Abstract
Background
Children undergoing leukemia treatment report co-occurring symptoms of fatigue, sleep disturbances, pain, nausea, and depression as a symptom cluster. Physical activity (PA) is essential for development and may influence symptom severity. Children with leukemia are at risk for cognitive impairments from CNS therapies. Using a longitudinal parallel-process model, relationships among function and symptom clusters were explored.
Objective
This study examined the longitudinal mediation effects of PA on cognition via a symptom cluster during leukemia treatment.
Methods
Symptoms, PA, and cognitive function of 327 children ages 3 to 18 were measured over 4 intervals during the first year of leukemia treatment. Children age 7 years and older self-reported and parents reported for younger children. Parents completed cognitive function measurements for all children. The influence of the first time-point and the subsequent change between all 4 time-points of PA on the symptom cluster was explored. Analysis determined whether the symptom cluster mediated the effect of cognition over the treatment period.
Results
Patients with a higher PA at Time 1 reduced their symptom cluster severity over the measurements. However, when PA increased over the measurements, symptom cluster severity also increased. When the symptom cluster was more severe at Time 1, cognitive function was lower at Time 1 and cognitive function decreased over time. When symptoms became more severe over time, cognitive function declined.
Conclusions
The symptom cluster acted as a mediator between physical activity and cognition.
Implications for Practice
Symptom management during treatment may be an additional strategy for protecting cognitive function.
INTRODUCTION
Children and adolescents undergoing treatment for cancer are at risk for experiencing multiple distressing symptoms from both the disease and its treatment.1 Symptoms rarely occur as a single event; instead, they are likely experienced concurrently resulting in a distinct symptom cluster.2 Some pediatric oncology researchers have identified several symptom clusters in children undergoing chemotherapy for a variety of cancer diagnoses using a single symptom self-report instrument while others have measured selected individual symptoms with separate scales.1
In a recent study, an a priori approach was used to select symptoms known to occur in children during treatment for acute lymphocytic leukemia (ALL). The symptoms of fatigue, sleep disturbances, pain, nausea, and depression were evaluated by child self-report or parent proxy in 236 children.3 Latent class growth analysis was used to categorize patients into mild, moderate, and severe symptom groups who followed distinct symptom trajectories over four intensive phases of ALL therapy.3 In an earlier study, Buckner and colleagues conducted a latent profile analysis of children with cancer analyzing both patient-reported symptoms and functional outcomes. They noted that evaluating functional impairments in relation to symptoms is essential to understanding how cancer impacts the child’s quality of life.4 Children with cancer must continue in their development over the trajectory of treatment which can last several years. Functional impairments that include physical and cognitive dimensions may negatively impact the child’s ongoing development during and after cancer treatment.
Longitudinal parallel-process (LPP) modeling is a form of analysis that can provide new insights into a person’s symptom experience and functioning. In this type of growth curve modeling, relationships between two or more longitudinal processes can be evaluated at the same time.5 Examination includes testing relationships between estimated intercepts (i.e. the initial level of a symptom or function) and growth trajectories (i.e. the slope of the change in symptoms or functions over time).5 The strength of this approach is that advanced longitudinal modeling addresses the trajectory of a symptom cluster that is likely to change during cancer treatment. Additionally, it allows for the examination of how concurrent symptoms may be influenced by a function such as physical activity and how symptom clusters may predict the trajectory of functional outcomes such as cognition and memory. Due to its modeling flexibility, LPP has been gaining popularity in the literature.5,6,7,8
Physical Activity
Physical activity, defined as any body movement other than resting, is a behavior that can be influenced and, therefore, explored in future research as an independent variable. Exercise is a subset of physical activity and is planned, structured, and repetitive.9 Physical activity includes any skeletal muscle movements typical of play behaviors in children.10 Improvement in physical activity can result in health benefits for children, even with modest changes.11 In reviews of children and adolescents with cancer, exercise and physical activity had a positive impact on fatigue, sleep, quality of life, and various aspects of physical functioning12 as well as cognitive function.13 However, during the first month through the first year of treatment for ALL, children remain sedentary and are significantly less active than their healthy peers.14,15,16
Cognitive Function
Cognitive function includes multiple dimensions: attention and concentration, executive function, information processing speed, language, visual-spatial skill, psychomotor ability, learning, and memory.17 Children with ALL receive central nervous system (CNS) directed therapy including intrathecal methotrexate and for some types of leukemia, craniospinal radiation therapy, to prevent an isolated CNS relapse. These interventions place the child at risk for academic and cognitive problems.17 Caregivers of children with ALL report an increase in learning problems after two years of therapy compared to ratings early in treatment.18 Little is known about cognitive function changes during the first year of treatment and how these changes are related to other symptoms.
Conceptual Framework
The conceptual framework for the study was an adaptation of a model from Sousa and colleagues who used longitudinal parallel-process modeling to examine symptoms longitudinally and evaluate their influence on quality of life in persons with HIV.5 We adapted the longitudinal parallel-process (LPP) model to first explore the influence of both the intercept and slope of physical activity on the symptom cluster and then assess the mediating influence of intercept and slope of the symptom cluster on the intercept and slope of cognitive function (Figure 1). The intercept of each variable was the initial (first) measurement. The slope was the rate of change in the symptom cluster and the rate of change in each function of physical activity and cognition over four study measurements. The study was informed by The Developmental Model for Children and Adolescents With Cancer19 which recognizes that symptoms from cancer and its treatment impacts multiple domains of a growing child’s development including cognitive, psychological, physiological, and physical components.
Figure 1.
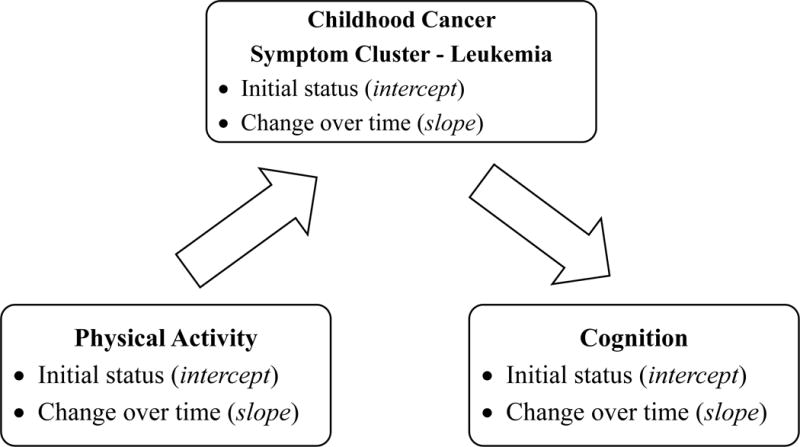
A Conceptual Longitudinal Mediation Model
Purpose
The purpose of this study was to examine the longitudinal mediation effects of physical activity on cognition via a symptom cluster during the first year of childhood ALL treatment.
METHODS
Design and Sample
In this longitudinal mediation study, self-report data were collected at four time points during ALL treatment: at the start of post-induction therapy (Time 1), four and eight months’ post-induction therapy (Times 2 and 3), and during the first cycle of maintenance/continuation therapy (Time 4). Participants were recruited from four pediatric oncology treatment centers in the United States. Eligible children and adolescents were ages 3-18 years, newly diagnosed and receiving ALL chemotherapy, without a cognitive disability pre-diagnosis, and fluent in English or Spanish. Children ages 7 to 17 provided assent to participate in the study and parents provided written consent. Children 18 years provided consent. The Institutional Review Board at each institutional site approved the study before implementation. Participants were included in the analysis if they had completed a minimum of two data collections.
Symptom Measurements
A data secure tablet PC (iPad) was used for all survey data collection and respondents were asked to reflect on symptoms and function during the previous week. Parents performed all proxy responses for children who were age 6 years or younger when starting the study. Measurements are all established instruments with published reliability and validity and were completed during a standard outpatient clinic visit.
Fatigue
The 10-item Childhood Fatigue Scale (CFS) was used to assess the fatigue-related symptoms in children 7-12 years. Children were asked to rate how much they were bothered by fatigue on a 4-point Likert scale ranging from “Not at all” to “A lot”.20 The Adolescent Fatigue Scale (AFS) was used in adolescents 13-18 years and is a 13-item self-report scale.21 The 17-item Parent Fatigue Scale (PFS) was be used to obtain proxy responses from parents of children 3 to 6 years of age.22 A T-score of total fatigue score with a range of 20-80 was used during analysis to combine the three groups’ scores with a higher score indicating more severe fatigue.
Sleep Disturbance
Sleep was measured using the Adolescent Sleep Wake Scale (ASWS) for subjects ages 13 to 1823 and the Child Sleep Wake Scale (CSWS) for subjects ages 3 to 12 years.24 Both instruments include 5 sub-scales including going to bed, falling asleep, maintaining sleep, going back to sleep and returning to wakefulness; responses were scored on a Likert scale ranging from 1 to 6. Scores were then averaged across subscales. For this study, scores were reversed so that a higher score indicated a more severe symptom which was consistent with the other symptom measures.
Pain
This symptom was measured using the Wong-Baker Faces Scale, a reliable and valid tool used for over 30 years to evaluate pain in children.25 The 6-point visual analogue scale (0, 2, 4, 6, 8, and 10) indicated the severity of pain with a higher score indicating worse pain.
Nausea
A Visual Analogue Scale (VAS) in the form of a thermometer was used to rate the severity of nausea from 0-100 with a higher score indicating worse nausea.26 The VAS included a statement at each end representing one extreme of the dimension being measured (e.g., no nausea).
Depression
The Child Depression Inventory (CDI-2)27 is a child self-report measure of depression that requires a low reading level and has established norms. Each of the 27 questions has three possible responses: 0 (absence of the symptom), 1 (mild symptom), or 2 (definite symptom).27 For this study, a T-score of total score with a range of 20- 80 was calculated with higher score indicating more severe depression.
Childhood Cancer Symptom Cluster
Because the five symptom measures (fatigue, sleep disturbance, pain, nausea, and depression) were highly correlated with each other at each time point,3 they were clustered and defined as the “Childhood Cancer Symptom Cluster- Leukemia” (CCSC-L) in this study. The composite score of the CCSC-L was calculated using exploratory factor analysis with maximum likelihood estimation which returned a one-factor solution with significant factor loadings from .37 to .91 and more than 50% of variance explained at each time point (Table 1).
Table 1.
Factor Loadings and Variance Explained in One-Factor Solution for the Childhood Cancer Symptom Cluster - Leukemia at Each Time Point
| Symptom | Factor | |||
|---|---|---|---|---|
| Childhood Cancer Symptom Cluster
| ||||
| Time 1 | Time 2 | Time 3 | Time 4 | |
| Fatigue | .74 | .79 | .91 | .79 |
| Sleep Disturbance | .56 | .51 | .54 | .65 |
| Pain | .65 | .53 | .62 | .57 |
| Nausea | .44 | .54 | .50 | .37 |
| Depression | .76 | .75 | .78 | .70 |
| Variance Explained | 52.2% | 51.1% | 56.4% | 50.8% |
Functional Measurements
Self-report of physical activity was also completed on a secure iPad with parents reporting for children age 6 years and under. Parents completed measurements of cognitive function for participants of all ages on a secure iPad.
Physical Activity
The Leisure Score Index (LSI) of the Godin-Leisure-Time Exercise Questionnaire was used to assess physical activity level by self-report. Respondents reported how many times on average they participated in strenuous, moderate, or mild exercise for more than 15 minutes during the previous 7 days.28,29 The total LSI was calculated by multiplying each frequency by its metabolic measurement unit as: (3 × mild) + (5 × moderate) + (9 × strenuous). A higher score indicates higher levels of physical activity. The questionnaire has been used in studies of children and adolescents with cancer.30,31
Cognition
The Parent-Perceived Child Cognitive Function (pedsPCF) is a 32-item scale that evaluates parent concerns regarding child’s memory and thought process.32 Each question asks the parent to rate the frequency and intensity on a 0-5 point scale. The scale has been evaluated in parents of children with cancer and is validated to identify children most at risk for attention, social, and thought problems.33 The total score was converted to a T-score with a range of 20- 80; a higher score indicates better cognitive function.
Analysis
Descriptive statistics were computed for sample characteristics and longitudinal variables (symptom measures, physical activity, and cognition). The initial sample consisted of 329 patients. There were no missing data in sociodemographic variables (age, sex, race/ethnicity). Only two (0.6%) of the patients had missing pieces of data on all longitudinal variables and were excluded from the analyses. Although the remaining 327 patients had missing data on a few of the longitudinal variables intermittently across the four time points such intermittent longitudinal missing data were missing completely at random33 (χ2(2061) = 2031.56, p = .67) and, thus, did not have negative impact on parameter estimation.35 Patients missed data measurement points for a variety of reasons including: being too ill, refusing a respond to a specific scale, and not reaching the point in treatment when a measurement was scheduled. Also, longitudinal missing data would be automatically handled in multilevel modeling within the longitudinal parallel process. Therefore, no further missing data treatment was necessary, which resulted in the final sample size of 327.
The longitudinal mediation effects of physical activity on cognition via the childhood cancer symptom cluster were examined using the two-step longitudinal parallel-process (LPP).36 In the first step, the intercept and slope of each of the longitudinal variables were estimated separately by using multilevel modeling with SAS Proc Mixed.37 This approach also helped deal with longitudinal missing data and control for sociodemographic variables. In the second step, structural equation modeling was employed for testing the longitudinal mediation effects of physical activity on cognition via the CCSC-L using IBM SPSS Amos.38
Because some of the intercepts and slopes of the variables were not normally distributed (e.g., skewed), the bootstrap resampling technique was implemented in the structural equation modeling to obtain more stable and valid standard errors of the estimates.39,40 In the bootstrap, the bias-corrected percentile method was used to calculate p-values, against a significance level of α = .05, for testing the significance of the path coefficients in the structural equation modeling. The structural equation model fit was evaluated using the following model-fit indices: Chi-square of the estimated model (χ2), goodness of fit index (GFI), normed fit index (NFI), incremental fit index (IFI), relative fit index (RFI), comparative fit index (CFI), and root mean square error of approximation (RMSEA). A nonsignificant Chi-square value (p > .05) suggests a good overall model fit to the data. For GFI, NFI, IFI, RFI, and CFI, values larger than .90 indicate that the model provides a good fit to the data, whereas RMSEA should be below .06. The fit indices and their criteria are commonly recommended in the literature.41,42
RESULTS
Descriptive Statistics
Table 2 shows that among 327 participants in the final sample, age was not equally distributed with 45.3%, 33.6%, and 21.1% for young children (3 to 6 years), children (7 to 12 years), and adolescents (13 to 18 years), respectively. Sex was fairly balanced with only a few more boys than girls (52.0% vs. 48.0%); whereas race/ethnicity was unbalanced with almost half being Hispanic (47.4%), 34.6% being non-Hispanic white, and fewer being non-Hispanic black (7.9%) or non-Hispanic others (10.1%).
Table 2.
Sample Characteristics (N = 327)
| Characteristic | Frequency (n) | Percent (%) | |
|---|---|---|---|
| Age | Young Child (3 - 6 years) | 148 | 45.3 |
| Child (7 - 12 Years) | 110 | 33.6 | |
| Adolescent (13 - 18 Years) | 69 | 21.1 | |
| Sex | Female | 157 | 48.0 |
| Male | 170 | 52.0 | |
| Race/ Ethnicity | Hispanic | 155 | 47.4 |
| Non-Hispanic White | 113 | 34.6 | |
| Non-Hispanic Black | 26 | 7.9 | |
| Non-Hispanic Other | 33 | 10.1 |
The means and standard deviations of the scores from the self-report measures of physical activity, individual symptoms in the CCSC-L, and cognition across the four time points are seen in Table 3. The scores on the self-report of physical activity increased over time, especially from Time 1 to Time 2 during which physical activity scores almost doubled. For the five symptom measures that make up the CCSC-L, fatigue, pain, and depression decreased over time; whereas sleep disturbances scores remained constant while nausea remained the same from Time 1 to Time 3 before decreasing from Time 3 to Time 4. The functional outcome of cognition slightly decreased from Time 1 to Time 3 and then remained constant at Time 4.
Table 3.
Means & Standard Deviations of the Longitudinal Variables by Time (N = 327)
| Variable | Time 1 | Time 2 | Time 3 | Time 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | M | SD | n | M | SD | n | M | SD | n | M | SD | |
| Physical Activity | 298 | 16.74 | 39.39 | 289 | 32.34 | 51.03 | 240 | 38.51 | 51.50 | 221 | 39.97 | 44.41 |
| Fatigue | 300 | 53.87 | 10.01 | 292 | 50.03 | 10.56 | 241 | 48.22 | 9.23 | 222 | 46.60 | 8.11 |
| Sleep Disturbance | 296 | 2.66 | 0.75 | 286 | 2.75 | 0.79 | 240 | 2.75 | 0.78 | 219 | 2.69 | 0.81 |
| Pain | 297 | 2.67 | 2.33 | 287 | 1.67 | 2.22 | 239 | 1.37 | 1.81 | 220 | 1.34 | 1.85 |
| Nausea | 295 | 15.12 | 21.67 | 286 | 14.83 | 21.84 | 237 | 15.33 | 21.35 | 221 | 11.24 | 18.33 |
| Depression | 297 | 51.46 | 9.88 | 287 | 50.66 | 10.52 | 234 | 49.33 | 10.23 | 217 | 47.92 | 8.75 |
| Cognition | 294 | 51.87 | 9.01 | 282 | 50.11 | 10.17 | 237 | 48.52 | 10.35 | 218 | 48.94 | 10.31 |
Longitudinal Mediation Effects
The model fit indices for the initial longitudinal mediation model were not all satisfactory (χ2(6) = 25.84, p < .001; GFI = .98, NFI = .96, IFI = .97, RFI = .90, CFI = .97; and RMSEA = .10), indicating that the model needed further improvement. To obtain a parsimonious, best-fit model, the initial model was modified by removing non-significant paths and adding significant paths based on statistical modification indices produced by IBM SPSS Amos as well as theoretical interpretability. A final model was reached as shown in Figure 2 in which all the standardized estimates of path coefficients were significant at the α = .05 level. The model fit indices for the final model improved and all were satisfactory (χ2(6) = 9.19, p = .163; GFI = .99, NFI = .99, IFI = .99, RFI = .96, CFI = 1.00; and RMSEA = .04).
Figure 2. The Final Growth Model of the Longitudinal Mediation Effects of Physical Activity on Cognition via the Childhood Cancer Symptom Cluster – Leukemia with Standardized Estimatesa.
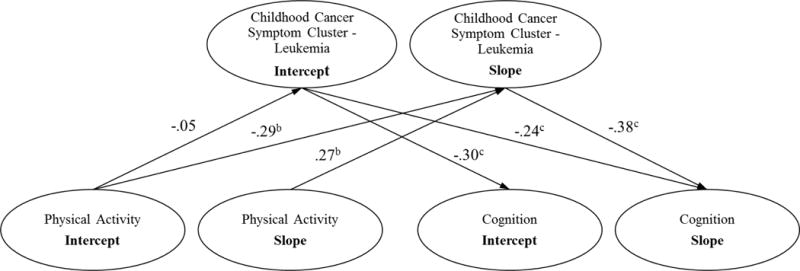
a Without loss of generality, the error terms and correlational paths are omitted
b p < .05
c p < .01
As seen in the mediational relationships diagramed in Figure 2, for patients with an initial high level of physical activity at Time 1 (intercept), the severity of the CCSC-L significantly decreased overtime (β = −.29, p < .033) as evidenced by the slope of its measurements over the four time points. However, the path coefficient from the first measurement of physical activity (intercept) to the first measurement of the CCSC-L (intercept) was not significant at the α = .05 level. In patients who had their physical activity increase over time (slope of the four physical activity measurements), the severity of their CCSC-L significantly worsened over the four measurements (β = .27, p < .017).
For patients who experienced a more severe level of the CCSC-L at the initial measurement (Time 1 intercept), their initial cognition function (Time 1 intercept) was lower (β = −.30, p < .005). Those children with a severe CCSC-L at the first measurement (intercept) also experienced a significant decrease in cognitive function over time (β = −.24, p < .007) as indicated by the decreasing slope of their cognitive scores. Moreover, if their CCSC-L became more severe over time (slope), their cognition decreased over time (slope) (β = −.38, p < .007).
DISCUSSION
To our knowledge, this is the first study to apply the longitudinal parallel-process to the analysis of longitudinal mediation effects of physical activity on cognition via symptom clusters in childhood cancer. From these results, we can broadly conclude that the CCSC-L acted as a mediator between physical activity and cognition. The longitudinal parallel-process provides researchers with a new approach to symptom research; findings provide insight into how symptoms change over the trajectory of treatment and how these changes are influenced by functions such as physical activity as well as how symptoms influence functional outcomes such as cognition. The findings of this study inform us on children undergoing treatment for leukemia; treatment of this type of cancer is most intensive in the early months of treatment. Application of the longitudinal parallel-process to symptoms experience by children during the first year of treatment for solid tumors would likely have a different outcome because differences in the patterns of treatment intensity as well as chemotherapy types.
In this study, self-report of higher levels of physical activity at the initial measurement was not related to a less severe symptom cluster at the first measurement, but was associated with a greater decrease in the CCSC-L severity over time demonstrating that those participants had a greater recovery from the distress of the symptom cluster. The first study measurement occurred just after patients completed induction chemotherapy. During induction chemotherapy, patients commonly experience muscle wasting and deconditioning due the high doses of corticosteroids.43 Children who were less impacted by these side effects may have been able to have higher physical activity levels at the first measurement or they possibly had been better conditioned pre-diagnosis which they then maintained into treatment and helped them recover from symptom distress. The relationship of early physical function positively impacting cancer symptoms and overall health is the premise of the concept of prehabilitation. Prehabilitation is a proactive intervention that occurs between the time of diagnosis and the initiation of treatment, such as an elective surgery for colorectal cancer or breast cancer.44 Improving physical activity before cancer treatment is not feasible with children diagnosed with leukemia however as treatment is initiated emergently to address the rising number of leukemia cells and symptoms of anemia and thrombocytopenia.
Analysis showed however that the patients who increased physical activity over the four measurements had an increase in the severity of the childhood leukemia symptom cluster over time; this was a surprising finding and merits further investigation. This study measured physical activity and symptoms over the natural course of treatment; there was not an intervention to impact activity levels. Future studies should evaluate if physical activity interventions can increase levels further as there may be a critical level that is needed to positively influence the symptoms in the CCSC-L.
Previous research reviews have reported on the impact of physical activity and exercise interventions on individual symptoms such as fatigue and sleep disturbance12,45 but this is the first study to explore its relationship as a non-directed function to a symptom cluster in children with cancer. Physical activity continues to be recommended for people of all ages with cancer during and after cancer treatment for improving health and quality of life.44 In children with cancer, physical activity is essential to their ongoing growth and development and needs to be supported by clinicians.13,46 Nurses are well-positioned in their clinical practice to encourage physical activity during hospitalization as well as coach patients and families on ways to increase their activity level in the home setting as a way to improve their health.
Study results demonstrated that the symptom cluster was associated as a mediator to cognitive function. At the first measurement post induction, a more severe CCSC-L was associated with a parent report of poorer cognition. This initial level of symptom cluster severity was also related to the child’s decrease in cognitive function over the first year of treatment. Those patients who had the cluster become more severe over time also decreased cognition over time. The identification of this relationship is an important finding and highlights the need to address symptom management early and throughout treatment as an additional strategy for protecting cognitive functioning.
Further research is needed to provide insight into the role biologic markers and genetics play in symptom cluster severity during leukemia treatment. The role of physical activity functioning pre-diagnosis also merits investigation as a true baseline measured through recall. Objective measurements of functional physical activity capacity such as actigraphy would add to our understanding of its role in symptom clusters. Parents’ report of their child’s cognitive function has been found to be a valid source of measurement in children with cancer.32,33 Testing the child directly using normative tests such as those in the NIH toolbox Cognition Measures47 administered by a trained assessor would have provided another dimension to functional outcomes but also increased the burden of study participation in a vulnerable population.
Physical activity is an important part of childhood yet during cancer treatment many pediatric patients and their families face barriers to becoming more active and report that they think exercising is unsafe.48 Future research needs to focus on how physical activity impact symptoms and how clinicians can decrease symptom distress and advance cognitive health for children with leukemia.
Acknowledgments
Disclosures: This was supported by a National Institutes of Health RO1CA1693398 and the Alex’s Lemonade Stand Foundation.
Footnotes
The authors declare no conflicts of interest to disclose.
Contributor Information
Mary C. Hooke, School of Nursing, University of Minnesota; Children’s Minnesota Cancer and Blood Disorders Program.
Cheryl Rodgers, School of Nursing, Duke University.
Olga Taylor, Texas Children’s Cancer and Hematology Centers/Baylor College of Medicine.
Kari M. Koerner, College of Nursing, University of Arizona.
Pauline Mitby, Children’s Minnesota Cancer and Blood Disorders Program.
Ida Moore, College of Nursing, University of Arizona.
Michael E. Scheurer, Texas Children’s Cancer and Hematology Centers/Baylor College of Medicine.
Marilyn J. Hockenberry, School of Nursing, Duke University.
Wei Pan, School of Nursing, Duke University.
References
- 1.Rodger C, Hooke MC, Ward J, Linder LA. Symptom clusters in children and adolescents with cancer. Semin Oncol Nurs. 2016;32(4):394–404. doi: 10.1016/j.soncn.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Barsevick A. Defining the symptom cluster: How far have we come? Semin Oncol Nurs. 2016;32(4):344–350. doi: 10.1016/j.soncn.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Hockenberry MJ, Hooke MC, Rodgers C, et al. Symptom trajectories in children receiving treatment for leukemia: A latent class growth analysis with multitrajectory modeling. J Pain Symptom Manage. 2017;54(1):1–8. doi: 10.1016/j.jpainsymman.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buckner TW, Wang J, DeWalt DA, Jacobs S, Reeve BB, Hinds PS. Patterns of symptoms and functional impairments in children with cancer. Pediatr Blood Cancer. 2014;61(7):1282–1288. doi: 10.1002/pbc.25029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sousa KH, Kwok OM, Schmiege SJ, West SG. A longitudinal approach to understanding the relationship between symptom status and QOL. West J Nurs Res. 2014;36(6):732–747. doi: 10.1177/0193945913510980. [DOI] [PubMed] [Google Scholar]
- 6.Dowling NM, Johnson SC, Gleason CE, Jagust WJ, Alzheimer’s Disease Neuroimaging Initiative The mediational effects of FDG hypometabolism on the association between cerebrospinal fluid biomarkers and neurocognitive function. Neuroimage. 2015;105:357–368. doi: 10.1016/j.neuroimage.2014.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winkler RM, Park J, Pan W, Brandon HD, Scher M, Holditch-Davis D. Does preterm period sleep development predict early childhood growth trajectories? J Perinatol. 2017;37(9):1047–1052. doi: 10.1038/jp.2017.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yıldırım M, Singh AS, te Velde SJ, et al. Mediators of longitudinal changes in measures of adiposity in teenagers using parallel process latent growth modeling. Obesity. 2013;21(11):2387–2395. doi: 10.1002/oby.20463. [DOI] [PubMed] [Google Scholar]
- 9.Physical Activity vs Exercise: What’s the Difference? American Council on Exercise; Web site. https://www.acefitness.org/education-and-resources/lifestyle/blog/5460/physical-activity-vs-exercise-what-s-the-difference. Published June 3, 2015. Accessed February 26, 2018. [Google Scholar]
- 10.How much physical activity do children need? Centers for Disease Control and Prevention; Web site. https://www.cdc.gov/physicalactivity/basics/children/index.htm. Published June 4, 2015. Accessed February 26, 2018. [Google Scholar]
- 11.Janssen I, Leblanc AG. Systematic review of the health benefits of physical activity and fitness in school-aged children and youth. Int J Behav Nutr Phys Act. 2010;7 doi: 10.1186/1479-5868-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baumann FT, Bloch W, Beulertz J. Clinical exercise interventions in pediatric oncology: a systematic review. Pediatr Res. 2013;74(4):366–374. doi: 10.1038/pr.2013.123. [DOI] [PubMed] [Google Scholar]
- 13.Götte M, Taraks S, Boos J. Sports in pediatric oncology: the role(s) of physical activity for children with cancer. J Pediatr Hematol Oncol. 2014;36(2):85–90. doi: 10.1097/MPH.0000000000000101. [DOI] [PubMed] [Google Scholar]
- 14.Winter C, Müller C, Brandes M, et al. Level of activity in children undergoing cancer treatment. Pediatr Blood Cancer. 2009;53(3):438–443. doi: 10.1002/pbc.22055. [DOI] [PubMed] [Google Scholar]
- 15.Fuemmeler BF, Pendzich MK, Clark K, et al. Diet, physical activity, and body composition changes during the first year of treatment for childhood acute leukemia and lymphoma. J Pediatr Hematol Oncol. 2013;35(6):437–43. doi: 10.1097/MPH.0b013e318279cd3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan SY, Poh BK, Chong HX, et al. Physical activity of pediatric patients with acute leukemia undergoing induction or consolidation chemotherapy. Leuk Res. 2013;37(1):14–20. doi: 10.1016/j.leukres.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Moore IM, Hockenberry MJ, Krull KR. Cancer-related cognitive changes in children, adolescents and adult survivors of childhood cancers. Semin Oncol Nurs. 2013;29(4):248–259. doi: 10.1016/j.soncn.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Jacola LM, Krull KR, Pui CH. Longitudinal assessment of neurocognitive outcomes in survivors of childhood acute lymphoblastic leukemia treated on a contemporary chemotherapy protocol. J Clin Oncol. 2016;34(11):1239–47. doi: 10.1200/JCO.2015.64.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hooke MC, Garwick AW. Gross CR Physical performance and fatigue in children and adolescents receiving chemotherapy. Oncol Nurs Forum. 2011;38(6):649–57. doi: 10.1188/11.ONF.649-657. [DOI] [PubMed] [Google Scholar]
- 20.Hinds PS, Yang J, Gattuso JS, et al. Psychometric and clinical assessment of the 10-item reduced version of the fatigue scale - child instrument. J Pain Symptom Manage. 2010;39(3):572–578. doi: 10.1016/j.jpainsymman.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mandrell BN, Yang J, Hooke MC. Psychometric and clinical assessment of the 13-item reduced version of the fatigue scale-adolescent instrument. J Pediatr Oncol Nurs. 2011;28(5):287–294. doi: 10.1177/1043454211418667. [DOI] [PubMed] [Google Scholar]
- 22.Hockenberry M, Hinds PS, Barrera P, et al. Three instruments to assess fatigue in children with cancer: the child, parent and staff perspectives. J Pain Symptom Manage. 2003;25(4):319–328. doi: 10.1016/s0885-3924(02)00680-2. [DOI] [PubMed] [Google Scholar]
- 23.LeBourgeois MK, Giannotti F, Cortesi F, Wolfson A, Harsh J. The relationship between reported sleep quality and sleep hygiene in Italian and American adolescents. Pediatrics. 2005;115(1 Suppl):S257–S265. doi: 10.1542/peds.2004-0815H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LeBourgeois M, Harsh JR. A new research measure for children’s sleep. Sleep. 2001;24:A213–A214. [Google Scholar]
- 25.Wong D, Baker C. Pain in children: comparison of assessment scales. Pediatr Nurs. 1988;14(1):9–17. [PubMed] [Google Scholar]
- 26.Scott J, Huskisson EC. Vertical or horizontal visual analogue scales. Ann Rheum Dis. 1979;38(6):560. doi: 10.1136/ard.38.6.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kovacs M. Children’s Depression Inventory 2 (CDI 2) San Antonio, TX: Pearson; 2010. [Google Scholar]
- 28.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10(3):141–146. [PubMed] [Google Scholar]
- 29.Sallis JF, Buono MJ, Roby JJ, Micale FG, Nelson JA. Seven-day recall and other physical activity self-reports in children and adolescents. Med Sci Sports Exerc. 1993;25(1):99–108. doi: 10.1249/00005768-199301000-00014. [DOI] [PubMed] [Google Scholar]
- 30.Keats MR. An Examination of Physical Activity Behaviors in a Sample of Adolescent Cancer Survivors. J Pediatr Oncol Nurs. 2006;23(3):135–142. doi: 10.1177/1043454206287304. [DOI] [PubMed] [Google Scholar]
- 31.Norris JM, Moules NJ, Pelletier G, Culos-Reed SN. Families of young pediatric cancer survivors: A cross-sectional survey examining physical activity behavior and health-related quality of life. J Pediatr Oncol Nurs. 2010;27(4):196–208. doi: 10.1177/1043454209358411. [DOI] [PubMed] [Google Scholar]
- 32.Lai JS, Zelko F, Butt Z, et al. Parent-perceived child cognitive function: results from a sample drawn from the US general population. Childs Nerv Syst. 2011;27(2):285–93. doi: 10.1007/s00381-010-1230-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lai JS, Zelko F, Krull KR, et al. Parent-reported cognition of children with cancer and its potential clinical usefulness. Qual Life Res. 2014;23(4):1049–58. doi: 10.1007/s11136-013-0548-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Little RJA. A test of missing completely at random for multivariate data with missing values. J Am Stat Assoc. 1988;83(404):1198–1202. [Google Scholar]
- 35.Little RJA, Rubin DB. Statistical Analysis with Missing Data. New York, NY: Wiley; 2002. [Google Scholar]
- 36.Cheong J, MacKinnon DP, Khoo ST. Investigation of mediational processes using latent growth curve modeling. Struct Equ Modeling. 2003;10(2):238–262. doi: 10.1207/S15328007SEM1002_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.SAS Institute Inc. SAS/STAT® 9.3 User’s Guide. Cary, NC: SAS Institute Inc; 2011. [Google Scholar]
- 38.Arbuckle JL. IBM® SPSS® Amos™ 23 User’s Guide. Armonk, NY: IBM Corp; 2014. 2014. [Google Scholar]
- 39.Bai H, Pan W. Resampling methods revisited: Advancing the understanding and applications in educational research. Int J Res Method Educ. 2008;31(1):45–62. [Google Scholar]
- 40.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. New York, NY: Chapman and Hall; 1998. [Google Scholar]
- 41.Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6(1):1–55. [Google Scholar]
- 42.Kline RB. Principles and Practice of Structural Equation Modeling. 2nd. New York, NY: The Guilford Press; 2005. [Google Scholar]
- 43.Ness KK, Kaste SC, Zhu L, et al. Skeletal, neuromuscular and fitness impairments among children with newly diagnosed acute lymphoblastic leukemia. Leuk Lymphoma. 2015;56(4):1004–1011. doi: 10.3109/10428194.2014.944519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silver JK. Cancer prehabilitation and its role in improving health outcomes and reducing health care costs. Semin Oncol Nurs. 2015;31(1):13–30. doi: 10.1016/j.soncn.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 45.Demark-Wahnefried W, Schmitz KH, Alfano CM, et al. Weight management and physical activity throughout the cancer care continuum. CA Cancer J Clin. 2018;68(1):64–89. doi: 10.3322/caac.21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Braam KI, van der Torre P, Takken T, Veening MA, van Dulmen-den Broeder E, Kaspers GJ. Physical exercise training interventions for children and young adults during and after treatment for childhood cancer. Cochrane Database Syst Rev. 2016;31(3):CD008796. doi: 10.1002/14651858.CD008796.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Denboer JW, Nicholls C, Corte C, Chestnut K. National Institutes of Health Toolbox Cognition Battery. Arch Clin Neuropsychol. 2014;29(7):692–694. [Google Scholar]
- 48.Ross WL, Le A, Zheng DJ, et al. Physical activity barriers, preferences, and beliefs in childhood cancer patients. Support Care Cancer. 2018 Jan 27; doi: 10.1007/s00520-017-4041-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


