Abstract
This study aims to retrospectively analyze the potential risk factors for uterine leiomyoma and prospectively examine whether these risk factors can increase the incidence of uterine leiomyoma.
Women who attended our outpatient department from January 1, 2010 to December 31, 2010 were enrolled. General demographical characteristics, personal information, and living habits were collected. Univariate and multivariate logistic regression analyses were used to identify the potential risk factors. Follow-up was regularly conducted to complete the prospective analysis.
A total of 1273 women were enrolled including 213 uterine leiomyomas (case group) and 1060 nonuterine leiomyoma women (control group). No statistical differences were found on the age, marital status, number and complication of pregnancy, frequent physical exercise, frequent alcohol consumption, and family history of uterine diseases between two groups (all P > .05). Mean body mass index (P = .043), high school education (P = .041), frequent smoking (P = .030), frequent caffeine consumption (P = .019), frequent milk or soybean consumption (P = .025), and frequent oral contraceptive use (P = .034) were statistically correlated with the onset of uterine leiomyoma. Multivariate analysis verified that frequent milk or soybean consumption (7.349 [5.081–9.454]; P = .039] and frequent oral contraceptive use (8.103 [4.486–12.583]; P = .018] were the independent risk factors for uterine leiomyoma.
Frequent milk or soybean consumption and frequent oral contraceptive use are associated with a high risk of uterine leiomyoma, and proper education on the prevention of uterine leiomyoma is highly recommended in clinical practice.
Keywords: education, milk, oral contraceptive use, risk factors, soybean consumption, uterine leiomyoma
1. Introduction
Uterine leiomyoma is one of the most common benign tumors originating from the female reproductive system, especially in women with the age 30 to 50 years old.[1] This disease is defined as benign tumors of smooth muscle cells in the uterus. Most patients can be asymptomatic, or nonspecific clinical manifestations, such as menorrhagia, pelvic pain, dysmenorrhoea, infertility, and recurrent abortion.[2] Currently, the main treatment for uterine leiomyoma is surgical removal, but this may impair the patients’ psychological and physiological health to a certain degree.[3]
Although great progress has been made on the management of this benign disease, the pathogenesis of uterine leiomyoma remains unclear.[4] It was reported that the incidence of uterine leiomyoma is on the increase with the process of aging.[5] For example, the prevalence of uterine leiomyoma in women aged over 35 years is 20%, which reaches 51.2% to 60.0% in women aged 40 to 50 years. Besides, it is well-acknowledged that uterine leiomyoma is an estrogen-dependent tumor.[6] Milk or soybean-rich diet is rich in estrogen-like nutrients, which may be associated with the development of uterine leiomyoma.[7] Thus, the identification of risk factors for uterine leiomyoma may help to prevent the occurrence of uterine leiomyoma, which could improve the general health of female population. However, most investigations on the risk factors for uterine leiomyoma were conducted in western countries,[8,9] and things may be different in China. Here, in this study, we first performed a retrospective analysis on the risk factors for uterine leiomyoma, which were used to define the high-risk cohort. The incidence of uterine leiomyoma in the certain high-risk cohort was prospectively evaluated by long-term follow-up. Our data could provide new insight into understanding on the prevention of uterine leiomyoma.
2. Participants and methods
2.1. Participants
Female outpatients who attended the Department of Gynecology and Obstetrics in Shangqiu First People's Hospital from January 1, 2010 to December 31, 2010 were enrolled. All the subjects were women before menopause of Han nationality from Shangqiu. The diagnosis of uterine leiomyoma was made by imaging (transvaginal ultrasound examination) and/or pathological examinations. The demographical and clinical data were collected. All the participants including uterine leiomyoma patients (case group, n = 213) and nonuterine leiomyoma patients (control group, n = 1060) signed the written informed consent. Our study was approved by the Ethic Committee of Shangqiu First People's Hospital and the methods used were in accordance with relevant regulations. The flowchart of our study was shown in Figure 1.
Figure 1.
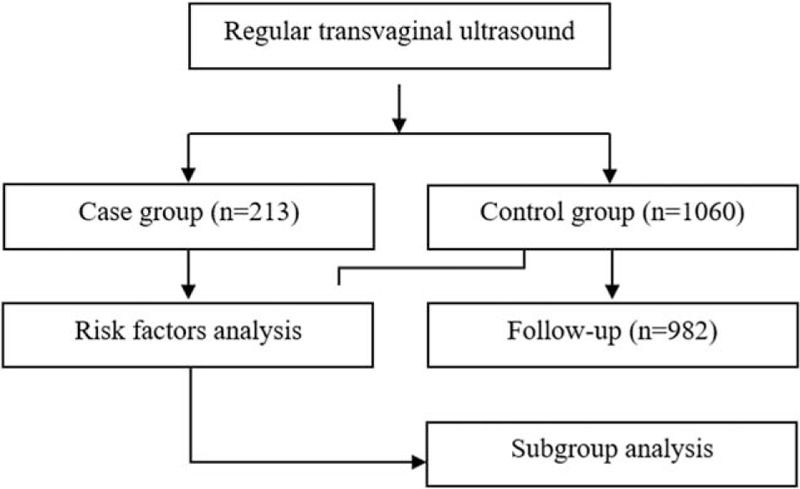
The flow diagram.
2.2. Questionnaire collection
A questionnaire was designed to collect related information and determine the risk factors for uterine leiomyoma based on the relevant literature. The questionnaires were filled by the participants when they were first enrolled and then in regular follow-ups. The contents of the questionnaire included general demographical characteristics, such as name, age, height, weight, and education; basic personal information, such as marital and reproductive history; present diseases; case history and family history; and living habits including smoking, drinking, and diet.
“Frequent smoking” was defined as the average of 4 cigarettes every week and “frequent physical exercise” was as at least 4 times every week. “Frequent alcohol consumption, frequent caffeine consumption, and frequent milk or soybean consumption” were referred to at least 4 times every week. The questionnaire was filled according to the own judgment of the participants on the average within the recent year.
2.3. Follow-up
All the participants in the control group were followed regularly every year. The final follow-up was conducted in December 31, 2015 and the follow-up period was 5 years. Physical examination and transvaginal ultrasound examination were conducted and the questionnaire was filled during follow-ups. The risk factor identified was used to define the high-risk group and the cumulative incidence of uterine leiomyoma was calculated every year.
2.4. Statistical analysis
All the statistical analysis was performed using SPSS 17.0 software (SPSS Inc., Chicago, IL). The continuous and categorical data were presented as mean (range) and percentage (%), respectively. Kolmogorov–Smirnov normality test was used to check the distribution of continuous variables. The differences between case group and control group were tested by independent student t test and chi-square test. Multivariable nonconditional logistic analysis was introduced to identify the risk factor associated with the onset of uterine leiomyoma. P value less than .05 was considered to be statistically significant.
3. Results
3.1. Demographical characteristics
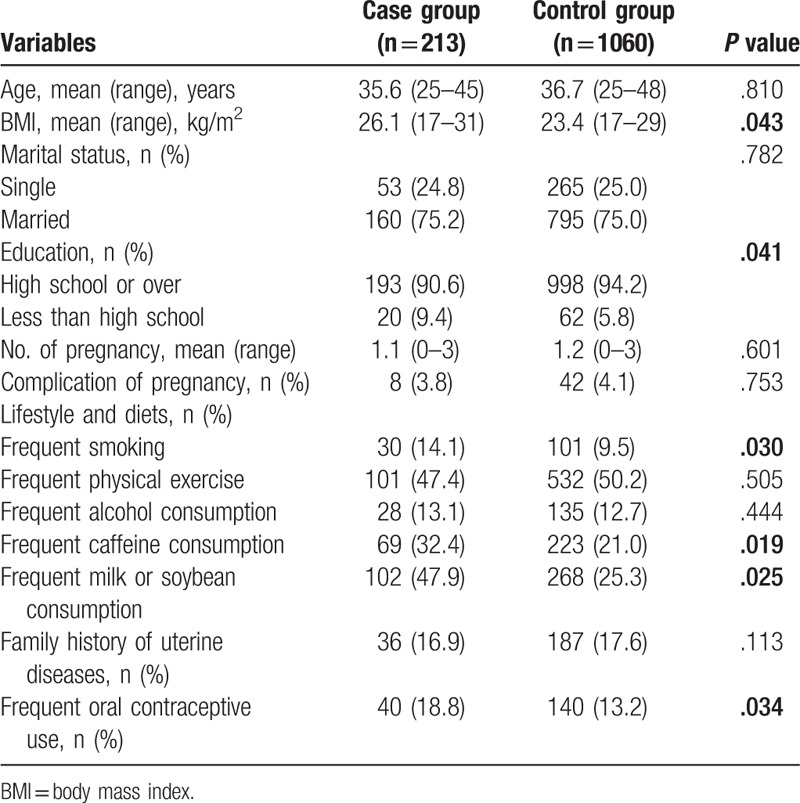
In the year of 2010, a total of 1273 women attended the Department of Gynecology and Obstetrics in Shangqiu First People's Hospital. The diagnosis of uterine leiomyoma was confirmed in 213 women (case group) and other 1060 participants served as control group. The mean age in case group and control group was 35.6 (25–45) and 36.7 (25–48) years old, respectively (Table 1).
Table 1.
Univariate analysis of the risk factors associated with the onset of uterine leiomyoma.
3.2. Univariate and multivariate risk factors for uterine leiomyoma
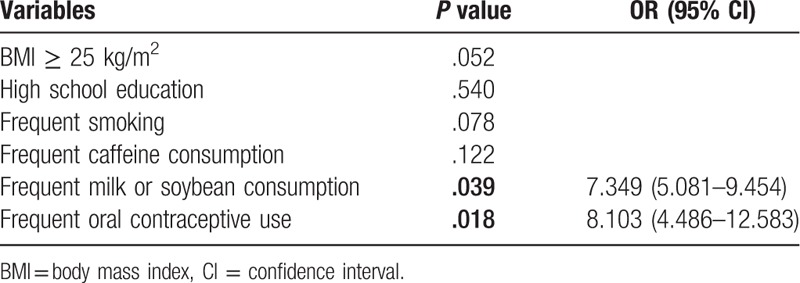
Univariate analysis showed that no differences were found on the age, marital status, No. of pregnancy, complication of pregnancy, frequent physical exercise, frequent alcohol consumption, and family history of uterine diseases between two groups (all P > .05). Mean body mass index in case group was higher than that in control group (26.1 [17–31] vs 23.4 [17–29]; P = .043). In addition, high school education (P = .041), frequent smoking (P = .030), frequent caffeine consumption (P = .019), frequent milk or soybean consumption (P = .025), and frequent oral contraceptive use (P = .034) were statistically correlated with the onset of uterine leiomyoma (Table 1), which were further enrolled into the multivariate analysis. Multivariate analysis verified that frequent milk or soybean consumption (7.349 [5.081–9.454]; P = .039) and frequent oral contraceptive use (8.103 [4.486–12.583]; P = .018) were the independent risk factors for the development of uterine leiomyoma (Table 2).
Table 2.
Multivariate analysis of the risk factors of uterine leiomyoma.
3.3. Follow-up
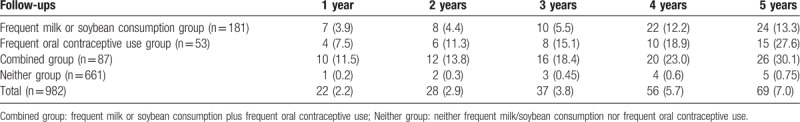
All the 5-year follow-ups were prospectively completed in 982 participants. The drop-off rate was 7.4% (78/1060). The overall cumulative incidence of uterine leiomyoma was calculated (Fig. 2). The overall cumulative incidence of uterine leiomyoma was increased. The incidence of uterine leiomyoma was significantly increased in subjects with frequent milk/soybean consumption plus oral contraceptive use compared within the subjects with frequent milk/soybean consumption or oral contraceptive use alone, and neither of them. Based on the determination of the risk factors mentioned above, subgroup analysis was also performed. More patients with uterine leiomyoma were diagnosed in frequent milk or soybean consumption plus frequent oral contraceptive use combined group on a yearly basis (Table 3). These data supported that frequent milk or soybean consumption and frequent oral contraceptive use were obviously correlated with higher risk for uterine leiomyoma in premenopausal women.
Figure 2.
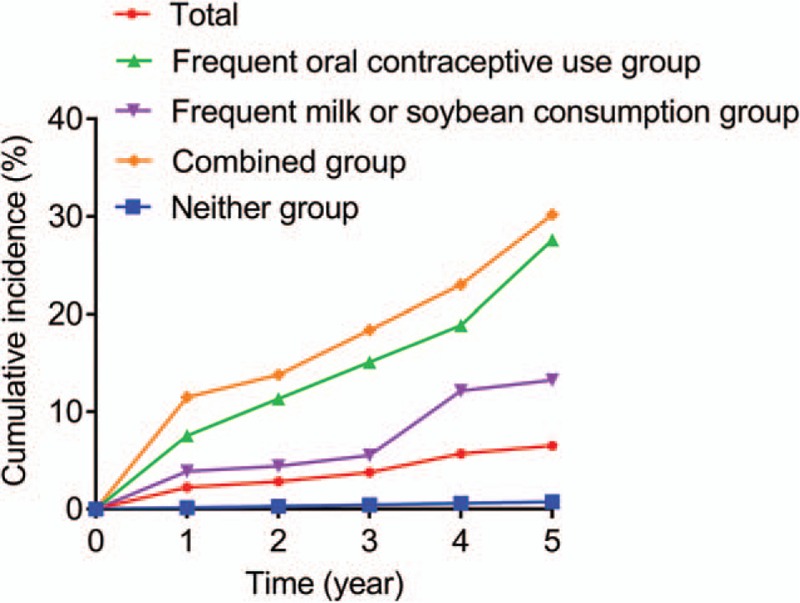
Cumulative incidence of uttering leiomyoma.
Table 3.
The incidence of uterine leiomyoma was increased during follow-up.
4. Discussion
Uterine leiomyoma is one of the most common threats to women's health, which could greatly impair their life quality.[10] This study for the first time established a combination of retrospective analysis and prospective cohort to validate the potential role of risk factors for uterine leiomyoma. These findings could help to develop effective preventive measurements for developing this disease.
Previous literature suggested that age might be considered as the most important risk factor for uterine leiomyoma, which could be explained by the increasing patient age, endogenous estrogen accumulation, changes in the immune system, and continuous exposure to environmental risk factors.[11–13] On the contrary, our study did not find that age was a risk factor associated with the development of uterine leiomyoma. The possible reason may be the insufficient sample size or the selected participants, for women who attended our outpatient department were enrolled in the year of 2010. Furthermore, no statistical differences were also found on the marital status as well as the number and complication of pregnancy (all P < .05).
There is no doubt that uterine leiomyoma is an estrogen-dependent benign tumor. Factors that could influence the function and level of estrogen within human body will be the potential candidate associated with uterine leiomyoma. Smoking, alcohol drinking, caffeine consumption, and physical exercise that are shown to be able to affect the metabolism of estrogen may influence the susceptibility to uterine leiomyoma in women.[14–17] In this study, things were a bit different. We found that frequent smoking and frequent caffeine consumption could significantly increase the risk of uterine leiomyoma (both P < .05), while frequent physical exercise and frequent alcohol consumption were not statistically associated with the onset of uterine leiomyoma. Besides, frequent milk or soybean consumption or oral contraceptive use was also prone to be associated with a relatively high risk of uterine leiomyoma. It is well-known that milk or soybean are rich in animal or plant estrogen-like substance, which may exert estrogen-like functions.[18,19] However, there is still a great controversy on the causal relationship between the use of oral contraceptives and the onset risk of uterine leiomyoma in previous publications.[20] One study proved that women who took oral contraceptives for a long time had a higher risk of uterine leiomyoma than those who did not. The other study indicated that women who took oral contraceptives for the first time at the age of 13 to 16 years old have a higher risk of uterine leiomyoma than those who never took, showing that early oral contraceptive use may contribute the high risk of uterine leiomyoma in such women.[21,22]
There were still limitations in our study. First, all the participants were from one single center and inpatients were not enrolled. Second, the consumption of alcohol, caffeine, cigarettes, and physical exercise was not quantified, which may complicate the completion of questionnaire and then decrease the compliance of the participants. Third, some valuable information is not yet included in this study, such as tea and meat consumption. We should analyze their effects on uterine leiomyoma in the future. A large-scale investigation based on advanced telecommunications will be conducted soon, which may further validate our present study.
Taken together, in this study, we first performed a retrospective analysis to identify the potential risk factors for uterine leiomyoma and then enrolled a prospective cohort to further consolidate the risk factors. Our findings support that frequent milk or soybean consumption and frequent oral contraceptive use are associated with a high risk of uterine leiomyoma. Proper education is highly recommended in clinical practice.
Author contributions
Conceptualization: Mei Gao
Data curation: Mei Gao
Formal analysis: Mei Gao
Investigation: Hui Wang
Methodology: Mei Gao and Hui Wang
Project administration: Mei Gao
Resources: Mei Gao and Hui Wang
Software: Mei Gao
Writing – original draft: Mei Gao and Hui Wang
Writing – review and editing: Mei Gao
Footnotes
Abbreviations: BMI = body mass index, CI = confidence interval.
The authors of this work have nothing to disclose.
References
- [1].Sparic R, Mirkovic L, Malvasi A, et al. Epidemiology of uterine myomas: a review. Int J Fertil Steril 2016;9:424–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dagur G, Suh Y, Warren K, et al. Urological complications of uterine leiomyoma: a review of literature. Int Urol Nephrol 2016;48:941–8. [DOI] [PubMed] [Google Scholar]
- [3].Gurusamy KS, Vaughan J, Fraser IS, et al. Medical therapies for uterine fibroids—a systematic review and network meta-analysis of randomised controlled trials. PLoS One 2016;11:e0149631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nothnick WB. Non-coding RNAs in uterine development, function and disease. Adv Exp Med Biol 2016;886:171–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Commandeur AE, Styer AK, Teixeira JM. Epidemiological and genetic clues for molecular mechanisms involved in uterine leiomyoma development and growth. Hum Reprod Update 2015;21:593–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Moravek MB, Bulun SE. Endocrinology of uterine fibroids: steroid hormones, stem cells, and genetic contribution. Curr Opin Obstet Gynecol 2015;27:276–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Shen Y, Xu Q, Xu J, et al. Environmental exposure and risk of uterine leiomyoma: an epidemiologic survey. Eur Rev Med Pharmacol Sci 2013;17:3249–56. [PubMed] [Google Scholar]
- [8].Baird DD, Harmon QE, Upson K, et al. A prospective, ultrasound-based study to evaluate risk factors for uterine fibroid incidence and growth: methods and results of recruitment. J Womens Health (Larchmt) 2015;24:907–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Korkmaz V, Ozkaya E, Ozer Kadife S, et al. Investigation of cardiovascular disease risk in women with uterine leiomyomas. Ir J Med Sci 2016;185:689–93. [DOI] [PubMed] [Google Scholar]
- [10].Aleksandrovych V, Bereza T, Sajewicz M, et al. Uterine fibroid: common features of widespread tumor (Review article). Folia Med Cracov 2015;55:61–75. [PubMed] [Google Scholar]
- [11].Flake GP, Andersen J, Dixon D. Etiology and pathogenesis of uterine leiomyomas: a review. Environ Health Perspect 2003;111:1037–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wise LA, Palmer JR, Harlow BL, et al. Reproductive factors, hormonal contraception, and risk of uterine leiomyomata in African-American women: a prospective study. Am J Epidemiol 2004;159:113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sakata R, Grant EJ, Ozasa K. Long-term follow-up of atomic bomb survivors. Maturitas 2012;72:99–103. [DOI] [PubMed] [Google Scholar]
- [14].Baird DD, Dunson DB, Hill MC, et al. Association of physical activity with development of uterine leiomyoma. Am J Epidemiol 2007;165:157–63. [DOI] [PubMed] [Google Scholar]
- [15].Wise LA, Palmer JR, Harlow BL, et al. Risk of uterine leiomyomata in relation to tobacco, alcohol and caffeine consumption in the Black Women's Health Study. Hum Reprod 2004;19:1746–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].He Y, Zeng Q, Dong S, et al. Associations between uterine fibroids and lifestyles including diet, physical activity and stress: a case-control study in China. Asia Pac J Clin Nutr 2013;22:109–17. [DOI] [PubMed] [Google Scholar]
- [17].Mahalingaiah S, Hart JE, Laden F, et al. Air pollution and risk of uterine leiomyomata. Epidemiology 2014;25:682–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lethaby A, Vollenhoven B. Fibroids (uterine myomatosis, leiomyomas). BMJ Clin Evid 2015;2015. [PMC free article] [PubMed] [Google Scholar]
- [19].Wise LA, Radin RG, Palmer JR, et al. Intake of fruit, vegetables, and carotenoids in relation to risk of uterine leiomyomata. Am J Clin Nutr 2011;94:1620–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Shen Q, Hua Y, Jiang W, et al. Effects of mifepristone on uterine leiomyoma in premenopausal women: a meta-analysis. Fertil Steril 2013;100:1722–6.e1-10. [DOI] [PubMed] [Google Scholar]
- [21].Qin J, Yang T, Kong F, et al. Oral contraceptive use and uterine leiomyoma risk: a meta-analysis based on cohort and case-control studies. Arch Gynecol Obstet 2013;288:139–48. [DOI] [PubMed] [Google Scholar]
- [22].Chiaffarino F, Parazzini F, La Vecchia C, et al. Use of oral contraceptives and uterine fibroids: results from a case-control study. Br J Obstet Gynaecol 1999;106:857–60. [DOI] [PubMed] [Google Scholar]


