Abstract
Acute kidney injury (AKI) is a critical determinant of outcomes in hospitalized patients with cirrhosis, but little is known of the impact of AKI in the outpatient setting. We analyzed 385 adult outpatients with cirrhosis listed for liver transplant at a single center; excluded were those with severe hepatic encephalopathy, hepatocellular carcinoma or on hemodialysis. Baseline serum creatinine (bCr) was defined as the lowest value recorded; peak creatinine (pCr) as the highest value; delta creatinine (ΔCr) as pCr minus bCr; AKI as a rise in sCR by ≥0.3 mg/dl from bCr; persistent kidney injury as elevation of sCR by ≥0.3 mg/dl from bCr on each subsequent clinical assessment. Among 385 outpatients with cirrhosis, bCr was ≤0.70, 0.70 – 0.97, and ≥0.97 mg/dL in 28%, 38%, and 34%, respectively. At a median follow-up of 16 (8 – 28) months, 143 (37%) had ≥1 AKI episode, which increased significantly by bCr group (24v.37v.48%, p=0.001). Of these 143 with AKI, 13% developed persistent kidney injury. A multivariable cox-regression analysis highlighted that bCr (HR 2.96) and ΔCr (HR 2.05) were the only factors independently associated with the development of persistent kidney injury [p<0.001]. The likelihood of death/delisting increased by bCr group (14v.19v.28%, p=0.03). A competing risk analysis demonstrated that each 1 mg/dL increase in bCr was independently associated with a 62% higher risk of death/delisting when accounting for transplantation and adjusting for confounders. In conclusion, not only is AKI common in outpatients with cirrhosis, but even “clinically normal” bCr levels significantly impact the risk of persistent kidney injury and waitlist mortality, supporting the need for a lower clinical threshold to initiate monitoring of renal function and implementation of renal-protective strategies.
Keywords: Renal Dysfunction, Acute Kidney Injury, Renal Recovery, Liver Disease, Waitlist Mortality, Liver Transplantation
INTRODUCTION
Renal dysfunction is a critical determinant of outcomes in patients with cirrhosis (1,2). It results from a broad spectrum of pathologies including functional etiologies (transient injury from alterations in perfusion) and structural causes (irreversible damage to the renal parenchyma) (3). Regardless of the cause, the development of acute kidney injury (AKI) has a profound impact on survival (4–7). This is particularly true in those with AKI who then progress to persistent kidney injury, where the 30-day mortality rate is nearly 10-fold higher (4,5,8). These findings highlight the importance of identifying the predictors of AKI, as it is a potentially preventable and reversible condition (9–11).
To date, most studies evaluating AKI in patients with cirrhosis have included only those who are hospitalized (5,12–16). While AKI is a critical determinant of short-term mortality in the inpatient setting, this setting limits the opportunity to implement strategies to prevent progression to persistent kidney injury. The one study that has focused on AKI in non-hospitalized patients with cirrhosis reported an association of AKI and mortality, but this was an observational study with only 90 patients and was under-powered to identify predictors of the development and progression of AKI (17).
To address this knowledge gap, we utilized a well-characterized prospective cohort of outpatients with cirrhosis awaiting liver transplantation to describe the prevalence of AKI, predictors of its persistence, and its impact on survival.
METHODS
Patients
We analyzed data from patients listed for liver transplantation at the University of California – San Francisco (UCSF) from March 2012 until December 2016. These patients were enrolled as part of a prospective study of adults (≥18 years) with cirrhosis who were seen as outpatients in the UCSF Liver Transplant Clinics (18,19). Patients with severe hepatic encephalopathy, as defined by the time to complete a Numbers Connection Test (20) of >120 seconds, were excluded because this may impair the patient’s ability to provide informed consent. For the purposes of this specific study, patients with hepatocellular carcinoma (HCC) were excluded, as these patients receive transplant at a rate that is not dependent upon their laboratory MELD score. We excluded patients on hemodialysis, as serum creatinine does not reflect renal function in this setting.
Comorbidities and Medications
We defined refractory ascites as fluid overload that was either unresponsive to sodium-restricted diet and high dose diuretic treatment, or recurred rapidly after therapeutic paracentesis and developed anytime during follow up (21). At the time of study enrollment, medical co-morbidities (e.g. hypertension and diabetes) were determined from the patient’s electronic health record, while hepatic encephalopathy was classified as none/mild versus moderate based on the patient’s performance on the Numbers Connection Test Score of < or >60 sec, respectively (22). The dose of loop and potassium-sparing diuretics were determined at the time of study enrollment. For the analysis, all doses of either the loop or potassium-sparing diuretic were converted to equivalent doses of furosemide and spironolactone, respectively.
Definition of Creatinine Values, Acute Kidney Injury, and Persistent Kidney Injury
Once enrolled, longitudinal data collection occurred at each subsequent clinic visit. Timing of clinical assessments was determined as clinically indicated by the treating physician. Data included demographics, vitals, and laboratory values drawn as part of clinical care in the outpatient setting. Baseline serum creatinine (bCr) was defined as the lowest value recorded during follow up. This was chosen because it allowed for fluctuations in serum creatinine typically seen while patients are awaiting liver transplantation. Patients were categorized by tertiles of bCr in our cohort using the creatinine cut-offs of <0.70, 0.70 – 0.97, ≥0.97 mg/dL. The most recent diagnostic criteria established by the International Club of Ascites were used for the following definitions (15,23):
Peak creatinine (pCr): the highest creatinine measured during follow up;
Delta creatinine (ΔCr): pCr minus bCr;
AKI: a rise in serum creatinine (sCr) by ≥0.3 mg/dl or >50% from baseline;
Persistent kidney injury: elevation of sCR by ≥0.3 mg/dl from baseline on each subsequent clinical assessment;
Transient AKI: return in sCr to within 0.3 mg/dL from baseline at any point during follow up
We defined AKI, as any qualifying change in sCr, regardless of time. Episodes of AKI were categorized by International Club of Ascites AKI stage (15). There are no clear consensus guidelines on the optimal estimate of glomerular filtration rates (eGFR) in patients with cirrhosis. Given that blood urea nitrogen levels – which are necessary for the Modification of Diet in Renal Disease Study (MDRD)-6 estimation – are not included in the MELDNa score and therefore not routinely drawn on all patients with every blood draw (only 31% had baseline blood urea nitrogen levels in our cohort), we calculated eGFR using both the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) and MDRD-4 formulas (24,25).
Statistical Analysis
Categorical data are presented as percentages and were compared between groups by chi-square test. Continuous variables are presented as medians with respective interquartile ranges (IQR) and were compared between groups by Wilcoxon Rank-Sum or Kruskall Wallis tests, as appropriate.
The primary outcome was persistent kidney injury. The secondary outcome was waitlist mortality defined as death on the waitlist or delisting for sickness. Unadjusted models assessed the association of all listed covariates with persistent kidney injury and waitlist mortality. All covariates with a p<0.2 in univariate analysis were considered for inclusion in multivariate models. Those not reaching significance of p<0.05 were sequentially eliminated (backwards selection). Cox regression assessed the risk of AKI occurrence and AKI persistence. Survival was compared between bCr groups using the log-rank test. A competing risks analysis assessed the association between kidney injury and other covariates with waitlist mortality, with liver transplantation as the competing risk (26). We censored patients who were removed from the waitlist for “social” reasons on the date of their removal, because these patients do not have the same risk for waitlist mortality or liver transplantation. We censored those who remained alive on the waitlist at the end of follow-up, because they remained at risk for transplant or waitlist mortality. We completed all analyses with both sCR and eGFR to ensure that there were no differences in the findings.
Two-sided p-values <0.05 were considered statistically significant. Analyses were performed using Stata 15.0 statistical software (College Station, TX). This study was approved by the institutional review board at the University of California, San Francisco.
RESULTS
Baseline characteristics of the entire cohort
A total of 385 patients with cirrhosis were followed for a median of 1.3 (0.67 – 2.3) years, with a median of 3 (2 – 4) clinical assessments. Baseline characteristics are shown in Table 1. Women comprised 42% of the cohort, 63% were Caucasian, and 40% were listed with cirrhosis secondary to chronic hepatitis C (HCV). Median age was 58 (50 – 63) years. Rates of diabetes and hypertension were 24 and 34%, respectively. Eighteen percent had refractory ascites and 18% had any hepatic encephalopathy. Median MELDNa score was 18 (15 – 22) and median Child-Pugh score (CPS) was 8 (7 – 9). Median bCr for the entire cohort was 0.80 mg/dL (0.68 – 1.04), occurring at the first clinical assessment in 167 (43%) patients.
Table 1. Baseline Demographics.
| Total | <0.7 mg/dL | Baseline Creatinine 0.70 – 0.97 mg/dL |
>0.97 mg/dL | P Value | |
|---|---|---|---|---|---|
| N (%) | 385 | 108 (28) | 148 (38) | 129 (34) | - |
|
| |||||
| Age (Years) | 58 (50 – 63) | 54 (47 – 60) | 59 (52 – 63) | 60 (53 – 64) | <0.001 |
|
| |||||
| Female (%) | 160 (42) | 57 (53) | 58 (39) | 45 (35) | 0.02 |
|
| |||||
| Race (%) | |||||
| Caucasian | 242 (63) | 54 (50) | 110 (74) | 78 (60) | <0.001 |
| African American | 13 (3) | 0 (0) | 7 (5) | 5 (4) | 0.08 |
| Hispanic | 94 (24) | 40 (37) | 21 (14) | 33 (26) | <0.001 |
| Asian | 13 (3) | 3 (3) | 6 (4) | 4 (3) | 0.84 |
| Others | 24 (6) | 11 (10) | 4 (3) | 9 (7) | 0.05 |
|
| |||||
| Etiology (%) | |||||
| HCV | 153 (40) | 42 (39) | 61 (41) | 50 (39) | 0.90 |
| Alcoholic Cirrhosis | 122 (32) | 23 (21) | 46 (31) | 53 (41) | 0.01 |
| NASH | 49 (13) | 9 (8) | 18 (12) | 22 (17) | 0.13 |
| Autoimmune* | 63 (16) | 24 (22) | 24 (16) | 15 (12) | 0.09 |
| HBV | 8 (2) | 3 (3) | 2 (1) | 3 (2) | 0.71 |
| Other | 40 (10) | 19 (18) | 16 (11) | 5 (4) | 0.003 |
|
| |||||
| Diabetes Mellitus (%) | 94 (24) | 19 (18) | 38 (26) | 37 (29) | 0.13 |
|
| |||||
| Hypertension (%) | 132 (34) | 28 (26) | 43 (29) | 61 (47) | 0.001 |
|
| |||||
| BMI kg/m2 (IQR) | 28.3 (25.1 – 33.1) | 27.5 (25.1 – 31.8) | 27.9 (25.2 – 32.8) | 28.8 (25.0 – 35.2) | 0.35 |
|
| |||||
| Refractory Ascites (%) | 69 (18) | 11 (10) | 24 (16) | 34 (26) | 0.005 |
| Any Hepatic Encephalopathy (%) | 70 (18) | 19 (18) | 27 (18) | 24 (18) | 0.99 |
|
| |||||
| Serum Na mmol/l (IQR) | 136 (134 – 139) | 136 (134 – 139) | 136 (133 – 138) | 136 (134 – 140) | 0.74 |
|
| |||||
| Albumin mg/dL (IQR) | 3.1 (2.6 – 3.5) | 3.00 (2.70 – 3.45) | 3.10 (2.50 – 3.50) | 3.20 (2.65 – 3.60) | 0.37 |
|
| |||||
| Baseline Creatinine mg/dL (IQR) | 0.80 (0.68 – 1.04) | 0.60 (0.51 – 0.63) | 0.8 (0.75 – 0.88) | 1.19 (1.04 – 1.30) | <0.001 |
|
| |||||
| MDRD 4-variable eGFR (ml/min/1.73 m2) | 75.5 (55.2 – 98.8) | 109.1 (91.2- 134.1) | 78.9 (66.5 – 91.6) | 51.2 (38.0 – 60.6) | <0.001 |
|
| |||||
| CKD-EPI eGFR (ml/min/1.73 m2) | 81.1 (58.3 – 99.0) | 104.6 (96.4 – 112.8) | 85.5 (71.6 – 97.1) | 55.0 (41.0 – 63.5) | <0.001 |
|
| |||||
| INR (IQR) | 1.4 (1.2 – 1.6) | 1.4 (1.2 – 1.6) | 1.4 (1.3 – 1.7) | 1.4 (1.2 – 1.6) | 0.31 |
|
| |||||
| Equivalent dose of Furosemide mg (IQR) | 20 (0 – 40) | 20 (0 – 40) | 20 (0 – 60) | 40 (0 – 40) | 0.049 |
|
| |||||
| Equivalent dose of Spironolactone mg (IQR) | 50 (0 – 100) | 25 (0 – 75) | 50 (0 – 100) | 50 (0 – 100) | 0.10 |
|
| |||||
| Total Bilirubin mg/dL (IQR) | 2.4 (1.7 – 3.9) | 2.8 (2.0 – 3.8) | 2.6 (1.8 – 4.5) | 2.0 (1.3 – 3.4) | <0.001 |
|
| |||||
| Albumin g/dL (IQR) | 3.1 (2.6 – 3.6) | 3.0 (2.7 – 3.5) | 3.1 (2.5 – 3.5) | 3.2 (2.7 – 3.6) | 0.37 |
|
| |||||
| Child-Pugh Score (IQR) | 8 (7 – 9) | 8 (7 – 9) | 8 (7 – 9) | 8 (7 – 9) | 0.57 |
|
| |||||
| MELDNa (IQR) | 18 (15 – 22) | 17 (15 – 21) | 19 (14 – 21) | 19 (16 – 23) | 0.009 |
Combined Autoimmune Hepatitis, Primary Sclerosing Cholangitis, and Primary Biliary Cholangitis; Hepatitis C (HCV); Non-Alcoholic Steatohepatitis (NASH); Hepatitis B (HBV); Sodium (Na); Modification of Diet in Renal Disease (MDRD); Estimated Glomerular Filtration Rate (eGFR); International Normalized Ratio (INR); Model for End-Stage Liver Disease with Serum Sodium (MELDNa)
Comparison of baseline characteristics by creatinine status
Categorizing patients by their bCr, 108 (28%) patients had a bCr of ≤0.70 mg/dL, 148 (38%) patients had a bCr of 0.70 – 0.97 mg/dL, and 129 (34%) patients had a bCr of ≥0.97 mg/dL. Demographics, clinical parameters, and laboratory findings for each of these three groups are listed in Table 1. Compared to the higher bCr groups, patients in the lowest bCr group were younger (54 v. 59 v. 60 years, p<0.001) and more likely to be female (53 v. 39 v. 35%, p=0.02) or Hispanic (37 v. 14 v. 26%, p<0.001). Those in the lowest bCr group were less likely to have alcoholic liver disease (21 v. 31 v. 41%, p=0.01), hypertension (26 v. 29 v. 47%, p=0.001), refractory ascites (10 v. 16 v. 26%, p<0.001), and be on any diuretics (67 v. 82 v. 85%, p=0.003). Those in the lowest bCr group had higher total bilirubin levels (2.8 v. 2.6 v. 2.0 mg/dL, p<0.001), but lower MELDNa scores (17 v. 19 v. 19, p=0.009). There were no significant differences in % with any hepatic encephalopathy (18 v. 18 v. 18%), albumin levels (3.0 v. 3.1 v. 3.2 g/dL) or CPS (8 v. 8 v. 8) [p>0.05 for each].
Incidence of and Risk Factors for Acute Kidney Injury
Using the International Club of Ascites staging criteria of AKI (15), 143 (37%) patients had at least one episode of AKI while on the liver transplantation waitlist. An episode of AKI was identified in all bCr groups, increasing significantly from the lowest to the highest bCr group (24 v. 37 v. 48%, p=0.001). Of these episodes of AKI, 117 (82%) were defined as AKI stage 1, 18 (13%) were AKI stage 2, and 8 (6%) were AKI stage 3. The median ΔCr during these episodes of AKI was 0.48 (0.38 – 0.76) mg/dL.
In univariable analysis, the factors associated with the development of AKI were refractory ascites (HR 1.95, p=0.001), Child-Pugh score (HR 1.14 per point, p=0.009), MELDNa (HR 1.08 per point, p<0.001), equivalent dose of furosemide at study enrollment (HR 1.20 per 25 equivalent mg, p<0.001), equivalent dose of spironolactone at study enrollment (HR 1.10 per 25 equivalent mg, p=0.01), and bCr (1.63 per 1 mg/dL, p<0.001). Relative to the group with the lowest bCr, the middle and higher categories of bCr had a higher risk of AKI (HR 2.32 for bCr 0.70-0.97 mg/dL, p<0.001; HR 2.93 for bCr >0.97 mg/dL, p<0.001). The final multivariable model for the development of AKI included bCr (HR 1.70 per 1 mg/dL, p<0.001), equivalent dose of furosemide (1.21 per 25 mg, p<0.001), and presence of refractory ascites (HR 1.91, p=0.001).
Prevalence of and Risk Factors for Persistent Kidney Injury
Of the 143 subjects with acute kidney injury in our cohort, 18 (13%) developed persistent kidney injury. Persistent kidney injury was identified in all bCr groups, but occurred predominantly in the highest bCr group (4 v. 4 v. 24%, p=0.001). Those with AKI who developed persistent kidney injury, compared to those with AKI who did not develop persistent kidney injury had significantly higher ΔCr (1.09 v. 0.45 mg/dL, p<0.001). The factors associated with persistent kidney injury in univariable analysis were etiology of NASH (HR 4.07, p=0.008), diabetes mellitus (HR 3.03, p=0.02), bCr (HR 3.00 per 1 mg/dL, p<0.001), and ΔCr (HR 2.11 per 1 mg/dL, p<0.001). The final multivariable model included bCr (HR 2.96 per 1 mg/dL, p<0.001) and ΔCr (HR 2.05 per 1 mg/dL, p<0.001). The complete univariable and multivariable analyses for development of persistent kidney injury are demonstrated in Table 2. A survival analysis was completed looking at the primary outcome of persistent AKI (Figure 1). This demonstrates that the likelihood of persistent kidney injury increases from the lowest to the highest bCr group (p<0.001).
Table 2.
Cox Regression Analysis for Persistent Kidney Injury
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age per Year | 1.02 | 0.96 – 1.09 | 0.46 | |||
| Female Sex | 1.25 | 0.48 – 3.25 | 0.65 | |||
| Autoimmune1 | 0.35 | 0.05 – 2.67 | 0.31 | |||
| NASH | 4.07 | 1.44 – 11.47 | 0.008 | |||
| BMI per 1 kg/m2 | 1.05 | 0.97 – 1.13 | 0.24 | |||
| Refractory Ascites | 2.20 | 0.80 – 5.99 | 0.13 | |||
| Any Hepatic Encephalopathy | 1.89 | 0.67 – 5.35 | 0.23 | |||
| Hypertension | 2.12 | 0.84 – 5.39 | 0.11 | |||
| Diabetes Mellitus | 3.03 | 1.19 – 7.71 | 0.02 | |||
| Albumin per 1 mg/dL | 0.95 | 0.69 – 1.31 | 0.74 | |||
| Baseline Creatinine per 1 mg/dL | 3.00 | 1.75 – 5.13 | <0.001 | 2.96 | 1.62 – 5.44 | <0.001 |
| Delta Creatinine per 1 mg/dL | 2.11 | 1.67 – 2.68 | <0.001 | 2.05 | 1.61 – 2.62 | <0.001 |
| Baseline Creatinine Group | 5.61 | 2.16 – 14.57 | <0.001 | |||
| MDRD4 eGFR per 1 ml/min/1.73 m2 | 0.96 | 0.94 – 0.99 | 0.001 | |||
| CKD-EPI eGFR per 1 ml/min/1.73 m2 | 0.95 | 0.93 – 0.97 | <0.001 | |||
| MELD-Na per 1 point | 1.14 | 1.06 – 1.24 | 0.001 | |||
| Child Pugh Score | 1.25 | 0.97 – 1.61 | 0.09 | |||
| Serum Na per 1 mmol/L | 0.96 | 0.85 – 1.09 | 0.54 | |||
| Total Bilirubin per 1 mg/dL | 1.04 | 0.88 – 1.23 | 0.62 | |||
| INR per 1 Unit | 0.86 | 0.23 – 3.19 | 0.82 | |||
| Equivalent dose of Furosemide per 25 mg | 0.89 | 0.64 – 1.26 | 0.52 | |||
| Equivalent dose of Spironolactone per 25 mg | 1.07 | 0.86 – 1.34 | 0.55 | |||
Combined Autoimmune Hepatitis, Primary Sclerosing Cholangitis, and Primary Biliary Cholangitis; Hepatitis C (HCV); Non-Alcoholic Steatohepatitis (NASH); Hepatitis B (HBV); Modification of Diet in Renal Disease (MDRD); Estimated Glomerular Filtration Rate (eGFR); Sodium (Na); International Normalized Ratio (INR); Model for End-Stage Liver Disease with Serum Sodium (MELDNa)
Figure 1. Cumulative Incidence Curve for Persistent Kidney Injury.
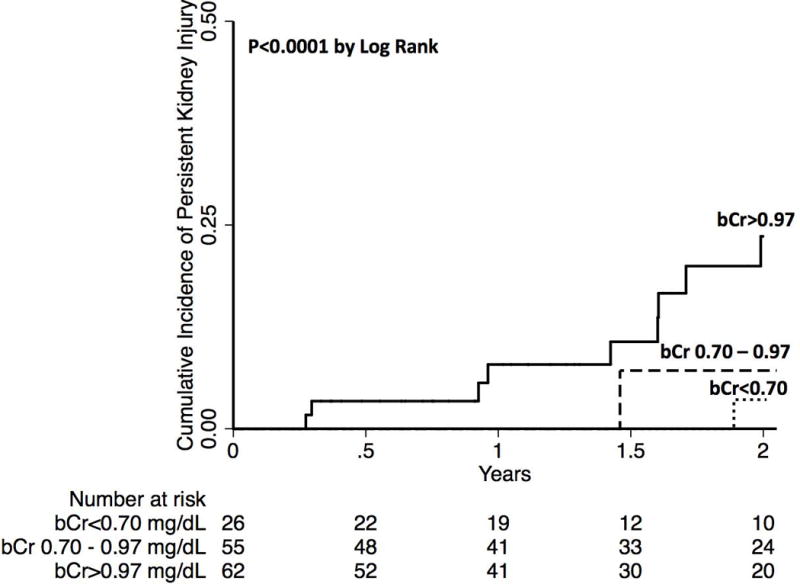
bCr = baseline serum creatinine
Waitlist Mortality and AKI
By the end of follow-up, 178 (46%) were still active on the waitlist or removed from the waitlist for social reasons, 79 (21%) either died on the waitlist or were delisted for sickness, and 127 (33%) received a liver transplant. Those in the highest baseline creatinine group experienced significantly increased waitlist mortality (14% v. 19% v. 28%, p=0.03). There was no difference in mortality in those patients with and without an episode of acute kidney injury (20 v. 22%, p=0.64). There was no difference in transplant rates between the three bCr groups (28 v. 35 v. 35%, p=0.43). Of those who received a liver transplant, the rates of simultaneous liver-kidney transplant were greatest in the highest bCr group (4 v. 0 v. 22%, p=0.002).
A competing risks analysis was completed to determine if there was an association between baseline renal function and episodes of acute kidney injury with waitlist mortality. In univariable analyses, the following were significantly associated with waitlist mortality: etiology of NASH (sub-hazard ratio [SHR] 2.27, p=0.002), bCr (SHR 1.60 per 1 mg/dL, p=0.004), age per year (SHR 1.03, p=0.03), dCr per 1 mg/dL (SHR 1.41, p<0.001), Child Pugh Score per 1 point (SHR 1.16, p=0.01) and MELDNa per 1 point (SHR 1.05, p=0.01). In the final multivariable model, only an etiology of NASH (SHR 2.24, p=0.003) and bCr (SHR 1.62 per 1 mg/dL, p=0.004) were significantly associated with waitlist mortality.
DISCUSSION
In this study evaluating the prevalence and impact of AKI in outpatients with cirrhosis listed for liver transplantation, we aimed to identify predictors of AKI, determinants of persistent kidney injury, and the impact of baseline renal function on waitlist survival. Confirming prior reports (12,27), we observed that higher baseline creatinine, refractory ascites, and dose of diuretics were associated with a higher risk of developing AKI. We further identified that this risk begins at lower baseline creatinine levels than previously appreciated – a 232% increased risk for those with a baseline creatinine of 0.70-0.97 mg/dl and a 293% increased risk for those with a baseline creatinine of greater than 0.97 mg/dL. Furthermore, we report two determinants of persistent kidney injury – degree of renal injury (ΔCr) and the baseline renal function (bCr). Lastly, we demonstrate that baseline creatinine levels >0.7 mg/dL, a threshold lower than what is currently used in the MELD score (>1 mg/dL), have a significant impact on waitlist mortality.
What might explain these findings? Patients with cirrhosis have clear reasons for a lower serum creatinine. First, they have decreased hepatic synthesis of creatine, the precursor of creatinine (28). Second, they have increased renal tubular secretion of creatinine (29). Third, they are known to have decreased muscle mass (28,30). We propose that these variations in biology lead to lower serum creatinine levels. This provides the basis of our findings that relatively low levels and subtle fluctuations in serum creatinine have a significant impact on not only the development of persistent kidney injury, but also waitlist mortality.
We acknowledge the following limitations to this study. First, the timing of laboratory assessments differed amongst patients, so it is possible that patients experienced episodes of AKI (that resolved spontaneously in between blood draws) that were not detected in our analyses. However, given that our laboratory assessments were collected as part of routine clinical care, our analyses reflect the information that would be available to a provider in clinical practice for real-life decision-making, making our results more generalizable to the outpatient practice setting. Second, the outpatient design of this study limited the information that could be collected regarding the etiology (e.g. infection, hypovolemia) of these episodes of acute kidney injury, phenotype of these episodes of acute kidney injury (e.g. hepatorenal syndrome, pre-renal, intrinsic acute kidney injury) and how these episodes were managed (e.g. vasopressors, diuretic withdrawal). Third, we used serum creatinine as the marker for renal function, which may be inaccurate in patients with cirrhosis (1,31); quantifications of eGFR may serve as more accurate assessments of renal function. To test for this possibility, we performed sensitivity analyses of our regression models using eGFR (calculated with CKD-EPI and MDRD4), but neither changed the qualitative interpretation of our analyses, as baseline creatinine and degree of change of renal function remained the strongest predictors of persistent kidney injury in these adjusted models.
Despite these limitations, this is one of the first studies describing renal outcomes in patients with cirrhosis listed for liver transplantation in the outpatient setting. Our finding that risk for persistent kidney injury is elevated even in outpatients with cirrhosis with baseline creatinine levels as low as 0.70 mg/dL – what would be considered “normal” based on laboratory reference ranges – has important implications for clinical practice. Specifically, our analyses identified patients listed for liver transplantation who should have closer monitoring of renal function or implementation of renal-protective strategies (i.e. diuretic adjustment, earlier fluid resuscitation). Furthermore, our data support previous work that serum creatinine levels not accounted for in the MELD score have important implications on waitlist outcomes (32). Lastly, our data suggest that clinicians should prepare their patients for the possibility of listing for simultaneous liver/kidney transplantation earlier in their disease course. Whether interventions to lower serum creatinine levels will reduce rates of persistent kidney injury or need for simultaneous liver/kidney transplantation warrants further study. Our data, focusing on patients with cirrhosis in the outpatient setting, when intervention is possible, provides the foundation for such investigation.
Table 3. Competing Risks Analysis for Waitlist Mortality.
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| SHR | 95% CI | P | SHR | 95% CI | P | |
| Age per Year | 1.03 | 1.00 – 1.06 | 0.03 | |||
| Female Sex | 0.96 | 0.62 – 1.51 | 0.87 | |||
| Autoimmune1 | 1.16 | 0.67 – 2.02 | 0.60 | |||
| NASH | 2.27 | 1.34 – 3.84 | 0.002 | 2.24 | 1.33 – 3.78 | 0.003 |
| Refractory Ascites | 1.30 | 0.75 – 2.24 | 0.35 | |||
| Any Hepatic Encephalopathy | 1.55 | 0.94 – 2.57 | 0.09 | |||
| Hypertension | 1.02 | 0.65 – 1.61 | 0.93 | |||
| Diabetes Mellitus | 1.18 | 0.74 – 1.90 | 0.48 | |||
| Baseline Creatinine per 1 mg/dL | 1.60 | 1.17 – 2.20 | 0.004 | 1.62 | 1.17 – 2.25 | 0.004 |
| Delta Creatinine per 1 mg/dL | 1.41 | 1.21 – 1.64 | <0.001 | |||
| Persistent AKI | 1.72 | 0.84 – 3.50 | 0.14 | |||
| MELD-Na per 1 point | 1.05 | 1.01 – 1.09 | 0.01 | |||
| Child Pugh Score | 1.16 | 1.03 – 1.31 | 0.01 | |||
Combined Autoimmune Hepatitis, Primary Sclerosing Cholangitis, and Primary Biliary Cholangitis; Hepatitis C (HCV); Non-Alcoholic Steatohepatitis (NASH); Hepatitis B (HBV); Sodium (Na); International Normalized Ratio (INR); Model for End-Stage Liver Disease with Serum Sodium (MELDNa)
Acknowledgments
Financial Support:This study was funded by K23AG048337 (Paul B. Beeson Career Development Award in Aging Research; Lai) and by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (UCSF Liver Center P30 DK026743, T32 DK060414; Cullaro), both of which played no role in the analysis of the data or the preparation of this manuscript
Abbreviations
- AKI
Acute Kidney Injury
- MELD
Model for End-Stage Liver Disease
- FrAILT
Functional Assessment in Liver Transplantation study
- HCC
Hepatocellular Carcinoma
- bCr
baseline serum creatinine
- pCr
peak serum creatinine
- ΔCr
delta serum creatinine
- sCr
serum creatinine
References
- 1.Ginès P, Schrier RW. Renal Failure in Cirrhosis. N Engl J Med. 2009;361(13):1279–90. doi: 10.1056/NEJMra0809139. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology [Internet] 2008 Dec 1;48(6):2064–77. doi: 10.1002/hep.22605. [cited 2017 Aug 9]; Available from: http://doi.wiley.com/10.1002/hep.22605. [DOI] [PubMed] [Google Scholar]
- 3.Wong F. Acute kidney injury in liver cirrhosis : new definition and application. Clin Mol Hepatol. 2016;22(4):415–22. doi: 10.3350/cmh.2016.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belcher JM, Garcia-Tsao G, Sanyal AJ, Bhogal H, Lim JK, Ansari N, et al. Association of AKI With mortality and complications in hospitalized patients with cirrhosis. Hepatology. 2013 doi: 10.1002/hep.25735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong F, O’Leary JG, Reddy KR, Patton H, Kamath PS, Fallon MB, et al. New consensus definition of acute kidney injury accurately predicts 30-day mortality in patients with cirrhosis and infection. Gastroenterology. 2013;145(6) doi: 10.1053/j.gastro.2013.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jenq C-C, Tsai M-H, Tian Y-C, Lin C-Y, Yang C, Liu N-J, et al. RIFLE classification can predict short-term prognosis in critically ill cirrhotic patients. Intensive Care Med [Internet] 2007 Oct 23;33(11):1921–30. doi: 10.1007/s00134-007-0760-6. [cited 2017 Aug 25] Available from: http://link.springer.com/10.1007/s00134-007-0760-6. [DOI] [PubMed] [Google Scholar]
- 7.Cholongitas E, Calvaruso V, Senzolo M, Patch D, Shaw S, O’Beirne J, et al. RIFLE classification as predictive factor of mortality in patients with cirrhosis admitted to intensive care unit. J Gastroenterol Hepatol [Internet] 2009 Oct 1;24(10):1639–47. doi: 10.1111/j.1440-1746.2009.05908.x. [cited 2017 Aug 25] Available from: http://doi.wiley.com/10.1111/j.1440-1746.2009.05908.x. [DOI] [PubMed] [Google Scholar]
- 8.Amathieu R, Al-khafaji A, Sileanu FE, Foldes E. Significance of Oliguria in Critically Ill Patients with Chronic Liver Disease. Hepatology. 2017:1–23. doi: 10.1002/hep.29303. [DOI] [PubMed] [Google Scholar]
- 9.Sort P, Navasa M, Arroyo V, Aldeguer X, Planas R, Ruiz-del-Arbol L, et al. Effect of Intravenous Albumin on Renal Impairment and Mortality in Patients with Cirrhosis and Spontaneous Bacterial Peritonitis. N Engl J Med [Internet] 1999 Aug 5;341(6):403–9. doi: 10.1056/NEJM199908053410603. [cited 2017 Aug 25] Available from: http://www.nejm.org/doi/abs/10.1056/NEJM199908053410603. [DOI] [PubMed] [Google Scholar]
- 10.Cardenas A, Gines P. Management of patients with cirrhosis awaiting liver transplantation. Gut [Internet] 2011 Mar 1;60(3):412–21. doi: 10.1136/gut.2009.179937. [cited 2017 Aug 25] Available from: http://www.ncbi.nlm.nih.gov/pubmed/21193458. [DOI] [PubMed] [Google Scholar]
- 11.Fernández J, Navasa M, Planas R, Montoliu S, Monfort D, Soriano G, et al. Primary Prophylaxis of Spontaneous Bacterial Peritonitis Delays Hepatorenal Syndrome and Improves Survival in Cirrhosis. Gastroenterology [Internet] 2007 Sep;133(3):818–24. doi: 10.1053/j.gastro.2007.06.065. [cited 2017 Aug 25] Available from: http://www.ncbi.nlm.nih.gov/pubmed/17854593. [DOI] [PubMed] [Google Scholar]
- 12.Wong F, O’Leary JG, Reddy KR, Garcia-Tsao G, Fallon MB, Biggins SW, et al. Acute Kidney Injury in Cirrhosis: Baseline Serum Creatinine Predicts Patient Outcomes. Am J Gastroenterol [Internet] 2017 Oct;2016:1–8. doi: 10.1038/ajg.2017.122. Available from: http://www.nature.com/doifinder/10.1038/ajg.2017.122. [DOI] [PubMed] [Google Scholar]
- 13.O’Leary JG, Levitsky J, Wong F, Nadim MK, Charlton M, Kim WR. Protecting the Kidney in Liver Transplant Candidates: Practice-Based Recommendations From the American Society of Transplantation Liver and Intestine Community of Practice. Am J Transplant [Internet] 2016 Sep 1;16(9):2516–31. doi: 10.1111/ajt.13790. [cited 2017 Sep 6] Available from: http://doi.wiley.com/10.1111/ajt.13790. [DOI] [PubMed] [Google Scholar]
- 14.Belcher JM, Garcia-Tsao G, Sanyal AJ, Bhogal H, Lim JK, Ansari N, et al. Association of AKI With mortality and complications in hospitalized patients with cirrhosis. Hepatology [Internet] 2013 Feb 1;57(2):753–62. doi: 10.1002/hep.25735. [cited 2017 Aug 25] Available from: http://doi.wiley.com/10.1002/hep.25735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angeli P, Ginès P, Wong F, Bernardi M, Boyer TD, Gerbes A, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: Revised consensus recommendations of the International Club of Ascites. Gut. 2015;62(4):968–74. doi: 10.1016/j.jhep.2014.12.029. [DOI] [PubMed] [Google Scholar]
- 16.Piano S, Rosi S, Maresio G, Fasolato S, Cavallin M, Romano A, et al. Evaluation of the Acute Kidney Injury Network criteria in hospitalized patients with cirrhosis and ascites. J Hepatol [Internet] 2013 Sep;59(3):482–9. doi: 10.1016/j.jhep.2013.03.039. [cited 2017 Oct 21] Available from: http://www.ncbi.nlm.nih.gov/pubmed/23665185. [DOI] [PubMed] [Google Scholar]
- 17.Tsien CD, Rabie R, Wong F. Acute kidney injury in decompensated cirrhosis. Gut [Internet] 2013;62(1):131–7. doi: 10.1136/gutjnl-2011-301255. Available from: http://gut.bmj.com/lookup/doi/10.1136/gutjnl-2011-301255. [DOI] [PubMed] [Google Scholar]
- 18.Lai JC, Covinsky KE, Dodge JL, Boscardin WJ, Segev DL, Roberts JP, et al. Development of a novel frailty index to predict mortality in patients with end-stage liver disease. Hepatology [Internet] 2017 Aug;66(2):564–74. doi: 10.1002/hep.29219. [cited 2017 Aug 18] Available from: http://doi.wiley.com/10.1002/hep.29219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai JC, Dodge JL, Sen S, Covinsky K, Feng S. Functional decline in patients with cirrhosis awaiting liver transplantation: Results from the functional assessment in liver transplantation (FrAILT) study. Hepatology [Internet] 2016 Feb 1;63(2):574–80. doi: 10.1002/hep.28316. [cited 2017 Aug 18] Available from: http://doi.wiley.com/10.1002/hep.28316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weissenborn K, Rückert N, Hecker H, Manns MP. The number connection tests A and B: interindividual variability and use for the assessment of early hepatic encephalopathy. J Hepatol [Internet] 1998 Apr;28(4):646–53. doi: 10.1016/s0168-8278(98)80289-4. [cited 2017 Aug 18] Available from: http://linkinghub.elsevier.com/retrieve/pii/S0168827898802894. [DOI] [PubMed] [Google Scholar]
- 21.Runyon BA. Management of Adult Patients with Ascites Due to Cirrhosis: Update 2012 [Internet] 2014:1–96. Available from: https://www.aasld.org/sites/default/files/guideline_documents/adultascitesenhanced.pdf.
- 22.Lai JC, Feng S, Terrault NA, Lizaola B, Hayssen H, Covinsky K. Frailty Predicts Waitlist Mortality in Liver Transplant Candidates. Am J Transplant [Internet] 2014 Aug;14(8):1870–9. doi: 10.1111/ajt.12762. [cited 2017 Aug 18] Available from: http://doi.wiley.com/10.1111/ajt.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angeli P, Ginès P, Wong F, Bernardi M, Boyer TD, Gerbes A, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: Revised consensus recommendations of the International Club of Ascites. J Hepatol [Internet] 2015;62:968–74. doi: 10.1016/j.jhep.2014.12.029. [cited 2018 Mar 21] Available from: http://www.journal-of-hepatology.eu/article/S0168-8278(14)00958-1/pdf. [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A More Accurate Method To Estimate Glomerular Filtration Rate from Serum Creatinine: A New Prediction Equation. Ann Intern Med [Internet] 1999 Mar 16;130(6):461. doi: 10.7326/0003-4819-130-6-199903160-00002. [cited 2017 Aug 29] Available from: http://annals.org/article.aspx?doi=10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 25.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med [Internet] 2009 May 5;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [cited 2017 Aug 29] Available from: http://www.ncbi.nlm.nih.gov/pubmed/19414839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fine JP, Gray RJ. Proportional Hazards Model for the Subdistribution of a Competing Risk A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc. 1999;1459:37–41. April 2013. [Google Scholar]
- 27.Francoz C, Glotz D, Moreau R, Durand F. The evaluation of renal function and disease in patients with cirrhosis. J Hepatol [Internet] 2010;52(4):605–13. doi: 10.1016/j.jhep.2009.11.025. Available from: [DOI] [PubMed] [Google Scholar]
- 28.Sherman DS, Fish DN, Teitelbaum I. Assessing renal function in cirrhotic patients: Problems and pitfalls. Am J Kidney Dis [Internet] 2003;41(2):269–78. doi: 10.1053/ajkd.2003.50035. Available from: [DOI] [PubMed] [Google Scholar]
- 29.Caregaro L, Menon F, Angeli P, Amodio P, Merkel C, Bortoluzzi A, et al. Limitations of Serum Creatinine Level and Creatinine Clearance as Filtration Markers in Cirrhosis. Arch Intern Med. 1994;154:201–5. [PubMed] [Google Scholar]
- 30.Baxmann AC, Ahmed MS, Marques NC, Menon VB, Pereira AB, Kirsztajn GM, et al. Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. Clin J Am Soc Nephrol. 2008;3(2):348–54. doi: 10.2215/CJN.02870707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papadakis MA, Arieff AI. Unpredictability of clinical evaluation of renal function in cirrhosis. Prospective study. AmJMed. 1987;82(0002–9343):945–52. doi: 10.1016/0002-9343(87)90156-2. [DOI] [PubMed] [Google Scholar]
- 32.Sharma P, Schaubel DE, Sima CS, Merion RM, Lok ASF. Re-weighting the model for end-stage liver disease score components. Gastroenterology [Internet] 2008 Nov 1;135(5):1575–81. doi: 10.1053/j.gastro.2008.08.004. [cited 2018 Mar 21] Available from: http://www.ncbi.nlm.nih.gov/pubmed/18835388. [DOI] [PubMed] [Google Scholar]


