CONCEPTUALIZING SLEEP IN A SOCIAL CONTEXT
Sleep represents an emergent set of many physiologic processes under primarily neurobiological regulation that impact many physiologic systems. As such, many advances have been made over the past several decades that have shed light on these neurobiologic mechanisms of sleep-wake,1–4 with especially exciting work in the area of functional genetics/genomics5,6 and molecular mechanisms of sleep-related regulation.7–9 Still, the phenomenon of sleep exists outside the nucleus and the cell membrane—sleep is experienced phenomenologically. Sleep is a biological requirement for human life, alongside food, water, and air. Like consumption of food and unlike breathing air, achieving this biological need requires the individual to engage in volitional behaviors. Although many of these behaviors are genetically and intrapersonally driven (eg, it is not a coincidence that most people prefer to sleep at night, and that most humans sleep in a stereotypical posturally recumbent manner), there is still much variability in sleep behaviors and practices. Because of this, sleep is also socially driven, dictated by the environment, and subject to interpersonal and societal factors.
Sleep in most humans occupies between 20% and 40% of the day. Even prehistoric evidence suggests the importance of sleep in human life10; this is consistent with archaeological and historical accounts of sleep having a prominent and important role in even early human society. Sleep was a universal phenomenon that was inescapable and thus was incorporated in social structures. In this way, sleep became not just a set of physiologic processes, but one represented in sociocultural structures. Thus, the timing, environment, and constraints surrounding sleep across human societies began to differ between rich and poor, powerful and powerless, rural and urban, and so forth. As sociologist, Simon Williams, writes, “Where we sleep, when we sleep, and with whom we sleep are all important markers or indicators of social status, privilege, and prevailing power relations.”11
Conceptualizing Downstream Consequences
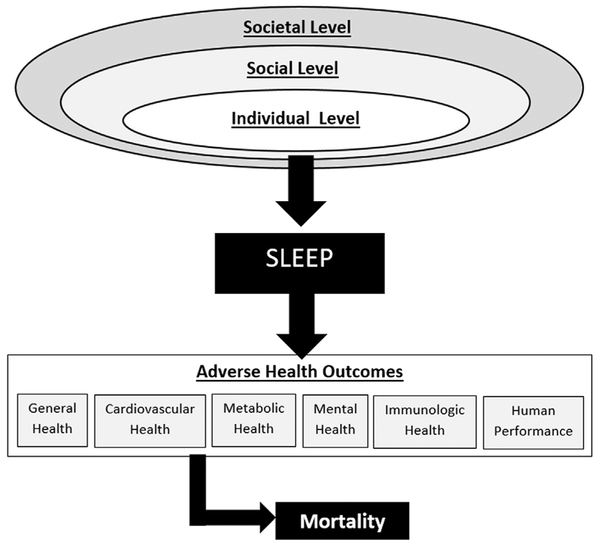
The downstream consequences of insufficient sleep duration and/or inadequate sleep quality (including sleep disorders and circadian misalignment of sleep) are varied and impact many physiologic systems. Conceptualizing these is therefore difficult. One way to do so is to acknowledge domains of outcomes and recognize the overlaps and relationships among those domains. The recent position statement from the American Academy of Sleep Medicine and Sleep Research Society12–15 broadly categorizes effects of insufficient sleep as pertaining to the following categories: general health, cardiovascular health, metabolic health, mental health, immunologic health, human performance, cancer, pain, and mortality.
Conceptualizing Upstream Influences: Social Ecological Models
Upstream social and environmental influences on sleep are also complex and overlapping and implicate many potential pathways. With this in mind, a social-ecological framework may be best suited to describe this relationship. The social-ecological model was originally developed to describe the complex ways that an individual’s behavior related to their health is a product of influences at the individual level, but that the individual operates in the context of social structures that they are a member of, but these structures exist outside of the individual.16 For example, an individual has genetic, psychological, and other reasons for consuming a healthy diet, but social structures that they are a part of but exist outside of that individual (like their neighborhood, which may have healthy food; their job, which may or may not have a cafeteria; their family, which may have other food restrictions, and so forth) play a role in that individual’s behavior.
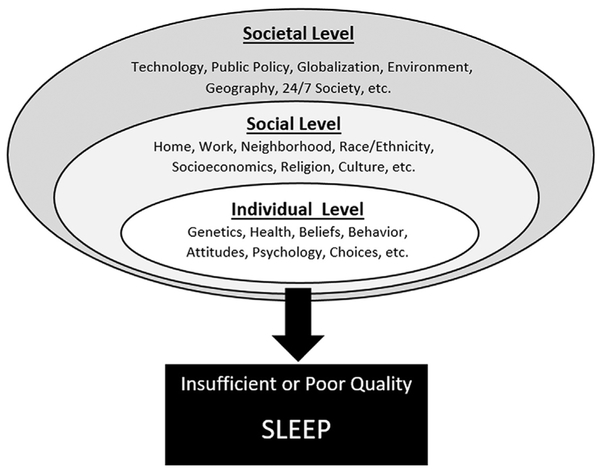
This model may also be appropriate for understanding sleep. At the individual level, factors that influence a person’s sleep include that person’s genetics, knowledge, beliefs, and attitudes about sleep, their overall health, and so forth. The individual level is embedded, though, within a social level, which includes the home (family, bedroom, and so forth), neighborhood, work/school, socioeconomics, religion, culture, race/ethnicity, and other factors. All of these factors influence sleep through the individual. Still, this social level is embedded within a societal level, which includes social forces that exist outside of things like work, family, and neighborhood, including globalization, geography, technology, public policy. These factors, at this high of a level, filter through the social structures that eventually come to bear on the individual. For example, as society embraced the Internet, it caused changes in jobs and families, which led to individual changes that play a role in sleep (such as social networking in bed or browsing the Internet late at night). Fig. 1 displays a social-ecological model of sleep, illustrating of sleep duration and quality are influenced by factors at the individual level, which is embedded within a social level, which itself is embedded within a societal level. Fig. 2 brings these models together, with sleep as the fulcrum (shown in Fig. 1) at the interface of upstream social-environmental influences (shown in more detail in Fig. 3) and downstream health and functional outcomes (shown in more detail in Fig. 2). This model brings all of these concepts together to describe how sleep is influenced by these societal factors and how those influences, through sleep, may play a role in health. The first version of this model was published in 2010,17 and it has appeared in several other publications since then.14,18–20 It may serve as a useful frame-work for conceptualizing the physiologic processes of sleep in a social context.
Fig. 1.
Social ecological model of sleep.
Fig. 2.
Social ecological model of sleep and health.
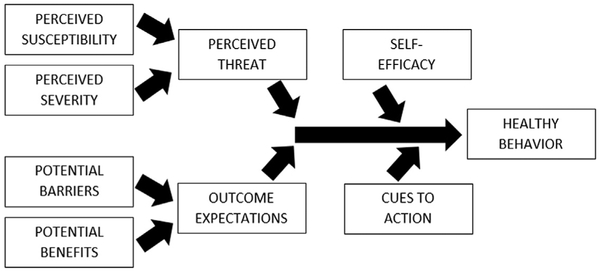
Fig. 3.
Health belief model.
POPULATION PREVALENCE OF SLEEP DURATION AND SLEEP DISTURBANCES
Sleep Duration
Population estimates of habitual sleep duration are variable, because few studies used identical methods to derive estimates. The best population-level estimates come from 1 of 3 sources: (1) self-reported time use data, (2) self-reported typical weeknight/work-night sleep, and (3) self-reported average sleep within 24 hours. For US-based data, the primary sources of these estimates come from the American Time Use Survey (ATUS) for time use data, the National Health Interview Survey or National Health and Nutrition Examination Survey (NHANES) for weeknight sleep, and the Behavioral Risk Factor Surveillance System (BRFSS) for 24-hour sleep.
Longitudinal analysis of time-use diaries by Knutson and colleagues21 found that the proportion of Americans reporting short (<6 hours) sleep was 7.6% in 1975 and 9.3% in 2006. Bin and colleagues22 examined similar time use data from several countries and showed that, in the United States, sleep duration has generally declined since the 1960s, if only by a small amount. The most comprehensive analysis of time use data related to sleep was recently undertaken by Basner and colleagues.23 They report that the age group that receives the most sleep is young adults (8.86 hours on weeknights and 10.02 hours on weekends) and that those aged 25 to 64 report about 0.70 to 0.99 fewer hours on weeknights and 0.62 to 1.16 fewer hours on weekends. Prevalence of sleep duration by hour is not reported, though.
Regarding weekday sleep duration, Grandner and colleagues24 reported census-weighted estimates of sleep duration using the 2007 to 2008 wave of the NHANES. They report that 6.2% of the population reports less than 5 hours of sleep, 33.78% reports 5 to 6 hours of sleep, 52.68% report 7 to 8 hours of sleep, and 7.38% report at least 9 hours of sleep per typical weeknight. These values from NHANES is similar to values reported from Krueger and Friedman,25 who assessed similar data from the NHIS using data from 2004 to 2007. They report prevalence of 5 hours or less being 7.8%, 6 hours being 20.5%, 7 hours being 30.8%, 8 hours being 32.5%, and 9 or more hours being 8.5%.
Regarding typical 24-hour sleep, which presumably includes napping, recent data from the Centers for Disease Control and Prevention (CDC) released data from the 2014 BRFSS, which included data from 444,306 American adults. Based on the recently published guidelines,12,15 the CDC calculated the prevalence of less than 7 hours of sleep duration across all 50 states.26 The median prevalence of less than 7 hours of sleep was 35.1%, with a range of 28.4% (South Dakota) to 43.9% (Hawaii). This report also documents that the prevalence of 5 hours or less was11.8%, with prevalence of 6, 7, 8, 9, and 10 or more hours being 23.0%, 29.5%, 27.7%, 4.4%, and 3.6%, respectively.
Taken together, the time diaries generally show more sleep than other retrospective reports, perhaps because they may better capture time in bed but not actual sleep. Indeed, most retrospective sleep reports have this issue,27 although perhaps it is particularly problematic for time diaries. In general, though, at least one-third of the population seems to be reporting habitual sleep of 6 hours or less. The proportion of those with 6 hours or less is salient, given the risk factors associated with sleep duration described in more detail in later discussion.
Sleep Disturbances
Sleep disturbances are difficult to measure at the population level. Often, population-level assessments of general sleep disturbances subsume insufficient sleep duration and/or sleep disorders that may not expressly fit into this category. The 2006 BRFSS asked the following question to more than 150,000 residents of 36 US states/territories: “Over the last 2 weeks, how many days have you had trouble falling asleep or staying asleep or sleeping too much?” In an analysis of these responses, values were coded in whole numbers ranging from 0 to 14, but responses aggregated at 0 and 14; therefore, responses were dichotomized as either endorsing or not endorsing “sleep disturbance.”28,29 For men, the prevalence of sleep disturbance ranged from13.7% (ages 70–74) to 18.1% (ages 18–24), and for women, the prevalence ranged from 17.7% (ages 80 or older) to 25.1% (ages 18–24).29 Interestingly, reports of sleep disturbance generally declined with age. This finding was recently replicated using data from the 2009 BRFSS, which showed a similar pattern of declining self-report of insufficient sleep with age.30
Regarding sleep symptoms, data from the 2007 to 2008 NHANES were examined with regards to prevalence of various sleep symptoms.31 Long sleep latency (more than 30 minutes) was reported by 18.8% of Americans. Self-reported difficulty falling asleep was reported at a rate of 11.71% for mild symptoms (1–3 times per week) and7.7% for moderate-severe symptoms (at least half of nights). Similarly, sleep maintenance difficulties were reported by 13.21% endorsing mild and 7.7% endorsing moderate-severe symptoms, and early morning awakenings were reported at a rate of 10.7% for mild and 5.8% for moderate-severe symptoms. Daytime sleepiness and nonrestorative sleep were reported at a rate of13.0% and 17.8% for mild symptoms, respectively, and 5.8% and 10.9% for moderate-severe symptoms, respectively. Frequent snoring was reported by 31.5% of adults and snorting/gasping during sleep was reported by 6.6% “occasionally” and 5.8% “frequently.”
SLEEP EFFECTS ON HEALTH AND LONGEVITY
Because sleep is involved with many physiologic systems, insufficient sleep duration and poor sleep quality have been associated with several adverse health outcomes. Separate literature texts have emerged describing some of the negative effects of insufficient sleep duration, sleep apnea, and insomnia.
Mortality
The first report documenting the relationship between sleep duration and mortality risk was published more than 50 years ago.32 This first study, an analysis of data from the American Cancer Society’s first Cancer Prevention Study of more than one million US adults, found that increased mortality risk was associated with both short (6 hours or less) and long (9 hours or more) sleep duration. Since that time, many other studies have been published, from both large and small cohorts, covering both short and long follow-up periods, from 6 continents. Taken together, this overall pattern of findings, that both short and long sleep are associated with mortality risk, has generally remained consistent across studies, although not all studies found this pattern.17 Two meta-analyses have been published, using slightly different methods and controls.33,34 Still, their findings were highly consistent, indicating a 10% to 12% increased risk for short sleep and a 30% to 38% increased risk associated with long sleep duration. Much controversy remains, though, regarding this issue. For example, the precision of measurement of sleep in these studies is often poor.17,27,35 Self-reported sleep time may better approximate time in bed, and although an acti-graphic study found a similar pattern,36 the cutoffs for short and long sleep indicated an overestimate among self-reports. Also, there is still a lack of clarity on the biological plausibility of the long sleep relationship, although some ideas have been proposed.37,38 For this reason, most of the attention has been focused on risks associated with short sleep duration, which may be far more prevalent.
Weight Gain and Obesity
Many studies have found associations between sleep duration and adiposity and obesity.39–41 Although most of these studies are cross-sectional, precluding causality, several other studies have longitudinally examined this relationship, demonstrating that short sleep duration is associated with increased weight gain over time.42–46 These studies include individuals with otherwise low obesity risk, diverse community samples, and samples where effectiveness of weight loss interventions was mitigated by sleep and circadian factors. Several important caveats seem to be present in this relationship. First, this relationship is dependent on age, with the strongest relationships among younger adults and U-shaped relationships more common in middle-aged adults.47 Also, this relationship may be moderated by race/ethnicity, with stronger relationships between sleep and obesity among non-Hispanic white and black/African American adults.24
Diabetes and Metabolism
Several studies have documented a cross-sectional relationship between insufficient sleep and diabetes risk.40,48–51 A recent meta-analysis showed that insufficient sleep is associated with a 33% increased risk of incident diabetes.52 These studies are supported by laboratory findings that show that physiologic sleep loss is associated with diabetes risk factors, including insulin resistance,53–56 and other diabetes risk factors, such as increased consumption of unhealthy foods.57–59 Physiologic studies also show that sleep loss can influence metabolism through changes in metabolic hormones,60,61 adipocyte function,62 and beta-cell function.63
Inflammation
Laboratory studies have shown that physiologic sleep restriction is associated with a proinflamma-tory state, including elevations in inflammatory cytokines, such as interleukin 1B (IL-1B),64,65 IL-6,64,66–68 IL-17,64,69 tumor necrosis factor-α,64,68,70–72 and C-reactive protein.64,69,73–76 Findings at the population level have been more difficult to assess,64 but similar relationships were found. A recent meta-analysis found no consistent relationship between sleep duration and inflammation,77 but this may be because it did not include some studies that were more generalizable and with larger samples (eg, Ref.76). Also, it is plausible that population-level samples did not optimally measure these markers, because relationships with sleep vary across 24 hours and single time-point blood draws may miss the window of difference.68
Cardiovascular Disease
In addition to increased likelihood of obesity, diabetes, and inflammation, insufficient sleep is associated with increased risk of cardiovascular disease. Many studies have found that short sleep duration is associated with hypertension.24,78–80 Although directionality is difficult to ascertain, several of these studies were longitudinal in nature. A meta-analysis of these longitudinal studies indicated that habitual short-sleep duration is associated with a 20% increased likelihood of hypertension, relative to normal sleep duration.81 Other studies have supported this association, showing increased 24-hour blood pressure in short sleepers.82 Other studies have also shown short sleep to be associated with hypercholesterolemia24,79 and atherosclerosis risk.83 Regarding cardiovascular endpoints, there is some evidence that habitual short sleep increases likelihood of cardiovascular events,84 although meta-analyses do not generally show short sleep to be associated with increased cardiovascular mortality.33,34,85
Neurocognitive Functioning
Many studies have examined the relationship between laboratory-induced sleep loss and neuro-cognitive function. The domain that is most often studied is vigilant attention,86,87 most often operationalized with the psychomotor vigilance task.87,88 These studies show that as sleep time declines, attentional lapses increase in a somewhat dose-dependent manner.86,89 Furthermore, these impairments often become cumulative over time90 and do not seem to level off even after weeks in a laboratory. Other domains of neurocognitive function have also been assessed. For example, reduced sleep duration has been shown to cause impairments in working memory,91 executive function,92 processing speed,93–95 and cognitive throughput.96 Although some of these effects may be rescued with stimulants such as caffeine, the effects on executive function particularly do not seem to be rescued.92 Although studies of this phenomenon in the general population are scarce, some studies show that reduced sleep time is associated with drowsy driving97 and occupational accidents.98–100
Mental Health
Many studies have shown that short sleep duration is associated with poor mental health. Sleep disruptions are a common diagnostic feature of many mental health disorders.101 Patients with mood disorders and anxiety disorders frequently experience short sleep duration. Sleep duration has also been identified as a suicide risk factor.102 In the general population, overall mental health has been identified as the leading predictor of self-reported insufficient sleep.30
BELIEFS AND ATTITUDES ABOUT SLEEP
Real-world sleep may be driven by many of the same factors that drive other health-related behaviors, such as diet and exercise. With this in mind, previous literature from health behavior researchers has identified several models that explain healthy behavior, identifying the roles of beliefs and attitudes.
The Health Belief Model and Application to Sleep
The Health Belief Model was originally developed in the 1960s,103,104 but has since been used in the study of many health-related behaviors. See Fig. 3 for a schematic of this model. This model can be applied to sleep behaviors. For example, a person will engage in healthy sleep behaviors (eg, making time for sufficient sleep or adhering to treatment) if they (1) believe that they are susceptible to the adverse effects of insufficient/poor sleep, (2) believe that the adverse effects are severe enough to warrant action, (3) believe that the action will mitigate the adverse effects,(4) believe that barriers to performing the action are sufficiently reduced, (5) are reminded to engage in the action, and (6) believe that performing the action is under their control. According to the health belief model, all of these are required for action. Therefore, just educating patients about the severity of outcomes of inaction, for example, is not sufficient to motivate behavior.
The Integrated Behavioral Model and Application to Sleep
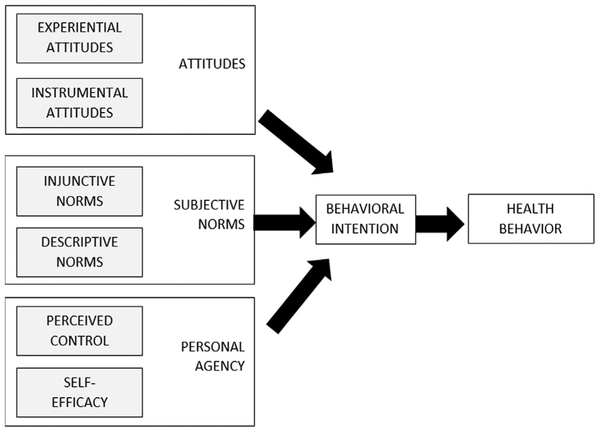
The Integrated Behavioral Model arose from the Theory of Planned Behavior and Theory of Reasoned Action105 to describe why people engage in behaviors. A schematic for this model is presented in Fig. 4. According to this model, attitudes, norms, and agency need to be addressed. Regarding attitudes, this would involve leading individuals to not only endorse helpful beliefs and attitudes about healthy sleep but also associate healthy sleep with positive feelings. Regarding norms, more research is needed to understand how the sleep of a person’s (perceived) peers and those to which that individual wishes to conform influences individual sleep behaviors.
Fig. 4.
Integrated behavior model.
Beliefs and Attitudes About Sleep
Across segments of society, sleep practices and beliefs can vary to a great extent. For example, bed-sharing with infants and other family members differentially exists across cultures.106–111 The cultural impact of dreaming also varies widely across cultures.112 As globalization and technology penetrate society, sleep-related beliefs and practices can change, including the provision of longer working hours,113–120 shift work,121–124 and discouraging otherwise culturally appropriate naps.125–127 There have been a few studies that examined beliefs and attitudes about sleep. In a sample from Brooklyn, New York, blacks/African Americans who were at high risk of obstructive sleep apnea had higher scores on the Dysfunctional Beliefs and Attitudes about Sleep scale, compared with those who were not at high risk.128 In a study in the Philadelphia area among older black and white women,129 participants were administered a questionnaire to evaluate sleep-related beliefs and practices. Black women were more likely to endorse incorrect and unhelpful statements. Sell and colleagues130 examined sleep knowledge among Mexican Americans in San Diego. Non-Hispanic whites were more likely than Mexican Americans to know what sleep apnea was, but when describing the symptoms, both groups had similar knowledge that such a problem existed. Taken together, the role of sleep and health in society is driven by healthy behavior choices. These behavioral decisions, as described in the models above, are largely influenced by beliefs and attitudes about sleep. These beliefs and attitudes, though, are differentially endorsed by racial/ethnic groups, which may underlie sleep difficulties in those populations.
GENDER AND AGE IMPACTS SLEEP IN THE POPULATION
Sleep Changes with Normal Aging in the Population
Physiologic changes in sleep have been well-documented. In a landmark meta-analysis by Ohayon and colleagues,131 polysomnographic sleep characteristics across the lifespan were examined across 65 studies spanning more than 40 years. This analysis found that with age, polysomnographic total sleep time, sleep efficiency, slow-wave sleep, rapid eye movement (REM) sleep, and REM latency decline, whereas sleep latency, wake after sleep onset, stage 1 sleep, and stage 2 sleep increase. This finding suggests a phenomenon of more disturbed and lighter sleep. In addition to these changes, melatonin secretion declines with age, which may also impact sleep consolidation in older adults.132 Risk for many sleep disorders also increases with age.133–135 In particular, sleep disorders, such as insomnia,136 restless legs syndrome,137,138 sleep apnea,139 and REM behavior disorder,140 include older age as a risk factor. However, a paradox exists, which was highlighted in a large, international cohort study by Soldatos and colleagues.141 In this study, older adults were more likely to report difficulties initiating and maintaining sleep. However, they did not endorse a greater level of dissatisfaction with their sleep. A lack of dissatisfaction is similar to results reported in Italy by Zilli and colleagues,142 who found that younger adults were more likely to report dissatisfaction with sleep than older adults. In the US population, general dissatisfaction with sleep associated with age was examined using the 2006 BRFSS. In a study of more than 150,000 US adults, general sleep disturbance (general difficulties with sleep) was most frequently reported in young adults, and rates generally declined with age.29 In controlled analyses, no age groups were statistically less likely to report sleep disturbances than the oldest adults, aged 80 or older, although many of the younger groups reported higher levels. These results were replicated using the 2009 BRFSS, which examined self-reported perceived insufficient sleep among greater than 350,000 US adults and found a decline in general sleep insufficiency associated with age.30 Thus, it appears that sleep objectively worsens with age, but that subjective dissatisfaction with sleep is not associated with normal aging. In fact, this may be a sign of illness or depression.143
Population-Level Differences in Sleep Between Men and Women
Differences in sleep between men and women have been widely reported in the literature for decades.144–149 Overall, in the general population, women report shorter sleep duration,150 more sleep symptoms,31 greater rates of insomnia,151 and lower rates of sleep apnea.152 In an analysis of sleep disturbances reported in the 2006 BRFSS, it was found that women reported more nighttime sleep disturbances and daytime tiredness than men. Across all age groups, sleep disturbance was reported by between 13.7% and 18.1% of men, depending on age group, and between 17.7% and 25.1% of women.29 Similarly, for daytime tiredness, rates were16.4% to 22.9% of men and 20.5% to 29.9% of women, depending on age. In all age groups, women reported nominally more disturbances than men. Statistically, after adjusting for demographics, socioeconomics, health variables, and depression, rates of sleep disturbances were more prevalent among women for all age groups between 25 and 69 years old and rates of daytime tiredness were more common in women for all age groups from 18 to 59 and 75 to 79.
Other issues regarding sleep differences exist between men and women. For example, sleep disturbances are common in pregnancy,153–155 especially the first and third trimesters. These sleep disturbances can include insomnia, short sleep duration, sleep fragmentation, and gestational sleep apnea. Sleep disturbance in pregnant women can result in adverse outcomes for both the mother and the fetus.156,157 Sleep in new parents (especially mothers) is also frequently disturbed,158,159 especially in the first few months after birth. Sleep disturbances among parents of infants are associated with increased postpartum depression,160–162 increased sleep disturbances among infants, and other adverse outcomes. Women also experience sleep disturbances around menopause. Sleep during the menopausal transition is often characterized by insomnia symptoms and increased sleep fragmentation.163 Hot flashes are also a common source of sleep disturbance around the menopausal transition.164
Some sleep disturbances are disproportionately experienced by men. For example, men are more likely to have obstructive sleep apnea,139 are more likely to have difficulty adhering to sleep apnea treatment,165,166 and are more likely to die as a result of complications or consequences of sleep apnea.167 In addition, men are more likely to be diagnosed with REM Behavior Disorder, which is typically diagnosed among older adults and likely predates neurodegenerative disorders.168 During the aging process, men are also more likely to demonstrate a steeper decline in slow-wave sleep generation,131 with lower amounts of slow-wave sleep among older man versus older women.
RACE, ETHNICITY, AND CULTURE ASSOCIATED WITH SLEEP
Insufficient Sleep Associated with Race/Ethnicity
Many studies have documented a “sleep disparity” in the population,19,39 such that racial/ethnic minorities, especially in the context of socioeconomic disadvantage, achieve less quality sleep. Most studies in this area have shown that, overall, blacks/African Americans are more likely to experience short sleep duration compared with non-Hispanic whites.19,39 One nationally representative study found that this pattern is robust even after adjustment for a large number of other demographic and socioeconomic covariates, such that the rate of very short sleep (≤4 hours) was 2.5 times those of non-Hispanic whites and the rate of short (5–6 hours) sleep was about twice as high.150 A similar pattern was seen for Asians/others, who reported very short sleep at a rate of 4 times that seen in non-Hispanic whites and a short sleep about twice as frequently. Among Hispanics/Latinos, there is less clear evidence of habitual short sleep, especially among Mexican Americans. In addition to epidemiologic studies, some laboratory studies have also examined this issue. For example, blacks/African Americans have been shown to sleep less in the laboratory.169–171 Also, this group has been shown to demonstrate less slow-wave sleep, compensated by increased stage 2 sleep. Other studies have shown similar patterns for sleep duration in other samples that included minority groups,172,173 and this topic was the subject of multiple recent reviews.19,174,175
Sleep Disturbances Associated with Race/Ethnicity in the Population
Less work has been done to characterize rates of sleep disturbances in racial/ethnic minorities. One previous study showed that racial/ethnic minorities demonstrated a lower sleep efficiency based on actigraphy.176 A study in the Philadelphia area found that race differences in poor sleep quality largely depended on socioeconomic status.177 A nationally representative study found that black/African Americans were 60% more likely than non-Hispanic whites to report sleep latency more than 30 minutes, although they (along with Hispanics/Latinos) were less likely to report “difficulty falling asleep.”31 This discrepancy between self-reported “problems” and computed long sleep latency suggests that symptom reports may vary based on the question asked. Overall, minority groups were less likely to report insomnia symptoms, nonrestorative sleep, and daytime sleepiness, although non-Mexican Hispanics/Latinos were more likely to endorse sleep apnea symptoms such as snoring.
Several studies have examined the role of racial discrimination as a unique stressor that impacts sleep. A study of residents in Michigan and Wisconsin found that exposure to racial discrimination was associated with sleep disturbances, above the effects of race, sociodemographics, and even depressed mood.178 This finding, that sleep disturbance is associated with exposure to racism was consistent with other findings that showed that exposure to discrimination was associated with shorter sleep and more sleep difficulties179 and that these findings are also seen in objective sleep assessments.169,170 Interestingly, polysomnographic differences in slow-wave sleep between black/African American and non-Hispanic white individuals (ie, reduced slow-wave sleep) were mediated by exposure to discrimination.
Sleep, Acculturation, and Immigration
Few studies have examined sleep related to acculturation. Sell and colleagues130 found that Mexican Americans who were more acculturated to American lifestyle were more familiar with information about sleep disorders. Also, in a nationally representative sample, speaking only Spanish at home was associated with a decreased likelihood of sleep duration in the short (5–6 hours) and very short (≤4 hours) categories compared with 7 to 8 hours. In this same sample, being born in Mexico (but not any other country) was associated with decreased likelihood of both short and very short sleep duration, but these effects were not significant after adjusting for other demographic and socioeconomic factors, which likely explain this finding.129
EMPLOYMENT, NEIGHBORHOOD, AND SOCIOECONOMICS
Although sleep is an important factor in overall health, society has incentivized insufficient sleep. Many of these incentives involve finances and employment. Because of this, there is evidence that one of the strongest societal determinants of sleep is work. The relationship between work and sleep is especially important for safety-sensitive occupations that not only incentivize insufficient sleep but also for which the associated fatigue also jeopardizes the public safety.
Trading Sleep for Work Hours
Replicating and extending prior work in this area, Basner and colleagues23 examined data from greater than 100,000 Americans over a 9-year period who participated in the ATUS, which is performed annually by the US Bureau of Labor Statistics and uses time diaries to determine work and other activities across 24 hours.180 In a recent report, Basner and colleagues23 show that work time, including actual work and other related activities (such as commuting), was the primary determinant of sleep duration. In addition, later start times of school and work were associated with longer sleep, such that each hour of delayed work or training start time was associated with 20 more minutes of sleep. Also, those holding multiple jobs were at greater risk for short sleep duration compared with those only working one job at a time. Although work is a strong determinant of sleep duration, other studies show that employed individuals report the lowest rates of self-reported sleep disturbance.28 Unemployment, on the other hand, is associated with more sleep problems.28,30
Sleep Deprivation and Sleep Disorders in Occupational Settings
Recognition of the role of sleep disorders and sleep deprivation in occupational settings is gaining increased attention. Rosekind and colleagues181 showed that the typical well-rested worker costs an employer about $1300 per year in lost sleep-related productivity, and this number increases to about $3000 for those with insomnia or insufficient sleep. Furthermore, the loss to productivity permeates many areas of functioning, including time management, mental and interpersonal demands, output demands, and physical job demands. Hui and Grandner182 show that not only is self-reported poor sleep quality associated with decreased work performance but also worsening sleep longitudinally predicts worsening performance over time. In addition, difficulty sleeping is associated with increased health care costs. Those with difficulty sleeping “often” or “always” were associated with additional health care costs of $3600 to $5200 per person per year more than those who “never” have sleep problems, and these costs increased over time if sleep became worse. Additional analyses from this dataset also showed that poor sleep may motivate employees to make healthy changes as part of a workplace wellness program, but it may also limit those employees’ ability to maintain healthy change.183
Regarding safety-sensitive occupations such as medicine, law enforcement, and transportation, sleep plays a critical role in safety. For example, sleep apnea occurs at high rates among commercial drivers184–186 and impairs their ability to drive safely. Accordingly, workplace programs to increase screening and treatment of sleep apnea may have financial benefits for companies.184 Similar efforts may show effectiveness in rail workers as well.187,188 Airline pilots face similar challenges, in addition to challenges presented by crossing many time zones. To address these concerns, sleep disorders screening in addition to circadian approaches and scheduled napping have shown effectiveness in improving safety.189–197 Among law enforcement and first responders, several studies have shown that sleep disturbances are common among police officers198–203 and firefighters.204,205 In particular, issues such as sleep apnea, insomnia, and shift work are the most common problems.206 In a landmark study by Rajaratnam and colleagues,198 police officers who were at greatest risk of sleep disorders were also more likely to be at risk for job-related problems, such as falling asleep at meetings and using unnecessary violence against citizens. Studies have shown that sleep disturbances in police officers and firefighters are associated with reduced ability to maintain job performance and safety.198,203,207
Several studies have been conducted among medical residents and nurses. For nurses, shift work and long work hours have been shown to be related to adverse health outcomes and indicators of reduced functioning.208–217 Among medical residents, long work hours and shift work have been shown to lead to insufficient sleep duration.218–221 Furthermore, longer work hours in medical residents have been associated with markers of reduced work performance, although impacts on actual work performance are more inconsistent.115,222 In a landmark study that compared 2 groups of residency programs, those that gave more time off for sleep did not show measurable changes in work performance.223 Paradoxically, residents given more time to sleep were more worried about decreases in their quality of work as a result of working less, yet they were more satisfied with the quality of their life and social functioning.
Sleep, Poverty, and Neighborhood Factors
Several studies have shown that poverty is associated with both shorter sleep duration and worse sleep quality.30 However, once the benefits of income are accounted for (by statistically covarying education, access to health care, and so forth), associations with income are often nonexistent and may go in the opposite direction. For example, in an analysis of data from greater than 350,000 US adults, insufficient sleep was associated with poverty before adjusting for covariates, but after adjustment, the opposite relationship was seen.30 A positive relationship between income and insufficient sleep after adjusting for covariates suggests that money may not buy sleep, but many of the benefits of income may contribute to healthy sleep. One aspect of this relationship is neighborhood quality. Several studies have investigated the role of the neighborhood in an individual’s sleep quality, showing that neighborhoods that are crime-ridden, not socially cohesive, and dirty, are associated with worse sleep quality.224–227 Furthermore, sleep quality may partially mediate the relationship between neighborhood quality and both mental227 and physical225 health. One way that a neighborhood may directly influence sleep would be via the physical environment. There is substantial literature showing that environmental noise228,229 and light230–233 can adversely impact sleep and that neighborhoods that are active at night may directly impact sleep through these.
INFLUENCES OF HOME, FAMILY, AND SCHOOL ENVIRONMENT
The home, family, and school environments also likely play important roles in an individual’s sleep. For example, household size is negatively associated with sleep, such that more crowded homes are more likely to foster insufficient sleep.30 Also, as mentioned above, the physical sleep environment can also play a role. Bedrooms that have levels of light, noise, and temperature that are not conducive to sleep may contribute to insufficient sleep.234–236 Although data on beds and other sleeping surfaces are relatively scarce, an uncomfortable sleeping environment may also reduce sleep ability.237–239
Another key issue of the home and family environment on sleep regards the marital relationship. Although most sleep research is performed on individuals sleeping alone in a laboratory, most adults do not sleep alone most nights.240,241 With this in mind, several studies have explored the important role of marital and relationship quality in sleep quality and how this relates to health. For example, relationship quality has been shown to be an important predictor of sleep health, especially among women, and relationship quality may be an important moderator between sleep quality and health.241–244
TECHNOLOGY IN AND OUT OF THE BEDROOM
In 2011, the National Sleep Foundation polled Americans regarding their use of technology in the bedroom. In a report of the findings of this survey, Gradisar and colleagues245 note that 90% of Americans use some sort of electronic device in the hour before bed. Also, more than two-thirds of adolescents and young adults used a Smartphone in the hour before bed, compared with approximately one-third of middle-aged adults and about one-fifth of older adults. Furthermore, the more engaging the technology application, the more the electronic device use was associated with difficulties falling asleep and nonrestorative sleep. This finding is supported by other work that shows that not only is electronic media use near bedtime prevalent246 but also the light emitted by the devices247 as well as the mental engagement248 can interfere with sleep. Growing awareness of the influence of mobile electronic device use on sleep is a key example of a societal-level change (use of technology) impacting an individual’s sleep.
GLOBALIZATION AND 24/7 SOCIETY
Another societal-level factor that impacts sleep is the advent of globalization and a 24/7 society. In the past, social interactions, commercial activities, and work responsibilities were dictated by more local factors. Now, though, the advent of globalization and 24/7 operations often impinge on sleep. Regarding globalization, individuals and organizations are connected across the globe. In combination with a society that institutes shift work and 24-hour operations, entire segments of the population are awake across all hours of the 24-hour day, and access to individuals across time zones is easier than ever. Because of this, social interactions (such as interactions with friends, family, and even online groups), commercial activities (such as eCommerce and availability of entertainment around the clock on demand), and work responsibilities (such as e-mails outside of business hours and business conducted across the globe) can impinge on sleep. The influence of globalization and 24/7 society on sleep behaviors is particularly relevant, because shift work has been repeatedly shown in both laboratory and field studies to be related to adverse health outcomes.
PUBLIC SAFETY AND PUBLIC POLICY
As mentioned above, many safety-sensitive occupations, such as those in transportation, law enforcement, and medicine, require healthy sleep for optimal performance. The problem is that these professions often institute policies that make healthy sleep difficult. As a result, the sleep of an individual in one of those occupations may have ramifications for others in the public. For example, when a large commercial truck crashes, it causes more damage and a greater likelihood of fatal injury.249,250 For this reason, several policy approaches to sleep and public safety have been proposed. The Accreditation Council for Graduate Medical Education has already instituted duty hour restrictions on medical residents, based on results from a report by the Institute of Medicine.218 These restrictions, although controversial,115,251–253 are likely resulting in increased sleep among medical residents.222 In the transportation industry, recommendations by the National Highway Transportation and Safety Administration address the need for sleep disorders screening and fatigue mitigation among commercial drivers,250 although formal regulations have not yet been passed. The Federal Aviation Administration also recently issued guidelines to address sleep issues in pilots.254 More work is needed in this area, and although regulations to ensure public safety have been proposed, they still have not yet been passed.
Another domain of public safety is drowsy driving. Even among non–commercial drivers, drowsy driving is an important public safety issue. Drowsy driving is prevalent, reported among about 5% of the US population over a 6-month period.255 Population-level data suggest that short sleep duration is an independent risk factor for drowsy driving, even if respondents believe that they are completely well rested.97
Another area of public policy related to sleep involves school start times. Existing evidence suggests that most US schools, especially high schools, start too early for most adolescents.256–259 Earlier start times not only promote shorter sleep duration among adolescents (who need more sleep than adults) but also do not take into account natural circadian delays that occur in adolescence.260 It has been proposed that delaying school start times can improve academic performance, improve mental health, and improve overall health in students.261–266
Other public policy initiatives have addressed the issue of environmental light and noise in neighborhoods. There are several policies in place, and more being proposed, that limit the brightness of street lights in neighborhoods, increase “quiet time” regulations at night, direct airplanes to avoid some residential areas at night, reduce traffic and train noise at night, and so forth. These approaches are usually regional, and many efforts are ongoing.
One more public policy implication relevant to sleep would be health policy legislation. For example, improving mental health parity laws will do much to intervene on perhaps the most important determinant of sleep health at the population level30 and will facilitate treatment of insomnia with the most well supported therapy.267 In addition, health equity legislation may help to address some of the disparities seen in sleep in the population.20 These and other future approaches may better promote healthy sleep from a policy standpoint.
IMPORTANT LIMITATIONS OF THE EXISTING LITERATURE
There are several important limitations to the existing literature, which constrain interpretations and generalizations of the data. The most important limitation is that there is a lack of consistency in sleep assessment methods across studies, and this is a problem for several reasons. First, retrospective self-report (eg, survey), prospective self-report (eg, diary), laboratory-based objective (eg, polysomnography), and field-based objective (eg, actigraphy) estimates of sleep tend to disagree with each other, because they capture different elements of sleep well. It is likely that physiologic sleep is substantially less than that which is self-reported.27 Even among survey methods, there seems to be systematic variation.35 Second, because there is still no nationally representative dataset that includes any well-validated estimate of sleep duration or quality, generalizability from one dataset to the next is limited. Third, cutoffs and categories used to describe sleep are often inconsistent across studies; for example, the cutoff for the shortest sleep duration category can be as low as 4 hours or less or as high as 7 hours.
Another important limitation is a general lack of physiologic sleep measures at the societal level. Because these measures are typically more expensive and require more infrastructure to implement, they are often infeasible for large studies that require assessment of thousands of people. Until sleep assessment becomes more of a priority, otherwise rich datasets will continue to have just a few nonvalidated survey items measuring sleep. Suboptimal measurement of sleep will make data interpretation difficult, because it is unclear the degree to which associations are referring to physiologic sleep or other factors that become subsumed in self-reported sleep experience.
A third important limitation regards the complexity of social environments. As shown in the Social-Ecological Model, the influences that may play a role in sleep are many, varied, and exist at several levels. Still, most studies do not address the complex nature of social-environmental influences on health. Also, future studies that will examine epigenetic effects will need to better account for gene-environment interactions, and this will require a better operationalization of environmental variables in many cases. An example of one study that brought these methodologies together is cited by Watson and colleagues,268 who combined geospatial neighborhood analyses with sleep genetic information to characterize a social-environmental influence on sleep duration.
A fourth important limitation to the existing literature is a lack of interventional studies. If, for example, sleep represents a modifiable factor in health disparities, it is plausible that improvements in sleep at the community level could reduce effects of health disparities. However, there is a lack of interventional studies that can demonstrate this; rather, the best examples of investigations in this area use mediational analysis to show that changes in sleep account for changes in health outcomes across groups, such as blood pressure.269 More sleep interventions at the community level are going to be needed in order to understand the causal role of sleep in these outcomes. Also, there is a general lack of empirically supported interventions for sleep health. Although many interventions exist to promote healthy diet, physical activity, and substance cessation, a lack of standardized sleep health interventions limits knowledge in this area.
FUTURE RESEARCH DIRECTIONS
Several potential future research directions may help advance knowledge in this area. First, expanded epigenetic studies are needed to explore gene-environment interactions. As the science of human sleep genetics develops, more research into how genetic vulnerabilities interact with environmental influences is needed. For example, although it is unlikely that genetics explains racial disparities in sleep, it is plausible that some genetic adaptations to one geographic region may confer risk in another region (eg, less sunlight, different food availability). Also, it may be possible that certain genetic vulnerabilities (eg, airway collapse) may differentially affect groups because body mass indexes increase in the presence of increasing obesity rates due to westernized diets.
Another important direction in research will be to clarify the sleep phenotypes and endophenotypes. Currently, typing of sleep at the community and population level is frequently based on broad sleep duration groups (eg, “short sleepers”) or sleep symptoms (“difficulty falling asleep”), although these groups can be highly heterogeneous. Genetic studies are limited by this limited clarity in sleep phenotypes. Short sleepers, for example, may comprise individuals who are “true” short sleepers and need less sleep, those who need more sleep but are able to tolerate less sleep for an extended period of time (“resilient”), and those who are insufficient sleepers. Still, insufficient sleepers may belong to groups that demonstrate neurocognitive and metabolic impairments at variable rates (eg, some individuals may demonstrate more metabolic impairments and some more cognitive). Perhaps more clarity regarding phenotypes will help move forward an agenda of better human sleep genetics.
More intervention studies are also needed at the laboratory, clinical, and community levels that address real-world sleep concerns. As mentioned above, there is a lack of healthy sleep interventions, relative to healthy diet or physical activity recommendations. Without these data, it is difficult to make recommendations in addition to just stating a problem. Also, interventions need to address issues that have generally been ignored yet carry real-world significance For example, despite many adults sleeping between 6 and 7 hours per night, this sleep duration is almost never included in the literature, either because epidemiologic studies categorize at the hour (including them in either 6- or 7-hour groups) or because laboratory studies try to maximize difference between groups (usually comparing 8–6, 5, or 4 hours but not between 6 and 7).14 These and other real-world issues need to be better captured in intervention studies.
Finally, intervention studies are needed that identify real-world approaches to increasing sleep time among chronically sleep-deprived individuals. Unlike traditional intervention study designs, where changing sleep is the intervention and some health marker is the outcome (which would address the question of whether changing sleep impacts health), study designs are needed whereby changing sleep itself is the outcome. For example, it is known that smoking cessation can positively impact health. However, how does one quit smoking? Just recommending that someone quit is not enough, and literature has emerged that proposes novel ways to achieve this difficult behavioral change. Likewise, changing sleep duration in a real-world setting (with home, work, and other societal pressures) may be difficult, and useful strategies besides simply making recommendations need to be explored.
KEY POINTS.
Insufficient sleep and sleep disorders are highly prevalent in the population and are associated with significant morbidity and mortality.
Adverse outcomes of insufficient sleep and/or sleep disorders are weight gain and obesity, cardiovascular disease, diabetes, accidents and injuries, stress, pain, neurocognitive dysfunction, psychiatric symptoms, and mortality.
Exposure to sleep difficulties varies by age, sex, race/ethnicity, and socioeconomic status; significant sleep health disparities exist in the population.
Societal influences, such as globalization, technology, and public policy, affect sleep at a population level.
Acknowledgments
Dr M.A. Grandner is supported by National Heart, Lung, and Blood Institute (K23HL110216).
REFERENCES
- 1.Cajochen C, Chellappa S, Schmidt C. What keeps us awake?–the role of clocks and hourglasses, light, and melatonin. Int Rev Neurobiol 2010;93: 57–90. [DOI] [PubMed] [Google Scholar]
- 2.Fuller PM, Lu J. Neurobiology of sleep In: Amlaner CJ, Fuller PM, editors. Basics of sleep guide. 2nd edition Westchester (IL): Sleep Research Society; 2009. p. 53–62. [Google Scholar]
- 3.Mackiewicz M, Naidoo N, Zimmerman JE, et al. Molecular mechanisms of sleep and wakefulness. Ann N Y Acad Sci 2008;1129:335–49. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz JR, Roth T. Neurophysiology of sleep and wakefulness: basic science and clinical implications. Curr Neuropharmacol 2008;6(4):367–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franken P A role for clock genes in sleep homeostasis. Curr Opin Neurobiol 2013;23(5):864–72. [DOI] [PubMed] [Google Scholar]
- 6.Feng D, Lazar MA. Clocks, metabolism, and the epigenome. Mol Cell 2012;47(2):158–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerstner JR, Lenz O, Vanderheyden WM, et al. Amyloid-beta induces sleep fragmentation that is rescued by fatty acid binding proteins in Drosophila. J Neurosci Res 2016. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu M, Chung S, Zhang S, et al. Basal forebrain circuit for sleep-wake control. Nat Neurosci 2015; 18(11):1641–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox J, Pinto L, Dan Y. Calcium imaging of sleep-wake related neuronal activity in the dorsal pons. Nat Commun 2016;7:10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park DA. The fire within the eye: a historical essay on the nature and meaning of light. Princeton (NJ): Princeton University Press; 1997. [Google Scholar]
- 11.Williams S Sleep and society: sociological ventures into the (Un)known. London: Taylor & Francis; 2005. [Google Scholar]
- 12.Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep 2015;38(6):843–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Consensus Conference Panel, Watson NF, Badr MS, et al. Joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society on the recommended amount of sleep for a healthy adult: methodology and discussion. J Clin Sleep Med 2015;11(8):931–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Consensus Conference Panel, Watson NF, Badr MS, et al. Joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society on the recommended amount of sleep for a healthy adult: methodology and discussion. Sleep 2015;38(8):1161–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Consensus Conference Panel, Watson NF, Badr MS, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. J Clin Sleep Med 2015; 11(6):591–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bronfenbrenner U Toward an experimental ecology of human development. Am Psychol 1977;32: 513–31. [Google Scholar]
- 17.Grandner MA, Patel NP, Hale L, et al. Mortality associated with sleep duration: the evidence, the possible mechanisms, and the future. Sleep Med Rev 2010;14:191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grandner MA. Addressing sleep disturbances: an opportunity to prevent cardiometabolic disease? Int Rev Psychiatry 2014;26(2):155–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grandner MA, Williams NJ, Knutson KL, et al. Sleep disparity, race/ethnicity, and socioeconomic position. Sleep Med 2016;18:7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grandner MA. Sleep disparities in the American population: prevalence, potential causes, relationships to cardiometabolic health disparities, and future drections for research and policy In: Kelly R, editor. Health disparities in America. Washington, DC: US Congress; 2015. p. 126–32. [Google Scholar]
- 21.Knutson KL, Van Cauter E, Rathouz PJ, et al. Trends in the prevalence of short sleepers in the USA: 1975–2006. Sleep 2010;33(1):37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bin YS, Marshall NS, Glozier N. Secular trends in adult sleep duration: a systematic review. Sleep Med Rev 2012;16(3):223–30. [DOI] [PubMed] [Google Scholar]
- 23.Basner M, Spaeth AM, Dinges DF. Sociodemo-graphic characteristics and waking activities and their role in the timing and duration of sleep. Sleep 2014;37(12):1889–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grandner MA, Chakravorty S, Perlis ML, et al. Habitual sleep duration associated with self-reported and objectively determined cardiometabolic risk factors. Sleep Med 2014;15(1):42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krueger PM, Friedman EM. Sleep duration in the United States: a cross-sectional population-based study. Am J Epidemiol 2009;169(9):1052–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Wheaton AG, Chapman DP, et al. Prevalence of healthy sleep duration among adults –United States, 2014. MMWR Morb Mortal Wkly Rep 2016;65(6):137–41. [DOI] [PubMed] [Google Scholar]
- 27.Kurina LM, McClintock MK, Chen JH, et al. Sleep duration and all-cause mortality: a critical review of measurement and associations. Ann Epidemiol 2013;23(6):361–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grandner MA, Patel NP, Gehrman PR, et al. Who gets the best sleep? Ethnic and socioeconomic factors related to sleep disturbance. Sleep Med 2010;11:470–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grandner MA, Martin JL, Patel NP, et al. Age and sleep disturbances among American men and women: data from the U.S. Behavioral Risk Factor Surveillance System. Sleep 2012;35(3): 395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grandner MA, Jackson NJ, Izci-Balserak B, et al. Social and behavioral determinants of perceived insufficient sleep. Front Neurol 2015;6:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grandner MA, Petrov MER, Rattanaumpawan P, et al. Sleep symptoms, race/ethnicity, and socioeconomic position. J Clin Sleep Med 2013;9(9): 897–905, 905A–D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hammond EC. Some preliminary findings on physical complaints from a prospective study of 1,064,004 men and women. Am J Public Health Nations Health 1964;54:11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gallicchio L, Kalesan B. Sleep duration and mortality: a systematic review and meta-analysis. J Sleep Res 2009;18(2):148–58. [DOI] [PubMed] [Google Scholar]
- 34.Cappuccio FP, D’Elia L, Strazzullo P, et al. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep 2010;33(5):585–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grandner MA, Patel NP, Gehrman PR, et al. Problems associated with short sleep: bridging the gap between laboratory and epidemiological studies. Sleep Med Rev 2010;14:239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kripke DF, Langer RD, Elliott JA, et al. Mortality related to actigraphic long and short sleep. Sleep Med 2011;12(1):28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Youngstedt SD, Kripke DF. Long sleep and mortality: rationale for sleep restriction. Sleep Med Rev 2004;8(3):159–74. [DOI] [PubMed] [Google Scholar]
- 38.Grandner MA, Drummond SP. Who are the long sleepers? Towards an understanding of the mortality relationship. Sleep Med Rev 2007;11(5):341–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adenekan B, Pandey A, McKenzie S, et al. Sleep in America: role of racial/ethnic differences. Sleep Med Rev 2013;17(4):255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morselli LL, Guyon A, Spiegel K. Sleep and metabolic function. Pflugers Arch 2012;463(1): 139–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knutson KL. Does inadequate sleep play a role in vulnerability to obesity? Am J Hum Biol 2012; 24(3):361–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watanabe M, Kikuchi H, Tanaka K, et al. Association of short sleep duration with weight gain and obesity at 1-year follow-up: a large-scale prospective study. Sleep 2010;33(2):161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chaput JP, Bouchard C, Tremblay A. Change in sleep duration and visceral fat accumulation over 6 years in adults. Obesity (Silver Spring) 2014; 22(5):E9–12. [DOI] [PubMed] [Google Scholar]
- 44.Chaput JP, Despres JP, Bouchard C, et al. The association between sleep duration and weight gain in adults: a 6-year prospective study from the Quebec Family Study. Sleep 2008;31(4): 517–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baron KG, Reid KJ, Kern AS, et al. Role of sleep timing in caloric intake and BMI. Obesity (Silver Spring) 2011;19(7):1374–81. [DOI] [PubMed] [Google Scholar]
- 46.Shechter A, Grandner MA, St-Onge MP. The role of sleep in the control of food intake. Am J Lifestyle Med 2014;8(6):371–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grandner MA, Schopfer EA, Sands-Lincoln M, et al. Relationship between sleep duration and body mass index depends on age. Obesity (Silver Spring) 2015;23(12):2491–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barone MT, Menna-Barreto L. Diabetes and sleep: a complex cause-and-effect relationship. Diabetes Res Clin Pract 2011;91(2):129–37. [DOI] [PubMed] [Google Scholar]
- 49.Aldabal L, Bahammam AS. Metabolic, endocrine, and immune consequences of sleep deprivation. Open Respir Med J 2011;5:31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bopparaju S, Surani S. Sleep and diabetes. Int J Endocrinol 2010;2010:759509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zizi F, Jean-Louis G, Brown CD, et al. Sleep duration and the risk of diabetes mellitus: epidemiologic evidence and pathophysiologic insights. Curr Diab Rep 2010;10(1):43–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shan Z, Ma H, Xie M, et al. Sleep duration and risk of type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care 2015;38(3):529–37. [DOI] [PubMed] [Google Scholar]
- 53.Buxton OM, Pavlova M, Reid EW, et al. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes 2010;59(9):2126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morselli L, Leproult R, Balbo M, et al. Role of sleep duration in the regulation of glucose metabolism and appetite. Best Pract Res Clin Endocrinol Metab 2010;24(5):687–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tasali E, Leproult R, Spiegel K. Reduced sleep duration or quality: relationships with insulin resistance and type 2 diabetes. Prog Cardiovasc Dis 2009;51(5):381–91. [DOI] [PubMed] [Google Scholar]
- 56.Spiegel K, Knutson K, Leproult R, et al. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol 2005;99(5):2008–19. [DOI] [PubMed] [Google Scholar]
- 57.Spaeth AM, Dinges DF, Goel N. Sex and race differences in caloric intake during sleep restriction in healthy adults. Am J Clin Nutr 2014;100(2):559–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim S, Deroo LA, Sandler DP. Eating patterns and nutritional characteristics associated with sleep duration. Public Health Nutr 2011;14(5):889–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nedeltcheva AV, Kilkus JM, Imperial J, et al. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr 2009;89(1): 126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van Cauter E, Spiegel K, Tasali E, et al. Metabolic consequences of sleep and sleep loss. Sleep Med 2008;9(Suppl 1):S23–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Spiegel K, Tasali E, Penev P, et al. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med 2004;141(11):846–50. [DOI] [PubMed] [Google Scholar]
- 62.Hayes AL, Xu F, Babineau D, et al. Sleep duration and circulating adipokine levels. Sleep 2011; 34(2):147–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perelis M, Ramsey KM, Marcheva B, et al. Circadian transcription from beta cell function to diabetes path-ophysiology. J Biol Rhythms 2016;31(4):323–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grandner MA,Sands-Lincoln MR, Pak VM, et al. Sleep duration, cardiovascular disease, and proinflamma-tory biomarkers. Nat Sci Sleep 2013;5:93–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Frey DJ, Fleshner M, Wright KP Jr. The effects of 40 hours of total sleep deprivation on inflammatory markers in healthy young adults. Brain Behav Immun 2007;21(8):1050–7. [DOI] [PubMed] [Google Scholar]
- 66.Ferrie JE, Kivimaki M, Akbaraly TN, et al. Associations between change in sleep duration and inflammation: findings on C-reactive protein and interleukin 6 in the Whitehall II Study. Am J Epidemiol 2013;178(6):956–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rohleder N, Aringer M, Boentert M. Role of interleukin-6 in stress, sleep, and fatigue. Ann N Y Acad Sci 2012;1261:88–96. [DOI] [PubMed] [Google Scholar]
- 68.Vgontzas AN, Zoumakis E, Bixler EO, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab 2004;89(5):2119–26. [DOI] [PubMed] [Google Scholar]
- 69.van Leeuwen WM, Lehto M, Karisola P, et al. Sleep restriction increases the risk of developing cardiovascular diseases by augmenting proinflammatory responses through IL-17 and CRP. PLoS One 2009; 4(2):e4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chennaoui M, Sauvet F, Drogou C, et al. Effect of one night of sleep loss on changes in tumor necrosis factor alpha (TNF-alpha) levels in healthy men. Cytokine 2011;56(2):318–24. [DOI] [PubMed] [Google Scholar]
- 71.Shearer WT, Reuben JM, Mullington JM, et al. Soluble TNF-alpha receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J Allergy Clin Immunol 2001; 107(1):165–70. [DOI] [PubMed] [Google Scholar]
- 72.Patel SR, Zhu X, Storfer-Isser A, et al. Sleep duration and biomarkers of inflammation. Sleep 2009; 32(2):200–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meier-Ewert HK, Ridker PM, Rifai N, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol 2004;43(4):678–83. [DOI] [PubMed] [Google Scholar]
- 74.Miller MA, Kandala NB, Kivimaki M, et al. Gender differences in the cross-sectional relationships between sleep duration and markers of inflammation: Whitehall II study. Sleep 2009;32(7):857–64. [PMC free article] [PubMed] [Google Scholar]
- 75.Matthews KA, Zheng H, Kravitz HM, et al. Are inflammatory and coagulation biomarkers related to sleep characteristics in mid-life women?: Study of Women’s Health across the Nation sleep study. Sleep 2010;33(12):1649–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grandner MA, Buxton OM, Jackson N, et al. Extreme sleep durations and increased C-reactive protein: effects of sex and ethnoracial group. Sleep 2013;36(5):769–779E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Irwin MR, Olmstead R, Carroll JE. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry 2016;80(1):40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.von Ruesten A, Weikert C, Fietze I, et al. Association of sleep duration with chronic diseases in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam study. PLoS One 2012;7(1):e30972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Altman NG, Izci-Balserak B, Schopfer E, et al. Sleep duration versus sleep insufficiency as predictors of cardiometabolic health outcomes. Sleep Med 2012;13(10):1261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Q, Xi B, Liu M, et al. Short sleep duration is associated with hypertension risk among adults: a systematic review and meta-analysis. Hypertens Res 2012;35(10):1012–8. [DOI] [PubMed] [Google Scholar]
- 81.Meng L, Zheng Y, Hui R. The relationship of sleep duration and insomnia to risk of hypertension incidence: a meta-analysis of prospective cohort studies. Hypertens Res 2013;36(11):985–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mezick EJ, Hall M, Matthews KA. Sleep duration and ambulatory blood pressure in black and white adolescents. Hypertension 2012;59(3):747–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.King CR, Knutson KL, Rathouz PJ, et al. Short sleep duration and incident coronary artery calcification. JAMA 2008;300(24):2859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Amagai Y, Ishikawa S, Gotoh T, et al. Sleep duration and incidence of cardiovascular events in a Japanese population: the Jichi Medical School cohort study. J Epidemiol 2010;20(2):106–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cappuccio FP, Cooper D, D’Elia L, et al. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J 2011;32(12):1484–92. [DOI] [PubMed] [Google Scholar]
- 86.Goel N, Rao H, Durmer JS, et al. Neurocognitive consequences of sleep deprivation. Semin Neurol 2009;29(4):320–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lim J, Dinges DF. Sleep deprivation and vigilant attention. Ann N Y Acad Sci 2008;1129:305–22. [DOI] [PubMed] [Google Scholar]
- 88.Dinges DF, Powell JW. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Beh Res Meth Instr Comp 1985;17:652–5. [Google Scholar]
- 89.Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med 2007;3(5):519–28. [PMC free article] [PubMed] [Google Scholar]
- 90.Van Dongen HP, Baynard MD, Maislin G, et al. Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep 2004;27(3):423–33. [PubMed] [Google Scholar]
- 91.Verweij IM, Romeijn N, Smit DJ, et al. Sleep deprivation leads to a loss of functional connectivity in frontal brain regions. BMC Neurosci 2014;15:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Killgore WD, Grugle NL, Balkin TJ. Gambling when sleep deprived: don’t bet on stimulants. Chronobiol Int 2012;29(1):43–54. [DOI] [PubMed] [Google Scholar]
- 93.Jackson ML, Croft RJ, Kennedy GA, et al. Cognitive components of simulated driving performance: sleep loss effects and predictors. Accid Anal Prev 2013;50:438–44. [DOI] [PubMed] [Google Scholar]
- 94.Saint Martin M, Sforza E, Barthelemy JC, et al. Does subjective sleep affect cognitive function in healthy elderly subjects? The Proof cohort. Sleep Med 2012;13(9):1146–52. [DOI] [PubMed] [Google Scholar]
- 95.Rupp TL, Wesensten NJ, Balkin TJ. Trait-like vulnerability to total and partial sleep loss. Sleep 2012; 35(8):1163–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Banks S, Van Dongen HP, Maislin G, et al. Neuro-behavioral dynamics following chronic sleep restriction: dose-response effects of one night for recovery. Sleep 2010;33(8):1013–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Maia Q, Grandner MA, Findley J, et al. Short and long sleep duration and risk of drowsy driving and the role of subjective sleep insufficiency. Accid Anal Prev 2013;59:618–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chiu HY, Tsai PS. The impact of various work schedules on sleep complaints and minor accidents during work or leisure time: evidence from a national survey. J Occup Environ Med 2013; 55(3):325–30. [DOI] [PubMed] [Google Scholar]
- 99.Lilley R, Day L, Koehncke N, et al. The relationship between fatigue-related factors and work-related injuries in the Saskatchewan Farm Injury Cohort Study. Am J Ind Med 2012;55(4):367–75. [DOI] [PubMed] [Google Scholar]
- 100.Kucharczyk ER, Morgan K, Hall AP. The occupational impact of sleep quality and insomnia symptoms. Sleep Med Rev 2012;16(6):547–59. [DOI] [PubMed] [Google Scholar]
- 101.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th edition Washington, DC: American Psychiatric Association; 2003. DSM-5. [Google Scholar]
- 102.Chakravorty S, Siu HY, Lalley-Chareczko L, et al. Sleep duration and insomnia symptoms as risk factors for suicidal ideation in a nationally representative sample. Prim Care Companion CNS Disord 2015;17(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rosenstock IM. Why people use health services. Milbank Mem Fund Q 1966;44(3 Suppl):94–127. [PubMed] [Google Scholar]
- 104.Champion VL, Skinner CS. The health belief model In: Glanz K, Rimer BK, Viswanath K, editors. Health behavior and health education: theory, research, and practice. San Francisco (CA): Jossey-Bass; 2008. p. 45–65. [Google Scholar]
- 105.Montano DE, Kasprzyk D. Theory of reasoned action, theory of planned behavior, and the integrated behavioral model In: Glanz K, Rimer BK, Viswanath K, editors. Health behavior and health education: theory, research, and practice. San Francisco (CA): Jossey-Bass; 2008. p. 68–96. [Google Scholar]
- 106.Hooker E, Ball HL, Kelly PJ. Sleeping like a baby: attitudes and experiences of bedsharing in northeast England. Med Anthropol 2001;19(3):203–22. [DOI] [PubMed] [Google Scholar]
- 107.Thoman EB. Co-sleeping, an ancient practice: issues of the past and present, and possibilities for the future. Sleep Med Rev 2006;10(6):407–17. [DOI] [PubMed] [Google Scholar]
- 108.Mindell JA, Sadeh A, Wiegand B, et al. Cross-cultural differences in infant and toddler sleep. Sleep Med 2010;11(3):274–80. [DOI] [PubMed] [Google Scholar]
- 109.Norton PJ, Grellner KW. A retrospective study on infant bed-sharing in a clinical practice population. Matern Child Health J 2011;15(4):507–13. [DOI] [PubMed] [Google Scholar]
- 110.Gettler LT, McKenna JJ. Evolutionary perspectives on mother-infant sleep proximity and breastfeeding in a laboratory setting. Am J Phys Anthropol 2011; 144(3):454–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jain S, Romack R, Jain R. Bed sharing in school-age children–clinical and social implications. J Child Adolesc Psychiatr Nurs 2011;24(3):185–9. [DOI] [PubMed] [Google Scholar]
- 112.Shulman D, Strousma GG. Dream cultures: explorations in the comparative history of dreaming. Oxford (United Kingdom): Oxford University Press; 1999. [Google Scholar]
- 113.Spurgeon A, Harrington JM, Cooper CL. Health and safety problems associated with long working hours: a review of the current position. Occup Environ Med 1997;54(6):367–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Goto A, Yasumura S, Nishise Y, et al. Association of health behavior and social role with total mortality among Japanese elders in Okinawa, Japan. Aging Clin Exp Res 2003;15(6):443–50. [DOI] [PubMed] [Google Scholar]
- 115.Lockley SW, Landrigan CP, Barger LK, et al. When policy meets physiology: the challenge of reducing resident work hours. Clin Orthop Relat Res 2006; 449:116–27. [DOI] [PubMed] [Google Scholar]
- 116.Ko GT, Chan JC, Chan AW, et al. Association between sleeping hours, working hours and obesity in Hong Kong Chinese: the ‘better health for better Hong Kong’ health promotion campaign. Int J Obes (Lond) 2007;31(2):254–60. [DOI] [PubMed] [Google Scholar]
- 117.Basner M, Dinges DF . Dubious bargain: trading sleep for Leno and Letterman. Sleep 2009;32(6): 747–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gangwisch JE. All work and no play makes Jack lose sleep. Commentary on Virtanen et al. Long working hours and sleep disturbances: the Whitehall II prospective cohort study. Sleep 2009;32: 737–45. Sleep 2009;32(6):717–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Virtanen M, Ferrie JE, Gimeno D, et al. Long working hours and sleep disturbances: the Whitehall II prospective cohort study. Sleep 2009;32(6):737–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nakata A Effects of long work hours and poor sleep characteristics on workplace injury among full-time male employees of small- and medium-scale businesses. J Sleep Res 2011;20(4):576–84. [DOI] [PubMed] [Google Scholar]
- 121.Mahan RP, Carvalhais AB, Queen SE. Sleep reduction in night-shift workers: is it sleep deprivation or a sleep disturbance disorder? Percept Mot Skills 1990;70(3 Pt 1):723–30. [DOI] [PubMed] [Google Scholar]
- 122.Rajaratnam SM, Arendt J. Health in a 24-h society. Lancet 2001;358(9286):999–1005. [DOI] [PubMed] [Google Scholar]
- 123.Nag PK, Nag A. Shiftwork in the hot environment. J Hum Ergol (Tokyo) 2001;30(1–2):161–6. [PubMed] [Google Scholar]
- 124.Costa G Shift work and health: current problems and preventive actions. Saf Health Work 2010; 1(2):112–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Owens J Sleep in children: cross-cultural perspectives. Sleep Biol Rhythms 2004;2:165–73. [Google Scholar]
- 126.Milner CE, Cote KA. Benefits of napping in healthy adults: impact of nap length, time of day, age, and experience with napping. J Sleep Res 2009;18(2): 272–81. [DOI] [PubMed] [Google Scholar]
- 127.Worthman CM, Brown RA. Sleep budgets in a globalizing world: biocultural interactions influence sleep sufficiency among Egyptian families. Soc Sci Med 2013;79:31–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pandey A, Gekhman D, Gousse Y, et al. Short sleep and dysfunctional beliefs and attitudes toward sleep among black men. Sleep 2011;34(Abstract Suppl): 261–2.21358843 [Google Scholar]
- 129.Grandner MA, Patel NP, Jean-Louis G, et al. Sleep-related behaviors and beliefs associated with race/ethnicity in women. J Natl Med Assoc 2013; 105(1):4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sell RE, Bardwell W, Palinkas L, et al. Ethnic differences in sleep-health knowledge. Sleep 2009; 32(Abstract Supplement):A392. [Google Scholar]
- 131.Ohayon MM, Carskadon MA, Guilleminault C, et al. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep 2004;27(7):1255–73. [DOI] [PubMed] [Google Scholar]
- 132.Hardeland R Melatonin in aging and disease-multiple consequences of reduced secretion, options and limits of treatment. Aging Dis 2012;3(2): 194–225. [PMC free article] [PubMed] [Google Scholar]
- 133.Neikrug AB, Ancoli-Israel S. Sleep disorders in the older adult - a mini-review. Gerontology 2010;56(2): 181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Roepke SK, Ancoli-Israel S. Sleep disorders in the elderly. Indian J Med Res 2010;131:302–10. [PubMed] [Google Scholar]
- 135.Martin J, Shochat T, Gehrman PR, et al. Sleep in the elderly. Respir Care Clin N Am 1999;5(3): 461–72, ix. [PubMed] [Google Scholar]
- 136.Ruiter ME, VanderWal GS, Lichstein KL. Insomnia in the elderly In: Pandi-Perumal SR, Monti JR, Monjan AA, editors. Principles and practice of geriatric sleep medicine. Cambridge (United Kingdom): Cambridge; 2010. p. 271–9. [Google Scholar]
- 137.Yeh P, Walters AS, Tsuang JW. Restless legs syndrome: a comprehensive overview on its epidemiology, risk factors, and treatment. Sleep Breath 2012;16(4):987–1007. [DOI] [PubMed] [Google Scholar]
- 138.Spiegelhalder K, Hornyak M. Movement disorders in the elderly In: Pandi-Perumal SR, Monti JR, Monjan AA, editors. Principles and practice of geriatric sleep medicine. Cambridge (United Kingdom): Cambridge; 2010. p. 233–40. [Google Scholar]
- 139.Peppard PE, Young T, Barnet JH, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013;177(9):1006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ferini Strambi L REM sleep behavior disorder in the elderly In: Pandi-Perumal SR, Monti JR, Monjan AA, editors. Principles and practice of geriatric sleep medicine. Cambridge (United Kingdom): Cambridge; 2010. p. 241–7. [Google Scholar]
- 141.Soldatos CR, Allaert FA, Ohta T, et al. How do individuals sleep around the world? Results from a single-day survey in ten countries. Sleep Med 2005;6(1):5–13. [DOI] [PubMed] [Google Scholar]
- 142.Zilli I, Ficca G, Salzarulo P. Factors involved in sleep satisfaction in the elderly. Sleep Med 2009; 10(2):233–9. [DOI] [PubMed] [Google Scholar]
- 143.Grandner MA, Patel NP, Gooneratne NS. Difficulties sleeping: a natural part of growing older? Aging Health 2012;8(3):219–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Roehrs T, Kapke A, Roth T, et al. Sex differences in the polysomnographic sleep of young adults: a community-based study. Sleep Med 2006;7(1): 49–53. [DOI] [PubMed] [Google Scholar]
- 145.Kimura M Minireview: gender-specific sleep regulation. Sleep Biol Rhythms 2005;3:75–9. [Google Scholar]
- 146.Vitiello MV, Larsen LH, Moe KE. Age-related sleep change: gender and estrogen effects on the subjective-objective sleep quality relationships of healthy, noncomplaining older men and women. J Psychosom Res 2004;56(5):503–10. [DOI] [PubMed] [Google Scholar]
- 147.Voderholzer U, Al-Shajlawi A, Weske G, et al. Are there gender differences in objective and subjective sleep measures? A study of insomniacs and healthy controls. Depress Anxiety 2003;17(3): 162–72. [DOI] [PubMed] [Google Scholar]
- 148.Mohsenin V Gender differences in the expression of sleep-disordered breathing: role of upper airway dimensions. Chest 2001;120(5):1442–7. [DOI] [PubMed] [Google Scholar]
- 149.Armitage R, Hudson A, Trivedi M, et al. Sex differences in the distribution of EEG frequencies during sleep: unipolar depressed outpatients. J Affect Disord 1995;34(2):121–9. [DOI] [PubMed] [Google Scholar]
- 150.Whinnery J, Jackson N, Rattanaumpawan P, et al. Short and long sleep duration associated with race/ethnicity, sociodemographics, and socioeconomic position. Sleep 2014;37(3):601–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Green MJ, Espie CA, Hunt K, et al. The longitudinal course of insomnia symptoms: inequalities by sex and occupational class among two different age cohorts followed for 20 years in the west of Scotland. Sleep 2012;35(6):815–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ye L, Pien GW, Weaver TE. Gender differences in the clinical manifestation of obstructive sleep apnea. Sleep Med 2009;10(10):1075–84. [DOI] [PubMed] [Google Scholar]
- 153.Del Campo F, Zamarron C. Sleep apnea and pregnancy. An association worthy of study. Sleep Breath 2013;17(2):463–4. [DOI] [PubMed] [Google Scholar]
- 154.Ibrahim S, Foldvary-Schaefer N. Sleep disorders in pregnancy: implications, evaluation, and treatment. Neurol Clin 2012;30(3):925–36. [DOI] [PubMed] [Google Scholar]
- 155.Facco FL, Kramer J, Ho KH, et al. Sleep disturbances in pregnancy. Obstet Gynecol 2010; 115(1):77–83. [DOI] [PubMed] [Google Scholar]
- 156.Chen YH, Kang JH, Lin CC, et al. Obstructive sleep apnea and the risk of adverse pregnancy outcomes. Am J Obstet Gynecol 2012;206(2):136, e1–5. [DOI] [PubMed] [Google Scholar]
- 157.Okun ML, Luther JF, Wisniewski SR, et al. Disturbed sleep, a novel risk factor for preterm birth? J Womens Health (Larchmt) 2012;21(1): 54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Moore M, Meltzer LJ, Mindell JA. Bedtime problems and night wakings in children. Sleep Med Clin 2007;2:377–85. [DOI] [PubMed] [Google Scholar]
- 159.Mindell JA, Kuhn B, Lewin DS, et al. Behavioral treatment of bedtime problems and night wakings in infants and young children. Sleep 2006;29(10): 1263–76. [PubMed] [Google Scholar]
- 160.Okun ML, Luther J, Prather AA, et al. Changes in sleep quality, but not hormones predict time to postpartum depression recurrence. J Affect Disord 2011;130(3):378–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chang JJ, Pien GW, Duntley SP, et al. Sleep deprivation during pregnancy and maternal and fetal outcomes: is there a relationship? Sleep Med Rev 2010;14(2):107–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pires GN, Andersen ML, Giovenardi M, et al. Sleep impairment during pregnancy: possible implications on mother-infant relationship. Med Hypotheses 2010;75(6):578–82. [DOI] [PubMed] [Google Scholar]
- 163.Ameratunga D, Goldin J, Hickey M. Sleep disturbance in menopause. Intern Med J 2012;42(7): 742–7. [DOI] [PubMed] [Google Scholar]
- 164.Regestein QR. Do hot flashes disturb sleep? Meno-pause 2012;19(7):715–8. [DOI] [PubMed] [Google Scholar]
- 165.Baron KG, Smith TW, Berg CA, et al. Spousal involvement in CPAP adherence among patients with obstructive sleep apnea. Sleep Breath 2011; 15(3):525–34. [DOI] [PubMed] [Google Scholar]
- 166.McDowell A Spousal involvement and CPAP adherence: a two-way street? Sleep Breath 2011; 15(3):269–70. [DOI] [PubMed] [Google Scholar]
- 167.Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med 2009;6(8):e1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mahowald MW, Schenck CH. REM sleep behaviour disorder: a marker of synucleinopathy. Lancet Neurol 2013;12(5):417–9. [DOI] [PubMed] [Google Scholar]
- 169.Tomfohr L, Pung MA, Edwards KM, et al. Racial differences in sleep architecture: the role of ethnic discrimination. Biol Psychol 2012;89(1):34–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Thomas KS, Bardwell WA, Ancoli-Israel S, et al. The toll of ethnic discrimination on sleep architecture and fatigue. Health Psychol 2006;25(5):635–42. [DOI] [PubMed] [Google Scholar]
- 171.Profant J, Ancoli-Israel S, Dimsdale JE. Are there ethnic differences in sleep architecture? Am J Hum Biol 2002;14(3):321–6. [DOI] [PubMed] [Google Scholar]
- 172.Ruiter ME, Decoster J, Jacobs L, et al. Normal sleep in African-Americans and Caucasian-Americans: a meta-analysis. Sleep Med 2011; 12(3):209–14. [DOI] [PubMed] [Google Scholar]
- 173.Ruiter ME, DeCoster J, Jacobs L, et al. Sleep disorders in African Americans and Caucasian Americans: a meta-analysis. Behav Sleep Med 2010; 8(4):246–59. [DOI] [PubMed] [Google Scholar]
- 174.Grandner MA, Knutson KL, Troxel W, et al. Implications of sleep and energy drink use for health disparities. Nutr Rev 2014;72(Suppl 1):14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Knutson KL. Sociodemographic and cultural determinants of sleep deficiency: implications for cardio-metabolic disease risk. Soc Sci Med 2013;79:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mezick EJ, Matthews KA, Hall M, et al. Influence of race and socioeconomic status on sleep: Pittsburgh SleepSCORE project. Psychosom Med 2008;70(4):410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Patel NP, Grandner MA, Xie D, et al. “Sleep disparity” in the population: poor sleep quality is strongly associated with poverty and ethnicity. BMC Public Health 2010;10(1):475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Grandner MA, Hale L, Jackson N, et al. Perceived racial discrimination as an independent predictor of sleep disturbance and daytime fatigue. Behav Sleep Med 2012;10(4):235–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Slopen N, Williams DR. Discrimination, other psychosocial stressors, and self-reported sleep duration and difficulties. Sleep 2014;37(1):147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bureau of Labor Statistics. American time use survey fact sheet. Washington, DC: Bureau of Labor Statistics; 2013. [Google Scholar]
- 181.Rosekind MR, Gregory KB, Mallis MM, et al. The cost of poor sleep: workplace productivity loss and associated costs. J Occup Environ Med 2010;52(1):91–8. [DOI] [PubMed] [Google Scholar]
- 182.Hui SK, Grandner MA. Trouble sleeping associated with lower work performance and greater health care costs: longitudinal data from Kansas State Employee Wellness Program. J Occup Environ Med 2015;57(10):1031–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hui SK, Grandner MA. Associations between poor sleep quality and stages of change of multiple health behaviors among participants of Employee Wellness Program. Prev Med Rep 2015;2:292–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gurubhagavatula I, Nkwuo JE, Maislin G, et al. Estimated cost of crashes in commercial drivers supports screening and treatment of obstructive sleep apnea. Accid Anal Prev 2008;40(1):104–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Pack AI, Maislin G, Staley B, et al. Impaired performance in commercial drivers: role of sleep apnea and short sleep duration. Am J Respir Crit Care Med 2006;174(4):446–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Xie W, Chakrabarty S, Levine R, et al. Factors associated with obstructive sleep apnea among commercial motor vehicle drivers. J Occup Environ Med 2011;53(2):169–73. [DOI] [PubMed] [Google Scholar]
- 187.Moore-Ede M, Heitmann A, Guttkuhn R, et al. Circadian alertness simulator for fatigue risk assessment in transportation: application to reduce frequency and severity of truck accidents. Aviat Space Environ Med 2004;75(3 Suppl):A107–18. [PubMed] [Google Scholar]
- 188.Paterson JL, Dorrian J, Clarkson L, et al. Beyond working time: factors affecting sleep behaviour in rail safety workers. Accid Anal Prev 2012; 45(Suppl):32–5. [DOI] [PubMed] [Google Scholar]
- 189.Darwent D, Dawson D, Roach GD. Prediction of probabilistic sleep distributions following travel across multiple time zones. Sleep 2010;33(2): 185–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Dorrian J, Darwent D, Dawson D, et al. Predicting pilot’s sleep during layovers using their own behaviour or data from colleagues: implications for biomathematical models. Accid Anal Prev 2012;45(Suppl):17–21. [DOI] [PubMed] [Google Scholar]
- 191.Drury DA, Ferguson SA, Thomas MJ. Restricted sleep and negative affective states in commercial pilots during short haul operations. Accid Anal Prev 2012;45(Suppl):80–4. [DOI] [PubMed] [Google Scholar]
- 192.Gander PH, Signal TL, van den Berg MJ, et al. In-flight sleep, pilot fatigue and Psychomotor Vigilance Task performance on ultra-long range versus long range flights. J Sleep Res 2013;22(6): 697–706. [DOI] [PubMed] [Google Scholar]
- 193.Holmes A, Al-Bayat S, Hilditch C, et al. Sleep and sleepiness during an ultra long-range flight operation between the Middle East and United States. Accid Anal Prev 2012;45(Suppl):27–31. [DOI] [PubMed] [Google Scholar]
- 194.Powell DM, Spencer MB, Petrie KJ. Fatigue in airline pilots after an additional day’s layover period. Aviat Space Environ Med 2010;81(11): 1013–7. [DOI] [PubMed] [Google Scholar]
- 195.Roach GD, Darwent D, Dawson D. How well do pilots sleep during long-haul flights? Ergonomics 2010;53(9):1072–5. [DOI] [PubMed] [Google Scholar]
- 196.Roach GD, Petrilli RM, Dawson D, et al. Impact of layover length on sleep, subjective fatigue levels, and sustained attention of long-haul airline pilots. Chronobiol Int 2012;29(5):580–6. [DOI] [PubMed] [Google Scholar]
- 197.Roach GD, Sargent C, Darwent D, et al. Duty periods with early start times restrict the amount of sleep obtained by short-haul airline pilots. Accid Anal Prev 2012;45(Suppl):22–6. [DOI] [PubMed] [Google Scholar]
- 198.Rajaratnam SM, Barger LK, Lockley SW, et al. Sleep disorders, health, and safety in police officers. JAMA 2011;306(23):2567–78. [DOI] [PubMed] [Google Scholar]
- 199.Charles LE, Gu JK, Andrew ME, et al. Sleep duration and biomarkers of metabolic function among police officers. J Occup Environ Med 2011;53(8): 831–7. [DOI] [PubMed] [Google Scholar]
- 200.Fekedulegn D, Burchfiel CM, Hartley TA, et al. Shift-work and sickness absence among police officers: the BCOPS study. Chronobiol Int 2013;30(7): 930–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Gu JK, Charles LE, Burchfiel CM, et al. Long work hours and adiposity among police officers in a US northeast city. J Occup Environ Med 2012;54(11): 1374–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.McCanlies EC, Slaven JE, Smith LM, et al. Metabolic syndrome and sleep duration in police officers. Work 2012;43(2):133–9. [DOI] [PubMed] [Google Scholar]
- 203.Neylan TC, Metzler TJ, Henn-Haase C, et al. Prior night sleep duration is associated with psychomotor vigilance in a healthy sample of police academy recruits. Chronobiol Int 2010;27(7):1493–508. [DOI] [PubMed] [Google Scholar]
- 204.Aisbett B, Wolkow A, Sprajcer M, et al. “Awake, smoky, and hot”: providing an evidence-base for managing the risks associated with occupational stressors encountered by wildland firefighters. Appl Ergon 2012;43(5):916–25. [DOI] [PubMed] [Google Scholar]
- 205.Vargas de Barros V, Martins LF, Saitz R, et al. Mental health conditions, individual and job characteristics and sleep disturbances among fire-fighters. J Health Psychol 2013;18(3):350–8. [DOI] [PubMed] [Google Scholar]
- 206.Grandner MA, Pack AI. Sleep disorders, public health, and public safety. JAMA 2011;306(23): 2616–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sharwood LN, Elkington J, Meuleners L, et al. Use of caffeinated substances and risk of crashes in long distance drivers of commercial vehicles: case-control study. BMJ 2013;346:f1140. [DOI] [PubMed] [Google Scholar]
- 208.Grundy A, Sanchez M, Richardson H, et al. Light intensity exposure, sleep duration, physical activity, and biomarkers of melatonin among rotating shift nurses. Chronobiol Int 2009;26(7):1443–61. [DOI] [PubMed] [Google Scholar]
- 209.Ruggiero JS, Redeker NS, Fiedler N, et al. Sleep and psychomotor vigilance in female shiftworkers. Biol Res Nurs 2012;14(3):225–35. [DOI] [PubMed] [Google Scholar]
- 210.Chang YS, Wu YH, Hsu CY, et al. Impairment of perceptual and motor abilities at the end of a night shift is greater in nurses working fast rotating shifts. Sleep Med 2011;12(9):866–9. [DOI] [PubMed] [Google Scholar]
- 211.Chung MH, Kuo TB, Hsu N, et al. Recovery after three-shift work: relation to sleep-related cardiac neuronal regulation in nurses. Ind Health 2012; 50(1):24–30. [DOI] [PubMed] [Google Scholar]
- 212.Demir Zencirci A, Arslan S. Morning-evening type and burnout level as factors influencing sleep quality of shift nurses: a questionnaire study. Croat Med J 2011;52(4):527–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Dorrian J, Paterson J, Dawson D, et al. Sleep, stress and compensatory behaviors in Australian nurses and midwives. Rev Saude Publica 2011; 45(5):922–30. [DOI] [PubMed] [Google Scholar]
- 214.Eldevik MF, Flo E, Moen BE, et al. Insomnia, excessive sleepiness, excessive fatigue, anxiety, depression and shift work disorder in nurses having less than 11 hours in-between shifts. PLoS One 2013; 8(8):e70882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Geiger-Brown J, Rogers VE, Han K, et al. Occupational screening for sleep disorders in 12-h shift nurses using the Berlin Questionnaire. Sleep Breath 2013;17(1):381–8. [DOI] [PubMed] [Google Scholar]
- 216.Geiger-Brown J, Rogers VE, Trinkoff AM, et al. Sleep, sleepiness, fatigue, and performance of 12-hour-shift nurses. Chronobiol Int 2012;29(2): 211–9. [DOI] [PubMed] [Google Scholar]
- 217.Geiger-Brown J, Trinkoff A, Rogers VE. The impact of work schedules, home, and work demands on self-reported sleep in registered nurses. J Occup Environ Med 2011;53(3):303–7. [DOI] [PubMed] [Google Scholar]
- 218.Ulmer C, Wolman DM, Johns MME. Institute of Medicine committee on optimizing graduate medical trainee (resident) hours and work schedules to improve patient safety Resident duty hours: enhancing sleep, supervision, and safety. Washington, DC: National Academies Press; 2009. [PubMed] [Google Scholar]
- 219.Amin MM, Graber M, Ahmad K, et al. The effects of a mid-day nap on the neurocognitive performance of first-year medical residents: a controlled interventional pilot study. Acad Med 2012;87(10):1428–33. [DOI] [PubMed] [Google Scholar]
- 220.Arora VM, Georgitis E, Woodruff JN, et al. Improving sleep hygiene of medical interns: can the sleep, alertness, and fatigue education in residency program help? Arch Intern Med 2007; 167(16):1738–44. [DOI] [PubMed] [Google Scholar]
- 221.Kim HJ, Kim JH, Park K-D, et al. A survey of sleep deprivation patterns and their effects on cognitive functions of residents and interns in Korea. Sleep Med 2011;12(4):390–6. [DOI] [PubMed] [Google Scholar]
- 222.Reed DA, Fletcher KE, Arora VM. Systematic review: association of shift length, protected sleep time, and night float with patient care, residents’ health, and education. Ann Intern Med 2010; 153(12):829–42. [DOI] [PubMed] [Google Scholar]
- 223.Bilimoria KY, Chung JW, Hedges LV, et al. National cluster-randomized trial of duty-hour flexibility in surgical training. N Engl J Med 2016;374(8):713–27. [DOI] [PubMed] [Google Scholar]
- 224.Hale L Do DP. Racial differences in self-reports of sleep duration in a population-based study. Sleep 2007;30(9):1096–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Hale L, Hill TD, Burdette AM. Does sleep quality mediate the association between neighborhood disorder and self-rated physical health? Prev Med 2010;51(3–4):275–8. [DOI] [PubMed] [Google Scholar]
- 226.Hale L, Hill TD, Friedman E, et al. Perceived neighborhood quality, sleep quality, and health status: evidence from the Survey of the Health of Wisconsin. Soc Sci Med 2013;79:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Hill TD, Burdette AM, Hale L. Neighborhood disorder, sleep quality, and psychological distress: testing a model of structural amplification. Health Place 2009;15(4):1006–13. [DOI] [PubMed] [Google Scholar]
- 228.Pirrera S, De Valck E, Cluydts R. Nocturnal road traffic noise: a review on its assessment and consequences on sleep and health. Environ Int 2010; 36(5):492–8. [DOI] [PubMed] [Google Scholar]
- 229.Noise Kawada T. and health: sleep disturbance in adults. J Occup Health 2011;53(6):413–6. [DOI] [PubMed] [Google Scholar]
- 230.Fonken LK, Kitsmiller E, Smale L, et al. Dim nighttime light impairs cognition and provokes depressive-like responses in a diurnal rodent. J Biol Rhythms 2012;27(4):319–27. [DOI] [PubMed] [Google Scholar]
- 231.Hu RF, Jiang XY, Zeng YM, et al. Effects of earplugs and eye masks on nocturnal sleep, melatonin and cortisol in a simulated intensive care unit environment. Crit Care 2010;14(2):R66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Wood B, Rea MS, Plitnick B, et al. Light level and duration of exposure determine the impact of self-luminous tablets on melatonin suppression. Appl Ergon 2013;44(2):237–40. [DOI] [PubMed] [Google Scholar]
- 233.Herljevic M, Middleton B, Thapan K, et al. Light-induced melatonin suppression: age-related reduction in response to short wavelength light. Exp Gerontol 2005;40(3):237–42. [DOI] [PubMed] [Google Scholar]
- 234.Pigeon WR, Grandner MA. Creating an optimal sleep environment In: Kushida CA, editor. Encyclopedia of sleep. Oxford (United Kingdom): Elsevier; 2013. p. 72–6. [Google Scholar]
- 235.Buxton OM, Ellenbogen JM, Wang W, et al. Sleep disruption due to hospital noises: a prospective evaluation. Ann Intern Med 2012;157(3):170–9. [DOI] [PubMed] [Google Scholar]
- 236.Parmeggiani PL. Sleep behaviour and temperature In: Parmeggiani PL, Velluti RA, editors. The physiologic nature of sleep. London: Imperial College Press; 2005. p. 387–405. [Google Scholar]
- 237.McCall WV, Boggs N, Letton A. Changes in sleep and wake in response to different sleeping surfaces: a pilot study. Appl Ergon 2012;43(2):386–91. [DOI] [PubMed] [Google Scholar]
- 238.Shanmugan B, Roux F, Stonestreet C, et al. Lower back pain and sleep: mattresses, sleep quality and daytime symptoms. Sleep Diagn Ther 2007; 2(5):36–40. [Google Scholar]
- 239.Verhaert V, Haex B, De Wilde T, et al. Ergonomics in bed design: the effect of spinal alignment on sleep parameters. Ergonomics 2011;54(2):169–78. [DOI] [PubMed] [Google Scholar]
- 240.Troxel WM. It’s more than sex: exploring the dyadic nature of sleep and implications for health. Psycho-som Med 2010;72(6):578–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Troxel WM, Robles TF, Hall M, et al. Marital quality and the marital bed: examining the covariation between relationship quality and sleep. Sleep Med Rev 2007;11(5):389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Troxel WM, Buysse DJ, Hall M, et al. Marital happiness and sleep disturbances in a multi-ethnic sample of middle-aged women. Behav Sleep Med 2009;7(1):2–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Troxel WM, Buysse DJ, Monk TH, et al. Does social support differentially affect sleep in older adults with versus without insomnia? J Psychosom Res 2010;69(5):459–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Troxel WM, Cyranowski JM, Hall M, et al. Attachment anxiety, relationship context, and sleep in women with recurrent major depression. Psycho-som Med 2007;69(7):692–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Gradisar M, Wolfson AR, Harvey AG, et al. The sleep and technology use of Americans: findings from the National Sleep Foundation’s 2011 Sleep in America poll. J Clin Sleep Med 2013;9(12):1291–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Orzech K, Grandner MA, Roane BM, et al. Electronic media use within 2 hours of bedtime predicts sleep variables in college students. Sleep 2012; 35(Abstract Suppl):A73. [Google Scholar]
- 247.Chang AM, Aeschbach D, Duffy JF, et al. Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proc Natl Acad Sci U S A 2015;112(4): 1232–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Weaver E, Gradisar M, Dohnt H, et al. The effect of presleep video-game playing on adolescent sleep. J Clin Sleep Med 2010;6(2):184–9. [PMC free article] [PubMed] [Google Scholar]
- 249.NHTSA. Drowsy driving. Wahinton, DC: US Department of Transportation; 2011. [Google Scholar]
- 250.Strohl KP, Blatt J, Council F, et al. Drowsy driving and automobile crashes: NCSDR/NHTSA expert panel on driver fatigue and sleepiness. Washington, DC: National Highway Traffic Safety Administration; 1998. [Google Scholar]
- 251.Borman KR, Biester TW, Jones AT, et al. Sleep, supervision, education, and service: views of junior and senior residents. J Surg Educ 2011;68(6): 495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Borman KR, Fuhrman GM. “Resident duty hours: enhancing sleep, supervision, and safety”: response of the Association of Program Directors in Surgery to the December 2008 report of the Institute of Medicine. Surgery 2009;146(3):420–7. [DOI] [PubMed] [Google Scholar]
- 253.Sataloff RT. Resident duty hours: concerns and consequences. Ear Nose Throat J 2009;88(3): 812–6. [PubMed] [Google Scholar]
- 254.Federal Aviation Administration. Fact sheet–sleep apnea in aviation. Washington, DC: FAA; 2015. [Google Scholar]
- 255.McKnight-Eily LR, Liu Y, Wheaton AG, et al. Unhealthy sleep-related behaviors—12 States, 2009. MMWR Morb Mortal Wkly Rep 2011;60(8):233–8. [PubMed] [Google Scholar]
- 256.Lufi D, Tzischinsky O, Hadar S. Delaying school starting time by one hour: some effects on attention levels in adolescents. J Clin Sleep Med 2011;7(2): 137–43. [PMC free article] [PubMed] [Google Scholar]
- 257.Moore M, Meltzer LJ. The sleepy adolescent: causes and consequences of sleepiness in teens. Paediatr Respir Rev 2008;9(2):114–20 [quiz: 120–1]. [DOI] [PubMed] [Google Scholar]
- 258.Wahlstrom K School start time and sleepy teens. Arch Pediatr Adolesc Med 2010;164(7):676–7. [DOI] [PubMed] [Google Scholar]
- 259.Wolfson AR, Spaulding NL, Dandrow C, et al. Middle school start times: the importance of a good night’s sleep for young adolescents. Behav Sleep Med 2007;5(3):194–209. [DOI] [PubMed] [Google Scholar]
- 260.Roenneberg T, Kuehnle T, Pramstaller PP, et al. A marker for the end of adolescence. Curr Biol 2004;14(24):R1038–9. [DOI] [PubMed] [Google Scholar]
- 261.Barnes M, Davis K, Mancini M, et al. Setting adolescents up for success: promoting a policy to delay high school start times. J Sch Health 2016; 86(7):552–7. [DOI] [PubMed] [Google Scholar]
- 262.Meltzer LJ, Shaheed K, Ambler D. Start later, sleep later: school start times and adolescent sleep in homeschool versus public/private school students. Behav Sleep Med 2016;14(2):140–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Millman RP, Boergers J, Owens J . Healthy school start times: can we do a better job in reaching our goals? Sleep 2016;39(2):267–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Minges KE, Redeker NS. Delayed school start times and adolescent sleep: a systematic review of the experimental evidence. Sleep Med Rev 2016;28:86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Thacher PV, Onyper SV. Longitudinal outcomes of start time delay on sleep, behavior, and achievement in high school. Sleep 2016;39(2): 271–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Wheaton AG, Chapman DP, Croft JB. School start times, sleep, behavioral, health, and academic outcomes: a review of the Literature. J Sch Health 2016;86(5):363–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Siebern AT, Manber R. Insomnia and its effective non-pharmacologic treatment. Med Clin North Am 2010;94(3):581–91. [DOI] [PubMed] [Google Scholar]
- 268.Watson NF, Horn E, Duncan GE, et al. Sleep duration and area-level deprivation in twins. Sleep 2016;39(1):67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Knutson KL, Van Cauter E, Rathouz PJ, et al. Association between sleep and blood pressure in midlife: the CARDIA sleep study. Arch Intern Med 2009;169(11):1055–61. [DOI] [PMC free article] [PubMed] [Google Scholar]