Abstract
Objective
It has been reported that a disintegrin and metalloproteinase with thrombospondin motifs-12 (ADAMTS-12) is a susceptibility gene for rheumatoid arthritis (RA) development, and its level is significantly increased in RA patients. In addition, ADAMTS-12 was also reported to be required for normal inflammation. This study aims to determine the role of ADAMTS-12 and the underlying mechanisms in the pathogenesis of inflammatory arthritis.
Methods
The collagen-induced arthritis (CIA) model was established in ADAMTS-12-deficient mice and their control littermates to determine the role of ADAMTS-12 in vivo; microCT scanning was used to demonstrate the destruction of ankle joint; histological analysis illustrated synovitis, pannus formation, as well as bone and cartilage destruction; ELISA was performed to measure serum levels of inflammatory cytokines; Protein-protein interaction assays were performed to detect the interactions of ADAMTS-12 and its various deletion mutants with connective tissue growth factor (CTGF).
Results
Deficiency of ADAMTS-12 leads to accelerated inflammatory arthritis in the CIA mouse model. Loss of ADAMTS-12 causes enhanced osteoclastogenesis. In vitro and in vivo protein-protein interaction assays demonstrate CTGF, a previously unrecognized substrate of ADAMTS-12, binding and processing by ADAMTS-12. In addition, deletion of ADAMTS-12 enhances, while overexpression of ADMATS-12 reduces, CTGF-mediated inflammation. Furthermore, ADAMTS-12 regulation of inflammation is largely lost in CTGF deficient macrophages. Importantly, blocking CTGF attenuates elevated inflammatory arthritis seen in ADAMTS-12-deficient CIA mouse model.
Conclusion
ADAMTS-12 proves to be a critical regulator of inflammatory arthritis through, at least in part, controlling CTGF turnover.
Keywords: ADAMTS-12, Metalloproteinase, Inflammatory arthritis, CTGF
Introduction
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease which is characterized by synovial inflammation, progressive bone erosion and cartilage destruction (1). Development of chronic, systemic inflammation is a central characteristic of RA that drives the emergence of pathological disease features, however, the mechanisms underlying interplays between aberrant matrix regulation and inflammation remain incompletely described(2).
Members of the “a disintegrin and metalloproteinase with thrombospondin motifs” (ADAMTS) family consist of extracellular proteases with a precisely ordered modular organization that includes at least one thrombospondin type I repeat (3–5). ADAMTS-12, an ADAMTS family member sharing a structure similar to ADAMTS-7 (3, 4), is expressed in the musculoskeletal system, placenta and fetal lung (6). ADAMTS-12 has been reported to play an important role in various biological processes, including inflammation, arthritis, development, atherosclerosis, cancer, and angiogenesis (6–12).
ADAMTS-12 was recently identified as a rheumatoid arthritis (RA) susceptibility gene (13). Specifically, the rs10461703 single nucleotide polymorphism (SNP) was found to correlate with RA incidence. Moreover, ADAMTS-12 was found to be significantly increased in the cartilage and synovium of RA patients (14, 15). Importantly, ADAMTS-12 is necessary for regulating normal inflammation through inducing neutrophil apoptosis (16), and deletion of ADAMTS-12 leads to enhanced allergen-induced inflammation and airway hyperresponsiveness (17). Interestingly, the release of interleukin-4 (IL-4), interleukin-5 (IL-5), interleukin-13 (IL-13) and interleukin-33 (IL-33) are remarkably increased in allergen-challenged ADAMTS-12-deficient mice compared to their control littermates (17).
Given that inflammation plays a critical role in RA and ADAMTS-12 is a RA- and inflammation-associated gene, we aim to determine whether ADAMTS-12 plays a role in the pathogenesis of RA and to define the mechanisms involved.
Materials and methods
Animals
All animal studies were performed in accordance with institutional guidelines and with approval from the Institutional Animal Care and Use Committee of New York University. Twelve-week-old male mice were used in the experiments. ADAMTS-12 mice on a B6 background were backcrossed at least 10 times before the mice were used for the experiments. Each group contained at least 6 mice and the experiments were replicated 3 times unless otherwise stated.
Collagen-induced arthritis (CIA) mouse model
The CIA mouse model was established as previously described (18). All steps were performed on ice or at 4°C in the dark to avoid the degradation of type II collagen (CII). 100 μg chicken CII (Chondrex, LLC, Seattle, WA) was emulsified with an equal volume of complete Freund’s adjuvant (CFA) (Chondrex, LLC, Seattle, WA) containing 4 mg/ml heat denatured mycobacterium (Chondrex, LLC, Seattle, WA). 0.1 ml of the chicken CII-CFA mixture was intradermally injected into 12-week-old male ADAMTS-12-deficient (KO) mice or wild type (WT) mice on day 0; injections were performed at a site about 1.5 cm distal from the base of the tail. A booster, comprised of 100 μg chicken CII emulsified with an equal volume of incomplete Freund adjuvant (IFA) (Chondrex, LLC, Seattle, WA), was intradermally applied on day 21.
Arthritis clinical score was evaluated using the following criteria: 0 = no evidence of erythema and swelling; 1 = erythema and mild swelling confined to the tarsals or ankle joint; 2 = erythema and mild swelling extending from the ankle to the tarsals; 3 = erythema and moderate swelling extending from the ankle to metatarsal joints; and 4 = erythema and severe swelling encompass the ankle, foot and digits, or ankylosis of the limb. CIA was considered to have been induced when the score reached higher than 1 in more than two limbs or higher than 2 in more than one limb. Incidence was taken as equal to the ratio of CIA mice to the total number of mice exposed to the emulsion injections, multiplied by 100.
To determine whether CTGF could mitigate the inflammation seen in ADAMTS-12-deficient CIA mice, commercially available hIgG or CTGF antibody (FG-3019, provided by FibroGen, Inc, San Francisco, CA) was intraperitoneally administrated at 30 mg/kg body weight twice per week for 6 weeks beginning at 3 weeks following the initial primary immunization.
Osteoclast differentiation
Osteoclastogenesis was performed as previously reported (19). Briefly, bone marrow cells were collected from wild type (WT) or ADAMTS-12-deficient (KO) mice. After that, cells were suspended in prepared culture medium of α-MEM with L-glutamine, penicillin, streptomycin and heat-inactivated 10% FBS, supplemented with 10 ng/ml M-CSF. Then the cells were incubated at 37°C overnight. On the next day, the non-adherent cells (bone marrow-derived macrophages, BMDM) were collected and seeded in 96-well plates for 7 days with 10 ng/ml M-CSF. TRAP staining was used to determine the osteoclast formation. Briefly, the cells were fixed with formalin and processed with regular TRAP staining. Red coloration indicated TRAP-positive cells. TRAP-positive multinucleated cells with more than three nuclei visualized by light microscopy were recorded as osteoclasts.
Yeast-Two-Hybrid (Y2H) assay
Yeast-Two-Hybrid assay was performed as previously described (20). Co-transformation of plasmids carrying the ADAMTS-12 mutants fused with the VP16 transactivation domain and plasmids expressing CTGF fused with GAL4 binding domain was performed in yeast strain CG 1945. Interaction was evaluated on the basis of the growth of co-transformants on selective media lacking tryptophan (Trp), leucine (Leu), and histidine (His) in the presence of 5 mmol/L 3-amino-1,2,4-triazole. The interaction was also verified by color change of co-transformants due to β-galactosidase activity converting X-Gal substrate into a blue product.
Statistical analysis
Results were expressed as mean values ±SD. For comparison of treatment groups, we performed unpaired t-tests (Mann-Whitney), paired t-tests, and one-way or two-way ANOVA (where appropriate). A p value of p<0.05 was considered statistically significant.
Detailed protocols and other procedures, including micro-CT, histological analysis and immunostaining, real-time PCR, generation of CTGF-deficient Raw264.7 macrophages, Co-IP and western blotting, are provided in online supplementary data.
Results
Deficiency of ADAMTS-12 leads to accelerated inflammatory arthritis
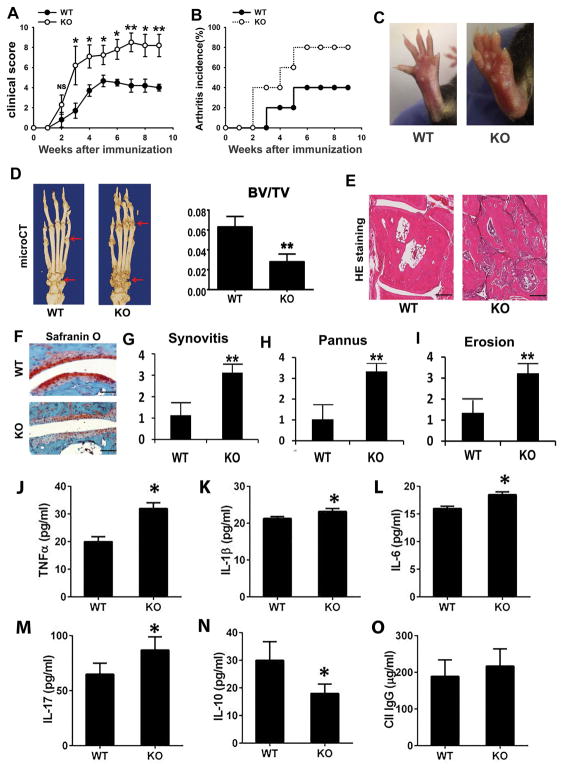
To determine the role of endogenous ADAMTS-12 in vivo in the pathogenesis of inflammatory arthritis, we investigated the clinical and histopathological features of ADAMTS-12-deficient mice and their control littermates in a mouse model of CIA. Accelerated disease onset, as well as significant increases in the arthritis severity score and arthritis incidence, were observed in ADAMTS-12-deficient mice as compared with control littermates (Fig. 1A&B). Representative images demonstrated that ADAMTS-12-deficient mice developed more severe paw swelling than WT mice (Fig. 1C). MicroCT imaging revealed ADAMTS-12-deficient mice exhibited more bone destruction (Fig. 1D). Additionally, statistical analysis indicated that ADAMTS-12-deficient mice exhibited significantly less bone volume to total volume (BV/TV) compared to WT mice (Fig. 1D). Histological analysis of whole ankle joints demonstrated a significant increase in synovitis, pannus formation, and destruction of bone in ADAMTS-12-deficient mice (Fig. 1E, 1G–I). In addition, Safranin O staining revealed that ADAMTS-12-deficient mice exhibited more cartilage loss as well (Fig. 1F). Given that inflammatory arthritis is a systemic inflammatory disease (19), we collected sera from CIA mice to investigate the level of inflammation-related cytokines by ELISA assay. Data indicated the pro-inflammatory cytokines in ADAMTS-12-deficient mice, including TNFα, IL-1β, IL-6, and IL-17, were significantly higher than those in control littermates (Fig. 1J–M). Interestingly, the level of IL-10, an anti-inflammatory cytokine, was significantly lower in ADAMTS-12-deficient mice than that in WT mice (Fig. 1N). These data indicate that ADAMTS-12-deficient mice exhibit enhanced release of pro-inflammatory and reduced secretion of anti-inflammatory cytokines in the CIA model. Given that CIA is also associated with anti-collagen IgG responses, we investigated whether ADAMTS-12 deficiency affected anti-collagen IgG response. The results revealed that ADAMTS-12-deficient CIA mice generated similar level of collagen specific IgG, as compared to WT CIA mice (Fig. 1O), suggesting that increased susceptibility of ADAMTS-12-deficient mice to CIA is not the consequence of an increased anti-collagen IgG response.
Fig. 1. ADAMTS-12-deficient mice are more susceptible to collagen-induced arthritis.
(A) Clinical arthritis scores in wildtype (WT) and knockout (KO) mice with CIA. The data are presented as the mean clinical score ± SEM.* p< 0.05, **p<0.01 versus the control WT group. (B) Incidence of arthritis in the indicated groups. (C) Paws of WT and KO mice were immunized with collagen II for 9 weeks. (D) Representative microCT images of paws of WT and KO collagen II–immunized mice. Arrow indicates areas of severe joint destruction. (E) Hematoxylin and eosin (H&E)–stained sections of ankle joints in WT and KO mice. (F) Safranin O staining of ankle joints in WT and KO mice. (G–I) Evaluation of synovitis, pannus, and erosion of ankle joints in WT and KO mice based on H&E staining. (J–O) Serum levels of TNFα, IL-1β, IL-6, IL-17, IL-10, and mouse anti-type II collagen IgG, as measured by ELISA. Six mice were used in each group and each experiment was repeated for 3 times. Values are mean ± SD *p<0.05, **p<0.01 versus the control WT group. Scale bar, 200μm.
Deficiency of ADAMTS-12 leads to accelerated osteoclastogenesis
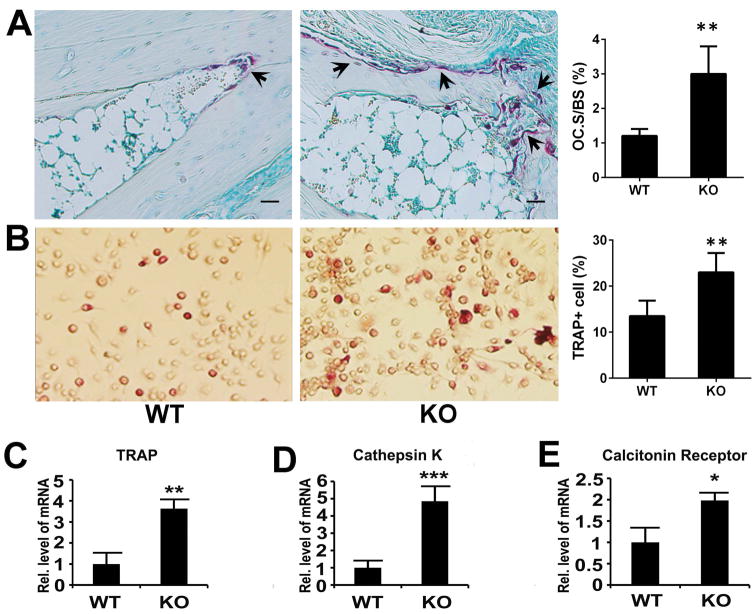
In the pathogenesis of inflammatory arthritis, osteoclastogenesis accompanies bone destruction. Accordingly, we next investigated the role of ADAMTS-12 in osteoclastogenesis during the inflammatory arthritis process. For this purpose, ankle joints collected from CIA mice were used for TRAP staining; more osteoclasts were observed in ADAMTS-12-deficient mice compared to WT mice (Fig. 2A). To further confirm this finding and investigate the mechanism involved, we examined the effects of ADAMTS-12 on osteoclastogenesis in vitro. We isolated primary bone marrow cells and induced differentiation of these cells into osteoclasts. TRAP staining demonstrated that ADAMTS-12 deficiency led to significantly more TRAP-positive osteoclasts, when compared to the control (Fig. 2B). In line with this, ADAMTS-12 deficiency significantly upregulated the expressions of marker genes of osteoclastogenesis, such as TRAP, cathepsin K and calcitonin receptor (Fig. 2C–E). Collectively, ADAMTS-12 deficiency resulted in enhanced osteoclastogenesis in the pathogenesis of inflammatory arthritis.
Fig. 2. Deficiency of ADAMTS-12 leads to accelerated osteoclastogenesis.
(A) Representative TRAP stained ankle joint sections and quantitative result of TRAP+ cell surface per bone surface (Oc.S/BS) from WT and KO mice immunized with collagen II for 9 weeks. Scale bar, 200μm. 6 mice were used in each group and each experiment was repeated 3 times. (B) TRAP staining and enumeration of TRAP+ cells from WT and KO cultures. TRAP-positive staining indicates osteoclast formation, n=3. (C–E) Transcriptional level of TRAP, Cathepsin K and Calcitonin Receptor in WT and KO BMDMs. Values are means ± SD. *p<0.05, **p < 0.01, ***p < 0.001 versus the control WT group. Scale bar, 200μm.
ADAMTS-12 interacts with and cleaves CTGF
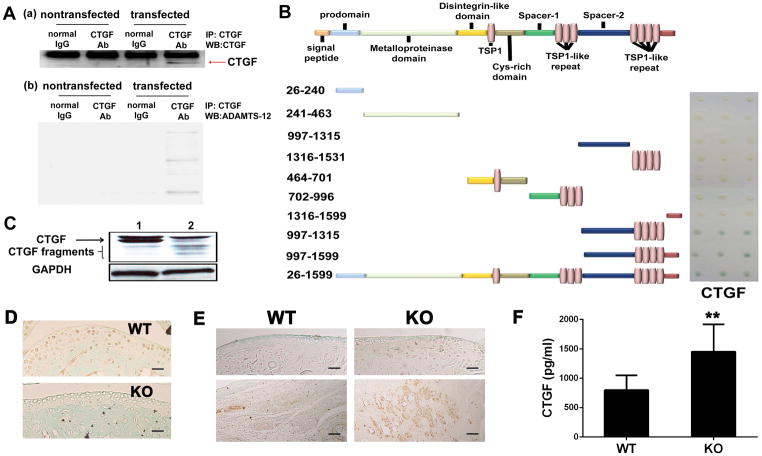
It is known that CTGF plays a pro-inflammatory and pro-osteoclastogenesis role in the pathogenesis of inflammatory arthritis (21). The facts that a) ADAMTS-7 and ADAMTS-12 share similar structure (12), and that b) ADAMTS-7 binds and digests CTGF (20), prompted us to determine whether ADAMTS-12 exerted its role in inflammatory arthritis through interacting with CTGF. To address this issue, we co-transfected CTGF and ADAMTS-12 into 293T cells and performed a Co-IP assay. As indicated in Fig. 3A, a specific CTGF band was immunoprecipitated by anti-CTGF antibodies in CTGF and ADAMTS-12-co-transfected cells; ADAMTS-12 bands were also immunoprecipitated by anti-CTGF antibodies under the same conditions (b), suggesting that ADAMTS-12 specifically associates with CTGF.
Fig. 3. ADAMTS-12 interacts with and cleaves CTGF.
(A) Co-IP assays detect specific associations of ADAMTS-12 with CTGF in immune complexes precipitated with CTGF antibody from protein lysates of 293T cells that were co-transfected with CTGF and ADAMTS-12 expression plasmids. (B) CTGF interaction with ADAMTS-12 or truncated forms in Y2H analyses. Schematic representation of ADAMTS-12 and its truncation mutants. Interaction was evaluated by color change of co-transformants in the colony lift assay resultant of β-galactosidase mediated conversion of X-Gal substrate into a blue product. (C) Western blotting of CTGF. CTGF was transfected into 293T cells (1) or CTGF and ADAMTS-12 were co-transfected into 293T cells together (2). Forty-eight hours later, cell lysates were prepared and CTGF processing was analyzed by Immunoblotting with anti-CTGF antibodies. Non-specific band recognized by antibody is indicated with “X”. (D) Immunohistochemistry (IHC) of ADAMTS-12 in ankle joints of WT and KO CIA mice. (E) IHC of CTGF in ankle joints of WT and KO CIA mice. Top panel, cartilage; Bottom panel, synovium. Scale bar, 200μm. (F) Serum CTGF level as measured by ELISA. Values are means ± SD. **p<0.01 versus the control WT group.
We next sought to identify the domains of ADAMTS-12 required for binding to CTGF. For this purpose, a series of truncated mutants of ADAMTS-12 were subcloned into a yeast expression pPC86 vector and a Y2H assay was performed. Like ADAMTS-7, which was reported to interact with CTGF (20), the C-terminal region containing a mucin domain (also called spacer-2) and thrombospondin type I repeats (TSRs) 5 to 8 of ADAMTS-12, are required and sufficient for interaction with CTGF (Fig. 3B).
More importantly, we observed the accumulation of CTGF fragments in western blot analysis using a rabbit polyclonal antibody that recognized C-terminal CTGF protein (Fig. 3C) in the lysate collected from co-transfected 293T cells, indicating binding and degradation of CTGF by ADAMTS-12 in mammalian cells. Furthermore, immunohistochemistry staining demonstrated that ADAMTS-12 was mainly expressed in cartilage, and CTGF was expressed in cartilage and synovium (Fig. 3D). In addition, the CTGF expression was highly upregulated in ADAMTS-12-deficient CIA mice as compared to WT CIA mice (Fig. 3E). Serum CTGF level was also markedly increased in ADAMTS-12-deficient CIA mice as compared to WT CIA mice (Fig. 3F). Taken together, all of the in vitro and in vivo evidence indicated that ADAMTS-12 interacted with and cleaved CTGF, and that ADAMTS-12 deficiency caused accumulation of CTGF during inflammatory arthritis.
Deletion of ADAMTS-12 enhanced CTGF-mediated inflammatory responses
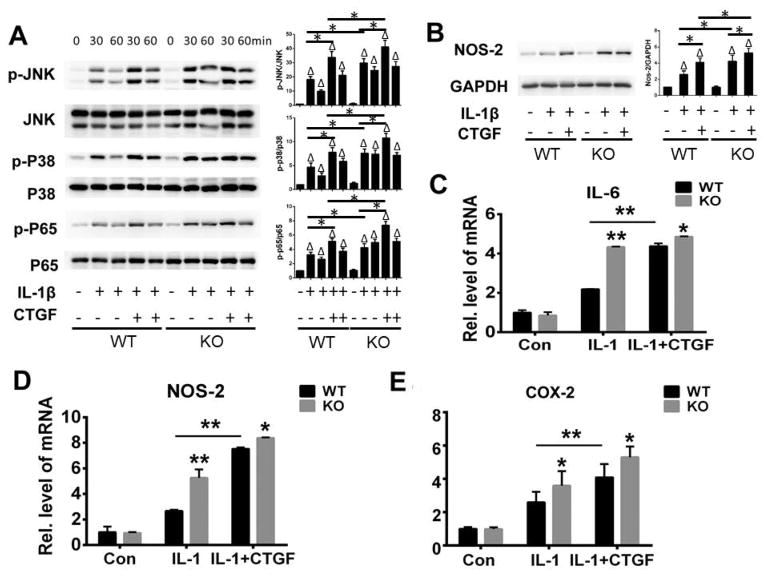
It is known that CTGF is a key pro-inflammatory mediator in fibroblast-like synoviocytes (FLSs) (22). Additionally, in FLSs, CTGF could promote expression of pro-inflammatory mediators in the presence of interleukin-1β (IL-1β) (23). Moreover, CTGF could enhance IL-1β-mediated signaling pathways. Given the fact that ADAMTS-12 interacts with and cleaves CTGF, we next determined whether ADAMTS-12 regulates CTGF-mediated inflammatory signaling. To address this issue, we isolated BMDMs from ADAMTS-12 knockout mice and their control littermates and cultured the cells in the absence or presence of IL-1β without and with CTGF. As indicated in Fig. 4A, IL-1β activated JNK, p38 and NF-kB signaling and CTGF further enhanced IL-1β-mediated inflammatory signaling pathways in WT BMDMs, which is in line with previous findings (23, 24). Interestingly, in ADAMTS-12-deficient BMDMs, the phosphorylation of these molecules was enhanced at each time point compared to the WT BMDMs. In addition, ADAMTS-12 knockout led to increased expression of NOS-2, an inflammation marker, in the presence of CTGF (Fig. 4B). To further investigate the mechanism involved, we cultured the BMDMs with the indicated stimulus for 6 hours and followed by real-time PCR. IL-1β could upregulate the expression of inflammatory factors such as Il-6, NOS-2, and COX-2. The upregulation of these genes was further enhanced in presence of CTGF. To be noted, the transcriptional level of IL-6, NOS-2, and COX-2 was significantly higher in the absence or presence of CTGF in ADAMTS-12-deficient BMDMs, as compared to WT BMDMs in response to IL-1β stimulation (Fig. 4D–4E). Additionally, the transcripts level of IL-6, NOS and COX-2 were significantly higher in combination of CTGF and IL-1β stimulation than IL-1β alone stimulation. Collectively, deficiency of ADAMTS-12 resulted in enhanced CTGF-mediated inflammatory responses.
Fig. 4. ADAMTS-12 deficiency leads to enhanced CTGF-mediated inflammatory responses.
(A) BMDMs from WT and KO mice were incubated with or without IL-1β in absence or presence of CTGF, and phosphorylation and expression of the indicated signaling molecules at various time points were determined by immunoblotting. Phosphorylated protein was quantified relative to total protein. Values are means ± SD. Δ p<0.05 relative expression of phosphorylated protein in Il-1β treated cells with or without CTGF as compared to untreated cells, * p<0.05 relative expression of phosphorylated protein in cells treated with CTGF and IL-1β compared with those treated with IL-1β only, or in KO cells compared to those in WT cells with the similar treatments. (B) BMDMs from WT and KO mice were incubated with or without IL-1β in absence or presence of CTGF for 48 hours, and expression of NOS-2 was examined by immunoblotting. NOS-2 expression was quantified relative to GAPDH. Values are means ± SD. Δ p<0.05 relative expression of NOS-2 in Il-1β treated cells with or without CTGF as compared to untreated cells, * p<0.05 relative expression of NOS-2 in cells treated with CTGF and IL-1β compared with that treated with IL-1β only, or in KO cells compared to those in WT cells with the same treatments. (C–E) Transcriptional level of IL-6, COX-2, and NOS-2 of BMDMs from WT and KO mice were incubated with or without IL-1β in absence or presence of CTGF. Values are means ± SD. *p<0.05, **p < 0.01 versus the control WT group. Unless noted, n=3.
CTGF is a critical mediator of ADAMTS-12 signaling during inflammation
Since loss of ADAMTS-12 increased CTGF-mediated signaling, next we determined whether CTGF is also a critical regulator of ADAMTS-12-mediated signaling. To address this issue, we generated CTGF-deficient Raw264.7 macrophages using the CRISPR-Cas9 technique, then transfected these CTGF-deficient macrophages with an ADAMTS-12 overexpression plasmid. As shown in Fig. 5A, the expression of CTGF was dramatically decreased in CTGF-deficient Raw264.7 macrophages. Interestingly, overexpression of ADAMTS-12 could decrease the activation of inflammatory signaling such as NF-kb, p38 and JNK in response to IL-1β. CTGF deficiency also resulted in the decreased activation of these signaling molecules, and this decrease reached an extent comparable to that observed in ADAMTS-12 overexpressing WT Raw264.7 macrophages. More importantly, overexpression of ADAMTS-12 in CTGF-deficient Raw264.7 macrophages failed to further inhibit the activation of these signal molecules as compared to CTGF-deficient Raw264.7 macrophages (Fig. 5B). Accordingly, the expression of NOS2, one of the inflammatory markers, was inhibited by overexpression of ADAMTS-12 in Raw264.7 macrophages and CTGF-deficient Raw264.7 macrophages at a comparable level in response to IL-1β. Overexpression of ADAMTS-12 in CTGF-deficient Raw264.7 macrophages failed to further inhibit the expression of NOS2 (Fig. 5C). In addition, the transcriptional level of inflammatory markers such as Il-6, NOS-2, and COX-2 exhibit the same trend demonstrated at the protein level for NOS-2 in response to IL-1β (Fig. 5D–F). Taken together, these results suggest that CTGF is a critical mediator of ADAMTS-12-mediated signaling during inflammation. In other words, ADAMTS-12 exerts its inhibition of inflammation through, at least partially, regulating CTGF turnover.
Fig. 5. ADAMTS-12-mediated signaling depends on CTGF during inflammation.
(A) Generation of CTGF-deficient (CTGF-def) Raw264.7 cells using the CRISPR-Cas9 technique. Expression of CTGF was determined by immunoblotting. (B, C) WT or CTGF-def Raw264.7 macrophages were transfected with pcDNA-ADAMTS12 (pcDNA-TS12) for 48hrs, then cells were treated with or without IL-1β for the indicated time. Cells were isolated to determine the phosphorylation of p65, p38 and JNK and the expression level of TS12 (B), or expression of NOS-2 and TS12 (C) by immunoblotting. (D–F) WT or CTGF-def Raw264.7 cells were transfected with pcDNA-ADAMTS12 (pcDNA-TS12) for 48hrs, then cells were treated with or without IL-1β. Transcriptional expression level of Il-6, NOS-2, and COX-2 were evaluated by real-time PCR. Values are means ± SD. *p<0.05, **p < 0.01 versus control group. Unless noted, n=3.
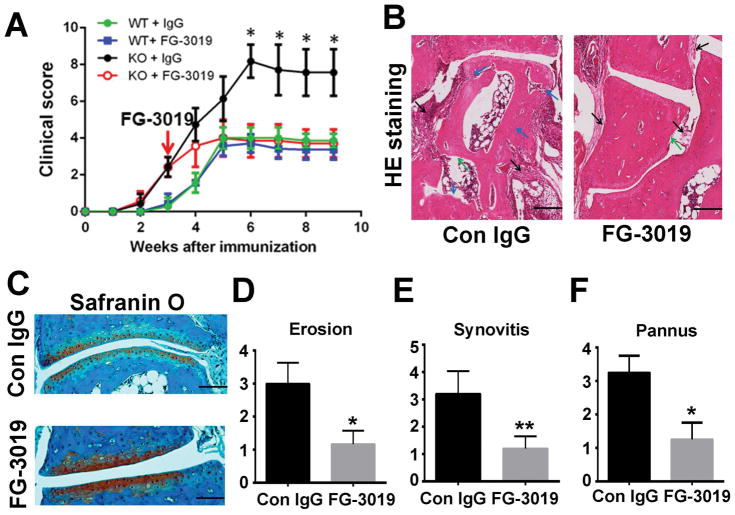
Blocking CTGF attenuates inflammatory arthritis in ADAMTS-12-deficient CIA mice model
To determine whether the enhanced inflammation in ADAMTS-12-deficient CIA mice resulted from accumulation of CTGF, we injected CTGF antibody, FG-3019, into ADAMTS-12-deficient CIA model after disease onset. The arthritis score in ADAMTS-12-deficient mice treated with FG-3019was significantly reduced to a similar level as observed in WT CIA mice (Fig. 6A). Moreover, histological analysis indicated that FG-3019significantly reduced inflammation and abrogated further tissue destruction in ADAMTS-12-deficient CIA mice (Fig. 6B, C). Specifically, the scores for erosion, synovitis, and pannus formation were significantly decreased in the presence of FG-3019 in ADAMTS-12-deficient mice (Fig. 6D–F). These results show that ADAMTS-12 deficiency led to accelerated inflammatory arthritis and blocking CTGF could attenuate elevated inflammatory response in these mice. Cumulatively, these findings hint that processing of CTGF by ADAMTS-12 could limit inflammation.
Fig. 6. Blocking CTGF attenuates inflammatory arthritis in ADAMTS-12-deficient CIA mice.
(A) WT and ADAMTS-12-deficient mice (n = 8 per group) were immunized with type II collagen to establish the CIA model; subsequently, mice were treated with hIgG or anti-CTGF antibodies (FG-3019) for a total of 6 weeks starting from the onset of disease. Clinical arthritis scores were recorded. (B) H&E stained sections of ankle joints in control IgG- or CTGF antibody-treated ADAMTS-12-deficient CIA mice. (C) Safranin O staining of ankle joints in control IgG- or CTGF antibody-treated ADAMTS-12-deficient CIA mice. (D–F) Evaluation of synovitis, pannus, and erosion of ankle joints in control IgG- or CTGF antibody-treated ADAMTS-12 deficient-CIA mice. Data are presented as means ± SD. *P < 0.05; **P < 0.01 vs. hIgG-treated control group. Scale bar, 200μm.
Discussion
RA is a chronic autoimmune disorder in which inflammatory cytokines play a critical role (25). So far, anti-inflammation drugs are widely employed to improve the disease symptoms (26). ADAMTS-12 was shown to be required for maintaining normal inflammation in a model of allergen-induced airway disease (17). Previous data indicated that ADAMTS-12 was an RA susceptibility gene (13) and that the level of ADAMTS-12 was significantly increased in RA patients (14, 15), suggesting that ADAMTS-12 might also play a role in RA pathology. Indeed, in this study we provide comprehensive evidences indicating that ADAMTS-12 plays a critical role in inflammatory arthritis in mice. We found that deletion of ADAMTS-12 in genetically-modified mice leads to enhanced inflammation under the CIA model. Higher clinical score of arthritis and disease incidence in ADAMTS-12-deficient mice indicated that ADAMTS-12-deficient mice are more susceptible to CIA. Given that inflammatory arthritis involves cartilage destruction, bone erosion, and synovitis, we examined the histological changes to bone and cartilage after deleting ADAMTS-12. Data suggested that loss of ADAMTS-12 resulted in more cartilage and bone loss. Importantly, the synovium inflammation and pannus formation were significantly increased in ADAMTS-12-deficient mice. Additionally, it’s well-known that inflammatory cytokines such as TNF-α, IL-1, IL-6, and IL-17 are upregulated, and the anti-inflammatory cytokine, IL-10, is down-regulated in the pathogenesis of RA. Interestingly, our data showed that loss of ADAMTS-12 resulted in increased levels of inflammatory cytokines and decreased levels of anti-inflammatory cytokine. All these data suggest ADAMTS-12 is required for limiting inflammatory arthritis.
Even though the potential involvements of ADAMTS-12 in arthritis was suggested in previous studies (3, 6, 14, 15, 27–29), the elucidation of a detailed mechanism was halted by defining its substrate. Importantly, ADAMTS-7 and ADAMTS-12 share a similar structure and form one ADAMTS sub-group. The high similarity between ADAMTS-7 and ADAMTS-12 suggests they could share a similar substrate. Indeed, both of them were reported to be able to interact with and digest COMP (14, 15, 27, 30–33). Interestingly, it was recently reported that ADAMTS-7 could directly interact with and processes connective tissue growth factor (CTGF). CTGF, also called CCN2, belongs to the CCN family and is associated with angiogenesis, fibrosis, atherosclerotic blood vessels, and erosive arthritis lesions (34). It is well-accepted that CTGF contributes to the pro-inflammatory and pro-atherogenic state in patients with RA (21, 35, 36). Given the importance of CTGF in RA and the similar structure between ADAMTS-7 and ADAMTS-12, we hypothesized ADAMTS-12 also interacts with and cleaves CTGF. Both Y2H and co-immunoprecipitation assays indicated that ADAMTS-12 and CTGF could bind together; the C-terminal mucin and TSP motifs of ADAMTS-12 were required and sufficient for binding CTGF. After overexpression of ADAMTS-12, the expression of full-length CTGF was reduced and the expression of CTGF fragments was increased. Importantly, CTGF appeared to accumulate in the synovium of ADMATS-12-deficient, inflammatory arthritis model mice, suggesting ADAMTS-12 is involved in CTGF turnover and ADAMTS-12 deficiency may slow CTGF clearance.
CTGF is a well-studied protein in arthritis, however, the role of CTGF in osteoarthritis (OA) and inflammatory arthritis is different. OA is considered to be a degenerative disease characterized by cartilage destruction, subchondral bone sclerosis, synovium inflammation, and osteophyte formation (37). Even though it is well-accepted that cartilage, plasma and synovial fluid CTGF concentrations positively correlate with radiographic severity in osteoarthritis patients (38), studies that have indicated a role of CTGF in osteoarthritis are controversial. Specifically, some studies using genetically modified mouse models and in vitro assays have demonstrated a protective role of CTGF against articular cartilage degeneration in OA progression through regulating chondrocyte differentiation and proliferation (39–43). However, given the importance of synovial lining cells in OA progression, some studies suggested that CTGF promoted inflammation and cartilage damage in the pathogenesis of OA (23, 44–46). Regardless of the controversy in OA, studies have consistently demonstrated that CTGF strongly promoted a catabolic effect in inflammatory arthritis progression (21, 47). The different roles of CTGF in OA and inflammatory arthritis may be due to its different roles in different cells and distinct micro-environments. In inflammatory arthritis progression, synovial fibroblasts play a critical role. In both OA and RA fibroblast cells, CTGF could upregulate pro-inflammatory cytokines such as IL-6 and monocyte chemoattractant protein-1 (MCP-1) to accelerate the disorder (45, 46, 48). Interestingly, studies have indicated that blocking CTGF largely ameliorated inflammatory arthritis in multiple murine models of inflammatory arthritis (21, 35, 48). In the present study, we found CTGF-enhanced expression of pro-inflammatory mediators such as IL-6, NOS-2, and COX-2 in response to IL-1β stimulation, and more importantly, ADAMTS-12 deficiency resulted in further enhanced up-regulation of these pro-inflammatory cytokines in response to IL-1β. Additionally, MAPK and NF-kB signaling pathways involved in inflammation were enhanced after deletion of ADAMTS-12. On the other hand, overexpression of ADAMTS-12 reduced CTGF-induced inflammation (Supplementary Fig. 1). ADAMTS-12 constraint of inflammation was lost in CTGF-deficient RAW264.7 macrophages cells (Fig. 5). Our data suggested that ADAMTS-12 deficiency enhanced inflammatory arthritis through, at least in part, the CTGF pathway. To further confirm this finding, we injected FG-3019 into ADAMTS-12-deficient CIA model mice. FG-3019 effectively rescued the symptoms and pathological changes caused by loss of ADAMTS-12. However, FG-3019 failed to further suppress inflammation in WT CIA mice (Fig. 6). These results, together with the finding that blocking CTGF could only bring the level of arthritis to the level of the WT CIA mice, suggest that targeting CTGF could be an effective approach for treating inflammatory arthritis, when the levels of CTGF were significantly elevated, for instance in the absence or deficiency of ADAMTS-12. It should be noted that the failure to suppress inflammation in WT CIA mice by CTGF antibody may be also be due to the insufficient dosage and/or frequency of antibody administration, as well as the genetic background of the mice used in the current study.
Besides inflammation, osteoclastogenesis also plays an important role in inflammatory arthritis by promoting bone erosion. Additionally, pro-inflammatory cytokines effectively promote osteoclast differentiation (19). Accordingly, we examined whether ADAMTS-12 was involved in osteoclast differentiation. The present study indicated that deletion of ADAMTS-12 caused accelerated osteoclast formation both in vivo and in vitro. Considering that CTGF strongly promoted osteoclastogenesis (36), and there is an interaction between CTGF and ADAMTS-12, the data suggest that ADAMTS-12 deficiency caused enhanced osteoclastogenesis at least partially through CTGF pathway. Collectively, in inflammatory arthritis progression, ADAMTS-12-deficient mice might lose the ability for inactivating CTGF, leading to enhanced susceptibility. Even though ADAMTS-12 and ADAMTS-7 share similar structure and substrate(s), the metalloproteinases play different roles in inflammatory arthritis. Previous data indicated overexpression of ADAMTS-7-promoted inflammation and cartilage destruction in transgenic ADAMTS-7 CIA model mice (49). Perhaps ADAMTS-7 exhibits better efficacy in degrading COMP relative to ADMATS-12. Furthermore, the transgenic mouse model may create an artificial environment that cannot fully demonstrate the real mechanism. Additionally, ADAMTS-7 is regarded as a pro-inflammatory metalloproteinase since it forms a positive loop with TNF-α (50). Although ADAMTS-7 could bind and cleave CTGF, TNFα is found to enhance CTGF production in synovial fibroblasts while inhibiting CTGF in chondrocytes. Therefore, the convergent CTGF levels in ADAMTS-7 deficient mice are still unknown and the role ADAMTS-7 in inflammatory arthritis remains to be delineated (48).
In conclusion, ADAMTS-12 appears to be a critical regulator of inflammatory arthritis through, at least partially, interacting with and inactivating CTGF. These findings not only provide novel insights into the role of ADAMTS-12 in the pathogenesis of inflammatory arthritis in vivo, but may also lead to the development of novel therapeutic intervention strategies for rheumatoid arthritis.
Supplementary Material
Acknowledgments
Funding source: This work was supported partly by NIH research grants R01AR062207, R01AR061484, R01NS103931, and a DOD research grant W81XWH-16-1-0482 (to C. J. Liu).
We thank FibroGen, Inc., (San Francisco, California, U.S.A.) for supplying the CTGF antibody (FG-3019) for the research use, and Dr. Santiago Cal from Universidad de Oviedo, Spain, for providing ADAMTS-12 expression constructs.
Footnotes
Contributors
J.W. and W.F. designed the experiments, obtained, analyzed and interpreted the data, and wrote the manuscript. W.H. obtained the data. A.H. obtained the data and edited the manuscript. K.E.L. kindly provided the antibodies and edited the manuscript. C.L. supervised the project, designed the experiments, and edited the manuscript. All authors drafted and reviewed the manuscript.
Conflict of interest
We herein declare that except for K.E.L., we have no conflict of interest. K.E.L. is an employee and shareholder of FibroGen, Inc.
References
- 1.van Steenbergen HW, Aletaha D, Beaart-van de Voorde LJ, Brouwer E, Codreanu C, Combe B, et al. EULAR definition of arthralgia suspicious for progression to rheumatoid arthritis. Ann Rheum Dis. 2017;76(3):491–96. doi: 10.1136/annrheumdis-2016-209846. [DOI] [PubMed] [Google Scholar]
- 2.Okada Y, Wu D, Trynka G, Raj T, Terao C, Ikari K, et al. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature. 2014;506(7488):376–81. doi: 10.1038/nature12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin EA, Liu CJ. The Emerging Roles of ADAMTS-7 and ADAMTS-12 Matrix Metalloproteinases. Open Acess Rheumatology: Research and Review. 2009;22(1):121–31. doi: 10.2147/oarrr.s6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin EA, Liu CJ. The role of ADAMTSs in arthritis. Protein Cell. 2010;1(1):33–47. doi: 10.1007/s13238-010-0002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stanton H, Rogerson FM, East CJ, Golub SB, Lawlor KE, Meeker CT, et al. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature. 2005;434(7033):648–52. doi: 10.1038/nature03417. [DOI] [PubMed] [Google Scholar]
- 6.Liu CJ. The role of ADAMTS-7 and ADAMTS-12 in the pathogenesis of arthritis. Nat Clin Pract Rheumatol. 2009;5(1):38–45. doi: 10.1038/ncprheum0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Llamazares M, Obaya AJ, Moncada-Pazos A, Heljasvaara R, Espada J, Lopez-Otin C, et al. The ADAMTS12 metalloproteinase exhibits anti-tumorigenic properties through modulation of the Ras-dependent ERK signalling pathway. J Cell Sci. 2007;120(Pt 20):3544–52. doi: 10.1242/jcs.005751. [DOI] [PubMed] [Google Scholar]
- 8.El Hour M, Moncada-Pazos A, Blacher S, Masset A, Cal S, Berndt S, et al. Higher sensitivity of Adamts12-deficient mice to tumor growth and angiogenesis. Oncogene. 2010;29(20):3025–32. doi: 10.1038/onc.2010.49. [DOI] [PubMed] [Google Scholar]
- 9.Moncada-Pazos A, Obaya AJ, Fraga MF, Viloria CG, Capella G, Gausachs M, et al. The ADAMTS12 metalloprotease gene is epigenetically silenced in tumor cells and transcriptionally activated in the stroma during progression of colon cancer. J Cell Sci. 2009;122(Pt 16):2906–13. doi: 10.1242/jcs.050468. [DOI] [PubMed] [Google Scholar]
- 10.Cal S, Arguelles JM, Fernandez PL, Lopez-Otin C. Identification, characterization, and intracellular processing of ADAM-TS12, a novel human disintegrin with a complex structural organization involving multiple thrombospondin-1 repeats. J Biol Chem. 2001;276(21):17932–40. doi: 10.1074/jbc.M100534200. [DOI] [PubMed] [Google Scholar]
- 11.Wei J, Richbourgh B, Jia T, Liu C. ADAMTS-12: a multifaced metalloproteinase in arthritis and inflammation. Mediators Inflamm. 2014;2014:649718. doi: 10.1155/2014/649718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelwick R, Desanlis I, Wheeler GN, Edwards DR. The ADAMTS (A Disintegrin and Metalloproteinase with Thrombospondin motifs) family. Genome Biol. 2015;16:113. doi: 10.1186/s13059-015-0676-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nah SS, Lee S, Joo J, Kim HK, Sohn DR, Kwon JT, et al. Association of ADAMTS12 polymorphisms with rheumatoid arthritis. Mol Med Rep. 2012;6(1):227–31. doi: 10.3892/mmr.2012.867. [DOI] [PubMed] [Google Scholar]
- 14.Guo F, Lai Y, Tian Q, Lin EA, Kong L, Liu C. Granulin-epithelin precursor binds directly to ADAMTS-7 and ADAMTS-12 and inhibits their degradation of cartilage oligomeric matrix protein. Arthritis Rheum. 2010;62(7):2023–36. doi: 10.1002/art.27491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu CJ, Kong W, Xu K, Luan Y, Ilalov K, Sehgal B, et al. ADAMTS-12 associates with and degrades cartilage oligomeric matrix protein. J Biol Chem. 2006;281(23):15800–8. doi: 10.1074/jbc.M513433200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moncada-Pazos A, Obaya AJ, Llamazares M, Heljasvaara R, Suarez MF, Colado E, et al. ADAMTS-12 metalloprotease is necessary for normal inflammatory response. J Biol Chem. 2012;287(47):39554–63. doi: 10.1074/jbc.M112.408625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paulissen G, El Hour M, Rocks N, Gueders MM, Bureau F, Foidart JM, et al. Control of allergen-induced inflammation and hyperresponsiveness by the metalloproteinase ADAMTS-12. J Immunol. 2012;189(8):4135–43. doi: 10.4049/jimmunol.1103739. [DOI] [PubMed] [Google Scholar]
- 18.Brand DD, Latham KA, Rosloniec EF. Collagen-induced arthritis. Nat Protoc. 2007;2(5):1269–75. doi: 10.1038/nprot.2007.173. [DOI] [PubMed] [Google Scholar]
- 19.Tang W, Lu Y, Tian QY, Zhang Y, Guo FJ, Liu GY, et al. The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice. Science. 2011;332(6028):478–84. doi: 10.1126/science.1199214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pi L, Jorgensen M, Oh SH, Protopapadakis Y, Gjymishka A, Brown A, et al. A disintegrin and metalloprotease with thrombospondin type I motif 7: a new protease for connective tissue growth factor in hepatic progenitor/oval cell niche. Am J Pathol. 2015;185(6):1552–63. doi: 10.1016/j.ajpath.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nozawa K, Fujishiro M, Kawasaki M, Yamaguchi A, Ikeda K, Morimoto S, et al. Inhibition of connective tissue growth factor ameliorates disease in a murine model of rheumatoid arthritis. Arthritis Rheum. 2013;65(6):1477–86. doi: 10.1002/art.37902. [DOI] [PubMed] [Google Scholar]
- 22.Hurvitz JR, Suwairi WM, Van Hul W, El-Shanti H, Superti-Furga A, Roudier J, et al. Mutations in the CCN gene family member WISP3 cause progressive pseudorheumatoid dysplasia. Nat Genet. 1999;23(1):94–8. doi: 10.1038/12699. [DOI] [PubMed] [Google Scholar]
- 23.Wang Z, Qiu Y, Lu J, Wu N. Connective tissue growth factor promotes interleukin-1beta-mediated synovial inflammation in knee osteoarthritis. Mol Med Rep. 2013;8(3):877–82. doi: 10.3892/mmr.2013.1570. [DOI] [PubMed] [Google Scholar]
- 24.Kular L, Pakradouni J, Kitabgi P, Laurent M, Martinerie C. The CCN family: a new class of inflammation modulators? Biochimie. 2011;93(3):377–88. doi: 10.1016/j.biochi.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 25.Noack M, Miossec P. Selected cytokine pathways in rheumatoid arthritis. Semin Immunopathol. 2017;39(4):365–83. doi: 10.1007/s00281-017-0619-z. [DOI] [PubMed] [Google Scholar]
- 26.Smolen JS, Breedveld FC, Burmester GR, Bykerk V, Dougados M, Emery P, et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis. 2016;75(1):3–15. doi: 10.1136/annrheumdis-2015-207524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luan Y, Kong L, Howell DR, Ilalov K, Fajardo M, Bai XH, et al. Inhibition of ADAMTS-7 and ADAMTS-12 degradation of cartilage oligomeric matrix protein by alpha-2-macroglobulin. Osteoarthritis Cartilage. 2008;16(11):1413–20. doi: 10.1016/j.joca.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu H, Zhu Y. Expression of ADAMTS-7 and ADAMTS-12 in the nucleus pulposus during degeneration of rat caudal intervetebral disc. J Vet Med Sci. 2012;74(1):9–15. doi: 10.1292/jvms.10-0556. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Q, Huang M, Wang X, Xu X, Ni M, Wang Y. Negative effects of ADAMTS-7 and ADAMTS-12 on endplate cartilage differentiation. J Orthop Res. 2012;30(8):1238–43. doi: 10.1002/jor.22069. [DOI] [PubMed] [Google Scholar]
- 30.Bai XH, Wang DW, Kong L, Zhang Y, Luan Y, Kobayashi T, et al. ADAMTS-7, a direct target of PTHrP, adversely regulates endochondral bone growth by associating with and inactivating GEP growth factor. Mol Cell Biol. 2009;29(15):4201–19. doi: 10.1128/MCB.00056-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu CJ, Kong W, Ilalov K, Yu S, Xu K, Prazak L, et al. ADAMTS-7: a metalloproteinase that directly binds to and degrades cartilage oligomeric matrix protein. FASEB J. 2006;20(7):988–90. doi: 10.1096/fj.05-3877fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang L, Wang X, Kong W. ADAMTS-7, a novel proteolytic culprit in vascular remodeling. Sheng Li Xue Bao. 2010;62(4):285–94. [PubMed] [Google Scholar]
- 33.Wang L, Zheng J, Bai X, Liu B, Liu CJ, Xu Q, et al. ADAMTS-7 mediates vascular smooth muscle cell migration and neointima formation in balloon-injured rat arteries. Circ Res. 2009;104(5):688–98. doi: 10.1161/CIRCRESAHA.108.188425. [DOI] [PubMed] [Google Scholar]
- 34.Rico MC, Rough JJ, Del Carpio-Cano FE, Kunapuli SP, DeLa Cadena RA. The axis of thrombospondin-1, transforming growth factor beta and connective tissue growth factor: an emerging therapeutic target in rheumatoid arthritis. Curr Vasc Pharmacol. 2010;8(3):338–43. doi: 10.2174/157016110791112296. [DOI] [PubMed] [Google Scholar]
- 35.Miyashita T, Morimoto S, Fujishiro M, Hayakawa K, Suzuki S, Ikeda K, et al. Inhibition of each module of connective tissue growth factor as a potential therapeutic target for rheumatoid arthritis. Autoimmunity. 2016;49(2):109–14. doi: 10.3109/08916934.2015.1113405. [DOI] [PubMed] [Google Scholar]
- 36.Nozawa K, Fujishiro M, Kawasaki M, Kaneko H, Iwabuchi K, Yanagida M, et al. Connective tissue growth factor promotes articular damage by increased osteoclastogenesis in patients with rheumatoid arthritis. Arthritis Res Ther. 2009;11(6):R174. doi: 10.1186/ar2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei J, Hettinghouse A, Liu C. The role of progranulin in arthritis. Ann N Y Acad Sci. 2016;1383(1):5–20. doi: 10.1111/nyas.13191. [DOI] [PubMed] [Google Scholar]
- 38.Honsawek S, Yuktanandana P, Tanavalee A, Chirathaworn C, Anomasiri W, Udomsinprasert W, et al. Plasma and synovial fluid connective tissue growth factor levels are correlated with disease severity in patients with knee osteoarthritis. Biomarkers. 2012;17(4):303–8. doi: 10.3109/1354750X.2012.666676. [DOI] [PubMed] [Google Scholar]
- 39.Itoh S, Hattori T, Tomita N, Aoyama E, Yutani Y, Yamashiro T, et al. CCN family member 2/connective tissue growth factor (CCN2/CTGF) has anti-aging effects that protect articular cartilage from age-related degenerative changes. PLoS One. 2013;8(8):e71156. doi: 10.1371/journal.pone.0071156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujisawa T, Hattori T, Ono M, Uehara J, Kubota S, Kuboki T, et al. CCN family 2/connective tissue growth factor (CCN2/CTGF) stimulates proliferation and differentiation of auricular chondrocytes. Osteoarthritis Cartilage. 2008;16(7):787–95. doi: 10.1016/j.joca.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 41.Woods A, Pala D, Kennedy L, McLean S, Rockel JS, Wang G, et al. Rac1 signaling regulates CTGF/CCN2 gene expression via TGFbeta/Smad signaling in chondrocytes. Osteoarthritis Cartilage. 2009;17(3):406–13. doi: 10.1016/j.joca.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 42.Nakao K, Kubota S, Doi H, Eguchi T, Oka M, Fujisawa T, et al. Collaborative action of M-CSF and CTGF/CCN2 in articular chondrocytes: possible regenerative roles in articular cartilage metabolism. Bone. 2005;36(5):884–92. doi: 10.1016/j.bone.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 43.Nishida T, Kubota S, Kojima S, Kuboki T, Nakao K, Kushibiki T, et al. Regeneration of defects in articular cartilage in rat knee joints by CCN2 (connective tissue growth factor) J Bone Miner Res. 2004;19(8):1308–19. doi: 10.1359/JBMR.040322. [DOI] [PubMed] [Google Scholar]
- 44.Blaney Davidson EN, Vitters EL, Mooren FM, Oliver N, Berg WB, van der Kraan PM. Connective tissue growth factor/CCN2 overexpression in mouse synovial lining results in transient fibrosis and cartilage damage. Arthritis Rheum. 2006;54(5):1653–61. doi: 10.1002/art.21795. [DOI] [PubMed] [Google Scholar]
- 45.Liu SC, Hsu CJ, Chen HT, Tsou HK, Chuang SM, Tang CH. CTGF increases IL-6 expression in human synovial fibroblasts through integrin-dependent signaling pathway. PLoS One. 2012;7(12):e51097. doi: 10.1371/journal.pone.0051097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu SC, Hsu CJ, Fong YC, Chuang SM, Tang CH. CTGF induces monocyte chemoattractant protein-1 expression to enhance monocyte migration in human synovial fibroblasts. Biochim Biophys Acta. 2013;1833(5):1114–24. doi: 10.1016/j.bbamcr.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki S, Morimoto S, Fujishiro M, Kawasaki M, Hayakawa K, Miyashita T, et al. Inhibition of the insulin-like growth factor system is a potential therapy for rheumatoid arthritis. Autoimmunity. 2015;48(4):251–8. doi: 10.3109/08916934.2014.976631. [DOI] [PubMed] [Google Scholar]
- 48.Nozawa K, Fujishiro M, Takasaki Y, Sekigawa I. Inhibition of rheumatoid arthritis by blocking connective tissue growth factor. World J Orthop. 2014;5(5):653–9. doi: 10.5312/wjo.v5.i5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y, Wei F, Liu CJ. Overexpression of ADAMTS-7 leads to accelerated initiation and progression of collagen-induced arthritis in mice. Mol Cell Biochem. 2015;404(1–2):171–9. doi: 10.1007/s11010-015-2376-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lai Y, Bai X, Zhao Y, Tian Q, Liu B, Lin EA, et al. ADAMTS-7 forms a positive feedback loop with TNF-alpha in the pathogenesis of osteoarthritis. Ann Rheum Dis. 2014;73(8):1575–84. doi: 10.1136/annrheumdis-2013-203561. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.