Abstract
Background:
Despite historically high rates of herpes zoster among people living with HIV (PLWH), comparative studies of herpes zoster by HIV serostatus are lacking since the advent of combination antiretroviral therapy and availability of zoster vaccine.
Methods:
Annual rates (2002–2015) of first-episode herpes zoster and zoster vaccination were calculated for PLWH and uninfected adults in the Veterans Aging Cohort Study and stratified by HIV serostatus and age. Herpes zoster was captured using ICD9 codes and vaccine receipt with procedural codes and pharmacy data.
Results:
Of 45,177 PLWH and 103,040 uninfected veterans, rates of herpes zoster decreased among PLWH (17.6 to 8.1/1000) over the study period but remained higher than uninfected adults (4.1/1000) at end of study period. Rates were higher in PLWH with lower CD4 (<200 vs >500 cells/μL: 18.0 vs 6.8/1000) and unsuppressed vs suppressed HIV-1 RNA (21.8 vs 7.1/1000). Restricted to virologically suppressed participants with CD4 >350 cells/μL, herpes zoster rates were similar among PLWH age <60 and ≥ 60 in 2015 (6.6 vs 6.7/1000) but higher than all uninfected age groups. At study end, cumulative receipt of zoster vaccine for PLWH ≥ 60 years was less than half that of uninfected veterans: 98.7 vs. 215.2/1000.
Conclusion:
Herpes zoster rates among PLWH have markedly decreased, but, even in cART-treated individuals, remain 50% higher than uninfected adults. Lower rates of zoster vaccine receipt combined with high rates of herpes zoster support the need for a safe and effective vaccine against herpes zoster for PLWH, formal zoster vaccine guidelines for PLWH, and consideration for expanded use at younger ages.
Keywords: HIV, herpes zoster, herpes zoster vaccine
Background:
Varicella-zoster virus is the causative agent of both varicella and herpes zoster diseases. Herpes zoster results from reactivation of latent virus within dorsal root ganglia 1. Herpes zoster is a unique vaccine-preventable disease in that risk of disease is not associated with exposure, but rather with host factors that influence reactivation, especially increasing age and immunosuppression. Declining varicella-zoster virus-specific cell-mediated immunity is thought to explain the increased incidence of herpes zoster in people >50 years of age or with immunosuppression 2,3.
In the era before combination antiretroviral therapy (cART), HIV infection was associated with a 12 to 17-fold increased risk of herpes zoster compared to uninfected adults 4. The subsequent decline in herpes zoster incidence over time has been attributed to improved immunologic function with cART, but the risk remains elevated, especially when the CD4 T-cell count remains depressed 5–7. Furthermore, even with cART, herpes zoster incidence continues to be higher in people living with HIV (PLWH) compared to uninfected individuals 8.
Prior to late 2017, there was only one licensed vaccine to prevent herpes zoster. The zoster vaccine live (ZVL) was recommended by the Advisory Committee on Immunization Practices (ACIP) in 2008 for use as a one-time vaccination in immune competent adults age ≥60 years with a warning to avoid in immunocompromised people 9. This recommendation was based on a large study that showed the vaccine reduced the risk of developing herpes zoster by 51.3% 10. Despite guidelines for ZVL (the only herpes zoster vaccine available until 2018), rates of vaccination in the United States were low in the general population, being less than 35.0% as of 2015 11 and even lower among HIV-uninfected veterans age ≥ 60 years, with only 6.4% vaccination coverage as of 2015 12.
The cardinal study evaluating efficacy of the ZVL, the Shingles Prevention Study, excluded immunosuppressed patients due to the vaccine being a live-attenuated vaccine 10. As a result, rates of zoster vaccination are even lower in immunocompromised populations and there is a paucity of literature regarding administration in these populations, including PLWH. However, a review of patients who received ZVL after hematopoietic cell transplant (HCT) (median time to vaccination was 482 and 1,323 days for autologous and allogeneic for HCT recipients, respectively) demonstrated no adverse events with ZVL and the authors concluded that ZVL can be administered to select patients with hematologic malignancy after HCT who are on minimal to no immunosuppressive therapy 13. Among PLWH, a small study demonstrated that the ZVL was safe and immunogenic in children with a CD4 T-cell % ≥15% 14. Recently, two doses of ZVL were also shown to be safe and immunogenic in adults living with HIV with a CD4 T-cell count ≥200cells/μL 15.
Despite data on safety and immunogenicity, vaccine uptake among PLWH has been low 16. Lack of or unclear guidelines regarding herpes zoster vaccination in PLWH have been implicated as one reason for low rates of vaccination in PLWH 16,17. The ACIP and World Health Organization (WHO) guidelines do not have a recommendation for zoster vaccination in PLWH, even with CD4 count ≥ 200 cells/uL 18,19. The Infectious Disease Society of America (IDSA) guidelines for immunocompromised individuals were corrected to include a recommendation on administration of ZVL to asymptomatic PLWH ≥60 years who are VZV seropositive with a CD4 T-cell count ≥200 cells/uL 20,21. Similarly, the British HIV association guidelines recommend administration of ZVL to asymptomatic PLWH ≥70 years who are VZV seropositive with a CD4 T-cell count ≥200 cells/uL 22. ZVL is contraindicated when the CD4 T-cell count is <200 cells/uL 20,21.
The objective of this study was to compare rates of herpes zoster incidence and vaccine incidence by HIV status over time. These data will assess the need for specific guidelines for herpes zoster vaccination in HIV.
Methods:
Study Population
VACS is a periodically updated prospective observational cohort identified from the US Department of Veterans Affairs that includes > 50,000 PLWH and > 100,000 uninfected adults. Our study population comprised adults living with and without HIV who were in VA care between 2002 and 2015.
Outcomes
Annual herpes zoster events were identified based on ICD9 codes (053.0, 053.11, 053.2x, 053.7x, 053.8, 053.9). Participants with a prior history of herpes zoster were excluded. Herpes zoster vaccine administration was captured using pharmacy data and CPT code 90736. The only herpes zoster vaccine available during this study time period was ZVL.
Covariates were captured both at study start (baseline) and for every fiscal year. Age was recorded in years. HIV-specific covariates were CD4 T-cell count (cells/μL) and HIV-1 viral load (RNA copies/mL). Virologic suppression was defined as ≤ 400 copies /mL to allow for a single definition across all years. Descriptive characteristics and co-morbidities were defined as in previously published VACS studies 23–26.
Statistical Analysis
Annual incidence rates (n/1000 and 95% confidence interval) of first event herpes zoster and herpes zoster vaccine receipt were determined from fiscal year 2002 (beginning 10/1/2001) to fiscal year 2015 (ending 9/30/2015). Eligible participants for each year were alive at the end of the fiscal year and had a VA outpatient visit within that fiscal year. Results were further stratified by age, CD4 T-cell category, and suppressed/unsuppressed HIV-1 viral load. A sub-analysis examining gender differences demonstrated no statistically significant differences, but was underpowered. For annual zoster vaccine rates, participants with prior history of zoster vaccine administration within another year during the study period were excluded from the annual analysis but included in cumulative analyses. Those with a CD4 T-cell count of <200 cells/μL were excluded from the zoster vaccine rate analysis.
Results:
Of 148,217 participants included in this study, 45,177 were PLWH and 103,040 were uninfected adults; median baseline ages were 48 (IQR 42,55) and 49 (IQR 42,55) years, respectively. At study entry, 46.4% of the cohort was ≥ 50 years old, 97.2% were male, 47.2% were black and 40.0% white (Table 1). PLWH were more likely to be current smokers, have hepatitis B or C virus, end-stage or chronic renal disease, and were more likely to have died by 09/30/2015. Alcohol-use disorders were similar by HIV serostatus. Only 49.9% of PLWH were on cART at study start in 2002, however, this increased to 81.7% by 2015. From 2002 until 2015, median CD4 T-cell count increased from 385 to 563 cells/μL and percent with a suppressed HIV-1 RNA level increased from 29.7% to 73.5%. HIV specific data by each year can be viewed in Table 2.
Table 1:
Patient Characteristics at Study Entry
| Overall | Uninfected | PLWH | |||||
|---|---|---|---|---|---|---|---|
| Variables | N=148217 | % | N=103040 | % | N=45177 | % | p value |
| Median Age, IQR | 49 (42, 55) | n/a | 49 (42, 55) | n/a | 48 (42, 55) | n/a | <0.001 |
| Age ≥50 | 68854 | 46.5 | 48096 | 46.7 | 20758 | 46.0 | 0.01 |
| Age ≥60 | 17709 | 12.0 | 12312 | 12.0 | 5397 | 12.0 | 0.99 |
| Male | 144085 | 97.2 | 100142 | 97.2 | 43943 | 97.3 | |
| Race/Ethnicity: | 0.0005 | ||||||
| White | 59211 | 40.0 | 41247 | 40.0 | 17964 | 39.8 | |
| Black | 69898 | 47.2 | 48299 | 46.9 | 21599 | 47.8 | |
| Latino | 12195 | 8.2 | 8636 | 8.4 | 3559 | 7.9 | |
| Other | 6913 | 4.7 | 4858 | 4.7 | 2055 | 4.6 | |
| Smoking status: | <0.001 | ||||||
| Never | 41909 | 28.3 | 29808 | 28.9 | 12101 | 26.8 | |
| Current | 77323 | 52.2 | 52035 | 50.5 | 25288 | 56.0 | |
| Former | 23763 | 16.0 | 17370 | 16.9 | 6393 | 14.2 | |
| Median BMI (kg/m2), IQR | 28 (24, 31) | n/a | 29 (25, 32) | n/a | 25 (23, 28) | n/a | <0.001 |
| Comorbidities: | |||||||
| Hepatitis B | 1967 | 1.3 | 581 | 0.6 | 1386 | 3.1 | <0.001 |
| Hepatitis C | 26828 | 18.1 | 13921 | 13.5 | 12907 | 28.6 | <0.001 |
| Cirrhosis | 651 | 0.4 | 454 | 0.4 | 197 | 0.4 | 0.90 |
| Diabetes | 42210 | 28.5 | 33362 | 32.4 | 8848 | 19.6 | <0.001 |
| Alcohol-related diagnosis | 17972 | 12.1 | 12462 | 12.1 | 5510 | 12.2 | 0.58 |
| Drug- related diagnosis | 16392 | 11.1 | 10445 | 10.1 | 5947 | 13.2 | <0.001 |
| CKD | 4915 | 3.3 | 2982 | 2.9 | 1933 | 4.3 | <0.001 |
| ESRD | 2133 | 1.4 | 1217 | 1.2 | 916 | 2.0 | <0.001 |
| History of malignancy | 868 | 0.6 | 582 | 0.6 | 286 | 0.6 | 0.11 |
| Died during follow-up | 32078 | 21.6 | 18771 | 18.2 | 13307 | 29.5 | <0.001 |
PLWH, people living with HIV; IQR, interquartile range; BMI, body mass index; ESRD, end stage renal disease; CKD, chronic kidney disease
Table 2:
HIV Specific Data by Year
| Year | Percent of PLWH on ART | CD4 T-Cell Count (cell/μL) | Percent with suppressed HIV-1 RNA |
|---|---|---|---|
| 2002 | 49.9 | 385 (217, 595) | 29.7 |
| 2003 | 51.8 | 396 (228, 596) | 31.2 |
| 2004 | 53.6 | 402 (237, 601) | 33.8 |
| 2005 | 56.5 | 410 (247, 607) | 37.8 |
| 2006 | 58.8 | 420 (258, 620) | 41.3 |
| 2007 | 61.0 | 435 (272, 638) | 45.1 |
| 2008 | 62.6 | 442 (278, 639) | 45.1 |
| 2009 | 64.2 | 462 (293, 665) | 53.4 |
| 2010 | 65.8 | 478 (308, 679) | 56.8 |
| 2011 | 68.7 | 494 (321, 695) | 60.6 |
| 2012 | 71.9 | 510 (332, 711) | 64.2 |
| 2013 | 75.1 | 525 (345, 731) | 66.4 |
| 2014 | 78.0 | 548 (366, 757) | 69.6 |
| 2015 | 81.7 | 563 (378, 780) | 73.5 |
PLWH, people living with HIV. ART, antiretroviral therapy. CD4 recorded as median (IQR).
Herpes zoster events
Between 2002 and 2015, 8,017 cases of first-time diagnoses of herpes zoster were reported. Average incidence rates over the study period were markedly higher among PLWH (4,749 cases of herpes zoster detected for an average rate 14.1/1000) compared to uninfected (3,268 cases for an average rate 3.4/1000). From 2002 to 2015, annual rates of herpes zoster decreased among PLWH (17.6 to 8.1/1000) and increased among uninfected (2.3 to 4.1/1000). By the end of the study period in 2015, rates remained significantly higher among PLWH compared to uninfected (8.1/1000 vs 4.1/1000, p<0.001), Table 3.
Table 3:
Rates of Herpes Zoster for Each Year by HIV serostatus and then by CD4 T-cell Count Strata and Viral Suppression in PLWH
| Year | Uninfected Rate/1000 (95% CI) | PLWH Rate/1000 (95% CI) | Rate/1000 (95% CI) by CD4 T-Cell Count (cells/μL) | Rate/1000 (95% CI) by HIV-1 RNA (copies/mL) | ||||
|---|---|---|---|---|---|---|---|---|
| ≤200 | 201–350 | 351–500 | >500 | Suppressed | Unsuppressed | |||
| 2002 | 2.3 (2.0, 2.7) | 17.6 (15.9, 19.2) | 38.0 (31.1, 44.8) | 21.7 (16.4, 26.9) | 20.2 (15.0, 25.5) | 14.9 (11.5, 18.3) | 16.4 (13.4, 19.4) | 29.7 (25.6, 33.8) |
| 2003 | 2.4 (2.0, 2.7) | 17.4 (15.7, 19.0) | 31.0 (24.5, 37.1) | 27.7 (21.8, 33.6) | 18.7 (13.9, 23.5) | 14.1 (10.9, 17.3) | 14.0 (11.3, 16.6) | 29.1 (25.1, 33.1) |
| 2004 | 2.7 (2.3, 3.1) | 17.0 (15.3, 18.6) | 33.1 (26.5, 39.6) | 22.3 (17.2, 27.3) | 14.7 (10.5, 19.0) | 13.5 (10.4, 16.4) | 13.9 (11.3, 16.5) | 26.5 (22.7, 30.3) |
| 2005 | 2.6 (2.2, 3.0) | 16.6 (15.0, 18.2) | 30.6 (24.2, 37.1) | 25.7 (20.3, 31.1) | 19.3 (14.6, 24.0) | 13.4 (10.5, 16.4) | 13.8 (11.3, 16.2) | 30.3 (26.1, 34.5) |
| 2006 | 2.6 (2.2, 2.9) | 16.6 (15.0, 18.2) | 29.7 (23.1, 36.3) | 24.4 (19.1, 29.7) | 17.7 (13.3, 22.0) | 14.7 (11.7, 17.8) | 13.2 (10.9, 15.5) | 31.1 (26.7, 35.6) |
| 2007 | 2.9 (2.5, 3.3) | 15.6 (14.0, 17.1) | 33.7 (26.4, 41.0) | 20.8 (15.9, 25.8) | 15.7 (11.6, 19.8) | 10.5 (8.0, 13.0) | 11.5 (9.4, 13.6) | 28.2 (23.7, 32.7) |
| 2008 | 3.6 (3.2, 4.1) | 15.7 (14.1, 17.2) | 31.8 (24.7, 38.9) | 15.3 (11.1, 19.6) | 19.6 (15.1, 24.1) | 13.3 (10.6, 16.1) | 13.9 (11.7, 16.1) | 28.6 (23.8, 33.3) |
| 2009 | 3.9 3.4, 4.3) | 14.4 (12.9, 15.9) | 34.6 (26.7, 42.4) | 18.9 (14.2, 23.6) | 14.7 (10.8, 18.6) | 9.5 (7.3, 11.6) | 10.7 (8.8, 12.5) | 31.0 (25.7, 36.3) |
| 2010 | 3.4 (3.0, 3.9) | 13.4 (11.9, 14.8) | 20.6 (14.2, 27.0) | 22.0 (16.9, 27.1) | 15.5 (11.6, 19.5) | 11.6 (9.3, 14.0) | 11.5 (9.7, 13.3) | 29.5 (24.0, 34.9) |
| 2011 | 3.6 (3.1, 4.0) | 13.3 (11.9, 14.8) | 31.6 (23.7, 39.6) | 17.5 (12.8, 22.2) | 12.1 (8.7, 15.6) | 11.5 (9.3, 13.7) | 11.6 (9.9, 13.4) | 29.1 (23.4, 34.8) |
| 2012 | 4.1 (3.6, 4.5) | 12.4 (11.0, 13.8) | 23.0 (16.0, 29.9) | 14.6 (10.2, 18.9) | 12.8 (9.3, 16.4) | 10.2 (8.2, 12.2) | 9.7 (8.1, 11.2) | 28.3 (22.4, 34.1) |
| 2013 | 4.7 (4.2, 5.2) | 10.0 (8.7, 11.2) | 19.8 (13.1, 26.5) | 12.2 (8.2, 16.2) | 12.8 (9.2, 16.4) | 7.0 (5.4, 8.6) | 9.0 (7.5, 10.4) | 16.8 (12.1, 21.5) |
| 2014 | 4.6 (4.0, 5.1) | 9.3 (8.0, 10.5) | 28.2 (19.7, 36.7) | 10.6 (6.7, 14.5) | 10.0 (6.8, 13.2) | 6.5 (5.0, 8.1) | 7.1 (5.8, 8.4) | 24.2 (18.2, 30.3) |
| 2015 | 4.1 (3.6, 4.6) | 8.1 (6.9, 9.2) | 18.0 (10.8, 25.1) | 14.4 (9.8, 19.0) | 7.5 (4.6, 10.3) | 6.8 (5.3, 8.3) | 7.1 (5.8, 8.3) | 21.8 (15.5, 28.1) |
PLWH, people living with HIV. Suppressed defined as a HIV-1 RNA ≤400 copies/mL and unsuppressed as a HIV-1 RNA >400 copies/mL. CI, confidence interval.
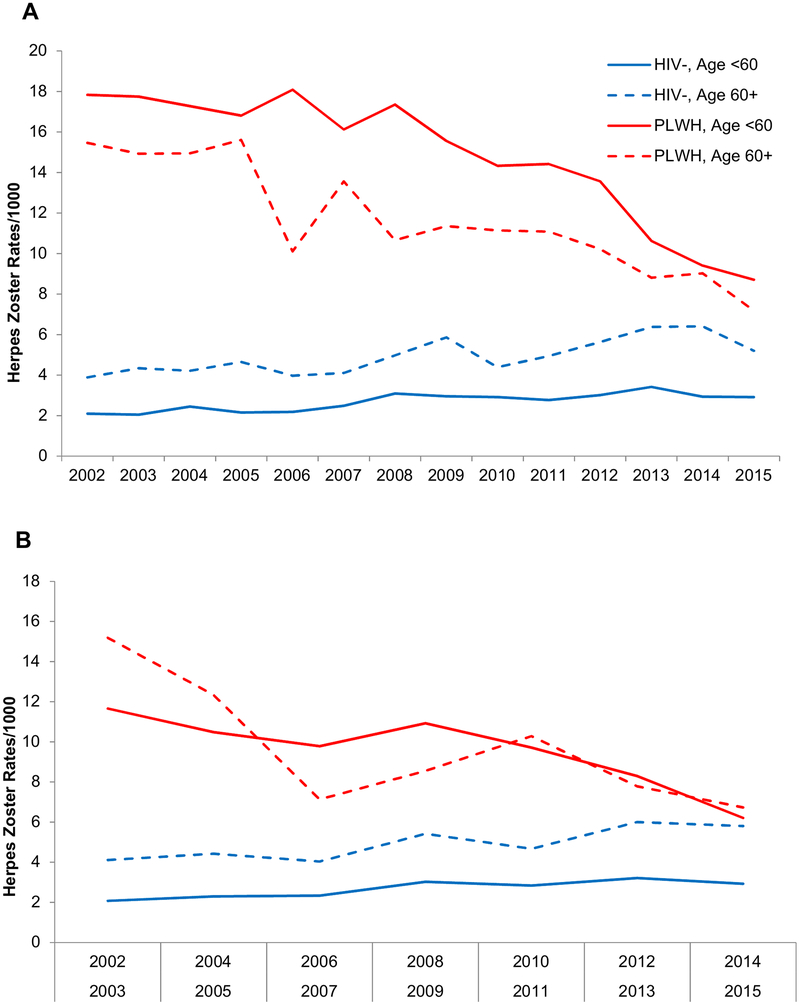
At end of study period (2015) the herpes zoster rates were highest among PLWH <60 years (8.7/1000) and lowest among uninfected adults <60 years (2.9/1000) (Figure 1, Panel A). In 2015, herpes zoster rates were highest among 50–59 year old PLWH (9.7/1000) and, among uninfected, in the 60–69 year old group (5.2/1000). In analyses restricted to PLWH, herpes zoster rates were highest among those with the lowest CD4 T-cell counts (CD4 <200 cells/μL: 18.0; CD4 201–350: 14.4; CD4 351–500: 7.5; >500: 6.8/1000) and higher among those with unsuppressed compared with suppressed HIV-1 RNA (21.8 vs 7.1/1000). In analyses restricted to virologically suppressed PLWH with CD4 T-cell >350 cells/μL, herpes zoster rates were similar among PLWH age <60 and age ≥ 60 (6.6 vs 6.7/1000) but remained higher than among uninfected (Figure 1, Panel B). The 2015 rates of herpes zoster by CD4 T-cell count strata and virologic suppression and then by HIV serostatus group by different age groups are shown in Tables 3 and 4, respectively.
Figure 1.
A, Herpes zoster rates by age and HIV serostatus over study period (2002–2015). B, Herpes zoster rates by age and HIV serostatus over study (2002–2015) when PLWH restricted to CD4 T-cell >350 cells/μL and HIV-1 RNA ≤400 copies/mL. PLWH: people living with HIV.
Table 4:
Herpes Zoster Rates by HIV Serostatus and Age Group in 2015
| Age | PLWH Rate/1000 (95% CI) | Uninfected Rate/1000 (95% CI) |
|---|---|---|
| 20–39 | 7.0 (3.5, 10.5) | 2.1 (0.5, 3.6) |
| 40–49 | 7.6 (4.6, 10.5) | 2.9 (1.6, 4.1) |
| 50–59 | 9.7 (7.5, 11.8) | 3.1 (2.3, 3.8) |
| 60–69 | 7.9 (5.9, 9.9) | 5.2 (4.3, 6.1) |
| ≥70 | 4.8 (2.0, 9.9) | 5.1 (3.5, 6.7) |
| Overall | 8.1 (6.9, 9.2) | 4.1 (3.6, 4.6) |
PLWH, people living with HIV. CI, confidence interval.
Zoster vaccine events
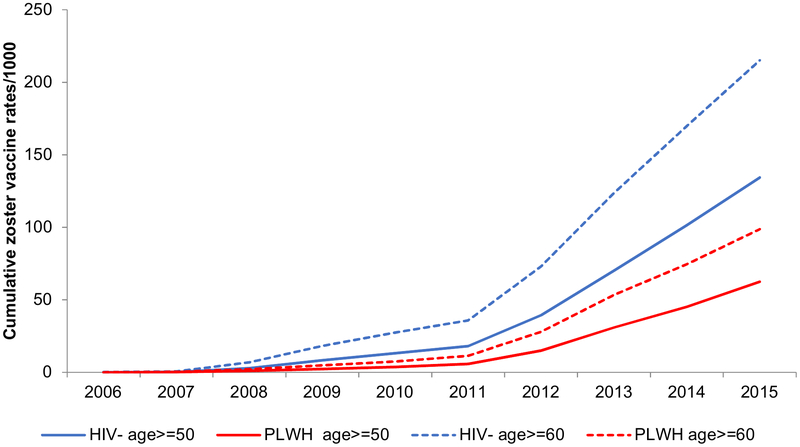
From 2006 to 2015, zoster vaccine was administered to 1,399 PLWH and 8,657 uninfected ≥ age 50 years. This included: 551 veterans between ages 50–59 years, 7440 between ages 60–69 years and 2064 aged ≥ 70 years. Vaccination rates increased steadily over the study period for both PLWH and uninfected (Figure 2), with the largest increase in rates of zoster vaccine seen between 2011 and 2012. By 2015, cumulative zoster vaccination was less than half for PLWH compared to uninfected: 62.5 vs 134.3/1000 among age ≥ 50 years and 98.7 vs 215.1/1000 among those ≥ 60 years (Figure 2).
Figure 2.
Cumulative herpes zoster vaccination by age and HIV serostatus. PLWH: people living with HIV.
Discussion:
Our study confirms and updates recent analyses showing that herpes zoster rates remain higher in PLWH compared to uninfected adults, despite virologic suppression and higher CD4 T-cell counts with the advent of cART 7,8,27,28. The striking inverse association between herpes zoster with CD4 T-cell count and viral suppression among PLWH (Table 3) emphasizes the importance of early cART initiation in decreasing the burden of herpes zoster. More importantly, we show that PLWH across all age groups have a greater risk of herpes zoster than uninfected, with the highest rates among those aged 50–59 years of age. Interestingly, when the analysis was restricted to those with HIV VL ≤400 copies/mL and CD4 T-cell count greater than 350 cells/μL, the age differences were attenuated, suggesting that among PLWH, HIV-related immunosuppression is a more important determinant of herpes zoster risk than age 29.
Our study demonstrated that rates of herpes zoster have steadily declined among adults living with HIV. Previous studies have also shown this trend, likely due to higher CD4 T-cell counts at HIV diagnosis, and improved virologic suppression with cART 7,8. The effect of vaccination on herpes zoster incidence among adults living with HIV has not been reported. Among adults without HIV, despite the introduction of the herpes zoster vaccine, rates of herpes zoster increased with increasing age, as has been previously reported in the literature 30–33. The cause of increasing rates of herpes zoster among adults without HIV is likely multifactorial, and may be the result of childhood varicella vaccination resulting in lower exposure among adults to primary varicella and subsequent boosting effect in addition to increasing use and strength of immunosuppressive medications 33.
Despite vaccine availability in the US since 2006, herpes zoster vaccination in the general population has been low, with the percentage of vaccinated, eligible adults ranging anywhere from 6–30% 11,12. Indeed, our study showed, among veterans, a cumulative vaccination coverage of 21.5% in uninfected adults ≥ 60 years and rates of < 10.0% among PLWH. There is a lack of published data regarding use of herpes zoster vaccination among PLWH though a recent study evaluating zoster vaccination rates at 6 different institution based clinics (three VA centers, a Canadian academic center, an University-based clinic, and an urban safety-net hospital) demonstrated zoster vaccine coverage rates anywhere from 1.5–42.5% among PLWH 16.
There are many potential reasons underlying the slow uptake of herpes zoster vaccination among PLWH. Lack of knowledge regarding the much higher incidence of herpes zoster in PLWH, safety concerns with ZVL, unclear guidelines, and practical barriers including billing and out-of-pockets costs have been implicated as impediments to herpes zoster vaccination among PLWH 17. Until recently, the only zoster vaccine available was ZVL, with a stated contraindication for use when CD4 T-cell count is less than 200 cells/uL 20,21. A recent publication has confirmed that 2 doses of ZVL are safe and likely immunogenic in PLWH with a suppressed viral load and CD4 T-cell count >200 cells/ uL 15.
A non-live, adjuvanted, recombinant zoster vaccine (RZV) against herpes zoster was approved in late 2017 for immune competent individuals 34. The recent approval and rollout of RZV is a stimulus to reinvigorate the push to vaccinate for this preventable disease. The ACIP recommends RZV for immunocompetent adults ≥ 50 years. RZV is a 2-dose vaccine with a reported efficacy in the general population of 97.2% 34. Further it retains excellent efficacy in people ≥70 years of age, with protection persisting for at least 4 years, whereas protection declines significantly after ZVL over a similar interval 35. A phase 1/2 study has already evaluated RZV in PLWH 36. The study included PLWH on cART with a CD4 T-cell count >200 cells/uL, cART-naïve PLWH with CD4 T-cell count >500 cells/uL, and PLWH on cART with a CD4 T-cell count between 50–199 cell/uL. RZV was safe and immunogenic compared to placebo but the study was insufficiently powered to make determinations about safety and immunogenicity among PLWH with a low CD4 T-cell counts or those not on cART 36. Additional studies are under way to evaluate the safety and immunogenicity of this vaccine in immunocompromised populations (NCT03493776, NCT01767467, NCT02058589, NCT01798056).
PLWH have diminished immunogenicity to certain vaccinations, and these effects are amplified by lack of viral suppression and low CD4 T-cell counts 37–40. Indeed, for many vaccinations it is advised to wait for the CD4 T-cell count to be >200 cells/uL or until there is a sustained rise in CD4 T-cell count prior to vaccinating to improve immune response to vaccination 41,42. As the greatest risk of herpes zoster among PLWH is in those with lower CD4 T-cell count and lack of viral suppression 5,6,43, an ideal vaccine candidate would be safe and provide lasting protection even when administered to those with lower CD4 T-cell counts. The immunogenicity of the RZV at age >70 years in the general population 35 may be a signal that this adjuvanted vaccine could provide protection against herpes zoster across a range of CD4 T-cell counts, though more studies are needed.
The strengths of this study include a large cohort for direct comparison of herpes zoster and herpes zoster vaccination rates among PLWH and uninfected adults, in an otherwise similar population. This study does have limitations: we used ICD9 codes to capture clinical events of first time zoster and a CPT code in addition to pharmacy data to capture herpes zoster vaccination and may have missed improperly coded clinical events or vaccination. A prior validation study of Veterans Affairs data in Atlanta demonstrated ICD9 codes were erroneous (PPV of 0.56) but still predicted true trends in incidence of zoster 32. The incidence of zoster vaccination may have been underestimated because our methods did not allow for inclusion of those who received vaccination outside of the VA system. Further, we were underpowered to evaluate efficacy of the ZVL due to the low numbers of vaccinated individuals. Additionally, data on adverse events or side effects related to administration of ZVL were not readily available. While our findings are likely generalizable, it is important to point out that the demographic and comorbid diseases in the veteran population may differ from patients who receive care outside Veterans Administration facilities.
In conclusion, this study highlights the continued higher rates of herpes zoster among PLWH compared with uninfected individuals. Rates of herpes zoster are high across all age groups of PLWH and are closely associated with both low CD4 T-cell counts and HIV-1 viremia. Herpes zoster vaccination uptake has been universally low, and rates of vaccination in PLWH are less than half of uninfected. The roll out of the RZV provides a new opportunity to study this vaccine in PLWH across all age and CD4 T-cell count spectra and develop vaccination guidelines for prevention of herpes zoster in PLWH.
Acknowledgments
Funding/Support and Conflicts of Interest:
This research was supported in part by the National Institutes of Health, through the National Institute on Alcohol Abuse and Alcoholism (U24-AA020794, U01-AA020790, U24-AA022001, and U10-AA13566-completed), the National Heart, Lung, and Blood Institute (T32HL116276 to KH, PI: James Hill and Bob Eckel), and the National Institute of Aging [K23AG050260] to KME. Additional funding was provided through the University of Colorado Department of Medicine Health Services Research Development Grant to KLH and in kind by the US Veterans Health Administration. The views expressed in this article are those of the authors and do not necessarily reflect the position or policies of the National Institutes of Health or the Department of Veterans Affairs.
MJL is on an Advisory Board and receives research funds from Merck & Co and GlaxoSmithKline, and shares intellectual property with Merck & Co. KME has received research funds (paid to the University of Colorado) from Merck & Co, Gilead Sciences, and EMD Serono.
References:
- 1.Weller TH. Varicella and herpes zoster. Changing concepts of the natural history, control, and importance of a not-so-benign virus. N Engl J Med. 1983;309(22):1362–1368. [DOI] [PubMed] [Google Scholar]
- 2.Weinberg A, Lazar AA, Zerbe GO, et al. Influence of age and nature of primary infection on varicella-zoster virus-specific cell-mediated immune responses. J Infect Dis. 2010;201(7):1024–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gershon AA, Gershon MD. Pathogenesis and current approaches to control of varicella-zoster virus infections. Clin Microbiol Rev. 2013;26(4):728–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchbinder SP, Katz MH, Hessol NA, et al. Herpes zoster and human immunodeficiency virus infection. J Infect Dis. 1992;166(5):1153–1156. [DOI] [PubMed] [Google Scholar]
- 5.Glesby MJ, Hoover DR, Tan T, et al. Herpes zoster in women with and at risk for HIV: data from the Women’s Interagency HIV Study. J Acquir Immune Defic Syndr. 2004;37(5):1604–1609. [DOI] [PubMed] [Google Scholar]
- 6.Levin MJ, Anderson JP, Seage GR 3rd, Williams PL. Short-term and long-term effects of highly active antiretroviral therapy on the incidence of herpes zoster in HIV-infected children. J Acquir Immune Defic Syndr. 2009;50(2):182–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moanna A, Rimland D. Decreasing incidence of herpes zoster in the highly active antiretroviral therapy era. Clin Infect Dis. 2013;57(1):122–125. [DOI] [PubMed] [Google Scholar]
- 8.Grabar S, Tattevin P, Selinger-Leneman H, et al. Incidence of herpes zoster in HIV-infected adults in the combined antiretroviral therapy era: results from the FHDH-ANRS CO4 cohort. Clin Infect Dis. 2015;60(8):1269–1277. [DOI] [PubMed] [Google Scholar]
- 9.Harpaz R, Ortega-Sanchez IR, Seward JF. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2008;57(Rr-5):1–30; quiz CE32–34. [PubMed] [Google Scholar]
- 10.Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352(22):2271–2284. [DOI] [PubMed] [Google Scholar]
- 11.Lu PJ, O’Halloran A, Williams WW, Harpaz R. National and State-Specific Shingles Vaccination Among Adults Aged >/=60 Years. Am J Prev Med. 2017;52(3):362–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moanna A, Rai R, Rimland D. Temporal Trends of Incidence of Herpes Zoster among Veterans: A National Analysis. IDWeek 2016; Abstract 628. [Google Scholar]
- 13.Naidus E, Damon L, Schwartz BS, Breed C, Liu C. Experience with use of Zostavax((R)) in patients with hematologic malignancy and hematopoietic cell transplant recipients. American J Hematol. 2012;87(1):123–125. [DOI] [PubMed] [Google Scholar]
- 14.Levin MJ, Gershon AA, Weinberg A, Song LY, Fentin T, Nowak B. Administration of live varicella vaccine to HIV-infected children with current or past significant depression of CD4(+) T cells. The J Infect Dis. 2006;194(2):247–255. [DOI] [PubMed] [Google Scholar]
- 15.Benson CA, Andersen JW, Macatangay BJC, et al. Safety and Immunogenicity of Zoster Vaccine Live in HIV-Infected Adults with CD4+ Cell Counts Above 200 Cells/mL Virologically Suppressed on Antiretroviral Therapy. Clin Infect Dis. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erlandson KM, Streifel A, Novin AR, et al. Low Rates of Vaccination for Herpes Zoster in Older People Living With HIV. AIDS Res Hum Retrovir. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aziz M, Kessler H, Huhn G. Providers’ lack of knowledge about herpes zoster in HIV-infected patients is among barriers to herpes zoster vaccination. Int J STD AIDS. 2013;24(6):433–439. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. Recommended Immunization Schedule for Adults Aged 19 Years or Older, United States, 2018. https://www.cdc.gov/vaccines/schedules/downloads/adult/adult-combined-schedule.pdf. Updated 2018. Accessed June 12, 2018. [Google Scholar]
- 19.HIV/AIDS treatment and care: clinical protocols for the WHO European region. World Health Organization, 2007, Copenhagen, Denmark. [Google Scholar]
- 20.Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58(3):309–318. [DOI] [PubMed] [Google Scholar]
- 21.Rubin LG et al. (Clin Infect Dis 2014; 58:e44–100). Clin Infect Dis. 2014;59(1):144–144. [Google Scholar]
- 22.Geretti AM. BHIVA Guidelines on the Use of Vaccines in HIV-Positive Adults 2015 British HIV Association, 2015. [DOI] [PubMed]
- 23.Butt AA, McGinnis K, Rodriguez-Barradas MC, et al. HIV infection and the risk of diabetes mellitus. AIDS. 2009;23(10):1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGinnis KA, Brandt CA, Skanderson M, et al. Validating smoking data from the Veteran’s Affairs Health Factors dataset, an electronic data source. Nicotine Tob Res. 2011;13(12):1233–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kraemer KL, McGinnis KA, Skanderson M, et al. Alcohol problems and health care services use in human immunodeficiency virus (HIV)-infected and HIV-uninfected veterans. Med Care. 2006;44(8 Suppl 2):S44–51. [DOI] [PubMed] [Google Scholar]
- 26.Goulet JL, Fultz SL, McGinnis KA, Justice AC. Relative prevalence of comorbidities and treatment contraindications in HIV-mono-infected and HIV/HCV-co-infected veterans. AIDS. 2005;19 Suppl 3:S99–105. [DOI] [PubMed] [Google Scholar]
- 27.Blank LJ, Polydefkis MJ, Moore RD, Gebo KA. Herpes zoster among persons living with HIV in the current antiretroviral therapy era. J Acquir Immune Defic Syndr. 2012;61(2):203–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hung CC, Hsiao CF, Wang JL, et al. Herpes zoster in HIV-1-infected patients in the era of highly active antiretroviral therapy: a prospective observational study. Int J STD AIDS. 2005;16(10):673–676. [DOI] [PubMed] [Google Scholar]
- 29.Chen SY, Suaya JA, Li Q, et al. Incidence of herpes zoster in patients with altered immune function. Infection. 2014;42(2):325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yawn BP, Saddier P, Wollan PC, St Sauver JL, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc. 2007;82(11):1341–1349. [DOI] [PubMed] [Google Scholar]
- 31.Johnson BH, Palmer L, Gatwood J, Lenhart G, Kawai K, Acosta CJ. Annual incidence rates of herpes zoster among an immunocompetent population in the United States. BMC Infect Dis. 2015;15:502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rimland D, Moanna A. Increasing incidence of herpes zoster among Veterans. Clin Infect Dis. 2010;50(7):1000–1005. [DOI] [PubMed] [Google Scholar]
- 33.Kawai K, Yawn BP, Wollan P, Harpaz R. Increasing Incidence of Herpes Zoster Over a 60-year Period From a Population-based Study. Clin Infect Dis. 2016;63(2):221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lal H, Cunningham AL, Godeaux O, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372(22):2087–2096. [DOI] [PubMed] [Google Scholar]
- 35.Cunningham AL, Lal H, Kovac M, et al. Efficacy of the Herpes Zoster Subunit Vaccine in Adults 70 Years of Age or Older. N Engl J Med. 2016;375(11):1019–1032. [DOI] [PubMed] [Google Scholar]
- 36.Berkowitz EM, Moyle G, Stellbrink HJ, et al. Safety and immunogenicity of an adjuvanted herpes zoster subunit candidate vaccine in HIV-infected adults: a phase 1/2a randomized, placebo-controlled study. J Infect Dis. 2015;211(8):1279–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malaspina A, Moir S, Orsega SM, et al. Compromised B cell responses to influenza vaccination in HIV-infected individuals. J Infect Dis. 2005;191(9):1442–1450. [DOI] [PubMed] [Google Scholar]
- 38.Kim HN, Harrington RD, Crane HM, Dhanireddy S, Dellit TH, Spach DH. Hepatitis B vaccination in HIV-infected adults: current evidence, recommendations and practical considerations. Int J STD AIDS. 2009;20(9):595–600. [DOI] [PubMed] [Google Scholar]
- 39.Overton ET, Nurutdinova D, Sungkanuparph S, Seyfried W, Groger RK, Powderly WG. Predictors of immunity after hepatitis A vaccination in HIV-infected persons. J Viral Hepat. 2007;14(3):189–193. [DOI] [PubMed] [Google Scholar]
- 40.Nunes MC, Madhi SA. Safety, immunogenicity and efficacy of pneumococcal conjugate vaccine in HIV-infected individuals. Hum Vaccin Immunother. 2012;8(2):161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crum-Cianflone NF, Sullivan E. Vaccinations for the HIV-Infected Adult: A Review of the Current Recommendations, Part I. Infect Dis Ther. 2017;6(3):303–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crum-Cianflone NF, Wallace MR. Vaccination in HIV-infected adults. AIDS Patient Care STDS. 2014;28(8):397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jansen K, Haastert B, Michalik C, et al. Incidence and risk factors of herpes zoster among hiv-positive patients in the german competence network for HIV/AIDS (KompNet): a cohort study analysis. BMC Infect Dis. 2013;13:372. [DOI] [PMC free article] [PubMed] [Google Scholar]