Abstract
Parkinson’s disease (PD) is the second most common neurodegenerative disease and is characterized by the formation of α-synuclein-containing protein aggregates called Lewy bodies within the brain. A crucial role for α-synuclein in the pathogenesis of PD is also suggested by the fact that point mutations, increased copy number, or polymorphisms in the α-synuclein gene SNCA all cause or contribute to the development of PD. In addition to SNCA, an increasing number of other genes have been implicated in PD. While mutations in at least some of these genes has been shown to cause the formation of Lewy bodies, the role of α-synuclein in these genetic forms of PD remains poorly defined. Since C. elegans do not have a homolog of α-synuclein, this organism provides the opportunity to identify synergism between α-synuclein and other genes implicated in PD. To do this, we generated a novel C. elegans model in which wild-type α-synuclein is ubiquitously expressed from a single copy transgene, and examined the resulting effect on phenotypic deficits in PD deletion mutants affecting PARK2/pdr-1, PINK1/pink-1, DJ-1/djr-1.1 and ATP13A2/catp-6. While the PD deletion mutants exhibit only mild phenotypic deficits in absence of α-synuclein, expression of wild-type α-synuclein caused increased sensitivity to multiple stresses, induced deficits in dopamine-dependent behavior, and accelerated loss of dopamine neurons. Overall, these results suggest that the recessive loss of function mutations act together with α-synuclein to cause PD, and that α-synuclein lowering strategies may be effective in genetic forms of PD.
Keywords: Parkinson’s disease, neurodegeneration, α-synuclein, C. elegans, genetics, animal model, parkin, PINK1, DJ-1, ATP13A2
1. Introduction
Parkinson’s disease (PD) is an adult-onset neurodegenerative disease currently affecting approximately 10 million patients worldwide. PD is characterized by deficiencies in movement including resting tremor, slowness of movement, postural instability, a shuffling gait, and rigidity that progressively worsen through the course of the disease. Patients with PD also experience non-motor symptoms, which can include dementia, sleep disturbances, anxiety, apathy, constipation, and depression, among others. In the brain, PD patients display a selective loss of dopamine neurons in a compartment of the basal ganglia called the substantia nigra (SN) and the formation of protein aggregates called Lewy bodies.
While PD was traditionally thought of as an entirely sporadic disorder, it is now clear that there is a strong genetic contribution to the disease. The gene encoding α-synuclein (SNCA) was the first gene to be implicated in PD (Polymeropoulos et al., 1997) and it was subsequently shown that Lewy bodies are composed of aggregated α-synuclein (Spillantini et al., 1998). Both mutations in the SNCA gene, and increased copy number of the SNCA gene have been implicated in PD (Singleton et al., 2003; Soldner et al., 2016). In addition, polymorphisms in the SNCA gene have been associated with idiopathic PD (Satake et al., 2009; Simon-Sanchez et al., 2009).
Since the discovery of the role of SNCA in PD, there have been multiple other genes implicated in monogenic PD, including PARK2/pdr-1, PINK1/pink-1, DJ-1/djr-1.1 and ATP13A2/catp-6. PARK2/pdr-1 encodes an E3 ubiquitin ligase that marks proteins for degradation by the proteasome (Kitada et al., 1998; Shimura et al., 2000). PINK1/pink-1 (PTEN-induced putative kinase 1) encodes a serine/threonine kinase that functions in the removal of damaged mitochondria (Valente et al., 2004; Vives-Bauza et al., 2010). DJ-1/djr-1.1 encodes a neuroprotective protein that acts to protect against oxidative stress (Bonifati et al., 2003; Taira et al., 2004). Finally, ATP13A2/catp-6 encodes a lysosomal cation transporting ATPase (Ramirez et al., 2006).
The extent to which other genes that have been implicated in PD act through α-synuclein to cause PD is currently uncertain. While the number of genetic PD patients that have been autopsied is limited, patients with mutations in other PD-associated genes, such as LRRK2 and PINK1, have been shown to have Lewy bodies indicating that these mutations can lead to the aggregation of α-synuclein. This suggests the possibility that the mechanism by which other genes implicated in PD lead to disease may involve α-synuclein. This idea is supported by the fact that multiple of these genes have activities that could obviously affect α-synuclein function or aggregation. For example, Parkin functions in the ubiquitin-proteasome system and thus disruption of Parkin could lead to difficulties in degrading α-synuclein leading to increased levels of α-synuclein.
Accordingly, we sought to determine the role of α-synuclein in mediating the detrimental effects of other PD-associated mutations. To address this question, we used Caenorhabditis elegans as a model organism. As worms do not express α-synuclein, using C. elegans enabled us to study α-synuclein toxicity in an organism that does not require normal α-synuclein function. Since previous α-synuclein models in C. elegans express α-synuclein only in specific tissues and from multiple copy arrays (Buttner et al., 2014; Cao et al., 2005; Cooper and Van Raamsdonk, 2018; Kuwahara et al., 2006; Kuwahara et al., 2008; Kuwahara et al., 2012; Lakso et al., 2003; Springer et al., 2005), we generated a new worm model that ubiquitously expresses wild-type α-synuclein from a single copy transgene and crossed these worms and another α-synuclein worm model to pdr-1, pink-1, djr-1.1 and catp-6 mutants. We found that the presence of α-synuclein decreased stress resistance and increased neurodegeneration in genetic models of PD suggesting that these other genetic defects act in conjunction with α-synuclein to cause PD.
2. Materials and Methods
2.1. Strains
The following strains were utilized in these experiments:
N2(wild-type),
BY250 vtIs7[Pdat-1::GFP(pRB490)],
NL5901 pkIs2386[Punc-54:: α-synuclein::YFP+unc-119(+)],
JVR047 rmIs126 [Punc-54::Q0:YFP],
JVR339 jerIs004[Peft-3:: α-synuclein:TagRFP:let-858_unc-119(+)]; unc-119(−);vtIs7[Pdat-1::GFP(pRB490)],
JVR 344 jerIs004[Peft-3::RFP:let-858_unc-119(+)]; unc-119(−);vtIs7[Pdat-1::GFP(pRB490)],
JVR043 pdr-1(gk448),
JVR036 pdr-1(gk448); vtIs7[Pdat-1:GFP(pRB490)],
MQ1722 pdr-1(gk448); pkIs2386[Punc-54:αsynuclein:YFP + unc-119(+)],
JVR366 pdr-1(gk448); jerIs004[Peft-3::a-syn:TagRFP:let-858_unc-119(+)]; unc-119(−);vtls7[Pdat-1::GFP(pRB490)],
MQ1775 pink-1(ok3538),
MQ1794 pink-1(ok3538);vtIs7[Pdat-1:GFP(pRB490)],
MQ1724 pink-1(ok3538); pkIs2386[Punc-54::αsynuclein:YFP + unc-119(+)],
JVR433 pink-1(ok3538); jerIs004[Peft-3::a-syn:TagRFP:let-858_unc-119(+)]; unc-119(−);vtIs7[Pdat-1::GFP(pRB490)],
JVR066 djr-1.1(tm918),
JVR220 djr-1.1(tm918); vtIs7[Pdat-1::GFP(pRB490)],
JVR154 djr-1.1(tm918); pkIs2386[Punc-54::αsynuclein:YFP + unc-119(+)],
JVR374 djr-1.2(tm1346); vtIs7[Pdat-1::GFP(pRB490)],
JVR367 djr-1.1(tm918);djr-1.2(tm1346);vtIs7[Pdat-1::GFP(pRB490)],
JVR381 djr-1.1(tm918);djr-1.2(tm1346);jerIs004[Peft-3::a-syn::TagRFP:let-858_unc-119(+)]; unc-119(−);vtIs7[Pdat-1::GFP(pRB490)],
MQ1736 catp-6 (ok3473),
MQ1793 catp-6 (ok3473); vtIs7[Pdat-1::GFP(pRB490)],
JVR364 catp-6 (ok3473); jerIs004[Peft-3::a-syn:TagRFP:let-858_unc-119(+)] II; unc-119(-);vtIs7[Pdat-1::GFP(pRB490)].
Strains were maintained on NGM plates seeded with OP50 bacteria at a temperature of 20°C.
2.2. Generation of new transgenic strains
Full length human α-synuclein cDNA (417 bp) lacking start and stop codons was PCR amplified using C. elegans genomic DNA isolated from Pdat-1::α-synuclein[wild-type] worms (Kuwahara et al., 2006). Primers included attB1 and attB2 sites, allowing for α-synuclein cDNA to undergo BP recombination into pDONR221 to form α-syn [2,1]; this construct was sequence verified and saved as pJVR015. Multi-site Gateway recombination was subsequently used to generate Peft-3::a-syn::tagRFP::let-858 UTR in the MosSCI pDEST backbone (pCFJ150, which contains the unc-119 rescue construct) to generate pJVR017. The start codon is contained in the 581bp eft-3 [4,1] promoter clone obtained through the C. elegans promoterome (GE Dharmacon), and the stop codon follows the in-frame RFP in the 3’ element pGH107 (tagRFP+let-858 UTR). The final strain (jerls004, JVR339) was constructed by injecting EG4322 (ttTi5605 II; unc-119(ed9) III) worms with pJVR017 (Peft-3::α-syn::tagRFP::let-858, unc-119(+)) at 25 ng/ul, with pCJF601 (50 ng/ul), pMA122 (10ng/ul), and markers including F25B3.3::GFP (5 ng/ul), lin-26::GFP (5 ng/ul), elt-7::GFP (5ng/ul) and direct Mos-mediated integration as previously described (Frokjaer-Jensen et al., 2008).
The control strain was constructed similarly, using multisite Gateway to combine pEft-3 [2,1], tagRFP+stop [2,1] (bsem1163) and pADA126(let-858 UTR [2,3]) into pCFJ150 to generate pJVR029. The construct was injected at 25 ng/ul, with appropriate co-injection constructs and integrated as above to generate jerIs015 (JVR344). All strains were outcrossed at least 4 times. At least 3 independent lines were generated per construct.
Single copy integration was confirmed using protocols developed by the Nonet lab (http://thalamus.wustl.edu/nonetlab/ResourcesF/Resources.html). Five plates of age synchronized worms were collected at day 1 of adulthood and washed 3x in M9 buffer. Samples were then frozen with liquid nitrogen and stored at −80°C. Genomic DNA was purified by phenol/chloroform extraction and ethanol precipitation. Samples were then diluted to 20 ng/μL and 100 ng of DNA was loaded into each PCR reaction and run using a long amplification PCR buffer and TAQ DNA polymerase (New England Biolabs MO323S). Primers targeted the flanking arms of the Mos1 site (NM3887 5' ACCGGAAACCAAAGGACGAGAG and NM3888 5' ACGCCCAGGAGAACACGTTAG) allowing the length of the inserted α-synuclein::RFP transgene to be determined. Genomic DNA isolated from the transgenic strain was compared to the pJVR017 plasmid used in the construction of the transgenic animals and the pCFJ150 backbone without an insert were also amplified for comparison using this protocol. The thermocycler conditions were set using a long amplification protocol: 30 seconds at 95°C, 35 cycles of 94°C for 10 seconds, 60°C for 50 seconds, 65°C for 12 minutes +10 second per cycle, 65°C for 10 minutes.
2.3. Lifespan
Lifespan was determined on nematode growth media (NGM) agar plates with 25 μM 5-fluoro-2′-deoxyuridine (FUdR) in order to reduce the development of progeny. While high concentrations of FUdR have been shown to impact longevity in specific strains, in our experience these differences, if any, are minimized by using 25 μM (Van Raamsdonk and Hekimi, 2011). Since this concentration of FUdR does not completely prevent the development of progeny to adulthood in the first generation, animals were transferred to fresh agar plates after 3 days. After the initial transfer, worms were moved to fresh plates weekly. Animal survival was observed every 2 days by gentle prodding. Three replicates of 30 animals each were completed.
2.4. Defecation rate
Defecation cycle length was measured by the length in time between consecutive pBoc contractions in day 1 adult worms. To reduce the impact of ambient laboratory temperature, defecation rate was quantified on 20 °C water filled chambers. Three replicates of 15 animals each were completed.
2.5. Postembryonic development time
Post-embryonic development (PED) was assessed by moving eggs to agar plates. After 3 h, newly hatched L1 worms were transferred to a new plate. The hours from hatching to the young adult transition was measured as the PED time. Three replicates of 30 animals each were completed.
2.6. Fertility
Brood size was determined by placing individual young adult staged animals onto agar plates with daily transfers to new plates until progeny production ceased. The resulting progeny was allowed to develop to adulthood before quantification. Three replicates of 10 animals each were completed.
2.7. Thrashing behavior
The thrashing rate was determined using video-tracking and computer analysis (Cooper et al., 2015). Videos were taken with an Allied Vision Tech Stingray F-145 B Firewire Camera (Allied Vision, Exton, PA, USA) using the MATLAB image acquisition toolbox. Analysis was performed using wrMTrck plugin for ImageJ (available at http://www.phage.dk/plugins). Three replicates of 50 animals each were completed.
2.8. Stress resistance assays: Heat stress, Oxidative stress, Osmotic stress
Stress resistance assays were performed as we have described previously (Dues et al., 2016). Heat stress sensitivity was assessed on the first day of adulthood at 37 °C. Sensitivity to chronic oxidative stress was determined through exposure to plates containing paraquat (Methyl viologen, Sigma) starting at day 1 of adulthood (Dues et al., 2017). Sensitivity to osmotic stress was determined in day 1 adult worms by transferring to plates containing 500 mM NaCl and measuring survival after 24 hours. Three replicates of 30 animals each were completed.
2.9. Basal slowing
Basal slowing is a measurement of dopamine dependent behavior. Animals at day 1 of adulthood were washed in M9 buffer to clean animals of bacteria. These animals were then moved to either unseeded agar plates or agar plates seeded with OP50 bacteria covering the whole plate. After approximately 5 minutes, videos of the entire plate were recorded for 1 minute with an Allied Vision Tech Stingray F-504 B Firewire Camera and a Navitar Zoom 7000 lens (Navitar, Tokyo, Japan) using the MATLAB image acquisition tool. Recordings were then processed using the wrMTrck plugin for ImageJ. Basal slowing was calculated as the difference in rate of movement on food versus off food divided by the rate of movement off food.
Crawling speed was determined from data collected from the unseeded off-food agar plate. Three replicates of 50 animals each were completed.
2.10. Degeneration of dopamine neurons and dendritic blebbing
Dopamine neuron neurodegeneration and dendritic blebbing was determined using worms that express GFP only in dopamine neurons under the dat-1 dopamine transporter promoter. Treatments that cause a loss of GFP-positive neurons cause a corresponding loss of dopamine neuron cell bodies (Nass et al., 2002). Pdat-1:GFP worms were aged on agar plates containing 25 μM FUdR to be observed at designated ages. At every time point, worms were mounted onto a 1% agar pad on a glass slide, paralyzed using 2 mM levamisole, and enclosed with a coverslip. Imaging of these animals was carried out with an Axioplan 2 inverted fluorescence microscope (Zeiss, Oberkochen, Germany). At each time point for the neurodegeneration assay remaining CEP, ADE, and PDE neurons were quantified. At each time point for the dendritic blebbing assay the total number of fluorescent blebs observed in the four rostral dopaminergic dendrites were quantified. Blebs, bead-shape protrusions from the neuronal processes, were defined as distortions of the dendrite structure with an area greater than approximately 5 μm2. Blebbing increases with age in several classes of neurons and correlates with the dysfunction of behaviors associated with those neurons (Pan et al., 2011). Three replicates of 10 animals per time point were completed.
2.11. Measurement of aggregation
Animals were mounted onto slides and imaged using a 63x objective on a Zeiss Axioplan 2 compound microscope (Zeiss, Oberkochen, Germany). Punctae, defined as clusters of at least 3 pixels whose intensity were at least 1 standard deviation above the background intensity, were quantified using MATLAB. Three replicates of 10 animals each were completed.
2.12. Quantitative real-time RT-PCR
Age-synchronized pre-fertile young adult worms from a limited lay were collected and washed three times in M9 buffer. Excess buffer was removed and 150 μl of Trizol was added prior to freezing. mRNA was isolated as described previously (Machiela et al., 2016) and converted to cDNA using a High Capacity cDNA Reverse Transcription kit (Life Technologies). Quantitative real-time RT-PCR was performed using a Fast SYBR Green Master Mix (Applied Biosystems) in a Bio-Rad iCycler Thermal Cycler.
2.13. Western blotting
Worms were picked from unseeded areas of NGM plates and collected into 10 μl of M9 buffer. Between 10 and 200 worms were utilized in an attempt to obtain comparable levels of α-synuclein based on the levels of α-synuclein mRNA. 3.5 μl of 6X Laemmli SDS Sample Buffer (GTX16357, GeneTex) and 1.5 μl 100 mM DTT were added to each sample. Samples were incubated at 95ΰC for 10 minutes in a BioRad T100 Thermal Cycler and then frozen until loading. Samples were boiled and loaded onto a 4-12% gel (Lonza). α-synuclein was detected using the H3C mouse anti-α-synuclein antibody (1:5000 dilution; Catalog#:h3c; RRID: AB_2618046) and anti-mouse HRP-conjugated secondary antibody (1:50,000 dilution) with enhanced chemiluminescence. Actin was detected using a mouse anti-actin antibody (JLA20, 1:5000 dilution).
2.14. Imaging
Young adult hermaphrodites were loaded on 2% agarose pads. 0.1% levamisole was used to anesthetize the worms. Images were captured with an IX-70 microscope (Olympus) fitted with a cooled CCD camera (CH350; Roper Scientific) driven by the Delta Vision system (Applied Precision). Images were deconvolved using the SoftWorx 3.0 deconvolution software from Applied Precision.
2.15. Statistical analysis
Experiments were performed such that the experimenter was blinded to the genotype of the worms being assayed. Survival plots were compared using the log-rank test. For analyses involving multiple groups and time points, a one-way or two-way ANOVA was used to assess significance followed by Bonferroni post-hoc tests for detecting specific differences between groups.
3. Results
3.1. Generation of characterization of a novel C. elegans model that express α-synuclein from a single copy transgene
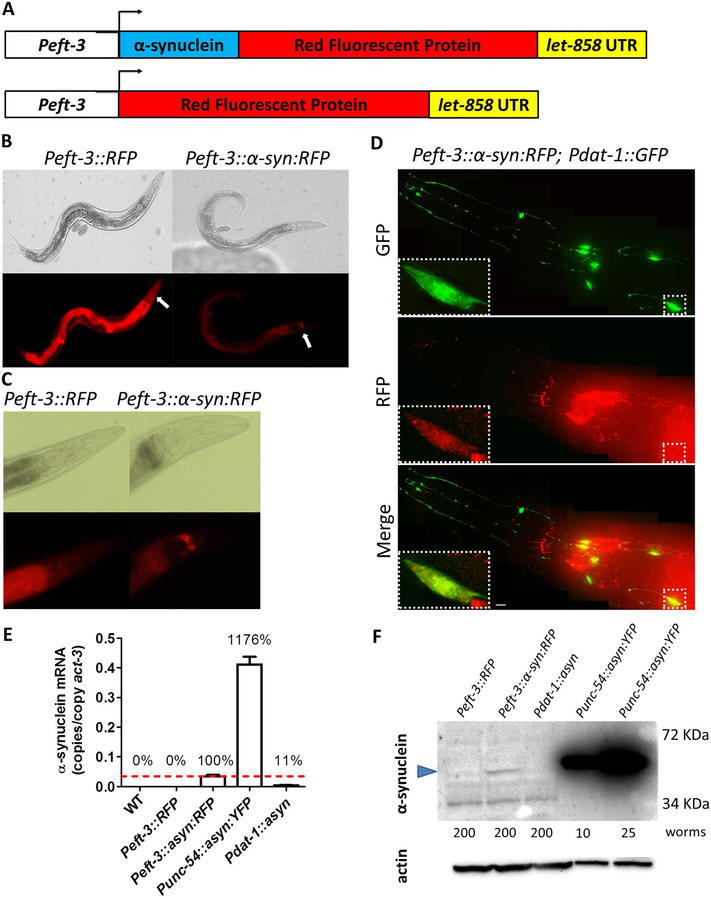
As worms do not express α-synuclein, C. elegans provides the opportunity to define the role of α-synuclein in the pathogenesis of PD in an organism that does not rely on α-synuclein’s normal biological function. To assess the extent to which α-synuclein contributes to the pathogenesis in other genetic forms of PD, we asked the question of whether expressing α-synuclein in genetic C. elegans models of PD would exacerbate phenotypic deficits already present in these strains or unmask new phenotypic deficits. To do this, we generated worms that express α-synuclein ubiquitously from a single copy transgene. Specifically, we cloned human wild-type α-synuclein and RFP next to a ubiquitous eft-3 promoter and used Mos-mediated integration to integrate a single copy of this transgene to approximate physiologic levels of expression (Frokjaer-Jensen et al., 2008) (Fig. 1A; these worms will be referred to as asyn:RFP worms). As a control, we also generated worms expressing a single copy RFP under the eft-3 promoter (these worms will be referred to as RFP worms).
Fig. 1. Ubiquitously expressed α-synuclein::RFP fusion protein is present in dopamine neurons and accumulates in the nerve ring.
A. Diagram illustrating constructs used to generate C. elegans models that ubiquitously express α-synuclein linked to RFP and control strains expressing RFP alone. Both transgenes are expressed behind a ubiquitous promoter eft-3. B,C. Peft-3::RFP and Peft-3::α-syn:RFP worms exhibit expression of RFP and α-syn:RFP, respectively, in all tissues. Peft-3::α-syn:RFP show an accumulation of α-syn:RFP in the nerve ring that is not present in the RFP control strain. The nerve ring is indicated by the white arrows. D. α-syn:RFP exhibits colocalization with GFP expressed in dopamine neurons using dat-1 promoter indicating that α-synuclein is being expressed in dopamine neurons. Scale bar indicates 5 αm. E. Quantitative real-time RT-PCR demonstrates that α-synuclein mRNA is being expressed at a much lower level than Punc-54::α-syn:YFP worms, which express α-synuclein from an integrated multi-copy array. F. Western blotting using H3C anti-α-synuclein antibody shows that α-synuclein is being expressed in Peft-3::α-syn:RFP worms at a much lower level than Punc-54::α-syn:YFP worms. Blue arrow indicates α-synuclein band. A non-specific band of lower molecular weight was observed (also present in Peft-3::RFP control worms). The number of worms used for each lysate is indicated. Error bars indicate SEM.
To confirm that only a single copy of the α-synuclein::RFP transgene was integrated at the Mos1 site, we performed long range PCR using primers that flanked the Mos1 site (Zheng et al., 2014). We compared genomic DNA from asyn:RFP worms, to the original pJVR017 plasmid which was used to generate the transgenic strain, and the pCFJ150 backbone without the asyn insert (Fig. S1). We found that the size of the fragment in the asyn:RFP strain was identical to the original pJVR017 plasmid and larger than the pCFJ150 backbone. This indicates that asyn:RFP worms have integrated a single copy of the asyn:RFP transgene.
To ensure that the eft-3 promoter was driving ubiquitous expression of asyn:RFP and RFP, we examined the worms under the fluorescent microscope. We found that both strains exhibited red fluorescence through the entire body (Fig. 1B). In all of the lines that we generated, we found that the red fluorescence is brighter in RFP worms compared to asyn:RFP suggesting that either the level of expression in the RFP control strain is higher than in asyn:RFP worms, or that the presence of α-synuclein diminishes the brightness of RFP. Interestingly, we found that the α-synuclein:RFP fusion protein accumulates in the nerve ring while RFP does not Fig. 1C).
In order to confirm that α-synuclein is expressed in the dopamine neurons on asyn:RFP worms, we crossed these worms to worms that express GFP only in the dopamine neurons (Pdat-1::GFP). As expected we found that the red fluorescence of asyn:RFP colocalizes with the GFP indicating that α-synuclein is being expressed in the dopamine neurons (Fig. 1D).
Next, we examined the levels of α-synuclein mRNA in our single copy model using quantitative real-time RT-PCR. We compared mRNA levels to a worm model that expresses wild-type α-synuclein linked to YFP in the body wall muscle from an integrated multicopy array (Punc-54::asyn:YFP (van Ham et al., 2008)) and a model that expresses wild-type α-synuclein exclusively in dopamine neurons from an integrated multicopy array (Pdat-1::asyn (Kuwahara et al., 2006)). We found that Peft-3::asyn:RFP worms have more than 10-fold less α-synuclein mRNA compared to Punc-54::asyn:YFP worms and approximately 10-fold more α-synuclein mRNA compared to the dopamine neurons model (Fig. 1E). Since the other models only express α-synuclein in a limited population of cells (95 body wall muscle cells or 8 dopa mine neurons), our single copy model has much less α-synuclein mRNA per α-synuclein-expressing cell than either of these previous models.
Finally, we sought to ensure that α-synuclein protein was being expressed in the asyn:RFP worms. To do this, we performed a Western blot using an anti-α-synuclein antibody (H3C). We found that this antibody detects a faint band in asyn:RFP worms but not RFP worms that is of the predicted size (Fig. 1F). Consistent with our quantitative real-time RT-PCR results, we found that asyn:RFP worms have much less α-synuclein protein than worms expressing α-synuclein from a multicopy array (Punc-54::asyn:YFP worms), while the levels of α-synuclein present in Pdat-1::asyn were below the level of detection.
3.2. Single copy expression of wild-type α-synuclein causes age-dependent behavioral deficits in movement
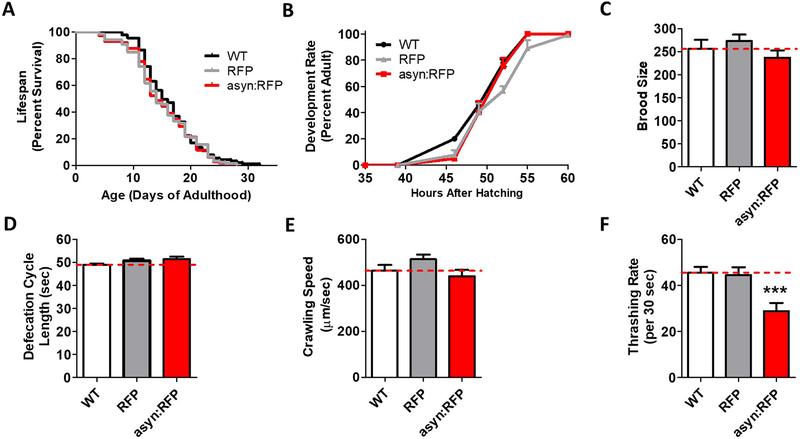
Before examining the effect of ubiquitous α-synuclein expression in genetic loss of function mutants, we first sought to determine whether ubiquitous expression of α-synuclein from a single copy transgene is sufficient to induce phenotypic deficits. We found that lifespan (Fig. 2A), post-embryonic development time (Fig. 2B), fertility (Fig. 2C), defecation cycle length (Fig. 2D) and crawling speed (Fig. 2E) were all equivalent to wild-type and RFP control worms in asyn:RFP worms. In contrast, the ubiquitous expression of wild-type α-synuclein caused a significant deficit in movement, which was not observed in the RFP control worms (Fig. 2F). Thus, while measures of overall health and physiologic rates are normal in asyn:RFP worms, these worms do exhibit a deficit in movement.
Fig. 2. Ubiquitous expression of α-synuclein causes a deficit in thrashing.
Worms expressing α-synuclein:RFP ubiquitously do not show deficits in lifespan (A), post-embryonic development time (B), brood size (C), defecation rate (D), or crawling speed (E). However, these worms show a significant deficit in their rate of movement in liquid (F). Peft-3::RFP worms are indicated as RFP. Peft-3::α-syn:RFP worms are indicated as asyn:RFP . Error bars indicate SEM. ***p<0.001.
3.3. α-synuclein increases dendritic blebbing and causes age-dependent deficits in basal slowing
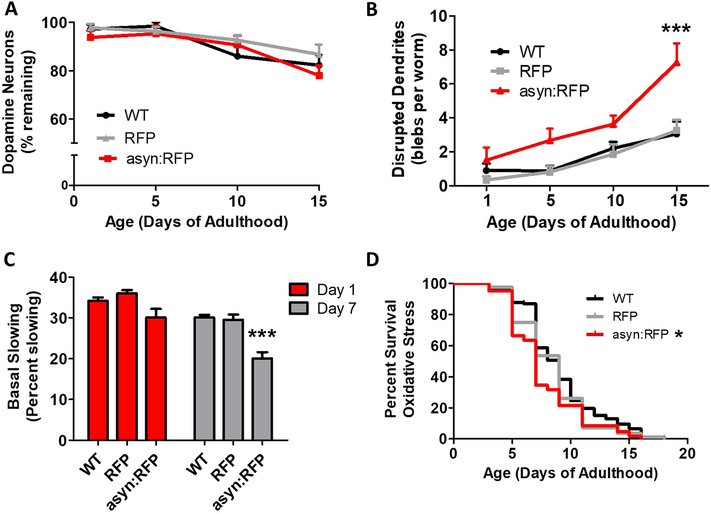
Since PD is characterized by the loss of dopamine neurons, we next examined the effect of ubiquitous expression of wild-type α-synuclein on neuronal survival and function. We found that while the number of dopamine neurons declined with age, asyn:RFP worms did not show accelerated loss of dopamine neurons compared to wild-type or RFP control worms (Fig. 3A). Since we did not observe gross changes in neuron survival, we explored morphology. Previous reports have shown that blebbing in neuronal processes correlates with neurological toxicity (Masoudi et al., 2014; Nass et al., 2002; Pan et al., 2011). Accordingly, we quantified dendritic blebbing in the anterior dendrites of dopaminergic neurons. We found that dendritic blebbing increased with age and that this increase was exacerbated by expression of α-synuclein (Fig. 3B). To determine if the increase in dendritic blebbing is associated with disruption of neuronal function, we quantified basal-slowing, which is a dopamine-dependent behavior. We found that asyn:RFP worms exhibit an age-dependent deficit in basal slowing (Fig. 3C). This suggests that while expression of α-synuclein is not sufficient to cause neuronal loss, it does lead to neuronal dysfunction with advancing age.
Fig. 3. Ubiquitous expression of α-synuclein causes an age-dependent deficit in dopamine-dependent behavior and dendritic blebbing.
Worms expressing α-synuclein:RFP ubiquitously do not show accelerated loss of dopamine neurons (A) but have increased dendritic blebbing (B), and an age-dependent deficit in basal slowing (C). D. Peft-3::α-syn:RFP worms exhibit mildly increased sensitivity to oxidative stress when treated with 4 mM paraquat. Peft-3::RFP worms are indicated as RFP. Peft-3::α-syn:RFP worms are indicated as asyn:RFP . Error bars indicate SEM. *p<0.05, ***p<0.001.
Since previous work has shown that α-synuclein increases oxidative stress in models of PD (Esteves et al., 2009; Pan et al., 2011; Perfeito et al., 2017; Tapias et al., 2017), we examined sensitivity to oxidative in stress by exposing worms to the superoxide-generating compound paraquat. We found that asyn:RFP worms showed a mild increase in sensitivity to oxidative stress compared to wild-type and RFP worms (Fig. 3D).
3.4. Single copy expression of wild-type α-synuclein exacerbates deficits in PD deletion mutants
To define the role of α-synuclein in the pathogenesis caused by loss of function PD mutations, we crossed the newly generated asyn:RFP model to pdr-1, pink-1, djr-1.1, and catp-6 deletion mutants. Since C. elegans also has a neuronally expressed version of DJ-1, we also generated a asyn:RFP;djr-1.1;djr-1.2 mutants.
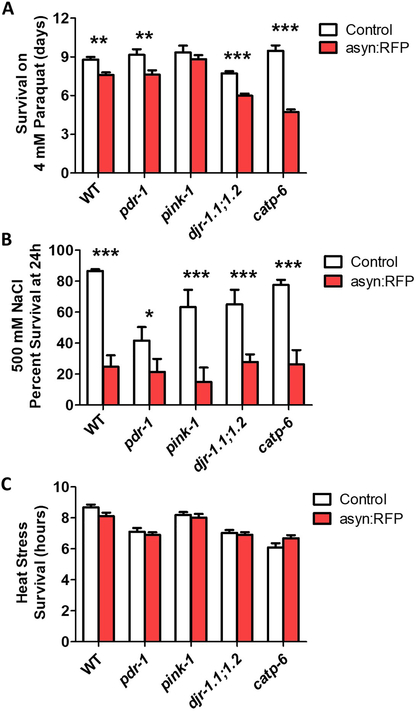
First, we examined sensitivity to oxidative, osmotic and heat stress, as all three stresses can increase protein aggregation (Gankam-Kengne et al., 2017; Mazzeo et al., 2012). After exposing worms to oxidative stress induced by 4 mM paraquat, we found that the ubiquitous expression of α-synuclein significantly increased stress sensitivity in wild-type, pdr-1, djr-1.1;djr-1.2 and catp-6 worms (Fig. 4A). Similarly, α-synuclein expression throughout the body markedly increased sensitivity to osmotic stress on plates containing 500 mM NaCl (Fig. 4B). In contrast, the asyn:RFP transgene had no effect on survival under 37°C heat stress (Fig. 4C). This may be because the heat stress is an acute assay occurring over a few hours while the osmotic and oxidative stress assays are chronic assays taking place over 24 hours or more.
Fig. 4. Ubiquitous expression of α-synuclein increases sensitivity to stress in recessive loss of function models of Parkinson’s disease.
α-synuclein expression throughout the body causes increased sensitivity to oxidative stress on 4 mM paraquat plates (A) and osmotic stress on 500 mM NaCl containing plates (B), but does not affect sensitivity to 37°C heat stress (C). Peft-3::α-syn:RFP worms are indicated as asyn:RFP . Error bars indicate SEM. *p<0.05, **p<0.01, ***p<0.001.
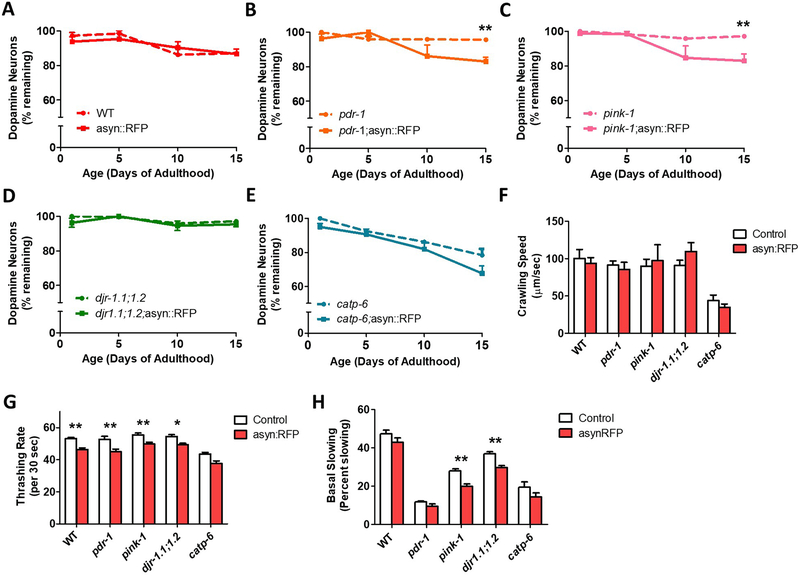
Since the loss of dopaminergic neurons is a hallmark feature of PD, we next sought to determine if the addition of α-synuclein would increase neurodegeneration in the recessive loss of function mutants. We found that while ubiquitous α-synuclein expression alone caused no degeneration with age compared to control (Fig. 5A), it significantly increases the rate of dopamine neuronal loss in pdr-1 and pink-1 mutants (Fig. 5B,C). The asyn:RFP transgene did not significantly accelerate neuronal loss in djr-1.1;djr-1.2 or catp-6 animals (Fig. 5D). Nonetheless, we did observe an increase in dendritic blebbing in asyn:RFP;djr-1.1;djr-1.2 animals compared to djr-1.1;djr-1.2 controls (Fig. S2).
Fig. 5. Ubiquitous expression of α-synuclein accelerates dopamine neuronal loss in pdr-1 and pink-1 mutants.
A. Worms expressing α-synuclein:RFP ubiquitously do not show an accelerated loss of dopamine neurons. B,C. In contrast, ubiquitous expression of α-synuclein does cause dopaminergic neurodegeneration in pdr-1 and pink-1 mutants. D,E. As in the WT background, α-synuclein expression throughout the body does not increase neuronal loss in djr-1.1;1.2 or catp-6 worms. The expression of α-synuclein does not decrease crawling speed on solid agar plates (F), but does decrease the rate of movement in liquid (G), and induce deficits in dopamine-dependent behavior as measured by basal slowing (H). Peft-3::α-syn:RFP worms are indicated as asyn:RFP. Error bars indicate SEM. *p<0.05, **p<0.01.
In order to determine whether the expression of α-synuclein would cause or exacerbate movement deficits in the PD deletion mutants, we measured crawling speed on solid plates and thrashing rate in liquid. While α-synuclein did not significantly alter crawling speed (Fig. 5F), ubiquitous expression of α-synuclein decreased thrashing rate in all strains (Fig. 5G). Finally, we examined the effect of α-synuclein on dopamine-dependent behavior. We found that α-synculein decreased basal slowing behavior in pink-1 and djr-1.1;djr-1.2 mutants (Fig. 6H), but did not significantly worsen the already reduced rate of basal slowing in pdr-1 and catp-6 mutants (Chakraborty et al., 2015; Cooper et al., 2017).
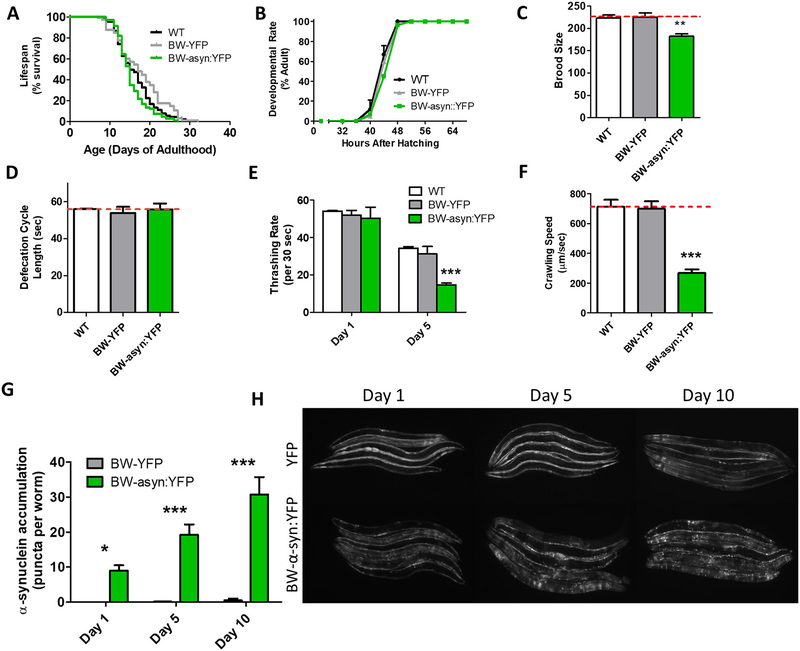
Fig. 6. Expression of α-synuclein in body wall muscle causes age-dependent deficits in movement and aggregation.
Worms expressing α-synuclein:YFP in body wall muscle exhibit a normal lifespan (A), normal development time (B), decreased fertility (C), a normal rate of defecation (D), an age-dependent decrease in movement in liquid (E), and decreased crawling speed (F). These deficits are associated with an age-dependent increase in the accumulation of α-synuclein (G,H). Punc-54::YFP worms are indicated as BW-YFP. Punc-54::α-syn:YFP worms are indicated as BW-asyn:YFP . Error bars indicate SEM. *p<0.05, **p<0.01, ***p<0.001.
3.5. Animals expressing α-synuclein in body wall muscle from a multi-copy array exhibit progressive aggregation leading to deficits in movement
Because C. elegans does not normally express α-synuclein, by expressing α-synuclein under tissue-specific promoters it is possible to examine the role of α-synuclein in specific tissues. As a proof-of-principle, we used a model expressing wild-type α-synuclein fused with YFP under the body wall specific unc-54 promoter, which has been previously used to screen for modifiers of aggregation (van Ham et al., 2008). While the official name for this strain in NL5901, we will refer to this strain as BW-asyn:YFP and its corresponding YFP control as BW-YFP. Unlike asyn:RFP worms that have a single copy of the transgene, BW-asyn:YFP worms were generated by the integration of a multi-copy extrachromosomal array resulting in higher levels of expression (Fig. 1E,F). Before examining the effect of this transgene in the recessive loss of function mutants, we first characterized the BW-asyn:YFP strain.
We found that BW-asyn:YFP exhibit a wild-type lifespan (Fig. 6A) and postembryonic development time (Fig. 6B), but show decreased fertility (Fig. 6C). Defecation cycle length was equivalent to wild-type in these worms (Fig. 6D). Examination of movement in these animals revealed an age-dependent deficit in thrashing rate (Fig. 6E), as well as significantly decreased crawling speed (Fig. 6F). As reported previously (van Ham et al., 2008), BW-asyn:YFP worms exhibit progressive aggregation with increasing age (Fig. 6G,H).
3.6. Expression of α-synuclein in the body wall muscle from a multi-copy array causes increased sensitivity to multiple forms of stress
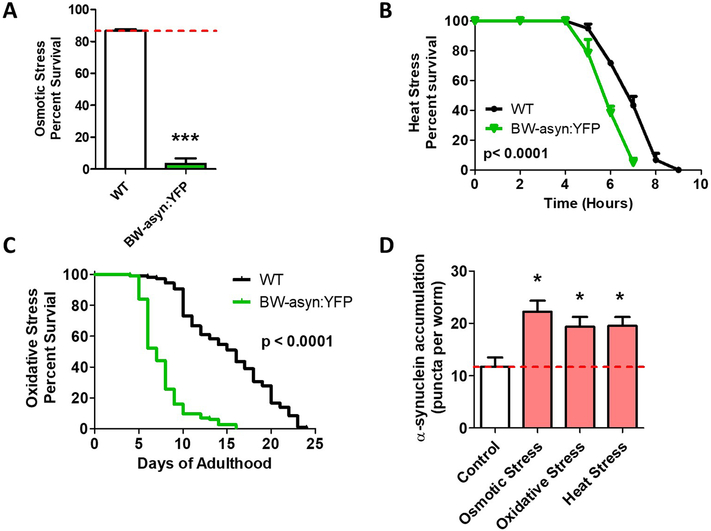
Since we observed that ubiquitous expression of α-synuclein causes increased sensitivity to stress, we examined the extent to which α-synuclein expressed in body wall muscle would also affect stress resistance as well as the effect of these stresses on aggregation. We found that BW-asyn:YFP have markedly increased sensitivity to osmotic stress compared to wild-type worms (Fig. 7A).These worms also show increased sensitivity to heat stress (Fig. 7B) and greatly increased sensitivity to oxidative stress (Fig. 7C). In testing the effect of these stresses on aggregation, we found that all of these stresses act to increase aggregation of α-synuclein (Fig. 7D).
Fig. 7. Expression of α-synuclein in body wall muscle causes increased sensitivity to multiple stresses.
Worms expressing α-synuclein:YFP in body wall muscle exhibit increased sensitivity to osmotic stress (A, 500 mM NaCl), heat stress (B, 37°C) and oxidative stress (C, 2 mM paraquat). D. All of these stresses were found to increase the accumulation of α-synuclein:YFP. Punc-54::α-syn:YFP worms are indicated as BW-asyn:YFP. Error bars indicate SEM. *p<0.05, ***p<0.001.
3.7. Expression of α-synuclein in the body wall muscle from a multi-copy array exacerbates multiple phenotypic deficits in loss of function PD mutants
Having performed an initial characterization of the BW-asyn:YFP strain, we crossed these worms to pdr-1, pink-1, djr-1.1 and catp-6 mutants to determine whether α-synuclein expression in just body wall muscle would induce or worsen deficits in the loss of function mutants. As we were unable to generate catp-6;BW-asyn:YFP worms, possibly due to synthetic lethality, we were only able to complete these experiments for pdr-1, pink-1 and djr-1.1.
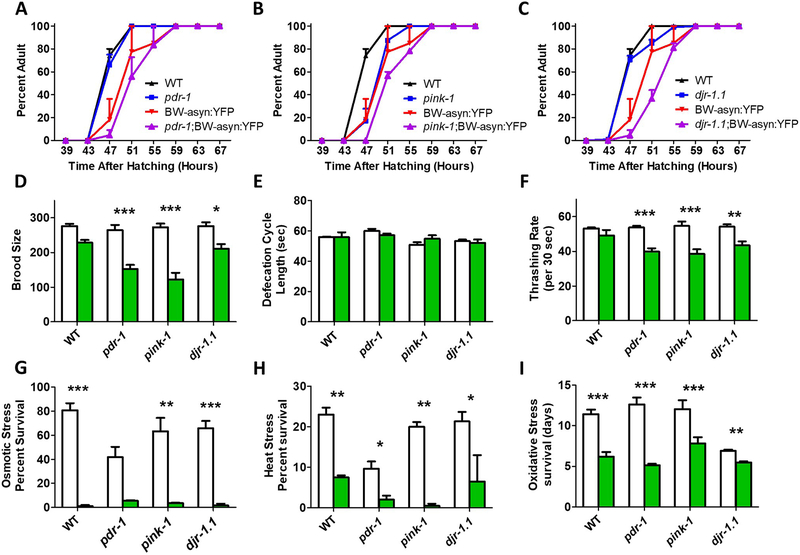
We found that the addition of α-synuclein in muscle cells resulted in slower post-embryonic development times (Fig. 8A-C). In each case the expression of asyn:YFP slowed development more in the PD mutant background than in wild-type worms. Similarly, while pdr-1, pink-1, and djr-1.1 mutants exhibit a normal brood size, in each case brood size was significantly decreased by the expression of α-synuclein in body wall muscle (Fig. 8D). As with the ubiquitous asyn:RFP transgene, expression of α-synuclein in body wall muscle did not affect defecation rate (Fig. 8E). In measuring the rate of movement, we found that the expression of α-synuclein in body wall muscle significantly decreased thrashing rate in pdr-1, pink-1 and djr-1.1 mutants but not in wild-type worms (Fig. 8F). As in the wild-type background, we found that the asyn:YFP transgene markedly increased sensitivity to osmotic stress (Fig. 8G), heat stress (Fig. 8H) and oxidative stress (Fig. 8I).
Fig. 8. Expression of α-synuclein in body wall muscle of recessive loss of function Parkinson’s disease mutants causes behavioral deficits and increased sensitivity to stress.
A-C. α-synuclein expression in body wall muscle caused a trend towards slowed development in pdr-1, pink-1 and djr-1.1 mutants. B. The expression of α-synuclein in body wall muscle decreased fertility in pdr-1 and pink-1 mutants to a greater extent than in WT (D), but had no effect on defecation rate (E) Green bars indicate BW-asyn:YFP background while white bars indicate wild-type background. F. Expressing α-synuclein in body wall muscle significantly decreased the rate of thrashing in pdr-1, pink-1 and djr-1.1 mutants but not in WT worms. Finally, α-synuclein expression in body wall muscle caused increased sensitivity to osmotic stress (G, 500 mM NaCl), heat stress (H, 37°C) and oxidative stress (I, 4 mM paraquat) independent of strain background. Punc-54::α-syn:YFP worms are indicated as BW-asyn:YFP . Error bars indicate SEM. *p<0.05, **p<0.01, ***p<0.001.
Since the formation of Lewy bodies is one of the hallmark features of PD, we examined the effect each PD-associated mutation on aggregation in BW-asyn:YFP worms. Using an unbiased, computer-based image analysis approach we quantified fluorescent puncta above a pre-specified size threshold. Using this approach, we found that the number of fluorescent puncta did not differ between the strains. Note that this result should be interpreted with much caution. This strain has additional small fluorescent puncta that are not large enough to quantify and likely more that are not large enough to visualize. Thus, it is quite possible that there is an increase or decrease in small aggregation intermediates, which may be toxic to the cell.
4. Discussion
4.1. Expression of wild-type α-synuclein causes phenotypic deficits in C. elegans
In this work, we characterize two transgenic worm models that express wild-type α-synuclein. Since C. elegans does not have an α-synuclein homolog, this presents the opportunity to determine the pathological role of α-synuclein and study the interaction of α-synuclein with other genetic defects implicated in PD. In addition to providing a more in depth characterization of an existing model in which asyn:YFP is highly expressed in body wall muscles, we have produced a novel model of PD by expressing a single-copy α-synuclein fused to RFP behind a globally expressed eft-3 promoter. Unlike previous α-synuclein worm models expressing α-synuclein from a single copy transgene should avoid possible artifacts of overexpression.
In comparing these two models (Table 1), we found that lifespan, development time, and defecation rate were not affected in either model. We found that both models exhibited deficits in movement, which exhibited an earlier onset in the ubiquitous model despite lower levels of expression. This may have resulted from the fact that α-synuclein is present in neurons in the ubiquitous strain. In the ubiquitous model, we observed dendritic blebbing and a deficit in dopamine-dependent behavior but no neuronal loss. This suggests that α-synuclein expression is inducing neuronal dysfunction but not death. Finally, we found that in both models expression of α-synuclein resulted in increased sensitivity to stress (discussed below). The fact that even wild-type α-synuclein can induce deficits is consistent with the fact that duplication or triplication of wild-type α-synuclein in humans can lead to PD (Singleton et al., 2003) and previous work showing that expression of wild-type α-synuclein in neurons can lead to deficits (Kuwahara et al., 2006; Lakso et al., 2003).
Table 1.
Comparison of α-synuclein worm models. NT = not tested.
| asyn:RFP | BW-asyn:YFP | |
|---|---|---|
| Promoter | eft-3 | unc-54 |
| Transgene | asyn(wild-type):RFP | asyn(wild-type):YFP |
| Expression pattern | Ubiquitous | Body wall muscle |
| Integration | Single copy | Multiple copy array |
| α-synuclein mRNA levels | Low | High |
| α-synuclein protein levels | Low | High |
| Lifespan | = | = |
| Development | = | = |
| Fertility | = | ↓ |
| Defecation | = | = |
| Movement (thrashing) | ↓ | ↓ |
| Movement (crawling) | = | ↓ |
| Dopamine neuron survival | = | NT |
| Dendritic blebbing | ↑ | NT |
| Dopamine-dependent behavior | ↓ | NT |
| Aggregation | Minimal | Widespread |
| Resistance to osmotic stress | ↓ | ↓ |
| Resistance to heat stress | = | ↓ |
| Resistance to oxidative stress | ↓ | ↓ |
4.2. Expression of α-synuclein decreases resistance to multiple stresses
In both worm models, we found that the expression of α-synuclein resulted in increased sensitivity to multiple types of stress. Both models were more sensitive to osmotic and oxidative stress, while the body wall muscle model was also more sensitive to heat stress. Given that α-synuclein is believed to function in synaptic transmission, it is uncertain whether the increase in stress sensitivity is related to α-synuclein’s normal function. It is possible that when an organism encounters stress there are signals between neurons that are involved in mounting an appropriate stress response. Consistent with this possibility, previous work has shown that α-synuclein can disrupt stress signaling through the inhibition of the kinase Cdc5/Plk2 (Wang et al., 2012).
An alternative possibility is that the increase in stress sensitivity is a result of increased aggregation. It has been shown that the expression of aggregation-prone polyglutamine protein leads to the misfolding of other proteins in the cell through the disruption of the protein folding environment, and that the expression of unstable proteins can accelerate the aggregation of polyglutamine proteins (Gidalevitz et al., 2006). In the α-synuclein models, it is possible that the aggregation of α-synuclein depletes the cell of chaperones that are required for the proper folding of other cellular proteins leading to an enhanced susceptibility to stress. Conversely, we observed that osmotic, heat and oxidative stress all increase the aggregation of α-synuclein. Similarly, previous work has shown that osmotic and heat stress cause increased aggregation of polyglutamine protein in a worm model of Huntington’s disease (Mazzeo et al., 2012), that exposure to the ROS-promoting toxin rotenone can cause α-synuclein aggregation (Sherer et al., 2003), and that oxidative stress can induce aggregation of α-synuclein (Goodwin et al., 2013; Hashimoto et al., 1999).
4.3. Role of α-synuclein in Parkinson’s disease loss of function mutants
In our previous work characterizing worm models of PD, we showed that pdr-1, pink-1 and djr-1.1 mutants exhibit mild phenotypic deficits that would not be amenable to high throughput screens for disease modifiers (Cooper et al., 2017). This result led us to ask whether such limited phenotypes result from the absence of α-synuclein in these animals, and more broadly to gain insight into the question as to whether mutations in these genes require α-synuclein to cause PD. To address this question, we took advantage of the fact that C. elegans lacks an α-synuclein homolog such that we could express α-synuclein in PD genetic loss of function mutants to determine if the presence of α-synuclein would induce or exacerbate phenotypic deficits. Using this approach, it is also possible to address the role of α-synuclein in specific tissues, as we have done here for body wall muscle.
In some cases, the interpretation of our results is complicated by the fact that the expression of α-synuclein by itself is sufficient to cause phenotypic deficits (e.g. resistance to stress). In those cases, it was necessary to show that the genetic mutant with α-synuclein expression was worse than both the genetic mutant alone and the α-synuclein model alone (ie. pdr-1;asyn:RFP worms are worse than pdr-1 worms and asyn:RFP worms). We found this to be true for basal slowing in the asyn:RFP model and development time and brood size in the BW-asyn:YFP model. In addition to these examples, there were also examples where the expression of α-synuclein unmasked deficits that were not present in either of the parent strains, including dopamine neuron loss in the asyn:RFP model and thrashing rate in the BW-asyn:YFP model. Combined, these results demonstrate that expression of α-synuclein can synergize for PD loss of function mutations to induce or exacerbate phenotypic deficits.
Previous studies have suggested that parkin can modulate the levels of α-synuclein. Increasing parkin expression using a lentiviral delivery system in rats decreased the levels of phosphorylated α-synuclein and prevented levels of inflammation and cell death compared to expressing α-synuclein alone (Khandelwal et al., 2010). Similarly, knocking out parkin expression in mice resulted in increased levels of phosphorylated α-synuclein (Van Rompuy et al., 2015). Further work has shown that the mechanism by which parkin affects the levels of α-synuclein is by modulating α-synuclein degradation (Lonskaya et al., 2013).
As with parkin, decreasing levels of PINK1 in mice leads to increased α-synuclein aggregation (Oliveras-Salva et al., 2014). This has also been demonstrated in cell culture experiments in which impairment of PINK1 function caused proteasome dysfunction, which was accompanied by an increase in α-synuclein aggregation (Liu et al., 2009). In Drosophila, overexpression of Pink1 was protective against α-synuclein induced motor deficits and defects in eye development (Todd and Staveley, 2008).
The levels of DJ-1 have also been shown to affect α-synuclein accumulation. Decreasing the levels of DJ-1 using RNA interference was shown to increase aggregation of α-synuclein (Batelli et al., 2008). Similarly, overexpression of DJ-1 has been shown to decrease α-synuclein aggregation and attenuate α-synuclein toxicity in yeast models (Zondler et al., 2014). DJ-1 has also been shown to protect against α-synuclein toxicity in cultured mouse dopaminergic neurons (Batelli et al., 2015).
Modulation of the levels of ATP13A2 also affects the levels of α-synuclein. Knockdown of ATP13A2 was shown to increase α-synuclein aggregation, while overexpression of ATP13A2 protects against α-synuclein toxicity in yeast and C. elegans models (Gitler et al., 2009). The effect of ATP13A2 on α-synuclein aggregation has also been demonstrated in cell culture (Lopes da Fonseca et al., 2016).
5. Conclusions
In this work we generated a novel C. elegans model to study the effects of wild-type α-synuclein that is expressed ubiquitously from a single copy transgene. Global expression of α-synuclein caused mild, age-dependent phenotypic deficits and induced or exacerbated deficits in the PD loss of function mutants pdr-1, pink-1, djr-1.1 and catp-6. This suggests that these mutations induce detrimental effects at least partially through α-synuclein, and that α-synuclein lowering strategies might be effective in these rare genetic forms of PD.
Supplementary Material
Acknowledgements
We would like to thank Mel Feany and Kit Tuen for performing the Western blot for α-synuclein protein. This work was supported by the National Institutes of Health (grant numbers R01 GM121756, R21 AG058241) and the Van Andel Research Institute. Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). We would also like to acknowledge the C. elegans knockout consortium and the National Bioresource Project of Japan for providing strains used in this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Batelli S, Albani D, Rametta R, Polito L, Prato F, Pesaresi M, Negro A, Forloni G, 2008. DJ-1 modulates alpha-synuclein aggregation state in a cellular model of oxidative stress: relevance for Parkinson's disease and involvement of HSP70. PloS one 3, e1884. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Batelli S, Invernizzi RW, Negro A, Calcagno E, Rodilossi S, Forloni G, Albani D, 2015. The Parkinson's disease-related protein DJ-1 protects dopaminergic neurons in vivo and cultured cells from alpha-synuclein and 6-hydroxydopamine toxicity. Neurodegener Dis 15, 13–23. [DOI] [PubMed] [Google Scholar]
- Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, van Dongen JW, Vanacore N, van Swieten JC, Brice A, Meco G, van Duijn CM, Oostra BA, Heutink P, 2003. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 299, 256–259. [DOI] [PubMed] [Google Scholar]
- Buttner S, Broeskamp F, Sommer C, Markaki M, Habernig L, Alavian-Ghavanini A, Carmona-Gutierrez D, Eisenberg T, Michael E, Kroemer G, Tavernarakis N, Sigrist SJ, Madeo F, 2014. Spermidine protects against alpha-synuclein neurotoxicity. Cell Cycle 13, 3903–3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao SS, Gelwix CC, Caldwell KA, Caldwell GA, 2005. Torsin-mediated protection from cellular stress in the dopaminergic neurons of Caenorhabditis elegans. Journal of Neuroscience 25, 3801–3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty S, Chen P, Bornhorst J, Schwerdtle T, Schumacher F, Kleuser B, Bowman AB, Aschner M, 2015. Loss of pdr-1/parkin influences Mn homeostasis through altered ferroportin expression in C-elegans. Metallomics 7, 847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JF, Dues DJ, Spielbauer KK, Machiela E, Senchuk MM, Van Raamsdonk JM 2015. Delaying aging is neuroprotective in Parkinson's disease: a genetic analysis in C. elegans models. NPJ Parkinson's disease 1, 15022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JF, Machiela E, Dues DJ, Spielbauer KK, Senchuk MM, Van Raamsdonk JM, 2017. Activation of the mitochondrial unfolded protein response promotes longevity and dopamine neuron survival in Parkinson's disease models. Scientific reports 7, 16441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JF, Van Raamsdonk JM, 2018. Modeling Parkinson's Disease in C. elegans. Journal of Parkinson's disease 8, 17–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dues DJ, Andrews EK, Schaar CE, Bergsma AL, Senchuk MM, Van Raamsdonk JM 2016. Aging causes decreased resistance to multiple stresses and a failure to activate specific stress response pathways. Aging (Albany NY) 8, 777–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dues DJ, Schaar CE, Johnson BK, Bowman MJ, Winn ME, Senchuk MM, Van Raamsdonk JM, 2017. Uncoupling of oxidative stress resistance and lifespan in long-lived isp-1 mitochondrial mutants in Caenorhabditis elegans. Free radical biology & medicine 108, 362–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteves AR, Arduino DM, Swerdlow RH, Oliveira CR, Cardoso SM, 2009. Oxidative stress involvement in alpha-synuclein oligomerization in Parkinson's disease cybrids. Antioxidants & redox signaling 11, 439–448. [DOI] [PubMed] [Google Scholar]
- Frokjaer-Jensen C, Davis MW, Hopkins CE, Newman BJ, Thummel JM, Olesen SP, Grunnet M, Jorgensen EM, 2008. Single-copy insertion of transgenes in Caenorhabditis elegans. Nature genetics 40, 1375–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gankam-Kengne F, Couturier BS, Soupart A, Brion JP, Decaux G, 2017. Osmotic Stress-Induced Defective Glial Proteostasis Contributes to Brain Demyelination after Hyponatremia Treatment. J Am Soc Nephrol 28, 1802–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidalevitz T, Ben-Zvi A, Ho KH, Brignull HR, Morimoto RI, 2006. Progressive disruption of cellular protein folding in models of polyglutamine diseases. Science 311, 1471–1474. [DOI] [PubMed] [Google Scholar]
- Gitler AD, Chesi A, Geddie ML, Strathearn KE, Hamamichi S, Hill KJ, Caldwell KA, Caldwell GA, Cooper AA, Rochet JC, Lindquist S, 2009. Alpha-synuclein is part of a diverse and highly conserved interaction network that includes PARK9 and manganese toxicity. Nature genetics 41, 308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin J, Nath S, Engelborghs Y, Pountney DL, 2013. Raised calcium and oxidative stress cooperatively promote alpha-synuclein aggregate formation. Neurochem Int 62, 703–711. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Hsu LJ, Xia Y, Takeda A, Sisk A, Sundsmo M, Masliah E, 1999. Oxidative stress induces amyloid-like aggregate formation of NACP/alpha-synuclein in vitro. Neuroreport 10, 717–721. [DOI] [PubMed] [Google Scholar]
- Khandelwal PJ, Dumanis SB, Feng LR, Maguire-Zeiss K, Rebeck G, Lashuel HA, Moussa CE, 2010. Parkinson-related parkin reduces alpha-synuclein phosphorylation in a gene transfer model. Mol Neurodegener 5, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N, 1998. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392, 605–608. [DOI] [PubMed] [Google Scholar]
- Kuwahara T, Koyama A, Gengyo-Ando K, Masuda M, Kowa H, Tsunoda M, Mitani S, Iwatsubo T, 2006. Familial Parkinson mutant alpha-synuclein causes dopamine neuron dysfunction in transgenic Caenorhabditis elegans. The Journal of biological chemistry 281, 334–340. [DOI] [PubMed] [Google Scholar]
- Kuwahara T, Koyama A, Koyama S, Yoshina S, Ren CH, Kato T, Mitani S, Iwatsubo T, 2008. A systematic RNAi screen reveals involvement of endocytic pathway in neuronal dysfunction in alpha-synuclein transgenic C. elegans. Human molecular genetics 17, 2997–3009. [DOI] [PubMed] [Google Scholar]
- Kuwahara T, Tonegawa R, Ito G, Mitani S, Iwatsubo T, 2012. Phosphorylation of alpha-Synuclein Protein at Ser-129 Reduces Neuronal Dysfunction by Lowering Its Membrane Binding Property in Caenorhabditis elegans. J Biol Chem 287, 7098–7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakso M, Vartiainen S, Moilanen AM, Sirvio J, Thomas JH, Nass R, Blakely RD, Wong G, 2003. Dopaminergic neuronal loss and motor deficits in Caenorhabditis elegans overexpressing human alpha-synuclein. Journal of neurochemistry 86, 165–172. [DOI] [PubMed] [Google Scholar]
- Liu W, Vives-Bauza C, Acin-Perez R, Yamamoto A, Tan Y, Li Y, Magrane J, Stavarache MA, Shaffer S, Chang S, Kaplitt MG, Huang XY, Beal MF, Manfredi G, Li C, 2009. PINK1 defect causes mitochondrial dysfunction, proteasomal deficit and alpha-synuclein aggregation in cell culture models of Parkinson"s disease. PloS one 4, e4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonskaya I, Desforges NM, Hebron ML, Moussa CE, 2013. Ubiquitination increases parkin activity to promote autophagic alpha-synuclein clearance. PloS one 8, e83914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes da Fonseca T, Pinho R, Outeiro TF, 2016. A familial ATP13A2 mutation enhances alpha-synuclein aggregation and promotes cell death. Human molecular genetics 25, 2959–2971. [DOI] [PubMed] [Google Scholar]
- Machiela E, Dues DJ, Senchuk MM, Van Raamsdonk JM, 2016. Oxidative stress is increased in C. elegans models of Huntington's disease but does not contribute to polyglutamine toxicity phenotypes. Neurobiology of disease 96, 1–11. [DOI] [PubMed] [Google Scholar]
- Masoudi N, Ibanez-Cruceyra P, Offenburger SL, Holmes A, Gartner A, 2014. Tetraspanin (TSP-17) Protects Dopaminergic Neurons against 6-OHDA-Induced Neurodegeneration in C. elegans. PLoS Genet 10, e1004767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzeo LEM, Dersh D, Boccitto M, Kalb RG, Lamitina T, 2012. Stress and aging induce distinct polyQ protein aggregation states. Proceedings of the National Academy of Sciences of the United States of America 109, 10587–10592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nass R, Hall DH, Miller DM 3rd, Blakely RD, 2002. Neurotoxin-induced degeneration of dopamine neurons in Caenorhabditis elegans. Proc Natl Acad Sci U S A 99, 3264–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveras-Salva M, Macchi F, Coessens V, Deleersnijder A, Gerard M, Van der Perren A, Van den Haute C, Baekelandt V, 2014. Alpha-synuclein-induced neurodegeneration is exacerbated in PINK1 knockout mice. Neurobiol Aging 35, 2625–2636. [DOI] [PubMed] [Google Scholar]
- Pan CL, Peng CY, Chen CH, McIntire S, 2011. Genetic analysis of age-dependent defects of the Caenorhabditis elegans touch receptor neurons. Proceedings of the National Academy of Sciences of the United States of America 108, 9274–9279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfeito R, Ribeiro M, Rego AC, 2017. Alpha-synuclein-induced oxidative stress correlates with altered superoxide dismutase and glutathione synthesis in human neuroblastoma SH-SY5Y cells. Arch Toxicol 91, 1245–1259. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL, 1997. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science 276, 2045–2047. [DOI] [PubMed] [Google Scholar]
- Ramirez A, Heimbach A, Grundemann J, Stiller B, Hampshire D, Cid LP, Goebel I, Mubaidin AF, Wriekat AL, Roeper J, Al-Din A, Hillmer AM, Karsak M, Liss B, Woods CG, Behrens MI, Kubisch C, 2006. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nature genetics 38, 1184–1191. [DOI] [PubMed] [Google Scholar]
- Satake W, Nakabayashi Y, Mizuta I, Hirota Y, Ito C, Kubo M, Kawaguchi T, Tsunoda T, Watanabe M, Takeda A, Tomiyama H, Nakashima K, Hasegawa K, Obata F, Yoshikawa T, Kawakami H, Sakoda S, Yamamoto M, Hattori N, Murata M Nakamura Y Toda T, 2009. Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson's disease. Nature genetics 41, 1303–1307. [DOI] [PubMed] [Google Scholar]
- Sherer TB, Kim JH, Betarbet R, Greenamyre JT, 2003. Subcutaneous rotenone exposure causes highly selective dopaminergic degeneration and alpha-synuclein aggregation. Experimental neurology 179, 9–16. [DOI] [PubMed] [Google Scholar]
- Shimura H, Hattori N, Kubo S, Mizuno Y, Asakawa S, Minoshima S, Shimizu N, Iwai K, Chiba T, Tanaka K, Suzuki T, 2000. Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nature genetics 25, 302–305. [DOI] [PubMed] [Google Scholar]
- Simon-Sanchez J, Schulte C, Bras JM, Sharma M, Gibbs JR, Berg D, Paisan-Ruiz C, Lichtner P, Scholz SW, Hernandez DG, Kruger R, Federoff M, Klein C, Goate A, Perlmutter J, Bonin M, Nalls MA, Illig T, Gieger C, Houlden H, Steffens M, Okun MS, Racette BA, Cookson MR, Foote KD, Fernandez HH, Traynor BJ, Schreiber S, Arepalli S, Zonozi R, Gwinn K, van der Brug M, Lopez G, Chanock SJ, Schatzkin A, Park Y, Hollenbeck A, Gao J, Huang X, Wood NW, Lorenz D, Deuschl G, Chen H, Riess O, Hardy JA, Singleton AB, Gasser T, 2009. Genome-wide association study reveals genetic risk underlying Parkinson's disease. Nature genetics 41, 1308–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K, 2003. alpha-synuclein locus triplication causes Parkinson's disease. Science 302, 841. [DOI] [PubMed] [Google Scholar]
- Soldner F, Stelzer Y, Shivalila CS, Abraham BJ, Latourelle JC, Barrasa MI, Goldmann J, Myers RH, Young RA, Jaenisch R, 2016. Parkinson-associated risk variant in distal enhancer of alpha-synuclein modulates target gene expression. Nature 533, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M, 1998. alpha-synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with lewy bodies. Proceedings of the National Academy of Sciences of the United States of America 95, 6469–6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer W, Hoppe T, Schmidt E, Baumeister R, 2005. A Caenorhabditis elegans Parkin mutant with altered solubility couples alpha-synuclein aggregation to proteotoxic stress. Human molecular genetics 14, 3407–3423. [DOI] [PubMed] [Google Scholar]
- Taira T, Saito Y, Niki T, Iguchi-Ariga SM, Takahashi K, Ariga H, 2004. DJ-1 has a role in antioxidative stress to prevent cell death. EMBO reports 5, 213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapias V, Hu X, Luk KC, Sanders LH, Lee VM, Greenamyre JT, 2017. Synthetic alpha-synuclein fibrils cause mitochondrial impairment and selective dopamine neurodegeneration in part via iNOS-mediated nitric oxide production. Cell Mol Life Sci 74, 2851–2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd AM, Staveley BE, 2008. Pink1 suppresses alpha-synuclein-induced phenotypes in a Drosophila model of Parkinson's disease. Genome 51, 1040–1046. [DOI] [PubMed] [Google Scholar]
- Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, Gonzalez-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B, Auburger G, Wood NW, 2004. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science 304, 1158–1160. [DOI] [PubMed] [Google Scholar]
- van Ham TJ, Thijssen KL, Breitling R, Hofstra RM, Plasterk RH, Nollen EA, 2008. C. elegans model identifies genetic modifiers of alpha-synuclein inclusion formation during aging. PLoS Genet 4, e1000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Raamsdonk JM, Hekimi S, 2011. FUdR causes a twofold increase in the lifespan of the mitochondrial mutant gas-1. Mech Ageing Dev 132, 519–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rompuy AS, Oliveras-Salva M, Van der Perren A, Corti O, Van den Haute C, Baekelandt V, 2015. Nigral overexpression of alpha-synuclein in the absence of parkin enhances alpha-synuclein phosphorylation but does not modulate dopaminergic neurodegeneration. Mol Neurodegener 10, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vives-Bauza C, Zhou C, Huang Y, Cui M, de Vries RL, Kim J, May J, Tocilescu MA, Liu W, Ko HS, Magrane J, Moore DJ, Dawson VL, Grailhe R, Dawson TM, Li C, Tieu K, Przedborski S, 2010. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc Natl Acad Sci U S A 107, 378–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Xu B, Liou LC, Ren Q, Huang S, Luo Y, Zhang Z, Witt SN, 2012. alpha-Synuclein disrupts stress signaling by inhibiting polo-like kinase Cdc5/Plk2. Proc Natl Acad Sci U S A 109, 16119–16124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q, Ahlawat S, Schaefer A, Mahoney T, Koushika SP, Nonet ML, 2014. The vesicle protein SAM-4 regulates the processivity of synaptic vesicle transport. PLoS Genet 10, e1004644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zondler L, Miller-Fleming L, Repici M, Goncalves S, Tenreiro S, Rosado-Ramos R, Betzer C, Straatman KR, Jensen PH, Giorgini F, Outeiro TF, 2014. DJ-1 interactions with alpha-synuclein attenuate aggregation and cellular toxicity in models of Parkinson's disease. Cell Death Dis 5, e1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.