Supplemental Digital Content is available in the text
Keywords: electrical stimulation, neurogenic bowel dysfunction, spinal cord injury
Abstract
Background:
This study aimed to perform a systematic literature review of the clinical trial evidence on electrical stimulation for the treatment of neurogenic bowel dysfunction (NBD) after spinal cord injury (SCI).
Methods:
Systematic electronic searches were carried out in the PubMed/Medline, EMBASE, Cochrane Central Register of Controlled Trials, and China National Knowledge Infrastructure databases, along with the reference lists in the include studies. Studies were eligible for inclusion if they adopted a controlled clinical design based on human population, the patients suffered from spinal cord injury, the main outcomes were the disorders of bowel function and the intervention was electrical stimulation. Also, the language was limited to English and Chinese.
Results:
Eleven studies were included in this systematic review, comprising transcutaneous electrical stimulation, transrectal bowel stimulation, sacral nerve stimulation, intravesical electrical stimulation, etc. Of the 11 studies, 3 were randomized controlled trials, 8 were controlled before-and-after trials. The quality of the included studies was moderate bias risk. Most studies revealed that the electrical stimulation was beneficial for the patient with NBD after SCI.
Conclusions:
Only 11 small clinical studies with 298 participants have evaluated the efficacy of electrical stimulation for NBD after SCI. Although some studies showed electrical stimulation was benefit for the patient with NBD after SCI, there was currently not enough evidence to support the use of electrical stimulation could improve the clinical symptoms of those patients. Thus, well-designed randomized controlled trials with larger patient population are warranted to establish its benefit in clinical practice in the future.
1. Introduction
Neurogenic bowel dysfunction (NBD) is a disease involving the loss or absence of normal bowel function due to nerve injury, neurological disease, or congenital defects of the nervous system.[1,2] Fecal incontinence, difficulty with evacuation, constipation, abdominal pain, and bloating are the common clinically symptoms of NBD.[3] Among the common causes of NBD, spinal cord injury (SCI) has been given more attention by clinical doctors. According to some reports, approximately 80% of SCI was accompanied by NBD.[3,4] It has been revealed that people with NBD often suffer from decreased quality of life, such as loss of independence, feeling of embarrassment, mental disorder, social isolation, etc., especially in SCI patients.[5,6]
The conservative treatments for NBD after SCI include oral laxatives, suppositories, and digital anorectal stimulation. With the increase of the research on NBD, new treatments have been found by clinicians, for example, colostomies, malone anterograde continence enema procedure, artificial bowel sphincters, and graciloplasties.[7] The mechanisms of those treatments are mainly through promoting intestinal fecal evacuation and strengthening the power of the anal sphincter to improve the function of bowel. Despite trying several measures, there were still numerous patients that either did not gain an acceptable level of therapeutic benefit or remained completely refractory to treatment.
Treatment was not frequently satisfactory; accordingly, other therapies should be explored. Recently, some studies have reported the use of electrical stimulation for the safe treatment of patients with NBD after SCI.[8–10] For instance, Worsoe et al[11] performed a stimulation, applied with plaster electrodes using an amplitude of twice the genito-anal reflex threshold (width: 200 μs; rate: 20 Hz), on patients with complete supraconal SCI, and found that the dorsal genital nerve stimulation led to an acute decrease of the rectal cross sectional area (CSA) and the rectal pressure CSA relation. Han et al[12] reported that the use of intravesical electrical stimulation therapy was effective in children aged 3.9 to 13.2 years old with NBD and spina bifida. However, few studies have been conducted on the assessment of the efficacy of randomized controlled trials of electrical stimulation in the patients with NBD after SCI. Besides, the type of SCI, different intervention, variable pathophysiology of NBD could also influence the interventional safety and efficacy of electrical stimulation.
Based on these uncertainties, the current systematic review was primarily aimed to rigorously examine the clinical evidence on the efficacy of electrical stimulation in the treatment of NBD after SCI.
2. Materials and methods
This review, which systematically evaluated the safety and efficacy of electrical stimulation therapy for patients with NBD after SCI, was designed using the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) checklist. We conducted a comprehensive literature search in PubMed/Medline (1966 to Nov 2017), EMBASE (1966 to Nov 2017), the Cochrane Central Register of Controlled Trials (CENTRAL) (1999 to Nov 2017), and China National Knowledge Infrastructure (1990 to Nov 2017). The search core terms included the following: neurogenic bowel dysfunction OR constipation OR fecal incontinence OR abdominal pain OR bloating OR colon transit time, spinal cord injury OR spinal cord trauma OR spinal cord laceration OR SCI, electrical stimulation OR electrotherapy. Additionally, we also searched the reference lists of the identified articles to determine the relevant studies. The complete search strategies were list in Supplemental Table 1.
In this review, all the selected studies were required to meet the following inclusion criteria: the study adopted a controlled clinical design based on human population; the subjects suffered from spinal cord injury, spina bifida, myelomeningocele, intervertebral disc, or foraminal stenosis; the intervention was electrical stimulation; the article reported the diagnostic criteria of NBD, especially described the information of colorectal and anal sphincter dysfunction; the outcomes included the colonic transit time, the stool consistency, stool frequency, anal-rectal pressure measurement, subjective satisfaction, score of neurogenic bowel function, and so on; the language was limited to English and Chinese. If a study did not meet the above-mentioned criteria, it was excluded.
The potential studies were independently selected by 2 reviewers according to the predetermined inclusion and exclusion criteria. In the process of retrieval, if divergences of opinion on the articles arose, a third reviewer evaluated the eligibility of the article in question.
In this review, a standardized form of risk of bias, which was adapted from the Cochrane Effective Practice and Organisation of Care (EPOC) Group, was used to identify the study quality.[13] The instrument recorded 9 criteria, including “was the allocation sequence adequately generated?,” “was the allocation adequately concealed?,” “were baseline outcome measurements similar?,” “were baseline characteristics similar?,” “were incomplete outcome data adequately addressed?,” “was knowledge of the allocated interventions adequately prevented during the study?,” “was the study adequately protected against contamination?,” “was the study free from selective outcome reporting?,” and “was the study free from other risks of bias?.” If an index was assessed “low risk,” it could have 1 score. If a study scored <4, it was considered of low quality; if a study scored 4 to 6, it was considered of moderate quality; and if a study scored >6, it was considered of high quality.
In this systematic review, ethical approval was not necessary as all the data were based on the previous published studies.
3. Results
3.1. Search results
In this systematic review, the search strategy initially identified 641 publications. For various reasons, 630 of these 641 articles were excluded. Accordingly, this process resulted in 11 articles being identified as meeting the rigid inclusion criteria. All the included studies were published between 1997 and 2015. Among the 11 articles, 3[9,14,15] were randomized controlled trials and 8[8,11,12,16–20] were self-controlled trials. One article[15] was published in Chinese. The screening process is summarized in Fig. 1.
Figure 1.
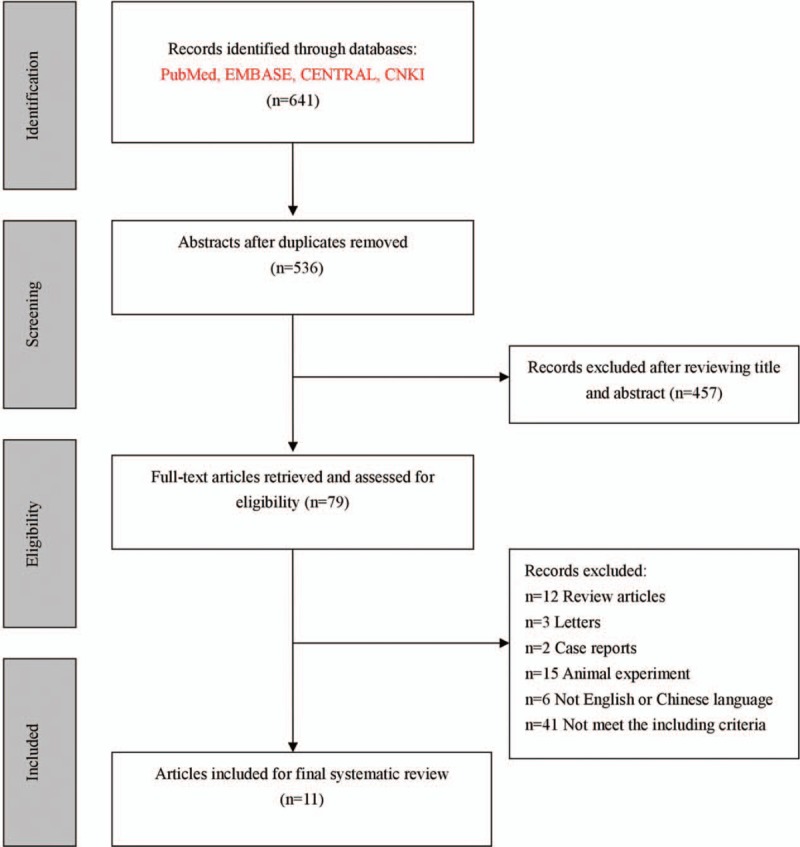
Flow diagram of the screening process.
3.2. Quality assessment of included studies
In this systematic review, the checklist of risk bias with EPOC (see Table 1) indicated that 1 study achieved 3 score, 4 studies achieved 4 scores, 4 studies achieved 5 scores, 2 studies achieved 6 scores. Overall, the quality of the included studies was moderate bias risk.
Table 1.
Quality assessment of included studies.
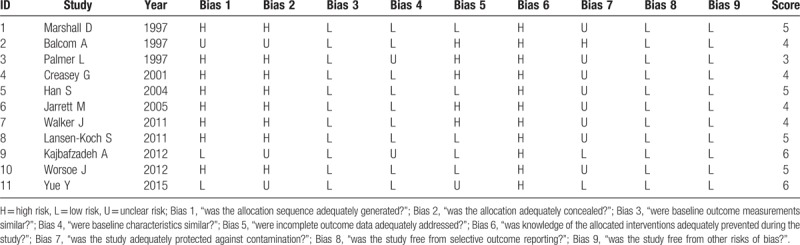
3.3. Characteristics of subjects in the included studies
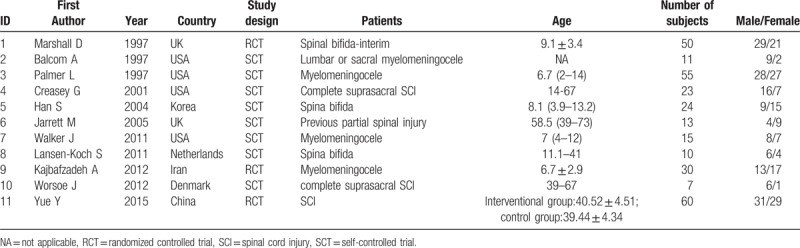
The subjects of 5 studies[8,9,12,14,17] were children (see Table 2). Most of the included studies[8,9,11,12,16,18–20] had a small sample size with <50 cases except for 3 studies.[14,15,17] The types of SCI were mainly focused on spinal bifida, myelomeningocele, and complete suprasacral SCI, but most of the studies did not provide the severity of the SCI.
Table 2.
Characteristics of the subjects in the included studies.
3.4. Interventional information on electrical stimulation
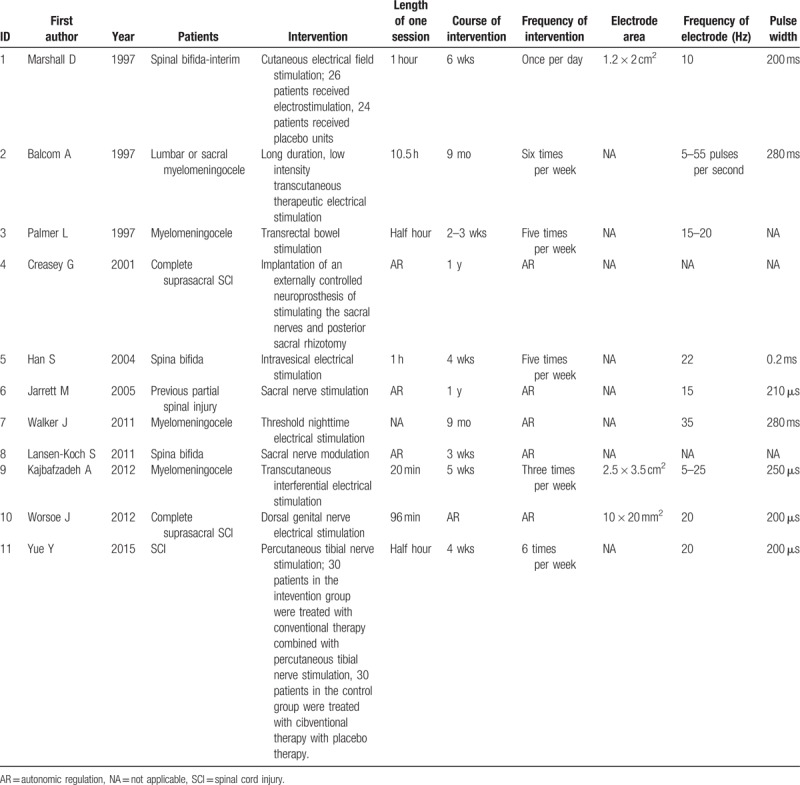
The basic interventional informations of electrical stimulation for patients with NBD after SCI were shown in Table 3. The types of electrical stimulation referred to transcutaneous electrical stimulation,[9,14,16] transrectal bowel stimulation,[17] intravesical electrical stimulation,[12] sacral nerve stimulation,[19,20] dorsal genital nerve electrical stimulation,[11] percutaneous tibial nerve stimulation,[15] threshold night-time electrical stimulation,[8] implantable neuroprosthesis for stimulating the sacral nerves and posterior.[18] With the different types of electrical stimulation used, the length of one session, course of intervention, frequency of the intervention, electrode area, frequency of electrode, and pulse width were variously different.
Table 3.
Interventional information on the electrical stimulation.
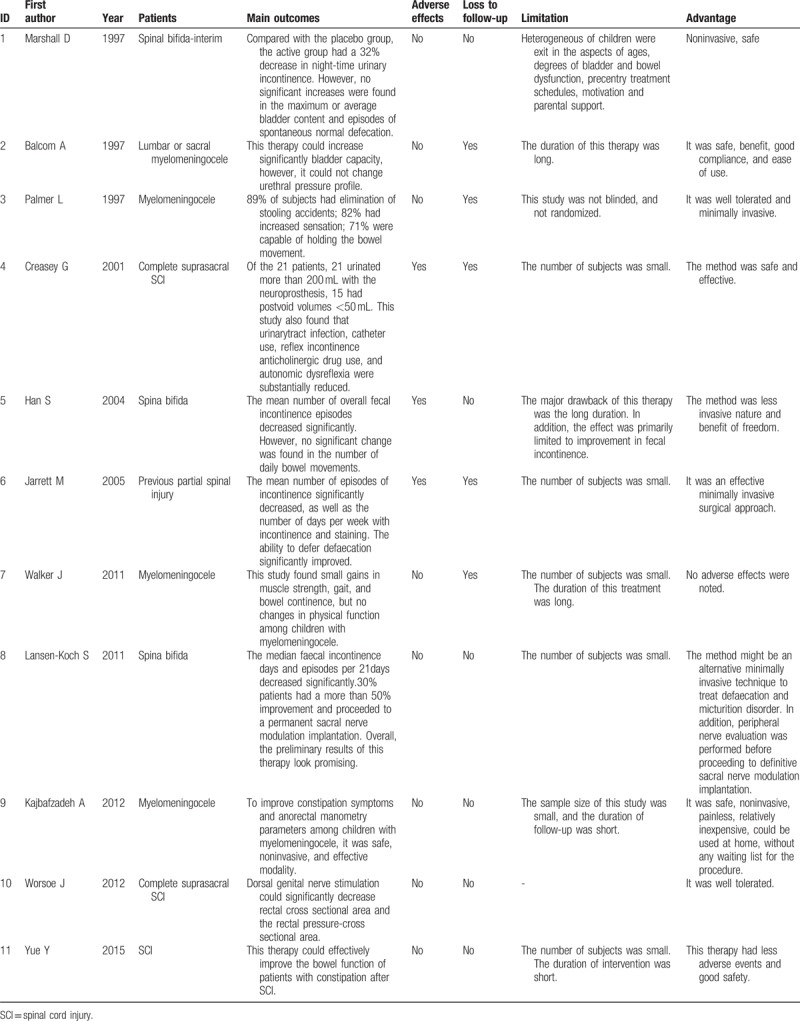
3.5. Main outcomes of the included studies
Despite disadvantages in 1 single study, on the whole, the main outcomes of electrical stimulation were safe and effective for the patients with NBD after SCI (see Table 4). However, due to the limitations regarding the sample size, study design, duration of intervention, etc., some studies suggested the authenticity and reliability of the electrical stimulation for NBD after SCI should be further verified in the future.
Table 4.
Main outcomes of the included studies.
4. Discussion
To the best of our knowledge, this systematic literature review is the first paper to evaluate the efficacy and safety of electrical stimulation therapy in the current clinical use for NBD after SCI. This review includes 11 articles represented on the most comprehensive systematic analysis of electrical stimulation therapy for this indication to date. In this study, we found that there were several methods of electrical stimulation for the treatment of NBD after SCI, and they mainly involved the transcutaneous electrical stimulation, transrectal bowel stimulation, intravesical electrical stimulation, sacral nerve stimulation, dorsal genital nerve electrical stimulation, percutaneous tibial nerve stimulation, etc. The main mechanism of the electrical stimulation therapy was the promotion of the healthy function of the intestinal function through improving blood flow, promoting protein synthesis, reinforcing muscular strength, and regulating nerve transmission.
In this review, 3 studies revealed the efficacy and safety of transcutaneous electrical stimulation for NBD after SCI. Transcutaneous electrical stimulation could stimulate sympathetic and parasympathetic nerve fibers in the bowel system and reduce the pressure of the internal and external sphincter in the anus. The outcomes of this treatment included a reduction of the difficulty of defecation and an increase of the frequency of defecation in SCI patients with NBD. These studies demonstrated that transcutaneous electrical stimulation therapy was safe, non-invasive, and painless for the patient with mild or moderate constipation. The results of the 3 studies were in line with other studies despite including different population.[21–24]
Regarding the transrectal bowel electrical stimulation, there were few study about their efficacy among patients with SCI. Palmer et al[17] carried out a self-control study with 55 children aged 2 to 14 to assess the efficacy of transrectal bowel stimulation for NBD. They found that 68% of the patients had significantly improved bowel function. This therapy could improve the ability to control the intestinal tract, enhance the sense of the need to defecate, and reduce intestinal peristalsis, so as to reduce the rate of fecal incontinence. In addition, it was suitable for patient with myelomeningocele and NBD with several advantages, such as being well tolerated, minimal invasion, and no adverse reaction. However, we could not find the results of the application in the adults.
Intravesical electrical stimulation was frequently used to improve the bladder function not only in the humans trials[25–27] but also in animal experiment.[28,29] The goal of this treatment could be achieved by decreasing intravesical pressure and increasing urinary bladder capacity, or by facilitating bladder emptying.[30] Similarly, intravesical electrical stimulation could also be used to treat the patients with NBD after SCI. Han et al[12] carried out a self-controlled study in 24 children with spina bifida to evaluate the efficacy of this therapy. After the intervention, the number of overall fecal incontinence episodes decreased significantly. Because of the advantages of the simple operation and excellent performance, he appraised that intravesical electrical stimulation was a viable option to control fecal incontinence in children with NBD and spina bifida.
In 1995, sacral nerve stimulation was introduced for idiopathic fecal incontinence, and then subsequently its indications had spread to include fecal incontinence of other etiologies.[31] For sacral nerve stimulation, the electrode was often placed through a sacral foramen between S2 and S4. Among the studies in this review, Jarrett et al[19] carried out a clinical study with 13 patients who had suffered from partial spinal injury to assess the efficacy of sacral nerve stimulation for fecal incontinence. He evaluated several indexes, such as the number of episodes of fecal incontinence per week, the number of days per week with staining or pad use, the ability to empty the bowel completely, and found that sacral nerve stimulation could benefit those patients. Javidan et al[32] also reported the beneficial effect of sacral nerve stimulation on bowel and bladder function in patients with SCI. To date, the mechanism of sacral nerve stimulation remained ambiguous. Most clinical data tended to show enhancement in striated muscular activity and neuromodulation of sacral reflexes.[33] However, sacral nerve stimulation, overall, was a relatively safe and effective technique for the patients with NBD and SCI despite the minimally invasive procedure.
The difference of causes and levels of SCI could also influence the effect of electrical stimulation for the NBD patients. For instance, Kim et al[34] had classified 33 SCI patients into 2 groups according to the level of cord injury: above T9 and T9 to L2. After 4 weeks of electrical stimulation treatment, this study found that the electrical stimulation to the sacral dermatomes could significantly increase the mean squeezing pressure of rectoanal manometry on the T9 to L2 SCI patients than the group of level above T9.
In this review, although several studies achieved a positive result about the safety and efficacy of the electrical stimulation procedure for those patients with NBD after SCI, the study inevitably still had several flaws. For instance, approximately 70% of the studies were designed as a self-controlled study, which resulted in an unclear data to assess the influence of the spontaneous recovery or the electrical stimulation during the process of rehabilitation. Moreover, randomized group division and blinding method were not designed in those studies. Even the length of the treatment course, sample size, withdrawal of the subjects from the study, the severity of the SCI, etc. could also affect the accuracy of the results. Therefore, in order to accomplish an accurate assessment of the efficacy of electrical stimulation for NBD after SCI, it is necessary to perform a study in the future with larger sample size and well-designed randomized control trial.
Author contributions
Yuling Deng and Yonghai Dong designed the study, Yun Liu, Qiong Zhang, and Xihong Guan wrote the manuscript. Xiaodan Chen performed the methodology. Meng Li, Lei Xu, and Cheng Yang discussed the results.
Data curation: Xiaodan Chen, Meng Li.
Formal analysis: Cheng Yang.
Investigation: Lei Xu.
Methodology: Xihong Guan, Xiaodan Chen, Meng Li.
Project administration: Yuling Deng, Yun Liu.
Resources: Xihong Guan, Cheng Yang.
Supervision: Xiaodan Chen.
Writing – original draft: Yonghai Dong, Yun Liu, Qiong Zhang.
Writing – review & editing: Yonghai Dong, Yun Liu, Xiaodan Chen, Lei Xu.
Supplementary Material
Footnotes
Abbreviations: CNKI = China National Knowledge Infrastructure, CSA = cross-sectional area, EPOC = Cochrane Effective Practice and Organisation of Care, NBD = neurogenic bowel dysfunction, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analysis, SCI = spinal cord injury.
YD, YD, YL, QZ, and XG have contributed equally to this work.
The research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Worsoe J, Rasmussen M, Christensen P, et al. Neurostimulation for neurogenic bowel dysfunction. Gastroenterol Res Pract 2013;2013:563294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Definitions. Definitions for neurogenic bowel. Available at: http://www.definitions.net/definition/neurogenic%20bowel. April 27th, 2017. [Google Scholar]
- [3].Krogh K, Nielsen J, Djurhuus JC, et al. Colorectal function in patients with spinal cord lesions. Dis Colon Rectum 1997;40:1233–9. [DOI] [PubMed] [Google Scholar]
- [4].Emmanuel A, Kumar G, Christensen P, et al. Long-term cost-effectiveness of transanal irrigation in patients with Neurogenic Bowel dysfunction. PLoS One 2016;11:e0159394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].DeLisa JA, Kirshblum S. A review: frustrations and needs in clinical care of spinal cord injury patients. J Spinal Cord Med 1997;20:384–90. [DOI] [PubMed] [Google Scholar]
- [6].Emmanuel A. Managing neurogenic bowel dysfunction. Clin Rehabil 2010;24:483–8. [DOI] [PubMed] [Google Scholar]
- [7].Paris G, Gourcerol G, Leroi AM. Management of neurogenic bowel dysfunction. Eur J Phys Rehabil Med 2011;47:661–76. [PubMed] [Google Scholar]
- [8].Walker JL, Ryan SW, Coburn TR. Does threshold nighttime electrical stimulation benefit children with spina bifida? A pilot study. Clin Orthop Relat Res 2011;469:1297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kajbafzadeh AM, Sharifi-Rad L, Nejat F, et al. Transcutaneous interferential electrical stimulation for management of neurogenic bowel dysfunction in children with myelomeningocele. Int J Colorectal Dis 2012;27:453–8. [DOI] [PubMed] [Google Scholar]
- [10].Rasmussen MM, Kutzenberger J, Krogh K, et al. Sacral anterior root stimulation improves bowel function in subjects with spinal cord injury. Spinal Cord 2015;53:297–301. [DOI] [PubMed] [Google Scholar]
- [11].Worsoe J, Fynne L, Laurberg S, et al. Acute effect of electrical stimulation of the dorsal genital nerve on rectal capacity in patients with spinal cord injury. Spinal Cord 2012;50:462–6. [DOI] [PubMed] [Google Scholar]
- [12].Han SW, Kim MJ, Kim JH, et al. Intravesical electrical stimulation improves neurogenic bowel dysfunction in children with spina bifida. J Urol 2004;171:2648–50. [DOI] [PubMed] [Google Scholar]
- [13].Cochrane Effective Practice And Organisation Of Care. Suggested risk of bias criteria for EPOC reviews. Available at: http://epoc.cochrane.org/sites/epoc.cochrane.org/files/public/uploads/Resources-for-authors2017/suggested_risk_of_bias_criteria_for_epoc_reviews.pdf Accessed November 20, 2017. [Google Scholar]
- [14].Marshall DF, Boston VE. Altered bladder and bowel function following cutaneous electrical field stimulation in children with spina bifida–interim results of a randomized double-blind placebo-controlled trial. Eur J Pediatr Surg 1997;7suppl 1:41–3. [DOI] [PubMed] [Google Scholar]
- [15].Yue Y. A randomized controlled trial to inverstigate percutaneous tibial nerve stimulation for the treatment of constipation after spinal cord injury, Nanjing University of Chinese Medicine; 2015. [Google Scholar]
- [16].Balcom AH, Wiatrak M, Biefeld T, et al. Initial experience with home therapeutic electrical stimulation for continence in the myelomeningocele population. J Urol 1997;158:1272–6. [DOI] [PubMed] [Google Scholar]
- [17].Palmer LS, Richards I, Kaplan WE. Transrectal electrostimulation therapy for neuropathic bowel dysfunction in children with myelomeningocele. J Urol 1997;157:1449–52. [PubMed] [Google Scholar]
- [18].Creasey GH, Grill JH, Korsten M, et al. An implantable neuroprosthesis for restoring bladder and bowel control to patients with spinal cord injuries: a multicenter trial. Arch Phys Med Rehabil 2001;82:1512–9. [DOI] [PubMed] [Google Scholar]
- [19].Jarrett ME, Matzel KE, Christiansen J, et al. Sacral nerve stimulation for faecal incontinence in patients with previous partial spinal injury including disc prolapse. Br J Surg 2005;92:734–9. [DOI] [PubMed] [Google Scholar]
- [20].Lansen-Koch SM, Govaert B, Oerlemans D, et al. Sacral nerve modulation for defaecation and micturition disorders in patients with spina bifida. Colorectal Dis 2012;14:508–14. [DOI] [PubMed] [Google Scholar]
- [21].Ladi-Seyedian SS, Sharifi-Rad L, Manouchehri N, et al. A comparative study of transcutaneous interferential electrical stimulation plus behavioral therapy and behavioral therapy alone on constipation in postoperative Hirschsprung disease children. J Pediatr Surg 2017;52:177–83. [DOI] [PubMed] [Google Scholar]
- [22].Ng RT, Lee WS, Ang HL, et al. Transcutaneous electrical stimulation (TES) for treatment of constipation in children. Cochrane Database Syst Rev 2016;11:D10873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Veiga ML, Costa EV, Portella I, et al. Parasacral transcutaneous electrical nerve stimulation for overactive bladder in constipated children: the role of constipation. J Pediatr Urol 2016;12:396.e1–6. [DOI] [PubMed] [Google Scholar]
- [24].Iqbal F, Thomas GP, Tan E, et al. Transcutaneous sacral electrical stimulation for chronic functional constipation. Dis Colon Rectum 2016;59:132–9. [DOI] [PubMed] [Google Scholar]
- [25].Choi EK, Hong CH, Kim MJ, et al. Effects of intravesical electrical stimulation therapy on urodynamic patterns for children with spina bifida: a 10-year experience. J Pediatr Urol 2013;9:798–803. [DOI] [PubMed] [Google Scholar]
- [26].Hagerty JA, Richards I, Kaplan WE. Intravesical electrotherapy for neurogenic bladder dysfunction: a 22-year experience. J Urol 2007;178(4 pt 2):1680–3. [DOI] [PubMed] [Google Scholar]
- [27].Boy S, Schurch B, Mehnert U, et al. The effects of tolterodine on bladder-filling sensations and perception thresholds to intravesical electrical stimulation: method and initial results. BJU Int 2007;100:574–8. [DOI] [PubMed] [Google Scholar]
- [28].Hong CH, Lee HY, Jin MH, et al. The effect of intravesical electrical stimulation on bladder function and synaptic neurotransmission in the rat spinal cord after spinal cord injury. BJU Int 2009;103:1136–41. [DOI] [PubMed] [Google Scholar]
- [29].De Bock F, De Wachter S, Wyndaele JJ. Influence of nerve transsections and combined bladder filling on intravesical electrostimulation-induced bladder contraction in the rat. Eur Urol 2009;56:527–32. [DOI] [PubMed] [Google Scholar]
- [30].Radziszewski K, Zielinski H, Radziszewski P, et al. Transcutaneous electrical stimulation of urinary bladder in patients with spinal cord injuries. Int Urol Nephrol 2009;41:497–503. [DOI] [PubMed] [Google Scholar]
- [31].Matzel KE, Stadelmaier U, Hohenfellner M, et al. Electrical stimulation of sacral spinal nerves for treatment of faecal incontinence. Lancet 1995;346:1124–7. [DOI] [PubMed] [Google Scholar]
- [32].Javidan AN, Mazel K, Latifi S, et al. Outcomes of implementation of sacral nerve stimulation on urination, defecation, and sexual function in patients with spinal cord injury. Int J Colorectal Dis 2014;29:1577–8. [DOI] [PubMed] [Google Scholar]
- [33].Brill SA, Margolin DA. Sacral nerve stimulation for the treatment of fecal incontinence. Clin Colon Rectal Surg 2005;18:38–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kim Y, Lee S, Choi K, et al. Effects of the electrical stimulation for the neurogenic bowel according to the level of spinal cord injury. J Korean Acad Rehab Med 2003;27:880–5. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


