Abstract
Diabetic kidney disease (DKD), defined as reduced glomerular filtration rate (GFR), elevated urine albumin excretion, or both that is clinically attributable to diabetes, is a common and morbid diabetes complication. Animal-experimental data, observational human studies, and short-term clinical trials suggest that vitamin D and omega-3 fatty acid supplements may be safe and inexpensive interventions to reduce the incidence and progression of DKD. The Vitamin D and Omega-3 Trial to Prevent and Treat DKD (VITAL-DKD) was designed as an ancillary study to the VITAL trial of 25,871 US adults. In a 2 × 2 factorial design, VITAL participants were randomly assigned to vitamin D3 (cholecalciferol, 2000 IU daily) or placebo and to marine omega-3 fatty acids (eicospentaenoic acid and docosahexaenoic acid, 1 g/d) or placebo. VITAL-DKD enrolled a subset of 1,326 VITAL participants with type 2 diabetes at baseline to test the effects of vitamin D and omega-3 fatty acids on changes in estimated GFR and urine albumin excretion. Over five years of follow-up, VITAL-DKD collected blood and urine samples to quantify changes in estimated GFR (the primary study outcome) and urine albumin excretion. At baseline, mean age of VITAL-DKD participants was 67.6 years, 46% were women, 30% were of racial or ethnic minority, and the prevalence of DKD (estimated GFR <60 mL/min/1.73m2 or urine albumin-creatinine ratio ≥30 mg/g) was 17%. In this type 2 diabetes population, VITAL-DKD will test the hypotheses that vitamin D and omega-3 fatty acids help prevent the development and progression of DKD.
Keywords: Diabetes, chronic kidney disease, diabetic kidney disease, vitamin D, omega-3 fatty acids
Introduction
Diabetic kidney disease (DKD), defined as albuminuria, reduced glomerular filtration rate (GFR), or both that is clinically attributable to diabetes, is a large and growing public health burden.1 Over the last 2-3 decades, the prevalence of DKD in the US has increased in direct proportion to the prevalence of diabetes itself, with an estimated 6.9 million people with DKD in 2005-2008.2 Intensive glucose control helps prevent DKD, and renin-angiotensin system (RAS) inhibitors help slow DKD progression.3–6 However, residual risks of DKD development and progression are high, and few new treatments targeting DKD have successfully been introduced in the last two decades. As a result, approximately 50,000 patients now progress from DKD to end stage kidney disease (ESKD) in the US each year.7 Moreover, patients with DKD are at markedly increased risk of cardiovascular disease and mortality.8 To mitigate the growing public health burden of DKD, new approaches are needed to prevent DKD, its progression, and cardiovascular sequelae. Ideally, such approaches should be sufficiently accessible, inexpensive, safe, and effective to apply to the large at-risk diabetes population.
Administration of vitamin D (cholecalciferol or ergocalciferol) is a promising therapeutic intervention for DKD prevention and treatment. While vitamin D has long been used to enhance bone health in selected populations, pleiotropic effects of vitamin D on other organ systems have more recently gained widespread attention.9,10 Vitamin D may have particularly important effects on the kidney, an organ that plays a central role in vitamin D metabolism.11 In animal-experimental models, 1,25-dihydroxyvitamin D (the active hormonal generated from cholecalciferol or ergocalciferol) prevents kidney damage by potently suppressing the RAS, reducing renal inflammation, and exerting direct pro-survival effects on podocytes.12 These effects reduce albuminuria and glomerulosclerosis, in synergy with RAS inhibitors.12–14 Human studies suggest that these effects may have clinical relevance. Lower circulating concentrations of 25-hydroxyvitamin D (used to assess vitamin D sufficiency) have been associated with increased risks of albuminuria and GFR loss in most (though not all) studies.15,16 In short-term clinical trials of people with DKD, 1,25-dihydroxyvitamin D and its analogues significantly reduced albuminuria but also possibly decreased GFR;17 whether this leads to progressive GFR loss over time or to long-term renoprotection, as with RAS inhibitors, is not known. Moreover, supplement forms of vitamin D (cholecalciferol and ergocalciferol) may be more appropriate for DKD prevention because they are less likely to cause hypercalcemia and are inexpensive, but effects of vitamin D supplements on human DKD are not known.
Omega-3 fatty acids, found naturally in high quantities in fatty fish, have vascular and anti-inflammatory properties that may help prevent and treat DKD.18 In animal models of diabetes, omega-3 fatty acids reduced renal inflammation, mesangial expansion, tubulo-interstitial fibrosis, and glomerulosclerosis.18–20 In epidemiologic studies, greater dietary fish intake was associated with decreased risks of albuminuria and reduced GFR, and higher plasma polyunsaturated omega-3 fatty acid concentrations were associated with slower loss of creatinine clearance.18 Short-term clinical trials provide proof of concept for beneficial vascular and anti-inflammatory effects in humans: at least 7 intervention studies suggest that omega-3 fatty acids improve vasodilation and/or decrease markers of endothelial cell activation, important determinants of intraglomerular pressure and urinary albumin filtration, and at least 3 studies of persons with type 2 diabetes found that omega-3 fatty acids reduced measures of oxidative stress, which is closely linked with tissue inflammation and DKD progression. Moreover, a meta-analysis of 17 trials in DKD, IgA nephropathy, or other glomerular diseases reported that omega-3 fatty acids reduced albuminuria by 19% reduction (95% CI 4-34% reduction); the effect estimate was similar though not statistically significant restricted to trials of DKD.21 Two subsequent trials provided additional supportive evidence that omega-3 fatty acids prevent loss of GFR.22,23
In summary, abundant data point to potential renal benefits of both vitamin D and omega-3 fatty acids, but existing evidence is inadequate to recommend routine vitamin D or omega-3 supplementation for the prevention or treatment of DKD. Therefore, we designed the Vitamin D and Omega-3 Trial to Prevent and Treat DKD (VITAL-DKD) to assess the efficacy and safety of vitamin D and omega-3 fatty acids for the prevention and treatment of DKD. Because both vitamin D and omega-3 fatty acid supplements may have beneficial effects that reduce cardiovascular morbidity and mortality among people with diabetes and DKD,11,18 secondary outcomes of VITAL-DKD will assess the effects of these interventions on cardiovascular risk.
Materials and methods
Study design
This study was designed as an ancillary study to the Vitamin D and Omega-3 Trial (VITAL), a randomized, double-blind, placebo-controlled trial of the benefits and risks of vitamin D and marine omega-3 fatty acids in the primary prevention of CVD and cancer (NCT01169259).24 The parent VITAL trial is a large study (N=25,871) that is conducted primarily by mail. The parent VITAL trial collected baseline blood samples on a subset of participants (N=16,954), but follow-up blood samples and urine samples were collected only among a subset of generally healthy participants. The VITAL-DKD trial was built into the parent VITAL trial. Specifically, a subset of VITAL participants with type 2 diabetes at baseline was identified, recruited, and enrolled into VITAL-DKD. Each VITAL-DKD participant was randomly assigned by the parent VITAL trial to vitamin D or placebo and omega-3 fatty acids or placebo. The VITAL-DKD trial collected baseline and follow-up outcome and covariate data specific to DKD to assess effects of study interventions on estimated GFR and albuminuria. The VITAL-DKD trial was approved by the Partners Human Research Committees, the Institutional Review Board of Brigham and Women’s Hospital, and registered with clinicaltrials.gov prior to enrolling participants (NCT01684722).
Study population
The parent VITAL trial is restricted to older individuals (men ages ≥ 50 years, women ages ≥ 55 years) because rates of chronic disease (including cardiovascular diseases, cancer, and kidney disease) increase substantially with age.24 VITAL excluded persons with clinically apparent cardiovascular disease or cancer (except non-melanoma skin cancer) because it is a trial for the primary prevention of these conditions. For the VITAL-DKD trial, we recruited a subset of VITAL participants with prevalent type 2 diabetes. Specifically, we targeted parent VITAL trial participants who reported a physician diagnosis of diabetes at screening and agreed to donate a blood sample to the VITAL study, From this group, we excluded (a) persons who reported a diagnosis of diabetes only during pregnancy (presumed gestational diabetes), and (b) persons who reported diabetes diagnosis prior to age 30 and first treated with insulin (likely type 1 diabetes). We also excluded participants with a known cause of kidney disease other than diabetes (Table 1).
Table 1.
Eligibility criteria for the Vitamin D and Omega-3 Trial to Prevent and Treat Diabetic Kidney Disease (VITAL-DKD)
| Inclusion criteria |
| Age ≥ 50 years (men), ≥ 55 years (women) (P) |
| Self-reported physician diagnosis of diabetes (DKD) |
| Blood and urine samples returned during placebo run-in (DKD) |
| Exclusion criteria |
| Prevalent cardiovascular disease or cancer (except non-melanoma skin cancer) (P) |
| Dialysis (P) or kidney transplant (DKD) |
| Hypercalcemia, hypo- or hyper-parathyroidism (P) |
| Severe liver disease (cirrhosis) or granulomatous disease (P) |
| Diagnosis of diabetes only during pregnancy (DKD) |
| Diabetes diagnosis prior to age 30 & first treated with insulin (DKD) |
(P) = parent VITAL trial criterion; (DKD) = criterion specific to VITALDKD
Enrollment
Potentially eligible individuals were identified during the parent VITAL trial placebo run-in period. Consecutive potentially eligible individuals were contacted for VITAL-DKD until the enrollment goal of 1,320 participants was met. Potentially eligible individuals were mailed a VITAL-DKD kit that included a separate ancillary study consent form, an ancillary study-specific questionnaire, and urine collection materials. Participants entered the VITAL-DKD ancillary study if they: (1) returned complete and valid DKD ancillary study materials; (2) returned a blood sample as part of the parent VITAL trial; (3) met all parent VITAL trial and DKD ancillary study eligibility criteria; and (4) were randomized into the parent VITAL trial.
Interventions
As part of the parent VITAL trial, DKD ancillary study participants were randomly assigned to one of four treatment groups in a 2×2 factorial design: (1) vitamin D plus placebo omega-3 fatty acids; (2) omega-3 fatty acids plus placebo vitamin D; (3) vitamin D plus omega-3 fatty acids; or (4) two placebos.24 Study medications were dispensed in calendar packs, with participants asked to take two pills (one vitamin D or placebo, plus one omega-3 fatty acids or placebo) each day. Vitamin D3 (cholecalciferol, 2000 IU) and matching inert placebo were provided by Pharmavite LLC. Omega-3 fatty acids (Omacor® fish oil, 1 g capsule containing 465 mg of EPA plus 375 mg of DHA) and matching inert placebo were provided by ProNova. Participants were asked to limit vitamin D intake from all supplemental sources combined to ≤800 IU daily and to forego use of non-study fish-oil supplements. Randomization occurred from November, 2011, through March, 2014. Assignments were assigned in blocks of eight, stratified by age, sex, and race. Treatment assignments were concealed to both participants and investigators.
Biosample and data collection
VITAL-DKD biosamples and data were collected at three time points: prior to randomization (baseline), two years after randomization, and five years after randomization. All blood samples, urine samples, and data were collected by mail. Each participant was mailed a DKD ancillary study kit that included paperwork and supplies for the local collection of blood (for follow-up collections only; blood was collected separately at baseline) and urine. Briefly, the full kit included a gel-filled freezer pack, a small box for returning biosamples and forms, and an overnight courier air bill; supplies and instructions for having blood drawn into prelabeled tubes; and a cup, plastic single-use pipette, and two 5-mL screw-top cryovials for urine. The DKD ancillary study questionnaire included detailed questions on diabetes treatments and complications and medication use relevant to diabetes care and hypertension treatment. Participants were instructed to identify a convenient day for phlebotomy (usually at their own healthcare provider or using an in-home testing service paid by the ancillary study) and freeze the gel pack overnight. On the sample collection day, they were instructed to collect a clean-catch first morning void, transfer urine to cryovials, obtain phlebotomy, and return all forms and biosamples with the frozen gel pack using FedEx overnight service. Upon receipt at the central laboratory at the Brigham Women’s Hospital Division of Preventive Medicine laboratory, blood was centrifuged to separate plasma, serum, red blood cells, and buffy coat, and aliquots of blood components and urine were stored at <−80ºC.
Outcomes
The primary outcome of VITAL-DKD is change in estimated GFR (eGFR) from baseline to study year 5. GFR will be estimated from the serum concentrations of creatinine and cystatin C using the CKD-EPI equation, which is the most accurate and precise available estimation method.25 Secondary outcomes include time to the composite outcome of 40% decrease in eGFR from baseline or the development of end stage renal disease;26 change in urine albumin-creatinine ratio (ACR) from baseline to study year 5; time to doubling of urine ACR to a final urine ACR ≥30 mg/g; change in hemoglobin A1c; changes in blood pressure; and changes in markers of cardiovascular risk (high sensitivity C-reactive protein, interleukin-6, and NT-pro brain natriuretic peptide). Changes in eGFR and urine ACR were considered co-primary outcomes until 2016, when funding was provided to extend monitoring for changes in eGFR from 2 to 5 years. Creatinine, cystatin C, albumin, and high sensitivity C-reactive protein are measured on a Beckman DXC chemistry analyzer. Creatinine is measured using a modified Jaffe reaction, cystatin C by immune-turbidimetric assay, and albumin by a timed endpoint method. Creatinine results are traceable to isotope dilution mass spectrometry, and cystatin C results are harmonized with ERM-DA471/IFCC.27 Interleukin-6 and NT-pro brain natriuretic peptide are measured using Mesoscale immunoassays.
Quality control
Laboratory drift and shift due to changes in reagents, calibrator lots, equipment, or equipment settings can introduce selective or nonselective bias into analyses.28,29 Therefore, we prospectively incorporated 5 levels of quality control (QC) into our measurements of creatinine, cystatin C, and urinary albumin in order to prevent lab error and, if present, to effectively detect and account for changes over time. First, assay controls provided by reagent manufacturers were run daily prior to clinical samples to verify proper analyzer function. Second, aliquots of “high” and “low” serum and urine standards, prepared by the hundreds and frozen at −80°C, were run in parallel with clinical samples each day to monitor for drift. Third, “QC sets” of 20 serum or urine samples, also prepared in numerous aliquots and frozen at −80°C and designed to span large ranges of serum creatinine/cystatin C or urine creatinine/albumin, were run periodically throughout the course of sample analysis. Fourth, blind replicates (5-10%) were analyzed to ensure the integrity of the entire assay and data management process, including the identification and labeling of samples and the incorporation of results into master databases. Fifth, national and international standards were measured periodically to verify that creatinine and cystatin C are consistent with NIST and ERM-DA471/IFCC standards. In the event that drift in assay results was identified over time, the “QC sets” can be used to harmonize measurements by generating regression equations and transforming drifted values as indicated.
Data analysis
Distributions of eGFR and urine ACR will be summarized by treatment group over time (baseline, year 2, and year 5), as will changes in eGFR and urine ACR from baseline to years 2 and 5. We will use linear mixed models to test whether changes in eGFR (primary outcome) and urine ACR (secondary outcome) differ by treatment assignment. For each model, either eGFR or urine ACR will be the dependent variable, and time and treatment x time interactions will be included as independent variables. In the event that any baseline characteristics are not balanced between treatment groups, sensitivity analyses will add to the model additional terms for such baseline characteristics and their interactions with time. Primary analyses will be performed in accordance with the intent-to-treat principle, meaning that all participants will be analyzed according to their assigned treatment group, regardless of adherence or follow-up. We will assess for interaction of the study interventions (vitamin D and omega-3 fatty acids) for the primary outcome. If we observe interaction, all analyses will separately compare each of the three active treatment groups to the treatment group that receives (placebo + placebo). If there is no evidence of interaction, all participants assigned to any active vitamin D will be compared to all participants assigned to placebo vitamin D, and all participants assigned to any active omega-3 fatty acids will be compared to all participants assigned to placebo omega-3 fatty acids. We will assess secondary categorical outcomes using discrete Cox proportional hazards models. We will describe the use of concomitant medications (such as glucose- and blood pressure-lowering medications) and laboratory values (such as hemoglobin A1c) over the course of the trial; if we observe large differences by treatment assignment, we will explore whether such differences mediate or attenuate treatment effects through multivariable adjustment. For the vitamin D intervention, prespecified subgroup analyses will be based on baseline total and bioavailable serum 25(OH)D concentrations, urine ACR, and eGFR as well as race and ethnicity. For the omega-3 fatty acid intervention, prespecified subgroup analyses will be based on baseline levels of EPA+DHA and hsCRP.
Power
The sample size of 1,320 participants was designed to provide 80% power to detect a ≥2.3 mL/min/1.73m2 difference in the change in eGFR, comparing each active treatment to placebo, at a two-sided alpha level of 0.05. At this sample size, there is likewise 80% power to detect a 15% or greater relative difference in change in urine ACR. Assumptions included independent and equal allocation of participants to each treatment; 80% of participants contributing at least one follow-up blood and urine sample; variation in baseline eGFR and urine ACR as observed in preliminary data (median (IQR) 80.7 (66.6-93.2) mL/min/1.73m2 and (1.3-10.3) mg/g, respectively); variation in change in eGFR and urine ACR as observed in preliminary data (mean (SD) −0.3 (13.6) mL/min/1.73m2 and −14.4 (98.2) mg/g, respectively); and no interaction between treatments.
Results
Enrollment
Of 3,244 potentially eligible individuals invited to join VITAL-DKD during the parent VITAL trial enrollment period, 1,750 (54%) consented to participate, and 1,326 (41% of those solicited) were randomized into the VITAL-DKD trial (Figure 1).
Figure 1.
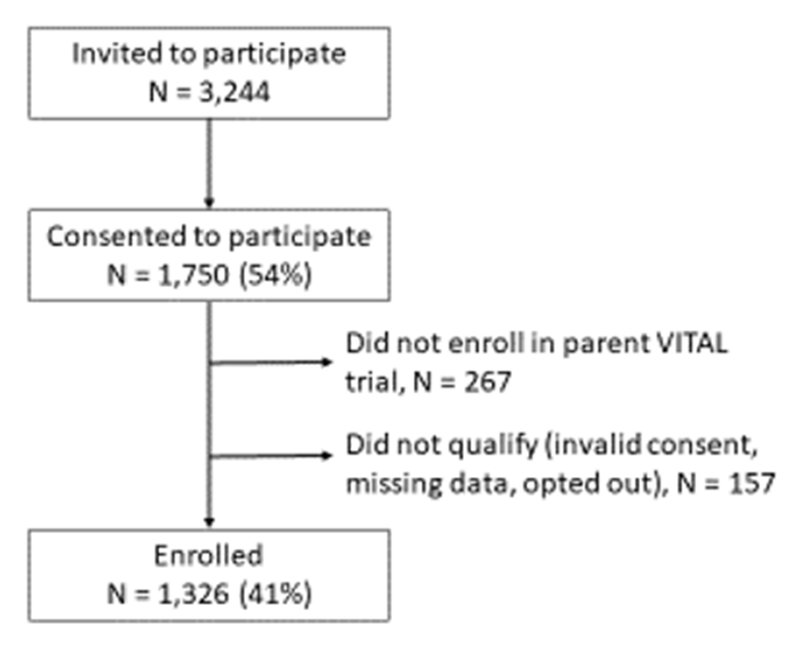
Enrollment in the VITAL-DKD trial.
Baseline characteristics
Distributions of age (mean 67.6 years), sex (46% female), and body mass index (mean 31.4 kg/m2) were similar to the parent VITAL trial and to all VITAL trial participants with diabetes (Table 2). The proportion of participants of minority race or ethnicity enrolled in VITAL-DKD (30%) was similar to that in the overall parent VITAL trial but slightly lower than that of all VITAL trial participants with diabetes. Among enrolled VITAL-DKD participants, the median duration of diagnosed diabetes was between 6 and 10 years, biguanides and sulfonylureas were the most commonly-used glucose-lowering medications, 20% were treated with insulin, 80% were treated with an antihypertensive agent (including 61% with an angiotensin converting enzyme inhibitor, an angiotensin II receptor blocker, or both), and 71% were treated with a lipid-lowering medication (Tables 2 and 3).
Table 2.
Baseline clinical characteristics of participants in the VITAL trial and the subset of VITAL participants who enrolled in the VITAL-DKD trial
| Baseline Characteristic | All enrolled VITAL participants (N=25,871) | All enrolled VITAL participants with diabetes (N=3,402) | Participants enrolled in VITAL-DKD (N=1,326) |
|---|---|---|---|
| Demographics | |||
| Mean age (SD), years | 66.6 (7.1) | 66.6 (7.0) | 67.6 (6.9) |
| Sex, % Female | 13,079 (51) | 1,760 (52) | 615 (46) |
| Race/ethnicity, % | |||
| Non-Hispanic White | 18,046 (71) | 1,799 (54) | 869 (67) |
| African American | 5,106 (20) | 1,163 (35) | 278 (21) |
| Hispanic | 1,013 (4) | 195 (6) | 76 (6) |
| Asian/Pacific Islander | 388 (2) | 76 (2) | 35 (3) |
| American Indian/Alaskan Native | 228 (1) | 35 (1) | 14 (1) |
| Other/unknown | 523 (2) | 72 (2) | 28 (2) |
| Greater than high school education, % | 22,514 (87) | 2,705 (80) | 1,112 (84) |
| Medical history and lifestyle | |||
| Diabetes, %f | 3,402 (13) | 3,402 (100) | 1,326 (100) |
| Smoking, % | 1,836 (7) | 308 (9) | 79 (6) |
| Any alcohol use, % | 17,443 (69) | 1,715 (52) | 702 (54) |
| Medication and supplement use | |||
| Antihypertensive medication, %e | 13,167 (51) | 2,752 (82) | 1,056 (80) |
| Cholesterol-lowering medication, % | 9,524 (37) | 2,148 (65) | 918 (71) |
| Supplemental vitamin D (≤800 IU/dayj), % | 11,030 (43) | 1,229 (36) | 545 (41) |
| Supplemental calcium (≤1200 mg/dayk), % | 6,831 (26) | 744 (22) | 319 (24) |
| Physical characteristics | |||
| Mean body mass index (SD), kg/m2 | 28.1 (5.7) | 32.0 (6.9) | 31.4 (6.7) |
For categorical variables, entries are the number of participants in category and percent of nonmissing responses. For continuous variables, columns contain mean (standard deviation) for nonmissing responses. Prevalence of missing responses for all variables ranged from 0 to 2%.
Table 3.
Additional baseline characteristics of participants in the VITAL-DKD trial
| Participant characteristic | N (%) or mean (SD) |
|---|---|
| Duration of diabetes | |
| < 1 year | 42 (3%) |
| 1-2 years | 177 (13%) |
| 3-5 years | 290 (22%) |
| 6-10 years | 360 (27%) |
| 11-20 years | 315 (24%) |
| > 20 years | 138 (10%) |
| Glucose-lowering medications | |
| Biguanides | 889 (67%) |
| Sulfonylureas | 393 (30%) |
| Thiazolidenediones | 124 (9%) |
| DPP-4 inhibitors | 115 (9%) |
| GLP1 receptor agonists | 48 (4%) |
| Meglitinides | 17 (1%) |
| Alpha-glucosidase inhibitors | 7 (1%) |
| Insulin | 271 (20%) |
| Antihypertensive medications | |
| ACE inhibitors | 573 (43%) |
| Angiotensin II receptor blockers | 262 (20%) |
| Diuretics | 365 (28%) |
| Calcium channel blockers | 276 (21%) |
| Beta blockers | 295 (22%) |
| Mineralocorticoid receptor antagonists | 9 (1%) |
| Number of antihypertensive medication classes | |
| 0 | 312 (24%) |
| 1 | 478 (36%) |
| 2 | 306 (23%) |
| 3 | 161 (12%) |
| ≥ 4 | 69 (5%) |
| Laboratory values | |
| Serum creatinine (mg/dL) | 0.9 (0.3) |
| Serum cystatin C (mg/L) | 0.9 (0.3) |
| Estimated GFR (mL/min/1.73m2) | |
| Using creatinine | 84.8 (19.5) |
| Using serum cystatin C | 87.6 (23.0) |
| Using creatinine and cystatin C | 88.0 (22.1) |
| Estimated GFR category* | |
| ≥60 mL/min/1.73m2 | 1170 (89%) |
| 45-<60 mL/min/1.73m2 | 110 (8%) |
| 30-<45 mL/min/1.73m2 | 35 (3%) |
| <30 mL/min/1.73m2 | 6 (<1%) |
| Urine ACR category* | |
| <30 mg/g | 1207 (91%) |
| 30-<300 mg/g | 94 (7%) |
| ≥300 mg/g | 24 (2%) |
| Serum calcium (mg/dL) | 8.5 (0.7) |
| Serum phosphorus (mg/dL) | 3.7 (1.5) |
| Serum albumin (g/dL) | 4.0 (0.5) |
For categorical variables, entries are the number of participants in category and percent of nonmissing responses. For continuous variables, columns contain mean (standard deviation) for nonmissing responses.
Mean GFR estimated from serum creatinine and cystatin C was 88.0 mL/min/1.73m2 (Figure 2 and Table 3). Estimated GFR was <60 mL/min/1.73m2 for 151 (11%) of participants, and urine ACR was ≥30 mg/g for 118 participants (9%). Combined, 229 participants (17%) had estimated GFR <60 mL/min/1.73m2 or urine ACR ≥30 mg/g.
Figure 2.
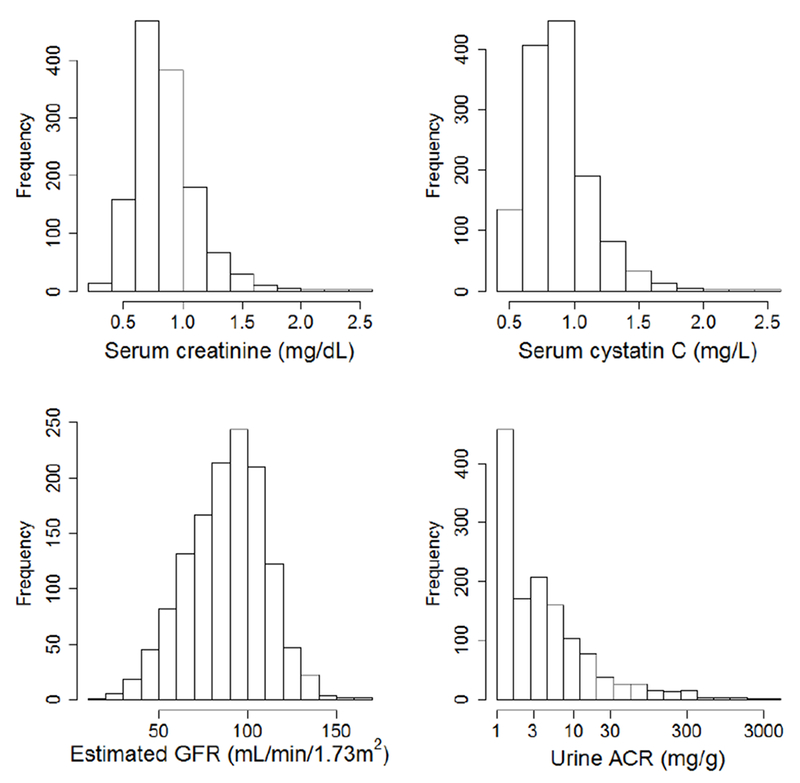
Baseline distributions of serum creatinine, serum cystatin C, estimated glomerular filtration rate (GFR), and urine albumin excretion at baseline in the VITAL-DKD trial. Estimated GFR is calculated from serum creatinine, serum cystatin C, age, sex, and race.
Quality control
Interassay CVs calculated from repeated measurements of matrix-appropriate standards spanning a range of concentrations were 2.4-6.9% for serum creatinine, 6.1-8.2% for serum cystatin C, and 1.9-2.9% for urine albumin. Measurements of serum and urine “QC sets” performed throughout the time period in which VITAL-DKD samples obtained at baseline or year 2 were measured demonstrated no evidence of laboratory drift or shift (Figure 3).
Figure 3. Quality control in the VITAL-DKD trial.
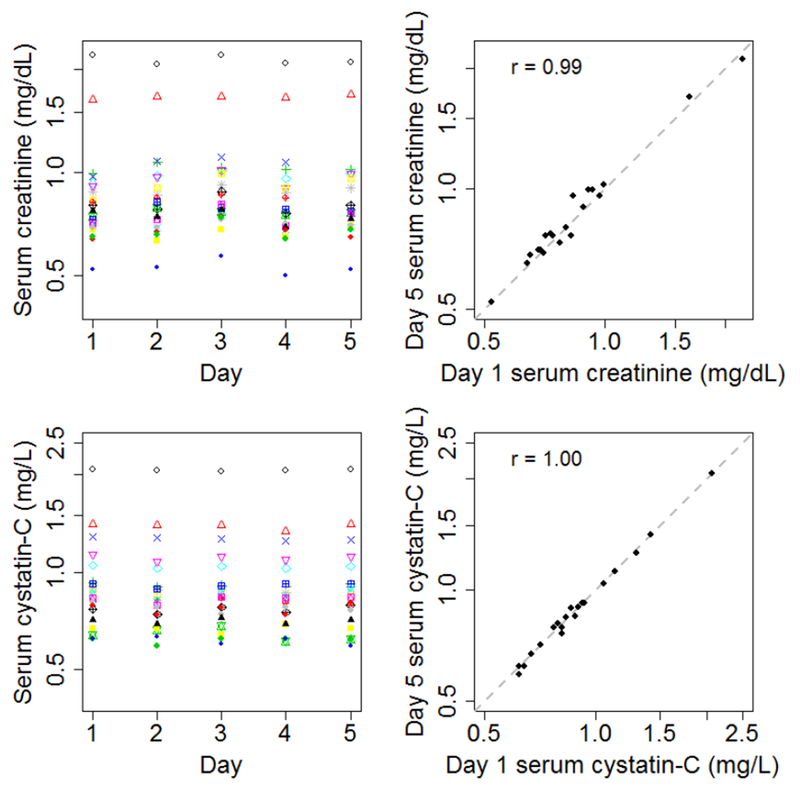
Left panels show serum concentrations of creatinine (top left panel) or cystatin C (bottom left panel) measured from multiple aliquots of 20 quality control samples spanning the time over which VITAL-DKD samples obtained at baseline or year 2 were measured. Each quality control sample is indicated with a distinct color and shape of data point. Right panels show serum concentrations of creatinine (top right panel) or cystatin C (bottom right panel) of the same 20 quality control samples measured on day 1 and day 7, along with the line of identity. Results show absence of shift or drift over the time period of measurement.
Discussion
The VITAL-DKD trial was initiated to test the effects of vitamin D and omega-3 fatty acid supplementation on the development and progression of DKD among people with type 2 diabetes. Potential beneficial effects of these agents on the kidney are supported by animal-experimental models, observational human studies, and short-term clinical trials. However, data on the long-term renal effects of vitamin D and omega-3 fatty acids are not known, and therefore routine use of these agents is not currently recommended by most clinical practice guidelines. The VITAL-DKD trial will help fill this gap in knowledge.
The VITAL-DKD trial built upon the foundation of the broader parent VITAL trial to provide an efficient means for evaluating the core hypotheses. In particular, from the parent VITAL trial, VITAL-DKD leveraged the large group of participants who had already expressed interest in participating in a clinical trial; provision of study medications; mechanisms to maintain retention and adherence; baseline and follow-up data; baseline blood samples; and database, personnel, and laboratory infrastructure. As a result, a relatively large ancillary study with 5 years of follow-up was integrated into a randomized trial with minimal additional expense. One trade-off with this efficient approach included collection of fewer follow-up measurements than many DKD trials, in order to minimize cost and participant burden for the broader parent trial. Fewer measurements increases the potential impact of missing data and makes it difficult to verify that changes in estimated GFR or urine ACR are persistent over time.
An uncommon feature of VITAL-DKD was collection of biosamples locally with overnight mailing to a central laboratory. This approach that allows geographically diverse participants to participate without excessive travel and is suitable for analytes that are stable to such transportation (including creatinine, cystatin C, and albumin),30 but not all analytes.
VITAL-DKD, through interventions provided by the parent VITAL trial, will test forms and doses of vitamin D and omega-3 fatty acids that may be appropriate for use on a wide scale. Vitamin D3 is recommended by many experts as the optimal form of vitamin D supplementation based on its known dose-response effects on 25-hydroxyvitamin D concentration, documented long-term safety, and proven benefits in fracture reduction when used with calcium in the setting of osteoporosis.9 The dose of vitamin D being tested by VITAL (2000 IU, or 50 µg), as well as the dosing schedule (once per day, rather than intermittent large boluses), was selected to provide an optimal balance of efficacy and safety based on evidence at the time of trial initiation and the recommended daily allowance and tolerable upper limit set by the Institute of Medicine.9 A wide range of vitamin D doses have been evaluated in prior trials, with our dose falling in the middle or low-middle of this range. The components and dose of omega-3 fatty acids (465 mg of EPA plus 375 mg of DHA) are consistent with those recommended by the American Heart Association31 and shown to be beneficial, with minimal side effects, in the GISSI trial.32 This dose of omega-3 fatty acids is lower than that used in many prior trials of kidney disease.21
The enrolled VITAL-DKD population is largely representative of the overall parent VITAL trial participants with diabetes, with the exception of race and ethnicity. A high proportion of potentially eligible individuals consented to participation in VITAL-DKD, which helped achieve this representation. Streamlined integration of VITAL-DKD procedures within the broader VITAL trial also likely facilitated participation.
VITAL-DKD participants are slightly less representative of people with diabetes in the broader United States. For example, in VITAL-DKD, the duration of diagnosed diabetes is longer, the prevalence of use of medications to control glycemia, blood pressure, and lipids is higher, and control of intermediate treatment targets (hemoglobin A1c and blood pressure) was better than in the broader US population with diabetes.33 Differences are likely due to the requirement of VITAL-DKD for a physician diagnosis of diabetes (whereas many US adults with diabetes are undiagnosed) and selection of a group with motivation sufficiently high to both participate in a clinical trial and attend carefully to their own health. Our results will apply directly only to adults with diagnosed type 2 diabetes.
The effects of vitamin D and omega-3 fatty acids on DKD may depend on baseline vitamin D and omega-3 fatty acid nutritional status, respectively. Baseline total and bioavailable serum 25(OH)D concentrations and EPA+DHA will be used to evaluate this possibility, but distributions of these biomarkers in our study population are not yet available.
In VITAL-DKD, the prevalence of kidney disease at baseline (17% with estimated GFR <60 mL/min/1.73m2 or urine ACR ≥30 mg/g, including 11% with estimated GFR <60 mL/min/1.73m2 and 9% with urine ACR ≥30 mg/g) was lower than that of the broader US population with diabetes (25%, 12%, and 16%, respectively).34 The relatively low proportion of VITAL-DKD participants with elevated urine ACR, in particular, may portend a low rate of GFR loss in the study, which could limit ability to detect differences by treatment assignment. In addition, while VITAL-DKD includes participants both with and without prevalent DKD, it will likely apply most clearly to patients without prevalent DKD, i.e. DKD prevention.
Consistent measurements of serum creatinine and cystatin C over time are essential to accurately determine treatment effects. Cystatin C results have been reported to shift with changes in reagents and calibrator lots.28,29 Should this problem arise, the prospective quality control approach developed for this project will allow calibration of future values to baseline values and international standards.
In conclusion, VITAL-DKD will test the hypotheses that vitamin D and omega-3 fatty acid supplementation help prevent the development and progression of DKD among people with type 2 diabetes. According to plan, study medications were discontinued on December 31, 2017, and VITAL-DKD has completed follow-up biosample and data collection. Laboratory analyses and quality control are expected to continue through 2018, with results presented in 2019.
Acknowledgements
We are indebted to the 25,871 VITAL participants and to the entire VITAL staff for their dedicated collaboration. The VITAL-DKD trial was funded by R01DK088762 from the National Institute of Diabetes and Digestive and Kidney Diseases. The parent VITAL trial was funded by grants U01CA138962 and R01CA138962, which include support from the National Cancer Institute; National Heart, Lung, and Blood Institute; Office of Dietary Supplements; National Institute of Neurological Disorders and Stroke; and the National Center for Complementary and Integrative Health. Additional support was provided by an unrestricted fund from the Northwest Kidney Centers. Voting members of the VITAL Data and Safety Monitoring Board include Lawrence S. Cohen, MD; Theodore Colton, ScD; Mark A. Espeland,PhD; Craig Henderson, MD; Alice H. Lichtenstein, ScD; Rebecca A. Silliman, MD, PhD; and Nanette K. Wenger, MD (chair). Ex-officio members include Josephine Boyington, PhD, MPH; Rebecca B. Costello, PhD; Cindy D. Davis, PhD; Peter Greenwald, MD; Gabriela Riscuta, MD; and Harold Seifried, PhD
Contributor Information
Ian H. de Boer, Division of Nephrology and Kidney Research Institute, University of Washington, Seattle, WA.
Leila R. Zelnick, Division of Nephrology and Kidney Research Institute, University of Washington, Seattle, WA.
Julie Lin, Renal Division, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA.
Debra Schaumberg, Real World Evidence, Evidera, PPD, Waltham MA; Department of Epidemiology, Harvard TH Chan School of Public Health, Boston MA; Department of Ophthalmology & Visual Sciences, University of Utah School of Medicine, Boston MA.
Lu Wang, Department of Global Epidemiology, Janssen Research & Development LLC, Titusville, NJ.
John Ruzinski, Kidney Research Institute, University of Washington, Seattle, WA.
Georgina Friedenberg, Division of Preventive Medicine, Brigham and Women’s Hospital, MAJulie Duszlak, Division of Preventive Medicine, Brigham and Women’s Hospital, MA.
Vadim Y. Bubes, Division of Preventive Medicine, Brigham and Women’s Hospital, MA.
Andrew N. Hoofnagle, Department of Laboratory Medicine and Kidney Research Institute, University of Washington, Seattle, WA.
Ravi Thadhani, Cedars-Sinai Medical Center, Los Angeles, CA.
Robert J. Glynn, Division of Preventive Medicine, Brigham and Women’s Hospital, Harvard Medical School, and Harvard T.H. Chan School of Public Health, Boston, MA.
Julie E. Buring, Division of Preventive Medicine, Brigham and Women’s Hospital, Harvard Medical School, and Harvard T.H. Chan School of Public Health, Boston, MA.
Howard Sesso, Division of Preventive Medicine, Brigham and Women’s Hospital, Harvard Medical School, and Harvard T.H. Chan School of Public Health, Boston, MA.
JoAnn E. Manson, Division of Preventive Medicine, Brigham and Women’s Hospital, Harvard Medical School, and Harvard T.H. Chan School of Public Health, Boston, MA.
References
- 1.Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic Kidney Disease: A Report From an ADA Consensus Conference. Diabetes Care October 2014;37(10):2864–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA : the journal of the American Medical Association. June 22 2011;305(24):2532–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Boer IH, Group DER. Kidney disease and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. January 2014;37(1):24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zoungas S, Arima H, Gerstein HC, et al. Effects of intensive glucose control on microvascular outcomes in patients with type 2 diabetes: a meta-analysis of individual participant data from randomised controlled trials. The lancet. Diabetes & endocrinology. June 2017;5(6):431–437. [DOI] [PubMed] [Google Scholar]
- 5.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. September 20 2001;345(12):851–860. [DOI] [PubMed] [Google Scholar]
- 6.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. September 20 2001;345(12):861–869. [DOI] [PubMed] [Google Scholar]
- 7.United States Renal Data System. 2016 USRDS annual data report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2016. [Google Scholar]
- 8.Fox CS, Matsushita K, Woodward M, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. November 10 2012;380(9854):1662–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dietary Reference Intakes for Calcium and Vitamin D, Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Washington, D.C.: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 10.Rosen CJ. Vitamin D Insufficiency. New England Journal of Medicine. 2010;364:248–254. [DOI] [PubMed] [Google Scholar]
- 11.Bosworth C, de Boer IH. Impaired vitamin D metabolism in CKD. Seminars in nephrology. March 2013;33(2):158–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Z, Zhang Y, Ning G, Deb DK, Kong J, Li YC. Combination therapy with AT1 blocker and vitamin D analog markedly ameliorates diabetic nephropathy: blockade of compensatory renin increase. Proc Natl Acad Sci U S A. October 14 2008;105(41):15896–15901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito I, Waku T, Aoki M, et al. A nonclassical vitamin D receptor pathway suppresses renal fibrosis. J Clin Invest. November 2013;123(11):4579–4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Deb DK, Zhang Z, et al. Vitamin D receptor signaling in podocytes protects against diabetic nephropathy. Journal of the American Society of Nephrology : JASN. December 2012;23(12):1977–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Boer IH, Katz R, Chonchol M, et al. Serum 25-hydroxyvitamin D and change in estimated glomerular filtration rate. Clinical journal of the American Society of Nephrology : CJASN. September 2011;6(9):2141–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Boer IH, Sachs MC, Cleary PA, et al. Circulating vitamin D metabolites and kidney disease in type 1 diabetes. The Journal of clinical endocrinology and metabolism. December 2012;97(12):4780–4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Borst MH, Hajhosseiny R, Tamez H, Wenger J, Thadhani R, Goldsmith DJ. Active Vitamin D Treatment for Reduction of Residual Proteinuria: A Systematic Review. Journal of the American Society of Nephrology : JASN. August 8 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shapiro H, Theilla M, Attal-Singer J, Singer P. Effects of polyunsaturated fatty acid consumption in diabetic nephropathy. Nature reviews. Nephrology. February 2011;7(2):110–121. [DOI] [PubMed] [Google Scholar]
- 19.Hagiwara S, Makita Y, Gu L, et al. Eicosapentaenoic acid ameliorates diabetic nephropathy of type 2 diabetic KKAy/Ta mice: involvement of MCP-1 suppression and decreased ERK1/2 and p38 phosphorylation. Nephrol Dial Transplant. March 2006;21(3):605–615. [DOI] [PubMed] [Google Scholar]
- 20.Garman JH, Mulroney S, Manigrasso M, Flynn E, Maric C. Omega-3 fatty acid rich diet prevents diabetic renal disease. Am J Physiol Renal Physiol. February 2009;296(2):F306–316. [DOI] [PubMed] [Google Scholar]
- 21.Miller ER 3rd, Juraschek SP, Appel LJ, et al. The effect of n-3 long-chain polyunsaturated fatty acid supplementation on urine protein excretion and kidney function: meta-analysis of clinical trials. Am J Clin Nutr. June 2009;89(6):1937–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoogeveen EK, Geleijnse JM, Kromhout D, et al. Effect of omega-3 fatty acids on kidney function after myocardial infarction: the Alpha Omega Trial. Clin J Am Soc Nephrol. October 7 2014;9(10):1676–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong CY, Yiu KH, Li SW, et al. Fish-oil supplement has neutral effects on vascular and metabolic function but improves renal function in patients with Type 2 diabetes mellitus. Diabet Med. January 2010;27(1):54–60. [DOI] [PubMed] [Google Scholar]
- 24.Manson JE, Bassuk SS, Lee IM, et al. The VITamin D and OmegA-3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemporary clinical trials. January 2012;33(1):159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. The New England journal of medicine. July 5 2012;367(1):20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levey AS, Inker LA, Matsushita K, et al. GFR decline as an end point for clinical trials in CKD: a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis. December 2014;64(6):821–835. [DOI] [PubMed] [Google Scholar]
- 27.Grubb A, Blirup-Jensen S, Lindstrom V, Schmidt C, Althaus H, Zegers I. First certified reference material for cystatin C in human serum ERM-DA471/IFCC. Clin Chem Lab Med. November 2010;48(11):1619–1621. [DOI] [PubMed] [Google Scholar]
- 28.White CA, Rule AD, Collier CP, et al. The impact of interlaboratory differences in cystatin C assay measurement on glomerular filtration rate estimation. Clinical journal of the American Society of Nephrology : CJASN. September 2011;6(9):2150–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Boer IH, Sun W, Cleary PA, et al. Longitudinal changes in estimated and measured GFR in type 1 diabetes. J Am Soc Nephrol. April 2014;25(4):810–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller WG, Bruns DE, Hortin GL, et al. Current issues in measurement and reporting of urinary albumin excretion. Clin Chem. January 2009;55(1):24–38. [DOI] [PubMed] [Google Scholar]
- 31.American Heart Association Nutrition C, Lichtenstein AH, Appel LJ, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. July 4 2006;114(1):82–96. [DOI] [PubMed] [Google Scholar]
- 32.Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet. August 7 1999;354(9177):447–455. [PubMed] [Google Scholar]
- 33.Afkarian M, Zelnick LR, Hall YN, et al. Clinical Manifestations of Kidney Disease Among US Adults With Diabetes, 1988–2014. JAMA. August 9 2016;316(6):602–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zelnick LR, Weiss NS, Kestenbaum BR, et al. Diabetes and CKD in the United States Population, 2009–2014. Clin J Am Soc Nephrol. December 7 2017;12(12):1984–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]


