Abstract
Erythropoietic protoporphyria (EPP) is a photodermatosis presenting in childhood with severe pain on sun exposure. The diagnosis is often delayed due to lack of awareness among pediatricians. We describe the diagnostic odyssey of 2 children presenting with symptoms of EPP and report results of a survey of 129 affected individuals.
Keywords: photodermatoses, porphyria, phototoxicity
Erythropoietic protoporphyria (EPP), an autosomal recessive photodermatosis, results from a deficiency of ferrochelatase, the last enzyme in the heme biosynthetic pathway (Figure 1; available at www.jpeds.com), due to mutations in the FECH gene.1 X-linked protoporphyria, a less common condition with essentially the same phenotype, results from gain-of-function mutations in the erythroid-specific ALAS2 gene.2 Both result in the accumulation of erythrocyte protoporphyrin, which is released in the plasma and taken up by the liver and vascular endothelium. Exposure to sunlight, particularly in the visible wavelength, results in photoactivation of the accumulated protoporphyrin leading to a singlet oxygen-mediated reaction resulting in tissue damage and severe pain.1
Figure 1. Heme Biosynthetic Pathway.
In erythropoietic protoporphyria (EPP), biallelic mutations in FECH lead to the deficiency of ferrochelatase, the last enzyme of the heme-biosynthetic pathway resulting in the accumulation of protoporphyrin. In X-linked protoporphyria (XLP), a gain-of-function mutation in ALAS2 results in more protoporphyrin than is required for hemoglobinization and it accumulates despite the normal ferrochelatase activity.
EPP is the most common porphyria in children and prevalence estimates range from 1:200,000 to 1:75,000.1 Patients develop severe, non-blistering phototoxicity within minutes of sun exposure.3,4 The pain is usually described as 10 on the pain scale. Most individuals with EPP experience tingling, itching and burning of the skin preceding the pain, followed by swelling and redness of sun-exposed areas. These prodromal symptoms serve as a warning sign to end sun exposure. Over time, patients develop a conditioned behavior of sun avoidance, which markedly limits their quality of life.5–7
Symptoms are preventable by sun-avoidance through the use of protective clothing, shade and tinted windows. These episodes can last up to seven days, causing missed school or work. Severity is highly variable, however, most patients are able to tolerate <30 minutes of sun exposure.5 With chronic exposure, the involved skin may become hyperkeratotic.3,8 Anemia is seen in ~40% of patients and 25% of patients will develop abnormal serum aminotransferases.1,4
About 2–5% of patients develop clinically significant liver dysfunction due to protoporphyrin deposition in bile and/or hepatocytes which can advance to cholestatic liver failure requiring transplantation. There is an increased risk of gallstones, which may present at a younger age.5
The diagnosis of EPP is made by detection of markedly increased erythrocyte protoporphyrin levels with a predominance of metal-free protoporphyrin. Plasma total porphyrins are also increased.1,5 In other erythrocyte disorders such as lead poisoning and anemia, the excess protoporphyrin accumulates mostly as zinc protoporphyrin. Genetic testing by sequencing the FECH or ALAS2 gene confirms the diagnosis.1,2
Clinical trials have shown that afamelanotide, a synthetic analog of α-melanocyte stimulating hormone, increased pain-free sun exposure and improved quality of life in adults with EPP.4,6,10,11 It has been approved for use in the European Union since 2014 and is currently being evaluated by the US Food and Drug Administration. Other therapies, such as beta carotene and cimetidine have not conclusively been proven to be effective for symptomatic management.9,13
Here we report 2 children with EPP, with a prolonged diagnostic odyssey. We highlight the challenges of identifying this rare, but important, disease which affects quality of life, not only for the patient, but also their families.
PATIENT 1
A 2-year-old white male presented to an urgent care clinic in severe pain, with swelling of the hands and feet after spending several hours at a lake with his family; his symptoms were initially thought to be an allergic reaction to the lake water, the sand, or his sunscreen. The swelling continued overnight, and the next day he was brought to the local emergency room, where his symptoms were attributed to geese feces exposure; he was given a dose of prednisone and discharged. The swelling and pain worsened, causing insomnia over the next several days. The symptoms subsided without additional intervention four days after onset.
The following summer, at age three, he had two similar episodes while swimming, and was given diphenhydramine without relief. During these episodes, he was noted to have intense itching of his skin. He was evaluated by a pediatrician, who discharged him without further testing or recommendations.
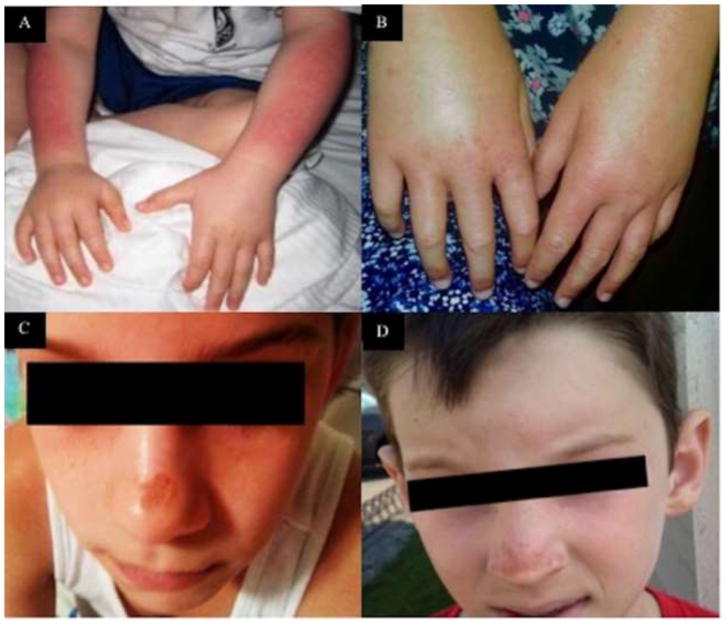
One year later, he was noted to have swelling of his hands and feet after spending time in a pool. He was inconsolable, with intense itching and redness on his extremities. (Figure 2, A) He was brought to the local emergency room, where he was given a dose of prednisone and discharged. After this episode, his family noted that he was hesitant to spend time outdoors and would seek shade whenever possible. He was evaluated by his pediatrician, who attributed his symptoms to a sunscreen allergy and referred him to a pediatric dermatologist, who recommended no further intervention. He then saw an allergist, who had no recommendations. A second pediatrician referred him to another pediatric dermatologist who ordered protoporphyrin testing, finally diagnosing EPP (Table I).
Figure 2. Skin Findings in Individuals with Erythropoietic Protoporphyria.
Skin findings in individuals with EPP include distal upper extremity edema and erythema (a, b), facial edema, erythema, and nasal hyperpigmentation and photodamage (c, d).
Table 1.
Clinical and Laboratory findings
| BIOCHEMICAL TESTS | NORMAL RANGE | PATIENT 1 | PATIENT 2 |
|---|---|---|---|
|
| |||
| Total plasma porphyrins | 0–0.9 mcg/dl | 4.9 mcg/dl | 3.7 mcg/dl |
| Erythrocyte protoporphyrin | 20–80 mcg/dl | 1,946 mcg/dl | 916 mcg/dl |
| % Zinc/Free protoporphyrin | --- | 4%/96% | 8%/92% |
|
| |||
| HEPATIC FUNCTION TESTS | |||
|
| |||
| Aspartate aminotransferase (AST) | 10–40 u/l | 27 u/l | 43 u/l |
| Alanine aminotransferase (ALT) | 1–45 u/l | 24 u/l | 14 u/l |
|
| |||
| HEMATOLOGIES | |||
|
| |||
| Hemoglobin | 10.6–14.4 g/dl | 10.4 g/dl | 11.7 g/dl |
| Hematocrit | 32.0–41.9 g/dl | 31.0 g/dl | 36.1 g/dl |
| Mean corpuscular volume (MCV) | 75.1–87.6 fl | 71.1 fl | 77 fl |
|
| |||
| IRON INDICES | |||
|
| |||
| Serum iron | 50–200 mcg/dl | 30 mcg/dl | 36 mcg/dl |
| Transferrin saturation | 15–50% | 9% | 11% |
| Total iron binding capacity (TIBC) | 250–400 mcg/dl | 339 mcg/dl | 334 mcg/dl |
| Ferritin | 20–200 ng/ml | 8 ng/ml | 13 ng/ml |
| 25-hydroxy Vitamin D | 30–100 ng/ml | 26.5 ng/ml | 24.5 ng/ml |
|
| |||
| GENETIC TESTING | |||
|
| |||
| FECH Mutation/Low Expression Allele* | --- | C411G/IVS3-48T>C | c.1113delT/IVS3-48T>C |
| Mutation type | --- | Missense | Frameshift |
|
| |||
| CLINICAL FINDINGS | |||
|
| |||
| Symptoms | --- | Itching and pain on sun-exposed areas | Tingling, burning and swelling of face, hands and feet |
| Physical exam | --- | Unremarkable | Unremarkable |
The IVS3-48T>C is a low expression allele in the FECH gene present in ~10% of the Caucasian population. EPP patients typically inherited a FECH mutation in trans with the low expression allele.
At 12 years of age, he continues to use photoprotection and minimizes sun exposure as the mainstay of his management. Currently, he experiences symptoms within five minutes of sun exposure.
PATIENT 2
A 5-year-old white female was noted to have pain with itching, burning and swelling on her hands and feet after one day on the beach; her symptoms were refractory to various antihistamines and resolved without intervention after one week. She was seen by a pediatrician who prescribed prednisone, without relief, and a dermatologist. Both suspected a seafood allergy or idiopathic angioedema. She experienced similar symptoms one year later again at the beach. At that time, her pediatrician performed bloodwork which showed a speckled ANA (anti-nuclear antibody) pattern, and she was referred to a pediatric rheumatologist, where her workup was negative. She was also referred to an allergist, where skin testing was negative. During this time, she found relief from avoidance of sun exposure and the use of photoprotective clothing.
At 7 years of age, she went to summer camp and noted scabbing on the tip of her nose in addition to swelling, redness, itching and burning of her hands, arms and face (Figure 2, B and C). This resolved without intervention after 5 days. A consultation with a dermatologist suggested the possibility of a diagnosis of EPP, but no testing was ordered as it was considered unlikely. Shortly after, her mother watched a television show featuring EPP and requested the dermatologist perform laboratory testing, which confirmed the diagnosis of EPP (Table 1).
She is now 11 years of age and reports that her symptoms continue to be worse during the spring and summer. Currently, her symptoms include tingling, burning and swelling of her face, hands and feet that last up to 4 days; they are not alleviated by any interventions.
RESULTS
To evaluate the diagnostic delay in the United States, we surveyed individuals through an anonymous online survey administered through the American Porphyria Foundation (APF) listserv and social media platform. This study was determined to be exempt by the Program for the Protection of Human Subjects at the Icahn School of Medicine at Mount Sinai. Eighty-eight (68%) adults with EPP and 41 (32%) parents of a child with EPP completed the survey. The majority (76%) presented with symptoms of EPP before four years of age (Figure 3; available at www.jpeds.com). This is consistent with a previous report of US patients5; 70% of patients saw their pediatrician or primary health care provider for symptoms, and 44% first saw a pediatric subspecialist; allergy/immunology and dermatology were the most commonly seen (Table 2; available at www.jpeds.com). The mean number of years between initial symptoms and diagnosis of EPP was 13 years (Figure 3). In 43% of total patients, a dermatologist suggested the diagnosis of EPP; a pediatric dermatologist suggested the diagnosis in 7% of cases (Table 3; available at www.jpeds.com). Thirty eight percent of patients saw five or more physicians prior to being diagnosed with EPP; of those, 22% saw more than 10 physicians. In 15% of cases, our respondents suggested the diagnosis of EPP to their physician; 16% first heard about EPP through the media. Prior to diagnosis, the majority of individuals were thought to have a sun allergy (50%), or another type of allergic reaction (16%); 15% of individuals were thought to have a psychological issue. A limitation of our study includes the lack of medical confirmation of the diagnosis. However, patients and family members are prescreened by the APF before joining these groups.
Figure 3.
Age at Onset of Erythropoietic Protoporphyria Symptoms Compared with Age at Erythropoietic Protoporphyria Diagnosis
Table 2.
| Type of Physicians Seen Prior to Diagnosis | ||
|---|---|---|
|
| ||
| Physician Type | # of Responses | Percentage |
| Pediatrician | 66 | 51.16% |
| Family Medicine | 61 | 47.29% |
| Pediatric Dermatologist | 50 | 38.76% |
| Allergist | 37 | 28.68% |
| Dermatologist | 29 | 22.48% |
| Emergency Medicine | 28 | 21.71% |
| Internal Medicine | 24 | 18.60% |
| Hematologist | 14 | 10.85% |
| Geneticist | 7 | 5.43% |
| Child Psychiatrist | 5 | 3.88% |
| Other* | 5 | 3.88% |
| General Practitioner | 3 | 2.33% |
| Pediatric Hepatologist | 2 | 1.55% |
| Cardiologist | 2 | 1.55% |
| Hepatologist | 2 | 1.55% |
| Endocrinologist | 2 | 1.55% |
| Neurologist | 1 | 0.78% |
| Nephrologist | 1 | 0.78% |
| Ophthalmologist | 1 | 0.78% |
|
| ||
| Total Number of Respondents | 129 | 100% |
“Other” comprised of one (1) Porphyria specialist, one (1) Army specialist and three (3) unspecified physician types.
Table 3.
| Physicians Suggesting the Diagnosis of EPP in Childhood (< 18 years of age) vs Adulthood (18 years of age or older)* | ||
|---|---|---|
|
| ||
| Type of Physician | Diagnosed in Childhood (< 18 years of age) | Percentage |
| Dermatologist** | 46*** | 69.69% |
| Primary Care Physician | 8 | 12.12% |
| Unspecified Specialist | 5 | 7.57% |
| Pediatrician | 2 | 3.03% |
| Allergist | 1 | 1.51% |
| Internal Medicine | 1 | 1.51% |
| Hepatologist | 1 | 1.51% |
| Emergency Medicine | 1 | 1.51% |
| Endocrinology | 1 | 1.51% |
|
| ||
| Total Number of Respondents | 66 | |
|
| ||
| Type of Physician | Diagnosed in Adulthood (≥ 18 years of age) | Percentage |
|
| ||
| Dermatologist | 18 | 66.67% |
| Unspecified Specialist | 3 | 11.11% |
| Primary Care Physician | 2 | 7.41% |
| Allergist | 1 | 3.70% |
| Hepatologist | 1 | 3.70% |
| Emergency Medicine | 1 | 3.70% |
| Hematologist | 1 | 3.70% |
|
| ||
| Total Number of Respondents | 27 | |
This table specifies what type of physician suggested the diagnosis of EPP in our surveyed cohort, stratified by age at diagnosis (childhood versus adulthood); respondents who reported that they suggested the diagnosis to a physician or had a family history of EPP (n=36) were excluded.
Includes both dermatologists (n=37) and pediatric dermatologists (n=9).
One respondent indicated that a pediatric dermatologist and a geneticist suggested the diagnosis of EPP.
DISCUSSION
Previous studies in Europe have shown a significant diagnostic delay for patients with EPP. Holme et al identified 389 patients with EPP in the UK; the median ages at symptom onset and diagnosis were one and 12 years, respectively.3 Wahlin et al identified 51 patients with EPP in Sweden; the median ages at onset and diagnosis were one and 22 years.12
Our data and previous reports suggest that the diagnostic delay can be over a decade.3,12 Difficulties in making the diagnosis include the lack of cutaneous lesions in between attacks and the absence of abnormalities on routine laboratory studies. Additionally, protoporphyrin testing needs to be sent to a specialty laboratory for an accurate diagnosis.11 Skin biopsy is not diagnostic as the findings are non-specific.14
Early diagnosis can lead to benefits of decreasing sun exposure and appropriate follow up including monitoring for hepatic complications which, though rare, can present in childhood requiring liver and/or bone marrow transplantation.15 Recommended annual monitoring includes erythrocyte protoporphyrin levels, hepatic function, hematologic indices, an iron profile, and vitamin D 25-OH levels (Table 4; available at www.jpeds.com).1
Table 4.
Key Features of Erythropoietic Protoporphyria and X-Linked Protoporphyria
| Key Features of EPP and XLP |
|---|
SYMPTOMS AFTER SUN-EXPOSURE*
|
| LABORATORY TESTS |
Diagnostic Testing
|
Yearly Monitoring
|
LONG TERM COMPLICATIONS
|
Symptoms may persist for several days after initial phototoxic reaction and can only be prevented by avoiding sun light
Additionally, early diagnosis of EPP is important for management, particularly as newer therapies such as afamelanotide may reach market authorization. Although there is limited published data on the psychosocial effects in children, a recent focus group study suggests that this disorder has a significant impact on children.8
We conclude that there is a need for educating pediatric providers about EPP to prevent a delay in diagnosis in children with phototoxicity, allowing for enhanced quality of life.
Acknowledgments
The Porphyrias Consortium (U54DK083909) is part of Rare Diseases Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), NCATS. This consortium is funded through collaboration between NCATS and NIDDK.M.B. received the NIDDK Career Development Award (K23 DK095946) and served on the advisory boards for Alnylam Pharma, Recordati rare diseases, and Mitsubishi Tanabe.
Abbreviations
- EPP
erythropoietic protoporphyria
Footnotes
The other authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Balwani M, Bloomer J, Desnick R . Porphyrias Consortium of the NIH-Sponsored Rare Diseases Clinical Research Network. Erythropoietic Protoporphyria, Autosomal Recessive. In: Pagon RA, Adam MP, Ardinger HH, et al., editors. GeneReviews® [Internet] Seattle (WA): University of Washington, Seattle; Sep 27, 1993–2017. 2012. [Updated 2014 Oct 16] Available from: https://www.ncbi.nlm.nih.gov/books/NBK100826/ [PubMed] [Google Scholar]
- 2.Balwani M, Bloomer J, Desnick R . Porphyrias Consortium of the NIH-Sponsored Rare Diseases Clinical Research Network. X-Linked Protoporphyria. In: Pagon RA, Adam MP, Ardinger HH, et al., editors. GeneReviews® [Internet] Seattle (WA): University of Washington, Seattle; Feb 14, 1993–2017. 2013. Available from: https://www.ncbi.nlm.nih.gov/books/NBK121284/ [Google Scholar]
- 3.Balwani M, Naik H, Anderson KE, Bissell DM, Bloomer J, Bonkovsky HL, et al. Clinical, Biochemical, and Genetic Characterization of North American Patients with Erythropoietic Protoporphyria and X-linked Protoporphyria. JAMA Dermatol. 2017;153(8):789–796. doi: 10.1001/jamadermatol.2017.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biolcati G, Marchesini E, Sorge F, Barbieri L, Schneider-Yin X, Minder EI. Long-term observational study of afamelanotide in 115 patients with erythropoietic protoporphyria. Br J Dermatol. 2015;172(6):1601–1612. doi: 10.1111/bjd.13598. [DOI] [PubMed] [Google Scholar]
- 5.Gou EW, Balwani M, Bissell DM, Bloomer JR, Bonkovsky HL, Desnick RJ, et al. Pitfalls in erythrocyte protoporphyrin measurement for diagnosis and monitoring of protoporphyrias. Clin Chem. 2015;61(12):1453–1456. doi: 10.1373/clinchem.2015.245456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holme S, Anstey A, Finlay A, Elder G, Badminton M. Erythropoietic protoporphyria in the U.K.: clinical features and effect on quality of life. Br J Dermatol. 2006;155:574–581. doi: 10.1111/j.1365-2133.2006.07472.x. [DOI] [PubMed] [Google Scholar]
- 7.Kim E, Garnock-Jones K. Afamelanotide: A Review in Erythropoietic Protoporphyria. Am J Clin Dermatol. 2016;17:179. doi: 10.1007/s40257-016-0184-6. [DOI] [PubMed] [Google Scholar]
- 8.Lane A, McKay J, Bonkovsky H. Advances in the management of erythropoietic protoporphyria – role of afamelanotide. App Clin Gen. 2016;9:179–189. doi: 10.2147/TACG.S122030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langendonk JG, Wilson JH. Insufficient Evidence of Cimetidine Benefit in Protoporphyria. JAMA Dermatol. 2017;153(2):237. doi: 10.1001/jamadermatol.2016.4049. [DOI] [PubMed] [Google Scholar]
- 10.Lecha M, Puy H, Deybach J-C. Erythropoietic protoporphyria. Orphanet J Rare Dis. 2009;4:19. doi: 10.1186/1750-1172-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marcus D, Halbrecht J, Bourque A, Lew G, Nadel H, Freedman M. Effect of cimetidine on δ-aminolevulinic acid synthase and microsomal heme oxygenase in rat liver. Biochem Pharmacol. 1984;33(13):2005–2008. doi: 10.1016/0006-2952(84)90565-3. [DOI] [PubMed] [Google Scholar]
- 12.Michaels B, Del Rosso J, Mobini N, Michaels J. Erythropoietic Protoporphyria: A Case Report and Literature Review. J Clin Aesth Dermatol. 2010;3(7):44–48. [PMC free article] [PubMed] [Google Scholar]
- 13.Minder E, Schneider-Yin X, Steurer J, Bachmann L. A systematic review of treatment options for dermal photosensitivity in erythropoietic protoporphyria. Cell Mol Biol (Noisy-le-grand) 2009;55(1):84–97. [PubMed] [Google Scholar]
- 14.Naik H, Shenbagam S, Go AM, Balwani M. Psychosocial Issues in Erythropoietic Protoporphyria: The Perspectives of Parents, Children, and Young Adults. Presented at the International Congress of Porphyrins and Porphyrias; Bordeaux, France. June 25–28, 2017. [Google Scholar]
- 15.Rand EB, Bunin N, Cochran W, Ruchelli E, Olthoff KM, Bloomer JR. Sequential liver and bone marrow transplantation for treatment of erythropoietic protoporphyria. Pediatrics. 2006 doi: 10.1542/peds.2006-0833. [DOI] [PubMed] [Google Scholar]
- 16.Wahlin S, Floderus Y, Stål P, Harper P. Erythropoietic protoporphyria in Sweden: demographic, clinical, biochemical and genetic characteristics. J Intern Med. 2011;269:278–288. doi: 10.1111/j.1365-2796.2010.02236.x. [DOI] [PubMed] [Google Scholar]