Abstract
Background:
The association of MMP-8 rs2012390 and rs11225394 polymorphisms with osteonecrosis of the femoral head (ONFH) risks was investigated in several studies with conflicting results. We performed the meta-analysis to evaluate the association between them.
Methods:
Potentially relevant literatures were searched from the electronic databases of PubMed, Web of Science, and Cochrane Library. All databases were searched up to May 6, 2018. The strength of associations of the MMP-8 rs2012390 and rs11225394 polymorphisms with ONFH risk was assessed by crude odds ratios (ORs) with their 95% confidence intervals (CIs) under different genetic models.
Results:
A total of 1469 cases diagnosed with ONFH and 1211 healthy controls were included in the current meta-analysis. A remarkable association between rs11225394 in the MMP-8 gene and an increased risk of ONFH was found (allele model: OR = 1.33, 95% CI = 1.09–1.61, P = .005; heterozygote model: OR = 1.39, 95% CI = 1.13–1.71, P = .002; dominant model: OR = 1.40, 95% CI = 1.14–1.73, P = .002, respectively). Meanwhile, a significant association between MMP-8 rs2012390 and the decreased risk of ONFH was found in heterozygote model (OR = 0.63, 95% CI = 0.51–0.77, P < .00001).
Conclusion:
The meta-analysis results showed a remarkable association between rs11225394 in MMP-8 gene and an increased risk of ONFH and a significant association between MMP-8 rs2012390 and the decreased risk of ONFH.
Keywords: matrix metalloproteinase, osteonecrosis of the femoral head, single-nucleotide polymorphism
1. Introduction
Osteonecrosis of the femoral head (ONFH), also known as avascular necrosis, is a challenging orthopedic disease characterized by both ischemia and bone necrosis. It severely damages the quality of life of patients in the advanced stage, especially to young patients with an active lifestyle. The incidence has been almost certainly on the rise, with a prevalence of approximately 20,000 to 30,000 new cases annually in the United States[1] and 100,000 to 200,000 new ones per year in China.[2] Nevertheless, several theories have been proposed to delineate the pathophysiology of ONFH, the exact pathogenic mechanisms remain poorly understood. The well-known risk factors include trauma, corticosteroid therapy, excessive alcohol consumption, coagulation disorders, autoimmune diseases, hemoglobinopathies, smoking, and so on.[3] However, no all smokers or divers experience this disease, illustrating that environmental and genetic background might contribute to the etiology of ONFH. Meanwhile, emerging lines of evidence suggested the matrix metalloproteinase (MMP) single-nucleotide polymorphisms (SNPs) were closely related to the susceptibility of ONFH.
MMPs, a family of more than twenty protein members, play pivotal roles in the process of degraduation of all structural components of the extracellular matrix. Multiple pieces of evidence have showed the abnormal expressed levels of MMPs in the in different orthopedic diseases. Green et al.[4] suggested that the high serum MMP-1 and MMP-3 levels predicted progression of joint damage. MMP-1, MMP-2, and MMP-9 proteins are overexpressed in the serum of osteoarthritis patients.[5] Also, MMP-3 protein is overexpressed in synovium of knee joint in osteoarthritis patients.[6] In contract, MMP-13 deficiency increases the fracture fragility of bone, indicating the normal need of MMP-13 for the fracture toughness.[7] What's more, both MMP-2 and MMP-9 are cleaved and activated by MMP-13.[8] As a result, MMP-13 deficient mice may show the loss of function of MMP-2 and MMP-9. Compared with other MMP members, MMP-8 has been less investigated for its biological roles and proteolytic substrates.[9] MMP-8 (also named collagenase-2 or neutrophil collagenase) is mainly expressed in neutrophils, and it is also produced by various other cell types including stem/progenitor cells.[10]
To date, several genetic association studies have examined the associations of MMP-8 rs2012390 and rs11225394 polymorphisms with ONFH risks, however, with controversial results. Du et al[11] shown that MMP-8 rs2012390 was associated with an increased risk of ONFH. However, Chen et al[12] reported that MMP-8 rs2012390 decreased the risk of ONFH. Thus far, no meta-analysis has yet been carried out to evaluate the relationships between MMP-8 rs2012390 and rs11225394 polymorphisms and ONFH. Therefore, we undertook this comprehensive meta-analysis to summarize these inconsistent results, aiming at providing a more precise evaluation whether MMP-8 rs2012390 and rs11225394 polymorphisms contribute to the risk of developing ONFH.
2. Methods
2.1. Search strategy
The review was approved by the review board of Linyi People's Hospital. All databases were searched up to May 6, 2018. Potentially relevant literatures were searched from the electronic databases of PubMed, Web of Science, and Cochrane Library using a combination of the following items: (“matirx metalloproteinase 8” OR “MMP 8” OR “collagenase 2” OR “neutrophil collagenase”) AND (“avascular necrosis of femoral head” OR “osteonecrosis of the femoral head” OR “ONFH” OR “femoral head necrosis”). No language restrictions were applied. The reference lists of all retrieved articles were also identified manually for additional possible studies.
2.2. Inclusion and exclusion criteria
Studies eligible for the meta-analysis need comply with the following criteria: with a case–control design; investigating the relationship between MMP-8 rs2012390 or rs11225394 polymorphisms and the risk of developing ONFH; providing sufficient data of genotype frequencies for MMP-8 rs2012390 or rs11225394; performed on human. Studies were excluded if they did not meet the inclusion criteria above and including the following aspects: reviews, letters to editors, case reports, comments, and animal studies; including overlapping data; obvious errors in research design.
2.3. Data extraction
Two reviewers independently extracted the following information from each collected study: first author, year of publication, country and ethnicity, numbers of cases and controls, genotype distributions in case and control groups, genotyping method. No disagreement about the data presented among all reviewers.
2.4. Quality assessment
The methodological quality of each selected study was evaluated according to the Clark Scores System which containing 10 items. The quality of studies with a score below 5 was regarded as low, between 5 and 7 was regarded as moderate, and above 7 was regarded as high.
2.5. Statistical analysis
All data were undertaken using Review Manager (version 5.1 for Windows; The Cochrane Collaboration, Oxford, UK). The strength of associations of the MMP-8 rs2012390 and rs11225394 polymorphisms with ONFH risk was assessed by crude odds ratios (ORs) with their 95% confidence intervals (CIs) under different genetic models (allele, homozygote, heterozygote, dominant, and recessive model). A test of heterogeneity across studies was measured using Chi-square-based Q and I2 statistics in each genetic model. The fixed effect (Mantel–Haenszel method) was employed to calculate the pooled OR if P > .1 or I2 < 50%.[13] Otherwise, the random effect (DerSimonian Laird) was chosen.[14] And then the publication bias was checked by Begg funnel plots and Egger linear test.[15,16] A sensitivity analysis was applied to assess the stability of results by omitting each study one at a time. All P values were 2-sided, and P < .05 was regarded as statistically significant.
3. Results
3.1. Study characteristics and quality assessment
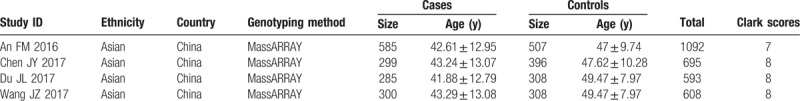
The procedure of selecting eligible studies is described in Fig. 1. Our search yielded 22 records in total. After screening the title and abstract, 7 articles were remained. And 3 more studies were rejected after further reviewing the full text according the selection criteria. Eventually, 4 studies with a total of 1469 cases diagnosed with ONFH and 1211 healthy controls were included in the current meta-analysis. Of the included studies, all were published in recent 3 years from 2016 to 2017 in English language with high quality. Two of them undertaken the same data of healthy participants as control.[11,17] All of them were performed in the Chinese population. The primary information of eligible studies is listed in Table 1.
Figure 1.
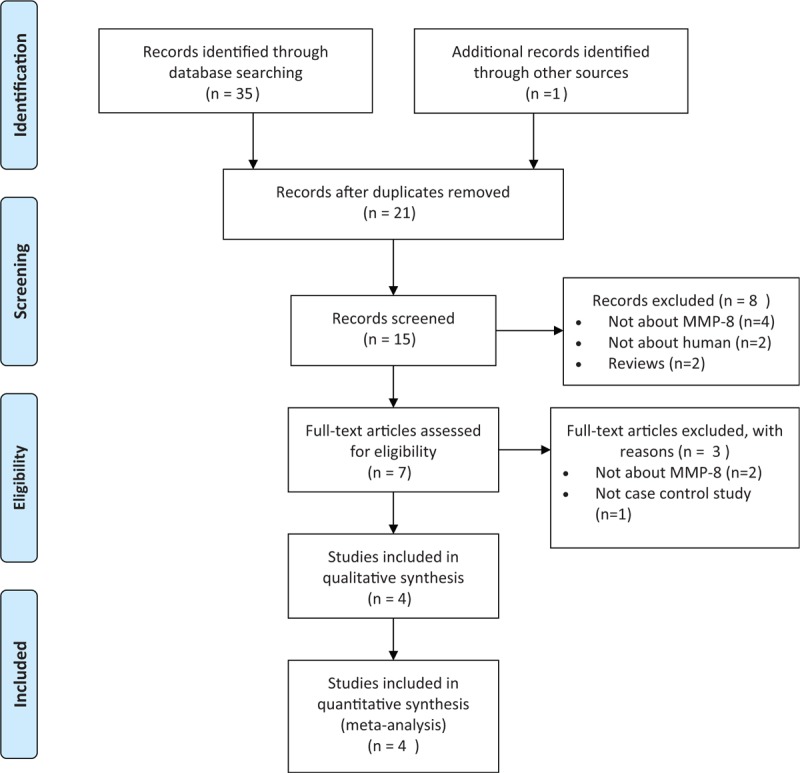
Flow diagram of the literature search.
Table 1.
Characteristics of the studies included in the meta-analysis.
3.2. Meta-analysis results
The meta-analysis results indicated that a remarkable association between rs11225394 in the MMP-8 gene and an increased risk of ONFH was found (allele model: OR = 1.33, 95% CI = 1.09–1.61, P = .005; heterozygote model: OR = 1.39, 95% CI = 1.13–1.71, P = .002; dominant model: OR = 1.40, 95% CI = 1.14–1.73, P = .002, respectively). Meanwhile, a significant association between MMP-8 rs2012390 and the decreased risk of ONFH was found in heterozygote model (OR = 0.63, 95% CI = 0.51–0.77, P < .01). No heterogeneity was observed among these studies, and therefore the fixed effect models were established above. As for allele model (OR = 1.03, 95% CI = 0.78–1.36, P = .85), homozygote model (OR = 1.04, 95% CI = 0.73–1.49, P = .82), dominant model (OR = 1.02, 95% CI = 0.72–1.49, P = .81), and recessive model (OR = 1.05, 95% CI = 0.74–1.49, P = .81), no statistical significance was identified in the association between MMP-8 rs2012390 and ONFH risk (Table 2 and Figs. 2–5).
Table 2.
Genotype and allelic distribution of rs2012390 and rs11225394 in the studies enrolled.
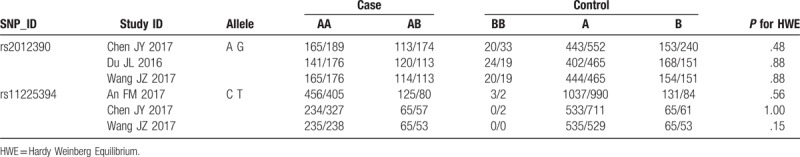
Figure 2.
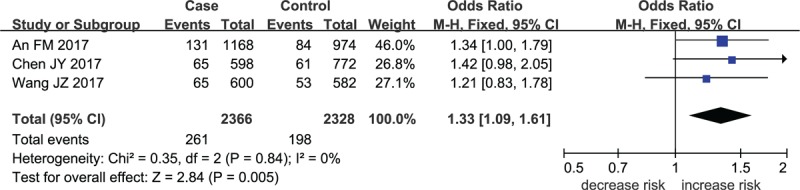
Forest plot for MMP-8 rs11225394 in allele model.
Figure 5.
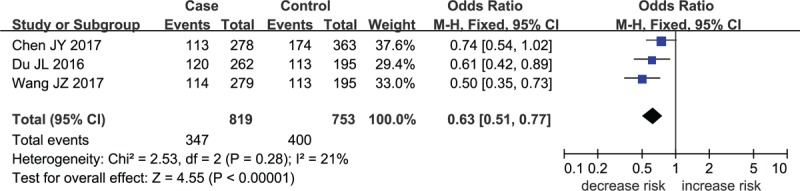
Forest plot for MMP-8 rs2012390 in heterozygote model.
Figure 3.
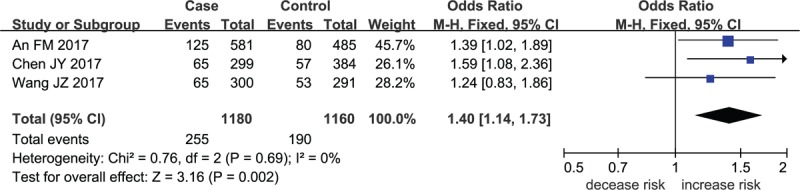
Forest plot for MMP-8 rs11225394 in heterozygote model.
Figure 4.
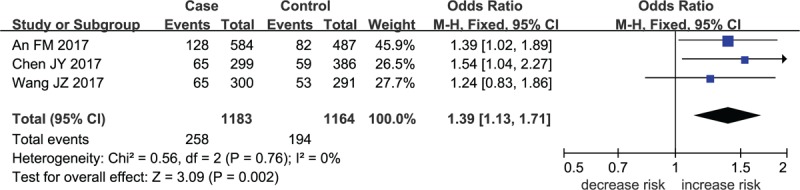
Forest plot for MMP-8 rs11225394 in dominant model.
3.3. Sensitivity analyses and publication bias
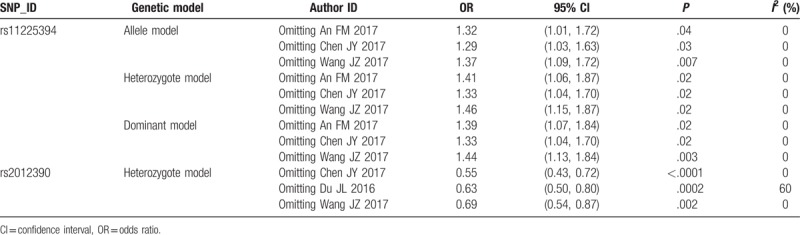
Sensitivity analyses were conducted by eliminating each eligible study in turn so as to reflect the influence of each individual study on the pooled ORs. For rs11225394 and rs2012390 polymorphisms, the corresponding pooled ORs were not dramatically altered, indicating that our results were statistically robust (Table 3).
Table 3.
Summary of sensitivity analyses.
The potential publication bias of eligible literatures was estimated by Begg funnel plot and Egger test. Neither Begg rank correlation method nor Egger regression method showed publication bias (Fig. 6).
Figure 6.
Funnel plot for publication bias among studies.
4. Discussion
ONFH is a kind of orthopedic disease confined to hip joint which is characterized by different pathologic stages of ischemia, necrosis, and eventually collapse. Ischemia is reversible, but the latter 2 stages are not.[18] It is widely believed that genes play an important role in the latter 2 processes. Besides, SNPs in several genes were found to be associated with the susceptibility to orthpedic diseases including ONFH.[19,20] Recently, several studies investigated the correlation between MMP-8 and the risk of ONFH.[12,17,21,22] Du et al[11] shown that MMP-8 rs2012390 was associated with an increased risk of ONFH. However, Chen et al[12] reported that MMP-8 rs2012390 decreased the risk of ONFH. And no meta-analysis has been undertaken to evaluate the results above. Their inconsistent results prompted our meta-analysis.
The present study was the first meta-analysis aimed to investigate the associations of MMP-8 rs2012390 and rs11225394 polymorphisms with ONFH risk. In the meta-analysis, obvious heterogeneity was observed throughout the studies enrolled. The meta-analysis results showed a remarkable association between rs11225394 in MMP-8 gene and an increased risk of ONFH, also a significant association between MMP-8 rs2012390 and the decreased risk of ONFH. No heterogeneity was observed among the eligible studies. Also, there was no evidence of publication bias was found in our meta-analysis. All tests above indicated that our results were stable and reliable.
MMP-8 is a neutrophil collagenase, which localizes to chromosome 11q22.3. It is known that MMP-8 is mostly produced by neutrophils, but chondrocytes and synovial fibroblasts could also produce this protein.[23,24] MMP-8 plays a pivotal role in the pathogenesis of inflammation. In inflammatory conditions, MMP-8 breaks down and remodels extracellular matrix to facilitate leukocyte transmigration into tissues. In periodontitis, high level of MMP-8 in gingival crevicular fluid might serve as a biomaker for periodontitis, indicating that MMP-8 might act as a promoting role in periodontitis.[25] In arthritis, lack of MMP-8 is associated with exacerbated joint inflammation, suggesting that MMP-8 might have a protective function in arthritis.[26] Previous studies also suggested that MMP-8 might have a promoting or protective role in arthritis.[27,28] However, the exact mechanism underlying the association between MMP-8 and ONFH susceptibility is still unclear. Chen et al[12] supposed that both risk and protective factors in ONFH were the results of MMP-8 in the regulations of the breakdown pathway of the extracellular matrix in bony tissue development, reproduction, and tissue remodeling. Du et al[11] speculated that polymorphisms of MMP-8 might have an effect on the inflammation or circulatory impairment of the femoral head. Furthermore, some studies suggested that the protective role of MMP-8 might be in part related to regulating the amount of estrogen receptors (ER) including both ER-α and ER-β.[29,30]
There are some limitations in the present work needed to be considered. First, we focused only on publications in the language of English, representing a potential source of bias. Second, stratified analyses were not performed based on ethnicities, as the studies enrolled were all Asians. However, the sample evaluated in the present study represented a certain population accounting for a large proportion worldwide. Third, the number of studies was small in our meta-analysis, which may have influenced the results. Despite those limitations listed aforementioned, our work also has its advantages. First, it contains the latest data on the association of MMP-8 rs11225394 and rs2012390 polymorphisms with ONFH risk. Moreover, it is the first eta-analysis that investigated the associations of MMP-8 rs2012390 and rs11225394 polymorphisms with ONFH risk.
5. Conclusions
To sum up, MMP-8 rs11225394 polymorphism increased ONFH risk and MMP-8 rs2012390 decreased ONFH risk. However, ethnicity-specific studies with lager sample size are still needed in the future to further estimate the effect size of the association.
Author contributions
Conceptualization: Biaofang Wei.
Software: Liangbin Jiang, Chungang Zhang.
Supervision: Biaofang Wei.
Validation: Biaofang Wei.
Visualization: Biaofang Wei.
Writing – original draft: Liangbin Jiang, Chungang Zhang.
Footnotes
Abbreviations: CI = confidence interval, MMP = matrix metalloproteinase, ONFH = osteonecrosis of the femoral head, OR = odds ratio, SNP = single-nucleotide polymorphism.
LJ and CZ contributed equally to this work.
The authors have no conflicts of interest to disclose.
References
- [1].Petrigliano FA, Lieberman JR. Osteonecrosis of the hip: novel approaches to evaluation and treatment. Clin Orthop Relat Res 2007;465:53–62. [DOI] [PubMed] [Google Scholar]
- [2].Yin JM, Liu Z, Zhao SC, et al. Relationship between the Apolipoprotein AI, B gene polymorphism and the risk of non-traumatic osteonecrosis. Lipids Health Dis 2014;13:149–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zalavras CG, Lieberman JR. Osteonecrosis of the femoral head: evaluation and treatment. J Am Acad Orthop Surg 2014;22:455–64. [DOI] [PubMed] [Google Scholar]
- [4].Green MJ, Gough AK, Devlin J, et al. Serum MMP-3 and MMP-1 and progression of joint damage in early rheumatoid arthritis. Rheumatology 2003;42:83–8. [DOI] [PubMed] [Google Scholar]
- [5].Zeng GQ, Chen AB, Li W, et al. High MMP-1, MMP-2, and MMP-9 protein levels in osteoarthritis. Genet Mol Res 2015;14:14811–22. [DOI] [PubMed] [Google Scholar]
- [6].Chen JJ, Huang JF, Du WX, et al. Expression and significance of MMP3 in synovium of knee joint at different stage in osteoarthritis patients. Asian Pac J Trop Med 2014;7:297–300. [DOI] [PubMed] [Google Scholar]
- [7].Tang SY, Herber RP, Ho SP, et al. Matrix metalloproteinase-13 is required for osteocytic perilacunar remodeling and maintains bone fracture resistance. J Bone Miner Res 2012;27:1936–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Inoue K, Mikuni-Takagaki Y, Oikawa K, et al. A crucial role for matrix metalloproteinase 2 in osteocytic canalicular formation and bone metabolism. J Biol Chem 2006;281:33814–24. [DOI] [PubMed] [Google Scholar]
- [9].Xiao Q, Zhang F, Grassia G, et al. Matrix metalloproteinase-8 promotes vascular smooth muscle cell proliferation and neointima formation. Arterioscler Thromb Vasc Biol 2014;34:90–8. [DOI] [PubMed] [Google Scholar]
- [10].Bedel A, Negre-Salvayre A, Heeneman S, et al. E-cadherin/beta-catenin/T-cell factor pathway is involved in smooth muscle cell proliferation elicited by oxidized low-density lipoprotein. Circ Res 2008;103:694–701. [DOI] [PubMed] [Google Scholar]
- [11].Du J, Jin T, Cao Y, et al. Association between genetic polymorphisms of MMP8 and the risk of steroid-induced osteonecrosis of the femoral head in the population of northern China. Medicine 2016;95:e4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chen J, Liu W, Cao Y, et al. MMP-3 and MMP-8 single-nucleotide polymorphisms are related to alcohol-induced osteonecrosis of the femoral head in Chinese males. Oncotarget 2017;8:25177–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719–48. [PubMed] [Google Scholar]
- [14].DerSimonian R. Meta-analysis in the design and monitoring of clinical trials. Stat Med 1996;15:1237–48. discussion 1249–1252. [DOI] [PubMed] [Google Scholar]
- [15].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [16].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wang J, Shi X, Yang H, et al. Association between alcohol-induced osteonecrosis of femoral head and risk variants of MMPS in Han population based on a case-control study. Oncotarget 2017;8:64490–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jacobs B. Epidemiology of traumatic and nontraumatic osteonecrosis. Clin Orthop Relat Res 1978;131:51–67. [PubMed] [Google Scholar]
- [19].Wang Y, Cao Y, Li Y, et al. Genetic association of the ApoB and ApoA1 gene polymorphisms with the risk for alcohol-induced osteonecrosis of femoral head. Int J Clin Exp Pathol 2015;8:11332–9. [PMC free article] [PubMed] [Google Scholar]
- [20].Kim TH, Baek JI, Hong JM, et al. Significant association of SREBP-2 genetic polymorphisms with avascular necrosis in the Korean population. BMC Med Genet 2008;9:94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Du J, Liu W, Jin T, et al. A single-nucleotide polymorphism in MMP9 is associated with decreased risk of steroid-induced osteonecrosis of the femoral head. Oncotarget 2016;7:68434–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].An F, Du J, Cao Y, et al. MMP8 polymorphism is associated with susceptibility to osteonecrosis of the femoral head in a Chinese Han population. Oncotarget 2017;8:21561–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cole AA, Chubinskaya S, Schumacher B, et al. Chondrocyte matrix metalloproteinase-8. Human articular chondrocytes express neutrophil collagenase. J Biol Chem 1996;271:11023–6. [DOI] [PubMed] [Google Scholar]
- [24].Hanemaaijer R, Sorsa T, Konttinen YT, et al. Matrix metalloproteinase-8 is expressed in rheumatoid synovial fibroblasts and endothelial cells. Regulation by tumor necrosis factor-alpha and doxycycline. J Biol Chem 1997;272:31504–9. [DOI] [PubMed] [Google Scholar]
- [25].Yuan C, Liu X, Zheng S. Matrix metalloproteinase-8 levels in oral samples as a biomarker for periodontitis in the Chinese population: an observational study. BMC Oral Health 2018;18:51–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Garcia S, Forteza J, Lopez-Otin C, et al. Matrix metalloproteinase-8 deficiency increases joint inflammation and bone erosion in the K/BxN serum-transfer arthritis model. Arthritis Res Ther 2010;12:R224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Itoh T, Matsuda H, Tanioka M, et al. The role of matrix metalloproteinase-2 and matrix metalloproteinase-9 in antibody-induced arthritis. J Immunol 2002;169:2643–7. [DOI] [PubMed] [Google Scholar]
- [28].Close DR. Matrix metalloproteinase inhibitors in rheumatic diseases. Ann Rheum Dis 2001;60suppl 3:iii62–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Korpi JT, Kervinen V, Maklin H, et al. Collagenase-2 (matrix metalloproteinase-8) plays a protective role in tongue cancer. Br J Cancer 2008;98:766–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Haukioja A, Tervahartiala T, Sorsa T, et al. Persistent oral human papillomavirus (HPV) infection is associated with low salivary levels of matrix metalloproteinase 8 (MMP-8). J Clin Virol 2017;97:4–9. [DOI] [PubMed] [Google Scholar]


