Abstract
Endotracheal tube (ETT) management is an essential technique in handling mice with mechanical ventilators. Malposition into bronchi causes not only lethal complications for them but also less efficient mechanical ventilation. However, in general, it is difficult to know whether the ETT is placed with appropriate depth into the trachea of mice. We measured the distance from incisors to the bifurcation of trachea of multiple mice, and created a new estimation formula to obtain the suitable ETT length for mice with a body weight range from 17 g to 25 g: length (mm) = 0.5 × bodyweight (g) + 7. However, millimeter step adjustments are impracticable. Thus, slightly shorter than 2 cm (18–20 mm) may be the universal ETT length for mice with bodyweight > 17 g. Furthermore, their foot size may be a good alternative to predict the individual optimal ETT length for mice.
Keywords: Mechanical ventilation, non-invasive endotracheal intubation, estimation, foot size, mice, tube position
Abstract
Endotrachealtubus (ETT)-Management ist eine wichtige Technik für Versuche mit Mäusen unter Einsatz mechanischer Beatmungsgeräte. Eine Fehlstellung der Bronchien führt nicht nur zu tödlichen Komplikationen, sondern auch zu einer schlechteren mechanischen Beatmung. Generell ist es jedoch schwierig zu beurteilen, ob der ETT entsprechend tief in die Luftröhre von Mäusen eingebracht wird. Wir haben den Abstand von den Schneidezähnen bis zur Bifurkation der Luftröhre mehrerer Mäuse gemessen und eine neue Schätzformel erstellt, um die passende ETT-Länge für Mäuse mit einem Körpergewicht im Bereich von 17 – 25 g zu erzielen: Länge (mm) = 0,5 × Körpergewicht (g) + 7. Anpassungen in Millimeterstufen sind jedoch nicht praktikabel. So könnte die universelle ETT-Länge für Mäuse mit dem Körpergewicht etwas kürzer als 2 cm (18 – 20 mm) sein. Darüber hinaus kann die Fußgröße von Mäusen eine gute Alternative sein, um die individuelle, für sie optimale ETT-Länge vorherzubestimmen.
Abstract
La gestión del Tubo Endotraqueal (ETT) es una técnica esencial a la hora de manipular ratones con ventiladores mecánicos. Una mala posición en los bronquios provoca no solo complicaciones letales para ellos sino también una ventilación mecánica menos eficaz. No obstante, en general, es difícil saber si la ETT está colocada a la profundidad necesaria dentro de la traquea del ratón. Medimos la distancia desde los incisivos a la bifurcación de la traquea de varios ratones y creamos una nueva fórmula de estimación para obtener la longitud adecuada de la ETT para ratones con un rango de peso corporal de 17 a 25 gr: longitud (mm) = 0,5 × peso corporal (gr) + 7. No obstante, los ajustes de pasos milimetrados son impracticables. Por tanto, una longitud ligeramente inferior a 2 cm (18 – 20 cm) puede ser la longitud universal de ETT para ratones con peso corporal. Asimismo, su tamao de pata puede ser una buena alternativa para predecir la longitud óptima de ETT individual para los ratones.
Abstract
La gestion des sondes endotrachéales (SET) est une technique essentielle dans le traitement des souris par ventilateur mécanique. Un mauvais positionnement dans les bronches provoque non seulement des complications mortelles, mais il rend également la ventilation mécanique moins efficace. Il est cependant généralement difficile de savoir si la SET est placeé á la bonne profondeur dans la tracheé de souris. Nous avons mesuré la distance entre la bifurcation des incisives et la tracheé de nombreuses souris, et créé´ une nouvelle formule d’estimation pour obtenir la longueur adéquate d’une SET destinée aux souris ayant un poids allant de 17 á 25 g : longueur (mm) = 0,5 × poids corporel (g) + 7. Il n’est toutefois pas possible dans la pratique d’effectuer des ajustements millimétriques. Ainsi, une sonde d’un peu moins de 2 cm de long (18 – 20 mm) peut s’avérer la mesure de SET universelle appropriée aux souris avec son poids corporel. En outre, le pied peut aussi s’avérer une bonne alternative pour prédire la longueur optimale d’une SET chez une souris individuelle.
Airway management is critical in animal experiments with mechanical ventilation. Malposition of the endotracheal tube (ETT) within the airway such as endobronchial intubation results in serious complications, including lung collapse, which could be lethal for mice. Although many technical reports have been published regarding non-invasive endotracheal intubation methods for mice, little is known about the optimal ETT depth for mice.1–11 How can we check the ETT position for intubated mice? In daily clinical care for intubated human patients, we routinely confirm the ETT position using several methods.12 Chest X-rays and lung auscultation are typical examples. For laboratory animals, however, they are generally inaccessible. Given the lack of ways to confirm ETT depth, determining a suitable ETT length for mice remains a priority.
Mice used in this work were included in research projects underway in our laboratory, with each project being approved by the Institutional Animal Care and Use Committee of the Cedars-Sinai Medical Center. All animal experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals of National Institute of Health and the Public Health Service Policy on Humane Care and Use of Laboratory Animals. The mice were purchased from The Jackson Laboratory (Bar Harbor, ME), and were housed under specific pathogen-free conditions at the Comparative Medicine Facility of Cedars-Sinai Medical Center. More specifically, animals were housed in individually ventilated cages (Allentown Inc., NJ, USA), five per cage, over autoclaved wood bedding (Sani-Chips, PJ Murphy, NJ, USA) under stable conditions (temperature 22–25̊ C, relative humidity 50–70%, 12-h light/dark cycle) with unrestricted access to water and diet. Nestlets (Ancare Corp, NY, USA) and a PVC pipe were provided for enrichment. We used three groups of mice; C57BL/6 male and female mice housed with standard diet; Balb/c male mice fed with standard diet; and C57BL6 female mice fed with high-fat diet (TD88137) (ENVIGO, Indianapolis, IN, USA) for 20 weeks (Table 1). All measurements were performed on mice after euthanasia by isoflurane inhalation overdose.
Table 1.
Characteristics of animals in this study.
| C57BL/6 mice With standard diet |
Balb/c mice With standard diet |
C57BL/6 mice With high-fat diet |
|||||
|---|---|---|---|---|---|---|---|
| All (n = 36) |
Male (n = 21) |
Female (n = 15) |
p-value1 | Male (n = 16) |
p-value2 | Female (n = 7) |
|
| Age (week) | 9.1 ± 2.8 | 8.2 ± 2.7 | 10.5 ± 2.4 | .01 | 8.3 ± 3.7 | .96 | 45.7 ± 5.1 |
| Bodyweight (g) | 20.9 ± 2.5 | 21.2 ± 2.7 | 20.4 ± 2.1 | .26 | 22.4 ± 3.2 | .29 | 47.4 ± 6.0 |
| Height (mm) | 89.1 ± 4.4 | 88.3 ± 5.2 | 90.2 ± 2.6 | .38 | 89.8 ± 4.5 | .37 | 106.9 ± 3.0 |
| I–B distance (mm) | 22.1 ± 1.3 | 22.1 ± 1.3 | 22.0 ± 1.3 | .72 | 22.1 ± 1.5 | .83 | 27.3 ± 1.9 |
| I–V distance (mm) | 11.6 ± 1.0 | 11.4 ± 1.0 | 11.8 ± 0.9 | .22 | 12.0 ± 1.1 | .08 | 14.4 ± 1.8 |
| V–B distance (mm) | 10.5 ± 1.1 | 10.7 ± 1.1 | 10.2 ± 1.2 | .23 | 10.2 ± 1.1 | .16 | 12.9 ± 1.6 |
| Foot size (mm) | 18.0 ± 0.5 | 18.0 ± 0.5 | 17.9 ± 0.5 | .64 | 18.0 ± 0.4 | .97 | 19.0 ± 0.0 |
Data are mean ± SD. p-values were calculated by Mann–Whitney’s U test between standard diet-fed C57BL/6 male and female mice1, or compared between standard diet-fed C57BL/6 male mice and Balb/c male mice2.
A standard disposable intravenous catheter (BD Insyte Autoguard, 20GA 1.00 in., Becton Dickinson Infusion Therapy Systems Inc, Sandy, UT, USA) was orotracheally intubated, and the distances between the lower incisors and the bifurcation of the trachea (I-B distance), or the center of the thyroid cartilage (an index of the level of vocal cord; I-V distance) were measured (Figure 1(a)). The relationship between these measurements and bodyweight or height (the distance between the nose to the joint of a tail) was plotted, and their linear approximation and the coefficient of determination (R2) were calculated (Excel for Mac 2011, Microsoft, Redmond, WA, USA). The formula used by Excel is: = the regression sum of squares / the total sum of squares = DEVSQ (explained I–B lengths 1, explained I–B length 2 . . .) / DEVSQ (measured I–B length 1, measured I–B length 2 . . .). In addition, using the same mice, their size of right foot (the distance from the tip of the heel to the tip of the middle toe) was measured to evaluate the association with appropriate length of ETT intubation. All other statistical analysis was performed using Prism 4.0 software (GraphPad Software, San Diego, CA, USA). A p value < 0.05 was considered statistically significant.
Figure 1.
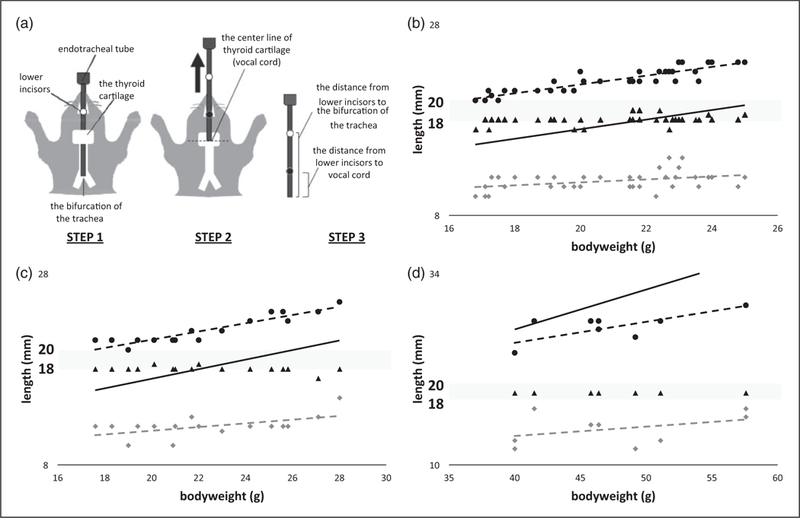
Strategy and results for measuring distances between lower incisors and the bifurcation of the carina, or the vocal cord. (a) To measure the distance between the lower incisors and the bifurcation of the trachea (I–B distance), the tip of intubated catheter was placed at the bifurcation, and the catheter was marked at the lower incisors level (Step 1). When the catheter was so short that it did not reach to the bifurcation of the trachea, a guidewire was inserted in the catheter to fill the gap. Subsequently, to measure distance between the lower incisors and the vocal code (I–V distance), the catheter was carefully withdrawn to the center of the thyroid cartilage, an index of the level of vocal cord, and catheter was marked again at the lower incisors level (Step 2). The catheter was finally pulled out and the distances between the tip of catheter and marked points were measured (Step 3). (b)–(d) Black and grey broken lines indicate I–B and I–V distance, respectively. Proposed target depth of endotracheal tube tip was indicated by a black solid line (y = 0.5× + 7). A grey band indicates 18–20 mm. Black triangles indicate the foot size of each mouse. Each graph shows the result of C57/BL6 mice with standard diet (b), Balb/c mice with standard diet (c), and obese female C57/BL6 mice fed with high-fat diet (d).
In the standard-diet C57BL/6 group, there was no significant difference between the sexes in height, or I–B and I–V distance (Table 1). Therefore, we decided to use both male and female data together in the subsequent analysis. We calculated the linear association between the I–B distance and body weight with a coefficient of determination (R2 = 0.81), which was better than with height (R2 = 0.25): I–B distance (mm) = 0.46 × bodyweight (g) + 12 (black broken line on Figure 1(b)). The length of trachea (distance from the vocal cord to the bifurcation of the trachea [V–B distance in Table 1], between black and grey broken lines on Figure 1(b)) was roughly 10 mm. The center of the trachea may be the safe ETT-tip position to prevent both accidental extubation and endobronchial intubation, which should be 5 mm inside from the vocal cord in our case.6 Hence, we created the following simplified formula to estimate optimal ETT length for mice: length (mm) × 0.5 bodyweight (g) + 7 (black solid line on Figure 1(b)). We also plotted foot sizes in Figure 1(b) (black triangles), all of which fell between the I–B and I–V distances. In addition, we confirmed that the simplified formula could be applied to the Balb/c mice group, and their foot size was also a good surrogate to estimate optimal ETT length (Figure 1(c)). On the other hand, our formula did not correlate well with mice that were older and overweight (Figure 1(d)); however, their foot size remained a good criterion of ETT length (Figure 1(d).
Our findings require some careful interpretation as these are not guarantees but instead are guides for appropriate ETT tip position. These findings may not be applicable to other strains of mice. Additionally, we did not analyze tube width in this study. Some investigations used 24-gauge catheters, while others 20-gauge for orotracheal intubation.4–9,13 While a wider endotracheal tube may cause injuries or edema to the airway, it allows a more accurate measure of airway pressure, ventilation with expected volume, or infallible drug administration by tight seal with the airway.6,9 Thus, it is ideal that the tube size be chosen according to purposes of intubation, which will be a good subject for future investigation. Based on these limitations, this report raised a critical issue of management of ETT length with ventilated mice, which has not been well addressed, and provided data to resolve it.
Evaluation of the success of endotracheal intubation is an important topic for researchers using intubated animals, and several methods have been reported in mice such as observing animal respiratory patterns and water drop movement inside the connected tube.2,4,13 However, these methods overlook extremely deep or shallow intubation, thus making them less reliable. Studies published by Vergari et al. clearly stipulate the appropriate ETT length as 3–6 mm to insert from the vocal cords, which corresponds with our findings. Brown et al. described the insertion length as 3 mm into the trachea; however, our results found that the trachea has more length to be occupied and the ~3 mm insertion length may be too shallow and cause accidental extubation.1–3 In this study, we have provided a new estimation formula to obtain a suitable ETT length, which could be applied to two different strains of mice within the general range of bodyweight. However, the bodyweight-based calculation may not always be applicable, especially in obese mice, as shown in Figure 1(d). Moreover, it is impractical to adjust the small tube precisely in 1 mm steps. Therefore, we concluded that slightly shorter than 2 cm (18–20 mm) is the optimal ETT length for mice within the range of bodyweight (Grey line on Figure 1(b)–(d)). It should be noted that this simple number (18–20 mm) can be applied safely to obese mice, while the calculated length by the formula can result in too deep intubation. These findings may indicate that the growth rate of the trachea along with increase in bodyweight becomes slower at a certain point. Furthermore, we found foot size to be a good alternative to estimate optimal ETT length. Measurement of foot size can be performed promptly and easily without expensive equipment. In addition to C57Bl/6 mice, this method could be applied to Balb/c mice as well as obese mice. Incidentally, foot size has been previously reported to be useful for estimating optimal nasotracheal tube length for human neonates.14 In conclusion, in addition to our formula, foot size may also accurately predict the distance for safe endotracheal intubation for mice. Further studies need to be performed to ensure the usefulness and safety of these estimation methods using mice under mechanical ventilation.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Institute of Health (NIH) grant 1R01-HL130353–01 to K.S.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflict of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Brown RH, Walters DM, Greenberg RS and Mitzner W. A method of endotracheal intubation and pulmonary functional assessment for repeated studied in mice. J Appl Physiol 1985; 87: 2362–2365. [DOI] [PubMed] [Google Scholar]
- 2.Vergari A, Polito A, Musumeci M, et al. Video-assisted orotracheal intubation in mice. Lab Anim 2003; 37: 204–206. [DOI] [PubMed] [Google Scholar]
- 3.Vergari A, Gunnella B, Rodola F, et al. A new method of orotracheal intubation in mice. Eur Rev Med Pharmacol Sci 2004; 8: 103–106. [PubMed] [Google Scholar]
- 4.Rivera B, Miller S, Brown E, et al. A novel method for endotracheal intubation of mice and rats used in imaging studies. Contemp Top Lab Anim Sci 2005; 44: 52–55. [PubMed] [Google Scholar]
- 5.Spoelstra EN, Ince C, Koeman A, et al. A novel and simple method for endotracheal intubation of mice. Lab Anim 2007; 41: 128–135. [DOI] [PubMed] [Google Scholar]
- 6.MacDonald KD, Chang HY and Mitzner W. An improved simple method of mouse lung intubation. J Appl Physiol 2009; 106: 984–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Das S, MacDonald K, Chang HY, et al. A simple method of mouse lung intubation. J Vis Exp 2013; e50318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Konno K, Itano N, Ogawa T, et al. New visible endotracheal intubation method using the endoscope system for mice inhalational anesthesia. J Vet Med Sci 2014; 76: 863–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas JL, Dumouchel J, Li J, et al. Endotracheal intubation in mice via direct laryngoscopy using an otoscope. J Vis Exp 2014; e50269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su CS, Lai HC, Wang CY, L, et al. Efficacious and safe orotracheal intubation for laboratory mice using slim torqueable guidewire-based technique: comparisons between a modified and a conventional method. BMC Anesthesiol 2016; 16: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vandivort TC, An D and Parks WC. An improved method for rapid intubation of the trachea in mice. J Vis Exp 2016; 53771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rudraraju P and Eisen LA. Confirmation of endotracheal tube position: a narrative review. J Intensive Care Med 2009; 24: 283–292. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe A, Hashimoto Y, Ochiai E, et al. A simple method for correct endotracheal intubation in mice. Lab Anim 2009; 43: 399–401. [DOI] [PubMed] [Google Scholar]
- 14.Embleton ND, Deshpande SA, Scott D, et al. Foot length, an accurate predictor of nasotracheal tube length in neonates. Arch Dis Child Fetal Neonatal Ed 2001; 85: F60–64. [DOI] [PMC free article] [PubMed] [Google Scholar]


