Abstract
Despite considerable advances in medicine, cardiovascular disease is still rising; with ischemic heart disease being the leading cause of death and disability worldwide. Thus extensive efforts are continuing to establish effective therapeutic modalities that would improve both quality of life and survival in this patient population. Novel therapies are now being investigated not only to protect the myocardium against ischemia-reperfusion injury but also regenerate the heart. Stem cell therapy, such as potential use of human mesenchymal stem cells, induced pluripotent stem cells and their exosomes will make it possible not only to address molecular mechanisms of cardiac conditioning, but also develop new therapies for ischemic heart disease.
Despite all the studies and progress made over the last 15 years on the use of stem cell therapy for cardiovascular disease, the efforts are still in their infancy. While the expectations have been high, the findings indicate that most of the clinical trials are generally small and the results are inconclusive. Because of many negative findings, there is certain pessimism that cardiac cell therapy is likely to yield any meaningful results over the next decade or so. Similar to other new technologies, early failures are not unusual and they may be followed by impressive success. Nevertheless, there has been considerable attention to safety by the clinical investigators since the adverse events of stem cell therapy have been impressively rare. In summary, while the regenerative biology might not help the cardiovascular patient in the near term, it is destined to do so over the next several decades.
Introduction
Cardiovascular disease is the leading global cause of death, accounting for over 17 million deaths per year. The number of cardiovascular deaths is expected to grow to more than 23 million by 2030, according to a report from the American Heart Association.1 In 2011 nearly 787,000 people died from heart disease, stroke and other cardiovascular diseases in the United States. Two new approaches have been identified that have the potential of added benefits to the current therapeutic strategies. The first focuses on enhancing the heart/myocardium’s tolerance to ischemia-reperfusion injury using cardiac conditioning that will be covered here only briefly as a historical background. The second approach is to create an environment within the heart muscle that will result in repair of the damaged myocardium; a topic of this review.
Considerable experimental evidence obtained in multiple models and species has demonstrated that all forms of myocardial ischemic conditioning (pre-conditioning, per-conditioning, post-conditioning and remote preconditioning) induce very potent cardioprotection in animal models.2–5 In healthy, young hearts, many of these conditioning methods can significantly increase the heart’s resistance against ischemia and reperfusion injury. However, essentially none of these forms of myocardial ischemic conditioning have been effective in patients. Remote ischemic pre-conditioning using transient arm ischemia–reperfusion did not improve clinical outcomes in the ERICCA study, with 1,612 patients undergoing elective on-pump coronary artery bypass grafting.6 Additionally, upper-limb remote ischemic preconditioning performed in 1,385 patients did not show any significant benefit among patients undergoing elective cardiac surgery.7 Therefore, these large multicenter trials have not only proved that ischemic conditioning was unsuccessful in cardiac surgeries; they also failed to confirm the presence of initial cardioprotection by ischemic conditioning-induced reduction of cardiac troponin release,8, 9 which is a standard diagnostic indicator of myocardial injury. The lack of clinical success most likely is due to underlying risk factors that interfere with cardiac conditioning, along with the use of cardioprotective agents that activate the endogenous cardioprotective mechanisms. Future preclinical validation of drug targets and cardiac conditioning will need to focus more on comorbid animal models (such as age, diabetes, and hypertension) and choosing the relevant endpoints for assessing the efficacy of cardioprotective procedures to have a successful, clinical translation.
While the existing therapies for the ischemic heart disease lower the early mortality rates, prevent additional damage to the heart muscle, and reduce the risk of further heart attacks, most of the patients are likely to have worse quality of life including frequent hospitalizations. Therefore, there is an ultimate need for a treatment to improve the clinical conditions by either replacing the damaged heart cells and/or improve cardiac performance. Thus, the cardiac tissue regeneration with the application of stem cells, or their exosomes, may be an effective therapeutic option.10 Stem cells, both adult and embryonic stem cells (ESCs) have the ability to self-replicate and transform into an array of specialized cells. Stem cells are becoming the most important tool in regenerative medicine since these cells have the potential to differentiate into cardiomyocytes. It would, therefore, be useful to find out if the differentiated cells can restore and improve cardiac function safely and effectively.
The purpose of this review is to present the current state of knowledge of potential use of human stem cells, induced human pluripotent stem cells (hiPSCs), and stem cell-derived exosomes as a cell based therapeutic strategy for the treatment of the damaged heart. These stem cells also provide feasibility to address fundamental research questions directly relevant to human health, including their challenges, limitations, and potential, along with future prospects. Human induced pluripotent stem cell technology, in particular, patient-specific hiPSC-derived cardiomyocytes (hiPSC-CMs) recently has enabled modeling of human diseases, offering a unique opportunity to investigate potential disease-causing genetic variants in their natural environment.
Stem Cells
Although there are many different kinds of stem cells, in this review we will include only those that have been used for most current cardiac regeneration studies.
Embryonic stem cells are obtained from the inner cell mass of the blastocyst that forms three to five days after an egg cell is fertilized by a sperm. They can give rise to every cell type in the fully formed body, but not the placenta and umbilical cord.
Tissue-specific stem cells (also referred to as somatic or adult stem cells) are more specialized than embryonic stem cells. The primary roles of adult stem cells in a living organism are to maintain and repair the tissue in which they are found.
Mesenchymal stem cells are multipotent stromal cells which can be isolated from the bone marrow. They are non-hematopoietic, multipotent stem cells with the capacity to differentiate into mesodermal lineage such as bone cells, cartilage cells, muscle cells and fat cells.
Induced pluripotent stem cells, or iPS cells, are cells taken from any tissue (usually skin or blood) and are genetically modified to behave like an embryonic stem cell. They are pluripotent, which means that they have the ability to form all adult cell types.
Umbilical cord blood stem cells are collected from the umbilical cord at birth and they can produce all of the blood cells in the body.
There is great potential with the recent advances in stem cell research and hiPSC-CMs, as these cells express the same ion channels and signaling pathways as primary human cardiomyocytes, can be cultured for a long time and are available in sufficient quantity. In addition, hiPSCs derived from diseased patients may be able to provide new forms of treatment of ischemic heart disease due to their potential for repairing damaged cardiac tissue, as shown in the Wu’s laboratory.11 Apart from their more direct role of tissue regeneration, stem cells may also have a clinical impact by secreting multiple growth factors and cytokines. Trophic mediators secreted by stem cells improve cardiac function by a combination of various mechanisms such as attenuating tissue injury, inhibiting fibrotic remodeling, promoting angiogenesis, mobilizing host tissue stem cells, and reducing inflammation. The cardioprotective panel of stem cell secreted factors are considerable and include, but not limited to bFGF/FGF-2, IL-1β, IL-10, PDGF, VEGF, HGF, IGF-1, SDF-1, thymosin-β4, Wnt5a, Ang-1 and Ang-2, MIP-1, EPO and PDGF.12–18 FGF-2 reduces ischemia-induced myocardial apoptosis, cell death and arrhythmias, and stimulates increased expression of anti-apoptotic Bcl-2.19, 20 HGF, bFGF, Ang-1 and -2, and VEGF secreted by BMMSCs lead to augmented vascular density and blood flow in the ischemic heart21–23, whereas SDF-1, IGF-1, HGF facilitate circulating progenitor cell recruitment to injury sites thereby promoting repair and regeneration.24–27 Stem cells also secrete ECM components including collagens, TGF-β, matrix metalloproteinases (MMPs) and tissue-derived inhibitors (TIMPs) that inhibit fibrosis.28–30
Therefore, the use of the right mediator may contribute to a better outcome in cell therapy. Many stem cell types have been used in regenerative cardiac research, including bone marrow-derived cells, myoblasts, endogenous cardiac stem cells, umbilical cord-derived mesenchymal stem cells and embryonic cells. However, an exciting new milestone in the field of regenerative and precision medicine was the development of hiPSCs. The therapeutic potential of hiPSCs is considerable, as they are patient-specific stem cells that do not face the immunologic barrier, in contrast to embryonic stem cells. Furthermore, there are sources of tissue to be reprogrammed into hiPSCs that are easily accessible, such as the donor’s skin, fat, or blood. Their use may avoid common legal and ethical problems that arise from the use of embryonic stem cells; they can differentiate into functional cardiomyocytes and they are now one of the most promising cell sources for cardiac regenerative therapy.
Pluripotent Stem Cells
Pluripotent stem cells (PSCs) have been derived by explanting cells from embryos at different stages of development under various growth conditions. PSCs can be classified into two distinct states, naive and primed, which are believed to represent successive snapshots of pluripotency as embryonic development proceeds.31, 32 Naïve pluripotent stem cells can be maintained in vitro by supplying leukocyte inhibitory factor combined with inhibition of mitogen-activated protein kinase/extracellular regulated kinase and glycogen synthase kinase 3 signaling, and are characterized by two active X chromosomes. Primed pluripotent stem cells are dependent on fibroblast growth factor 2 signaling and transforming growth factor-β signaling, and display inactivation of one X chromosome.31 Human embryonic stem cells and induced PSCs (iPSCs) are considered to share some properties of naïve mouse embryonic stem cells.33 Naïve human iPSCs can be derived by reversion of primed iPSCs into a state that resembles naïve mouse ESCs.34
Fibroblasts are the most commonly used primary somatic cell type for the generation of iPSCs. Fibroblasts can be reprogrammed to stable self-renewing iPSCs which resemble ESCs by enforced expression of a cocktail of transcription factors consisting of octamer-binding protein (Oct4), SRY-box containing gene 2 (Sox2), Kruppel-like factor 4 (Klf4), c-myelocytomatosis oncogene (c-Myc), Lin28, and Nanog gene.35, 36 iPSCs can be generated, expanded, and then differentiated into any cell types including endothelial cells (ECs) and cardiomyocytes for in vitro studies or, ultimately, cell therapy.37, 38
In recent years, it has been shown that somatic cells can be directly converted to cardiomyocytes, although the efficacy is extremely low. Transgenic expression of three cardiac-specific transcription factors (Gata4, Mef2c, and Tbx5) resulted in the trans-differentiation of fibroblasts into contracting cardiomyocytes referred to as induced cardiomyocytes (iCMs). In addition, other reports have also shown that direct reprogramming of somatic cells to iCMs is also feasible using various small molecules and microRNAs (miRNAs), such as Hand2, Mesp1, Myocardin, ESRRG, miR-1, and miR-133.39–42 Subsequently, alternative approaches have succeeded in generating human iCMs with gene expression profiles and functional characteristics similar to those detected in ESC-CMs.43
Induced Pluripotent Stem Cells
Due to the aforementioned advances in iPSC-derived CMs, it is now possible to generate an unlimited quantity of a patient’s own heart cells. This new model allows researchers to study and understand the molecular and cellular mechanisms of inherited cardiomyopathies, channelopathies, as well as model acquired heart diseases. Although additional studies are needed to test their safety and efficacy, these heart cells may be also used for regenerative medicine applications following myocardial infarction.
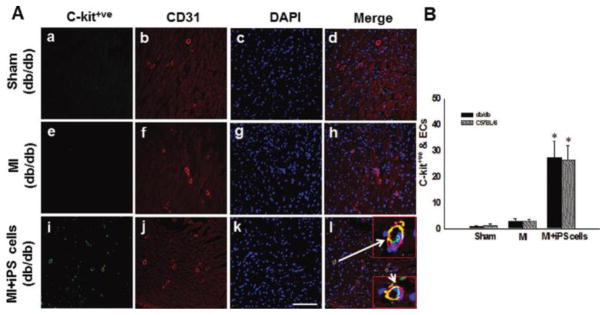
It was shown that hiPSCs may lose their pluripotency when transplanted into a border zone of infarcted cardiac tissue, and engraft into native myocardium where they only partially differentiate into cardiac myocytes. In Yan’s study, they reported that iPS cell transplantation in the infarcted diabetic db/db and nondiabetic mice resulted in an increase in vascular smooth muscle and endothelial cells in the infarcted heart, leading to a significantly improved cardiac function (Figure 1).44
Figure 1.
iPS cell transplantation in the infarcted diabetic db/db and nondiabetic mice resulted in an increase in vascular smooth muscle and endothelial cells in the infarcted heart, leading to a significantly improved cardiac function. Photomicrographs show anti-CD-31 in red (A, panels a, e, i), anti-red fluorescence protein (RFP) in green (A, panels b, f, j) and total nuclei stained with diamidino-phenylindole (DAPI) in blue (A, panels c, g, k). Merged images are shown in A, panels d, h, i. Scale bar=100 μm. Panel B shows quantitative analysis of total endothelial cells generation from transplanted iPS cells in both C57BL/6 and db/db mouse hearts two weeks post-MI,*P < 0.001 vs MI.44
Another study demonstrated that iPSC derived progenitor cells differentiated into a cardiomyocyte phenotype and developed contracting areas in mice heart tissue. Beneficial remodeling and improved ventricular function were observed despite the lack of well-aligned mature donor cardiomyocytes.45
In regards to safety, an important obstacle to the clinical use of hiPSCs for the regenerative purposes is their great heterogeneity in terms of plasticity and epigenetic landscape. There is a potential that allogeneic hiPSC transplantation into the heart may cause in situ tumorigenesis.46 In addition, the heterogeneity of the cardiac cells produced from pluripotent hiPSCs administration and their random implantation is likely to cause cardiac arrhythmias. One of the main limitations of the hiPSC-derived cardiomyocytes is that they are embryonic in nature as compared to adult cells. Many laboratories are still trying to make these myocytes more mature and to make lineage-specific cells so as to obtain a pure population of atrial cells, nodal cells, or ventricular cells. iPSC-derived cardiomyocytes exhibit an immaturity of the sarcoplasmic reticulum, and a β-adrenergic response that is significantly different from native ventricular tissue of a comparable age. Once the cells are mature, it is also likely that investigators will be able to test the effects of various drugs using hiPSC-CMs from a diverse population of patients with different sexes, ethnicities, and cardiovascular diseases.
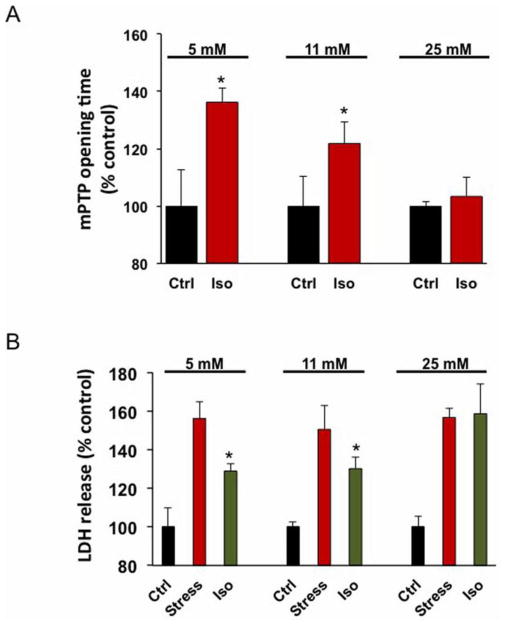
We are utilizing a model of the patient-specific hiPSCs differentiated into cardiac lineage in order to delineate the environmental and cellular mechanisms responsible for impaired cardioprotection in diabetes. The advantage of this approach is that the effect of cardioprotection can be evaluated in human cells, thereby capturing the complex physiologic interactions at the patient-specific myocyte level. Our results indicate that iPSC-derived cardiomyocytes are not only a viable model to investigate the underlying mechanisms of anesthetic cardioprotection,47 but they also respond similarly to human myocytes48 and human embryonic stem-cell-derived cardiomyocytes.49 Isoflurane preconditioning protected hiPSC-derived cardiomyocytes from oxidative stress-induced lactate dehydrogenase release and mitochondrial permeability transition pore opening at normal glucose concentrations (Figure 2).50
Figure 2.
Isoflurane delayed mitochondrial permeability transition pore (mPTP) opening and protected induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs) from oxidative stress in 5 mM and 11 mM glucose. mPTP opening was induced by photoexcitation-generated oxidative stress. Isoflurane delayed mPTP opening in iPSC-CMs in the presence of 5 mM and 11 mM glucose concentrations (A). Isoflurane did not delay mPTP opening in the presence of 25 mM glucose concentrations (A). *P < 0.001 versus control, n=18 cells/group. H2O2-induced oxidative stress increased lactate dehydrogenase (LDH) release from iPSC-CMs in 5 mM, 11 mM and 25 mM glucose concentrations (B). In iPSC-CMs, isoflurane reduced stress-induced LDH release in 5 mM and 11 mM glucose, but not in 25 mM glucose (B). *P < 0.05 versus stress, n=3 experiments/group. Ctrl = Control; Iso = Isoflurane treatment; and Stress = H2O2 + 2-Deoxyglucose.50
Anesthetic preconditioning also protects cardiomyocytes indirectly through its action on other cell types in the heart, such as endothelial cells,51 or by modulation of inflammatory response. However, hyperglycemia undermines the effectiveness of anesthetic-induced cardioprotection by dysregulating cellular signaling.47 In addition, this study demonstrated that the cardioprotective effects of isoflurane in elevated glucose conditions can be restored by scavenging reactive oxygen species or inhibiting mitochondrial fission. These findings may contribute to further understanding and guidance for restoring pharmacological cardioprotection in hyperglycemic patients. Cardiomyocytes derived from healthy donors and patients with a particular disease (such as diabetes) open new possibilities of studying genotype and phenotype related pathologies in a human-relevant model. Such diseases were nearly impossible to investigate in the past due to the lack of human cardiac cells available for experimental investigation.
Intra-myocardial Cell-Based Therapy
Preclinical studies
Some preclinical studies provide evidence that bone marrow stem cells contribute to cardiac function and reverse remodeling after ischemic damage52 acting both locally53 and remotely.54 In studies to date, bone marrow stem cells have been either infused55 or injected56 in areas that were undergoing revascularization. In preclinical studies, Bolli’s group reported that multiple treatments are necessary to properly evaluate the full therapeutic potential of cell therapy.57, 58 In their study on mice with a myocardial infarction received one or three doses of cardiac mesenchymal cells through the percutaneous infusion into the left ventricular cavity, 14 days apart. The single-dose group showed improved left ventricular ejection fraction after the 1st infusion but not after the 2nd and 3rd-vehicle infusion. In contrast, in the multiple-dose group, left ventricular ejection fraction improved after each cardiac mesenchymal cell-infusion. The multiple-dose group also exhibited less collagen in the non-infarcted region vs. the single-dose group. Engraftment and differentiation of cardiac mesenchymal cells were negligible in both groups, indicating paracrine effects.58 There appear to be at least three dominant mechanisms that underlie the cardiac reparative response: reduction in tissue fibrosis, neovascularization, and neomyogenesis.54, 59
Human ESC-CMs isolated from embryoid bodies have also been used for replacing myocardial scar tissue with new, functional cardiac cells, and therefore achieving actual myocardial regeneration. The ESC-CMs behave structurally and functionally like cardiomyocytes, expressing characteristic morphology, cell marker and transcription factor expression, sarcomeric organization and electrophysiological properties, including spontaneous action potentials and beating activity.60 Mouse and human cardiac-committed ESCs have been transplanted into small and large animal models of acute and old MI. Although these studies have demonstrated durable in vivo engraftment, proliferation and differentiation of ESC-CMs, as well as electromechanical integration with host cardiomyocytes61, they have not universally shown improvement in myocardial remodeling and function. While the reported benefits can thus be attributed to a potential synergy62 between the favorable environment created by the revascularization of the region and the mesenchymal stem cells, the precise delineation of each contribution, however, remains unknown. In addition, so far there is no evidence that critical number of new cells is regenerated or injected stem cells survive following the transplant. Animal studies have shown that only 1% of the stem cells injected into the heart tissue are detectable after 1 month. Nevertheless, in one of the recent systemic reviews and meta-analysis studies of preclinical work, cardiac stem cells treatment resulted in significant improvement of ejection fraction compared with placebo.63 In addition, there was a reduction in the magnitude of effect in large compared with small animal models.
A cell-based therapy could offer additional clinical benefits for post-ischemic heart by improving revascularization along with structural and functional properties.12, 64, 65 There are several limitations for effective stem cell therapy, but the major problems deal with their delivery, type of cells to be used, limited retention of the cells in the heart and the risk of immune rejection. Direct injection of stem cells into the heart muscle results in significant cell death and washout resulting in majority of cells being removed from the heart soon after the injection. Many preclinical studies have reported that intravenously administered MSCs for acute myocardial infarction attenuate the progressive deterioration in LV function and adverse remodeling in mice with large infarcts, and in ischemic cardiomyopathy, they improve LV function.63 Moreover, the cardiac phenotype of human embryonic stem cell-derived cardiac myocytes and human induced pluripotent stem cell-derived cardiac myocytes salvages the injured myocardium better than undifferentiated stem cells through their differential paracrine effects.66
Clinical studies
In the clinical studies, the investigators have used mainly two approaches of cell administration: intramyocardial delivery and intracoronary injection. Direct cardiac muscle injections can be performed either surgically or using percutaneous endocardial injection catheters, while coronary injection of stem cells can be done using an antegrade intracoronary artery injection or a retrograde sinus injection. The antegrade intracoronary artery injection is more attractive because it is the least invasive but some microvascular plugging can occur as a result of stem cell injection leading to microinfactions when the cells injected are too large for the capillary bed. Since the stem cells also need to cross the capillary wall, this approach has been found to be less effective as compared to intramyocardial delivery. Although the cell type, dosage, concentration, and delivery modalities are important considerations for regenerative cell therapy clinical trials, the available data are inconclusive and additional early phase studies will be needed before proceeding to pivotal clinical trials.67
The stem cells derived from the bone marrow of the healthy donors have been used in majority of clinical trials as briefly summarized in Table 1. So far, the clinical trials for cardiac regeneration have mainly used cell-based therapies, including bone marrow-derived cells, mesenchymal stem cells and cardiac progenitor cells. While the listed studies have met safety end points either with autologous or allogeneic cell sources68, the effect on cardiac function has been somewhat disappointing. One of the largest multicenter clinical trials using bone-marrow cells given via intracoronary injection for myocardial infarct patients, failed to reinforce the notion that these therapies are efficacious since it did not meet its primary goal.69 A recently published Cochrane review of bone-marrow trials for heart attack patients also found no benefits for various primary goals such as mortality, morbidity, life quality and LVEF.70 An additional Cochrane review using bone-marrow-derived stem/progenitor cells as a treatment for chronic ischemic heart disease and congestive heart failure identified low-quality evidence of reduction in mortality and improvement of LVEF.71
Table 1.
Selected list of landmark clinical trials using mostly bone marrow-derived mesenchymal stem cells conducted to treat acute myocardial infarction and heart failure.
| Study | n | Type of patient | Cell type; Delivery | Follow up (months) | Outcome LVEF % | Ref. |
|---|---|---|---|---|---|---|
| Janssens et al. | 67 | AMI | BMD; Intracoronary | 4 | N.S. | 106 |
| BOOST | 60 | AMI | BMD; Intracoronary | 61 | N.S. | 107 |
| ASTAMI | 100 | AMI | BMD; Intracoronary | 36 | N.S. | 108 |
| REGENT | 200 | AMI | BMD; Intracoronary | 6 | N.S. | 109 |
| REPAIR-AMI | 204 | AMI | BMD; Intracoronary | 24 | 5% | 110 |
| MAGIC | 97 | HF | SMB | 6 | N.S. | 92 |
| FOCUS-CCTRN | 92 | HF | BMD; Transendocardial | 6 | N.S. | 111 |
| POSEIDON | 30 | ICM | BMD; Transendocardial | 12 | N.S. | 112 |
| SCIPIO | 33 | IHF | CPCs; Intracoronary | 12 | 8% | 70 |
| CADUCEUS | 25 | AMI | CDCs; Intracoronary | 12 | N.S. | 71 |
| TOPCARE-AMI | 55 | AMI | BMD; Intracoronary | 60 | 11% | 113 |
| TAC-HFT | 65 | ICM | BMD; Transendocardial | 12 | N.S. | 114 |
| MSC-HF | 55 | IHF | BMD; Transendocardial | 6 | 5% | 115 |
| MESAMI | 10 | ICM | BMD; Transendocardial | 12 | 6% | 116 |
| SWISS-AMI | 200 | AMI | BMD; Intracoronary | 4 | N.S. | 117 |
| REGENERATE-AMI | 100 | AMI | BMD; Intracoronary | 12 | N.S. | 118 |
| PreSERVE-AMI | 161 | AMI | BMD; Intracoronary | 6 | N.S. | 67 |
| MiHeart-AMI | 121 | AMI | BMD; Intracoronary | 6 | N.S. | 119 |
| RIMECARD | 30 | HF | UC-MSC; Intravenous | 12 | 5% | 74 |
| TIME | 120 | AMI | BMD; Intracoronary | 24 | N.S. | 120 |
n: Number of patients in the study; LVEF: Changes in Left ventricular Ejection Fraction; IHF: Ischemic Heart Failure; CPCs: Cardiac Progenitor Cells; AMI: Acute Myocardial Infarction; CDCs: Cardiosphere-derived Cells; BMD: Bone Marrow Derived; HF: Heart Failure; SMB: Skeletal Myoblasts; ICM: Ischemic Cardiomyopathy; UC-MSCs: Umbilical cord-derived mesenchymal stem cells. N.S.: Not Significant
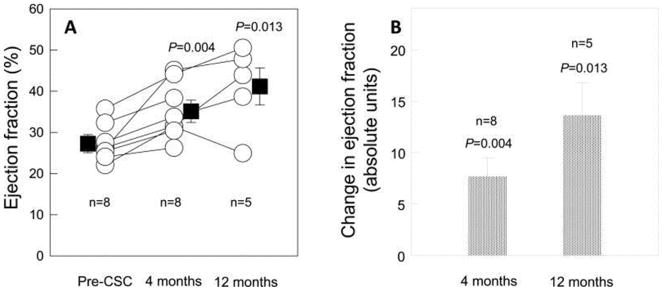
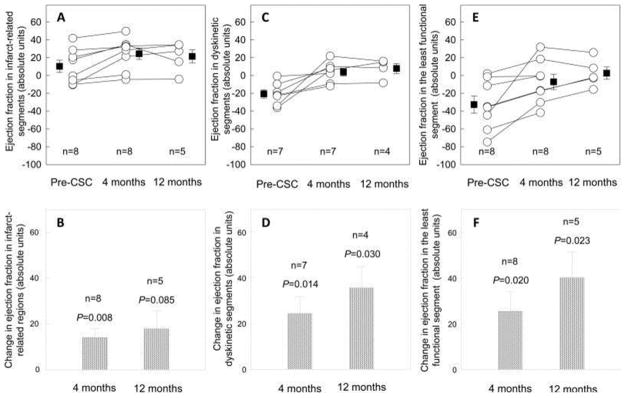
The limited clinical success of stem-cell injections for the treatment of myocardial infarction or heart failure has been mainly attributed to the low retention and survival of injected cells. One of the clinical trials for treatment of heart failure resulting from ischemic heart disease used autologous c-kit(+) cardiac stem cells and produced a significant improvement in both global (Figure 3) and regional LV function (Figure 4), a reduction in infarct size, and an increase in viable tissue that persisted at least 1 year after cardiac infusion (SCIPIO trial).72 Another study, also involving small number of patients, used intracoronary administration of autologous cardiosphere-derived cells and the treatment led to a decreased scar size, increased viable myocardium, and improved regional function of infarcted myocardium at 1 year post-injection (CADUCEUS trial) (Figure 5).73
Figure 3.
Administration of Cardiac Stem Cells (CSC) in Patients with Ischemic Cardiomyopathy. Panel A: The mean baseline LVEF in the eight treated patients who were included in the cardiac magnetic resonance analysis was 27.5% at baseline (4 months after CABG surgery and before CSC infusion), and increased markedly to 35.1% (P=0.004, n=8) at 4 months and 41.2% (P=0.013, n=5) at 12 months after CSC infusion. Panel B: Change in LVEF at 4 months and 12 months after CSC infusion (absolute EF units). Data are means ± SEMs. 72
Figure 4.
Panel A: Regional EF at baseline and 4 and 12 months after CSC infusion in the infarct-related regions. Panel B: Change in regional EF in the infarct-related regions at 4 and 12 months after CSC infusion (absolute EF units). Panel C: Regional EF in the dyskinetic segments of the infarct-related regions at baseline and 4 and 12 months after CSC infusion. Panel D: Change in regional EF in the dyskinetic segments of the infarct-related regions at 4 and 12 months after CSC infusion (absolute EF units). Panel E: Regional EF in the least functional segment of the infarct-related regions at baseline and 4 and 12 months after CSC infusion. Panel F: Change in regional EF in the least functional segment of the infarct-related regions at 4 and 12 months after CSC infusion (absolute EF units). Data are means ± SEMs. 72
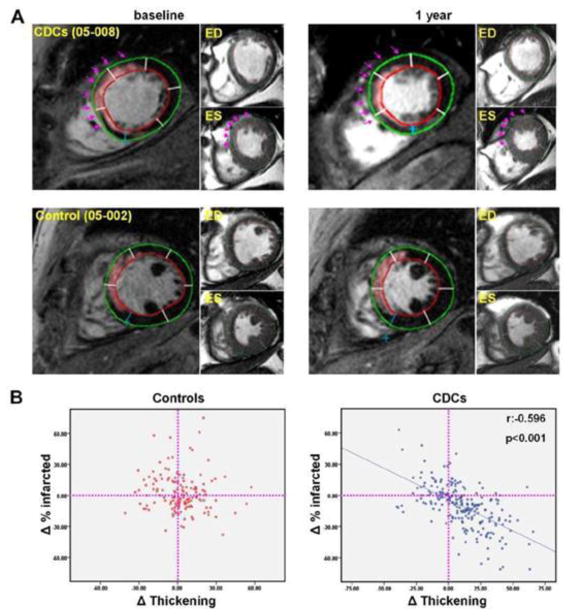
Figure 5. Intracoronary Cardiosphere-Derived Cells After Myocardial Infarction.
(A) Representative matched, delayed contrast-enhanced magnetic resonance images and their corresponding cine short-axis images (at end-diastole [ED] and end-systole [ES]) at baseline and 1 year. In the pseudocolored, delayed contrast-enhanced images, infarct scar tissue, as determined by the full width half maximum method, appears pink. Each cardiac slice was divided into 6 segments and the infarcted segments were visually identified from delayed contrast enhanced images. Scar size (percentage of infarcted tissue per segment) and systolic thickening were calculated for each individual infarcted segment at baseline and 1 year. Endocardial (red) and epicardial (green) contours of the left ventricle are shown. In the CDC-treated patient (top row), scar decreased, viable mass increased and regional systolic function improved over the period of 1 year in the treated infarcted segments (highlighted by arrows). In contrast, no major changes in scar mass, viable myocardial mass or regional systolic function were observed in the control patient (bottom row). (B) Scatterplots of the changes in the percentage of infarcted tissue and the changes in systolic thickening for every infarcted segment of treated and control patients. ED = end-diastole. 73
Cells harvested from the umbilical cord
Umbilical cord blood has been demonstrated as a very useful and rich source of stem and progenitor cells, capable of restoring blood formation and immunological functions after transplantation. Cord blood stem cells are currently used to treat a range of blood disorders and immune system conditions such as leukemia, anemia and autoimmune diseases. These stem cells are used largely in the treatment of children but have also started being used in adults following chemotherapy treatment. Another type of cell that can also be collected from umbilical cord blood is mesenchymal stromal cells. These cells can grow into bone, cartilage and other types of tissues and are being used in many research studies to see if patients could benefit from these cells too. The fact that cord blood can be frozen and stored for later use led, in 1991, to the establishment of the first cord blood bank from voluntary donors in New York. To date, there are over 54 public cord blood banks in different parts of the world with more than 350,000 units frozen and ready to be used.74 Indeed cord blood transplantation is being used as an alternative to bone marrow transplantation, and more than 14,000 transplants have been documented. Cord blood stem cell treatments differ from bone marrow stem cell treatments in three key areas: increased tolerance of the human leukocyte antigen-mismatching, decreased risk of graft-versus-host disease, and enhanced proliferation ability.75 Recent results of the RIMECARD study by Bartolucci et al. in human subjects using umbilical cord-derived MSCs as potential heart failure therapy are quite encouraging.76 The patients had stable heart failure (HF), with reduced ejection fraction of less than 40. Although the sample size was small (15 controls and 15 HF patients treated with UC-MSCs) to establish either safety or efficacy, the echocardiographic and cardiac MRI evaluations demonstrated improvements in ejection fraction, starting at 3 months, and persisting through 12 months. The patients treated with placebo did not improve in either left ventricular ejection fraction or clinical functional class. As indicated by the authors, it is tempting to speculate that the robust paracrine secretion of various factors, including hepatocyte growth factor, might play an important role in mediating the therapeutic effects of the UC-MSCs.
The main disadvantage of cord blood use is that the volume collected is fixed and relatively small. Therefore, the number of stem cells available for transplantation is low compared to the number of cells that can be collected in customizable bone marrow or peripheral blood stem cell harvests. Nevertheless, there are many opportunities for further development of this technology such as the cord blood selection algorithms that are currently heavily weighted toward maximizing cell doses at the expense of the human leukocyte antigen-matching.77
Tissue Engineering
Beside the stem cell injection therapy, currently there are non-cardiogenic and cardiogenic stem-cell tissue patches, for the repair of myocardial infarction. Recent studies have utilized non-cardiogenic tissue patches made of skeletal myoblasts78–81, bone marrow-derived stem cells82, 83, or endothelial progenitor cells84 for the repair of damaged heart. Compared with the injection of a cell suspension, the implantation of tissue sheets composed of skeletal myoblasts has been proven more advantageous for the treatment of myocardial infarction in rats78, 85, and dilated cardiomyopathy in hamsters.86 Moreover several cardiogenic cardiac tissue-engineering methodologies have been developed for use with primary neonatal cardiomyocytes. These include: injection of a mixture of bioactive hydrogels and cells followed by cell-hydrogel polymerization in situ87 and the epicardial implantation of a tissue-engineered cardiac patch.88, 89
Generally, implantation of the engineered myoblast sheets over an infarction site yielded improved neovascularization, attenuated left ventricular dilatation, decreased fibrosis, improved fractional shortening, and prolonged animal survival compared to the delivery of the same number of myoblasts by cell injection.85 In addition, the bone marrow-derived, spatially arranged SMC-endothelial progenitor bi-level cell sheet interactions between SMCs and endothelial progenitor cells augment mature neovascularization, limit adverse remodeling, and improve ventricular function after myocardial infarction.90 In diabetic patients treatment of diabetes mellitus-induced cardiomyopathy with tissue-engineered smooth muscle cell-endothelial progenitor cell bi-level cell sheets prevented cardiac dysfunction and microvascular disease associated with diabetes mellitus-induced cardiomyopathy.91 As indicated before, the main disadvantage of injecting the cell-suspensions directly into the heart muscle compare to engineered heart tissue technique is that most of the injected cells are washed out of the heart or do not survive the injection. This is inefficient and can also be dangerous if some cells have not yet fully developed into myocardial cells and are therefore still pluripotent. These cells could survive in other parts of the body and form tumors. The advantage of the tissue patches is that significantly fewer of the stem cell-derived heart cells are required and fewer cells undergo apoptosis. Some of the major drawbacks currently encountered with regeneration using tissue patches, include the problems with electrical continuity and patch vascularization. Using a similar tissue-engineering strategy, Shimko et al. formed cardiac constructs using pure differentiated cardiomyocytes derived by genetic selection from D3 mouse ESCs with a neomycin-resistance gene driven by the α-MHC promoter.92 They found that 10% cyclic stretch at rate of 1–3 Hz for 3 days increased the expression of cardiac markers such as α-cardiac actin, α-MHC and Mef-2c, but the resulting cardiac tissues were noncontractile. Immunostaining showed that pure cardiomyocytes were present, but they had disorganized sarcomeres and a relatively rounded appearance.92
Recently, in a study published by Nummi et al., they reported that during on-pump coronary artery bypass graft surgery, part of the right atrial appendage can be excised upon insertion of the right atrial cannula of the heart-lung machine and the removed tissue can be easily cut into micrografts for transplantation.93 Appendage tissue is harvested during cannulation of the right atrium, and therefore, no additional procedure is needed. Isolation of the cells and preparing the matrix for transplantation is done simultaneously with the coronary artery bypass graft operation in the operating room, so the perfusion time and the aorta clamp time are not increased. After the bypass anastomoses, the atrial appendage sheet is placed on the myocardium with three to four sutures allowing the myocardium to contract without interference. They believe that atrial appendage-derived cells therapy administered during CABG surgery will have an impact on patient treatment in the future.93
While some of the outcomes of these trials have been modest at best, it is now evident that the success of future cardiac cell therapies will be highly dependent on the ability to overcome the problem of low retention and survival of implanted cells.94 Potential approaches to address this issue include: coinjecting cells with bioactive, in situ polymerizable hydrogels87, preconditioning cells with hypoxia or prosurvival factors95, genetic engineering of cells to enhance their angiogenic and/or antiapoptotic action96 and the epicardial implantation of a preassembled tissue-engineered patches.27, 85, 97 In particular, tissue patch implantation, although surgically more complex than cell or cell/hydrogel injections, may support long-term survival of transplanted cells and exert a more efficient structural and functional cardiac tissue reconstruction at the infarct site.98
Cellular Reprogramming
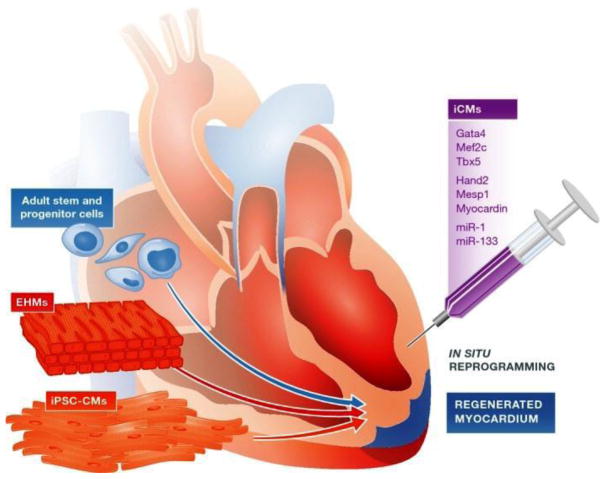
The adult human heart lacks sufficient ability to replenish the damaged cardiac muscles since the rate of cardiomyocyte renewal activity is less than 1% per year. The mechanical and electrical engraftment of injected cardiomyocytes is largely not feasible at the scale that would be necessary for cardiac improvement. On the other hand, the human heart contains large population of fibroblasts that could be used for direct reprograming. As such, direct fibroblasts reprogramming in vivo has emerged as a possible approach for cardiac regeneration. With considerably better understanding of the various molecular mechanisms, direct fibroblast reprogramming has improved considerably but still lacks sufficient efficacy using human cells (Figure 6).
Figure 6. Cell therapy and tissue engineering approaches for cardiovascular disease therapy.
There are various novel treatment options that have been tested for the heart failure due to ischemic heart disease or genetic disorders. Previous clinical trials have employed various adult stem cell and progenitor cell populations to test their efficacy for therapeutic applications. Additional approaches are being explored, including implantation of in vitro constructed cell sheets of engineered heart muscles (EHMs) as well as direct in vivo reprogramming of cardiac fibroblasts in the scar region to cardiomyocytes. The regenerative capacity of adult stem and progenitor cell populations is also being evaluated along with administration of exosomes and small vesicles secreted by the stem cells.36
Stem Cell-Derived Exosomes and Small Vesicles
As indicated earlier, the survival of transplanted stem cells is dismal and the beneficial effects of stem cell therapies is not due to their differentiation into new cardiomyocytes but instead because they are the temporary source of the exosomal growth factors. Therefore, despite the stem cells’ early demise, there are some limited cardiac benefits from this treatment, including decreased cardiomyocyte apoptosis, reduced fibrosis, enhanced neovascularization and improved left ventricular ejection fraction. It is for that reason why the exosome therapy recapitulates the benefits of stem cell therapy,99 and many studies have shown that the activation of cardioprotective pathways obtained by stem cell therapy can be reproduced by the injection of exosomes produced by the stem cells.100 An additional benefit of using exosomes for cardioprotection and regeneration is the lack of tumor-forming potential of exosomes. However, the underlying mechanisms of stem cells or hiPSC-derived exosome therapy are still unclear. Numerous scientific investigations have identified recent applications of exosomes in the development of molecular diagnostics, drug delivery systems and therapeutic agents.
Exosomes are small membrane vesicles (30–100 nm) of endocytic origin that are secreted by most cells after being formed in the cellular multivesicular bodies. The fusion of multivesicular bodies into the plasma membrane leads to the release of their intraluminal vesicles as exosomes. Once released in the extracellular environment, their cargo of functional molecules can be taken up by recipient cells via several mechanisms including fusion with the plasma membrane, phagocytosis and endocytosis. The formation and release can be upregulated through different steps based on environmental stimuli such as stress or hypoxia. There are two main mechanisms responsible for exosome release. First, there is a constitutive mechanism that is mediated by specific proteins involved in membrane trafficking, such as RAB heterotrimeric G-proteins and protein kinase D. Second, there exists an inducible mechanism that can be activated by several stimuli including increased intracellular Ca2+ and DNA damage. Studies have used different approaches to also increase the angiogenic potential of exosomes released by the stem cells.101 Exosome release with a basal angiogenic potential can be substantially increased in vitro using stress conditions that mimic organ injury, such as hypoxia, irradiation, or drug treatments. Changes in exosomal composition facilitate angiogenesis and tissue repair most likely via enhanced level of growth factors and cytokines.
Exosomes contain various molecular constituents of their cell of origin, including proteins and RNA. The cargo of mRNA and miRNA in exosomes was first discovered at the University of Gothenburg in Sweden.102 In that study, the differences in cellular and exosomal mRNA and miRNA content were described, as well as the functionality of the exosomal mRNA cargo. Exosomes facilitate cell-cell communication to the recipient cell membrane and deliver effectors including transcription factors, oncogenes, small and large non-coding regulatory RNAs (such as microRNAs) and mRNAs into recipient cells and can be used for cardiac protection and repair. Exosomes have also been shown to carry double-stranded DNA. Exosomes can be derived from many different types of stem cells including umbilical cord, cardiosphere-derived cells, cardiac stem cells, embryonic, induced pluripotent, mesenchymal and endothelial progenitor cells. They can carry and deliver mRNAs, miRNAs and proteins to the injured heart muscle and play a significant role in resident cardiac stem cell activity, cardiomyocyte proliferation, beneficial cardiac remodeling, apoptosis reduction, angiogenesis, anti-inflammatory response and a decrease in infarct size. The advantages for effective exosome therapy include the cell free component, log-term stability and low or no immune response. Some of the limitations include the necessity of repeated injections, target cell selection and the random packing of the exosome cargo.
Some preliminary reports have demonstrated that exosomes released from cardiac progenitor cells can improve cardiac function in the damaged heart.103, 104 It has been proposed that exosomes released from transplanted cardiomyocytes are involved in metabolic events in target cells by facilitating an array of metabolism-related processes, including modulation of gene expression. Moreover, exosomes secreted from the hiPSC-derived cardiomyocytes exert protective effects by transferring endogenous molecules to salvage injured neighboring cells by regulating angiogenesis, apoptosis, fibrosis, and inflammation. It has been shown that ischemic preconditioned hearts promote exosome release and help spread cardioprotective signals within the myocardium.105, 106 Also, the administration of mesenchymal stromal cell-secreted exosomes demonstrated improved cardiac function in the acute myocardial infarction mouse model. Mesenchymal stem cell-derived exosomes increased adenosine triphosphate levels, reduced oxidative stress, and activated the PI3K/Akt pathway to enhance cardiomyocyte viability after ischemia-reperfusion (I/R) injury.107 Recently, it was shown that ischemic preconditioning of mesenchymal stromal cells increased levels of miR-21, miR-22, miR-199a-3p, miR-210, and miR-24 in exosomes released by the cells, and the administration of mesenchymal stromal cell-ischemic preconditioning exosomes resulted in a reduction of cardiac fibrosis and apoptosis compared with the hearts treated with control exosomes. Stem cell-derived exosomes possess the ability to modulate cardiomyocyte survival and confer protection against angiotensin II-induced hypertrophy by activating PI3K/Akt/eNOS pathways via RNA enriched within the exosomes. Additionally, it has been shown that exosome treatment increased levels of adenosine triphosphate and NADH, decreased oxidative stress, increased phosphorylated-Akt and phosphorylated- glycogen synthase kinase 3, and reduced phosphorylated-c-JNK in hearts after I/R. Subsequently, both local and systemic inflammations were significantly reduced 24 hours after reperfusion.107 Intact exosomes restore bioenergetics, reduce oxidative stress, and activate pro-survival signaling, thereby enhancing cardiac function and geometry after myocardial I/R injury.107 Clearly, stem cell-derived exosomes may be a potential adjuvant to reperfusion therapy for myocardial infarction and heart failure.
Conclusions
The existing therapies for the ischemic heart disease have many limitations and efforts are underway for new treatments using the stem cell therapy to improve the clinical conditions by either replacing the damaged heart cells and/or improve cardiac performance. The cardiac tissue regeneration with stem cells, their exosomes or small vesicles and tissue engineering may be effective therapeutic options. Although the expectations have been high, the results from majority of clinical trials are negative. Due to very low engraftment and survival of stem cells injected into a cardiac muscle, there is convincing evidence that the release of paracrine factors from the stem cells contributes to myocardial cardioprotection and regeneration. It is likely that the future research will be focused on the biology of these endogenous signaling pathways, and will lead the way for different applications of exosomes and small vesicles in regenerative medicine. In the future there might be more successful approaches that would utilize stem cell technology with various bioengineering constructs having not only cardiomyocytes but other cardiac cells.
Regarding the regulatory agencies, one would need a significant efficacy/safety data and meaningful end points when compared with standard-of-care drugs that are used today for heart attacks and heart failures. So where are we today since most of the clinical trials did not achieve their primary efficacy end points? Because the cell-therapy studies for heart disease did not achieve this so far, more preclinical work might be necessary using current and other approaches in order to demonstrate compelling rationale for new clinical trials. Although the regenerative biology might not be very helpful to the cardiovascular patient in the near term, it is most likely that we will witness very impressive and exciting results over the next several decades.
Acknowledgments
This work was supported in part by grants P01GM066730 and T32 GM089586 from the National Institutes of Health, Bethesda, MD.
Abbreviations
- Ca2+
calcium ion
- Ecs
endothelial cells
- eNOS
endothelial nitric oxide synthase
- eNOS/PKG
endothelial nitric oxide synthase/protein kinase G
- ERK1/2
Extracellular signal-regulated protein kinases 1 and 2
- hiPSCs
human stem cells, induced pluripotent stem cells
- HiPSC-CMs
human stem cells, induced pluripotent stem cells-derived cardiomyocytes
- IPSC
induced pluripotent stem cells
- NADH
Nicotinamide adenine dinucleotide
- JAK/STAT
Janus kinase and Signal Transducer and Activator of Transcription pathways
- p42/p44 MAPK/ERK
specific isoforms of mitogen-activated protein kinase and extracellular regulated kinase
- PI3K/Akt/mTOR
Phosphatidylinositol 3-kinase, serine/threonine kinase also known as protein kinase B, and mammalian target of rapamycin pathway
- PKC
protein kinase C
- PKG
cGMP protein kinase G pathway
Footnotes
Disclosure
The authors declare that they have no disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Maia Terashvili, Department of Physiology, Medical College of Wisconsin, 8701 Watertown Plank Road, Milwaukee, Wisconsin, 53226, USA.
Zeljko J. Bosnjak, Departments of Medicine and Physiology, Medical College of Wisconsin, 8701 Watertown Plank Road, Milwaukee, Wisconsin, 53226, USA.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P American Heart Association Statistics C, Stroke Statistics S. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heusch G, Botker HE, Przyklenk K, Redington A, Yellon D. Remote Ischemic Conditioning. Journal of the American College of Cardiology. 2015;65:177–195. doi: 10.1016/j.jacc.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heusch G. Cardioprotection: chances and challenges of its translation to the clinic. Lancet. 2013;381:166–175. doi: 10.1016/S0140-6736(12)60916-7. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz Longacre L, Kloner RA, Arai AE, Baines CP, Bolli R, Braunwald E, Downey J, Gibbons RJ, Gottlieb RA, Heusch G, Jennings RB, Lefer DJ, Mentzer RM, Murphy E, Ovize M, Ping PP, Przyklenk K, Sack MN, Vander Heide RS, Vinten-Johansen J, Yellon DM. New Horizons in Cardioprotection Recommendations From the 2010 National Heart, Lung, and Blood Institute Workshop. Circulation. 2011;124:1172–1179. doi: 10.1161/CIRCULATIONAHA.111.032698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heusch G. Molecular Basis of Cardioprotection Signal Transduction in Ischemic Pre-, Post-, and Remote Conditioning. Circulation Research. 2015;116:674–699. doi: 10.1161/CIRCRESAHA.116.305348. [DOI] [PubMed] [Google Scholar]
- 6.Hausenloy DJ, Candilio L, Evans R, Ariti C, Jenkins DP, Kolvekar S, Knight R, Kunst G, Laing C, Nicholas J, Pepper J, Robertson S, Xenou M, Clayton T, Yellon DM Investigators ET. Remote Ischemic Preconditioning and Outcomes of Cardiac Surgery. New England Journal of Medicine. 2015;373:1408–1417. doi: 10.1056/NEJMoa1413534. [DOI] [PubMed] [Google Scholar]
- 7.Meybohm P, Kohlhaas M, Stoppe C, Gruenewald M, Renner J, Bein B, Albrecht M, Cremer J, Coburn M, Schaelte G, Boening A, Niemann B, Sander M, Roesner J, Kletzin F, Mutlak H, Westphal S, Laufenberg-Feldmann R, Ferner M, Brandes IF, Bauer M, Stehr SN, Kortgen A, Wittmann M, Baumgarten G, Meyer-Treschan T, Kienbaum P, Heringlake M, Schoen J, Treskatsch S, Smul T, Wolwender E, Schilling T, Fuernau G, Bogatsch H, Brosteanu O, Hasenclever D, Zacharowski K RIPHeart (Remote Ischemic Preconditioning for Heart Surgery) Study Collaborators. RIPHeart (Remote Ischemic Preconditioning for Heart Surgery) Study: Myocardial Dysfunction, Postoperative Neurocognitive Dysfunction, and 1 Year Follow-Up. J Am Heart Assoc. 2018 Mar 26;7(7) doi: 10.1161/JAHA.117.008077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hausenloy DJ, Mwamure PK, Venugopal V, Harris J, Barnard M, Grundy E, Ashley E, Vichare S, Di Salvo C, Kolvekar S, Hayward M, Keogh B, MacAllister RJ, Yellon DM. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomised controlled trial. Lancet. 2007;370:575–9. doi: 10.1016/S0140-6736(07)61296-3. [DOI] [PubMed] [Google Scholar]
- 9.Thielmann M, Kottenberg E, Kleinbongard P. Cardioprotective and prognostic effects of remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: a single-centre randomised, double-blind, controlled trial. Lancet. 2013;382:597–604. doi: 10.1016/S0140-6736(13)61450-6. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez-Aviles F, Sanz-Ruiz R, Climent AM, Badimon L, Bolli R, Charron D, Fuster V, Janssens S, Kastrup J, Kim HS, Luscher TF, Martin JF, Menasche P, Pinto FJ, Simari RD, Stone GW, Terzic A, Willerson JT, Wu JC, Group TW. Global Overview of the Transnational Alliance for Regenerative Therapies in Cardiovascular Syndromes (TACTICS) Recommendations: A Comprehensive Series of Challenges and Priorities of Cardiovascular Regenerative Medicine. Circ Res. 2018;122:199–201. doi: 10.1161/CIRCRESAHA.117.312099. [DOI] [PubMed] [Google Scholar]
- 11.Wu JX, Li J, Zhang NN, Zhang CH. Stem cell-based therapies in ischemic heart diseases: a focus on aspects of microcirculation and inflammation. Basic Research in Cardiology. 2011;106:317–324. doi: 10.1007/s00395-011-0168-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3:e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korf-Klingebiel M, Kempf T, Sauer T, Brinkmann E, Fischer P, Meyer GP, Ganser A, Drexler H, Wollert KC. Bone marrow cells are a rich source of growth factors and cytokines: implications for cell therapy trials after myocardial infarction. Eur Heart J. 2008;29:2851–8. doi: 10.1093/eurheartj/ehn456. [DOI] [PubMed] [Google Scholar]
- 14.Kubal C, Sheth K, Nadal-Ginard B, Galinanes M. Bone marrow cells have a potent anti-ischemic effect against myocardial cell death in humans. Journal of Thoracic and Cardiovascular Surgery. 2006;132:1112–1118. doi: 10.1016/j.jtcvs.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 15.Xu M, Uemura R, Dai Y, Wang Y, Pasha Z, Ashraf M. In vitro and in vivo effects of bone marrow stem cells on cardiac structure and function. J Mol Cell Cardiol. 2007;42:441–8. doi: 10.1016/j.yjmcc.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circulation Research. 2006;98:1414–1421. doi: 10.1161/01.RES.0000225952.61196.39. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi M, Li TS, Suzuki R, Kobayashi T, Ito H, Ikeda Y, Matsuzaki M, Hamano K. Cytokines produced by bone marrow cells can contribute to functional improvement of the infarcted heart by protecting cardiomyocytes from ischemic injury. American Journal of Physiology-Heart and Circulatory Physiology. 2006;291:H886–H893. doi: 10.1152/ajpheart.00142.2006. [DOI] [PubMed] [Google Scholar]
- 18.Pallante BA, Duignan I, Okin D, Chin A, Bressan MC, Mikawa T, Edelberg JM. Bone marrow Oct3/4+ cells differentiate into cardiac myocytes via age-dependent paracrine mechanisms. Circ Res. 2007;100:e1–11. doi: 10.1161/01.RES.0000253487.02398.85. [DOI] [PubMed] [Google Scholar]
- 19.Xie Z, Koyama T, Suzuki J, Fujii Y, Togashi H, Sawa H, Nagashima K. Coronary reperfusion following ischemia: different expression of bcl-2 and bax proteins, and cardiomyocyte apoptosis. Jpn Heart J. 2001;42:759–70. doi: 10.1536/jhj.42.759. [DOI] [PubMed] [Google Scholar]
- 20.Nishida S, Nagamine H, Tanaka Y, Watanabe G. Protective effect of basic fibroblast growth factor against myocyte death and arrhythmias in acute myocardial infarction in rats. Circulation Journal. 2003;67:334–339. doi: 10.1253/circj.67.334. [DOI] [PubMed] [Google Scholar]
- 21.Schinkothe T, Bloch W, Schmidt A. In vitro secreting profile of human mesenchymal stem cells. Stem Cells Dev. 2008;17:199–206. doi: 10.1089/scd.2007.0175. [DOI] [PubMed] [Google Scholar]
- 22.Schenke-Layland K, Strem BM, Jordan MC, Deemedio MT, Hedrick MH, Roos KP, Fraser JK, Maclellan WR. Adipose tissue-derived cells improve cardiac function following myocardial infarction. J Surg Res. 2009;153:217–23. doi: 10.1016/j.jss.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Estrada R, Li N, Sarojini H, An J, Lee MJ, Wang E. Secretome from mesenchymal stem cells induces angiogenesis via Cyr61. J Cell Physiol. 2009;219:563–71. doi: 10.1002/jcp.21701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD, DiCorleto PE, Topol EJ, Penn MS. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 25.Neuss S, Becher E, Woltje M, Tietze L, Jahnen-Dechent W. Functional expression of HGF and HGF receptor/c-met in adult human mesenchymal stem cells suggests a role in cell mobilization, tissue repair, and wound healing. Stem Cells. 2004;22:405–14. doi: 10.1634/stemcells.22-3-405. [DOI] [PubMed] [Google Scholar]
- 26.Mourkioti F, Rosenthal N. IGF-1, inflammation and stem cells: interactions during muscle regeneration. Trends Immunol. 2005;26:535–42. doi: 10.1016/j.it.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Cabrera-Fuentes HA, Aragones J, Bernhagen J, Boening A, Boisvert WA, Botker HE, Bulluck H, Cook S, Di Lisa F, Engel FB, Engelmann B, Ferrazzi F, Ferdinandy P, Fong A, Fleming I, Gnaiger E, Hernandez-Resendiz S, Kalkhoran SB, Kim MH, Lecour S, Liehn EA, Marber MS, Mayr M, Miura T, Ong SB, Peter K, Sedding D, Singh MK, Suleiman MS, Schnittler HJ, Schulz R, Shim W, Tello D, Vogel CW, Walker M, Li QOY, Yellon DM, Hausenloy DJ, Preissner KT. From basic mechanisms to clinical applications in heart protection, new players in cardiovascular diseases and cardiac theranostics: meeting report from the third international symposium on “New frontiers in cardiovascular research”. Basic Research in Cardiology. 2016;111(6):69. doi: 10.1007/s00395-016-0586-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu X, Xu Z, Xu Y, Cui G. Effects of mesenchymal stem cell transplantation on extracellular matrix after myocardial infarction in rats. Coron Artery Dis. 2005;16:245–55. doi: 10.1097/00019501-200506000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Ohnishi S, Yasuda T, Kitamura S, Nagaya N. Effect of hypoxia on gene expression of bone marrow-derived mesenchymal stem cells and mononuclear cells. Stem Cells. 2007;25:1166–77. doi: 10.1634/stemcells.2006-0347. [DOI] [PubMed] [Google Scholar]
- 30.Ohnishi S, Ohgushi H, Kitamura S, Nagaya N. Mesenchymal stem cells for the treatment of heart failure. International Journal of Hematology. 2007;86:17–21. doi: 10.1532/IJH97.07041. [DOI] [PubMed] [Google Scholar]
- 31.Nichols J, Smith A. Naive and Primed Pluripotent States. Cell Stem Cell. 2009;4:487–492. doi: 10.1016/j.stem.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 32.Kumari D. States of Pluripotency: Naïve and Primed Pluripotent Stem Cells. INTECH; 2016. ( ) [DOI] [Google Scholar]
- 33.Stadtfeld M, Hochedlinger K. Induced pluripotency: history, mechanisms, and applications. Genes & Development. 2010;24:2239–2263. doi: 10.1101/gad.1963910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo G, Yang J, Nichols J, Hall JS, Eyres I, Mansfield W, Smith A. Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development. 2009;136:1063–1069. doi: 10.1242/dev.030957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelaini S, Cochrane A, Margariti A. Direct reprogramming of adult cells: avoiding the pluripotent state. Stem Cells Cloning. 2014;7:19–29. doi: 10.2147/SCCAA.S38006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ebert AD, Diecke S, Chen IY, Wu JC. Reprogramming and transdifferentiation for cardiovascular development and regenerative medicine: where do we stand? Embo Molecular Medicine. 2015;7:1090–1103. doi: 10.15252/emmm.201504395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rufaihah AJ, Huang NF, Kim J, Herold J, Volz KS, Park TS, Lee JC, Zambidis ET, Reijo-Pera R, Cooke JP. Human induced pluripotent stem cell-derived endothelial cells exhibit functional heterogeneity. American Journal of Translational Research. 2013;5(1):21–35. [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang JH, Wilson GF, Soerens AG, Koonce CH, Yu JY, Palecek SP, Thomson JA, Kamp TJ. Functional Cardiomyocytes Derived From Human Induced Pluripotent Stem Cells. Circulation Research. 2009;104:E30–E41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jayawardena TM, Egemnazarov B, Finch EA, Zhang L, Payne JA, Pandya K, Zhang Z, Rosenberg P, Mirotsou M, Dzau VJ. MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circ Res. 2012;110:1465–73. doi: 10.1161/CIRCRESAHA.112.269035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nam YJ, Song K, Luo X, Daniel E, Lambeth K, West K, Hill JA, DiMaio JM, Baker LA, Bassel-Duby R, Olson EN. Reprogramming of human fibroblasts toward a cardiac fate. Proc Natl Acad Sci U S A. 2013;110:5588–93. doi: 10.1073/pnas.1301019110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Protze S, Khattak S, Poulet C, Lindemann D, Tanaka EM, Ravens U. A new approach to transcription factor screening for reprogramming of fibroblasts to cardiomyocyte-like cells. J Mol Cell Cardiol. 2012;53:323–32. doi: 10.1016/j.yjmcc.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 42.Wada R, Muraoka N, Inagawa K, Yamakawa H, Miyamoto K, Sadahiro T, Umei T, Kaneda R, Suzuki T, Kamiya K, Tohyama S, Yuasa S, Kokaji K, Aeba R, Yozu R, Yamagishi H, Kitamura T, Fukuda K, Ieda M. Induction of human cardiomyocyte-like cells from fibroblasts by defined factors. Proc Natl Acad Sci U S A. 2013;110:12667–72. doi: 10.1073/pnas.1304053110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fu JD, Stone NR, Liu L, Spencer CI, Qian L, Hayashi Y, Delgado-Olguin P, Ding S, Bruneau BG, Srivastava D. Direct reprogramming of human fibroblasts toward a cardiomyocyte-like state. Stem Cell Reports. 2013;1:235–47. doi: 10.1016/j.stemcr.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan B, Abdelli LS, Singla DK. Transplanted induced pluripotent stem cells improve cardiac function and induce neovascularization in the infarcted hearts of db/db mice. Mol Pharm. 2011;8:1602–10. doi: 10.1021/mp2003576. [DOI] [PubMed] [Google Scholar]
- 45.Mauritz C, Martens A, Rojas SV, Schnick T, Rathert C, Schecker N, Menke S, Glage S, Zweigerdt R, Haverich A, Martin U, Kutschka I. Induced pluripotent stem cell (iPSC)-derived Flk-1 progenitor cells engraft, differentiate, and improve heart function in a mouse model of acute myocardial infarction. European Heart Journal. 2011;32:2634–2641. doi: 10.1093/eurheartj/ehr166. [DOI] [PubMed] [Google Scholar]
- 46.Gutierrez-Aranda I, Ramos-Mejia V, Bueno C, Munoz-Lopez M, Real PJ, Macia A, Sanchez L, Ligero G, Garcia-Parez JL, Menendez P. Human induced pluripotent stem cells develop teratoma more efficiently and faster than human embryonic stem cells regardless the site of injection. Stem Cells. 2010;28:1568–70. doi: 10.1002/stem.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Canfield SG, Sepac A, Sedlic F, Muravyeva MY, Bai XW, Bosnjak ZJ. Marked Hyperglycemia Attenuates Anesthetic Preconditioning in Human-induced Pluripotent Stem Cell-derived Cardiomyocytes. Anesthesiology. 2012;117:735–744. doi: 10.1097/ALN.0b013e3182655e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mio Y, Bienengraeber MW, Marinovic J, Gutterman DD, Rakic M, Bosniak ZJ, Stadnicka A. Age-related attenuation of isoflurane preconditioning in human atrial cardiomyocytes - Roles for mitochondrial respiration and sarcolemmal adenosine triphosphate-sensitive potassium channel activity. Anesthesiology. 2008;108:612–620. doi: 10.1097/ALN.0b013e318167af2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sepac A, Sedlic F, Si-Tayeb K, Lough J, Duncan SA, Bienengraeber M, Park F, Kim J, Bosnjak ZJ. Isoflurane Preconditioning Elicits Competent Endogenous Mechanisms of Protection from Oxidative Stress in Cardiomyocytes Derived from Human Embryonic Stem Cells. Anesthesiology. 2010;113:906–916. doi: 10.1097/ALN.0b013e3181eff6b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Canfield SG, Zaja I, Godshaw B, Twaroski D, Bai X, Bosnjak ZJ. High Glucose Attenuates Anesthetic Cardioprotection in Stem-Cell-Derived Cardiomyocytes: The Role of Reactive Oxygen Species and Mitochondrial Fission. Anesth Analg. 2016;122:1269–79. doi: 10.1213/ANE.0000000000001254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Novalija E, Varadarajan SG, Camara AKS, An JZ, Chen Q, Riess ML, Hogg N, Stowe DF. Anesthetic preconditioning: triggering role of reactive oxygen and nitrogen species in isolated hearts. American Journal of Physiology-Heart and Circulatory Physiology. 2002;283:H44–H52. doi: 10.1152/ajpheart.01056.2001. [DOI] [PubMed] [Google Scholar]
- 52.Schuleri KH, Feigenbaum GS, Centola M, Weiss ES, Zimmet JM, Turney J, Kellner J, Zviman MM, Hatzistergos KE, Detrick B, Conte JV, McNiece I, Steenbergen C, Lardo AC, Hare JM. Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. European Heart Journal. 2009;30:2722–2732. doi: 10.1093/eurheartj/ehp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao YS, Li TL, Wei XF, Bianchi G, Hu JP, Sanchez PG, Xu K, Zhang P, Pittenger MF, Wu ZJJ, Griffith BP. Mesenchymal Stem Cell Transplantation Improves Regional Cardiac Remodeling Following Ovine Infarction. Stem Cells Translational Medicine. 2012;1(9):685–95. doi: 10.5966/sctm.2012-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suzuki G, Iyer V, Lee TC, Canty JM. Autologous Mesenchymal Stem Cells Mobilize cKit(+) and CD133(+) Bone Marrow Progenitor Cells and Improve Regional Function in Hibernating Myocardium. Circulation Research. 2011;109:1044–1054. doi: 10.1161/CIRCRESAHA.111.245969. [DOI] [PubMed] [Google Scholar]
- 55.Hu SS, Liu S, Zheng Z, Yuan X, Li LH, Lu MJ, Shen R, Duan FJ, Zhang XL, Li J, Liu XW, Song YH, Wang W, Zhao SH, He ZX, Zhang H, Yang KM, Feng W, Wang X. Isolated Coronary Artery Bypass Graft Combined With Bone Marrow Mononuclear Cells Delivered Through a Graft Vessel for Patients With Previous Myocardial Infarction and Chronic Heart Failure A Single-Center, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Journal of the American College of Cardiology. 2011;57:2409–2415. doi: 10.1016/j.jacc.2011.01.037. [DOI] [PubMed] [Google Scholar]
- 56.Stamm C, Kleine HD, Choi YH, Dunkelmann S, Lauffs JA, Lorenzen B, David A, Liebold A, Nienaber C, Zurakowski D, Freund M, Steinhoff G. Intramyocardial delivery of CD133(+) bone marrow cells and coronary artery bypass grafting for chronic ischemic heart disease: Safety and efficacy studies. Journal of Thoracic and Cardiovascular Surgery. 2007;133:717–25. doi: 10.1016/j.jtcvs.2006.08.077. [DOI] [PubMed] [Google Scholar]
- 57.Tokita Y, Tang XL, Li QH, Wysoczynski M, Hong KU, Nakamura S, Wu WJ, Xie W, Li D, Hunt G, Ou QH, Stowers H, Bolli R. Repeated Administrations of Cardiac Progenitor Cells Are Markedly More Effective Than a Single Administration: A New Paradigm in Cell Therapy. Circulation Research. 2016;119:635–651. doi: 10.1161/CIRCRESAHA.116.308937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guo YR, Wysoczynski M, Nong YB, Tomlin A, Zhu XP, Gumpert AM, Nasr M, Muthusamy S, Li H, Book M, Khan A, Hong KU, Li QH, Bolli R. Repeated doses of cardiac mesenchymal cells are therapeutically superior to a single dose in mice with old myocardial infarction. Basic Research in Cardiology. 2017;112(2):18. doi: 10.1007/s00395-017-0606-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karantalis V, DiFede DL, Gerstenblith G, Pham S, Symes J, Zambrano JP, Fishman J, Pattany P, McNiece I, Conte J, Schulman S, Wu K, Shah A, Breton E, Davis-Sproul J, Schwarz R, Feigenbaum G, Mushtaq M, Suncion VY, Lardo AC, Borrello I, Mendizabal A, Karas TZ, Byrnes J, Lowery M, Heldman AW, Hare JM. Autologous Mesenchymal Stem Cells Produce Concordant Improvements in Regional Function, Tissue Perfusion, and Fibrotic Burden When Administered to Patients Undergoing Coronary Artery Bypass Grafting. Circulation Research. 2014;114:1302–1310. doi: 10.1161/CIRCRESAHA.114.303180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mummery CL, Zhang JH, Ng ES, Elliott DA, Elefanty AG, Kamp TJ. Differentiation of Human Embryonic Stem Cells and Induced Pluripotent Stem Cells to Cardiomyocytes A Methods Overview. Circulation Research. 2012;111:344–358. doi: 10.1161/CIRCRESAHA.110.227512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shiba Y, Fernandes S, Zhu WZ, Filice D, Muskheli V, Kim J, Palpant NJ, Gantz J, Moyes KW, Reinecke H, Van Biber B, Dardas T, Mignone JL, Izawa A, Hanna R, Viswanathan M, Gold JD, Kotlikoff MI, Sarvazyan N, Kay MW, Murry CE, Laflamme MA. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature. 2012;489:322–5. doi: 10.1038/nature11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gokhale AG, Chelluri LK, Kumaresan K, Subramanyam G, Sudhakar K, Vemuri S, Debnath T, Ratnakar KS. Evaluation of the autologous bone marrow mononuclear therapy and functional restoration in the scarred myocardium by imaging analysis. J Cardiovasc Dis Res. 2011;2:133–6. doi: 10.4103/0975-3583.83037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zwetsloot PP, Vegh AM, Jansen of Lorkeers SJ, van Hout GP, Currie GL, Sena ES, Gremmels H, Buikema JW, Goumans MJ, Macleod MR, Doevendans PA, Chamuleau SA, Sluijter JP. Cardiac Stem Cell Treatment in Myocardial Infarction: A Systematic Review and Meta-Analysis of Preclinical Studies. Circ Res. 2016;118:1223–32. doi: 10.1161/CIRCRESAHA.115.307676. [DOI] [PubMed] [Google Scholar]
- 64.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–19. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Burchfield JS, Dimmeler S. Role of paracrine factors in stem and progenitor cell mediated cardiac repair and tissue fibrosis. Fibrogenesis Tissue Repair. 2008;1:4. doi: 10.1186/1755-1536-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tachibana A, Santoso MR, Mahmoudi M, Shukla P, Wang L, Bennett M, Goldstone AB, Wang M, Fukushi M, Ebert AD, Woo YJ, Rulifson E, Yang PC. Paracrine Effects of the Pluripotent Stem Cell-Derived Cardiac Myocytes Salvage the Injured Myocardium. Circ Res. 2017;121:e22–e36. doi: 10.1161/CIRCRESAHA.117.310803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Golpanian S, Schulman IH, Ebert RF, Heldman AW, DiFede DL, Yang PC, Wu JC, Bolli R, Perin EC, Moye L, Simari RD, Wolf A, Hare JM Cardiovascular Cell Therapy Research N. Concise Review: Review and Perspective of Cell Dosage and Routes of Administration From Preclinical and Clinical Studies of Stem Cell Therapy for Heart Disease. Stem Cells Transl Med. 2016;5:186–91. doi: 10.5966/sctm.2015-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bouchard A. Stem Cells in the Treatment of Heart Failure. MyHeartnet. 2017;1:4. ( https://myheart.net/articles/stem-cells-in-the-treatment-of-heart-failure) [Google Scholar]
- 69.Quyyumi AA, Vasquez A, Kereiakes DJ, Klapholz M, Schaer GL, Abdel-Latif A, Frohwein S, Henry TD, Schatz RA, Dib N, Toma C, Davidson CJ, Barsness GW, Shavelle DM, Cohen M, Poole J, Moss T, Hyde P, Kanakaraj AM, Druker V, Chung A, Junge C, Preti RA, Smith RL, Mazzo DJ, Pecora A, Losordo DW. PreSERVE-AMI: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial of Intracoronary Administration of Autologous CD34+ Cells in Patients With Left Ventricular Dysfunction Post STEMI. Circ Res. 2017;120:324–331. doi: 10.1161/CIRCRESAHA.115.308165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fisher SA, Zhang H, Doree C, Mathur A, Martin-Rendon E. Stem cell treatment for acute myocardial infarction. Cochrane Database Syst Rev. 2015:CD006536. doi: 10.1002/14651858.CD006536.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fisher SA, Doree C, Mathur A, Taggart DP, Martin-Rendon E. Stem cell therapy for chronic ischaemic heart disease and congestive heart failure. Cochrane Database Syst Rev. 2016;12:CD007888. doi: 10.1002/14651858.CD007888.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chugh AR, Beache GM, Loughran JH, Mewton N, Elmore JB, Kajstura J, Pappas P, Tatooles A, Stoddard MF, Lima JA, Slaughter MS, Anversa P, Bolli R. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation. 2012;126:S54–64. doi: 10.1161/CIRCULATIONAHA.112.092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Malliaras K, Makkar RR, Smith RR, Cheng K, Wu E, Bonow RO, Marban L, Mendizabal A, Cingolani E, Johnston PV, Gerstenblith G, Schuleri KH, Lardo AC, Marban E. Intracoronary cardiosphere-derived cells after myocardial infarction: evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction) J Am Coll Cardiol. 2014;63:110–22. doi: 10.1016/j.jacc.2013.08.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mummery CL, Stolpe Avd, Roelen BAJ, Clevers H. Stem cells: scientific facts and fiction. 2. London: Elsevier/AP, Academic Press Elsevier; 2014. [Google Scholar]
- 75.Thomas ED. A history of haemopoietic cell transplantation. British Journal of Haematology. 1999;105:330–339. doi: 10.1111/j.1365-2141.1999.01337.x. [DOI] [PubMed] [Google Scholar]
- 76.Bartolucci J, Verdugo FJ, Gonzalez PL, Larrea RE, Abarzua E, Goset C, Rojo P, Palma I, Lamich R, Pedreros PA, Valdivia G, Lopez VM, Nazzal C, Alcayaga-Miranda F, Cuenca J, Brobeck MJ, Patel AN, Figueroa FE, Khoury M. Safety and Efficacy of the Intravenous Infusion of Umbilical Cord Mesenchymal Stem Cells in Patients With Heart Failure: A Phase 1/2 Randomized Controlled Trial (RIMECARD Trial [Randomized Clinical Trial of Intravenous Infusion Umbilical Cord Mesenchymal Stem Cells on Cardiopathy]) Circ Res. 2017;121:1192–1204. doi: 10.1161/CIRCRESAHA.117.310712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Van Besien K, Liu H, Jain N, Stock W, Artz A. Umbilical cord blood transplantation supported by third-party donor cells: rationale, results, and applications. Biol Blood Marrow Transplant. 2013;19:682–91. doi: 10.1016/j.bbmt.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Memon IA, Sawa Y, Fukushima N, Matsumiya G, Miyagawa S, Taketani S, Sakakida SK, Kondoh H, Aleshin AN, Shimizu T, Okano T, Matsuda H. Repair of impaired myocardium by means of implantation of engineered autologous myoblast sheets. Journal of Thoracic and Cardiovascular Surgery. 2005;130:1333–1341. doi: 10.1016/j.jtcvs.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 79.Hata H, Matsumiya G, Miyagawa S, Kondoh H, Kawaguchi N, Matsuura N, Shimizu T, Okano T, Matsuda H, Sawa Y. Grafted skeletal myoblast sheets attenuate myocardial remodeling in pacing-induced canine heart failure model. J Thorac Cardiovasc Surg. 2006;132:918–24. doi: 10.1016/j.jtcvs.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 80.Siepe M, Giraud MN, Pavlovic M, Receputo C, Beyersdorf F, Menasche P, Carrel T, Tevaearai HT. Myoblast-seeded biodegradable scaffolds to prevent post-myocardial infarction evolution toward heart failure. J Thorac Cardiovasc Surg. 2006;132:124–31. doi: 10.1016/j.jtcvs.2006.01.052. [DOI] [PubMed] [Google Scholar]
- 81.Giraud MN, Ayuni E, Cook S, Siepe M, Carrel TP, Tevaearai HT. Hydrogel-based engineered skeletal muscle grafts normalize heart function early after myocardial infarction. Artif Organs. 2008;32:692–700. doi: 10.1111/j.1525-1594.2008.00595.x. [DOI] [PubMed] [Google Scholar]
- 82.Chen CH, Wei HJ, Lin WW, Chiu I, Hwang SM, Wang CC, Lee WY, Chang Y, Sung HW. Porous tissue grafts sandwiched with multilayered mesenchymal stromal cell sheets induce tissue regeneration for cardiac repair. Cardiovasc Res. 2008;80:88–95. doi: 10.1093/cvr/cvn149. [DOI] [PubMed] [Google Scholar]
- 83.Potapova IA, Doronin SV, Kelly DJ, Rosen AB, Schuldt AJ, Lu Z, Kochupura PV, Robinson RB, Rosen MR, Brink PR, Gaudette GR, Cohen IS. Enhanced recovery of mechanical function in the canine heart by seeding an extracellular matrix patch with mesenchymal stem cells committed to a cardiac lineage. Am J Physiol Heart Circ Physiol. 2008;295:H2257–63. doi: 10.1152/ajpheart.00219.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Derval N, Barandon L, Dufourcq P, Leroux L, Lamaziere JM, Daret D, Couffinhal T, Duplaa C. Epicardial deposition of endothelial progenitor and mesenchymal stem cells in a coated muscle patch after myocardial infarction in a murine model. Eur J Cardiothorac Surg. 2008;34:248–54. doi: 10.1016/j.ejcts.2008.03.058. [DOI] [PubMed] [Google Scholar]
- 85.Bursac N. Cardiac tissue engineering using stem cells. IEEE Eng Med Biol Mag. 2009;28:80, 82, 84–6, 88–9. doi: 10.1109/MEMB.2009.931792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kondoh H, Sawa Y, Miyagawa S, Sakakida-Kitagawa S, Memon MA, Kawaguchi N, Matsuura N, Shimizu T, Okano T, Matsuda H. Longer preservation of cardiac performance by sheet-shaped myoblast implantation in dilated cardiomyopathic hamsters. Cardiovascular Research. 2006;69:466–475. doi: 10.1016/j.cardiores.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 87.Davis ME, Hsieh PC, Takahashi T, Song Q, Zhang S, Kamm RD, Grodzinsky AJ, Anversa P, Lee RT. Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proc Natl Acad Sci U S A. 2006;103:8155–60. doi: 10.1073/pnas.0602877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zimmermann WH, Didie M, Doker S, Melnychenko I, Naito H, Rogge C, Tiburcy M, Eschenhagen T. Heart muscle engineering: An update on cardiac muscle replacement therapy. Cardiovascular Research. 2006;71:419–429. doi: 10.1016/j.cardiores.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 89.Nguyen PK, Neofytou E, Rhee JW, Wu JC. Potential Strategies to Address the Major Clinical Barriers Facing Stem Cell Regenerative Therapy for Cardiovascular Disease: A Review. JAMA Cardiol. 2016;1:953–962. doi: 10.1001/jamacardio.2016.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Luger D, Lipinski MJ, Westman PC, Glover DK, Dimastromatteo J, Frias JC, Albelda MT, Sikora S, Kharazi A, Vertelov G, Waksman R, Epstein SE. Intravenously Delivered Mesenchymal Stem Cells: Systemic Anti-Inflammatory Effects Improve Left Ventricular Dysfunction in Acute Myocardial Infarction and Ischemic Cardiomyopathy. Circ Res. 2017;120:1598–1613. doi: 10.1161/CIRCRESAHA.117.310599. [DOI] [PubMed] [Google Scholar]
- 91.Kawamura M, Paulsen MJ, Goldstone AB, Shudo Y, Wang H, Steele AN, Stapleton LM, Edwards BB, Eskandari A, Truong VN, Jaatinen KJ, Ingason AB, Miyagawa S, Sawa Y, Woo YJ. Tissue-engineered smooth muscle cell and endothelial progenitor cell bi-level cell sheets prevent progression of cardiac dysfunction, microvascular dysfunction, and interstitial fibrosis in a rodent model of type 1 diabetes-induced cardiomyopathy. Cardiovasc Diabetol. 2017;16:142. doi: 10.1186/s12933-017-0625-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shimko VF, Claycomb WC. Effect of mechanical loading on three-dimensional cultures of embryonic stem cell-derived cardiomyocytes. Tissue Eng Part A. 2008;14:49–58. doi: 10.1089/ten.2007.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nummi A, Nieminen T, Patila T, Lampinen M, Lehtinen ML, Kivisto S, Holmstrom M, Wilkman E, Teittinen K, Laine M, Sinisalo J, Kupari M, Kankuri E, Juvonen T, Vento A, Suojaranta R, Harjula A consortium A. Epicardial delivery of autologous atrial appendage micrografts during coronary artery bypass surgery-safety and feasibility study. Pilot Feasibility Stud. 2017;3:74. doi: 10.1186/s40814-017-0217-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Menasche P, Alfieri O, Janssens S, McKenna W, Reichenspurner H, Trinquart L, Vilquin JT, Marolleau JP, Seymour B, Larghero J, Lake S, Chatellier G, Solomon S, Desnos M, Hagege AA. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117:1189–200. doi: 10.1161/CIRCULATIONAHA.107.734103. [DOI] [PubMed] [Google Scholar]
- 95.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O’Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–24. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 96.Zhang M, Methot D, Poppa V, Fujio Y, Walsh K, Murry CE. Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J Mol Cell Cardiol. 2001;33:907–21. doi: 10.1006/jmcc.2001.1367. [DOI] [PubMed] [Google Scholar]
- 97.Eschenhagen T, Zimmermann WH. Engineering myocardial tissue. Circ Res. 2005;97:1220–31. doi: 10.1161/01.RES.0000196562.73231.7d. [DOI] [PubMed] [Google Scholar]
- 98.Sekine H, Shimizu T, Dobashi I, Matsuura K, Hagiwara N, Takahashi M, Kobayashi E, Yamato M, Okano T. Cardiac cell sheet transplantation improves damaged heart function via superior cell survival in comparison with dissociated cell injection. Tissue Eng Part A. 2011;17:2973–80. doi: 10.1089/ten.tea.2010.0659. [DOI] [PubMed] [Google Scholar]
- 99.Kishore R, Khan M. Cardiac cell-derived exosomes: changing face of regenerative biology. Eur Heart J. 2017;38:212–215. doi: 10.1093/eurheartj/ehw324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Davidson SM, Takov K, Yellon DM. Exosomes and Cardiovascular Protection. Cardiovasc Drugs Ther. 2017;31:77–86. doi: 10.1007/s10557-016-6698-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Alcayaga-Miranda F, Varas-Godoy M, Khoury M. Harnessing the Angiogenic Potential of Stem Cell-Derived Exosomes for Vascular Regeneration. Stem Cells Int. 2016;2016:3409169. doi: 10.1155/2016/3409169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature Cell Biology. 2007;9:654–9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 103.Barile L, Lionetti V, Cervio E, Matteucci M, Gherghiceanu M, Popescu LM, Torre T, Siclari F, Moccetti T, Vassalli G. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovascular Research. 2014;103:530–541. doi: 10.1093/cvr/cvu167. [DOI] [PubMed] [Google Scholar]
- 104.Gray WD, French KM, Ghosh-Choudhary S, Maxwell JT, Brown ME, Platt MO, Searles CD, Davis ME. Identification of Therapeutic Covariant MicroRNA Clusters in Hypoxia-Treated Cardiac Progenitor Cell Exosomes Using Systems Biology. Circulation Research. 2015;116:255–63. doi: 10.1161/CIRCRESAHA.116.304360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Giricz Z, Varga ZV, Baranyai T, Sipos P, Paloczi K, Kittel A, Buzas EI, Ferdinandy P. Cardioprotection by remote ischemic preconditioning of the rat heart is mediated by extracellular vesicles. Journal of Molecular and Cellular Cardiology. 2014;68:75–78. doi: 10.1016/j.yjmcc.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 106.Li J, Rohailla S, Gelber N, Rutka J, Sabah N, Gladstone RA, Wei C, Hu PZ, Kharbanda RK, Redington AN. MicroRNA-144 is a circulating effector of remote ischemic preconditioning. Basic Research in Cardiology. 2014;109(5):423. doi: 10.1007/s00395-014-0423-z. [DOI] [PubMed] [Google Scholar]
- 107.Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor ENE, Timmers L, van Rijen HV, Doevendans PA, Pasterkamp G, Lim SK, de Kleijn DP. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Research. 2013;10:301–312. doi: 10.1016/j.scr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 108.Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, Kalantzi M, Herbots L, Sinnaeve P, Dens J, Maertens J, Rademakers F, Dymarkowski S, Gheysens O, Van Cleemput J, Bormans G, Nuyts J, Belmans A, Mortelmans L, Boogaerts M, Van de Werf F. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367:113–21. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 109.Meyer GP, Wollert KC, Lotz J, Pirr J, Rager U, Lippolt P, Hahn A, Fichtner S, Schaefer A, Arseniev L, Ganser A, Drexler H. Intracoronary bone marrow cell transfer after myocardial infarction: 5-year follow-up from the randomized-controlled BOOST trial. Eur Heart J. 2009;30:2978–84. doi: 10.1093/eurheartj/ehp374. [DOI] [PubMed] [Google Scholar]
- 110.Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, Endresen K, Ilebekk A, Mangschau A, Fjeld JG, Smith HJ, Taraldsrud E, Grogaard HK, Bjornerheim R, Brekke M, Muller C, Hopp E, Ragnarsson A, Brinchmann JE, Forfang K. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199–209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 111.Tendera M, Wojakowski W, Ruzyllo W, Chojnowska L, Kepka C, Tracz W, Musialek P, Piwowarska W, Nessler J, Buszman P, Grajek S, Breborowicz P, Majka M, Ratajczak MZ Investigators R. Intracoronary infusion of bone marrow-derived selected CD34+CXCR4+ cells and non-selected mononuclear cells in patients with acute STEMI and reduced left ventricular ejection fraction: results of randomized, multicentre Myocardial Regeneration by Intracoronary Infusion of Selected Population of Stem Cells in Acute Myocardial Infarction (REGENT) Trial. Eur Heart J. 2009;30:1313–21. doi: 10.1093/eurheartj/ehp073. [DOI] [PubMed] [Google Scholar]
- 112.Assmus B, Rolf A, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Tillmanns H, Yu J, Corti R, Mathey DG, Hamm CW, Suselbeck T, Tonn T, Dimmeler S, Dill T, Zeiher AM, Schachinger V Investigators R-A. Clinical outcome 2 years after intracoronary administration of bone marrow-derived progenitor cells in acute myocardial infarction. Circ Heart Fail. 2010;3:89–96. doi: 10.1161/CIRCHEARTFAILURE.108.843243. [DOI] [PubMed] [Google Scholar]
- 113.Perin EC, Willerson JT, Pepine CJ, Henry TD, Ellis SG, Zhao DX, Silva GV, Lai D, Thomas JD, Kronenberg MW, Martin AD, Anderson RD, Traverse JH, Penn MS, Anwaruddin S, Hatzopoulos AK, Gee AP, Taylor DA, Cogle CR, Smith D, Westbrook L, Chen J, Handberg E, Olson RE, Geither C, Bowman S, Francescon J, Baraniuk S, Piller LB, Simpson LM, Loghin C, Aguilar D, Richman S, Zierold C, Bettencourt J, Sayre SL, Vojvodic RW, Skarlatos SI, Gordon DJ, Ebert RF, Kwak M, Moye LA, Simari RD Cardiovascular Cell Therapy Research N. Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: the FOCUS-CCTRN trial. JAMA. 2012;307:1717–26. doi: 10.1001/jama.2012.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, Breton E, Davis-Sproul J, Schulman IH, Byrnes J, Mendizabal AM, Lowery MH, Rouy D, Altman P, Wong Po Foo C, Ruiz P, Amador A, Da Silva J, McNiece IK, Heldman AW, George R, Lardo A. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308:2369–79. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Leistner DM, Fischer-Rasokat U, Honold J, Seeger FH, Schachinger V, Lehmann R, Martin H, Burck I, Urbich C, Dimmeler S, Zeiher AM, Assmus B. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI): final 5-year results suggest long-term safety and efficacy. Clin Res Cardiol. 2011;100:925–34. doi: 10.1007/s00392-011-0327-y. [DOI] [PubMed] [Google Scholar]
- 116.Heldman AW, DiFede DL, Fishman JE, Zambrano JP, Trachtenberg BH, Karantalis V, Mushtaq M, Williams AR, Suncion VY, McNiece IK, Ghersin E, Soto V, Lopera G, Miki R, Willens H, Hendel R, Mitrani R, Pattany P, Feigenbaum G, Oskouei B, Byrnes J, Lowery MH, Sierra J, Pujol MV, Delgado C, Gonzalez PJ, Rodriguez JE, Bagno LL, Rouy D, Altman P, Foo CW, da Silva J, Anderson E, Schwarz R, Mendizabal A, Hare JM. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. JAMA. 2014;311:62–73. doi: 10.1001/jama.2013.282909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mathiasen AB, Qayyum AA, Jorgensen E, Helqvist S, Fischer-Nielsen A, Kofoed KF, Haack-Sorensen M, Ekblond A, Kastrup J. Bone marrow-derived mesenchymal stromal cell treatment in patients with severe ischaemic heart failure: a randomized placebo-controlled trial (MSC-HF trial) Eur Heart J. 2015;36:1744–53. doi: 10.1093/eurheartj/ehv136. [DOI] [PubMed] [Google Scholar]
- 118.Guijarro D, Lebrin M, Lairez O, Bourin P, Piriou N, Pozzo J, Lande G, Berry M, Le Tourneau T, Cussac D, Sensebe L, Gross F, Lamirault G, Huynh A, Manrique A, Ruidavet JB, Elbaz M, Trochu JN, Parini A, Kramer S, Galinier M, Lemarchand P, Roncalli J. Intramyocardial transplantation of mesenchymal stromal cells for chronic myocardial ischemia and impaired left ventricular function: Results of the MESAMI 1 pilot trial. Int J Cardiol. 2016;209:258–65. doi: 10.1016/j.ijcard.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 119.Surder D, Manka R, Lo Cicero V, Moccetti T, Rufibach K, Soncin S, Turchetto L, Radrizzani M, Astori G, Schwitter J, Erne P, Zuber M, Auf der Maur C, Jamshidi P, Gaemperli O, Windecker S, Moschovitis A, Wahl A, Buhler I, Wyss C, Kozerke S, Landmesser U, Luscher TF, Corti R. Intracoronary injection of bone marrow-derived mononuclear cells early or late after acute myocardial infarction: effects on global left ventricular function. Circulation. 2013;127:1968–79. doi: 10.1161/CIRCULATIONAHA.112.001035. [DOI] [PubMed] [Google Scholar]
- 120.Choudry F, Hamshere S, Saunders N, Veerapen J, Bavnbek K, Knight C, Pellerin D, Locca D, Westwood M, Rakhit R, Crake T, Kastrup J, Parmar M, Agrawal S, Jones D, Martin J, Mathur A. A randomized double-blind control study of early intra-coronary autologous bone marrow cell infusion in acute myocardial infarction: the REGENERATE-AMI clinical trialdagger. Eur Heart J. 2016;37:256–63. doi: 10.1093/eurheartj/ehv493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nicolau JC, Furtado RHM, Silva SA, Rochitte CE, Rassi A, Jr, Moraes J, Jr, Quintella E, Costantini CR, Korman APM, Mattos MA, Castello HJ, Jr, Caixeta A, Dohmann HFR, de Carvalho ACC MiHeart AMII. Stem-cell therapy in ST-segment elevation myocardial infarction with reduced ejection fraction: A multicenter, double-blind randomized trial. Clin Cardiol. 2018;41(3):392–99. doi: 10.1002/clc.22882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Traverse JH, Henry TD, Pepine CJ, Willerson JT, Chugh A, Yang PC, Zhao DXM, Ellis SG, Forder JR, Perin EC, Penn MS, Hatzopoulos AK, Chambers JC, Baran KW, Raveendran G, Gee AP, Taylor DA, Moye L, Ebert RF, Simari RD. TIME Trial: Effect of Timing of Stem Cell Delivery Following ST-Elevation Myocardial Infarction on the Recovery of Global and Regional Left Ventricular Function: Final 2-Year Analysis. Circ Res. 2018;122:479–488. doi: 10.1161/CIRCRESAHA.117.311466. [DOI] [PMC free article] [PubMed] [Google Scholar]