Abstract
Objective
To estimate the prevalence of metabolic syndrome (MetS) and examine its association with chronic kidney disease (CKD) progression in children enrolled in the Chronic Kidney Disease in Children (CKiD) study.
Study design
MetS was defined as being overweight or obese and having ≥2 cardiometabolic risk factors (CMRFs). Incidence, and prevalence of MetS were assessed using pairs of visits approximately two years apart.
Results
A total of 799 pairs of person-visits (contributed by 472 children) were included in the final analysis. Of these, 70% had a normal body mass index (BMI), 14% were overweight and 16% were obese. At the first visit, the prevalence of MetS in the overweight group was 40% and in the obese group was 60%. In adjusted models, annual percent eGFR decline in those who had normal BMI and incident or persistent multiple CMRFs or those with persistent MetS was −6.33%, nus;6.46%, and nus;6.08% (respectively) compared with children who never had multiple CMRFs (nus;3.38%, P = .048, 0.045, and 0.036 respectively). Children with normal BMI and incident multiple CMRFs and those with persistent MetS had approximately twice the odds of fast eGFR decline (>10% per year) compared with those without multiple CMRFs and normal BMI.
Conclusion
Children with CKD have a high prevalence of MetS. These children as well as those with normal BMI but multiple CMRFs experience a faster decline in kidney function.
Keywords: Chronic kidney disease, children, metabolic syndrome, cardiovascular risk
Cardiovascular and metabolic health are important in the management of pediatric chronic kidney disease (CKD). Children with CKD have excess cardiovascular risk factors, and many suffer from metabolic conditions such as obesity.1 The interaction between cardiovascular and metabolic health in the context of CKD presents a challenge in terms of stratifying risk. The clustering of cardiometabolic risk factors (CMRFs) such as insulin resistance, dyslipidemia, hypertension and obesity, is termed metabolic syndrome (MetS). The presence of MetS is associated with a higher incidence of CKD and, among adult patients with established CKD, with a more rapid decline in glomerular filtration rate (GFR).2,3 MetS is relatively uncommon in children. A recent systemic review estimated the median prevalence to be 3.3% (range 0–19.2%).4 We previously demonstrated that nearly 40% of pediatric kidney transplant patients met criteria for MetS at one year after renal transplantation and a substantial percentage (28%) of these patients have developed MetS during the first post-transplant year, mostly due to an increased prevalence of obesity and associated cardiovascular risk factors.5 Although the prevalence of obesity in children with CKD prior to kidney transplant is similar to that in the general population, patients with CKD have a higher prevalence of other traditional cardiovascular risk factors. For example, almost half of the children enrolled in the CKiD multicenter observational study of children with CKD stage 2–4, had hypertension and dyslipidemia, and about one-third of them had a combination of at least 2 CMRFs.6 These data suggest that as in kidney transplant recipients, MetS is likely more frequent in children with CKD than in the general population of children.
The goal of this study was to estimate the prevalence and incidence of MetS in children with mild-moderate CKD and to examine its association with CKD progression in children enrolled in the CKiD study. We hypothesized that a significant proportion of children will meet criteria for the diagnosis of metabolic syndrome and that the presence of MetS will be associated with a more rapid decline in kidney function.
METHODS
CKiD is a multicenter observational prospective cohort study of the natural history and progression of pediatric CKD. A total of 891 children have been enrolled, ages between 1–16 years and an estimated GFR (eGFR) 30–90 ml/min/1.73m2 at 54 clinical sites in the United States and Canada. Full details of the study design, structure and research procedures have been previously published.7 Briefly, during the annual CKiD study visit, each subject had height, weight, and manual blood pressure measured. The laboratory data included a basal metabolic profile, cystatin C, urine protein to creatinine ratio, lipid profile, and fasting blood glucose. The study design and protocols were approved by Institutional Review Boards at each site and by an external study monitoring board appointed by the National Institute of Diabetes and Digestive and Kidney diseases.
Metabolic syndrome
MetS was defined as the presence of overweight or obesity as defined by an age- and sex-specific body mass index (BMI) >85th percentile, as well as having 2 or more CMRFs (high triglycerides; low HDL cholesterol, hypertension and impaired fasting plasma glucose levels).8 Abnormally high triglycerides were defined as being greater than or equal to the age- and sex-specific 95th percentiles, and abnormally low HDL was defined as being less than or equal to the age- and sex-specific 5th percentile.8 Elevated systolic or diastolic blood pressure or hypertension was defined as being greater than or equal to the age- and sex-specific 90th percentile or self-reported hypertension with use of antihypertensive therapies. Impaired fasting plasma glucose was defined as being > 100 mg/dl.9 Triglycerides and cholesterol were measured at the second annual CKiD study visit and every two years thereafter. Based upon the BMI, the MetS phenotypes were categorized as mild MetS phenotype (BMI 85–95th percentile and ≥2 CMRFs) and severe MetS phenotype (BMI ≥ 95th percentile and ≥ 2 CMRFs). In addition to categorizing MetS as the presence of high BMI with at least two CMRFs, we also categorized the presence of multiple CMRFs among children who had a normal BMI (i.e., age and sex-specific BMI between 5th and 85th percentiles). This allowed us to evaluate the two distinct groups in addition to those with elevated BMI and MetS: the “metabolically healthy overweight or obese” ie, high BMI without ≥ 2 CMRFs and the “metabolically unhealthy normal BMI” i.e. normal BMI with ≥ 2CMRFs.
To characterize different patterns of multiple CMRFs and MetS, we used pairs of person-visits (approximately two years apart) as the unit of observation. Specifically, if a participant contributed three visits, two pairs of consecutive person-visits were obtained. BMI categories (normal, overweight or obese) for pairs of visits were restricted to the index (or first) visit of each pair as the children’s BMI z-score were stable from the index visit to the follow-up visit (Pearson correlation coefficient = 0.89). Among children who had an elevated BMI at their first visit, the classifications of MetS were defined as never MetS (in which the index and subsequent visits were free of multiple CMRFs), incident MetS (in which the index visit, but not the subsequent visit, was free of multiple CMRFs), resolved MetS (in which the index visit had multiple CMRFs, but the subsequent visit did not) and persistent MetS (in which both visits had multiple CMRFs). Similar categories were constructed for those with normal BMI at index visit but were denoted as multiple CMRFs.
Outcomes
In addition to describing the prevalence and incidence of MetS and multiple CMRFs, we also characterized change in eGFR in our sample of person-visits. Estimated GFR was measured at each annual visit and based on the full 2012 CKiD equation using data on serum creatinine, cystatin c and blood urea nitrogen10, 11.
Annual change in kidney function was calculated as the difference in eGFR in the log scale between the index visit (i) and the follow-up visit (i +1) divided by the difference in time (in years) between the two visits:
The annual percent change in eGFR was calculated as [exp(annual change in log(eGFR)-1] × 100%. In addition to describing the annual percent change in eGFR as a continuous variable, we dichotomized this variable to define fast progression as an annual decline in eGFR greater than 10%.
Statistical analyses
The analytic approach comprised three main comparisons. First, the distributions of demographic and clinical characteristics, as well as prevalence of each CMRF, were compared by BMI categories at the index visit to describe differences by BMI. Pair-wise comparisons with normal BMI as the reference group were calculated using the Wilcoxon rank sum test for continuous characteristics and were calculated using the chi-squared test for categorical (including binary) characteristics. Second, distributions of demographic and clinical characteristics were compared within each BMI category by the presence of at least two CMRFs to describe associations related to multiple CMRFs, using the same methods as described above. Lastly, we formally compared children who ever had multiple CMRFs (among children with normal BMI at index visit) or MetS at either visit (pooling individuals who were overweight or obese at the index visit) to those with normal BMI at the index visit and who did not have multiple CMRFs at either visit, which resulted in a total of 8 groups (1 reference group and 7 comparison groups). For formal statistical comparisons, linear regression models were fit with annual difference in log(eGFR) as the dependent variable and group membership as categorical independent variables (with normal BMI and never multiple CMRFs as the reference group). Logistic regression models were fit with the dependent variable being fast progression (i.e., percent decline in GFR > 10% per year). Univariate (i.e., unadjusted) models were reported as well as models adjusted for CKD diagnosis (glomerular vs. non-glomerular; Pierce et al9), sex, self-reported African-American race, CKD duration (in years), corticosteroid use, and maternal college education as a proxy for socioeconomic status. These covariates were expected to be associated with high BMI and metabolic abnormalities and accelerated disease progression. Adjustments for these variables were incorporated using inverse probability of exposure weights. Generalized Estimation Equations (GEE) was used to account for within subject correlations. Statistical significance was assessed at the p < 0.05 level. All statistical analyses were performed in SAS 9.4 and R 3.1.1.
RESULTS
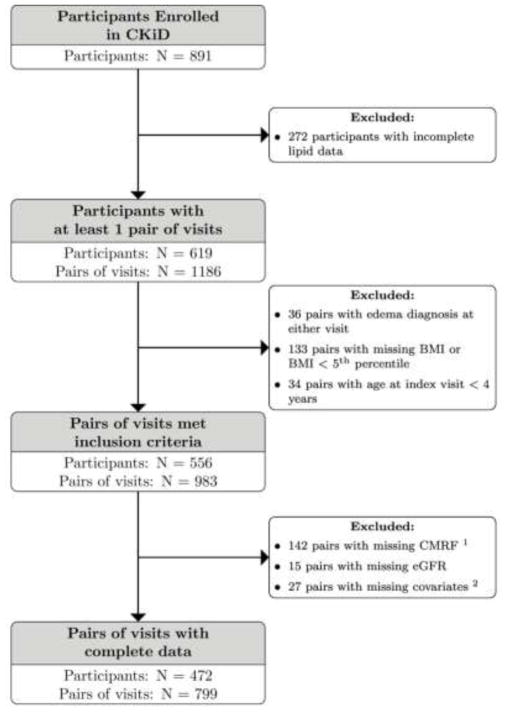
Of the 891 participants, a total of 472 contributed 799 pairs of person-visits with complete data. Figure 1 displays the inclusion of the participants and their corresponding pairs of person-visits. Those included in the final analysis had a BMI > 5th percentile and complete data for CMRF, eGFR and covariates. Among these, 560 (70%) had a normal BMI,112 (14%) were overweight, and 127 (16%) were obese at the index visit.
Figure 1.
Description of the selection of participants and pairs of visits that contributed to the analytic study population.
1CMRF: cardiometabolic risk factors
2Covariktes: CKD diagnosis, maternal education, gender, race. CKD duration (in years) and corticosteroid use
Table 1 presents the characteristics at the index visit by BMI categories among 799 pairs of person-visits. The median age and proportion of boys among different BMI groups were similar. The proportion of household income less than $36,000 was approximately 40% across the three groups, but lower level of maternal education was observed in the obese group as compared with the normal BMI group (p = 0.07).
Table 1.
Distribution of characteristics at index visit by BMI categories, % (N) or Median [IQR]
| Variable | Normal BMI | Overweight | Obese | ||
|---|---|---|---|---|---|
| N = 560 | N = 112 | P Valuea | N = 127 | P Valuea | |
| N of unique Children | 340 | 88 | 86 | ||
| Age at index visit | 11.16 [8.11, 14.25] | 12.11 [9.26, 14.54] | 0.017 | 12.04 [9.51, 15.12] | 0.002 |
| Year since CKD onset at index visit | 10.05 [7.27, 13.26] | 10.12 [7.20, 13.07] | 0.797 | 9.94 [6.22, 13.38] | 0.378 |
| Male | 63% (351) | 61% (68) | 0.695 | 65% (83) | 0.572 |
| Black Race | 16% (88) | 20% (22) | 0.305 | 26% (33) | 0.006 |
| Household income < $36,000 | 36% (200) | 45% (50) | 0.074 | 44% (56) | 0.078 |
| Maternal education: college or more | 33% (183) | 29% (33) | 0.506 | 24% (31) | 0.069 |
| Glomerular diagnosis | 12% (65) | 21% (24) | 0.005 | 28% (35) | <0.001 |
| eGFR (ml/min|1.73m2) | 48.8 [37.7, 62.0] | 53.4 [42.3, 66.1] | 0.023 | 54.8 [41.8, 66.2] | 0.010 |
| Urine protein creatinine ratio | 0.26 [0.11, 0.71] | 0.33 [0.11, 1.05] | 0.242 | 0.40 [0.11, 0.88] | 0.233 |
| Currently taking corticosteroid (systemic) | 2.3% (13) | 4.5% (5) | 0.200 | 7.1% (9) | 0.006 |
| High triglycerides | 37% (208) | 54% (60) | 0.005 | 65% (83) | <0.001 |
| Low HDL cholesterol | 8% (42) | 18% (20) | <0.001 | 24% (30) | <0.001 |
| Hypertension | 51% (284) | 49% (55) | 0.448 | 69% (88) | <0.001 |
| High glucose | 14% (77) | 14% (16) | 0.881 | 18% (23) | 0.208 |
| Counts of CMRFs | |||||
| 0 | 28% (157) | 18% (20) | 0.107 | 9% (12) | <0.001 |
| 1 | 43% (240) | 42% (47) | 31% (39) | ||
| 2 | 22% (121) | 29% (32) | 36% (46) | ||
| 3 | 7% (39) | 11% (12) | 21% (27) | ||
| 4 | 1% (3) | 1% (1) | 2% (3) | ||
Based-on comparison with normal BMI group
The prevalence of glomerular CKD diagnoses was higher with increasing BMI: 12% among those with normal BMI, 21% among overweight, and 28% among obese. The time since CKD onset at the index visit was approximately 10 years for children across the three BMI groups. The eGFR was significantly higher in the heavier groups: the median eGFR was 48.8 ml/min/1.73m2 in the normal BMI group, 53.4 ml/min/1.73m2 in the overweight group, and 54.8 ml/min/1.73m2 in the obese group. Although the urine protein creatinine ratio was higher for the overweight and obese children compared with those with normal BMI, these differences were not statistically significant.
Among CMRFs, the prevalence of high triglycerides and low HDL cholesterol were significantly higher in the overweight and obese groups (Table 1). Hypertension was higher only in the obese group. The distributions of cumulative CMRFs (i.e., the cumulative frequencies of these four CMRFs) differed between the normal BMI and obese groups. Although the overweight group had a higher burden of cumulative CMRFs compared with the normal BMI group, this difference was not significant.
Table II presents the characteristics at index visit by number of CMRFs, stratified by BMI categories. For those with a normal BMI, 29% had at least two CMRFs. In contrast, the prevalence of multiple CMRFs was 51% in the overweight/obese participants: 40% in the overweight group and 60% in the obese group (these two groups are defined as having MetS). Regardless of BMI category at the index visit, children with ≥ 2 CMRFs were similar to children with none or one CMRF in terms of age, CKD duration, race, and maternal education. Among overweight children, those with MetS had a higher prevalence of lower household income (i.e., < $36,000; p = 0.002). Within each BMI category, eGFR was significantly lower and urine protein creatinine ratio was significantly higher in the ≥ 2 CMRFs group compared with < 2 CMRFs group.
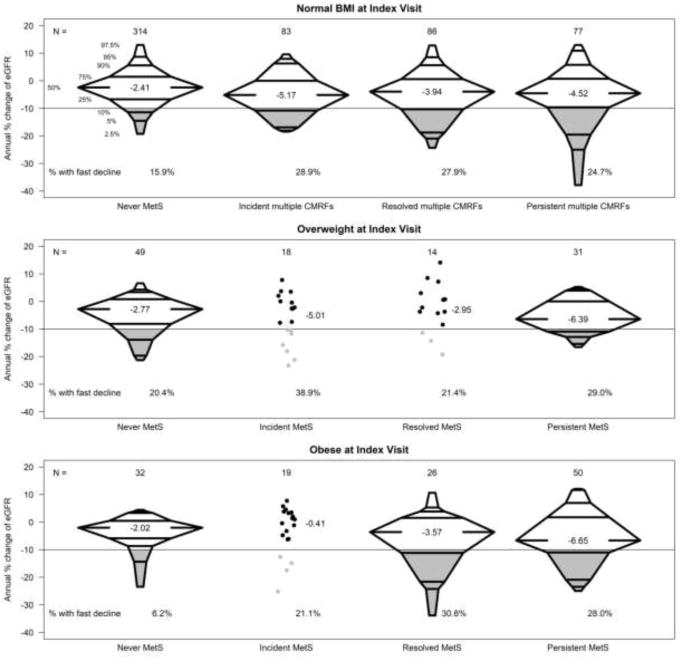
Figure 2 displays the annual percent changes in eGFR from index visit to follow-up visit with corresponding changes in MetS or multiple CMRFs classifications, stratified by BMI status at the index visit. Among participants with a normal BMI who never had multiple CMRFs, the median percent change in eGFR was −2.4% per year. In this group, the proportion of those who declined at least 10% per year (i.e. fast decline) was 15.9%. Participants with persistent multiple CMRFs, regardless of BMI category (normal or MetS), had a consistently faster decline(25–29%) than those who had no multiple CMRFs at any time. Within each BMI category, there were similar distributions of change between incident and resolved multiple CMRFs/MetS, although there were few people contributing to these classifications in the overweight and obese categories. Indeed, those who had MetS were most likely to have persistent MetS between the two visits. Within each BMI category, the median percent eGFR change was most substantial among those with multiple CMRFs ranging from −4.52% per year (normal BMI group) to −6.65% per year (obese BMI group).
Figure 2.
Distributions of the annual percent change of eGFR (as percentile boxplots) by categories of visit pairs stratified by BMI category at the index visit: free of metabolic syndrome, incident multiple cardiometabolic risk factors or metabolic syndrome, resolved cardiometabolic risk factors or metabolic syndrome, and persistent multiple cardiometabolic risk.
• Indicate individual data points in which the group size is less than 20 pairs of person-visits.
—— Indicate 10% or more decrease in eGFR per year; proportion below this threshold is shaded in grey
Table 3, A presents unadjusted and adjusted comparisons of annual percent change in eGFR for those with multiple CMRFs or MetS compared with children with normal BMI and no multiple CMRFs. The group with normal BMI and without multiple CMRFs over two years (considered lowest risk) was used as the reference for the analyses described in Table 3. For this group, the average annual percent change in eGFR was −3.19% (95%CI: −4.11%, −2.27%). Those who had a normal BMI and incident multiple CMRFs had a faster decline of eGFR (annual change −5.62%, p for difference with reference group =0.044). Those with persistent MetS had an eGFR change of −6.18% per year (95%CI: −8.48%, −3.83%) and this was also a significantly faster decline than the reference group (p for difference = 0.020). Those with incident and resolved MetS also had faster eGFR declines (estimated annual percent change = −5.11% and −5.89%, respectively), but these estimates were not significantly different from the reference group (p = 0.231 and 0.208, respectively). In adjusted models, those who had normal BMI and incident multiple CMRFs had a faster decline of eGFR compared with children who never had multiple CMRFs (−6.33% vs. −3.38%, p = 0.048). Among children who were overweight or obese, those with persistent MetS had the fastest GFR decline (6.08% per year), a value that was significantly different from the reference group (p = 0.036). Those with incident or resolved MetS also experienced a faster estimated GFR decline compared with the reference group, but these differences were not statistically significant.
Table 3.
Comparing pairs of person visits in children ever developed MetS to those who never developed MetS
| Table 3a. Annual percent change of eGFR
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Adjustment | Parameters | Normal BMI | Overweight or obese | ||||||
| Never ≥2 CMRFs |
Incident ≥2 CMRFs |
Resolved ≥2 CMRFs |
Persistent ≥2 CMRFs |
Never MetS |
Incident MetS |
Resolved MetS |
Persistent MetS |
||
| Unadjusted | Estimate | −3.19 | −5.62 | −5.41 | −6.40 | −4.56 | −5.11 | −5.89 | −6.18 |
| 95% CI | (−4.11, −2.27) | (−7.73, −3.46) | (−7.63, −3.14) | (−9.09, −3.63) | (−6.44, −2.65) | (−8.03, −2.10) | (−9.85, −1.77) | (−8.48, −3.83) | |
| P value | Ref. | 0.044 | 0.092 | 0.032 | 0.204 | 0.231 | 0.208 | 0.020 | |
| Adjusteda | Estimate | −3.38 | −6.33 | −5.42 | −6.46 | −4.53 | −4.84 | −5.44 | −6.08 |
| 95% CI | (−4.34, −2.40) | (−8.98, −3.59) | (−7.66, −3.12) | (−9.23, −3.60) | (−6.59, −2.42) | (−7.81, −1.78) | (−10.0, −0.61) | (−8.36, −3.74) | |
| P value | Ref. | 0.048 | 0.123 | 0.045 | 0.325 | 0.368 | 0.406 | 0.036 | |
| Table 3b. Fast decline of eGFR (annual percent decline > 10)
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Adjustment | Parameters | Normal BMI | Overweight or obese | ||||||
| Never ≥2 CMRFs |
Incident ≥2 CMRFs |
Resolved ≥2 CMRFs |
Persistent ≥2 CMRFs |
Never MetS |
Incident MetS |
Resolved MetS |
Persistent MetS |
||
| Unadjusted | Estimate | 1 | 2.06 | 1.98 | 1.81 | 0.94 | 2.30 | 1.94 | 2.12 |
| 95% CI | NA | (1.15, 3.69) | (1.15, 3.41) | (0.98, 3.32) | (0.47, 1.87) | (1.09, 4.83) | (0.94, 3.99) | (1.18, 3.81) | |
| P value | Ref. | 0.016 | 0.013 | 0.056 | 0.862 | 0.028 | 0.071 | 0.012 | |
| Adjusteda | Estimate | 1 | 2.13 | 1.86 | 1.76 | 1.07 | 2.03 | 1.81 | 2.11 |
| 95% CI | NA | (1.15, 3.94) | (1.07, 3.24) | (0.94, 3.30) | (0.51, 2.28) | (0.92, 4.49) | (0.86, 3.85) | (1.13, 3.95) | |
| P value | Ref. | 0.016 | 0.028 | 0.076 | 0.852 | 0.080 | 0.121 | 0.019 | |
CI: Confidence Interval;
Adjusted for CKD diagnosis, maternal education, gender, race, CKD duration (in years) and corticosteroid use.
To characterize the relationship between these MetS categories and the prevalence of fast progression defined as an eGFR decline of greater than 10% per year, a binary outcome was defined (represented as the shaded portions in Figure 2) and used in logistic regression models presented in Table 3, B. In unadjusted analyses, those with a normal BMI and incident or resolved multiple CMRFs had about 2 times higher odds of fast progression compared with the reference group (p = 0.016 and 0.013). Children with a normal BMI and persistent multiple CMRFs also had higher odds of fast eGFR decline compared with the reference group (p=0.056). In unadjusted analyses, among children with a higher BMI, those with incident, persistent, or resolved MetS were more likely to have a fast (>10%) eGFR decline than the reference group (OR = 2.30, 2.12, and 1.94, respectively). In adjusted analyses, the associated were similar: all the comparison groups had greater odds of a fast eGFR decline than the reference group, and the differences reached significance for children with a normal BMI and with incident or resolved multiple CMRFs, or children with an elevated BMI and with persistent MetS but not incident or resolved MetS.
DISCUSSION
Our study demonstrated that about half of the CKiD participants who were overweight or obese had multiple CMRFs and met criteria for MetS and that the presence of MetS/multiple CMRFs was associated with a faster decline of kidney function. A higher prevalence of MetS in children with CKD compared with the general population of children is not surprising in the context of a high prevalence of individual traditional cardiovascular risk factors (i.e. hypertension, dyslipidemia, hyperglycemia/insulin resistance) seen in this population.6 The frequency of MetS in our study was similar to that of adults with CKD12, a population with a high frequency of diabetes, hypertension and preexisting cardiovascular disease. These results are important because MetS in adults with CKD is associated with increased cardiovascular morbidity and mortality13. In children, the presence of multiple CMRFs is associated with early atherosclerosis as is evident from non-invasive 14–16 and autopsy17,18 studies in children.
Children who had MetS at their initial visit were more likely to continue to have MetS subsequently. This group with persistent MetS had the fastest rate of CKD progression. The common theory for worsening of CKD in the presence of MetS is attributed partly to specific CMRFs (hypertension and insulin resistance, in particular). However, previous studies have shown that obesity is independently associated with the development and progression of CKD, including those patients without diabetes and hypertension2,3,10–12 suggesting that metabolically healthy but obese patients have an increased risk of CKD.20, 22 This is also supported by pathologic changes, including glomerular sclerosis, tubular atrophy, and interstitial fibrosis found in patients with severe obesity and the metabolic syndrome prior to the onset of overt kidney disease.23,24
Although a higher frequency of MetS in children who are overweight or obese is expected, we also found that approximately one-third of the CKiD participants with a normal weight had ≥ 2 CMRFs (non-obese but metabolically unhealthy group). More importantly, participants with multiple CMRFs in both the normal and high BMI categories had a consistently faster and similar decline in kidney function compared with those who had no history of multiple CMRFs. These findings suggest that the presence of multiple CMRFs rather than obesity per se might be more important for CKD progression in our cohort. However, because the prevalence of multiple CMRFs was significantly higher in a group of overweight and obese children versus normal weight group, children with obesity should still be considered as a high-risk group for CKD progression.
We also observed that those with resolved multiple CMRFs experienced an accelerated decline in eGFR compared with those who never developed multiple CMRFs (Table 3). This suggests that the risk conferred during the time when multiple CMRFs were present continued even after the observed resolution. It should be noted that the time frame of this analysis was approximately two years and that the change in multiple CMRFs and eGFR decline should be interpreted in that context. It is possible that longer follow-up may divulge longer-term benefits related to CMRF resolution.
Our study has important strengths including a large sample size and standardized demographic, clinical, and laboratory measurements over time. Our definition of “MetS”, although consistent with ATPIII criteria, has an exception that instead of using any 3/5 CMRFs, we have divided groups using BMI as a consistent variable as recommended by an Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents.8 This allowed us to compare the presence and effects of multiple CMRFs in the normal BMI group with those that are overweight or obese and have multiple CMRFs. We have included BMI instead of waist circumference (WC) as a measure of obesity because BMI has discriminated cardiovascular risks among adolescents in a previous study25 and offered robust associations with cardiometabolic variables.26
However, there are limitations to our study. Because our primary aim was investigating the pediatric metabolic syndrome, our analysis described the burden of conditions as a total count such that each condition is given equal weight. This is limitation as specific comorbidities to define MetS (e.g. hypertension, dyslipidemia, or abnormal glucose metabolism) may confer different levels of risk. However, there is no currently established hierarchy that describes the potentially different levels of risk. Future research should investigate how each component of MetS is related to risk, because it is not likely that each condition confers anequal risk. Identifying risk contribution and clustering of variables will help clinicians prioritize treatment for children with MetS. Although the eGFR in our study was calculated using combined serum creatinine and cystatin C by the CKiD estimating equation10, we cannot exclude that the performance of eGFR was affected by obesity. Creatinine-based equations have demonstrated significant variability among obese patients.27 There are studies suggesting that cystatin C-based formulas may provide a more accurate kidney function estimation irrespective of body composition 28, 29, however others argue that cystatin C might underestimate eGFR, especially in obese populations.30
Table 2.
Distribution of characteristics by multiple cardiometabolic risk factors (CMRFs) status and metabolic syndrome (MetS) status at index visit, stratified by BMI categories. % (N) or Median [IQR]*
| Variable | Normal BMI | Overweight | Obese | ||||||
|---|---|---|---|---|---|---|---|---|---|
| < 2 CMRFs | ≥2 CMRFs | P Valuea | < 2 CMRFs | ≥2 CMRFs (MetS) | P Valuea | < 2 CMRFs | ≥2 CMRFs (MetS) | P Valuea | |
| N | 397 | 163 | 67 | 45 | 51 | 76 | |||
| N of unique ID | 269 | 128 | 55 | 38 | 42 | 55 | |||
| Age at index visit | 11.27 [8.27, 14.44] | 10.73 [7.87, 13.37] | 0.200 | 11.90 [9.03, 14.76] | 13.22 [10.01, 14.32] | 0.386 | 11.57 [9.15, 14.65] | 12.83 [9.86, 15.63] | 0.165 |
| Years since CKD onset at index visit | 10.32 [7.32, 13.56] | 9.58 [7.02, 12.48] | 0.185 | 9.42 [6.40, 12.11] | 10.78 [8.19, 13.70] | 0.086 | 9.32 [5.16, 12.04] | 9.96 [6.36, 14.09] | 0.264 |
| Male | 61% (242) | 67% (109) | 0.189 | 52% (35) | 73% (33) | 0.025 | 63% (32) | 67% (51) | 0.613 |
| Black Race | 17% (68) | 12% (20) | 0.151 | 22% (15) | 16% (7) | 0.372 | 31% (16) | 22% (17) | 0.257 |
| Household income < $36,000 | 36% (144) | 34% (56) | 0.667 | 33% (22) | 62% (28) | 0.002 | 41% (21) | 46% (35) | 0.587 |
| Maternal education: college or more | 34% (133) | 31% (50) | 0.517 | 31% (21) | 27% (12) | 0.595 | 20% (10) | 28% (21) | 0.302 |
| Glomerular diagnosis | 10% (39) | 16% (26) | 0.040 | 25% (17) | 16% (7) | 0.214 | 27% (14) | 28% (21) | 0.982 |
| eGFR (mL/min|1.73m2) | 50.5 [40.0, 63.5] | 44.7 [33.2, 57.7] | <0.001 | 58.8 [43.9, 75.0] | 47.6 [38.3, 57.2] | 0.001 | 60.3 [46.8, 70.8] | 51.3 [41.3, 62.4] | 0.033 |
| Urine protein creatinine ratio | 0.23 [0.09, 0.68] | 0.35 [0.16, 0.75] | 0.004 | 0.24 [0.08, 0.73] | 0.56 [0.20, 1.13] | 0.008 | 0.17 [0.09, 0.53] | 0.47 [0.11, 1.16] | 0.002 |
| Currently taking corticosteroid (systemic) | 1.5% (6) | 4.3% (7) | 0.047 | 6.0% (4) | 2.2% (1) | 0.346 | 3.9% (2) | 9.2% (7) | 0.255 |
| High triglycerides | 17% (68) | 86% (140) | <0.001 | 31% (21) | 87% (39) | <0.001 | 27% (14) | 91% (69) | <0.001 |
| Low HDL cholesterol | 1% (4) | 23% (38) | <0.001 | 0% (0) | 44% (20) | <0.001 | 0% (0) | 39% (30) | <0.001 |
| Hypertension | 38% (150) | 82% (134) | <0.001 | 31% (21) | 76% (34) | <0.001 | 49% (25) | 83% (63) | <0.001 |
| High glucose | 5% (18) | 36% (59) | <0.001 | 7% (5) | 24% (11) | 0.012 | 0% (0) | 30% (23) | <0.001 |
Those who were overweight or obese, ≥ 2 CMRFs were classified as having MetS; those with normal BMI, ≥ 2 CMRFs were defined as having multiple CMRFs.
P values compared to children in each BMI group with < 2 CMRFs.
Acknowledgments
Supported by the National Institutes of Health (NIH) (U01-DK-66143, U01-DK-66174, U01DK-082194, U01-DK-66116).
Data in this manuscript were collected by the Chronic Kidney Disease in children prospective cohort study (CKiD) with clinical coordinating centers (Principal Investigators) at Children’s Mercy Hospital and the University of Missouri - Kansas City (Bradley Warady, MD) and Children’s Hospital of Philadelphia (Susan Furth, MD, PhD), Central Biochemistry Laboratory (George Schwartz, MD) at the University of Rochester Medical Center, and data coordinating center (Alvaro Muñoz, PhD) at the Johns Hopkins Bloomberg School of Public Health. The CKiD Study is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases, with additional funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Heart, Lung, and Blood Institute (U01-DK-66143, U01-DK-66174, U01-DK-082194, U01-DK-66116). The CKiD website is located at http://www.statepi.jhsph.edu/ckid.
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Furth SL, Abraham AG, Jerry-Fluker J, Schwartz George J, Benfiel Mark, Kaskel Frederick, et al. Metabolic abnormalities, cardiovascular disease risk factors, and GFR decline in children with chronic kidney disease. Clinical journal of the American Society of Nephrology: CJASN. 2011;6(9):2132–2140. doi: 10.2215/CJN.07100810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurella M, Lo JC, Chertow GM. Metabolic syndrome and the risk for chronic kidney disease among nondiabetic adults. Journal of the American Society of Nephrology: JASN. 2005;16(7):2134–2140. doi: 10.1681/ASN.2005010106. [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Muntner P, Hamm LL, Jones DW, Batuman V, Fonseca V, et al. The metabolic syndrome and chronic kidney disease in U.S. adults. Annals of internal medicine. 2004;140(3):167–174. doi: 10.7326/0003-4819-140-3-200402030-00007. [DOI] [PubMed] [Google Scholar]
- 4.Friend A, Craig L, Turner S. The prevalence of metabolic syndrome in children: a systematic review of the literature. Metab Syndr Relat Disord. 2013;11(2):71–80. doi: 10.1089/met.2012.0122. [DOI] [PubMed] [Google Scholar]
- 5.Wilson AC, Greenbaum LA, Barletta GM, Chand D, Lin JJ, Patel HP, et al. High prevalence of the metabolic syndrome and associated left ventricular hypertrophy in pediatric renal transplant recipients. Pediatric transplantation. 2010;14(1):52–60. doi: 10.1111/j.1399-3046.2009.01141.x. [DOI] [PubMed] [Google Scholar]
- 6.Wilson AC, Schneider MF, Cox C, Greenbaum Larry A, Saland Jeffrey, White Colin T, et al. Prevalence and correlates of multiple cardiovascular risk factors in children with chronic kidney disease. Clinical journal of the American Society of Nephrology: CJASN. 2011;6(12):2759–2765. doi: 10.2215/CJN.03010311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furth SL, Cole SR, Moxey-Mims M, Kaskel Frederick, Robert Mak, Schwartz George, et al. Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clinical journal of the American Society of Nephrology: CJASN. 2006;1(5):1006–1015. doi: 10.2215/CJN.01941205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128(Suppl 5):S213–256. doi: 10.1542/peds.2009-2107C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012 Jan;35(Suppl 1):S64–S71. doi: 10.2337/dc12-s064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz GJ, Schneider MF, Maier PS, Moxey-Mims M, Dharnidharka V, Warady BA, et al. Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int. 2012;82:445–453. doi: 10.1038/ki.2012.169. (Commentary by Delanaye P and Ebert N in Nat Rev Nephrol 2012; 8:503–504) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pierce CB, Cox C, Saland JM, Furth SL, Munoz A. Methods for characterizing differences in longitudinal glomerular filtration rate changes between children with glomerular chronic kidney disease and those with nonglomerular chronic kidney disease. American journal of epidemiology. 2011;174(5):604–612. doi: 10.1093/aje/kwr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Townsend RR, Anderson AH, Chen J, Gadebegku Crystal A, Feldman Harold I, Fink Jeffrey C, et al. Metabolic syndrome, components, and cardiovascular disease prevalence in chronic kidney disease: findings from the Chronic Renal Insufficiency Cohort (CRIC) Study. American journal of nephrology. 2011;33(6):477–484. doi: 10.1159/000327618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prasad GV. Metabolic syndrome and chronic kidney disease: Current status and future directions. World J Nephrol. 2014 Nov 6;3(4):210–9. doi: 10.5527/wjn.v3.i4.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Litwin M, Niemirska A. Metabolic syndrome in children with chronic kidney disease and after renal transplantation. Pediatr Nephrol. 2014 Feb;29(2):203–16. doi: 10.1007/s00467-013-2500-1. Epub 2013 Jun 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juonala M, Järvisalo MJ, Mäki-Torkko N, Kähönen M, Viikari JS, Raitakari OT. Risk factors identified in childhood and decreased carotid artery elasticity in adulthood: the Cardiovascular Risk in Young Finns Study. Circulation. 2005;112:1486–1493. doi: 10.1161/CIRCULATIONAHA.104.502161. [DOI] [PubMed] [Google Scholar]
- 16.Iannuzzi A, Licenziati MR, Acampora C, Renis M, Agrusta M, Romano L, Valerio G, Panico S, Trevisan M. Carotid artery stiffness in obese children with the metabolic syndrome. Am J Cardiol. 2006;97:528–531. doi: 10.1016/j.amjcard.2005.08.072. [DOI] [PubMed] [Google Scholar]
- 17.Berenson GS, Srinivasan SR, Bao W, Newman WP, III, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults: the Bogalusa Heart Study. N Engl J Med. 1998;338:1650–1656. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 18.McGill HC, Jr, McMahan CA, Herderick EE, Zieske AW, Malcom GT, Tracy RE, Strong JP Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Obesity accelerates the progression of coronary atherosclerosis in young men. Circulation. 2002;105:712–2718. doi: 10.1161/01.cir.0000018121.67607.ce. [DOI] [PubMed] [Google Scholar]
- 19.Chen J, Muntner P, Hamm LL, Fonseca V, Batuman V, Whelton PK, et al. Insulin resistance and risk of chronic kidney disease in nondiabetic US adults. Journal of the American Society of Nephrology: JASN. 2003;14(2):469–477. doi: 10.1097/01.asn.0000046029.53933.09. [DOI] [PubMed] [Google Scholar]
- 20.Chang Y, Ryu S, Cho J, Pastor-Barriuso R, Guallar E. Metabolically Healthy Obesity and Development of Chronic Kidney Disease. Annals of internal medicine. 2016;165(10):744–745. doi: 10.7326/L16-0405. [DOI] [PubMed] [Google Scholar]
- 21.Bakker SJ, Gansevoort RT, de Zeeuw D. Metabolic syndrome: a fata morgana? Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2007;22(1):15–20. doi: 10.1093/ndt/gfl581. [DOI] [PubMed] [Google Scholar]
- 22.Jung CH, Lee MJ, Kang YM, Hwang JY, Kim EH, Park JY, et al. The risk of chronic kidney disease in a metabolically healthy obese population. Kidney international. 2015;88(4):843–850. doi: 10.1038/ki.2015.183. [DOI] [PubMed] [Google Scholar]
- 23.Alexander MP, Patel TV, Farag YM, Florez A, Rennke HG, Singh AK. Kidney pathological changes in metabolic syndrome: a cross-sectional study. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2009;53(5):751–759. doi: 10.1053/j.ajkd.2009.01.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Serra A, Romero R, Lopez D, Navarro M, Esteve A, Perez N, et al. Renal injury in the extremely obese patients with normal renal function. Kidney international. 2008;73(8):947–955. doi: 10.1038/sj.ki.5002796. [DOI] [PubMed] [Google Scholar]
- 25.Katzmarzyk PT, Srinivasan SR, Chen W, Malina RM, Bouchard C, Berenson GS. Body mass index, waist circumference, and clustering of cardiovascular disease risk factors in a biracial sample of children and adolescents. Pediatrics. 2004;114(2):e198–205. doi: 10.1542/peds.114.2.e198. [DOI] [PubMed] [Google Scholar]
- 26.Patel HP, Saland JM, Ng DK, Jiang Shuai, Warady Bradley A, Furth Susan L, et al. Waist Circumference and Body Mass Index in Children with Chronic Kidney Disease and Metabolic, Cardiovascular, and Renal Outcomes. The Journal of pediatrics. 2017;191:133–139. doi: 10.1016/j.jpeds.2017.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verhave JC, Gansevoort RT, Hillege HL, De Zeeuw D, Curhan GC, De Jong PE. Drawbacks of the use of indirect estimates of renal function to evaluate the effect of risk factors on renal function. J Am Soc Nephrol. 2004;15(5):1316–1322. [PubMed] [Google Scholar]
- 28.Fadrowski JJ, Neu AM, Schwartz GJ, Furth SL. Pediatric GFR estimating equations applied to adolescents in the general population. Clinical journal of the American Society of Nephrology: CJASN. 2011;6(6):1427–1435. doi: 10.2215/CJN.06460710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bokenkamp A, Domanetzki M, Zinck R, Schumann G, Byrd D, Brodehl J. Cystatin C--a new marker of glomerular filtration rate in children independent of age and height. Pediatrics. 1998;101(5):875–881. doi: 10.1542/peds.101.5.875. [DOI] [PubMed] [Google Scholar]
- 30.Vupputuri S, Fox CS, Coresh J, Woodward M, Muntner P. Differential estimation of CKD using creatinine- versus cystatin C-based estimating equations by category of body mass index. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2009;53(6):993–1001. doi: 10.1053/j.ajkd.2008.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]