% CI, 29.8%–43.8%), hemorrhage, 8.6% (95% CI, 3.2%–14%), thrombosis, 1.4% (95% CI, 0.1%–2.8%), and heart failure, 2.9% (95% CI, 0.3%–5.6%). The incidence of high-grade cardiovascular events were: hypertension, 9.9% (95% CI, 7.4%–12.4%), hemorrhage, 1.2% (95% CI, 0.3%–2.2%), thrombosis, 1.6% (95% CI, 0.2%–3.4%), and heart failure, 2.9% (95% CI, 0.3%–5.6%). Random-effects models were adopted for the pooled analysis of hypertension, hemorrhage, and thrombosis due to the significant heterogeneities for the events of all-grade and high-grade, while fixed-effects model explored for event of heart failure at all-grade and high-grade because of the insignificant heterogeneities, forest plots of which were showed in appendix 1–8 (see figure 1–8, Supplemental Digital Content, which present the forest plots of adverse events including hypertension, hemorrhage, thrombosis, and heart failure at all/high grade).
3.5. Relative risk of cardiovascular events
A meta-analysis of the RRs of cardiovascular events at all-grade and high-grade were performed in the 6 randomized, placebo-controlled clinical trials. Thus, a total of 2065 patients were enrolled. The RRs and their 95% CIs of all-grade cardiovascular events were: hypertension, 4.10 (95% CI, 3.07–5.46; P < .00001), hemorrhage, 2.71 (95% CI, 1.45–5.08; P = .002), thrombosis, 1.27 (95% CI, 0.49–3.27; P = .62), and heart failure, 0.79 (95% CI, 0.16–3.94; P = .77). The RRs and their 95% CIs of high-grade cardiovascular events were: hypertension, 5.82 (95% CI, 3.46–9.78; P < .00001), hemorrhage, 0.90 (95% CI, 0.50–1.61; P = .72), thrombosis, 1.28 (95% CI, 0.48–3.41; P = .62), and heart failure, 1.15 (95% CI, 0.23–5.69; P = .86), respectively (Figs. 4A–D and 5A–D).
Figure 4.
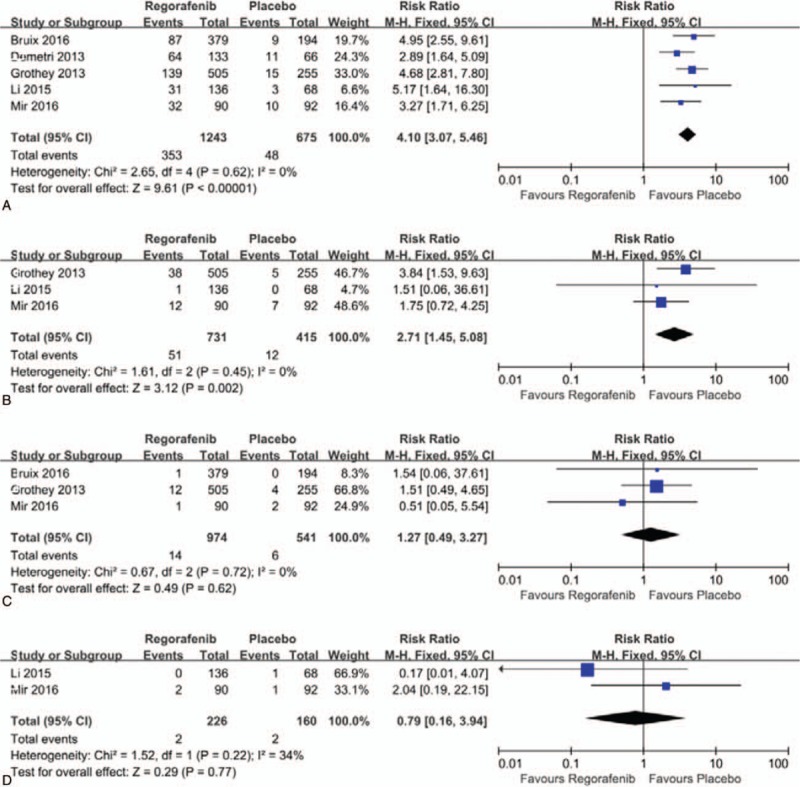
(A–D). Forest plots of relative risk of cardiovascular events of all-grade associated with regorafenib versus control. A. Hypertension; B. Hemorrhage; C. Thrombosis; D. Heart failure.
Figure 5.
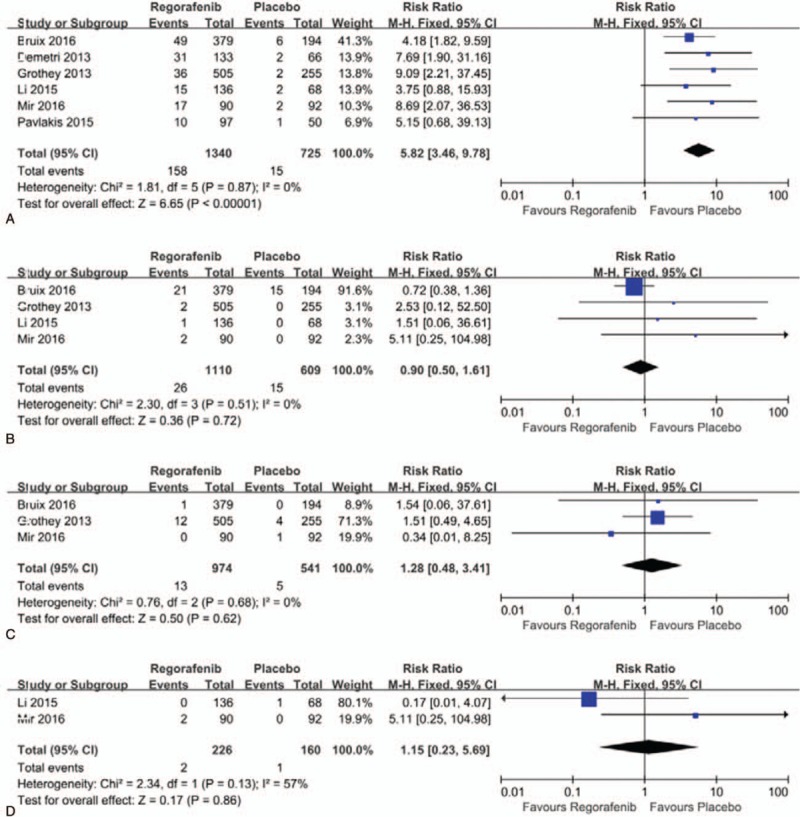
(A–D). Forest plots of relative risk of cardiovascular events of high-grade associated with regorafenib versus control. A. Hypertension; B. Hemorrhage; C. Thrombosis; D. Heart failure.
3.6. Publication bias
Funnel plot analysis has revealed no evidence of a publication bias for RRs of cardiovascular events at all-grade or high-grade (appendix 9–16, see figure 9–16, Supplemental Digital Content, which present the funnel plots of hypertension, hemorrhage, thrombosis, and heart failure at all/high grade at all/high grade for publication bias).
4. Discussion
To the best of our knowledge, here is the most updated and largest scaled systematic review and meta-analysis to evaluate the incidence and RR of the cardiovascular events for regorafenib in patients with solid carcinomas. Our analysis of available data revealed the incidence of cardiovascular events at all-grade and high-grade (grade 3 and 4) related to regorafenib were: hypertension at all-grade, 36.8% (95% CI, 29.8%–43.8%), and hypertension at high-grade, 9.9% (95% CI, 7.4%–12.4%); hemorrhage at all-grade, 8.6% (95% CI, 3.2%–14%), and hemorrhage at high-grade, 1.2% (95% CI, 0.3%–2.2%); thrombosis at all-grade, 1.4% (95% CI, 0.1%–2.8%), and thrombosis at high-grade, 1.6% (95% CI, 0.2%–3.4%); heart failure at all-grade, 2.9% (95% CI, 0.3%–5.6%), and heart failure at high-grade, 2.9% (95% CI, 0.3%–5.6%). Additionally, the present analysis demonstrated a significantly increased risk of hypertension at all-grade and high-grade, as well as hemorrhage at all-grade with the treatment of regorafenib compared with control.
With the extension of treatment duration and survival time, antitumor-induced cardiotoxicity has emerged as the second cause of death in patients who had received antitumor therapy.[12] Approximately, 2% to 3% patients had been reported suffering antitumor-induced cardiotoxicities during treatment in perspective clinical trials, while nearly 26% of which in prospective ones accounted.[13] A majority of cytotoxic agents have already been revealed with substantial cardiovascular events, such as adriamycin,[14] cyclophosphamide,[15] cisplatin,[16] fluorouracil,[17,18] and paclitaxel,[19] among which adriamycin comes to be the most outstanding one. The widely accepted hypothesis for adriamycin-induced cardiotoxicity were the generation of excess reactive oxygen species (ROS),[20] Topoisomerase (Top) 2β inhibition,[21] and the activation of p53 with its apoptotic pathway.[22] As the clinical application for decades with those agents, sufficient attention for cardiovascular toxicity has been paid, and relevant monitoring procedure, as well as precautionary measures established. However, cardiovascular toxicities of targeted drugs, the emerging effective and convenient ones in recent years, have also been reported in clinical researches, such as anti-Her-2 drugs,[12,20–22] anti-VEGFR drugs,[13,23] EGFR-TKIs,[24–26] and multi-target agents,[27,28] with mechanism of which still remains controversial.
Regorafenib, the multi-targeted agent, has also been reported considerable cardiovascular toxicities, and even fatal events.[4,6] According to the present pooled analysis, hypertension and hemorrhage seem to be the most significant cardiovascular events, with their incidence rate at all-grade as 36.8% and 8.6%, respectively, which was accordance with the data from former researches on other multi-targeted drugs, such as pazopanib, vandetanib, and axitinib. The incidence rate of hypertension with those multi-targeted drugs ranges from 30% to 50% of dose-dependent, of which the elevation of systemic blood pressure (SBP) from 20 to 30 mmHg, and increasing of diastolic blood pressure (DBP) from 9 to 17 mmHg.[28] Likewise, hypertension induced or aggravated intracranial hemorrhage (ICH), should been cared in clinical application. Additionally, other than ICH, regorafenib-induced hemorrhage has also been shown as nosebleed,[3] gastrointestinal bleeding,[3] and esophagorrhagia,[6] which might should not attributed to the exacerbation of hypertension merely. Results from several researches have revealed that hypertension and bleeding caused by those agents was resulted from endothelial dysfunction, dysfunctional nitric oxide metabolism, and vascular rarefaction, which was parallel to bevacizumab.[29] In addition, the destruction of pericytes, which are essential for blood vessel formation and maintenance, should also be considered responsible for agents-induced hypertension and hemorrhage. However, specific mechanism of hypertension or hemorrhage caused by regorafenib and multi-targeted drugs still remains doubtful.
Comparatively, relative risks of the other cardiovascular events induced by regorafenib, thrombosis, and heart failure, appear no statistical significance compared with placebo in present study, which seems better tolerated compared with other analogous agents. Sunitinib has been reported with a particularly high risk of congestive heart failure (CHF) (8.0%–12.5%), with a decrease in left ventricular ejection fraction (LVEF) of 1.5% to 2.0% after each cycle of treatment.[30,31] Besides, a former pooled analysis of randomized controlled trials involving 10,255 patients was conducted to evaluate the risk of arterial thrombotic events in patients treated with sorafenib or sunitinib, the results of which suggested that the RR of arterial thrombotic events related to sorafenib and sunitinib was 3.03 (95% CI: 1.25–7.37; P = .015) compared with the control,[32] result of which has been further identified by another meta-analysis regarding the cardiotoxicity of sorafenib and sunitinib.[33] Cabozantinib, another multi-targeted agent, targeting FLT3, KIT, MET, RET, and VEGFR2, was showed with severe pulmonary embolism at an incidence rate of 6% according to a phase II randomized discontinuation trial in patients with advanced prostate cancer.[34] Pazopanib, was even reported companied with pulmonary embolism at an incidence rate up to 10%, including 3% fatal events of that, in patients with advanced GIST.[35] However, as the diversity of carcinomas and characteristic of patients between regorafenib and other multi-targeted drugs, the superiority of thrombosis and heart failure with regoranib should be judged more prudent further.
As the apparent adverse events of hypertension of targeted agents, some solutions have been attempted for the prevention and treatment. Angiotensin-converting enzyme inhibitors (ACEI) and β-blockers have been shown to improve myocardial energetics, and further attenuate the degree of cell death, which results from sunitinib-induced apoptosis.[36] Additionally, thalidomide was also reported that it could protect pericyte survival, and reduce sunitinib-induced cardiovascular events without influencing its anticancer efficacy.[37] Metformin, the historic antidiabetic, was showed with preventing stress-induced left ventricular dysfunction as well according to vivo researches.[38] More studies on protective agents and deepgoing researches on mechanism of cardiovascular events of multi-targeted drugs including regorafenib have been conducted to provide alternative therapies.
It should be acknowledged that a number of limitations were existed in present meta-analysis, the most obvious one is the heterogeneity, which caused by the diversity of dose of regorafenib and tumor types in the included patients. We have tried to perform a meta-regression to solve the problem. However, we cannot specify the relevant coefficient, especially among studies of single-armed model (24/30), which comes to be one of the limitations of our study. Furthermore, the value of the present pooled analysis was limited by the respective limitations of included researches, which comes to be the common deficiency of meta-analysis. Finally, RR of heart failure was based on merely 2 placebo-controlled trials due to the missing data of other literatures, which should has brought controversial outcome of that. Thus, we could not establish more convinced results with the limitations.
In conclusion, the present meta-analysis has demonstrated that regorafenib is associated with an increasing risk of hypertension at all-grade and high-grade, as well as hemorrhage at all-grade compared with control. Adequate awareness of cardiovascular adverse events of regorafenib should be established for clinicians.
Author contributions
Data curation: Jianxin Chen.
Investigation: Junhui Wang.
Methodology: Jianxin Chen.
Project administration: Jianxin Chen.
Software: Jianxin Chen, Junhui wang.
Supervision: Jianxin Chen, Junhui Wang.
Visualization: Jianxin Chen.
Writing – original draft: Jianxin Chen.
Writing – review & editing: Jianxin Chen.
Supplementary Material
Footnotes
Abbreviations: ACEI = angiotensin-converting enzyme inhibitors, aGC = advanced gastric cancer, AM = America, aSTS = advanced soft tissue sarcoma, CHP = congestive heart failure, CI = confidence intervals, DBP = diastolic blood pressure, ECOG PS = European cooperative oncology group performance status, FGFR = fibroblast growth factor receptor, FLT1 = fms-related tyrosine kinase 1, GIST = gastrointestinal stromal tumor, HCC = hepatocellular carcinoma, ICH = intracranial hemorrhage, KDR = kinase insert domain receptor, LVEF = left ventricular ejection fraction, mCRC = metastatic colorectal cancer, NCI CTC = National Cancer Institute Common Toxicity Criteria, NR = not reported, PDGFR = platelet-derived growth factor, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, PTK = protein tyrosine kinase, RC = renal carcinoma, Reg = regorafenib, RR = relative risk, SBP = systemic blood pressure, STs = solid tumors, TKI = tyrosine kinase inhibitors, VEGFR = vascular endothelial growth factor receptor.
Funding: This study was not funded.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Authors declare that they have no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer 2011;129:245–55. [DOI] [PubMed] [Google Scholar]
- [2].Strumberg D, Schultheis B. Regorafenib for cancer. Exp Opin Investig Drugs 2012;21:879–89. [DOI] [PubMed] [Google Scholar]
- [3].Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303–12. [DOI] [PubMed] [Google Scholar]
- [4].Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389:56–66. [DOI] [PubMed] [Google Scholar]
- [6].Li J, Qin S, Xu R, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2015;16:619–29. [DOI] [PubMed] [Google Scholar]
- [7].Mir O, Brodowicz T, Italiano A, et al. Safety and efficacy of regorafenib in patients with advanced soft tissue sarcoma (REGOSARC): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol 2016;17:1732–42. [DOI] [PubMed] [Google Scholar]
- [8].Adenis A, de la Fouchardiere C, Paule B, et al. Survival, safety, and prognostic factors for outcome with Regorafenib in patients with metastatic colorectal cancer refractory to standard therapies: results from a multicenter study (REBECCA) nested within a compassionate use program. BMC Cancer 2016;16:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Liu B, Ding F, Liu Y, et al. Incidence and risk of hypertension associated with vascular endothelial growth factor receptor tyrosine kinase inhibitors in cancer patients: a comprehensive network meta-analysis of 72 randomized controlled trials involving 30013 patients. Oncotarget 2016;7:67661–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yu J, Zhang Y, Leung LH, et al. Efficacy and safety of angiogenesis inhibitors in advanced gastric cancer: a systematic review and meta-analysis. J Hematol Oncol 2016;9:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lenihan D, Suter T, Brammer M, et al. Pooled analysis of cardiac safety in patients with cancer treated with pertuzumab. Ann Oncol 2012;23:791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Economopoulou P, Kotsakis A, Kapiris I, et al. Cancer therapy and cardiovascular risk: focus on bevacizumab. Cancer Manag Res 2015;7:133–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Outomuro D, Grana DR, Azzato F, et al. Adriamycin-induced myocardial toxicity: new solutions for an old problem? Int J Cardiol 2007;117:6–15. [DOI] [PubMed] [Google Scholar]
- [15].Floyd JD, Nguyen DT, Lobins RL, et al. Cardiotoxicity of cancer therapy. J Clin Oncol 2005;23:7685–96. [DOI] [PubMed] [Google Scholar]
- [16].Demkow U, Stelmaszczyk-Emmel A. Cardiotoxicity of cisplatin-based chemotherapy in advanced non-small cell lung cancer patients. Respir Physiol Neurobiol 2013;187:64–7. [DOI] [PubMed] [Google Scholar]
- [17].Polk A, Vaage-Nilsen M, Vistisen K, et al. Cardiotoxicity in cancer patients treated with 5-fluorouracil or capecitabine: a systematic review of incidence, manifestations and predisposing factors. Cancer Treat Rev 2013;39:974–84. [DOI] [PubMed] [Google Scholar]
- [18].Polk A, Vistisen K, Vaage-Nilsen M, et al. A systematic review of the pathophysiology of 5-fluorouracil-induced cardiotoxicity. BMC Pharmacol Toxicol 2014;15:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mukku RB, Fonarow GC, Watson KE, et al. Heart failure therapies for end-stage chemotherapy-induced cardiomyopathy. J Card Fail 2016;22:439–48. [DOI] [PubMed] [Google Scholar]
- [20].Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 2011;365:1273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dang C, Guo H, Najita J, et al. Cardiac outcomes of patients receiving adjuvant weekly paclitaxel and trastuzumab for node-negative, ERBB2-positive breast cancer. JAMA Oncol 2016;2:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Baselga J, Gelmon KA, Verma S, et al. Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapy. J Clin Oncol 2010;28:1138–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tsai HT, Marshall JL, Weiss SR, et al. Bevacizumab use and risk of cardiovascular adverse events among elderly patients with colorectal cancer receiving chemotherapy: a population-based study. Ann Oncol 2013;24:1574–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Scheffel RS, Dora JM, Siqueira DR, et al. Toxic cardiomyopathy leading to fatal acute cardiac failure related to vandetanib: a case report with histopathological analysis. Eur J Endocrinol 2013;168:K51–4. [DOI] [PubMed] [Google Scholar]
- [25].Ma W, Xu M, Liu Y, et al. Safety profile of combined therapy inhibiting EFGR and VEGF pathways in patients with advanced non-small-cell lung cancer: a meta-analysis of 15 phase II/III randomized trials. Int J Cancer 2015;137:409–19. [DOI] [PubMed] [Google Scholar]
- [26].Kloth JS, Pagani A, Verboom MC, et al. Incidence and relevance of QTc-interval prolongation caused by tyrosine kinase inhibitors. Br J Cancer 2015;112:1011–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ewer MS, Suter TM, Lenihan DJ, et al. Cardiovascular events among 1090 cancer patients treated with sunitinib, interferon, or placebo: a comprehensive adjudicated database analysis demonstrating clinically meaningful reversibility of cardiac events. Eur J Cancer 2014;50:2162–70. [DOI] [PubMed] [Google Scholar]
- [28].Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 2013;369:722–31. [DOI] [PubMed] [Google Scholar]
- [29].Veronese ML, Mosenkis A, Flaherty KT, et al. Mechanisms of hypertension associated with BAY 43-9006. J Clin Oncol 2006;24:1363–9. [DOI] [PubMed] [Google Scholar]
- [30].Chu TF, Rupnick MA, Kerkela R, et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet 2007;370:2011–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hutson TE, Figlin RA, Kuhn JG, et al. Targeted therapies for metastatic renal cell carcinoma: an overview of toxicity and dosing strategies. Oncologist 2008;13:1084–96. [DOI] [PubMed] [Google Scholar]
- [32].Choueiri TK, Schutz FA, Je Y, et al. Risk of arterial thromboembolic events with sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials. J Clin Oncol 2010;28:2280–5. [DOI] [PubMed] [Google Scholar]
- [33].Schutz FA, Je Y, Richards CJ, et al. Meta-analysis of randomized controlled trials for the incidence and risk of treatment-related mortality in patients with cancer treated with vascular endothelial growth factor tyrosine kinase inhibitors. J Clin Oncol 2012;30:871–7. [DOI] [PubMed] [Google Scholar]
- [34].Smith DC, Smith MR, Sweeney C, et al. Cabozantinib in patients with advanced prostate cancer: results of a phase II randomized discontinuation trial. J Clin Oncol 2013;31:412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Mir O, Cropet C, Toulmonde M, et al. Pazopanib plus best supportive care versus best supportive care alone in advanced gastrointestinal stromal tumours resistant to imatinib and sunitinib (PAZOGIST): a randomised, multicentre, open-label phase 2 trial. Lancet Oncol 2016;17:632–41. [DOI] [PubMed] [Google Scholar]
- [36].Bhave M, Akhter N, Rosen ST. Cardiovascular toxicity of biologic agents for cancer therapy. Oncology (Williston Park) 2014;28:482–90. [PubMed] [Google Scholar]
- [37].Chintalgattu V, Rees ML, Culver JC, et al. Coronary microvascular pericytes are the cellular target of sunitinib malate-induced cardiotoxicity. Sci Transl Med 2013;5:187ra69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Gundewar S, Calvert JW, Jha S, et al. Activation of AMP-activated protein kinase by metformin improves left ventricular function and survival in heart failure. Circ Res 2009;104:403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


