Abstract
Bombesin-like receptor 3 (BRS3) is an orphan G protein-coupled receptor that regulates energy homeostasis and heart rate. We report that acute activation of Brs3-expressing neurons in the dorsomedial hypothalamus (DMHBrs3) increased body temperature (Tb), brown adipose tissue temperature, energy expenditure, heart rate and blood pressure, with no effect on food intake or physical activity. Conversely, activation of Brs3 neurons in the paraventricular nucleus of the hypothalamus (PVHBrs3) had no effect on Tb or energy expenditure, but suppressed food intake. Inhibition of DMHBrs3 neurons decreased Tb and energy expenditure, suggesting a necessary role in Tb regulation. We found that the preoptic area provides major input (excitatory and inhibitory) to DMHBrs3 neurons. Optogenetic stimulation of DMHBrs3 projections to the raphe pallidus (RPa) increased Tb. Thus, DMHBrs3→RPa neurons regulate Tb, energy expenditure and heart rate, and PVHBrs3 neurons regulate food intake. Brs3 expression is a useful marker for delineating energy metabolism regulatory circuitry.
Introduction
Bombesin-like receptor 3 (BRS3) is an orphan G-protein coupled receptor (GPCR) that is phylogenetically well-conserved and highly expressed in specific brain regions 1,2. BRS3 is a member of a GPCR subfamily that includes the neuromedin B and gastrin releasing peptide receptors. Mammalian BRS3 lacks a known endogenous high-affinity ligand and has a low affinity for bombesin, a frog skin peptide that is not found in mammals 3–6. Targeted deletion of Brs3 in mice causes obesity, mediated both by a decrease in energy expenditure and an increase in food intake, with a reduced resting body temperature (Tb) and resting heart rate 7–11. Concordantly, BRS3 agonists increase resting Tb, energy expenditure, heart rate, blood pressure, activate brown adipose tissue (BAT) and decrease food intake 4,12–15. The global BRS3 null phenotype is replicated by selective loss of Brs3 in neurons expressing vesicular glutamate transporter 2 (Vglut2) 16. Both the location of the responsible Brs3 neuron populations and the specific circuits directing this physiology remain to be elucidated.
Brs3 is found in a limited number of brain nuclei, mostly in the hypothalamus 1,12. Brs3-expressing nuclei implicated in the regulation of Tb, energy expenditure and food intake, include the dorsomedial hypothalamus (DMH), paraventricular nucleus of the hypothalamus (PVH), parabrachial nucleus (PBN) and preoptic area (POA) 1,12,17. Thus, Brs3 expression could be a valuable marker for elucidating the circuitry important for the treatment of metabolic diseases.
The central nervous system tightly regulates body temperature in mammals, with the purpose of maintaining homeostasis while adapting to changes in the external or internal milieu. Thermal physiology depends on the size of the organism. Large mammals are oriented towards heat loss, through environmental adaptation, vasodilation, sweating and/or panting. Small mammals focus on heat generation, including BAT activation and shivering. They also conserve heat using behavioral thermoregulation and vasoconstriction. Physiological changes to regulate Tb are initiated by sensation of the thermal environment, relayed via ascending sensory signals to the POA, which also harbors temperature-sensing neurons 18–21. The DMH is a pivotal nucleus in the output pathway of POA neurons, receiving direct preoptic input in response to cold 22–24, or warm 18,21 ambient temperatures to regulate sympathetic activation of BAT 22,24.
Acute manipulation of defined populations of DMH neurons (eg., expressing LepRb, Vglut2, Vgat, Chat) in awake animals can alter Tb, BAT temperature, physical activity and food intake 18,21,25–27. A thermoregulatory output pathway of the DMH is to the raphe pallidus (RPa), likely also driving cardiovascular sympathetic outflow 28–30. This sympathoexcitatory population of DMH→RPa neurons is located in the dorsal DMH (dDMH) and extends further dorsally into dorsal hypothalamic area (DHA) 31. Vglut2 and LepRb are neuroanatomical markers for DMH→RPa neurons 22,28,31,32. Here we elucidate the functions of Brs3 neurons in the DMH and PVH, focusing on regulation of Tb and energy expenditure, and also examining food intake and heart rate.
Results
Brs3 expression pattern
To study the functions of cells expressing Brs3, Brs3-2A-CreERT2 (Brs3-Cre) mice were produced by targeted insertion of tamoxifen-inducible Cre recombinase gene into the native Brs3 locus (Supplementary Fig. 1a). Brs3-Cre expression was visualized using a Cre-dependent tdTomato reporter line (Ai14). The concentration of tdTomato-positive neurons was highest in the POA, PVH, DMH, bed nucleus of the stria terminalis (BNST), medial posterodorsal amygdala (MePD) and lateral parabrachial nucleus (LPB) (Supplementary Fig. 1b,c, Supplementary Table 1). No tdTomato expression was observed without tamoxifen treatment. In situ hybridization demonstrated that most neurons expressing Brs3 mRNA also express tdTomato mRNA and vice versa (Supplementary Fig. 1d). The tdTomato expression pattern is consistent with that previously reported for Brs3 mRNA 1 and ligand binding 12. We conclude that the Cre expression in Brs3-Cre mice faithfully reproduces the endogenous Brs3 expression pattern.
Brs3 is located on the X chromosome and Brs3Cre undergoes X-inactivation (see Methods and Supplementary Fig. 2a). Due to the X-inactivation, all subsequent studies used male mice.
We next investigated the phenotype of mice carrying the Brs3Cre allele. Hypothalamic Brs3 mRNA expression was reduced by 41 ± 3 % (Supplementary Fig. 2b). Brs3-Cre mice showed no difference in baseline Tb (data not shown). However, on a chow diet, the Brs3-Cre mice showed a slight but statistically significant increase in body weight, fat mass, and lean mass, compared to littermate controls; these differences were magnified by a high fat diet (Supplementary Fig. 2c). This phenotype suggests that the Brs3Cre allele has reduced BRS3 function. However, the Brs3-Cre mice did respond to the selective BRS3 agonist MK-5046, by increasing Tb and reducing food intake (Supplementary Fig. 2d-e). Taken together, these data indicate that the Brs3Cre allele is a hypomorph, not a null.
Activation of Brs3 neurons by cold exposure, refeeding and leptin treatment
We examined the activation of Brs3 neurons in response to interventions that affect energy homeostasis. Cold exposure stimulated Fos expression in Brs3 neurons in the dDMH/DHA, medial preoptic area (MPA) and median preoptic area (MnPO), but not in the PVH, vDMH, or PBN (Fig. 1a,b). In contrast, refeeding after a fast increased Fos expression in Brs3 neurons in the PVH and PBN, but not in the dDMH/DHA, vDMH, MPA or MnPO (Fig. 1c,d). Thus, different subsets of Brs3 neurons are activated by cold exposure and by refeeding.
Figure 1. Brs3 neurons are differentially activated by exposure to a cold environment and by refeeding.
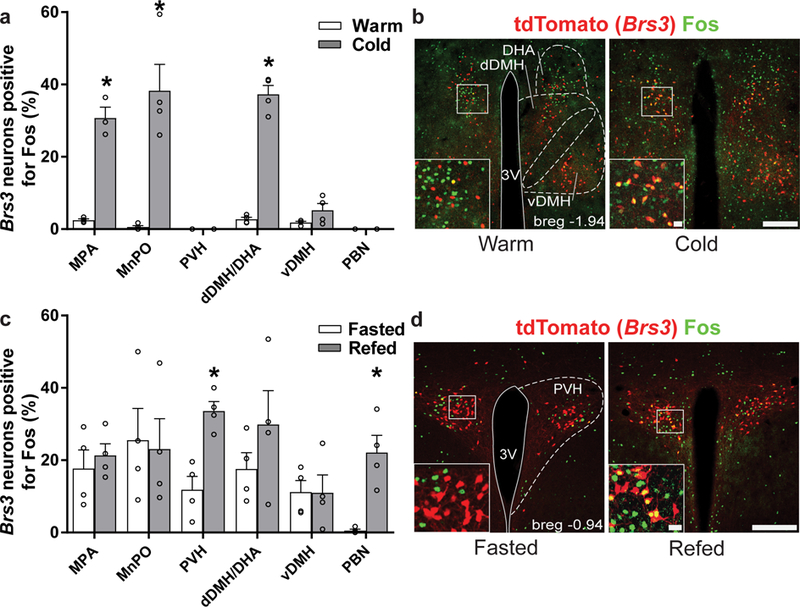
(a) Percentage of Brs3 neurons positive for Fos following cold (4 °C) or warm (30 °C) exposure. The environmental temperature did not affect total number of Brs3-positive neurons (100% is 171 ± 15 in the MPA, 125 ± 12 in the MnPO, 181 ± 15 in the PVH, 134 ± 10 in the dDMH/DHA, 255 ± 26 in the vDMH, and 214 ± 18 in the PBN; mean ± SEM). * indicates p<0.05 by unpaired two-sided t-test within region: MPA, p = 0.0001; MnPO, p = 0.002; dDMH/DHA, p = 0.0005. (b) Examples of DMH/DHA sections from Brs3-Cre;Ai14 mice stained for Fos after warm or cold exposure. (c) Percentage of Brs3 neurons positive for Fos following a 20h fast or fast and 2h of refeeding. The total number of Brs3-positive neurons was not affected by fasting or refeeding (100% is 166 ± 20 in the MPA, 99 ± 17 in the MnPO, 190 ± 9 in the PVH, 137 ± 12 in the dDMH/DHA, 232 ± 25 in the vDMH and 225 ± 18 in the PBN). * indicates p<0.05 by unpaired two-sided t-test within region: PVH, p = 0.003; PBN, p = 0.004. (d) Examples of Brs3-Cre;Ai14 PVH sections stained for Fos after fasting or refeeding. a,c. n = 4/group, with 2–3 sections per mouse. Data are mean + SEM. Scale bar in b,d 200 µm; 20 µm in inset.
Brs3 mRNA is enriched in leptin receptor-expressing neurons 33, so we investigated if leptin activates Brs3 neurons. Leptin treatment increased STAT3 phosphorylation in Brs3 neurons in the MPA, MnPO, dDMH/DHA and vDMH, but not the PVH or PBN (Supplementary Fig. 3a,b), indicating that these neurons can be activated by leptin, likely directly.
We selected the PVH and DMH for further investigation, based on the high density of Brs3 neurons, responses to stimuli and known role in energy balance regulation. Micro-infusion of the selective BRS3 agonist, MK-5046, into the DMH increased BAT temperature (TBAT), while drug infusion into the PVH did not (Supplementary Fig. 3c,d). No effect on TBAT was seen with vehicle infusion into either the DMH or PVH.
Activation of DMHBrs3 neurons increases Tb and energy expenditure; PVHBrs3 neuron activation suppresses food intake
The Fos and agonist data suggest distinct functions for the DMHBrs3 and PVHBrs3 populations. To further assess their roles in energy metabolism, we used chemogenetics to selectively activate either DMHBrs3 or PVHBrs3 neurons. Viral expression and function of the excitatory designer receptor exclusively activated by designer drug (DREADD) hM3Dq (Fig. 2a,b) was confirmed in ex vivo brain slices, where the agonist clozapine-N-oxide (CNO) appropriately depolarized and/or increased firing of DMHBrs3 or PVHBrs3 neurons (Supplementary Fig. 4a,b).
Figure 2. Activation of DMHBrs3 neurons increases Tb and activation of PVHBrs3 neurons suppresses food intake.

(a, b) Schematic of virus injection (left) and representative expression of hM3Dq-mCherry (right) in DMH (a) and PVH (b). Scale bar, 200 µm. (c, d) Tb, total energy expenditure (TEE), respiratory exchange ratio (RER: vCO2/vO2) and physical activity response to CNO (1 mg/kg) or vehicle in DMHBrs3::hM3Dq (c, n = 8) and PVHBrs3::hM3Dq (d, n = 7) mice at 30°C. For quantification, Pre is mean from −150 to −30 and Post from 60 to 180 min. * p values, paired two-sided t-test on change from baseline, CNO vs vehicle: DMH Tb, p = 0.003; DMH TEE, p = 0.005. (e, g) Blood glucose response to CNO (1 mg/kg) in ad lib fed DMHBrs3::hM3Dq (e; n = 8/group) and PVHBrs3::hM3Dq (g; n = 6/group) mice during light cycle. * indicates p = 0.02 by paired two-sided t-test, CNO vs vehicle. (f, h) Effect of CNO (1 mg/kg) on food intake (using average of two trials/mouse) at onset of dark cycle after 5 h fast in DMHBrs3::hM3Dq (f; n = 8/group) and PVHBrs3::hM3Dq (h; n = 6/group) mice. * indicates p = 0.008 by paired two-sided t-test, CNO vs vehicle. (c-h) Crossover design. Data are mean ± SEM in left panels of (c-e, g). Black (vehicle) and red (CNO) bars represent means.
In vivo, activation of DMHBrs3 neurons with CNO (1 mg/kg) increased light phase Tb by 1.13 ± 0.18 °C (vs 0.13 ± 0.09 °C with vehicle, p = 0.003; Fig. 2c). A higher CNO dose (3 mg/kg) produced a similar response, while a lower dose (0.3 mg/kg) elicited a shorter duration increase in Tb. BRS3 agonist MK-5046 increased Tb to a similar level as CNO (Supplementary Fig. 4c). CNO treatment of control mice lacking hM3Dq did not affect Tb or energy expenditure (data not shown). Neither MK-5046 nor CNO affected physical activity beyond the increase caused by handling and seen with vehicle treatment (Fig. 2c,d; Supplementary Fig. 4d). Chemogenetic activation of DMHBrs3 neurons increased energy expenditure by 33% (0.082 ± 0.017 kcal/h with CNO vs −0.003 ± 0.007 kcal/h with vehicle, p = 0.005; Fig. 2c). The duration of the increase in energy expenditure was shorter than the increase in Tb. Activation of DMHBrs3 neurons caused higher glucose levels (Fig. 2e, p = 0.02), but had no effect on food intake at the onset of the dark cycle following 5h of food deprivation (Fig. 2f). CNO did not significantly change plasma non-esterified fatty acid or glycerol levels (data not shown).
In contrast, activation of PVHBrs3 neurons did not alter Tb, energy expenditure, or blood glucose (Fig. 2 d,g). However, activation of PVHBrs3 neurons decreased food intake by 30.0 ± 3.6 % (Fig. 2h, p = 0.008). We also tested if inhibition of PVHBrs3 neurons could increase food intake, via viral expression of the inhibitory DREADD hM4Di (Supplementary Fig. 4e,f). Inhibition of PVHBrs3 neurons with CNO in calorically-replete mice during the light cycle increased food intake by 69 ± 16% (Supplementary Fig. 4g).
Taken together, these data demonstrate that activation of PVHBrs3 neurons does not change Tb or energy expenditure, but PVHBrs3 neurons can bi-directionally regulate food intake. In contrast, activation of DMHBrs3 neurons has no effect on food intake, but increased Tb and energy expenditure.
Adulthood ablation and acute inhibition of DMHBrs3 neurons reduce cold-induced thermogenesis
We next examined if ablation of the DMHBrs3 neurons in adulthood could affect long term body weight regulation and food intake. DMHBrs3 neurons were ablated by injections of a Cre-dependent diphtheria toxin A (DTA)-expressing AAV into Brs3-Cre mice (Supplementary Fig. 5a-c). In the EYFP control mice, we counted 531 ± 97 (range: 267 to 862) EYFP-positive (Brs3) neurons in the DMH vs 44 ± 19 (range: 0 to 132) in the EYFP+DTA mice, a 92% reduction. Body weight, food intake, energy expenditure, and fat mass were not different between the groups (Supplementary Fig. 5d-g). No difference was seen in baseline Tb or physical activity at 22 °C (Supplementary Fig. 5h,i). There was a modest reduction in Tb during cold (7° C) exposure in EYFP+DTA, compared to EYFP mice (Supplementary Fig. 5j). There was no difference in the Tb increase produced by the stress of placing the mouse in the empty cage previously occupied by an unfamiliar male (Supplementary Fig. 5k). The BRS3 agonist MK-5046 increased Tb to similar levels in both groups (Supplementary Fig. 5l). The mild Tb phenotype of the DMHBrs3-ablated mice may be due to incomplete ablation, adaptation to neuronal loss, and/or the existence of other Tb-regulating Brs3 neurons. Thus, adulthood loss of DMHBrs3 neurons did not affect body weight, food intake, or energy expenditure, but modestly impaired Tb defense during cold exposure.
To determine if acute inhibition of DMHBrs3 neurons affects cold-induced thermogenesis, we virally expressed the inhibitory DREADD hM4Di (Fig. 3a). In ex vivo brain slices, CNO decreased the firing rate and hyperpolarized DMHBrs3 neurons (Supplementary Fig. 6a). In vivo, CNO reduced Tb by 1.03 ± 0.27 °C vs a 0.21 ± 0.12 °C increase with vehicle (p = 0.02; Fig. 3b). CNO also reduced energy expenditure by 17 ± 2% compared to vehicle, with no effect on physical activity. No effect of CNO was observed in wild type mice injected with the DIO-hM4Di virus in the DMH (Supplementary Fig. 6c). No significant reduction in Tb occurred at an ambient temperature of 34 °C (Supplementary Fig. 6b), at which there is no cold-induced thermogenesis to inhibit. These data suggest that under standard housing conditions (~ 22 °C) DMHBrs3 neurons contribute to the neural tone regulating cold-induced thermogenesis.
Figure 3. Inhibition of DMHBrs3 neurons reduces TEE and Tb.

(a) Schematic of virus injection (left) and representative example of hM4Di-mCherry expression in DMH of Brs3-Cre;Ai6 mice (right). Scale bar, 500 µm. (b) Tb, TEE, RER (vCO2/vO2), and physical activity response to CNO (1 mg/kg) or vehicle in DMHBrs3::hM4Di mice at 22 °C (n = 6/group). Data are mean ± SEM, crossover design. * p values, paired two-sided t-test of the change from baseline, CNO vs vehicle: Tb, p = 0.02; TEE, p = 0.03. (c) Effect of CNO (1 mg/kg) in DMHBrs3::hM4Di mice on response to lipopolysaccharide (LPS) treatment (50 µg/kg, ip). (d) Effect of CNO (1 mg/kg) in DMHBrs3::hM4Di mice in response to cage switch, (n = 5 /group). Black rectangles at the top of the graph indicate p<0.05 by paired two-sided t-test, CNO vs vehicle, for individual data points, without multiplicity correction. (b,c) For quantification, Pre is mean from −150 to −30 and Post is 60 to 180 min. Black (vehicle) and red (CNO) bars represent means. (c,d) Data are mean + SEM, crossover design.
In addition to cold, other situations can increase Tb. We studied inhibition of DMHBrs3 neurons during lipopolysaccharide treatment, which causes fever via a systemic inflammatory response, and found a blunted Tb increase in CNO-treated mice (Fig. 3c). We next combined inhibition of DMHBrs3 neurons with the stress of being placed in a cage previously occupied by an unfamiliar male. The initial handling and cage switch similarly increased Tb in both groups, after which the CNO-treated mice showed a greater Tb reduction (Fig. 3d). These results are consistent with DMHBrs3 neurons being part of a common effector pathway for regulating Tb. Alternatively, they may supply a parallel signal that is integrated with other signals. This result rules out that intervention-activated circuits completely bypass or dominate over the capacity of DMHBrs3 neurons to regulate Tb.
Optogenetic activation of DMHBrs3 neurons increases Tb, heart rate, and blood pressure
Optogenetic activation of DMHBrs3 neurons allowed studying rapid physiological responses without handling the mice, which particularly confounds measurement of heart rate and blood pressure. We transduced DMHBrs3 neurons with AAV-Flex-ChR2-tdTomato (Fig. 4a) or control (AAV-DIO-mCherry) viruses. Stimulation of ChR2 in DMHBrs3 neurons increased neuronal firing in an ex vivo slice experiment (Supplementary Fig. 7a). In vivo, awake mice in their home cage were studied during five cycles of 20 minutes of photostimulation, followed by 60 minutes with the laser off (Fig. 4b). The photostimulation triggered a rapid and robust increase in Tb with no effect on physical activity. There was no attenuation or systematic difference in Tb as a function of cycle number, so the five cycles were averaged in all further experiments. No effect was observed in mCherry-expressing control mice. Increasing stimulation frequency produced dose-dependent increases in Tb (Supplementary Fig. 7b).
Figure 4. Optogenetic activation of DMHBrs3 neurons increases Tb, TBAT, HR and MAP, but not physical activity.

(a) Schematic of laser stimulation (left) and representative expression of ChR2 and optic fiber placement (dotted line) in DMH (right). Scale bar represents 200 µm. (b) Tb and physical activity during repeated cycles with 20 min epochs of optogenetic stimulation (blue interval) of DMHBrs3::ChR2 (n = 8) and DMHBrs3::mCherry (n = 6) mice. Data are mean + SEM. (c) Infrared camera interscapular (TBAT, top left) and lumbar (Tlumbar, middle left) skin temperature measurements during optogenetic stimulation (blue interval) of DMHBrs3::ChR2 (n = 8) and DMHBrs3::mCherry (n = 7) mice. Data are mean + SEM. For quantification of TBAT (top right) and Tlumbar (middle right), Off is −10 to −2 and On is 10 to 18 min. * p = 0.0001, paired two-sided t-test. Bottom left panel, difference between TBAT and Tlumbar. * p<0.03 at t = 4,6,8 min, unpaired two-sided t-test, DMHBrs3::ChR2 vs DMHBrs3::mCherry. Bottom right panel: initial rate of increase in TBAT and Tlumbar in DMHBrs3::ChR2 mice (from 0 to 4 min of laser on). * p = 0.002, paired two-sided t-test. (d) Heart rate (HR), mean arterial pressure (MAP), Tb and physical activity responses to laser (blue interval) in DMHBrs3::ChR2 and DMHBrs3::mCherry mice (n = 4/group) in left panels (data are mean ± SEM). Temperature data from subcutaneously and intraperitoneally implanted DSI transmitters were pooled and represented as Tb. Data (mean of 5 epochs) is normalized to −20 to 0 min (baseline). For quantification in right panels, Off is −10 to 0 and On is 11 to 20 min. * p values, paired two-sided t-test, Off vs On: ∆HR, p = 0.006; ∆MAP, p = 0.047; ∆Tb, p = 0.007. (e) Summary of Tb and physical activity in DMHBrs3::mCherry (black), DMHBrs3::ChR2 (red), DMH::ChR2 (violet), and DMHnon-Brs3::ChR2 (blue) mice. Data are means + SEM of data from Fig. 4b and Supplementary Fig. 9b. For quantification, the mean difference is between −10 to 0 and 11 to 20 min is used. * p<0.05 one-way ANOVA, with Tukey’s multiple comparison test. Laser stimulation (blue intervals) was alternating 1 s on (20 Hz, 10 ms pulses) with 3 s off for 20 min. (c, d) Bars are means (red: mCherry; black: ChR2).
The 20-minute stimulation duration allowed measurement of the full effect on Tb; shorter durations did not allow Tb to stabilize and longer durations did not increase it further (Supplementary Fig. 7c). Our standard stimulation used a 1 s on / 3 s off protocol (20 Hz, 10 ms pulses). Doubling the light dose (to 1 s on / 1 s off), doubled the initial rate of increase in Tb (Supplementary Fig. 7c). Thus, our standard stimulation protocol is not a maximal stimulus.
A primary effector mechanism for increasing Tb is sympathetic activation of brown adipose tissue, with other mechanisms being vasoconstriction and behavioral adaptation. To examine the effect of DMHBrs3 optogenetic activation on BAT thermogenesis, we used infrared imaging. At baseline, TBAT was 35.7 ± 0.08 °C and lumbar temperature (Tlumbar) was 34.6 ± 0.09 °C. Optogenetic stimulation of DMHBrs3 neurons increased TBAT by 1.36 ± 0.16 °C (p = 6.8 × 10−5) and Tlumbar by 1.24 ± 0.15 °C (p = 8.9 × 10−5) (Fig. 4c, Supplementary Fig. 7d). TBAT initially increased 1.90 ± 0.18 times faster than did Tlumbar. These results implicate BAT as a driver of the increase in Tb.
Sympathetic stimulation of adipose tissue causes lipolysis 34. After optogenetic stimulation of DMHBrs3 neurons, we quantified phosphorylated hormone sensitive lipase (pHSL), which indicates activation of lipolysis. In BAT, but not inguinal white adipose tissue (iWAT), higher levels of pHSL were detected, demonstrating stimulation of BAT (Supplementary Fig. 7e, 11).
Photostimulation of DMHBrs3 neurons increased heart rate (HR) by 109 ± 15 bpm (p = 0.006), mean arterial blood pressure (MAP) by 10.2 ± 3.4 mmHg (p = 0.047) and Tb by 0.48 ± 0.09 °C (p = 0.007), without an effect on physical activity (Fig. 4d). The increase in heart rate and MAP was rapid, with HR reaching near-maximum levels in under 30s (Supplementary Fig. 7f). In contrast, the change in Tb was slower, due to the heat capacity of the body. Photostimulation during the dark phase produced similar increases in heart rate, MAP and Tb (Supplementary Fig. 8a). Doubling the laser duty cycles (from 1s on, 3s off to 1s on, 1s off) produced greater increases in HR (185 ± 35 bpm) and MAP (17.3 ± 3.2 mmHg) (Supplementary Fig. 8b).
DMHnon-Brs3 neurons increase Tb and physical activity
Next, we probed the possibility that additional DMH neurons besides DMHBrs3 neurons regulate Tb. We selectively expressed ChR2 in DMHnon-Brs3 neurons using a ‘Cre off’ virus in Brs3-Cre mice. These mice will express ChR2 in all DMH neurons except those expressing Cre (Supplementary Fig. 9a). We also expressed this virus in wild type mice, lacking Cre, in which all infected DMH neurons (both DMHBrs3 and DMHnon-Brs3) express ChR2 (DMH::ChR2). Upon optogenetic stimulation, DMH::ChR2 mice increased their Tb by 1.80 ± 0.17 °C (p = 0.002) and physical activity by 74 ± 15 counts (p = 0.02; Supplementary Fig. 9b). DMHnon-Brs3::ChR2 mice increased their Tb by 1.08 ± 0.15 °C (p = 0.0002) and physical activity by 75 ± 17 counts (p = 0.005). The massive activity increases contrast with the lack of activity increase upon stimulation of DMHBrs3 neurons and likely accounts for at least some of the Tb increase in the DMHnon-Brs3::ChR2 mice (Fig. 4b,e and Supplementary Fig. 9b). Optogenetic activation of DMHnon-Brs3 neurons did not warm up TBAT significantly faster than Tlumbar (Supplementary Fig. 9c,d).
DMHBrs3→RPa pathway increases Tb and receives input from POA and other nuclei
We anterogradely traced the projections of DMHBrs3 neurons using AAV-DIO-synaptophysin-mCherry. Major projections were to the RPa, PVH and various POA nuclei (Supplementary Fig. 10). The DMHBrs3 neurons also projected to the ventrolateral periaqueductal grey (vlPAG), nucleus accumbens, LH, locus coeruleus, and caudal, but not rostral, ventrolateral medulla. Other less densely innervated brain regions included BNST, paraventricular nucleus of the thalamus (PVT) and arcuate nucleus (Arc).
DMH projections to the RPa can stimulate BAT and increase Tb in anesthetized rats 28,29,35. We tested the ability of the DMHBrs3→RPa projections to increase Tb, by infecting DMH cell bodies with AAV-Flex-ChR2 and photostimulating the RPa. Stimulating the DMHBrs3→RPa terminals increased Tb by 0.43 ± 0.12 °C (p = 0.006), with no effect on physical activity and no effect on Tb or activity in mCherry controls (Fig. 5a-c). Thus, selective activation of the DMHBrs3→RPa pathway is sufficient to increase Tb, most likely through glutamatergic projections 1,16,28,29,32. Indeed, DMHBrs3→RPa neurons rarely expressed Gad2 (2.6 ± 0.5%), a marker for GABAergic neurons (Fig. 5d). Thus, we hypothesize that the DMHBrs3→RPa neurons are glutamatergic.
Figure 5. Optogenetic stimulation of DMHBrs3 →RPa terminals increases Tb, possibly via glutamate.

(a) Schematic of virus (AAV-Flex-ChR2) injection in the DMH and laser stimulation of the RPa. (b) Tb response to optogenetic stimulation (blue interval) of DMHBrs3→RPa terminals in DMHBrs3::ChR2 (n = 6) and control DMHBrs3::mCherry (n = 6) mice. Data are change from −20 to 0 min and are mean + SEM (left). For quantification, Off is mean from −10 to 0 and On from 11 to 20 min. * p = 0.006, paired two-sided t-test, Off vs On. Black (mCherry) and red (ChR2) bars represent means. (c) Representative section showing DMHBrs3→RPa terminals (left; fiber indicated by dotted lines) and schematic of fiber placement (right, fiber positions indicated by x). Scale bar represents 100 µm. (d) Schematic of retrograde Cre-dependent EYFP-expressing HSV injected into the RPa of Brs3-Cre;Gad2-mCherry mice. EYFP-positive neurons are DMHBrs3→RPa. mCherry-positive neurons express Gad2. Arrowhead indicates colocalization. We counted 72 ± 13 EYFP neurons per animal (4 mice, two slices per mouse). Scale bar overview, 250 µm, inset 100 µm. Abbreviations: DHA - dorsal hypothalamic area; d/vDMH - dorsal/ventral dorsomedial hypothalamus; f - fornix; mt - mammillothalamic tract; RE - nucleus of reuniens; VMH - ventromedial hypothalamus; 3v - 3rd ventricle
To identify the neuronal inputs to the DMHBrs3→RPa pathway, we used projection-specific rabies monosynaptic retrograde tracing. DMHBrs3→RPa neurons were mostly located in the dDMH/DHA and not the vDMH (Fig. 6a,b). We found 28 ± 13 DMHBrs3→RPa starter neurons and 304 ± 142 input neurons projecting to the starter neurons. Areas with the highest concentration of input neurons were the MPA, Arc and portions of the DMH (vDMH and posterior DMH). Notably, most of the Arc input neurons were located in the posterior Arc (ArcP; caudal to Br −2.06). Brain regions with intermediate to high density levels of input were the parabrachial nucleus (mostly LPB) and raphe interpositus nucleus and raphe magnus (RIP/RMg). Areas with intermediate input numbers included several preoptic and other hypothalamic nuclei, as well as the zona incerta and vlPAG (Fig. 6c-i). Sparse input areas include the cortex (anterior portion of cingulate cortex), nucleus accumbens (shell and core), medial and lateral septum, ventral pallidum, lBNST and central nucleus of the amygdala. Other areas with sparse input neurons were the suprachiasmatic nucleus, subfornical organ, PVT, medial tuberal region, lateral habenula and ventral premammillary nucleus. In the medulla, sparse GFP neurons were found in the nucleus of the solitary tract and the rostral ventrolateral medulla. No GFP neurons were found at the injection site when only EnvA-G-deleted-Rabies-GFP was injected in the RPa (without RG or TVA viruses). We did not observe any collaterals of DMHBrs3→RPa neurons in other DMHBrs3 projection areas, suggesting that the DMHBrs3→RPa neurons project only to the RPa.
Figure 6. DMHBrs3→RPa neurons receive input from POA and other nuclei.

(a) Schematic of projection-specific rabies tracing. (b) Brs3-Cre;Ai14 mice injected with AAV-DIO-TVA-mCherry in the DMH and EnvA-G-deleted-Rabies-GFP in the RPa, showing the dDMH/DHA localization of DMHBrs3→RPa neurons. (c-i) Brs3-Cre mice were injected with Flex-TVA-mCherry and Flex-RG viruses in the DMH and EnvA-G-deleted-Rabies-GFP in the RPa. (c,d) DMH showing DMHBrs3→RPa starter neurons expressing TVA-mCherry and GFP. (d) higher magnification of inset. (e-h) Examples of regions with higher numbers of input neurons, which express only GFP. (i) Areas with input neurons to DMHBrs3→RPa neurons, percentage of total. Only areas with input neurons in at least 3 of the 4 mice are shown. Scale bar, b,c,h 200 µm, d-g 100 µm. Abbreviations: 3V – third ventricle; ARCp – arcuate nucleus, posterior part; AVPe – periventricular nucleus, anterior ventral part; DHA – dorsal hypothalamic area; LH – lateral hypothalamus; ml – medial lemniscus; MnPO – median preoptic area; MPA – medial preoptic area; py – pyramidal tract; RIP – raphe interpositus nucleus; RMg – raphe magnus; v/dDMH – dorsomedial hypothalamus (ventral/dorsal); scp – superior cerebellar peduncle.
Most Tb regulation models implicate GABAergic projections from various POA regions to DMH to control Tb 18,21,22. To evaluate the neurotransmitter input from the preoptic area to DMHBrs3→RPa neurons we performed ChR2-assisted circuit mapping (CRACM). We transduced POA neurons with AAV-ChR2-EYFP and enabled identification of DMHBrs3→RPa neurons with HSV-lsl-mCherry injected in the RPa. We found that the majority of DMHBrs3→RPa neurons recorded receive both GABAergic and glutamatergic input from the POA (Fig. 7). This indicates that the DMHBrs3→RPa circuit is under dynamic control by the POA.
Figure 7. DMHBrs3→RPa neurons receive inhibitory and excitatory input from the POA.

(a) Schematic showing injection of 1) Cre-dependent mCherry-expressing HSV in the RPa (marking DMHBrs3→RPa neurons) and 2) Cre-independent ChR2-EYFP-expressing AAV in the POA. Scale bar represents 200 µm (left image) and 25 µm (right image). (b) Voltage-clamp trace of a DMHBrs3→RPa neuron in a DMH-containing brain slice. Recording was made in the presence of TTX (500 nM) and 4-AP (100 µM), showing monosynaptic inhibitory and excitatory input from the POA. Picrotoxin (25 mM) and kynurenic acid (3 mM) blocked inhibitory postsynaptic currents (IPSCs) and excitatory postsynaptic currents (EPSCs), respectively. All 15 DMHBrs3→RPa neurons recorded from (2 mice) had PSCs in response to light. IPSCs recorded at +10mV and EPSCs at −55mV. (c) Venn diagram of POA input to DMHBrs3→RPa neurons, showing the number of each type of PSC.
Discussion
Genetic and pharmacologic experiments have demonstrated a role for BRS3 in the control of Tb, energy expenditure, food intake and heart rate. We investigated the responsible neurons, showing that DMHBrs3 neurons regulate Tb, energy expenditure and heart rate, while not affecting food intake or physical activity. More specifically, DMHBrs3 neurons use a DMHBrs3→RPa pathway to bidirectionally control Tb, including via action on brown adipose tissue. In contrast, PVHBrs3 neurons control food intake and do not affect Tb, energy expenditure, or physical activity. These results suggest a mechanistic basis for the phenotype of Brs3 knockout mice. Loss of BRS3 from the DMHBrs3 neurons could contribute to the reduced resting Tb, energy expenditure and heart rate. Loss of BRS3 from PVHBrs3 neurons could promote the increased food intake. Together, these mechanisms contribute to the obese phenotype of the global null mice.
The simplicity of this model notwithstanding, a caveat is that genetic Brs3 ablation nullifies only the contribution of one receptor (and its elusive ligand), while optogenetic and chemogenetic manipulations probe the properties of the neuron expressing this receptor. The current study does not address whether BRS3 in neurons in other areas also contribute, possibly redundantly, to the regulation of Tb, energy expenditure, heart rate and food intake. For example, we observed that PBNBrs3 neurons are activated during refeeding, which could indicate a role in regulating food intake or signaling nutritional status. Similarly, the activation of MPABrs3 and MnPOBrs3 neurons by a cold environment suggests the possibility of contribution to the regulation of thermogenesis by these populations. Recently, Brs3 mRNA was found to be enriched in a warm-sensitive population of POA (predominantly Gad2/BDNF/PACAP-expressing) neurons 18. Paradoxically, we observed very low Fos expression in MPABrs3 and MnPOBrs3 neurons after warm exposure. Possibly, Brs3 neurons in different preoptic nuclei provide complementary contributions to Tb regulation. This discrepancy could also be due to the different warm conditions (30 °C vs 37 °C).
The Brs3-Cre allele is hypomorphic, as are others in widely used knock-in Cre lines (e.g., somatostatin-IRES-Cre 36, DTA-Cre, https://www.jax.org/strain/006660). Our observations about DMHBrs3 and PVHBrs3 neurons are likely not compromised by the Brs3-Cre’s hypomorphism since the mice do respond to BRS3 agonist. Additionally, we used Brs3 as a neuronal marker, focusing on the circuitry and function of Brs3 neurons, and not the function of the BRS3 receptor per se.
Multiple lines of evidence suggest that the DMH→RPa pathway that activates BAT is glutamatergic. Glutamate receptor antagonist administration in the RPa inhibits increased BAT sympathetic nerve activity and temperature, evoked by pharmacological disinhibition 29 or optogenetic 28 stimulation of the DMH. Brs3 and Vglut2 colocalize in the DMH by in situ hybridization 1. Also, germline deletion of Brs3 in glutamatergic, but not GABAergic, neurons recapitulates the Tb phenotype of the global Brs3 knockout and re-expression of Brs3 in glutamatergic, but not GABAergic, neurons reversed the null phenotype 16. Indeed, we found that DMHBrs3→RPa projecting neurons rarely express Gad2.
The PVH contains multiple neuronal types that regulate food intake, including those expressing MC4R 37, BDNF 38, AVP 39 and non-Oxt Nos1 40. The PVHBrs3 neurons could be a subpopulation of any of these, except the appetite-regulating BDNF neurons, which appear to be located more rostrally 38. The downstream circuits through which the PVHBrs3 neurons exert their functions are not known. Since re-expression of BRS3 in Vglut2-positive neurons normalizes the increased food intake of Brs3 null mice and is sufficient for suppression of food intake by BRS3 agonist 16, the relevant PVHBrs3 neurons are probably glutamatergic.
The predominant mechanism of cold-induced thermogenesis in mice is sympathetic activation of BAT, causing heat production via substrate oxidation uncoupled from ATP generation 22,41. We demonstrated that DMHBrs3 neurons increase BAT temperature, preceding the Tb increase, suggesting that BAT is driving the Tb increase. A subpopulation of DMH neurons contributes to the sympathetic regulation of both BAT and WAT 42. DMHBrs3 stimulation caused BAT activation, also demonstrated by increased phosphorylation of HSL. DMHBrs3 neuronal activation did not produce a detectable increase in plasma non-esterified fatty acid or glycerol levels. It is possible that longer duration of stimulation 34 would demonstrate WAT activation. We have not investigated if activation of DMHBrs3 neurons also modulates other thermoregulatory mechanisms, such as vasodilation/vasoconstriction, shivering, or behavioral adaptation 23.
Brs3 knockout mice have a reduced resting heart rate, which increases disproportionally with physical activity, reaching a similar upper limit as in wild type mice. Blood pressure in Brs3 knockout and wild type mice is similar at rest, but increases more with activity and stress in the Brs3 knockout mice 7,10. A working model is that Brs3 knockout mice have a reduced resting sympathetic tone and heart rate, which increase disproportionally with activity. The resting blood pressure is defended by feedback regulation and overshoots during physical activity. BRS3 agonists acutely increase heart rate and blood pressure, effects that attenuate with continued treatment over hours to days 10,13,14. Optogenetics allows study of acute cardiovascular parameters without handling perturbations. Optogenetic activation increased heart rate and arterial pressure, demonstrating that DMHBrs3 neurons are sufficient to produce these effects. This result suggests that, from a therapeutic standpoint, it will be difficult to dissociate the desired activation of BAT by BRS3 agonists from the undesired increases in heart rate and blood pressure. The obesity-associated increase in mouse blood pressure may implicate leptin signaling, possibly through leptin receptors in the DMH 43. DMHBrs3 neurons, a large fraction of which are activated by leptin, might overlap with the DMHLepRb neurons involved in blood pressure increases in obesity.
DMHBrs3 neurons contribute to cold-induced thermogenesis and their activation increased Tb and energy expenditure, without altering physical activity. In contrast, activation of DMHnon-Brs3 neurons greatly increased activity, which likely accounts for at least some of the concomitant increase in Tb. Other studies of the DMH have identified DMHLepRb, DMHVglut2 and DMHVgat neurons as increasing physical activity, Tb, and energy expenditure 21,26. These observations suggest that Brs3 is a marker for a distinct subpopulation, regulating the sympathetic nervous system independent of physical activity.
The heart rate increase from DMHBrs3 neuron activation may be important for increasing blood flow to activated BAT, bringing fuel (glucose, fatty acids) and distributing heat to the rest of the body. It is also plausible that heat from the increased cardiac work contributes to increasing Tb.
Nakamura and colleagues investigated projections of DMH neurons and found that only the DMH neurons projecting to the RPa (and not the PVH or vlPAG) activated BAT 28. DMHLepRb neurons also project to RPa 31. We found that some dDMH/DHABrs3 neurons are activated by leptin, although we did not determine the overlap with DMHBrs3→RPa neurons. The DMHBrs3→RPa projections are sufficient to increase Tb and these neurons do not appear to have collaterals. However, it remains possible that DMHBrs3 pathways in addition to DMHBrs3→RPa increase Tb.
Multiple areas of the brain project to the DMH 44 and we found that many provide input to DMHBrs3→RPa neurons. Suprahypothalamic input to the DMH possibly mediates autonomic responses to stress 35,45,46. However, we did not observe substantial cortical input to DMHBrs3→RPa neurons, suggesting that they are not central to this response. We found that RIP/RMg neurons project to DMHBrs3→RPa neurons; the RIP/RMg to DMH input was not previously reported44. In confirmation, we have also observed labeled RIP/RMg neurons with Rabies-GFP injected in the DMH (unpublished observations, Ramón Piñol). An interesting function for LPB input to DMHBrs3→RPa neurons could be to relay ascending thermosensory information directly to the DMH, independent of the LPB to POA pathway 47. Finally, significant numbers of labeled neurons in the vDMH and posterior DMH suggest intra-DMH circuitry.
DMHBrs3→RPa neurons receive abundant synaptic input from preoptic nuclei. DMH-regulated BAT thermogenesis is under inhibitory preoptic control 18,23,30,48,49, and possibly excitatory as well 50. Our data suggest that DMHBrs3→RPa neurons are regulated by both GABAergic and glutamatergic preoptic neurons. Further research is needed to determine which POA neurons provide the inhibitory and excitatory inputs to DMHBrs3→RPa neurons.
In summary, we identify two Brs3 populations with separate functions. PVHBrs3 neurons can robustly regulate food intake without affecting Tb. DMHBrs3 neurons regulate Tb, energy expenditure, BAT activity, heart rate and blood pressure, with no effect on food intake or physical activity. The data demonstrate the existence of a DMHBrs3→RPa population dedicated to thermogenesis. Brs3 is a neuronal marker useful for dissecting the neural circuits controlling energy homeostasis.
Materials and Methods
Animals.
Animal studies were approved by the NIDDK/NIH Animal Care and Use Committee. Mice were singly housed at 22 C with lights on 6 am-6 pm and fed chow (7022 NIH-07 diet, 15% kcal fat, energy density 3.1 kcal/g, Envigo Inc., Indianapolis, IN). Food and water were available ad libitum, including during drug treatments, indirect calorimetry and optogenetic experiments. Mice: Brs3-Cre (see below), B6.Cg-Gt.(Rosa)26Sortm6(CAG-ZsGreen1)Hze/J, with Cre-dependent ZsGreen (Ai6; Jax# 007906) 51, B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J, with Cre-dependent tdTomato (Ai14; Jax# 007914) 51, Gad2–2a-NLS-mCherry (Gad2-mCherry; Jax# 023140)52. Male mice were used, unless otherwise noted. For all behavioral and physiological studies, male mice were between 8 and 45 weeks of age. Mice for electrophysiological studies to test DREADD and ChR2 function (male and female) and for CRACM studies (male) were between 5 and 13 weeks of age. Mice were on a mixed C57BL/6J and 129SvEv background. Mice from multiple litters were used for all studies.
Brs3–2A-CreERT2 mice.
Generation.
A targeting vector using C57BL/6 targeting arms and DTA negative selection was designed to insert a 2A peptide, CreERT2 recombinase, thymidine kinase polyA signal and Frt-flanked neo selection cassette, replacing the TAG stop codon in the third exon of the Brs3 gene (Cyagen, Sunnyvale, CA). The 2A sequence adds EGRGSLLTCGDVEENPG to the C-terminus of BRS3 and a proline to the N-terminus of CreERT2; it is expected that the two proteins are translated in a 1:1 ratio 53. This construct was electroporated into TC1 ES cells (129SvEv) and correctly targeted cells were injected into Black Swiss blastocysts (Mouse Knockout Core, NIDDK). Chimeric mice were bred with FLPo10 mice (#011065, Jackson Laboratory) to delete the neo cassette and then to C57BL/6J mice to remove the FLPo10 transgene to obtain Brs3–2A-CreERT2 (Brs3-Cre) mice.
Tamoxifen.
Cre-mediated recombination was achieved by treatment with tamoxifen (110 mg/kg ip in corn oil, unless otherwise noted; Sigma Aldrich) daily for 5 consecutive days, with mice studied ?2 weeks after the last dose. Lower tamoxifen doses produced fewer tdTomato-positive neurons in Brs3Cre/+;Ai14/+ mice and higher doses were occasionally lethal.
Brs3-Cre mice validation.
Fluorescent in situ hybridization.
Mice were anesthetized (chloral hydrate, 500 mg/kg ip). Next, brains were removed, frozen on dry ice, embedded in M1 Embedding Matrix (Thermoscientific Fisher, Waltham, MA), cut on a cryostat (Leica CM 1860), adhered to SuperFrost Plus slides (VWR) and immediately refrozen. Samples were fixed in fresh 10% neutral buffered formalin solution (Sigma Aldrich), processed according to RNAscope fluorescent Multiplex Assay manual (Advanced Cell Diagnostics; probe 454111 for Brs3 and 317041-C2 for tdTomato) and cover slipped with HardSet antifade mounting medium with DAPI.
BRS3 function.
To quantify Brs3 mRNA levels, hypothalamic tissue RNA was extracted (Qiagen Allprep DNA/RNA micro Kit, Germantown, MD). RNA was reverse transcribed (Roche Transcriptor High Fidelity cDNA Synthesis Kit, Indianapolis, IN). cDNA was quantified by real-time polymerase chain reaction (q-PCR, Applied Biosystems QuantStudio Real-Time PCR System, Foster City, CA) using SYBR green, normalized to 18S RNA.
To test the possibility of hypomorphism in Brs3-Cre mice, 6-week-old Brs3Cre/Y and Brs3+/Y littermates were singly housed and monitored for body weight, body composition (EchoMRI, EMR-122, Echo Medical Systems) and food intake. Mice were fed high-fat diet (60% kcal fat, 5.24 metabolizable kcal/g; D12492, Research Diets) at 8 weeks. Energy expenditure was estimated by energy balance 54. To test functionality of BRS3 in Brs3-Cre mice, Tb and food intake response to BRS3 agonist MK-5046 55 (generous gift from Merck; 10 mg/kg in saline) was tested.
X-inactivation.
Brs3 is located on the X chromosome, so the Brs3Cre allele could undergo X-inactivation 56. Neurons expressing tdTomato were counted in heterozygous (Brs3Cre/+;Ai14/+) and homozygous (Brs3Cre/Cre;Ai14/+) female mice, carrying one vs two copies, respectively, of the Brs3Cre allele. In the three regions studied (PVH, BNST and MePD) the heterozygous females had half the number of tdTomato-positive neurons as the homozygous females (Supplementary Fig. 2a). The number of tdTomato-expressing neurons was similar in hemizygous male (Brs3Cre/Y;Ai14/+) and homozygous female mice.
Experimental paradigms to probe neuronal activity.
Warm/Cold Exposure.
Mice were habituated for 2–3 days at 22 C in a temperature-controlled chamber in their home cages. For the warm- (cold-) exposed group, chow was removed and the chamber was set to 30 C (4 C) at 8:30 AM. Cage interior temperature was continuously monitored using a temperature logger (HOBO data logger) and reached the target temperature in 60 minutes. After 4 hours in the chamber, the mice were perfused, their brains processed for immunohistochemistry and neurons scored for Brs3 (tdTomato) and Fos expression.
Fast/refeed.
Mice began fasting in their home cages at 3:00 PM. After 20 hours, they were either perfused immediately or given access to chow for 2 hours and then perfused and scored for Brs3 (tdTomato) and Fos expression.
Leptin treatment.
Mice began fasting in their home cages at 3:00 PM. After 20 hours, they were injected with either saline or leptin (4 mg/kg ip). After 1 hour, mice were perfused and neurons scored for Brs3 (tdTomato) and pStat3 expression.
Immunohistochemistry.
Tissue Preparation.
Mice were anesthetized (chloral hydrate, 500 mg/kg ip), perfused transcardially with 0.9% saline followed by 10% neutral buffered formalin, and the brain was removed. Brains were post-fixed (10% formalin, room temperature, 4 hours) and incubated in 20% sucrose in 0.1M PBS (4?C, overnight), sectioned coronally into three equal series (50 ?m sections) on a sliding microtome (SM2010 R, Leica) and collected in 0.1M PBS. Sections were washed 3×10 minutes in 0.1M PBS and the following steps were performed with shaking at room temperature and in 0.1M PBS solutions, unless noted otherwise:
Fos.
Sections were blocked for 2 hours in 0.5% Triton X-100, 2% normal horse serum (NHS) and then incubated (48 hours, 4?C) with 1:2000 rabbit-anti-phospho-cFos (Ser32; #5348, Cell Signaling Technology)57 in 0.5% Triton X-100, 2% NHS. Sections were washed, incubated (4 hours) with secondary antibody (1:1000 488-donkey-anti-rabbit secondary, Abcam, Cambridge, MA, in 0.5% Triton X-100, 2% NHS) and washed, mounted and cover slipped with hardset antifade mounting medium containing DAPI (Vectashield).
pSTAT3.
Sections were incubated for 20 minutes in 1% NaOH and 0.3% H2O2, 10 minutes in 0.3% glycine in 0.1M PBS, 10 minutes in 0.03% SDS and blocked for 1 hour in 3% normal donkey serum (NDS), 0.3% TritonX-100. Sections were then incubated overnight in 3% NDS, 1:250 rabbit anti pSTAT3 (Tyr705; #9131S, Cell Signaling Technology)58, 1:250 goat anti DsRed (L-18) (sc-33353, Santa Cruz)59 and 0.3% Triton X-100 diluted and washed 6×8 minutes. Next, sections were incubated 1 hour with secondary antibodies (1:250 488-donkey-anti-rabbit, 1:250 594-donkey-anti-goat (both Abcam), 3% NDS and 0.3% Triton X-100). Sections were then rinsed, mounted and cover slipped with hardset antifade mounting medium containing DAPI.
mCherry.
Sections were incubated for 1 hour in 0.3% Triton X-100, 3% normal donkey serum (NDS) and then incubated overnight with 1:2000 rabbit-anti-DsRed (ab 632496, Clontech)60 in 0.3% Triton X-100, 3% NDS. Sections were washed, incubated (2 hours) with secondary antibody (1:500 Alexa-594 donkey-anti-rabbit; Invitrogen) in 0.5% Triton X-100 and 2% NHS and washed, mounted and cover slipped with hardset antifade mounting medium containing DAPI (Vectashield).
Stereotaxic injections.
General surgical procedures.
Mice were anesthetized with 0.5–1.5% isoflurane (1 L per minute of oxygen) or a ketamine/xylazine mix (80/10 mg/kg) and placed in a stereotaxic instrument (Digital Just for Mouse Stereotaxic Instrument, Stoelting). Ophthalmic ointment (Puralube, Dechra) was applied. All injections were done with pulled-glass pipettes (pulled 20–40 m tip diameter; 0.275 ID, 1 mm OD, Wilmad Lab Glass ) at a visually controlled rate of 50 nl per min with an air pressure system regulator (Grass Technologies, Model S48 Stimulator). The pipette was kept in place for 5 min after injection. Post-surgery mice received subcutaneous sterile saline injections to prevent dehydration and analgesic (buprenorphine; 0.1 mg/kg).
Viral vectors.
We used the following viruses AAV8-hSyn-DIO-hM3Dq-mCherry, AAV8-hSyn-DIO-hM4Di-mCherry, AAV8-Ef1a-FLEX-TVA-mCherry, AAV8-Eff1a-DIO-mCherry and AAV8-ef1a-mCherry-FLEX-DTA (University of North Carolina Vector Core)61; AAV8-hSyn-DIO-hM3Dq-mCherry and AAV8-hSyn-DIO-hM4Di-mCherry (Addgene); AAV2-ef1a-DIO-GFP, AAV9-Ef1a-ChR2-Eyfp, AAV9-CAG-FLEX-ChR2-tdTomato (gift from Scott Sternson, Addgene plasmid #18917; University of Pennsylvania Viral Vector Core); AAV8-Ef1a-DIO-synaptophysin-mCherry (Virovek, Inc), AAV8-CAG-FLEX-RabiesG(Y733F) (Stanford University Vector Core); HSV-Ef1a-lsl-mCherry and HSV-Ef1a-lsl-Eyfp (Massachusetts Institute of Technology, Viral Core Facility); EnvA-G-Deleted-Rabies-Egfp (Gene Transfer, Targeting and Therapeutics Core at Salk Institute); AAV9-Ef1a-DO-hChR2(H134R)-mCherry (gift from Bernardo Sabatini; Addgene plasmid 37082; Vigene Biosciences, Inc)62
Nucleus-specific effect of MK-5046 on TBAT.
Mice (Brs3-Cre;Ai9 or WT) were transiently anesthetized with 1.5% isoflurane followed by ip urethane (1.5 g/kg; U2500, Sigma) and placed in a stereotaxic instrument. Tb was kept constant at 35 C with a homeothermic blanket system (Stoelting). TBAT was recorded with a 4600 Thermometer and a small surface temperature probe (Measurement Specialties; Fisher Scientific). 50 nl of BRS3 agonist MK-5046 (1 mg/ml in saline) or vehicle (saline) was injected (rate: 25 nl/min). After injections, all mice received 300 nl of 1g/ml PGE2 (Tocris) injections in the POA to confirm intact BAT thermogenesis. All injection sites were marked with 25 nl 0.2% Fluospheres (Life Technologies). After experiments the brains were removed, drop-fixed in 10% formalin, sectioned and mounted to verify injection sites. Mice that did not respond to PGE2 or had missed injections were excluded from analysis.
Chemogenetics.
Virus injections.
Brs-Cre mice received bilateral injections of 100 nl AAV8-hSyn-DIO-hM3Dq-mCherry in the DMH (AP: −1.85; ML: +/− 0.3; DV: −5.05, mm from bregma) or 50 nl in the PVH (AP: −0.90; ML: +/− 0.25; DV: −4.75, mm from bregma). For chemogenetic inhibition experiments we injected 150 nl AAV8-hSyn-DIO-hM4Di-mCherry bilaterally in the DMH of Brs3-Cre or Brs3-Cre;Ai6 and, as a control group, C57BL/6J mice, or in the PVH of Brs3-Cre mice.
General experimental procedure.
Mice received ip injections of 1.0 mg/kg CNO in sterile 0.9% saline, unless noted otherwise. After completion of all experiments mice were perfused and brains were collected and processed for mCherry immunohistochemistry. Mice with uni- and bilateral hits were used. Mice without hM3Dq or hM4Di expression or with expression outside of the target nuclei were excluded from analysis and served as additional controls for CNO.
hM3Dq physiology: Food intake (DMH/PVH).
Mice were fasted five hours before the onset of their dark cycle. Mice were dosed with CNO or vehicle 15 minutes prior to lights out. Food intake was measured two hours following dosing.
hM3Dq physiology: blood glucose and plasma lipids (DMH/PVH).
Blood glucose, triglycerides and glycerol were measured using tail blood samples in ad-lib fed chow mice during the first part of the light cycle after veh or CNO dosing. Serum was collected by tail bleeding. Blood glucose was measured with a Glucometer Contour (Bayer) and a serum sample was frozen until assayed for free fatty acids as per the assay kit manufacturer’s manual (Pointe Scientific).
hM4Di physiology: Food intake (PVH).
Ad-lib fed mice were dosed with CNO or vehicle four hours into the light cycle and food was removed. After 15 minutes food was returned and food intake was measured two hours following return. Data is averages of three trials.
hM4Di physiology interventions(DMH):
Low doses of lipopolysaccharide cause systemic inflammation and mild hyperthermia 63. Mice were ip injected with lipopolysaccharide (50 g/kg). Cage switch-stress mediated increases in Tb 64,65. Mice that had been living > 4d in a cage were switched with their neighbor’s, 90 minutes after injection of Veh/CNO.
Body temperature telemetry experiments.
Surgical procedure and recording
Animals were anesthetized as above and E-Mitters (Starr Life Sciences) were implanted intraperitonially 66. Two days before experiments mice were housed in temperature controlled chamber in their home cages on ER4000 energizer/receivers (Starr Life Sciences). Tb and activity were continuously measured by telemetry and 1 min means collected with VitalView software (Starr Life Sciences). Experiments were performed at 22 C, unless indicated otherwise.
Indirect calorimetry.
An Oxymax/CLAMS (Columbus Instruments) was used to measure Tb, total energy expenditure (TEE), RER (respiratory exchange ratio, O2 consumed:CO2 produced), and activity by beam break simultaneously in mice implanted with E-mitters 66. Experiments were performed at 22 C or 30 C, as indicated. Sampling was every 13 minutes, measuring from 12 chambers.
DTA ablation experiment
At 11 weeks of age, Brs3-Cre mice were housed individually. Body weight, food intake, and body composition were measured, from which energy expenditure was calculated by mass balance (as above in Brs3-Cre mice validation). At 14 weeks, the DMHBrs3 ablation group (EYFP+DTA) was injected with Cre-dependent EYFP virus (AAV2-ef1a-DIO-EYFP) plus mCherry-Cre-dependent diphtheria toxin A virus (AAV8-ef1a-mCherry-FLEX-DTA) 62, 1:1 mix, 150 nl per side. The control group (EYFP) was only injected with the Cre-dependent EYFP virus, 1:2 dilution, 150 nl per side. At 15 weeks mice received tamoxifen treatment. At age 25 weeks mice were implanted E-Mitters (see above; Body temperature experiments), which precluded further body composition measurements. Tb experiments were performed at 27–29 weeks. From week 32 to 38 mice were housed at thermoneutrality (30 C) and received high-fat diet (60% kcal fat, 5.24 metabolizable kcal/g; D12492, Research Diets).
EYFP neuron quantification.
After completion of the experiments brains were processed for histological analysis. Brs3-positive (i.e., EYFP positive) neurons in the DMH/DHA were counted in both groups in every third 40 m coronal section. Mice in the DTA+EYFP group with no or unilateral mCherry expression in the DMH were excluded from analysis, as were unilateral EYFP-expressing mice in the control group.
Cold exposure experiments and cage switch experiments.
Mice were housed in their home cages in a thermal chamber, two days before experiments to acclimate. At the beginning of the light cycle the thermal chamber temperature was switched from 22 C to 7 C for 6 h. In cage switch experiments, mice that had been living > 4d in a cage were switched with their neighbor’s or lifted by their tail and placed back in their own as a handling control in a crossover design.
Optogenetics experiments.
Virus injections and fiber implant.
Brs-Cre or Brs3-Cre;Ai6 mice were injected with 75 nl AAV9-CAG-FLEX-ChR2-tdTomato, AAV9-ef1a-DIO-ChR2-eyfp or, as controls, AAV8-Eff1a-DIO-mCherry. Following virus injection, optical fibers (200 ?m diameter core; NA 0.22; Nufern), glued to ceramic zirconia ferrules (230 ?m bore; 1.25 OD diameter; Precision Fiber Products), were implanted unilaterally over the DMH (AP: −1.85; ML: 0.3; DV: −4.5, mm from bregma) or the RPa (AP: −6.0; ML: 0; DV: −5.5, mm from bregma). Fibers were fixed to the skull using C&B Metabond Quick Cement and dental acrylic.
Experimental procedures.
Mice were allowed to adapt to the fiber patch cord for at least three days prior to experiments and typically not handled on the day of the experiment. Fiber optic cables (200 m diameter; NA: 0.22, 1 m long; Doric Lenses; or, 0.5m long, ThorLabs) were connected to the implanted fiber optic cannulas with zirconia sleeves (Doric Lenses) and coupled to lasers via a fiber optic rotary joint (Doric Lenses). We adjusted the light power of the laser (473 nm; Laserglow or Opto Engine) such that the light power (measured with a fiber optic power meter; PM20A; ThorLabs) at the end of the fiber optic cable was ~10 mW. Using an online light transmission calculator for brain tissue (http://web.stanford.edu/group/dlab/cgi-bin/graph/chart.php), we estimated the light power at the DMH or RPa between 3 and 6 mW/mm2. This is an upper limit due to possible light loss between the fiber optic cable and the implanted optic fiber. Light pulses were controlled by a waveform generator (Arduino) programmed to deliver light pulses. In most experiments (unless otherwise indicated), stimulation was on for 1 s, followed by 3 s off, pulses were 10 ms delivered at 20 Hz. After the completion of experiments, fiber placement and ChR2 expression were assessed. Animals without ChR2 expression or incorrect placement of optic fibers were excluded from analysis.
Western blot analysis.
Mice received 1h of photostimulation and were euthanized and BAT and iWAT were dissected and stored at −80oC. Protein from BAT and iWAT was prepared using RIPA buffer, quantified (Pierce BCA Protein Assay Kit, Thermo Scientific, Rockford, IL), separated in 4–20% SDS-PAGE gels (30 g/lane), transferred to PVDF membrane, and probed with anti-pHSL (1:1000, 4126L, Cell Signaling Technology), anti- HSL (1:1000, 4107S, Cell Signaling Technology), anti-UCP1 (1:5000, U6382, Sigma-Aldrich), or anti-?-TUBULIN (1:5000, T6074, Sigma-Aldrich). Signals were detected with SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific), images were scanned with ChemiDocTM Touch Imaging System (BIO-RAD).
Electrophysiology.
Verification of DREADD function.
Brs3-Cre mice (5–6 weeks) received injections of 150 nl AAV8-syn-DIO-hM3Dq-mCherry in PVH and DMH. Mice were used 4–6 weeks later. Brain slices were obtained and stored at approximately 30?C in a heated, oxygenated holding chamber containing aCSF (in mmol/l) 124 NaCl, 4.4 KCl, 2 CaCl2, 1.2 MgSO4, 1 NaH2PO4, 10.0 glucose, and 26.0 sodium bicarbonate before being transferred to a submerged recording chamber maintained at approximately 30?C (Warner Instruments, Hamden, CT). Recording electrodes (3–5?M?) were pulled with a Flaming-Brown Micropipette Puller (Sutter Instruments, Novato, CA) using thin-walled borosilicate glass capillaries. Current-clamp recordings monitoring shifts in resting membrane potential were obtained using electrodes filled with an intracellular recording solution containing (in mM): 135 K+-gluconate, 5 NaCl, 2 MgCl2, 10 HEPES, 0.6 EGTA, 4 Na2APT, 0.4 Na2GPT.
CRACM.
Brs3-Cre mice (5–10 weeks) received injections of 300 nl HSV-Ef1a-lsl-mCherry in the RPa (AP: −6.0; ML: 0; DV: −6.0, mm from bregma) and 100 nl of AAV1-Ef1a-ChR2-Eyfp bilaterally in the anterior POA (AP: 0.5; ML: +/− 0.4; DV: −5.25, mm from bregma). Mice were used 2 to 6 weeks later. Light evoked excitatory and inhibitory postsynaptic currents (EPSCs and IPSCs, respectively) were measured in voltage-clamp mode using electrodes filled with an intracellular recording solution containing (in mM): 135 Cs-methanesulfonate, 10 KCl, 10 HEPES, 1 MgCl2, 0.2 EGTA, 4 Mg-ATP, 0.3 GTP, 20 phosphocreatine. Lidocaine N-ethyl bromide (1 mg/ml) was included in the intracellular solution to block postsynaptic sodium currents. Neurons were held at ?55 mV to isolate glutamatergic synaptic transmission and record EPSCs, or +10 mV to isolate GABAergic synaptic transmission and record spontaneous IPSCs within individual neurons. Tetrodotoxin (TTX, 500 nM) and 4-Aminopyridine (4-AP, 100 M) were included in the bath aCSF (aCSF as above). The last cell’s IPSCs were blocked with picrotoxin (25 mM) and EPSCs with kynurenic acid (3mM).
Quantitative thermal imaging in freely moving mice
We measured the emissivity of shaved and unshaved skin from C57BL/6J mice as follows: A piece of dorsal skin was taped in tight contact with a 40 C metal surface in a black insulated vessel and equilibrated for 30 min, which achieved stable temperatures for >10 minutes. Emissivity was calculated as the skin temperature determined by the IR camera (FLIR Systems T650sc with emissivity set to 1, using ResearchIR 4 software) divided by the skin temperature measured with a digital thermometer. The emissivity was 0.97 0.02 for shaved skin and 0.88 0.01 for unshaved skin.
Shaved skin temperature in the interscapular and dorsal lumbar regions were used as measures of BAT and core body temperature, respectively, as others have done67,68. One day prior to study, a 2 × 2 cm midline area 2 cm above the tail and the interscapular region were shaved under isoflurane anesthesia. After housing overnight with the optical fiber attached in their home cage, mice were placed in a 20 × 20 cm cage (with bedding), 170 cm below the IR camera. Mice were allowed to acclimate for 3 h in the experimental enclosure. Mice then underwent 3 cycles of 20 min baseline, 20 min laser on, and 40 min post-stimulation, with IR images collected continuously (7.5 frames per second) during the 3 cycles. The maximum interscapular and lumbar temperatures were determined by a blinded observer every 2 minutes using ResearchIR (see supplementary Figs. 7d and 9d for sample stills).
Blood pressure and heart rate telemetry.
In a subset of mice with a Tb response to optogenetic stimulation of the DMH, we replaced the Emitter with an intra-arterial pressure telemetry probe. Continuous ambulatory intra-arterial blood pressure, heart rate, physical activity and subcutaneous (model HD-X10) or core (model HD-X11) temperature were measured with radio transmitters (Data Sciences International, St Paul, MN), after implantation in the carotid artery as described 69. Data were sampled at 1000 Hz and processed using a PhysioTel RPC-1 receiver, and collected with Ponemah v6.30 (Data Sciences International). Unless noted, 1 min averages were used for analysis.
Retrograde HSV tracing.
300 nl of HSV-ef1a-lsl-EYFP or HSV-ef1a-lsl-mCherry was injected in the RPa of Brs3-Cre;Gad2-mCherry (colocalization, Fig. 5) or Brs3-Cre (CRACM, Fig.7) mice. Experiments were performed at least 2 weeks after tamoxifen treatment finished. Colocalization with mCherry in Brs3-Cre;Gad2-mCherry mice was quantified with confocal images.
Projection-specific monosynaptic rabies tracing.
Viral surgeries.
100 nl of a 1:1 mixture of AAV8-Ef1a-Flex-TVA-mCherry and AAV8-CAG-FLEX-RG was injected in the DMH (AP: −1.85; ML: +/− 0.3; DV: −5.05, mm from bregma) of Brs3-Cre mice. 4 weeks after tamoxifen treatment we injected 150 nl EnvA-G-deleted-Rabies-GFP in the RPa70,71. Some mice received 100 nl of EnvA-G-deleted-Rabies-GFP or EnvA-G-deleted-Rabies-ChR2-mCherry in the DMH as a control for retrograde infection of nuclei near the RPa and were not included in quantitative analysis. Two mice received 200 nl EnvA-G-deleted-Rabies-GFP in the RPa as a control for non-specific Egfp expression. Two mice (Brs3-Cre;Ai14) received AAV8-Ef1a-Flex-TVA-mCherry in the dDMH/DHA and EnvA-G-deleted-Rabies-GFP in the RPa as a control for non-RG-mediated retrograde infection upstream of starter cells.
Analysis.
Six days after Rabies virus injection, mice were euthanized. Brains were dissected and fixed in 10% formalin for 2 days, transferred to 30% sucrose overnight, sliced (50 m) on a freezing microtome (Leica), and collected in three series. For counting, one series of slices was mounted and cover slipped with hard set mounting medium containing DAPI. Slides were imaged with a VS120 slide scanner Olyvia software (v2.9, Olympus). Brain regions were defined according to Mouse Brain Atlas 72. Neurons were counted as starter neurons when mCherry (and/or tdTomato in Brs3-Cre;Ai14 mice) fluorescence was detected in GFP-expressing neurons.
Image capture and processing.
General procedure.
Overview images were captured using an Olympus BX61 motorized microscope with Olympus BX-UCB hardware (VS120 slide scanner) and processed using Olympus OlyVIA software (Olympus). Confocal imaging of individual nuclei for quantification of Brs3-positive neurons, pSTAT3-positive neurons and Fos-positive neurons was performed with an upright Zeiss Axio Observer Z1 microscope with a 10X objective, Zeiss 700 confocal hardware, and Zen software (2012; Zeiss). Z-stacks (9–12 ?m) of slices processed for fluorescence in situ hybridization were taken using the same confocal with a 40X objective and collapsed into a maximum intensity projection using Zen software. Images to illustrate expression of tdTomato, ChR2, DREADD, GCaMP or GFP were taken with VS120 slidescanner or Zeiss 700 confocal. Images were minimally processed to adjust brightness and contrast.
Analysis.
Overview images of every third coronal brain section were acquired using a slide-scanning microscope and used to qualitatively describe Brs3 neuron distribution in two Brs3Cre/Y;Ai14/+ mice. Neuron counting and colocalization analysis was performed using neuroanatomical landmarks 72. Neurons were counted manually with the experimenter blinded to the experimental group. Data is represented as mean SEM
Quantification and statistics.
All data are represented as mean SEM or mean + SEM, unless otherwise indicated. No statistical methods were used to pre-determine sample sizes but our sample sizes are similar to those reported in previous publications3266. Data distribution was assumed to be normal, but this was not formally tested. All t-tests were two-sided. All n numbers refer to number of mice used for the experiment.
In experiments in which mice are handled, there is an increase in physical activity, Tb, and energy expenditure that lasts for about 1 h. Taking this into account, our standard quantification intervals for baseline were from 150 to 30 min before intervention (Pre), which was compared with 60 to 180 min after intervention (Post). These intervals avoid disturbances due to personnel in the animal room and the confounding effects of handling. Mice were tested in crossover experiments, using randomized order. Paired t-tests were used to analyze the within-mouse change from baseline effect between control and experimental interventions. Data collection and analysis were not performed blind to the conditions of the experiments.
Optogenetic experiments do not involve handling the mouse. Our standard analysis protocol is to average (within each mouse) five stimulation epochs done during a single day. This averaging minimizes the effects of spontaneous physical activity. For each mouse, data is normalized to the 20-min baseline immediately before laser on. Because of the body’s heat capacity, Tb increases are not instant, so we used 10 to 20 min from laser on as the response period. We used paired t-tests for within-mouse comparison of the effect of stimulation. For comparison between more than 2 groups we used a one-way ANOVA with multiple comparison testing. Data collection and analysis were not performed blind to the conditions of the experiments
Supplementary Material
Acknowledgments
We thank Alexxai Kravitz for input throughout the project and critical reading of the manuscript, Alice Franks for assitance with animal husbandry, Yuning Huang and Yinyan Ma for assistance with surgeries and Audrey Noguchi and Danielle Springer of the NHLBI Murine Phenotyping Core for the cardiovascular telemetry implantation surgeries. Scott Sternson provided the AAV-ChR plasmid construct and Rachael Neve HSVs. MK-5046 was generously donated by Merck. Rabies virus was obtained from the GT3 Core Facility of the Salk Institute, which was funded by NIH-NCI CCSG: P30 014195 and NINDS R24 Core Grant and funding from NEI. This research was supported by the Intramural Research Program (DK075057 MLR, DK075062 MLR, DK075063 MLR, DK07002 MJK) of the National Institute of Diabetes and Digestive and Kidney Diseases, NIH.
Footnotes
Accession Codes
No accession codes were used for this study
Data availability. The data that support the findings of this study are available from the corresponding authors on request.
Competing Interest Statement
The authors declare no competing interests.
References
- 1.Zhang L et al. Anatomical characterization of bombesin receptor subtype-3 mRNA expression in the rodent central nervous system. J Comp Neurol 521, 1020–1039, doi: 10.1002/cne.23216 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Jensen RT, Battey JF, Spindel ER & Benya RV International Union of Pharmacology. LXVIII. Mammalian bombesin receptors: nomenclature, distribution, pharmacology, signaling, and functions in normal and disease states. Pharmacol Rev 60, 1–42 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weber HC, Hampton LL, Jensen RT & Battey JF Structure and chromosomal localization of the mouse bombesin receptor subtype 3 gene. Gene 211, 125–131, doi: 10.1016/S0378-1119(98)00050-X (1998). [DOI] [PubMed] [Google Scholar]
- 4.Xiao C & Reitman ML Bombesin-Like Receptor 3: Physiology of a Functional Orphan. Trends in endocrinology and metabolism: TEM 27, 603–605, doi: 10.1016/j.tem.2016.03.003 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mo C et al. Characterization of NMB, GRP and their receptors (BRS3, NMBR and GRPR) in chickens. J Mol Endocrinol 59, 61–79, doi: 10.1530/JME-17-0020 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y et al. Receptor-specific crosstalk between prostanoid E receptor 3 and bombesin receptor subtype 3. FASEB J 32, 3184–3192, doi: 10.1096/fj.201700337RR (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohki-Hamazaki H et al. Mice lacking bombesin receptor subtype-3 develop metabolic defects and obesity. Nature 390, 165–169 (1997). [DOI] [PubMed] [Google Scholar]
- 8.Ladenheim EE et al. Factors contributing to obesity in bombesin receptor subtype-3-deficient mice. Endocrinology 149, 971–978, doi: 10.1210/en.2007-1319 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brommage R et al. High-throughput screening of mouse knockout lines identifies true lean and obese phenotypes. Obesity 16, 2362–2367, doi: 10.1038/oby.2008.361 (2008). [DOI] [PubMed] [Google Scholar]
- 10.Lateef DM et al. Bombesin-like receptor 3 regulates blood pressure and heart rate via a central sympathetic mechanism. Am J Physiol Heart Circ Physiol 310, H891–898, doi: 10.1152/ajpheart.00963.2015 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lateef DM, Abreu-Vieira G, Xiao C & Reitman ML Regulation of body temperature and brown adipose tissue thermogenesis by bombesin receptor subtype-3. American journal of physiology. Endocrinology and metabolism 306, E681–687, doi: 10.1152/ajpendo.00615.2013 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan XM et al. Regulation of energy homeostasis by bombesin receptor subtype-3: selective receptor agonists for the treatment of obesity. Cell metabolism 11, 101–112, doi: 10.1016/j.cmet.2009.12.008 (2010). [DOI] [PubMed] [Google Scholar]
- 13.Guan XM et al. Antiobesity effect of MK-5046, a novel bombesin receptor subtype-3 agonist. J Pharmacol Exp Ther 336, 356–364, doi: 10.1124/jpet.110.174763 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Reitman ML et al. Pharmacokinetics and pharmacodynamics of MK-5046, a bombesin receptor subtype-3 (BRS-3) agonist, in healthy patients. J Clin Pharmacol 52, 1306–1316, doi: 10.1177/0091270011419854 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nio Y et al. A Novel Selective Bombesin Receptor Subtype 3 Agonist Promotes Weight Loss in Male Diet-Induced Obese Rats with Circadian Rhythm Change. Endocrinology, doi: 10.1210/en.2016-1825 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Xiao C et al. Bombesin-like receptor 3 ( Brs3 ) expression in glutamatergic, but not GABAergic, neurons is required for regulation of energy metabolism. Molecular metabolism 6, 1540–1550, doi: 10.1016/j.molmet.2017.08.013 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J et al. Molecular Basis of the Pharmacological Difference between Rat and Human Bombesin Receptor Subtype-3 (BRS-3). Biochemistry 41, 8954–8960, doi: 10.1021/bi0202777 (2002). [DOI] [PubMed] [Google Scholar]
- 18.Tan CL et al. Warm-Sensitive Neurons that Control Body Temperature. Cell 167, 47–59 e15, doi: 10.1016/j.cell.2016.08.028 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song K et al. The TRPM2 channel is a hypothalamic heat sensor that limits fever and can drive hypothermia. Science 353, 1393–1398, doi: 10.1126/science.aaf7537 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boulant JA Role of the Preoptic-Anterior Hypothalamus in Thermoregulation and Fever. Clinical Infectious Diseases 31, S157–S161, doi: 10.1086/317521 (2000). [DOI] [PubMed] [Google Scholar]
- 21.Zhao ZD et al. A hypothalamic circuit that controls body temperature. Proc Natl Acad Sci U S A 114, 2042–2047, doi: 10.1073/pnas.1616255114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrison SF Central neural control of thermoregulation and brown adipose tissue. Auton Neurosci 196, 14–24, doi: 10.1016/j.autneu.2016.02.010 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamura K & Morrison SF Central efferent pathways for cold-defensive and febrile shivering. J Physiol 589, 3641–3658, doi: 10.1113/jphysiol.2011.210047 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cano G et al. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J Comp Neurol 460, 303–326, doi: 10.1002/cne.10643 (2003). [DOI] [PubMed] [Google Scholar]
- 25.Jeong JH et al. Cholinergic neurons in the dorsomedial hypothalamus regulate mouse brown adipose tissue metabolism. Molecular metabolism 4, 483–492, doi: 10.1016/j.molmet.2015.03.006 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rezai-Zadeh K et al. Leptin receptor neurons in the dorsomedial hypothalamus are key regulators of energy expenditure and body weight, but not food intake. Molecular metabolism 3, 681–693, doi: 10.1016/j.molmet.2014.07.008 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garfield AS et al. Dynamic GABAergic afferent modulation of AgRP neurons. Nat Neurosci 19, 1628–1635, doi: 10.1038/nn.4392 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kataoka N, Hioki H, Kaneko T & Nakamura K Psychological stress activates a dorsomedial hypothalamus-medullary raphe circuit driving brown adipose tissue thermogenesis and hyperthermia. Cell metabolism 20, 346–358, doi: 10.1016/j.cmet.2014.05.018 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Cao WH & Morrison SF Glutamate receptors in the raphe pallidus mediate brown adipose tissue thermogenesis evoked by activation of dorsomedial hypothalamic neurons. Neuropharmacology 51, 426–437, doi: 10.1016/j.neuropharm.2006.03.031 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Dimicco JA & Zaretsky DV The dorsomedial hypothalamus: a new player in thermoregulation. Am J Physiol Regul Integr Comp Physiol 292, R47–63, doi: 10.1152/ajpregu.00498.2006 (2007). [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y et al. Leptin-receptor-expressing neurons in the dorsomedial hypothalamus and median preoptic area regulate sympathetic brown adipose tissue circuits. J Neurosci 31, 1873–1884, doi: 10.1523/JNEUROSCI.3223-10.2011 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Machado NLS et al. A Glutamatergic Hypothalamomedullary Circuit Mediates Thermogenesis, but Not Heat Conservation, during Stress-Induced Hyperthermia. Current Biology, doi: 10.1016/j.cub.2018.05.064 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allison MB et al. TRAP-seq defines markers for novel populations of hypothalamic and brainstem LepRb neurons. Molecular metabolism 4, 299–309, doi: 10.1016/j.molmet.2015.01.012 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeng W et al. Sympathetic neuro-adipose connections mediate leptin-driven lipolysis. Cell 163, 84–94, doi: 10.1016/j.cell.2015.08.055 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dampney RA Central mechanisms regulating coordinated cardiovascular and respiratory function during stress and arousal. Am J Physiol Regul Integr Comp Physiol 309, R429–443, doi: 10.1152/ajpregu.00051.2015 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Viollet C et al. Somatostatin-IRES-Cre Mice: Between Knockout and Wild-Type? Frontiers in endocrinology 8, doi: 10.3389/fendo.2017.00131 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shah BP et al. MC4R-expressing glutamatergic neurons in the paraventricular hypothalamus regulate feeding and are synaptically connected to the parabrachial nucleus. Proc Natl Acad Sci U S A 111, 13193–13198, doi: 10.1073/pnas.1407843111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.An JJ, Liao GY, Kinney CE, Sahibzada N & Xu B Discrete BDNF Neurons in the Paraventricular Hypothalamus Control Feeding and Energy Expenditure. Cell metabolism 22, 175–188, doi: 10.1016/j.cmet.2015.05.008 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pei H, Sutton AK, Burnett KH, Fuller PM & Olson DP AVP neurons in the paraventricular nucleus of the hypothalamus regulate feeding. Molecular metabolism 3, 209–215, doi: 10.1016/j.molmet.2013.12.006 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sutton AK et al. Control of food intake and energy expenditure by Nos1 neurons of the paraventricular hypothalamus. J Neurosci 34, 15306–15318, doi: 10.1523/JNEUROSCI.0226-14.2014 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cannon B & Nedergaard J Brown adipose tissue: function and physiological significance. Physiol Rev 84, 277–359, doi: 10.1152/physrev.00015.2003 (2004). [DOI] [PubMed] [Google Scholar]
- 42.Nguyen NL et al. Separate and shared sympathetic outflow to white and brown fat coordinately regulates thermoregulation and beige adipocyte recruitment. Am J Physiol Regul Integr Comp Physiol 312, R132–R145, doi: 10.1152/ajpregu.00344.2016 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simonds SE et al. Leptin mediates the increase in blood pressure associated with obesity. Cell 159, 1404–1416, doi: 10.1016/j.cell.2014.10.058 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson RH & Swanson LW Organization of inputs to the dorsomedial nucleus of the hypothalamus: a reexamination with Fluorogold and PHAL in rat. Brain Research Reviews 27, 89–118 (1998). [DOI] [PubMed] [Google Scholar]
- 45.Fontes MA et al. Asymmetric sympathetic output: The dorsomedial hypothalamus as a potential link between emotional stress and cardiac arrhythmias. Auton Neurosci, doi: 10.1016/j.autneu.2017.01.001 (2017). [DOI] [PubMed] [Google Scholar]
- 46.Nakamura K Neural circuit for psychological stress-induced hyperthermia. Temperature (Austin) 2, 352–361, doi: 10.1080/23328940.2015.1070944 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakamura K & Morrison SF Central efferent pathways mediating skin cooling-evoked sympathetic thermogenesis in brown adipose tissue. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology 292, R127–R136, doi: 10.1152/ajpregu.00427.2006 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morrison SF Central control of body temperature. F1000Res 5, doi: 10.12688/f1000research.7958.1 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cao WH, Fan W & Morrison SF Medullary pathways mediating specific sympathetic responses to activation of dorsomedial hypothalamus. Neuroscience 126, 229–240, doi: 10.1016/j.neuroscience.2004.03.013 (2004). [DOI] [PubMed] [Google Scholar]
- 50.Dimitrov EL, Kim YY & Usdin TB Regulation of hypothalamic signaling by tuberoinfundibular peptide of 39 residues is critical for the response to cold: a novel peptidergic mechanism of thermoregulation. J Neurosci 31, 18166–18179, doi: 10.1523/JNEUROSCI.2619-11.2011 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Madisen L et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13, 133–140, doi: 10.1038/nn.2467 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peron SP, Freeman J, Iyer V, Guo C & Svoboda K A Cellular Resolution Map of Barrel Cortex Activity during Tactile Behavior. Neuron 86, 783–799, doi: 10.1016/j.neuron.2015.03.027 (2015). [DOI] [PubMed] [Google Scholar]
- 53.Szymczak-Workman AL, Vignali KM & Vignali DA Generation of 2A-linked multicistronic cassettes by recombinant PCR. Cold Spring Harb Protoc 2012, 251–254, doi: 10.1101/pdb.prot067884 (2012). [DOI] [PubMed] [Google Scholar]
- 54.Ravussin Y, Gutman R, LeDuc CA & Leibel RL Estimating energy expenditure in mice using an energy balance technique. Int J Obes 37, 399–403 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sebhat IK et al. Discovery of MK-5046, a Potent, Selective Bombesin Receptor Subtype-3 Agonist for the Treatment of Obesity. ACS Med Chem Lett 2, 43–47, doi: 10.1021/ml100196d (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Graves JA Did sex chromosome turnover promote divergence of the major mammal groups?: De novo sex chromosomes and drastic rearrangements may have posed reproductive barriers between monotremes, marsupials and placental mammals. Bioessays 38, 734–743, doi: 10.1002/bies.201600019 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qi J et al. VTA glutamatergic inputs to nucleus accumbens drive aversion by acting on GABAergic interneurons. Nat Neurosci 19, 725–733, doi: 10.1038/nn.4281 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stratigopoulos G et al. Hypomorphism of Fto and Rpgrip1l causes obesity in mice. J Clin Invest 126, 1897–1910, doi: 10.1172/JCI85526 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fox DA et al. Gestational lead exposure selectively decreases retinal dopamine amacrine cells and dopamine content in adult mice. Toxicol Appl Pharmacol 256, 258–267, doi: 10.1016/j.taap.2011.05.021 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Geerling JC, Yokota S, Rukhadze I, Roe D & Chamberlin NL Kolliker-Fuse GABAergic and glutamatergic neurons project to distinct targets. J Comp Neurol 525, 1844–1860, doi: 10.1002/cne.24164 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu Z, Autry AE, Bergan JF, Watabe-Uchida M & Dulac CG Galanin neurons in the medial preoptic area govern parental behaviour. Nature 509, 325–330, doi: 10.1038/nature13307 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saunders A, Johnson CA & Sabatini BL Novel recombinant adeno-associated viruses for Cre activated and inactivated transgene expression in neurons. Front Neural Circuits 6, 47, doi: 10.3389/fncir.2012.00047 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rudaya AY, Steiner AA, Robbins JR, Dragic AS & Romanovsky AA Thermoregulatory responses to lipopolysaccharide in the mouse: dependence on the dose and ambient temperature. Am J Physiol Regul Integr Comp Physiol 289, R1244–1252, doi: 10.1152/ajpregu.00370.2005 (2005). [DOI] [PubMed] [Google Scholar]
- 64.Lee DL, Webb RC & Brands MW Sympathetic and angiotensin-dependent hypertension during cage-switch stress in mice. Am J Physiol Regul Integr Comp Physiol 287, R1394–1398, doi: 10.1152/ajpregu.00306.2004 (2004). [DOI] [PubMed] [Google Scholar]
- 65.Lateef DM, Abreu-Vieira G, Xiao C & Reitman ML Regulation of body temperature and brown adipose tissue thermogenesis by bombesin receptor subtype-3. American journal of physiology. Endocrinology and metabolism 306, E681–687, doi: 10.1152/ajpendo.00615.2013 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lute B et al. Biphasic effect of melanocortin agonists on metabolic rate and body temperature. Cell metabolism 20, 333–345, doi: 10.1016/j.cmet.2014.05.021 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vianna DML & Carrive P Stress-induced hyperthermia is not mediated by brown adipose tissue in mice. Journal of Thermal Biology 37, 125–129, doi: 10.1016/j.jtherbio.2011.11.010 (2012). [DOI] [Google Scholar]
- 68.Gachkar S et al. 3-Iodothyronamine Induces Tail Vasodilation Through Central Action in Male Mice. Endocrinology 158, 1977–1984, doi: 10.1210/en.2016-1951 (2017). [DOI] [PubMed] [Google Scholar]
- 69.Kim SM et al. Salt sensitivity of blood pressure in NKCC1-deficient mice. Am J Physiol Renal Physiol 295, F1230–1238, doi: 10.1152/ajprenal.90392.2008 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stornetta RL, Inglis MA, Viar KE & Guyenet PG Afferent and efferent connections of C1 cells with spinal cord or hypothalamic projections in mice. Brain Struct Funct 221, 4027–4044, doi: 10.1007/s00429-015-1143-3 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Betley JN, Cao ZF, Ritola KD & Sternson SM Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell 155, 1337–1350, doi: 10.1016/j.cell.2013.11.002 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Paxinos G & Franklin KBJ The mouse brain in stereotaxic coordinates. Compact 3rd edn, (Elsevier Academic Press, 2008). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


