Abstract
We aim to establish that accelerated aging and premature cellular senescence seen in individuals with Down syndrome is related to reduced DNA polymeraseβ. We report here that primary fibroblasts from Down syndrome individuals exhibit greater SA-β-gal staining (4-fold increase, p<0.001), increased p16 transcript abundance (3-fold increase, p<0.01), and reduced HMGB1 nuclear localization (1.5-fold lower, p<0.01). We also find that DNA polymerase β expression is significantly reduced in Down syndrome primary fibroblasts (53% decline, p<0.01). To evaluate whether DNA polymerase β might be causative in senescence induction, we evaluated the impact of murine DNA polymerase β nullizygosity on senescence. We find that unexposed DNA polymerase β -null primary fibroblasts exhibit a robust increase in the number of senescent cells compared to wild-type (11-fold, p< 0.001), demonstrating that loss DNA polymerase β is sufficient to induce senescence. We also see an additional increase in response to hydroxyurea (3-fold greater than WT-HU, p<0.05). These data demonstrate that loss of DNA polymerase β is sufficient to induce senescence. Additionally, we report a significant induction in spontaneous DNA double strand breaks in DNA polymerase β null MEFs (5-fold increase from wild-type, p< 0.0001). Our findings strongly suggest that DNA polymerase β is causative in senescence induction, reasonably pointing to DNA polymerase β as a likely factor driving the premature senescence in Down syndrome.
1. INTRODUCTION
A link between DNA repair capacity and longevity was first proposed by Hart and Setlow (Hart and Setlow, 1974), who correlated mammalian DNA repair capacity to maximum lifespan. This association launched an entire field of study into the relative roles of DNA repair capacity on lifespan. In turn, many human conditions of premature and/or accelerated aging have been directly linked to defective DNA repair (Brosh and Bohr, 2007). Down syndrome (DS) is a genetic disorder caused by Trisomy 21 and is characterized by a shortened lifespan and biomarkers of precocious aging (Esbensen, 2010; Hermon et al., 2001; Hill et al., 2003). When cultured, the proliferative potential of primary fibroblasts and lymphocytes from individuals with Down syndrome is reduced and senescence-associated beta-galactosidase (SA-β-gal) activity is increased (Cristofalo et al., 2004; Kalanj-Bognar et al., 2002). This early appearance of SA-β-gal activity is also observed in both cultured fibroblasts and skin tissue from TS65Dn mice (Contestabile et al., 2009), and in skin tissue from individuals with Trisomy 21 (Rodriguez-Sureda et al., 2015). While other disorders of accelerated aging have been directly connected to specific DNA repair defects, Down syndrome has not. There is evidence for reduced base excision repair (BER) in Down syndrome, which includes: accumulation of DNA repair intermediates in the form of strand breaks (Maluf and Erdtmann 2001; Athanasiou et al., 1980), increased chromosomal damage (Caria et al., 2001; Shafiket al., 1988), reduced in vitro measures of DNA repair capacity (Agarwal et al., 1970; Raji et al., 1998), and reduced expression of DNA base excision repair genes (Cabelof et al., 2009; Raji et al., 1998), in particular, DNA polymerase β (POLβ).
Defective or reduced base excision repair has been linked to aging, on observations that old mice (24 months) exhibit an approximate 50% reduction in base excision repair capacity, POLβ protein and Polβ mRNA expression (Cabelof et al., 2002). Further, reduction in base excision repair capacity is linked to age-related disorders including dementia and cancer (Sykora et al., 2015; Weissman et al., 2007; Yamtich et al., 2012). These aging phenotypes are, in turn, linked specifically to reduced POLβ (Cabelof et al., 2002; Kisby et al., 2010; Krishna et al., 2005; Rao et al., 1994; Rao et al., 2001; Subba Rao and Subba Rao, 1984). Mouse models developed to evaluate the functions of Polβ, show peri-embryonic lethality for homozygous Polβ null (Polβ−/−) mice (Sobol, 1996), while heterozygous mice (Polβ+/−) are both viable and fertile. These heterozygous mice express gene-dosage reductions in Polβ, which quantitatively mimic the reductions in POLβ reported for Down syndrome. The increased mortality rate seen in Polβ+/− mice (Cabelof et al., 2006) was the first observation that a DNA base excision repair protein might be directly linked to accelerated aging phenotypes. Mechanistically, POLβ is induced in response to DNA damage (Cabelof et al., 2002; Cabelof et al., 2003; Cabelof, 2007), so we should anticipate an adaptive upregulation in POLβ in response to the accumulation of endogenous DNA damage in Down syndrome. The absence of this adaptive response could conceivably contribute to senescence. Our objective in this work is to establish direct evidence that loss of POLβ promotes cellular senescence, connecting its role directly to this mechanism of aging.
EXPERIMENTAL PROCEDURES
Tissue Culture.
DS primary fibroblasts (AG06872, AG05397) and their age- and sex-matched controls (GM00969, GM05659) were acquired from Coriell Cell Repositories. Primary cells were maintained in MEM medium (Life Technologies, Cat#10370–021) supplemented with 15% fetal bovine serum (Hyclone, Cat# SH3007003) and 1% penicillin/streptomycin (Gibco, Cat# 15240–062). Passage number was carefully documented and cells of similar passage were used in comparisons. Where noted, data were pooled for both non-Down syndrome (NDS) cells and both DS cells. In the Polβ experiments, primary cells of wild-type or DNA polymeraseβ deficient fibroblasts were isolated from heterozygous females (Polβ+/−) at day 10.5 of gestation, as described in Sobol et al. (1996), and were a generous gift from Samuel H. Wilson. The cells we used were named WT-48, WT-53 (herein designated WT when data are pooled), and Polβ−/−47, Polβ−/−51 (herein designated Polβ−/− when data are pooled). These primary mouse embryonic fibroblasts were maintained in DMEM (Gibco, Cat# 11995081) and supplemented with 10% FBS. Passage number was carefully documented and cells of similar passage were used in comparisons.
SA-β-gal:
Senescence-associate β-galactosidase activity was assessed using a senescence detection kit from BioVision (Milpitas, CA), according to the manufacturer’s instructions. Briefly, cells were seeded at 5 × 104 density and left untreated or treated for 10–14 days with either hydroxyurea (HU; 300μM) or methyl methanesulfonate (MMS; 600μM). Media was changed every three days, and fresh dose of HU or MMS was given. Cells were fixed for 5 minutes at room temperature and incubated overnight at 37°C at ambient CO2 with staining solution containing X-gal and staining supplement. Blue-stained cells were identified as senescent. A minimum of 300 cells in at least 5 fields of view were photographed and counted at 20X magnification, by two blinded counters. Data shown here represent the average of three independent pooled experiments. Results are expressed as percentage of stained cells in the total number of cells. Cells cultured and treated concomitantly with those used to assess senescence were passaged for additional 2 weeks to evaluate their ability to recover/emerge from senescence.
Western blotting:
Nuclear and cytoplasmic fractions were extracted using the NucBuster™ Protein Extraction Kit (Novagen, Darmstadt, Germany) and total protein in each fraction was quantified using the Pierce BCA Protein Assay Kit (Thermo Scientific), as per the manufacturer protocols. 40 μg of protein was run on 10% Mini-PROTEAN TGX Precast Protein els (BioRad) and transferred to 0.2 μm PVDF by using the Trans-Blot Turbo Transfer System (BioRad), according to the manufacturer’s recommendations. Loading and transfer consistency was verified by UV-activation of the gel and membrane. Following activation, blots were blocked for 30 minutes in 5% BSA in PBST and incubated in HMGB1 primary antibody (1:500) (Sigma, Cat# H9664) at 4C overnight. Blots were subsequently washed in PBST and incubated for 90min at room temperature in anti-mouse IgG-HRP secondary antibody (Cell Signaling, #7076), diluted at 1:5000 in PBS. Bands were detected using a ChemiImager, following activation with the SuperSignal Chemiluminescent Substrate luminol/enhancer (BioRad). Optical density was determined using ImageJ (Schneider et al., 2012). HMGB1 values for nuclear and cytoplasmic fractions are calculated as band intensity normalized to total protein intensity.
Immunofluorescence staining:
4 × 104 cells were seeded on coverslips in 12-well plates and fixed in ethyl: acetate (80:20) for 5 min. After three washes, cells were blocked in PBST containing 1% BSA for 30 min at room temperature and incubated with combined primary anti-γH2AX (1:100) (Millipore, Cat# 05–636) and anti-53BP1 antibodies (1:100) (NovusBio, Cat # NB100–340) for 1.5 hr at room temperature or overnight at 4°C. Cells were then incubated with combined Alexa Fluor 488-conjugated anti-mouse IgG (1:400) (Invitrogen, Cat# A-11001) and Alexa Fluor 568-conjugated anti-rabbit IgG (Invitrogen, Cat # A-11011) for one hour at room temperature and mounted with Pro-Long Gold anti-fade reagent (Invitrogen, CA). Slides were photographed under the Nikon Eclipse 80i microscope (Nikon, CA) and processed using the Nikon Elements built-in software. Approximately 100 cells were selected in more than 10 fields of view and the number of foci positive for both proteins were counted for each cell.
Expression analysis:
cDNA was synthesized, as described previously (Cabelof et al., 2006), from 2μg RNA using random hexamer primers and purified with the QIAquick PCR Purification columns (Qiagen, Valencia, CA). Transcripts were amplified and quantitated with a LightCycler 480 real-time PCR machine (Roche). PCR reactions contained 2μl purified cDNA, 0.5 μM of each sense and antisense primer, and 2μl FastStart DNA Master SYBR Green I enzyme-SYBR reaction mix (Roche). For all amplifications, PCR conditions consisted of an initial denaturation step at 95°C for 5 min, followed by 40–45 cycles at 95°C for 10s, primer specific annealing temperatures for 10s, and elongation at 72°C for 10s. Melting curves from 65°C to 95°C confirmed specificity. External standards were prepared for all genes from cDNA amplicons cloned into pCRII TOPO cloning vector (Invitrogen, Carlsbad, CA). All transcripts were quantitated and normalized to GAPDH or Rpl4 expression as stated. Primer sequences are detailed in Table 1, with the exception of primer sets used for amplification of human POLβ and human p16, which were purchased from SA Biosciences (Frederick, MD) (Cat#PPH13735F-200 and Cat#PPH00207C, respectively). Human CBS, BACH1 and SOD1, all Chromosome 21-localized genes, were used to confirm DS status of the primary fibroblasts.
Table 1:
Quantitative RT-PCR Primer sequences
| Gene | Sense primer 5′–3′ | Anti-sense primer 5′–3′ |
|---|---|---|
| Human | ||
| CBS | ggggctgagattgtgaggac | cggtactggtctaggatgtga |
| SOD1 | ggtgggccaaaggatgaagag | ccacaagccaaacgacttcc |
| BACH1 | ctcagccttaatgaccagcgg | gcctacgattcttgagtggaag |
| Mouse | ||
| p16 UPL probe #91 | aatctccgcgaggaaagc | gtctgcagcggactccat |
| Polβ (exon 12–13) | agcgagaaggatggaaaggaa | cgtgcgctctcatgttcttat |
| Housekeeping genes | ||
| Gapdh | aggtcggtgtgaacggatttg | tgtagaccatgtagttgaggtca |
| GAPDH | aaggtgaaggtcggagtcaac | ggggtcattgatggcaacaata |
| Rpl4 | ccgtcccctcatatcggtgta | gcatagggctgtctgttgttttt |
| Rpl4 UPL probe # 75 | tggtggttgaagataaggttga | ccaagctttgagtttcttgagc |
Data Analysis:
Results are expressed as mean ± SEM and were analyzed using Student’s t-test. Statistical comparisons between groups were conducted using one-way ANOVA, using GraphPad Prism 5.0 (Graph Pad Software, La Jolla, CA). Values of p < 0.05 were considered statistically significant and individual p-values are shown in the figures.
RESULTS
Premature cellular senescence in Down syndrome primary fibroblasts
Primary fibroblasts from individuals with Down syndrome were assessed for premature senescence phenotypes by several different senescence markers. Cells from Down syndrome individuals have been reported to exhibit many features of early senescence, including enlarged and flattened morphology, increased numbers of multinucleated cells, and early appearance of SA-β-gal activity (Cristofalo et al., 2004; Kalanj-Bognar et al., 2002). These in vitro findings are consistent with the accelerated aging that is consistently observed in Down syndrome (Patterson & Cabelof, 2012). Senescence is an irreversible cell cycle state that can be induced by multiple endogenous and exogenous signals. Widely used biomarkers of senescence are SA-β-gal, p16 expression (Alcorta et al., 1996), and HMGB1 cellular localization, all of which we have quantified here.
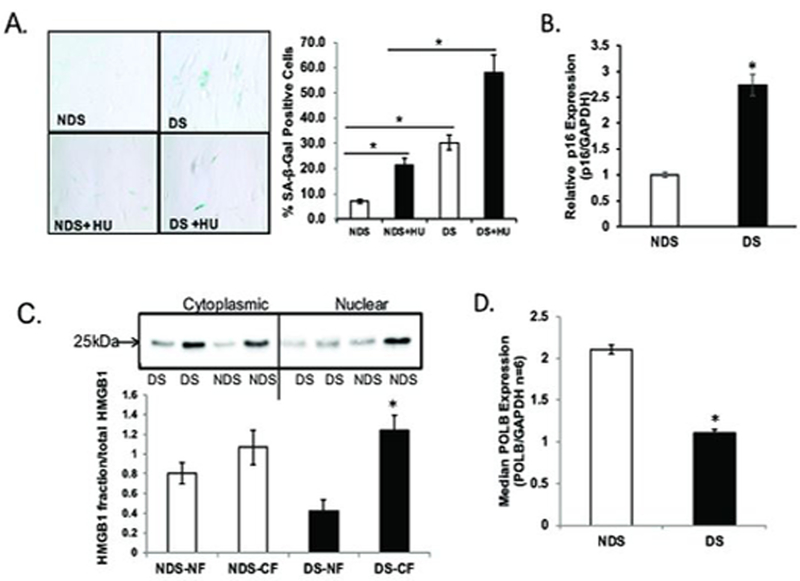
SA-β-gal is expressed only in senescent cells and is not observed in pre-senescent, quiescent, or transformed cells (Dimri et al., 1995). We confirm the early appearance of SA-β-gal activity in DS in the absence of any exogenous stressors: 5% of NDS fibroblasts stain positive for SA-β-gal while 20% of DS fibroblasts stain positive for SA-β-gal (Figure 1A, 4-fold increase, p<0.001). In addition, we evaluated the senescence response to HU and find only 21.4% of NDS fibroblasts are senescent (very similar to senescence response we see in unexposed DS fibroblasts), while 58.2% of the DS fibroblasts are senescent (Figure 1A, >2-fold, p<0.01). Thus, DS fibroblasts have an increased level of senescence at baseline and also in response to the replication stress induced by HU.
Figure 1: Premature cellular senescence in Down syndrome primary fibroblasts.
All experiments were conducted in primary fibroblasts from age- and sex-matched donors either without Down syndrome (NDS, GM00696 and GM05659) or with Down syndrome (DS, AG06872 and AG05397) as described in Experimental. Graphs are labeled DS or NDS. A. Down syndrome fibroblasts exhibit a premature senescence phenotype. Senescence-associated β-galactosidase (SA-β-Gal) activity was measured in primary fibroblasts from donors with or without Down syndrome as described in Experimental. A minimum of 300 cells was counted in random fields by a technician blinded to genotype to quantify the proportion of SA-β-Gal-positive cells. Quantification is presented as the average of three independent experiments ± SEM and is expressed as a percent of positive cells [(SA-β-Gal-positive cells/total cells)*100]. Data is expressed as mean ± SEM for three independent pooled experiments. *Value significantly different from control at p < 0.01. B. Upregulation in p16 in Down syndrome primary fibroblasts. p16 transcript abundance was determined by quantitative RT-PCR and normalized to GAPDH as described in methods. Data is expressed as mean ± SEM for three independent experiments. *Value significantly different from control at p < 0.01. C. HMGB1 is excluded from the nuclear fraction in Down syndrome primary fibroblasts. Nuclear and cytoplasmic fractions were extracted from cells, separated on 10% TGX Stain-free precast gels (BioRad) and transferred to PVDF. Membrane was incubated in 1:500 dilution of HMGB1 (Sigma, Cat# H9664). HMGB1 in nuclear and cytoplasmic fractions was normalized to total protein as described in Experimental. Normalization (loading control) was done for both nuclear and cytoplasmic fractions by calculating optical density of HMGB1/total protein. Graph is labeled for genotype (DS or NDS) and fraction (NF, nuclear fraction; CF, cytoplasmic fraction). Data is expressed as mean ± SEM for three independent experiments from pooled averages. *Value significantly different from control at p < 0.01. D. Primary Down syndrome fibroblasts exhibit reduced DNA polymeraseβ transcript abundance. Gene expression was evaluated in primary fibroblasts from donors with or without Down syndrome as described in Experimental. POLβ transcript levels were determined by quantitative real-time RT-PCR and normalized to GAPDH. Data are presented as the average of pooled samples from each genotype ± SEM. *Values significantly different from control (NDS) at p < 0.01.
Elevated p16 expression maintains the senescence phenotype and is an established marker of premature aging (Alcorta et al., 1996). Further, removal of p16-expressing cells (i.e., senescent cells) from tissues can reverse senescent phenotypes (Baker et al., 2011). We evaluated p16 transcript abundance and find a significant increase in p16 in DS (Figure 1B, 3-fold increase, p<0.01). As a third marker of cellular senescence, we evaluated HMGB1 levels in the nucleus. HMGB1 is a nuclear non-histone DNA-binding protein shown to have a role both in regulating DNA repair and in senescence (Enokido et al., 2008; Lange & Vasquez, 2009; Menon et al., 2016; Prasad et al., 2007). Davalos et al., demonstrated that HMGB1 redistribution from the nucleus is crucial to the senescence-associated secretory phenotype (SASP) (Davalos et al. 2013). We have quantitated both nuclear and cytoplasmic fractions of HMGB1 and show clearly that the DS fibroblasts have disproportionately less HMGB1 in the nucleus as compared to their NDS counterparts (Figure 1C, >1.5-fold, ±SEM, p<0.01). In NDS cells, 44% of total HMGB1 is nuclear, while in DS cells, only 25% of HMGB1 is nuclear.
Reduced DNA base excision repair, and in particular POLβ has been reported and appreciated as a phenotype of DS for some time (Fry et al., 1984; Raji & Rao, 1998). More recently, we evaluated this question in blood samples from acute megakaryoblastic leukemia (AMkL) patients and in fetal liver tissue. We reported that both AMkL patients with DS and fetal liver tissue from DS abortuses expressed lower levels of POLβ than did the NDS counterparts (Cabelof et al., 2009). As such, we chose to evaluate POLβ in primary NDS and DS fibroblasts and determine whether senescence could be a function of impaired POLβ. We evaluated POLβ expression in primary fibroblast cells from six individuals with DS and six without DS (DS and NDS), and find a significant reduction in POLβ expression in Down syndrome cells (Figure 1D, 53% reduced, ±SEM, p<0.01). In light of the well-established role of Polβ in processing damage induced by methyl methanesulfonate (MMS) (Sobol et al., 2002) we anticipated that Down syndrome cells would senesce in response to MMS exposure. We attempted a range of dosing and timing schedules to induce senescence with MMS but never saw an increase in the number of senescent cells as a result Down syndrome (data not shown). This suggests that the mechanism of senescence in DS may not be an increase in DNA damage (MMS) per se, but an inability to respond to replication stress (HU).
Homozygous loss of DNA polymeraseβ induces cellular senescence
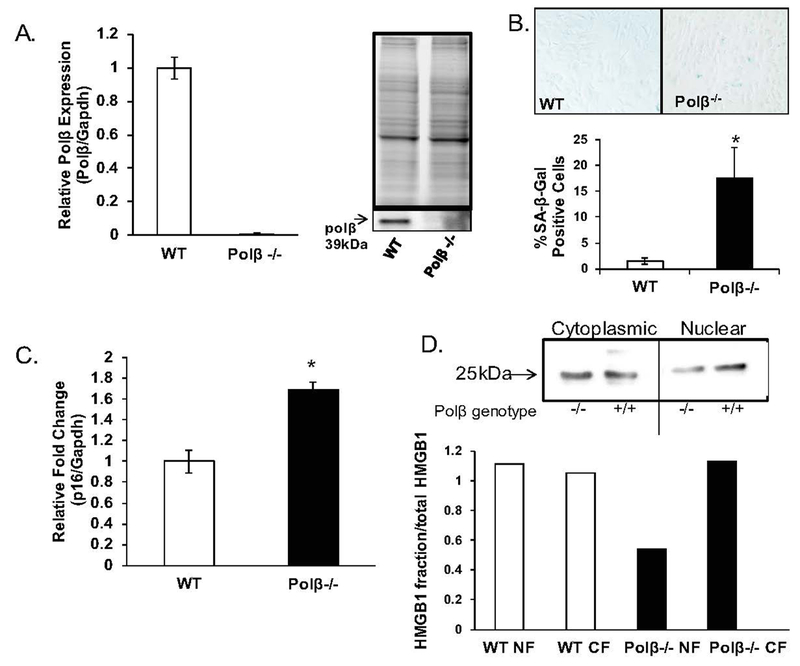
To elucidate a possible role for POLβ in cellular senescence, we evaluated DNA polymeraseβ null (Polβ−/−) primary mouse embryonic fibroblasts gifted by Samuel H. Wilson (Sobol et al., 1996). Senescence evaluation is best conducted in primary cells. While the use of primary cells limits the types of questions we can experimentally address, it does provide the best possible information on the key question under investigation here, which is senescence. We first verified the known reduction in Polβ expression in Polβ−/− and wildtype (WT) cells (Sobol et al., 1996). In two different cell lines from each genotype, we confirmed that mRNA and protein levels were undetectable in the Polβ−/− cells (Figure 2A). Next, we evaluated these cells for the same biomarkers of senescence used in the DS experiments, both at baseline and in response to exogenous DNA damage. Cells grown and treated in parallel were maintained in culture for an additional two weeks, but never reentered the cell cycle. In the absence of HU or other DNA damaging agents, we find robust SA-β-Gal staining in the Polβ−/− cells, a much greater senescence response due to loss of Polβ than we had anticipated (Figure 2B, > 11-fold, p< 0.001). Notably, Polβ−/− cells and DS cells exhibit very similar SA-β-Gal staining (~20% of cells stain positive). In addition to significant increase in SA-β-Gal positive cells induced by loss of Polβ, p16 was also significantly upregulated in the Polβ−/− cells, further supporting that senescence is induced when Polβ is absent (Figure 2C, 1.69-fold, p<0.001). We find that HMGB1 appears to be reduced in the nuclear fraction of the Polβ−/− cells as well (Figure 2D).
Figure 2: Homozygous loss of DNA polymeraseβ induces cellular senescence.
Fibroblasts were isolated from heterozygous DNA polymeraseβ (polβ+/−) female mice at day 10.5 of gestation to create primary wild-type (WT) or DNA polymeraseβ null (polβ−/−) embryonic fibroblasts (Sobol et al., 1996), as described in Experimental. The primary embryonic fibroblasts used are designated WT-48, WT-53 (WT), null-47, null-51 (polβ−/−). All experiments have been conducted in triplicate in all four groups of fibroblasts between passages 10–12 (except where noted), and data has been pooled for statistical analysis. A. Validation of polB null status in primary polβ wildtype and homozygous null fibroblasts. Both mRNA and protein expression levels were measured in two isogenic wildtype and two isogenic polβ−/− fibroblasts. We confirm the null status of both primary polβ−/− fibroblasts, as expected. Data is expressed as mean ± SEM for three independent experiments. *Value significantly different from control at p < 0.01. B. Loss of polβ induces accumulation of SA-β-gal positive cells. Senescence-associated β-galactosidase (SA-β-Gal) activity was measured in primary mouse embryonic fibroblasts from WT and polβ−/− fibroblasts as described in Experimental. 300 cells were counted in random fields by a technician blinded to genotype to quantify the proportion of SA-β-Gal-positive cells. Image is a representative staining of WT and polβ−/− fibroblasts. We consistently found SA-β-gal staining to be lesser in the WT fibroblasts and greater in the polβ−/− fibroblasts. Quantification is presented as the average of three pooled independent experiments ± SEM and is expressed as a percent of positive cells [(SA-β-Gal-positive cells/total cells)*100]. *Value significantly different from control at p < 0.01. C. Loss of polB induces p16 expression. p16 expression was measured in wildtype and DNA polymeraseβ null primary embryonic fibroblasts, using a probe-based system as described in Experimental. Data is expressed as mean ± SEM for three independent experiments from pooled averages. *Value significantly different from control at p < 0.01. D. HMGB1 is excluded from the nuclear fraction in passage polβ−/− fibroblasts. Nuclear and cytoplasmic fractions were extracted from cells grown at passage number 5 and passage number 30 as described in Experimental. Protein was separated on 10% TGX Stain-free precast gels (BioRad) and transferred to PVDF. Membrane was incubated in 1:500 dilution of HMGB1 (Sigma, Cat# H9664). HMGB1 in nuclear and cytoplasmic fractions was normalized to total protein as described in Experimental. Normalization (loading control) was done for both nuclear and cytoplasmic fractions by calculating optical density of HMGB1/total protein. Data presented as compilation of two independent experiments. Graph is labeled for genotype (WT or null) and fraction (NF, nuclear fraction; CF, cytoplasmic fraction).
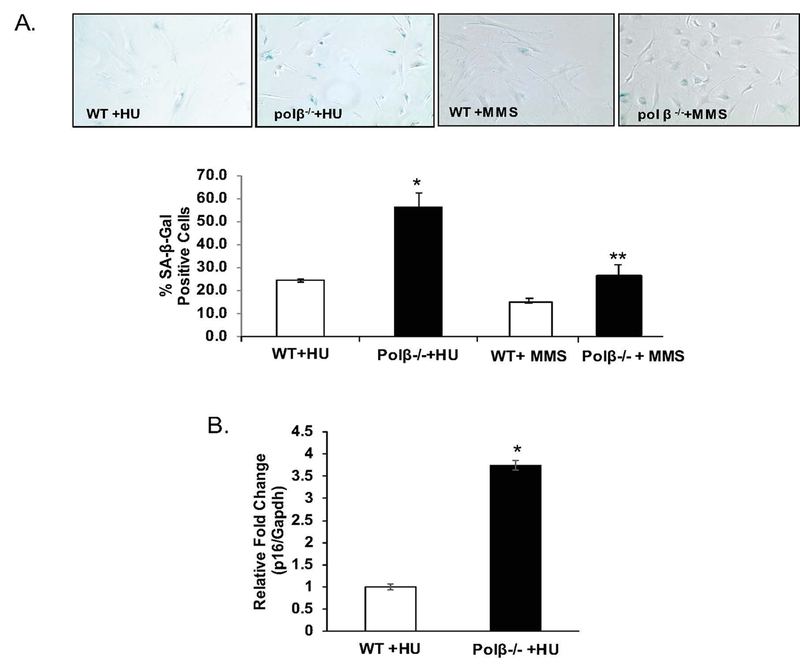
Loss of DNA polymeraseβ amplifies the senescence-inducing effects of hydroxyurea
Treatment of Polβ null cells with HU resulted in a greater increase in both SA-β-gal positive cells (Figure 2A, >2-fold, p<0.05) and p16 expression (Figure 2B, > 3.5-fold, p<0.001).
We consistently found less SA-β-gal staining in the WT fibroblasts than in the Polβ−/− fibroblasts in response to both HU and MMS exposure, but the genotype effect in response to MMS is minimal. In response to HU, Polβ loss induces 56% positive-staining cells, nearly equivalent to the impact of HU on DS cells, but in response to MMS we find only 26% positive staining cells, only minimally more than the 18% positive cells in Polβ−/− cells. This was surprising to us as Polβ has a clear role in processing MMS-induced damage (Sobol et al., 1996), and suggests that senescence induction as a function of Polβ may not be due to DNA alkylation damage, but rather to an inability to handle replication stress.
DNA polymeraseβ may protect cells from spontaneous DNA double strand breaks.
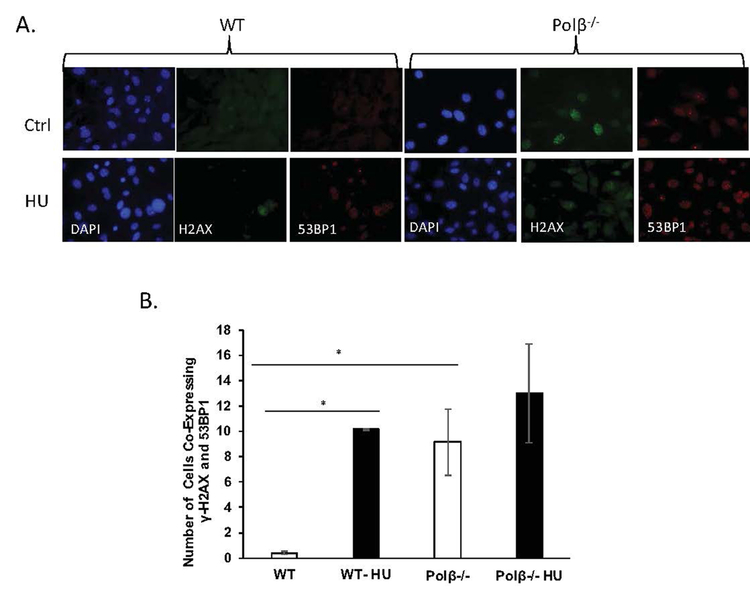
Hydroxyurea inhibits ribonucleotide reductase, which alters dNTP/NTP ratios. This imbalance generates strand breaks and stalled replication forks, both known drivers of senescence (Yeo et al., 2000). As such, we quantified foci expressing both γ-H2AX and 53BP1to evaluate the impact of HU on DNA double strand break (DSB) formation, both in the presence and absence of Polβ. While not all foci will represent DSB directly, co-localized foci are informative for both DSB accumulation, and for stalled replication forks that could be induced by HU (Sirbu et al., 2011). Co-localized foci in WT cells unexposed to HU were essentially undetectable, as expected. In response to HU, WT cells expressed about 10 co-localized foci/100 cells imaged (Fig 3B, p<0.001). Unexpectedly, the Polβ−/− cells expressed a similar level of co-localized foci in the absence of HU exposure, about 9/100 cells imaged (Fig 4B, p<0.001, compared to WT). If we presume these foci represent DSB, this demonstrates that loss of Polβ alone is sufficient to induce DSB. It is potentially interesting that HU-treated WT cells and untreated Polβ−/− cells express very similar DSB phenotypes. However, what is difficult to reconcile is that the Polβ−/− cells treated with HU do not exhibit a statistically significant genotype effect on foci formation, while there is a significant genotype effect on senescence induction (Fig 2A). This could be a function simply of the sensitivity or precision of this assay for DSB detection. But it is also possible that the elevated baseline of DSB in the Polβ−/− cells could generate stalled forks once a DSB threshold has been exceeded, preventing cell death and driving senescence. The concept of loss of Polβ as a protective mechanism is not new (Ventrella-Lucente et al., 2010), nor is a protective role of senescence new (Campisi, 2013).
Figure 4. DNA polymeraseβ may protect cells from spontaneous DNA double strand breaks.
WT and polβ−/− primary fibroblasts were exposed to HU as described in Experimental. A. γ-H2AX foci are amplified by loss of DNA polβ. Overlappping γ-H2AX/53BP1 foci serve as a marker for DNA double strand breaks. Foci were detected by γ-H2AX and 53BP1 immunostaining in primary mouse embryonic fibroblasts as described in Experimental. All four groups of cells either remained untreated or were treated with 300uM HU for 24 hours, fixed and stained with DAPI (blue). Cells were probed with antibody against γ-H2AX (green; anti-γ H2AX antibody) and 53BP1 (red; anti-53BP1) as described in Experimental. Slides were photographed under the Nikon Eclipse 80i microscope (Nikon, CA) and processed using the Nikon Elements built-in software. B. Quantification of DNA double strand breaks. Approximately 100 cells were selected in more than 10 fields of view and the number of foci positive for both γ-H2AX and 53BP1 were counted for each cell. Data are presented as mean ±SEM. *Values significantly different from control at p < 0.01.
Figure 3: Loss of DNA polymeraseβ amplifies the senescence-inducing effects of hydroxyurea.
WT and polβ−/− primary fibroblasts were exposed to either hydroxyurea (300uM) or methyl methanesulfonate (MMS; 600uM) every 3 days for 10–14 days to induce senescence as described in Experimental. A. Loss of DNA polymeraseβ amplifies the accumulation of SA-β-gal positive cells in response to hydroxyurea (HU) but not methyl methanesulfonate (MMS). All four groups of cells either remained untreated, or were exposed to a standard HU senescence-inducing protocol, or MMS as described in Experimental. Briefly, cells were seeded 24 hours prior to treatment and exposed every 2 days over a 14 day period. 300 cells were counted in random fields by a technician blinded to both genotype and treatment. Image is a representative staining of WT and polβ−/− fibroblasts. Quantification is presented as the average of three pooled independent experiments ± SEM and is expressed as a percent of positive cells [(SA-β-Gal-positive cells/total cells)*100]. *Value significantly different from control at p < 0.01. B. Upregulation of p16 by hydroxyurea is amplified by loss of polβ. p16 expression was measured in WT and polβ−/− fibroblasts using a probe-based system as described in Experimental. Data is expressed as mean ± SEM for three independent pooled experiments. *Value significantly different from control at p < 0.01. **Value significantly different from control at p<0.05.
DISCUSSION
The Polβ heterozygous mouse expresses 50% less Polβ and ages at a slightly faster rate than its wildtype littermates (Cabelof et al., 2006). This is quantitatively similar to the approximate 50% decline in POLβ levels reported previously in Down syndrome (Raji et al., 1998), a condition likewise characterized by a faster rate of aging. Cultured Down syndrome fibroblasts exhibit enlarged and flattened morphology, increased number of multinucleated cells and early appearance of SA-β-gal activity (Cristofalo et al., 2004; Kalanj-Bognar et al., 2002). We confirm a robust, early cellular senescence in our Down syndrome model system through multiple measures of senescence (SA-β-gal activity, p16 expression and HMGB1 subcellular localization, Figure 1 A, B and C). The source of early aging in Down syndrome is likely due to a combination of increased endogenous damage and reduced DNA base excision repair capacity. Both the accumulation of damage and the reduction in repair capacity in Down syndrome begin in utero (Cabelof et al., 2009; Domenico et al., 2015; Nizetic and Groet, 2012; Pogribna et al., 2001) and create a constitutive environment of DNA stress. Under typical conditions POLβ would be induced in response to endogenous DNA damage. We suggest that the lack of POLβ (53% reduction, Figure 1D) and absence of a POLβ-driven DNA repair response could explain early senescence in Down syndrome.
We test this hypothesis by determining whether loss of Polβ is sufficient to induce senescence. We find that homozygous loss of murine Polβ results in an 11.7-fold increase in SA-β-gal activity (Figure 2B) and a nearly 2-fold increase in p16 expression (Figure 2C). This is direct evidence for Polβ in senescence induction. The increase in p16 in both model systems may be therapeutically interesting, as elimination of p16 expressing cells can reverse some age associated phenotypes (Baker et al. 2011). Following treatment with HU and MMS, we find a robust senescence response to hydroxyurea (18% positive cells in unexposed Polβ−/− cells, Figure 2B ; 56% positive cells in HU-treated Polβ−/− cells, Figure 2A), but a much smaller effect of MMS and genotype on senescence (18% positive cells in unexposed Polβ−/− cells, Figure 2B; 26% positive cells in MMS-treated Polβ−/− cells, Figure 2A). Thus, as compared to untreated Polβ−/− cells, HU treatment results in a 310% increase in senescent cells (3.1-fold change) while MMS treatment only results in a 40% increase (1.4- fold change). Loss of Polβ is well-understood to increase sensitivity to alkylating agents (Horton et al., 2002; Ochs et al., 1999; Pascucci et al., 2005; Podlutsky et al., 2001; Sobol et al., 1996; Cabelof et al., 2003) through a strand break mechanism driven by Polβ dRPase-activity (Sobol et al., 2002). We suggest that in the absence of Polβ−/− those breaks are largely eliminated and that the senescence observed by MMS in the Polβ−/− cells primarily reflects the endogenous SA-β-gal activity seen in untreated Polβ−/− cells, with minimal additive effect of MMS.
Hydroxyurea induces senescence directly through a stalled fork/strand break-mediated mechanism (Yeo et al., 2000), known as replication stress. As such, we chose to evaluate DNA double strand breaks induced by hydroxyurea and replication stress, in senescing cells as a function of Polβ deficiency. We find increased number of colocalized γH2AX and p53BP1foci in senescing cells, which is amplified by loss of Polβ. We should note that DSB have been shown to accumulate in a knock-in mouse model of PolB variant R137Q (Pan et al., 2016), Polβ−/− cells exposed to MMS (Pascucci et al., 2005), and an Alzheimer’s/Polβ+/− mouse (3xTgAD/Polβ) (Sykora et al., 2015). But this is the first report that loss of Polβ alone results in a significant accumulation of colocalized γH2AX and p53BP1, presumed to be DSB. Further research is needed into whether the disparate effects of MMS and HU on senescence may be informative about the molecular mechanisms of senescence induction.
It is well-known that complementation of Polβ null cells with purified Polβ restores wildtype phenotypes (Sobol et al., 1996). It would be highly informative to restore Polβ levels in the Down syndrome primary fibroblasts and evaluate the ability to complement the senescence phenotypes. Unfortunately, we have been unable to successfully complete these experiments, as our attempts at manipulating the primary Down syndrome fibroblasts did not generate viable cells that could withstand the senescence protocol. As such, the question of whether Polβ provides a definitive causative explanation for senescence in Down syndrome remains open. But a collection of evidences points to a role, if only partial. Another open question is why POLβ is systematically reduced in Down syndrome (beginning in utero), and whether the failure to respond properly to DNA damage might make these individuals uniquely susceptible to environmental exposures. The incidence of Down syndrome is currently 14.47 per 10,000 live births (Parker et al., 2010), making susceptibililty to environmental exposures in Down syndrome a relevant population-level question. Given the known impact of POLβ deficiency on sensitivity to exogenous exposures, Down syndrome presents a known human model of POLβ haploinsufficiency, which could potentially be exploited to elucidate molecular mechanisms of senescence as a function of environmental exposures.
ACKNOWLEDGEMENTS
This work was supported by the National Institute on Aging at the National Institutes of Health, R21AG044625 (DCC). DNA polymeraseβ homozygous null mouse embryonic primary fibroblasts were provided by Samuel H. Wilson, NIEHS. We would like to thank Judith Campisi for help with SA-β-gal, p16 and HMGB1 experiments.
REFERENCES
- Agarwal SS, Blumberg BS, Gerstley BJ, London WT, Sutnick AI, and Loeb LA. 1970. DNA polymerase activity as an index of lymphocyte stimulation: studies in Down’s syndrome. J Clin Invest, 49: 161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcorta DA, Xiong Y, Phelps D, Hannon G, Beach D, and Barrett JC. 1996. Involvement of the cyclin-dependent kinase inhibitor p16 (INK4a) in replicative senescence of normal human fibroblasts. Proc Natl Acad Sci U S A, 93: 13742–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasiou K, Sideris EG, and Bartsocas C. 1980. Decreased Repair of X-ray Induced DNA Single-Strand Breaks in Lymphocytes in Down’s Syndrome. Pediatr Res, 14: 336–38. [DOI] [PubMed] [Google Scholar]
- Baker Darren J., Wijshake Tobias, Tchkonia Tamar, LeBrasseur Nathan K., Childs Bennett G., Bart van de Sluis, Kirkland James L., and van Deursen Jan M.. 2011. Clearance of p16(Ink4a)-positive senescent cells delays ageing-associated disorders. Nature, 479: 232–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosh RM Jr., and Bohr VA. 2007. Human premature aging, DNA repair and RecQ helicases. Nucleic Acids Res, 35: 7527–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabelof DC 2007. Aging and base excision repair: in need of a comprehensive approach. DNA Repair (Amst), 6: 1399–402. [DOI] [PubMed] [Google Scholar]
- Cabelof DC, Guo Z, Raffoul JJ, Sobol RW, Wilson SH, Richardson A, and Heydari AR. 2003. Base excision repair deficiency caused by polymerase beta haploinsufficiency: accelerated DNA damage and increased mutational response to carcinogens. Cancer Res, 63: 5799–807. [PubMed] [Google Scholar]
- Cabelof DC, Ikeno Y, Nyska A, Busuttil RA, Anyangwe N, Vijg J, Matherly LH, Tucker JD, Wilson SH, Richardson A, and Heydari AR. 2006. Haploinsufficiency in DNA polymerase beta increases cancer risk with age and alters mortality rate. Cancer Res, 66: 7460–5. [DOI] [PubMed] [Google Scholar]
- Cabelof DC, Patel HV, Chen Q, van Remmen H, Matherly LH, Ge Y, and Taub JW. 2009. Mutational spectrum at GATA1 provides insights into mutagenesis and leukemogenesis in Down syndrome. Blood, 114: 2753–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabelof DC, Raffoul JJ, Yanamadala S, Ganir C, Guo Z, and Heydari AR. 2002. Attenuation of DNA polymerase beta-dependent base excision repair and increased DMS-induced mutagenicity in aged mice. Mutat Res, 500: 135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J 2013. Aging, Cellular Senescence, and Cancer. Annu Rev Physiol. 75: 685–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caria H, Chaveca T, and Rueff J. 2001. Aneuploidy induced in lymphocytes of parents of trisomic 21 children. Teratog Carcinog Mutagen, 21: 369–82. [DOI] [PubMed] [Google Scholar]
- Contestabile A, Fila T, Cappellini A, Bartesaghi R, and Ciani E. 2009. Widespread impairment of cell proliferation in the neonate Ts65Dn mouse, a model for Down syndrome. Cell Prolif, 42: 171–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristofalo Vincent J., Lorenzini Antonello, Allen RG, Torres Claudio, and Tresini Maria. 2004. Replicative senescence: a critical review’, Mechanisms of Ageing and Development. 125: 827–48. [DOI] [PubMed] [Google Scholar]
- Davalos Albert R., Kawahara Misako, Malhotra Gautam K., Schaum Nicholas, Huang Jiahao, Ved Urvi, Beausejour Christian M., Coppe Jean-Philippe, Rodier Francis, and Campisi Judith. 2013. p53-dependent release of Alarmin HMGB1 is a central mediator of senescent phenotypes. The Journal of Cell Biology, 201: 613–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Arruda Cardoso Smith M, Borsatto-Galera B, Feller RI, Goncalves A, Oyama RS, Segato R, Chen E, Carvalheira GM, Filho AS, Burbano RR, and Payao SL. 2004. Telomeres on chromosome 21 and aging in lymphocytes and gingival fibroblasts from individuals with Down syndrome. J Oral Sci, 46: 171–7. [DOI] [PubMed] [Google Scholar]
- Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, and et al. 1995. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A, 92: 9363–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenico Fabio Di, Pupo Gilda, Mancuso Cesare, Barone Eugenio, Paolini Francesca, Arena Andrea, Blarzino Carla, Schmitt Frederick A., Head Elizabeth, Butterfield D. Allan, and Marzia Perluigi. 2015. Bach1 overexpression in Down syndrome correlates with the alteration of the HO-1/BVR-A system: insights for transition to Alzheimer Disease. Journal of Alzheimer’s disease: JAD, 44: 1107–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enokido Y, Yoshitake A, Ito H, and Okazawa H. 2008. Age-dependent change of HMGB1 and DNA double-strand break accumulation in mouse brain. Biochem Biophys Res Commun, 376: 128–33. [DOI] [PubMed] [Google Scholar]
- Esbensen AJ 2010. Health conditions associated with aging and end of life of adults with Down syndrome. Int Rev Res Ment Retard, 39: 107–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry M, Silber J, Loeb LA, and Martin GM. 1984. Delayed and reduced cell replication and diminishing levels of DNA polymerase-alpha in regenerating liver of aging mice. J Cell Physiol, 118: 225–32. [DOI] [PubMed] [Google Scholar]
- Hart RW, and Setlow RB. 1974. Correlation between deoxyribonucleic acid excision-repair and life-span in a number of mammalian species. Proc Natl Acad Sci U S A, 71: 2169–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermon C, Alberman E, Beral V, and Swerdlow AJ. 2001. Mortality and cancer incidence in persons with Down’s syndrome, their parents and siblings. Ann Hum Genet, 65: 167–76. [DOI] [PubMed] [Google Scholar]
- Hill DA, Gridley G, Cnattingius S, Mellemkjaer L, Linet M, Adami HO, Olsen JH, Nyren O, and Fraumeni JF Jr. 2003. Mortality and cancer incidence among individuals with Down syndrome. Arch Intern Med, 163: 705–11. [DOI] [PubMed] [Google Scholar]
- Horton JK, Baker A, Berg BJ, Sobol RW, and Wilson SH. 2002. Involvement of DNA polymerase beta in protection against the cytotoxicity of oxidative DNA damage. DNA Repair (Amst), 1: 317–33. [DOI] [PubMed] [Google Scholar]
- Kalanj-Bognar S, Rundek T, Furac I, Demarin V, and Cosovic C. 2002. Leukocyte lysosomal enzymes in Alzheimer’s disease and Down’s syndrome. J Gerontol A Biol Sci Med Sci, 57: B16–21. [DOI] [PubMed] [Google Scholar]
- Kisby GE, Kohama SG, Olivas A, Churchwell M, Doerge D, Spangler E, de Cabo R, Ingram DK, Imhof B, Bao G, and Kow YW. 2010. Effect of caloric restriction on base-excision repair (BER) in the aging rat brain. Exp Gerontol, 45: 208–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna TH, Mahipal S, Sudhakar A, Sugimoto H, Kalluri R, and Rao KS. 2005. Reduced DNA gap repair in aging rat neuronal extracts and its restoration by DNA polymerase beta and DNA-ligase. J Neurochem, 92: 818–23. [DOI] [PubMed] [Google Scholar]
- Lange SS, and Vasquez KM. 2009. HMGB1: the jack-of-all-trades protein is a master DNA repair mechanic. Mol Carcinog, 48: 571–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharbel Weidner Maluf, and Erdtmann Bernardo. 2001. Genomic instability in Down syndrome and Fanconi anemia assessed by micronucleus analysis and single-cell gel electrophoresis. Cancer Genet Cytogenet, 124: 71–75. [DOI] [PubMed] [Google Scholar]
- Menon R, Behnia F, Polettini J, Saade GR, Campisi J, and Velarde M. 2016. Placental membrane aging and HMGB1 signaling associated with human parturition. Aging (Albany NY), 8: 216–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizetic D, and Groet J. 2012. Tumorigenesis in Down’s syndrome: big lessons from a small chromosome. Nat Rev Cancer, 12: 721–32. [DOI] [PubMed] [Google Scholar]
- Ochs K, Sobol RW, Wilson SH, and Kaina B. 1999. Cells deficient in DNA polymerase beta are hypersensitive to alkylating agent-induced apoptosis and chromosomal breakage. Cancer Res, 59: 1544–51. [PubMed] [Google Scholar]
- Pan F, Zhao J, Zhou T, Kuang Z, Dai H, Wu H, Sun H, Zhou X, Wu X, Hu Z, He L, Shen B, and Guo Z. 2016. Mutation of DNA Polymerase beta R137Q Results in Retarded Embryo Development Due to Impaired DNA Base Excision Repair in Mice. Sci Rep, 6: 28614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker SE, Mai CT, Canfield MA, Rickard R, Wang Y, Meyer RE, Anderson P, Mason CA, Collins JS, Kirby RS, and Correa A. 2010. Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res A Clin Mol Teratol, 88: 1008–16. [DOI] [PubMed] [Google Scholar]
- Pascucci B, Russo MT, Crescenzi M, Bignami M, and Dogliotti E. 2005. The accumulation of MMS-induced single strand breaks in G1 phase is recombinogenic in DNA polymerase beta defective mammalian cells. Nucleic Acids Res, 33: 280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podlutsky AJ, Dianova II, Wilson SH, Bohr VA, and Dianov GL. 2001. DNA synthesis and dRPase activities of polymerase beta are both essential for single-nucleotide patch base excision repair in mammalian cell extracts. Biochemistry, 40: 809–13. [DOI] [PubMed] [Google Scholar]
- Pogribna Marta, Melnyk Stepan, Pogribny Igor, Chango Abalo, Yi Ping, and James S. Jill. 2001. Homocysteine Metabolism in Children with Down Syndrome: In Vitro Modulation. American Journal of Human Genetics, 69: 88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad R, Liu Y, Deterding LJ, Poltoratsky VP, Kedar PS, Horton JK, Kanno S, Asagoshi K, Hou EW, Khodyreva SN, Lavrik OI, Tomer KB, Yasui A, and Wilson SH. 2007. HMGB1 is a cofactor in mammalian base excision repair. Mol Cell, 27: 829–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raji NS, Surekha A, and Rao KS. 1998. Improved DNA-repair parameters in PHA-stimulated peripheral blood lymphocytes of human subjects with low body mass index. Mech Ageing Dev, 104: 133–48. [DOI] [PubMed] [Google Scholar]
- Rao KS, Annapurna VV, and Raji NS. 2001. DNA polymerase-beta may be the main player for defective DNA repair in aging rat neurons. Ann N Y Acad Sci, 928: 113–20. [DOI] [PubMed] [Google Scholar]
- Rao KS, Vinay Kumar D, Bhaskar MS, and Sripad G. 1994. On the ‘active’ molecules of DNA-polymerase beta in aging rat brain. Biochem Mol Biol Int, 34: 287–94. [PubMed] [Google Scholar]
- Rodriguez-Sureda V, Vilches A, Sanchez O, Audi L, and Dominguez C. 2015. Intracellular oxidant activity, antioxidant enzyme defense system, and cell senescence in fibroblasts with trisomy 21. Oxid Med Cell Longev, 2015: 509241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, and Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat Methods, 9: 671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafik HM, Au WW, and Legator MS. 1988. Chromosomal radiosensitivity of Down syndrome lymphocytes at different stages of the cell cycle. Hum Genet, 78: 71–5. [DOI] [PubMed] [Google Scholar]
- Sirbu BM, Couch FB, Feigerle JT, Bhaskara S, Hiebert SW and Cortez D. 2011. Analysis of protein dynamics at active, stalled, and collapsed replication forks. Genes & Development, 25: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobol RW, Horton JK, Kuhn R, Gu H, Singhal RK, Prasad R, Rajewsky K, and Wilson SH. 1996. Requirement of mammalian DNA polymerase-beta in base-excision repair. Nature, 379: 183–6. [DOI] [PubMed] [Google Scholar]
- Sobol RW, Watson DE, Nakamura J, Yakes FM, Hou E, Horton JK, Ladapo J, Van Houten B, Swenberg JA, Tindall KR, Samson LD, and Wilson SH. 2002. Mutations associated with base excision repair deficiency and methylation-induced genotoxic stress. Proceedings of the National Academy of Sciences of the United States of America, 99: 6860–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subba Rao KV, and Subba Rao K. 1984. Increased DNA polymerase beta-activity in different regions of aging rat brain. Biochem Int, 9: 391–7. [PubMed] [Google Scholar]
- Sykora P, Misiak M, Wang Y, Ghosh S, Leandro GS, Liu D, Tian J, Baptiste BA, Cong WN, Brenerman BM, Fang E, Becker KG, Hamilton RJ, Chigurupati S, Zhang Y, Egan JM, Croteau DL, Wilson DM 3rd, Mattson MP, and Bohr VA. 2015. DNA polymerase beta deficiency leads to neurodegeneration and exacerbates Alzheimer disease phenotypes. Nucleic Acids Res, 43: 943–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventrella-Lucente LF, Unnikrishnan A, Pilling AB, Patel HV, Kushwaha D, Dombkowski AA, Schmelz EM, Cabelof DC, and Heydari AR. 2010. Folate deficiency provides protection against colon carcinogenesis in DNA polymerase beta haploinsufficient mice. J Biol Chem, 285: 19246–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weirich-Schwaiger H, Weirich HG, Gruber B, Schweiger M, and Hirsch-Kauffmann M. 1994. Correlation between senescence and DNA repair in cells from young and old individuals and in premature aging syndromes. Mutat Res, 316: 37–48. [DOI] [PubMed] [Google Scholar]
- Weissman L, Jo DG, Sorensen MM, de Souza-Pinto NC, Markesbery WR, Mattson MP, and Bohr VA. 2007. Defective DNA base excision repair in brain from individuals with Alzheimer’s disease and amnestic mild cognitive impairment. Nucleic Acids Res, 35: 5545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamtich Jennifer, Nemec Antonia A., Keh Agnes, and Sweasy Joann B.. 2012. A Germline Polymorphism of DNA Polymerase Beta Induces Genomic Instability and Cellular Transformation. PLOS Genetics, 8: e1003052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo EJ, Hwang YC, Kang CM, Kim IH, Kim DI, Parka JS, Choy HE, Park WY, and Park SC. 2000. Senescence-like changes induced by hydroxyurea in human diploid fibroblasts. Exp Gerontol, 35: 553–71. [DOI] [PubMed] [Google Scholar]