Abstract
Nucleotide-binding and oligomerization domain-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome, which is composed of an NLRP3 domain, the adaptor molecule apoptosis-associated speck-like protein containing a CARD (ASC) domain, and procaspase-1, plays an important role in the immune pathophysiology of the secondary damage induced by intracerebral hemorrhage (ICH). This study aims to investigate whether pre-stroke treatment with fimasartan, an angiotensin II receptor blocker, has anti-inflammatory effects on ICH by inhibiting the activation of the NLRP3 inflammasome. Sprague-Dawley rats were divided into five groups: sham, vehicle, low-dose (0.5 mg/kg) and regular-doses (1.0 and 3.0 mg/kg) fimasartan. These rats were treated for 30 days before the induction of collagenase-induced ICH and continuously 3 days after surgery. The mean blood pressure (BP) in the low-dose fimasartan group was not significantly different from that of control, and BP in the regular-dose groups was decreased in a dose-dependent manner. Pretreatment with low-dose fimasartan attenuated ICH-induced edema and improved neurological functions. Activation of the NLRP3/ASC/caspase-1 and the NF-κB pathways after ICH was markedly reduced by low-dose fimasartan. The double immunofluorescence staining of brain cells showed a significant decrease in the co-localization of NLRP3 with Iba1 (microglia marker) positive cells by fimasartan treatment. Cultured microglia cells stimulated by hemolysate demonstrated significant activation of the inflammasome, which was reduced by fimasartan. Pretreatment with a low-dose fimasartan alleviated brain damage after acute ICH by inhibiting the NLRP3 inflammasome without lowering MBP. Our study suggests pre-stroke administration of fimasartan could potentially attenuate ICH-induced secondary brain injury by targeting the inflammasome.
Keywords: Stroke, Intracerebral hemorrhage, NLRP3 inflammasome, Brain injury
1. Introduction
Intracerebral hemorrhage (ICH) is one of the subtypes of stroke that is characterized by high mortality and morbidity, and represents approximately 10–15% of strokes worldwide each year (Keep et al., 2012; Lan et al., 2017). The number of hospital admissions for ICH has increased in the past ten years. Despite the recent advance in stroke therapy, the mortality rate has not decreased due to the increased elderly population, the racial differences in the incidence of ICH, and the increased use of anticoagulants (Qureshi et al., 2009; Qureshi et al., 2001). Currently, there are no effective therapies for ICH because of the complex and poorly understood mechanisms. Accumulating evidence suggests that inflammatory factors are involved in the secondary brain damage induced by ICH (Yang et al., 2017); however, the molecular mechanisms underlying the innate immune response in ICH are not fully understood (Iadecola and Anrather, 2011; Wang and Doré, 2007).
Recently, the so-called inflammasome has been found to regulate diverse inflammatory responses in all organs, including the brain (Strowig et al., 2012). The inflammasome plays a key role in the innate immune response in the central nervous system (CNS) diseases (Heneka et al., 2017). Typically, the inflammasome is composed of at least one member of the cytosolic innate immune sensor family, the NOD-like receptors (NLRs), coupled with the adaptor apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), and caspase-1 (Schroder and Tschopp, 2010). The nucleotide binding and oligomerization domain-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome is the best characterized and the most clinically relevant inflammasome to date. Activation of the NLRP3 inflammasome subsequently activates caspase-1 and induces the secretion of mature cytokines, such as interleukin-1β (IL-1β) (Zhu et al., 2017). The processing of mature cytokines is mediated by two distinct signals. The first signal is the priming signal, which involves activation of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathway. The second signal involves the formation of the NLRP3 inflammasome complex with cleaved caspase-1 (Franchi et al., 2012). An increasing number of studies have suggested that the NLRP3 inflammasome contributes to brain inflammation in stroke, especially in ICH (Fann et al., 2013; Ma et al., 2014).
It is well-known that chronic hypertension is by far the most common risk of bleeding that can lead to ICH and that angiotensin II receptor antagonists (ARBs) are one of the most effective management options for hypertension (Saavedra, 2012). ARBs collectively have neuroprotective effects, although the underlying mechanisms of action may differ. Previous studies found that patients treated with ARBs or angiotensin-converting enzyme inhibitors (ACEI) prior to ischemic stroke onset are associated with lower stroke severity and better outcome, which may be indirectly related to effect due to the lowered BP (Fuentes et al., 2010; Selim et al., 2005). These drugs may have a potential neuroprotective effect and lower the stroke risk for stroke patients. A recent study found pre-stroke ARB treatments might reduce the 30-day mortality of ischemic stroke patients (Sundboll et al., 2015). Fimasartan (BR-A-657), a new ARB drug, has a stronger affinity for the AT1 receptor subtype and presents superior inhibitory activity compared to other ARBs (Chi et al., 2011). Pre-stroke use of fimasartan has been found to be protective in ischemic stroke and myocardial infarction (Han et al., 2013; Kim et al., 2015). However, the effect of pre-treatment of fimasartan on ICH has remained unknown.
Therefore, this study aims to evaluate the dose-dependent effect of fimasartan administration in acute ICH stroke and investigate the mechanism of neuroinflammatory attenuation that is beyond BP control. We conducted a 30-day pretreatment with different doses of fimasartan and validated its ability to regulate BP prior to ICH. We decided to move forward with the low-dose fimasartan as its effects on BP were statistically indistinguishable from controls, thus allowing us to isolate BP-independent mechanisms. Specifically, we investigated the role of low-dose fimasartan on two inflammatory signal pathways induced by ICH: the NLRP3 inflammasome and the NF-κB pathways.
2. Materials and methods
2.1. Fimasartan administration
Fimasartan (Boryung Pharmaceutical Company, Republic of Korea), was dissolved in phosphate-buffered saline (1 mg/mL) and diluted with sterile water to constitute either the low dose (0.5 mg/kg, p.o.) or regular doses (1.0 and 3.0 mg/kg, p.o.) according to our previous study (Kim et al., 2015). Fimasartan or distilled water (DW) was administered orally for 30 days prior to the induction of ICH, and continuously for 3 days after the surgery at the same time every morning.
2.2. Induction of intracerebral hemorrhage
All animal experimental protocols were performed in accordance with the relevant guidelines and regulations approved by the National Institutes of Health Animal Care and Use Committee of the Biomedical Research Institute at Seoul National University Hospital. Total 113 four-week-old male Sprague-Dawley rats (Koatech, Seoul, Republic of Korea) weighing 65–75 g were randomly separated into five groups: sham, ICH + DW, ICH + low-dose fimasartan (0.5 mg/kg, p.o.) and ICH + regular-doses fimasartan (1.0 and 3.0 mg/kg, p.o.). After one month, we performed the ICH surgery on adult rats at 8 weeks of age (250 – 300 g) as previously published (Song et al., 2003). The rats were then injected intrastriatally (3.0 mm left, 0.2 mm posterior to the bregma and 6.0 mm in depth) with collagenase IV (0.6 U in 1 μL saline, Sigma) in a stereotaxic frame (David Kopf Instruments, Tujunga, CA, USA). The forelimb flexion and contralateral circling were observed to confirm the success of the ICH surgery. Sham-operated rats only underwent needle insertion without collagenase injection. Male rats were used to avoid the neuroprotective effects of estrogens which may affect stroke outcome (Dubal and Wise, 2002).
2.3. Monitoring of blood pressure
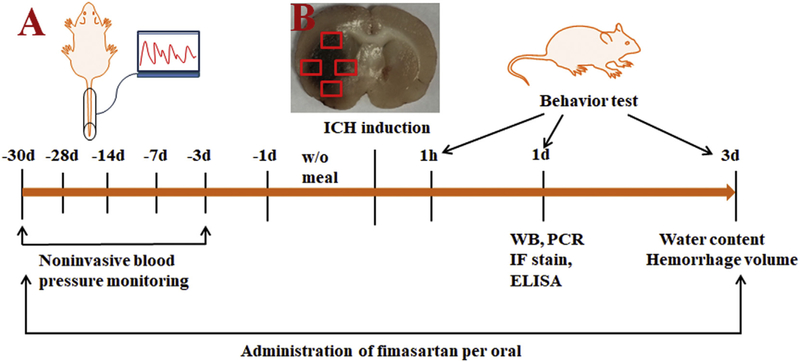
Mean BP levels were noninvasively monitored at the same time from the rat’s tail (homologated by Bland Altman Testing) (Ciocoiu et al., 2013) with a CODA Noninvasive Blood Pressure System (Kent Scientific Corporation, Torrington, CT) throughout the whole experiment including days 28, 21, 14, 7 and 3 prior to the surgery and at baseline with the administration of fimasartan (n = 10 per group) (Fig. 1A).
Fig. 1.
Schematic diagram of the study design and brain areas where positive cells are quantified. (A) A low-dose (0.5 mg/kg), regular-doses (1 or 3 mg/kg) of fimasartan, or DW was administrated to Sprague-Dawley rats 30 days before the induction of intracerebral hemorrhage (ICH) and 3 days after surgery. Noninvasive mean BP was monitored during the course pretreated with fimasartan. Western blot analysis, RT-PCR, ELISA and immunofluorescence staining were performed 1 day after ICH. Brain water content and hemorrhage volume were evaluated 3 days after ICH. The recovery of neurological function was assessed on 1 h, 1 day and 3 days after ICH. (B) High-power field images fully covering the perihematomal areas were obtained from slices through the center of the hemorrhagic lesion for counting NLRP3 inflammasome components on activated microglia cells. The square markers indicate Iba-1-positive cells in the perihematomal region.
2.4. Measuring the brain water content and hemorrhage volume
We analyzed the brain water content and hematoma volume 3 days after surgery because edema is the most severe three days after ICH (Xi et al., 2006). Rats were euthanized 72 h after ICH. The brains (n = 12 per group) were then divided into two hemispheres along the midline and immediately weighed using an electric analytical balance to obtain the wet weight. The brain samples were then dried in a gravity oven at 100 °C for 24 h to obtain the dry weight. Water content = (wet content-dry content)/(wet content) × 100% (Song et al., 2003).
To evaluate the hemorrhagic lesion volume, the brains (n = 8 per group) were serially sectioned at 1-mm intervals in the coronal plane through the needle entry site. The hematoma area of each section was assessed by Image J (National Institutes of Health, Bethesda, MD). The total hematoma volume (mm3) was calculated by summing the hematoma area in each section and multiplying by the thickness of the sections (Kim et al., 2013).
2.5. Behavioral testing
Two behavioral tests, the modified Neurological Severity Score (mNSS) and forelimb placing test, were performed by investigators blinded to the groups. The rats were assessed before and 1 h, 1 day and 3 days after ICH (n = 17 per group). The mNSS test includes a composite of the motor (six points), sensory (two points), and beam balance (six points) tests, in addition to the absence of reflexes and presence of abnormal movements (four points). The mNSS is graded on a scale of 0–18 (normal score = 0; maximal deficit score = 18) to determine impairment (Chen et al., 2001).
The vibrissae-elicited forelimb placing test was used to assess the asymmetry between forelimbs (Schallert et al., 2000). The rats were held to allow their forelimbs hanging free. Each forelimb was tested by stimulating their ipsilateral vibrissae on the edge of the countertop once per trial for 10 trials. Intact rats stretch each forelimb quickly on the edge of the table in response to vibrissae stimulation. The total scores were determined by the percentage of successful placing responses.
2.6. Western blotting
The brain tissue samples from the sham, control and low-dose fimasartan groups (n = 5 per group), were homogenized in ice-cold RIPA buffer (Biosesang, Seoul, Korea) after euthanasia 1-day post-ICH. The protein concentration was determined using a Bradford Protein Assay Kit (Bio-Rad, Hercules, CA, USA). Protein (60 μg) was separated on 8% and 15% sodium dodecyl sulfate-polyacrylamide gels using an electrophoresis gel and then transferred to polyvinylidene-difluoride membranes. The membranes were blocked with 5% skim milk and incubated with primary antibodies (Table 1) overnight. After blocking the secondary antibodies with horseradish peroxidase-conjugated anti-rabbit IgG (Santa Cruz Biotechnology), the blots were detected using an enhanced chemiluminescence detection system (Thermo Fisher Scientific, Waltham, MA). Image J (National Institute of Health, Bethesda, MD) was used to analyze the density of the signals. The results are representative of at least three independent experiments from every brain sample.
Table 1.
List of antibodies.
| Antibody | Supplier, catalog no. | Host | WB | IF |
|---|---|---|---|---|
| NLRP3 | Santa Cruz, sc-66,846 | Rabbit | 1:200 | 1:50 |
| ASC | Santa Cruz, sc-22,514 | Rabbit | 1:200 | 1:100 |
| Caspase-1 | Abcam, ab1872 | Rabbit | 1:200 | 1:50 |
| IL-1β | Abcam, ab9722 | Rabbit | 1:1000 | − |
| IL-18 | Santa Cruz, sc-7954 | Rabbit | 1:200 | − |
| NFκB | Abcam, ab7970 | Rabbit | 1:1000 | − |
| IκB | Cell Signaling, 9242 | Rabbit | 1:1000 | − |
| COX-2 | Abcam, ab15191 | Rabbit | 1:1000 | − |
| GFAP | Cell Signaling, 3670 | Mouse | − | 1:200 |
| Iba-1 | Santa Cruz, sc-28,528 | Goat | − | 1:50 |
| Actin | Bioworld, BSAP0060 | Rabbit | 1:10,000 | − |
WB, Western Blot; IF, Immunofluorescence.
2.7. Quantitative Real-Time PCR
The brain tissue was rinsed with cold PBS, and total RNA was isolated from the left striatum of every group (n = 5 per group) using TRIzol ® reagent (Invitrogen, La Jolla, CA, USA). A total of 2 μg of RNA was converted into cDNA using TOPscript RT DryMIX (Enzynomics Inc., Korea) according to the manufacturer’s instructions. The Taqman probes for NLRP3 mRNA (Assay ID: Rn04244620_m1) and caspase-1 mRNA (Assay ID: Rn00562725_g1) were purchased from Applied Biosystems (Foster City, CA). The sequence-specific primers were as follows: ASC, forward, 5’-GCT CAC AAT GTC TGT GCT TAG AG-3′; reverse, 5′-GCA GTA GCC ACA GCT CCA G-3′ and rS6, forward 5’-TGC TCT TGG TGA AGA GTG GA-3′; reverse 5′-CAA GAA TGC CCC TTA CTC AAA-3′. Quantitative polymerase chain reaction (qPCR) was performed using SYBR Premix Ex Taq™ (Takara Bio Inc., Shiga, Japan) on an ABI 7500 Real-Time PCR System (Perkin-Elmer Applied Biosystems, Lincoln, CA, USA). The results are presented as the mean ± standard error of the relative mRNA expression normalized to GAPDH or rS6 using the comparative ΔΔCt method.
2.8. Cytokine measurement
The levels of cytokines including interleukin-1 beta (IL-1β) and TNF-α were quantified by single-plex ELISA kit specific for rat tissue (n = 5 per group) according to the manufacturer’s instructions (R&D systems). The results are reported based on standards and expressed as pictograms of the measured molecule per mL of tissue (pg/mL).
2.9. Double-labeled immunofluorescence staining and cell quantification
Rats (n = 6 per group) were transcardially perfused with saline and 4% paraformaldehyde in 0.1 mol/L (PH 7.4) phosphate-buffered saline at 1 day after ICH. The cryopreserved brain samples were sectioned in the coronal plane at a thickness of 10 μm and mounted onto silane-coated slides (Dako, Glostrup, Denmark). The sections were incubated with primary antibodies (summarized in Table 1) at 4 °C overnight. For double immunofluorescence (IF) staining, specific cellular markers including ionized calcium binding adaptor protein-1 (Iba-1) for microglia were used to identify the inflammasome derived from different cell types. The slides were mounted with DAKO Paramount (DAKO Corporation, Carpinteria, CA) to stain the cell nuclei. The primary antibody was omitted as a negative control for the IF staining. Bright-field and fluorescence micrographs were acquired using a Leica DM5500B microscope (Leica Microsystems) and LAS-AF image acquisition software (Leica Microsystems, Rijswijk, Netherlands).
The number of positive cells in the perihematomal regions was counted in 3 axial sections and at least 4 fields for each section per animal through the center of the hemorrhagic lesion by two independent investigators who were masked to the group identification according to established protocols (Jung et al., 2004). To count the number of positive microglia, 16 high-power fields were obtained from the stained sections through the center of the ICH lesion (Fig. 1B) 1 day after ICH. The total counts in the measured sections were converted into cell densities for comparison between the ICH groups.
2.10. Microglia cell culture and treatment
The mouse microglia cell line BV2 (ATCC, Manassas, VA) was cultured in DMEM with 10% FBS and 1% penicillin-streptomycin at 37 °C in 5% CO2. Microglia cells were divided into four groups. One is the control group and the second group was stimulated by hemolysate alone. The third group was pretreated with fimasartan (30 ng/mL) alone for 6 h and the last group was stimulated with fimasartan before incubation with 10% hemolysate for 2 h, and then harvested for western blotting analysis.
2.11. Statistical analysis
The results are presented as the mean ± SEM. Statistical analysis was performed using Prism (GraphPad Software, La Jolla, CA). The results from the different groups were analyzed using an unpaired sample t-test or repeated measures of analysis of variance followed by Bonferroni’s post hoc test. The nonparametric Mann-Whitney U test or Wilcoxon signed-rank test was used for unpaired and paired samples, respectively. The nonparametric Kruskal-Wallis H test was used to test multiple groups. A two-tailed value of p < .05 was considered significant.
3. Results
3.1. No difference in the mean blood pressure levels in the low-dose fimasartan-treated group
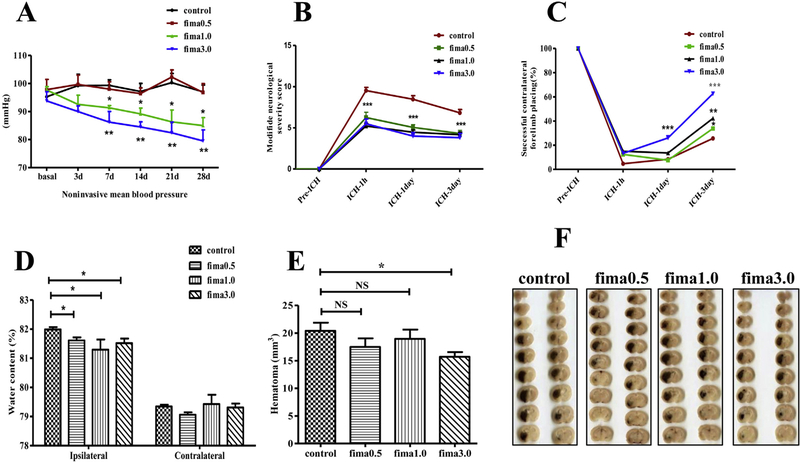
To determine whether fimasartan (fima) regulate BP in a dose-dependent manner, we monitored the mean BP level during the one month of pretreatment. The noninvasive mean BP in the low-dose fimasartan group was not significantly different from that of the control group. After treatment for 30 days, the mean BP was 97 ± 2.4 for the control group and 97 ± 3.1 mmHg for low-dose fimasartan group (p > .05). The regular-doses fimasartan groups showed a reduction in the mean BP as early as 3 days after treatment and led to significantly lower BP starting at 7 days in a dose-dependent manner (91 ± 0.8, p < .05, for fima 1.0 group, and 86 ± 3.8 mmHg, p < .01 for fima 3.0 group, respectively), compared to the control group (99 ± 2.0). (Fig. 2A).
Fig. 2.
Fimasartan regulated blood pressure, improved neurological functional recovery, and reduced brain water content. Noninvasive mean BP (A) decreased in the regular-doses fimasartan groups 3 days after the fimasartan administration, whereas in the low-dose fimasartan group, the mean BP was not different from that of the control group (n = 10 per group). Fimasartan improved the neurologic functional recovery in ICH injury. All rats (n = 17 per group) were subjected to the modified Neurological Severity Score (mNSS) (B) and the forelimb placing test (C). The mNSS was 0, and the forelimb placing test scores were 100, indicating normal neurological function. In contrast to the control group, (B) demonstrates that the mNSS was decreased in the fimasartan groups from day 1 to day 3 compared with the score at the 1 h time point after ICH. (C) demonstrates the forelimb placing test scores were increased in the fimasartan group from day 1 to day 3 compared with the scores at the 1 h time point after ICH. (D) The brain water content of the fimasartan groups was lower than that of the control group in the hemorrhagic hemisphere (n = 8 for the control group and n = 12 for fimasartan groups). (E, F) The hemorrhage volume was not different in the low-dose and regular-dose 1.0 mg/kg fimasartan group compared to that in the control group, but a reduction in the 3.0 mg/kg fimasartan group. n = 8 per group; * p < .05, ** p < .01, *** p < .001.
3.2. Improved neurological functioning outcomes
Functional recovery was significantly improved in the fimasartan-treated group compared to that in the control group at 1 h, 1 day and 3 days after ICH. In all rats, the mNSS score was 0 before ICH, which indicated normal neurological functioning. In the control group, the mNSS score peaked (9.5 ± 0.4) 1 h after ICH and decreased to 8.5 ± 0.4 on day 1 and 6.8 ± 0.4 on day 3 after ICH. In the fimasartan-treated group, the mNSS score also decreased from day 1 to day 3 after ICH compared with the score at the 1 h time point. Compared to the control group, the fimasartan-treated group demonstrated a significant decrease in the mNSS score at all of the time points indicating the improved outcome, p < .001 (Fig. 2B). In the fima 0.5 mg/kg group, the mNSS score peaked (6.3 ± 0.7) 1 h after ICH and decreased to 5.1 ± 0.3 on day 1 and 4.3 ± 0.4 on day 3; In the fima 1.0 mg/kg group, the mNSS score peaked (5.2 ± 0.3) 1 h after ICH and decreased to 4.5 ± 0.3 on day 1 and 4.2 ± 0.3 on day 3; in the fima 3.0 mg/kg group, the mNSS score peaked (5.5 ± 0.6) 1 h after ICH and decreased to 4.0 ± 0.4 on day 1 and 3.8 ± 0.5 on day 3.
For the forelimb placing test, the score was 100 in all of the rats before ICH, which indicated normal forelimb function. In the control group, the minimal improvement was observed from 1 h after ICH 4.7 ± 0.3 to 8.4 ± 0.4 on day 1 and 25.6 ± 1.2 on day 3 after ICH In the fimasartan-treated groups, there was an improvement in the score from day 1 to day 3 compared to that from 1 h after ICH in a dose-dependent manner. The fimasartan-treated group demonstrated a significantly higher score than the control group on day 3 (control vs. fima 0.5 mg/kg, 25.6 ± 1.2 vs. 33.8 ± 1.2, p < .05; control vs. fima 1.0 mg/kg, 25.6 ± 1.2 vs. 42.3 ± 1.4, p < .01; control vs. fima 3.0 mg/kg, 25.6 ± 1.2 vs. 62.7 ± 1.4, p < .001) (Fig. 2C). These results suggest that pretreatment with fimasartan improved the neurological function recovery after ICH.
3.3. Decreased brain water content and attenuated hematoma volume
Compared to the control group, both the low-dose and regular-dose fimasartan-treated groups showed a significant attenuation in the brain water content at 3 days after ICH (control vs. fima 0.5 mg/kg, 82.0 ± 0.08% vs. 81.6 ± 0.10%, p < .05; control vs. fima 1.0 mg/kg, 81.3 ± 0.35% vs. 79.4 ± 0.32, p < .05; control vs. fima 3.0 mg/kg, 81.5 ± 0.16 vs. 79.3 ± 0.14, p < .05) (Fig. 2D).
There was no difference in the hemorrhagic lesion volume in the low-dose and regular-dose 1.0 mg/kg fimasartan group compared to that in the control group (control vs. fima 0.5 mg/kg, 20.41 ± 1.47 mm3 vs. 17.5 ± 1.55 mm3, p > .05), which indicated that these doses of fimasartan did not affect bleeding. There was a reduction in the lesion volume in the fimasartan 3.0 mg/kg group (control vs. fima 3.0 mg/kg 20.41 ± 1.47 mm3 vs. 15.71 ± 0.85 mm3, p < .05) 1 day after ICH (Fig. 2E, F).
3.4. Activation of NF-κB/IκB and the downstream molecule COX-2 was suppressed
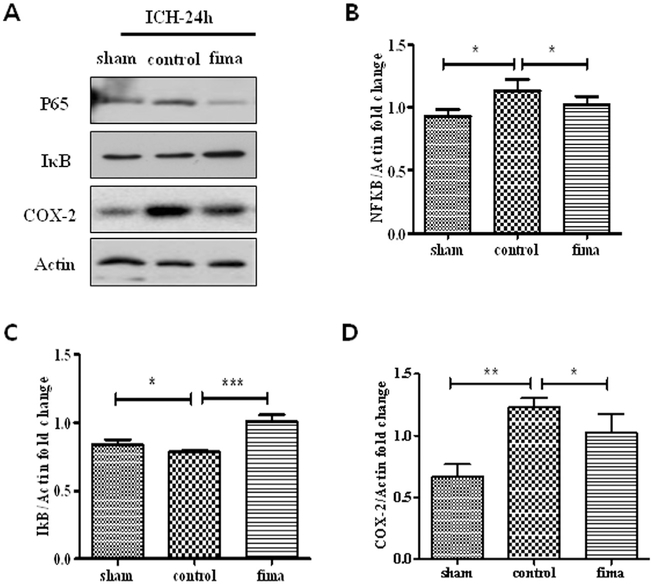
Based on the preliminary experiments, we determined that the low-dose fimasartan (0.5 mg/kg) was the effective dose in the present study without significant BP modulation effect, and then investigated the anti-inflammatory mechanism of the low-dose fimasartan effect on ICH rats. We first evaluated the activation of the NF-κB pathway, which is the priming signal for the inflammasome process. Compared to the sham group, NF-κB (1.13 ± 0.05 vs. 0.93 ± 0.03 in sham, p < .05) and COX-2 (1.23 ± 0.04 vs. 0.67 ± 0.06 in sham, p < .01) in control group were activated, and IκB (0.78 ± 0.01 vs. 0.84 ± 0.02 in sham, p < .05) was degraded in the control group. However, compared to control group, these changes were significantly attenuated by low-dose fimasartan (NF-κB 1.02 ± 0.04, p < .05; COX-2, 1.03 ± 0.09, p < .05, and IκB 1.01 ± 0.03, p < .001) (Fig. 3A–D).
Fig. 3.
Fimasartan attenuated the ICH-induced NF-κB pathway activation and downstream molecular COX-2. Twenty-four hours after ICH, the density of NF-κB (B) and COX-2 (D) in the control group was higher than in the sham group. After administration of low-dose fimasartan, the density of these activations in the fimasartan group was lower than in the control group. The density of IκB (C) in the control group was lower than in the sham group, and that of the fimasartan group was higher than in the control group. Data are presented as the mean ± SEM from three independent experiments n = 4 per group; * p < .05, ** p < .01, *** p < .001.
3.5. Activation of NLRP3/ASC/caspase-1 and the subsequent release of IL-1β were inhibited
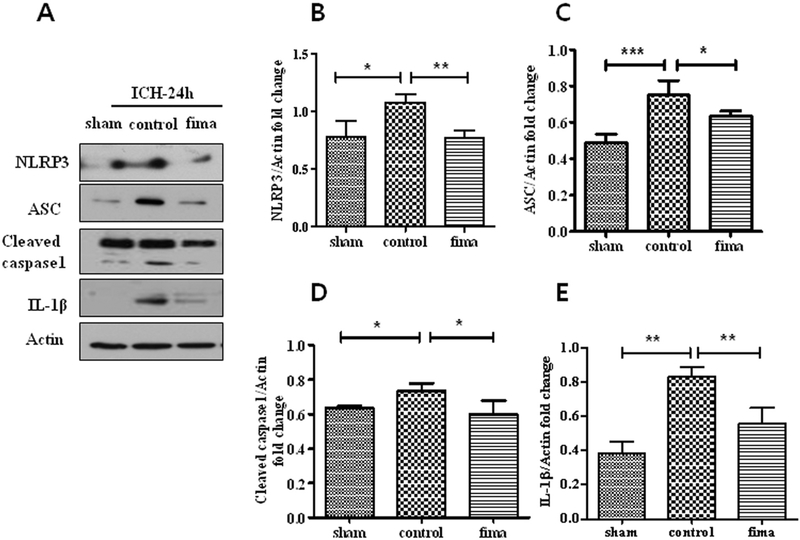
As the second signal for the inflammasome process, the expression of the NLRP3/ASC/caspase-1 complex was evaluated. We further analyzed the role of low-dose fimasartan in the activation of the NLRP3 inflammasome. Compared to their levels in the sham group, NLRP3 (1.08 ± 0.04 vs. 0.78 ± 0.08 in sham, p < .05), ASC (0.75 ± 0.05 vs. 0.49 ± 0.03 in sham, p < .001) and cleaved caspase-1 (0.73 ± 0.03 vs. 0.64 ± 0.01 in sham, p < .05) were upregulated in the control group (Fig. 4B–D). The production of mature IL-1β (0.83 ± 0.03 vs. 0.38 ± 0.04 in sham, p < .01) was also increased (Fig. 4E). However, compared to control group, these effects were inhibited by low-dose fimasartan (NLRP3, 0.77 ± 0.04, p < .01; ASC, 0.64 ± 0.01, p < .05; cleaved caspase-1, 0.60 ± 0.05, p < .05; mature IL-1β, 0.56 ± 0.05, p < .01).
Fig. 4.
Fimasartan attenuated ICH-induced NLRP3 inflammasome activation. Twenty-four hours after ICH, the density of NLRP3 (B), ASC (C), caspase-1 (D) and IL-1β (E) in the control group was higher than in the sham group. After administration of low-dose fimasartan, the density of these inflammasome components in the fimasartan group was significantly lower than in the control group. Data are presented as the mean ± SEM from three independent experiments. n = 4 per group; * p < .05, ** p < .01, *** p < .001.
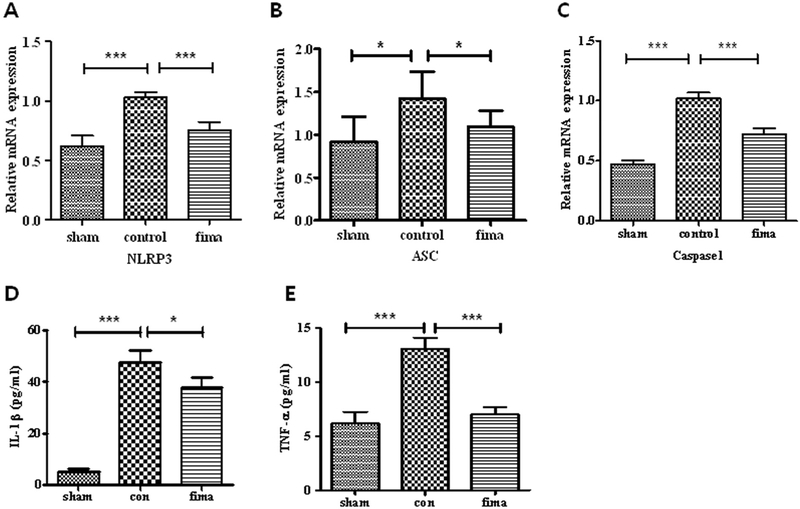
3.6. The expression of NLRP3/ASC/caspase-1 mRNA and the subsequent release of pro-inflammatory cytokines were reduced
To confirm our findings, we determined the contribution of fimasartan on the mRNA levels of NLRP3 inflammasome involved in ICH. Compared to Sham group, ICH induced a 1.7-fold increase in NLRP3 mRNA (p < .001), a 1.5-fold increase in ASC mRNA (p < .05) and a 1.2-fold increase in caspase-1 mRNA (p < .001). However, compared to control group, low-dose fimasartan significantly decreased these mRNA expression levels with a 0.3-fold decrease in NLRP3 mRNA (p < .001), a 0.2-fold decrease in ASC mRNA (p < .05) and a 0.3-fold decrease in caspase-1 mRNA (p < .001) (Fig. 5A, B, C). Compared with the sham group, the subsequent secretion of the cytokines IL-1β and TNF-α were increased in the control group (9.4-fold in IL-1β and 2.1-fold in TNF-α, both p < .001). Fimasartan effectively reduced the secretion with a 0.2-fold decrease in IL-1β (p < .05 compared to control) and a 0.5-fold decrease in TNF-α (p < .001 compared to control) (Fig. 5D, E).
Fig. 5.
Fimasartan inhibited ICH-induced expression of NLRP3 inflammasome mRNA and release of pro-inflammatory cytokines. Twenty-four hours after intracerebral hemorrhage (ICH), the mRNA expression of NLRP3 (A), ASC (B), and caspase-1 (C) and the release of pro-inflammatory cytokines IL-1β (D) and TNF-α (E) were higher in the control group than in the sham group. 30-day administration of low-dose fimasartan reduced the expression of NLRP3 mRNA and subsequent release of cytokines. The RT-PCR results are expressed as a fold change relative to the control group. Data are presented as the mean ± SEM from three independent experiments. n = 5 per group; * p < .05, *** p < .001.
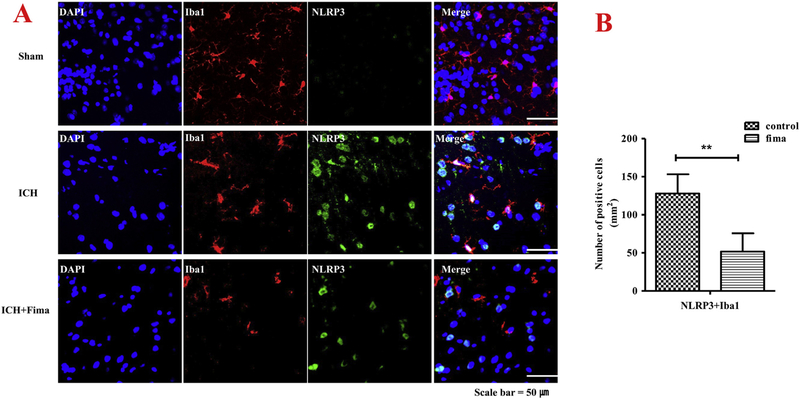
3.7. The expression of ICH-induced NLRP3/ASC/caspase-1 in microglia was downregulated
Increased number of active microglia is found in acute ICH (Chang et al., 2017). To better understand fimasartan’s effect on the innate immune response after ICH, we performed double IF staining using Iba-1+ and NLRP3 inflammasome (Fig. 6A). NLRP3 inflammasome was found double-stained in most Iba-1 positive cells in the area around the hematoma. Almost all the Iba-1+ cells exhibited larger cell bodies in the control group at one day after ICH, compared to the sham group where there is nearly no NLRP3 expression in Iba-1+ cells. However, the activated expression was downregulated by fimasartan compared to that of the control group. The quantitative analysis showed that fimasartan decreased the number of double-stained cells with active NLRP3 components combined with microglia (NLRP3: 124 ± 12 vs. 44 ± 7, p < .01) (Fig. 6B).
Fig. 6.
Fimasartan reduced activation of NLRP3 inflammasome largely in activated microglia within one day of ICH. Double labeling immunofluorescence staining images showed co-labeling for NLRP3 (A), and Iba-1 merged with DAPI around the hematoma in brain cryosections. Representative microphotographs show NLRP3 was activated in microglia cells labeled by Iba-1. Analysis of NLRP3 inflammasome immunoreactivity in the microglia expressed as a number of NLRP3 and Iba-1 positive cells (B). An increase in the proportion of the area positively stained with the Iba-1 antibody was used to measure microglia activation. Data are presented as mean ± SEM. Scale bar = 50 μm. n = 5 per group; ** p < .01.
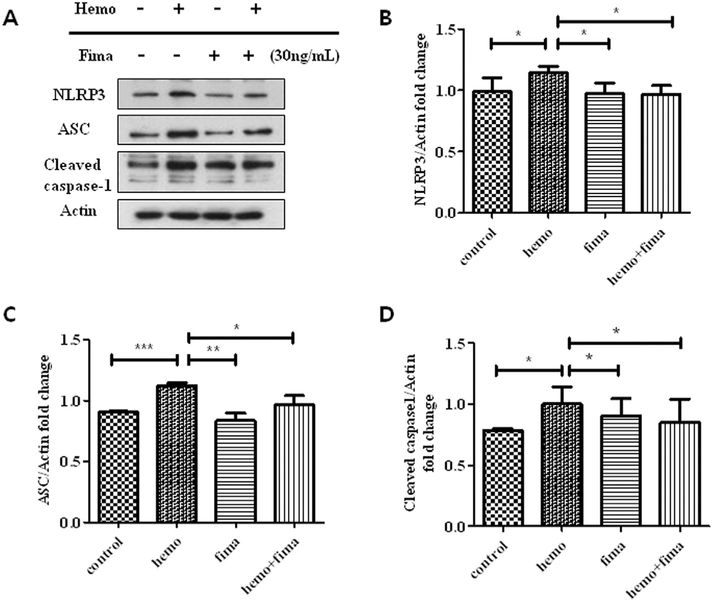
3.8. The expression of ICH-induced NLRP3/ASC/caspase-1 in microglia was modulated in vitro
To directly measure the effects of fimasartan on microglia, we used hemolysate to stimulate cells, which is considered to mimic ICH in vitro. Consistent with our in vivo results, we found hemolysate significantly increased NLRP3 inflammasome expression in the microglia. Compared to control group, 1.2-fold increase in NLRP3 (p < .05), 1.2-fold increase in ASC (p < .001) and 1.3-fold increase in cleaved caspase-1 (p < .05). In contrast, compared to hemo group, these activations were decreased by pretreatment with fimasartan with a 0.1-fold decrease in NLRP3 (p < .05), a 0.2-fold decrease in ASC (p < .05) and a 0.2-fold decrease in cleaved caspase-1 (p < .05) (Fig. 7A–D). These data indicated that the microglia cell may contribute to the activation of NLRP3 inflammasome following ICH, which could be attenuated by fimasartan treatment.
Fig. 7.
Fimasartan downregulated the hemolysate-induced activation of the NLRP3 inflammasome in microglia. The density of NLRP3 (B), ASC (C) and caspase-1 (D) in the hemolysate alone group were higher than in the control group. After pretreatment with fimasartan, the density in the fimasartan group was significantly lower than in the hemolysate alone group. Data are presented as the mean ± SD from three independent experiments. * p < .05, ** p < .01, *** p < .001.
4. Discussion
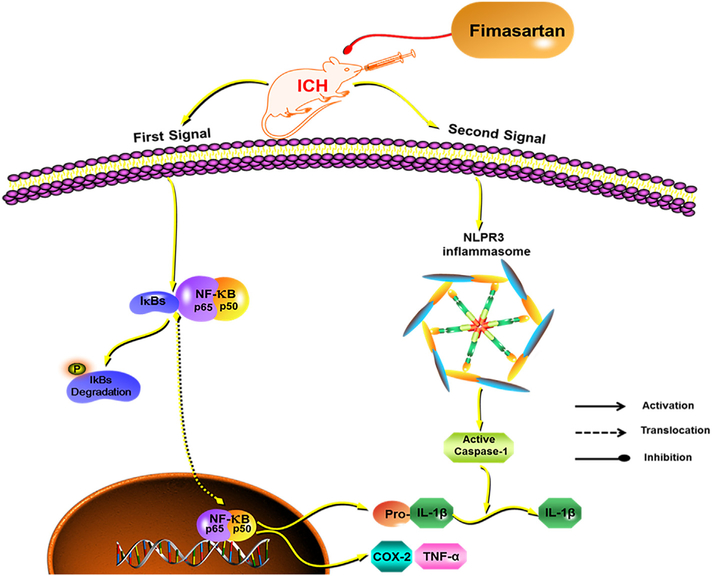
Pre-stroke treatment with different doses of fimasartan improved the outcome of ICH stroke and inhibited the inflammatory response related to the secondary injury of ICH. Our study suggested that the components of the NLRP3 inflammasome may contribute to the innate immune response following ICH, and that low-dose fimasartan inhibited the activation of the NLRP3 inflammasome without lowering the BP in ICH rats (Fig. 8). Regular doses (1.0 and 3.0 mg/kg, p.o.) of fimasartan can regulate the BP and improve the neurologic function in a dose-dependent effect.
Fig. 8.
A hypothetical mechanism for the fimasartan attenuation of NLRP3 inflammasome activation in response to ICH. Intracerebral hemorrhage (ICH) induces the inflammatory response of microglia via activating 2 signals. The priming signal, that is the NF-κB pathway, releases the cytokines pro-IL-1β and TNFα. The second signal assembles the NLRP3 inflammasome and cleaves caspase-1 into the active form, which subsequently promotes the pro-IL-1β into the maturation IL-1β. Pretreatment with low-dose fimasartan reduces the secondary damage induced by acute ICH via downregulating the activation of the two signals.
The inflammatory response is a key mechanism responsible for secondary brain damage after ICH. Recently, the inflammasome has been recognized as a vital player in the innate immune response in stroke (Fann et al., 2013). The discovery of the inflammasome in 2002 is considered a milestone in immunology (Martinon et al., 2002). NLRP3 is the most intensively studied inflammasome and has been broadly investigated in the CNS immune system, especially in its involvement with the ICH-induced inflammatory response (Feng et al., 2015; Ma et al., 2014). IL-1β, which is the downstream product of NLRP3 processing, is also an important therapeutic target in ICH (Lok et al., 2012). This leads to our hypothesis that targeting the formation of the NLRP3 inflammasome may be a potential therapy for ICH. Therefore, in this study, we focused on the effects of low-dose fimasartan treatment on ICH by inhibiting two IL-1β related signal pathways. Consistent with our hypothesis, both signal pathways were activated 1-day post-ICH. Low-dose fimasartan ameliorated these activations without affecting the BP, which suggests that the anti-inflammasome effects of fimasartan are independent of its effect on BP. Furthermore, inhibition of inflammasome activation by fimasartan was associated with better recovery of neurological function and a reduction in brain edema.
ARBs have been proven to exacerbate protective effects in numerous pre-clinical studies. Fimasartan is a selective AT1 receptor antagonist, for which the safety, efficacy, and dose-response have been investigated in human and rodent studies (Chi et al., 2013; Kim et al., 2015, 2012). On healthy subjects, fimasartan is well tolerated with single oral doses of 20–480 mg (Chi et al., 2011). For patients, 60 mg and 120 mg are commonly selected as the regular doses, while a dose < 30 mg is selected as the low dose (Lee et al., 2012a, b). Following the guide for dose conversion between animals and humans (Nair and Jacob, 2016), fimasartan was given orally at 1 and 3 mg/kg as regular dose to reduce BP in hypertensive rats (Chi et al., 2013). We previously found the neuroprotective effects on ischemic stroke after oral administration of fimasartan (dose, 0.5, 1 and 3 mg/kg) (Kim et al., 2015; Shin et al., 2011). In addition, our previous in-vitro study that mimicked hemorrhagic brain injury, found that pre-administration of fimasartan ameliorated the hemolysate-induced immune response in astrocytes (Yang et al., 2016). Based on these pieces of evidence, we selected the current doses of fimasartan to investigate the dose-dependent effects of fimasartan on ICH rats. Accumulating evidence indicates that ARBs can penetrate the blood-brain barrier (BBB), especially with chronic treatment or some pathologic conditions that make the BBB more permissive (Michel et al., 2013; Noda et al., 2012). Therefore, we speculate that fimasartan is able to pass through the BBB. In particular, pretreatment with low-dose ARBs, such as telmisartan and candesartan, have neuroprotective effects in normotensive rodents (Groth et al., 2003; Tsukuda et al., 2009). These studies suggest ARBs, including fimasartan, may be given to the patients at risk for ICH chronically.
Previous studies found systematic pretreatment with the candesartan decreased neuronal injury after the ischemic stroke on rats. Furthermore, pre-administration use of ARBs was found to be associated with the reduced mortality of patients with ischemic stroke (Groth et al., 2003; Sundboll et al., 2015). Compared to these studies, this study supported that pre-stroke administration of low-dose fimasartan has favorable effects on acute ICH injury without affecting BP. Pre-stroke administration of low-dose fimasartan may confer anti-inflammatory effects on ICH by targeting the NLRP3 inflammasome. Future studies may be needed to investigate strategies to offer ARB-based therapies for ICH.
The classical immune cells of the CNS are associated with the inflammatory response after ICH. It is well known that the activations of these cells result in the release of inflammatory cytokines, destruction of the BBB and further development of edema within the first 24 h (Zhou et al., 2014). Different types of inflammasomes generate innate immune responses in different brain cells. Accumulating evidence suggests that the NLRP3 inflammasome is activated in microglia (de Rivero Vaccari et al., 2014; Santoni et al., 2015). Microglia cells are the resident macrophage in the brain and play an important role in modulating inflammatory response induced by ICH (Lan et al., 2017). Therefore, the amelioration of the microglia activation in the acute stage of ICH could have a neuroprotective value (Hanisch and Kettenmann, 2007). Previous studies have demonstrated that levels of NLRP3 proteins, especially IL-1β, were significantly increased 24 h after injury of different stroke models (Abulafia et al., 2009; Tomura et al., 2012). Therefore, in this study, we selected 24 h as the target time point of inflammation induced by ICH. Double-labeled immunostaining suggested that high expression levels of the NLRP3/ASC/caspase-1 complex were colocalized with the reactive microglia cells in the acute stage of the ICH rat model. We also confirmed the effects of fimasartan on cultured microglia cell. Using the hemolysate stimulation, the levels of active NLRP3 inflammasome decreased with pretreatment of fimasartan.
Our study presents in vitro and in vivo evidence confirming the therapeutic effects of fimasartan on the secondary injury after ICH. Fimasartan is mainly used as an antihypertensive medication. Although our study focused on the pre-stroke administration of low-dose fimasartan neuroprotection on ICH without BP decrease, the effects of regular doses of fimasartan in hypertensive models or post-treatment of ICH may need to be studied further to determine its clinical and translational relevance. ICH is still primarily a disease of the elderly and age is an important risk factor for stroke. However, very few studies were conducted on the aged rodents because of the many physiological and neurochemical changes that happen with the aging (Anyanwu, 2007; Liu et al., 2010, 2009). In addition, previous studies showed inconsistent and complex effects on aging stroke rats (Kharlamov et al., 2000; Shapira et al., 2002; Sutherland et al., 1996). Future studies may be required to validate the translational potential of ICH stroke models in elder animals.
5. Conclusions
In summary, pretreatment with low-dose fimasartan is a potent candidate drug for protecting secondary damage from acute ICH by inhibiting the NLRP3 inflammasome. The activation of the NLRP3/ASC/caspase-1 inflammasome and the subsequent cytokines may contribute to the innate immune response of ICH. Further clinical studies may be needed to confirm the neuroprotective effects of fimasartan on stroke.
Acknowledgments
The authors wish to thank Dr. Sang-Kyu Ye from the Department of Pharmacology, Seoul National University College of Medicine, for providing the BV2 microglia cell. The research was supported by the Korean Health Technology R&D Projects funded by the Ministry of Health & Welfare, Republic of Korea (HI14C1277) and (HI15C2311). This work was partially supported by the grants from the Health Fellowship Foundation, Seoul, Republic of Korea and the Boryung Pharmaceutical Company. XY and XJ were supported in part by R01HL118084 and R01NS110387from United States National Institutes of Health (NIH) (both to XJ) and Maryland Stem Cell Research Fund, USA (2018-MSCRFD-4271) (to XJ). The funding organization had no role in the design, conduct, or analysis conducted during this study, or in the preparation of this report.
Footnotes
Compliance with ethical standards
All animal experimental protocols were performed in accordance with the relevant guidelines and regulations approved by the National Institutes of Health Animal Care and Use Committee of the Biomedical Research Institute at Seoul National University Hospital.
Conflicts of interest
The authors declare no conflict of interest.
References
- Abulafia DP, de Rivero Vaccari JP, Lozano JD, Lotocki G, Keane RW, Dietrich WD, 2009. Inhibition of the inflammasome complex reduces the inflammatory response after thromboembolic stroke in mice. J. Cereb. Blood Flow Metab. 29, 534–544. [DOI] [PubMed] [Google Scholar]
- Anyanwu EC, 2007. Neurochemical changes in the aging process: implications in medication in the elderly. TheScientificWorldJOURNAL 7, 1603–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CF, Wan J, Li Q, Renfroe SC, Heller NM, Wang J, 2017. Alternative activation-skewed microglia/macrophages promote hematoma resolution in experimental intracerebral hemorrhage. Neurobiol. Dis 103, 54–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M, 2001. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke 32, 1005–1011. [DOI] [PubMed] [Google Scholar]
- Chi YH, Lee H, Paik SH, Lee JH, Yoo BW, Kim JH, Tan HK, Kim SL, 2011. Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of Fimasartan Following Single and Repeated Oral Administration in the Fasted and Fed States in Healthy Subjects. Am. J. Cardiovasc. Drugs 11, 335–346. [DOI] [PubMed] [Google Scholar]
- Chi YH, Lee JH, Kim JH, Tan HK, Kim SL, Lee JY, Rim HK, Paik SH, Lee KT, 2013. Pharmacological characterization of BR-A-657, a highly potent nonpep-tide angiotensin II receptor antagonist. Biol. Pharm. Bull 36, 1208–1215. [DOI] [PubMed] [Google Scholar]
- Ciocoiu M, Badescu L, Miron A, Badescu M, 2013. The involvement of a polyphenol-rich extract of black chokeberry in oxidative stress on experimental arterial hypertension. Evid. Based Complement. Alternat. Med 2013, 912769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubal DB, Wise PM, 2002. Estrogen and neuroprotection: from clinical observations to molecular mechanisms. Dialogues Clin. Neurosci 4, 149–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fann DY-W, Lee S-Y, Manzanero S, Chunduri P, Sobey CG, Arumugam TV, 2013. Pathogenesis of acute stroke and the role of inflammasomes. Ageing Res. Rev 12, 941–966. [DOI] [PubMed] [Google Scholar]
- Feng L, Chen Y, Ding R, Fu Z, Yang S, Deng X, Zeng J, 2015. P2X7R blockade prevents NLRP3 inflammasome activation and brain injury in a rat model of intracerebral hemorrhage: involvement of peroxynitrite. J. Neuroinflammation 12, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi L, Munoz-Planillo R, Nunez G, 2012. Sensing and reacting to microbes through the inflammasomes. Nat. Immunol 13, 325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes B, Fernandez-Dominguez J, Ortega-Casarrubios MA, Sanjose B, Martinez-Sanchez P, Diez-Tejedor E, 2010. Treatment with angiotensin receptor blockers before stroke could exert a favourable effect in acute cerebral infarction. J. Hypertens 28, 575–581. [DOI] [PubMed] [Google Scholar]
- Groth W, Blume A, Gohlke P, Unger T, Culman J, 2003. Chronic pretreatment with candesartan improves recovery from focal cerebral ischaemia in rats. J. Hypertens 21, 2175–2182. [DOI] [PubMed] [Google Scholar]
- Han J, Park S-J, Thu VT, Lee S-R, Long LT, Kim HK, Kim N, Park SW, Jeon E-S, Kim E-J, Yoon C-H, Cho G-Y, Choi D-J, 2013. Effects of the novel angiotensin II receptor type I antagonist, fimasartan on myocardial ischemia/reperfusion injury. Int. J. Cardiol 168, 2851–2859. [DOI] [PubMed] [Google Scholar]
- Hanisch U-K, Kettenmann H, 2007. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci 10, 1387–1394. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Kummer MP, Latz E, 2017. Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol 14, 463–477. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Anrather J, 2011. The immunology of stroke: from mechanisms to translation. Nat. Med 17, 796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung KH, Chu K, Jeong SW, Han SY, Lee ST, Kim JY, Kim M, Roh JK, 2004. HMG-CoA Reductase Inhibitor, Atorvastatin, Promotes Sensorimotor Recovery, Suppressing Acute Inflammatory Reaction After Experimental Intracerebral Hemorrhage. Stroke 35, 1744–1749. [DOI] [PubMed] [Google Scholar]
- Keep RF, Hua Y, Xi G, 2012;Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol. 11, 720–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharlamov A, Kharlamov E, Armstrong DM, 2000. Age-dependent increase in infarct volume following photochemically induced cerebral infarction: putative role of astroglia. J. Gerontol. Series A Biol. Sci. Med. Sci 55, B135–B141 (discussion B142–133). [DOI] [PubMed] [Google Scholar]
- Kim JH, Lee JH, Paik SH, Kim JH, Chi YH, 2012. Fimasartan, a novel angiotensin II receptor antagonist. Arch. Pharm. Res 35, 1123–1126. [DOI] [PubMed] [Google Scholar]
- Kim CK, Ryu WS, Choi IY, Kim YJ, Rim D, Kim BJ, Jang H, Yoon BW, Lee SH, 2013. Detrimental effects of leptin on intracerebral hemorrhage via the STAT3 signal pathway. J. Cereb. Blood Flow Metab. 33, 944–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CK, Yang XL, Kim YJ, Choi IY, Jeong HG, Park HK, Kim D, Kim TJ, Jang H, Ko SB, Yoon BW, 2015. Effect of long-term treatment with fimasartan on transient focal ischemia in rat brain. Biomed. Res. Int 2015, 295925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan X, Han X, Li Q, Yang Q-W, Wang J, 2017. Modulators of microglial activation and polarization after intracerebral haemorrhage. Nat. Rev. Neurol 13, 420–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Yang HM, Lee HY, Kim JJ, Choi DJ, Seung KB, Jeon ES, Ha JW, Rim SJ, Park JB, Shin JH, Oh BH, 2012a. Efficacy and tolerability of once-daily oral fimasartan 20 to 240 mg/d in Korean Patients with hypertension: findings from Two Phase II, randomized, double-blind, placebo-controlled studies. Clin. Ther 34, 1273–1289. [DOI] [PubMed] [Google Scholar]
- Lee SE, Kim YJ, Lee HY, Yang HM, Park CG, Kim JJ, Kim SK, Rhee MY, Oh BH, 2012b. Efficacy and tolerability of fimasartan, a new angiotensin receptor blocker, compared with losartan (50/100 mg): a 12-week, phase III, multicenter, prospective, randomized, double-blind, parallel-group, dose escalation clinical trial with an optional 12-week extension phase in adult Korean patients with mild-to-moderate hypertension. Clin. Ther 34, 552–568 (568 e551–559). [DOI] [PubMed] [Google Scholar]
- Liu F, Yuan R, Benashski SE, McCullough LD, 2009. Changes in experimental stroke outcome across the life span. J. Cereb. Blood Flow Metab. 29, 792–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Akella P, Benashski SE, Xu Y, McCullough LD, 2010. Expression of Na-K-Cl cotransporter and edema formation are age dependent after ischemic stroke. Exp. Neurol 224, 356–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok J, Zhao S, Leung W, Seo JH, Navaratna D, Wang X, Whalen MJ, Lo EH, 2012. Neuregulin-1 effects on endothelial and blood–brain barrier permeability after experimental injury. Transl Stroke Res 3, 119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Chen S, Hu Q, Feng H, Zhang JH, Tang J, 2014. NLRP3 inflammasome contributes to inflammation after intracerebral hemorrhage. Ann. Neurol 75, 209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon F, Burns K, Tschopp J, 2002. The Inflammasome: A Molecular Platform Triggering Activation of Inflammatory Caspases and Processing of proIL-β. Mol. Cell 10, 417–426. [DOI] [PubMed] [Google Scholar]
- Michel MC, Foster C, Brunner HR, Liu L, 2013. A systematic comparison of the properties of clinically used angiotensin II type 1 receptor antagonists. Pharmacol. Rev 65, 809–848. [DOI] [PubMed] [Google Scholar]
- Nair AB, Jacob S, 2016. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm 7, 27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda A, Fushiki H, Murakami Y, Sasaki H, Miyoshi S, Kakuta H, Nishimura S, 2012. Brain penetration of telmisartan, a unique centrally acting angiotensin II type 1 receptor blocker, studied by PET in conscious rhesus macaques. Nucl. Med. Biol 39, 1232–1235. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF, 2001. Spontaneous Intracerebral Hemorrhage. N. Engl. J. Med 344, 1450–1460. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Mendelow AD, Hanley DF, 2009. Intracerebral haemorrhage. Lancet 373, 1632–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rivero Vaccari JP, Dietrich WD, Keane RW, 2014. Activation and regulation of cellular inflammasomes: gaps in our knowledge for central nervous system injury. J. Cereb. Blood Flow Metab. 34, 369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra JM, 2012. Angiotensin II AT1 receptor blockers as treatments for in-flammatory brain disorders. Clin. Sci 123, 567–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoni G, Cardinali C, Morelli MB, Santoni M, Nabissi M, Amantini C, 2015. Danger- and pathogen-associated molecular patterns recognition by pattern-recognition receptors and ion channels of the transient receptor potential family triggers the inflammasome activation in immune cells and sensory neurons. J. Neuroinflammation 12, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST, 2000. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology 39, 777–787. [DOI] [PubMed] [Google Scholar]
- Schroder K, Tschopp J, 2010. The inflammasomes. Cell 140, 821–832. [DOI] [PubMed] [Google Scholar]
- Selim M, Savitz S, Linfante I, Caplan L, Schlaug G, 2005. Effect of pre-stroke use of ACE inhibitors on ischemic stroke severity. BMC Neurol. 5, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira S, Sapir M, Wengier A, Grauer E, Kadar T, 2002. Aging has a complex effect on a rat model of ischemic stroke. Brain Res. 925, 148–158. [DOI] [PubMed] [Google Scholar]
- Shin BS, Kim TH, Paik SH, Chi YH, Lee JH, Tan HK, Choi Y, Kim M, Yoo SD, 2011. Simultaneous determination of fimasartan, a novel antihypertensive agent, and its active metabolite in rat plasma by liquid chromatography-tandem mass spectrometry. Biomed. Chromatogr 25, 1208–1214. [DOI] [PubMed] [Google Scholar]
- Song EC, Chu K, Jeong SW, Jung KH, Kim SH, Kim M, Yoon BW, 2003. Hyperglycemia exacerbates brain edema and perihematomal cell death after intracerebral hemorrhage. Stroke 34, 2215–2220. [DOI] [PubMed] [Google Scholar]
- Strowig T, Henao-Mejia J, Elinav E, Flavell R, 2012. Inflammasomes in health and disease. Nature 481, 278–286. [DOI] [PubMed] [Google Scholar]
- Sundboll J, Schmidt M, Horvath-Puho E, Christiansen CF, Pedersen L, Botker HE, Sorensen HT, 2015. Preadmission use of ACE inhibitors or angiotensin receptor blockers and short-term mortality after stroke. J. Neurol. Neurosurg. Psychiatry 86, 748–754. [DOI] [PubMed] [Google Scholar]
- Sutherland GR, Dix GA, Auer RN, 1996. Effect of age in rodent models of focal and forebrain ischemia. Stroke 27, 1663–1667 (discussion 1668). [DOI] [PubMed] [Google Scholar]
- Tomura S, de Rivero Vaccari JP, Keane RW, Bramlett HM, Dietrich WD, 2012. Effects of therapeutic hypothermia on inflammasome signaling after traumatic brain injury. J. Cereb. Blood Flow Metab. 32, 1939–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukuda K, Mogi M, Iwanami J, Min LJ, Sakata A, Jing F, Iwai M, Horiuchi M, 2009. Cognitive deficit in amyloid-beta-injected mice was improved by pretreatment with a low dose of telmisartan partly because of peroxisome proliferator-activated receptor-gamma activation. Hypertension 54, 782–787. [DOI] [PubMed] [Google Scholar]
- Wang J, Doré S, 2007. Inflammation after intracerebral hemorrhage. J. Cereb. Blood Flow Metab. 27, 894–908. [DOI] [PubMed] [Google Scholar]
- Xi G, Keep RF, Hoff JT, 2006. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 5, 53–63. [DOI] [PubMed] [Google Scholar]
- Yang B, Wang S, Yu S, Chen Y, Li L, Zhang H, Zhao Y, 2017. C1q/tumor necrosis factor-related protein 3 inhibits oxidative stress during intracerebral hemorrhage via PKA signaling. Brain Res. 1657, 176–184. [DOI] [PubMed] [Google Scholar]
- Yang XL, Kim CK, Kim TJ, Sun J, Rim D, Kim YJ, Ko SB, Jang H, Yoon BW, 2016. Anti-inflammatory effects of fimasartan via Akt, ERK, and NFkappaB pathways on astrocytes stimulated by hemolysate. Inflamm. Res 65, 115–123. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Wang Y, Wang J, Anne Stetler R, Yang QW, 2014. Inflammation in intracerebral hemorrhage: from mechanisms to clinical translation. Prog. Neurobiol 115, 25–44. [DOI] [PubMed] [Google Scholar]
- Zhu W, Cao F-S, Feng J, Chen H-W, Wan J-R, Lu Q, Wang J, 2017. NLRP3 inflammasome activation contributes to long-term behavioral alterations in mice injected with lipopolysaccharide. Neuroscience 343, 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]