Abstract
Coronary microvascular dysfunction predicts and may be a proximate cause of cardiac dysfunction and mortality in diabetes; however, few effective treatments exist for these conditions. We recently demonstrated that mineralocorticoid receptor (MR) antagonism reversed cardiovascular dysfunction in early-stage obesity/insulin resistance. The mechanisms underlying this benefit of MR antagonism and its relevance in the setting of long-term obesity complications like diabetes; however, remain unclear. Thus, the present study evaluated the impact of MR antagonism on diabetes-related coronary dysfunction and defines the MR-dependent vascular transcriptome in the Otsuka Long-Evans Tokushima Fatty (OLETF) rat recapitulating later stages of human diabetes. OLETF rats were treated with spironolactone (Sp) and compared to untreated OLETF and lean Long-Evans Tokushima Otsuka rats. Sp treatment attenuated diabetes-associated adipose and cardiac inflammation/fibrosis and improved coronary endothelium-dependent vasodilation but did not alter enhanced coronary vasoconstriction, blood pressure, or metabolic parameters in OLETF rats. Further mechanistic studies using RNA deep sequencing of OLETF rat aortas revealed 157 differentially expressed genes following Sp including upregulation of genes involved in the molecular regulation of nitric oxide bioavailability (Hsp90ab1, Ahsa1, Ahsa2) as well as novel changes in α1D adrenergic receptors (Adra1d), cyclooxygenase-2 (Ptgs2), and modulatory factors of these pathways (Ackr3, Acsl4). Further, Ingenuity Pathway Analysis predicted inhibition of upstream inflammatory regulators by Sp and inhibition of ‘migration of endothelial cells’, ‘differentiation of smooth muscle’, and ‘angiogenesis’ biological functions by Sp in diabetes. Thus, this study is the first to define the MR-dependent vascular transcriptome underlying treatment of diabetes-related coronary microvascular dysfunction by Sp.
Keywords: fibrosis, inflammation, cardiac, LETO
1. Introduction
Coronary microvascular dysfunction has recently been proposed as a precipitating cause of cardiac diastolic dysfunction and heart failure with preserved ejection fraction in the setting of co-morbid conditions such as obesity and diabetes [1]. Indeed, in the absence of coronary atherosclerosis, impaired coronary flow reserve (CFR; i.e., microvascular dysfunction) is associated with cardiac damage (i.e., positive troponin) [2] and is a powerful independent predictor of cardiac mortality in diabetic patients [3]. Few effective treatments exist, however, for coronary microvascular dysfunction in diabetes or other disease states [4]. In light of these known associations, delineation of such treatments and underlying mechanisms holds promise to improve coronary blood flow regulation in diabetes and reduce diabetes-associated cardiac diastolic dysfunction, a condition also for which no treatment currently exists.
We recently demonstrated that mineralocorticoid receptor (MR) blockade reversed obesity-associated cardiovascular dysfunction in the obese, insulin resistant Zucker rat model [5]. The MR is a ligand-activate1d transcription factor that is functionally expressed in both vascular smooth muscle and endothelial cells [6]. Accumulating evidence implicates vascular MR signaling underlying gene expression of vascular ion channels [7–9], receptors for vasoactive ligands [8–11], adhesion/inflammatory molecules [12–16], and fibrosis/oxidant stress signaling [14, 15, 17]; pathways critical for microvascular (dys)function and coronary blood flow control [18]. Importantly, it was recently demonstrated that MR antagonism improved CFR in patients with diabetes, independent of blood pressure [19, 20]. The nature of MR-dependent gene transcription and MR-regulated gene networks in the setting of obesity and diabetes, however, has not been assessed and holds promise for the identification of novel mechanisms of diabetes-related coronary dysfunction. Accordingly, we evaluated the hypothesis that treatment of coronary microvascular dysfunction in obesity and type 2 diabetes by MR antagonism is associated with pronounced alterations in the vascular transcriptome.
This hypothesis was evaluated utilizing the Otsuka Long-Evans Tokushima Fatty (OLETF) rat model of obesity and diabetes and their lean Long-Evans Tokushima Otsuka (LETO) counterparts [21]. The hyperphagic OLETF rat recapitulates adult-onset human obesity and type 2 diabetes by exhibiting late onset hyperglycemia (>13 wks of age), mild obesity, and hyperlipidemia transitioning to overt diabetes by 40 wk of age [22, 23]. In this way, the OLETF rat model is a more translationally relevant model of the slow onset later stages of human diabetes as opposed to the Zucker rat, used in our prior study [5], that models earlier stages of obesity (i.e., insulin resistance) from very early in life. In addition, the OLETF rat exhibits impaired CFR and structural remodeling of the coronary microcirculation [24]; however, the time course of obesity/diabetes-associated changes in coronary endothelial function and vasoconstrictor responses has not previously been evaluated in this model. Thus, we evaluated the time course of obesity/diabetes-associated changes in coronary microvascular function and whether MR antagonism could reverse established coronary vasomotor dysfunction. Furthermore, assessment of the vascular transcriptome in diabetes with and without MR antagonism was accomplished by RNA deep sequencing (RNA-Seq) to globally investigate potential mechanisms underlying the vascular benefits of MR antagonism in diabetes. The impact of MR antagonism on cardiac and adipose fibrosis/inflammation was also assessed.
2. Methods
2.1. Animals.
All animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Missouri. Animals were housed in a temperature-controlled room with a 12:12-h light:dark cycle and provided ad libitum water and standard rodent chow (Formulab 5008, Purina Mills, St. Louis, MO). Male LETO and OLETF rats (4 wk of age) were obtained from Japan SLC, Inc. The progression of coronary dysfunction was assessed in batches of both strains studied at 5–8 (LETO: n=4, OLETF: n=5), 20 (LETO: n=5, OLETF, n=4), and 32 (LETO: n=11, OLETF: n=12) weeks of age. Involvement of MR signaling was assessed in 32 week old OLETF rats randomly assigned to placebo (n=4) or spironolactone (Sp; n=5) treatment paradigms. Under isoflurane anesthesia (2–4% in 100% O2), rats were implanted with time-release, matrix-driven delivery pellets (Innovative Research of America, Sarasota, FL) containing either placebo or Sp (sc; 20 mg·kg−1·d−1) for 21 days beginning at 29 weeks of age, as we previously published [5, 25]. This dose was chosen to elicit complete systemic MR inhibition in vivo, based on a previous report [26]. Animals were fasted 5 hours prior to euthanization, anesthetized with pentobarbital sodium (100 mg/kg), blood was collected from the abdominal aorta, processed to serum, and frozen at −80°C. Animals were euthanized by exsanguination.
2.2. Blood pressure.
Blood pressure (BP) was determined in placebo and Sp-treated OLETF rats by tail-cuff (Coda 8, Kent Scientific, Torrington, CA) during the final week of treatment. Rats were acclimated to restraints and tail cuffs for 3 days. Blood pressure was determined on the fourth day with 10–25 cuff inflations averaged for each animal, as previously reported [5].
2.3. Plasma chemistries.
Blood glucose (AlphaTrak, Abbott) was determined immediately prior to euthanasia. Serum total cholesterol, triglycerides, and potassium were measured using an Olympus AU680 automated chemistry analyzer (Beckman-Coulter, Brea, CA) and plasma insulin was determined using a rat-specific ELISA by Comparative Clinical Pathology Services (Columbia, MO). Plasma aldosterone were determined in duplicate by radioimmunoassay at the Vanderbilt Hormone Assay & Analytical Services Core (Nashville, TN).
2.4. Coronary arteriolar function.
Coronary function was assessed as previously described [5, 27]. Briefly, the heart was removed and immediately placed in ice-cold physiological salt solution (PSS) containing (in mM): 145 NaCl, 4.7 KCl, 1.2 NaH2PO4, 1.17 MgSO4, 2 CaCl2, 5 glucose, 2 pyruvate, 0.02 EDTA, 3 MOPS, and 1% bovine serum albumin, pH 7.4. Coronary arterioles (<200 μm internal diameter) from the septum or left ventricular (LV) free wall were dissected, secured to glass micropipettes, pressurized to 60cmH2O at 37°C, and visualized with an inverted microscope for determination of internal diameter with a video micrometer recorded using Chart software with a PowerLab data acquisition system (ADInstruments, Colorado Springs, CO). Vessels free from leaks were equilibrated for 1 hr and viability was assessed by presence of vasoconstriction to 80 mM KCl. Prior to assessment of vasodilator responses, arterioles were preconstricted to 25–45% tone with the thromboxane A2 analog U46619 (0.1–1 μM). Preconstrictor doses of U46619 were not different among groups (LETO: 0.5±0.1 μM; OLETF: 0.5±0.1 μM; OLETF Sp: 0.4±0.2 μM). Vasodilator responses to acetylcholine (ACh; 1 nM – 10 mM), insulin (Novolin R, Novo Nordisk; 0.1 – 100 ng/ml), or the nitric oxide (NO) donor sodium nitroprusside (SNP; 1 nM – 10 mM) were assessed by cumulative addition of agonists to the vessel bath. In addition, vasoconstrictor responses to U46619 (10 nM – 1 μM), endothelin-1 (ET-1; 0.1 – 10 nM), or the L-type Ca2+ channel activator BayK-8644 (0.1 – 100 nM) were assessed. Maximum passive diameter was determined at the end of experiments by replacing bath PSS with Ca2+-free PSS. Coronary vasodilator responses are presented as percent maximal dilation, calculated as [(Dd – Db)/(Dmax – Db)] × 100, and constrictor responses are presented as percent vasoconstriction, calculated as [(Db – Dd)/Db] × 100 where Dd is diameter after a drug intervention, Db is baseline diameter, and Dmax is maximal passive diameter. No differences in coronary arteriolar function were found between untreated and placebo-treated OLETF rats so these data were combined.
2.5. Real time PCR.
Cardiac and retroperitoneal adipose tissue samples were frozen immediately at sacrifice and subsequently homogenized using a tissue homogenizer (TissueLyser LT, Qiagen, Valencia, CA) followed by total RNA isolation (RNeasy Fibrous or Lipid Tissue Kit, Qiagen) and assessment of RNA purity and concentration using a Nanodrop spectrophotometer (Thermo Scientific, Wilmington, DE). First-strand cDNA was synthesized from total RNA using the Improm-II Reverse Transcription System (Promega, Madison, WI). In placebo- and Sp-treated OLETF rats the cardiac septum was separated from the LV free wall and placed in RNAlater solution after which the septal coronary artery was dissected and frozen until processed for RNA isolation (NucleospinXS, Clontech, Mountain View, CA) and assessment of RNA purity and concentration. As expected, these single vessels had limited RNA yield so total RNA was amplified (ExpressArt mRNA amplification kit; AMS Biotechnology, United Kingdom) to provide sufficient RNA for PCR analysis. First-strand cDNA was synthesized from total RNA as above. Quantitative real-time PCR was performed using the CFX Connect™ Real-Time PCR Detection System (BioRad, Hercules, CA), as previously described [5]. Primer sequences (Supplemental Table 1) were purchased from IDT (Coralville, IA) and duplicate PCR reactions were performed using iTaq UniverSYBR Green SMX (BioRad, Hercules, CA). GAPDH primers were used to amplify the endogenous control product and mRNA expression values were calculated as 2ΔCT whereby ΔCT = GAPDH CT – gene of interest CT and are presented normalized to the appropriate control groups, which were set at 1.
2.6. RNA Sequencing (RNA-Seq).
Total RNA was extracted from thoracic aorta of placebo and Spiro treated OLETF rats and high RNA integrity confirmed for each sample using the Qubit assay (ThermoFisher). RNA-Seq was performed on aortic total RNA due to the limitation of obtaining adequate RNA yield from rat coronary artery for RNA-Seq. Genes that were differentially expressed following Sp treatment were examined in coronary samples by RT-PCR, as described above. Samples underwent ribosomal RNA reduction (Ribo-Zero Gold Kit, Illumina) and cDNA library preparation via manufacturer’s protocols including purification of poly-A containing mRNA, RNA fragmentation, generation of double-stranded cDNA, and ligation of index containing sample identifier adapters. Final constructs were evaluated using the Qubit assay and diluted according to Illumina’s standard sequencing protocol for sequencing on the HiSeq 4000. For sequencing, samples were loaded onto one flow cell where clusters of each oligo were replicated and fluorescently-labeled bases were attached to the complementary bases of each sequence in the sequencer. The Illumina Genome Analyzer recorded paired end 100 base pair reads with at least 79 million reads per sample (range: 79–116 million reads per sample; ~80% matched to the USCS rn5 rat reference genome). Reads were trimmed to ensure adaptor sequence removal and tiled to the reference rat genome (UCSC rn5) using Bowtie2 2.2.6 (Johns Hopkins University) and annotated with BEDTools 2.17.0. Following statistical determination of differentially expressed transcripts (described below), Ingenuity Pathway Analysis (IPA; Qiagen) was utilized for examination of the top up- and down-regulated genes and the corresponding top networks and pathways.
2.7. Statistical Analysis.
Coronary function as well as cardiac and adipose gene expression data were evaluated with one- or two-way ANOVA followed by Fishers LSD post hoc analysis, as appropriate. For RNA-Seq data, nonspecific filtering of genes prior to statistical testing was carried out to increase detection power, based on the requirement that a gene have an expression level greater than 220 raw counts per million (CPM) output for at least 5 libraries (5 was chosen because it was the size of each treatment group). This CPM cutoff was established empirically based on the point at which the ERCC Spike-Ins at different concentrations were no longer distinguishable in the samples. In this study, ERCC Spike-Ins were no longer distinguishable at 75 CPM and we chose to utilize a more conservative cutoff of 220 CPM. Adjustment to the P values was made by EdgeR 3.4 software to account for multiple testing with a 10% false discovery rate (FDR) and based on the method of Benjamini and Hochberg [28].
3. Results
3.1. Differential progression of coronary arteriolar vasodilator and vasoconstrictor dysfunction with age in OLETF rats.
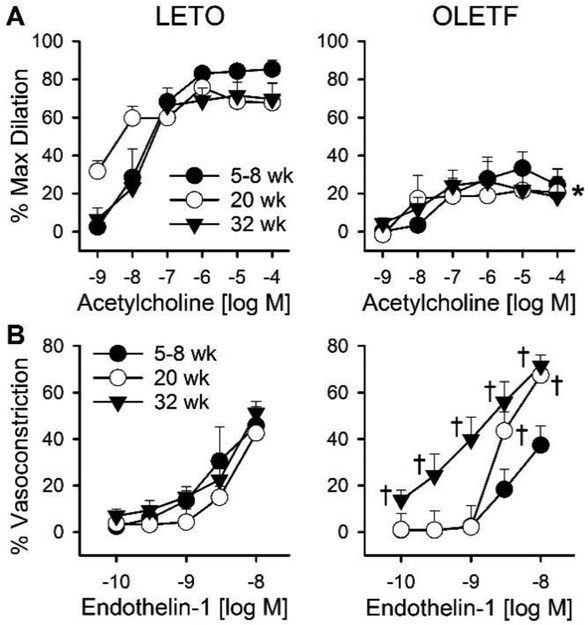
Similar to previous reports from our group [22, 29], OLETF rats exhibited increased body weight and adipose expansion at all ages studied compared to LETO rats (Table 1 and Supplemental Table 2). Average passive diameters and percent preconstriction of isolated coronary arterioles studied were similar within age groups (Supplemental Table 3). Examination of isolated coronary arterioles revealed impaired endothelium-dependent vasodilation to ACh in OLETF rats at all ages studied (5–8, 20, and 32 weeks) (Figure 1). Vasodilation to SNP was similar in 32 week old OLETF and LETO rats (data not shown). Vasoconstriction to ET-1, however, was progressively increased with age in OLETF rats. Specifically, compared to LETO rats, constriction to ET-1 was unchanged in 5–8 week old OLETF rats, increased at the highest doses in 20 week old OLETF rats, and increased at all doses examined in 32 week old OLETF rats (Figure 1).
Table 1.
Phenotypic characteristics of 32 week old LETO and OLETF rats
| Parameter | LETO | OLETF | OLETF | ||||||
|---|---|---|---|---|---|---|---|---|---|
| + Spironolactone | |||||||||
| Body Weight (g) | 471 | ± | 14 | 674 | ± | 21* | 617 | ± | 28* |
| Heart Weight (g) | 1.46 | ± | 0.04 | 1.65 | ± | 0.07* | 1.58 | ± | 0.04* |
| RP Adipose Weight (g) | 7.6 | ± | 0.8 | 22.7 | ± | 2.6* | 20.9 | ± | 2.7* |
| Systolic Blood Pressure (mmHg) | 141 | ± | 6 | 138 | ± | 5 | 146 | ± | 9 |
| Plasma Glucose (mg·dl−1) | 153 | ± | 4 | 236 | ± | 19* | 223 | ± | 14* |
| Plasma Insulin (ng·ml−1) | 3.4 | ± | 0.4 | 3.5 | ± | 1.5 | 3.9 | ± | 1.3 |
| Plasma Cholesterol (mg·dl−1) | 94 | ± | 3 | 153 | ± | 8* | 147 | ± | 19* |
| Plasma Triglycerides (mg·dl−1) | 41 | ± | 3 | 301 | ± | 33* | 267 | ± | 65* |
| Serum Potassium (mEq·L−1) | 5.0 | ± | 0.1 | 4.5 | ± | 0.4 | |||
| Plasma Aldosterone (pg·ml−1) | 252 | ± | 16 | 135 | ± | 14* | 215 | ± | 32** |
Values are mean ± SE, n = 5–10,
p<0.05 versus LETO,
p<0.05 versus OLETF; RP, retroperitoneal
Figure 1.
Differential progression of coronary vasomotor dysfunction in OLETF rats. Vasomotor responses of coronary arterioles from Long-Evans Tokushima Otsuka (LETO; left panels) control and Otsuka Long-Evans Tokushima Fatty (OLETF; right panels) rats to (A) the endothelium-dependent vasodilator acetylcholine and (B) the vasoconstrictor endothelin-1 at 5–8, 20, and 32 weeks of age. Values are mean±SE; n=3–5; *p<0.05 versus LETO, †p<0.05 versus 5–8 wk OLETF and age-matched LETO.
3.2. MR inhibition attenuates cardiac and adipose pro-inflammatory and cardiac fibrosis-related gene expression in OLETF rats independent of blood pressure.
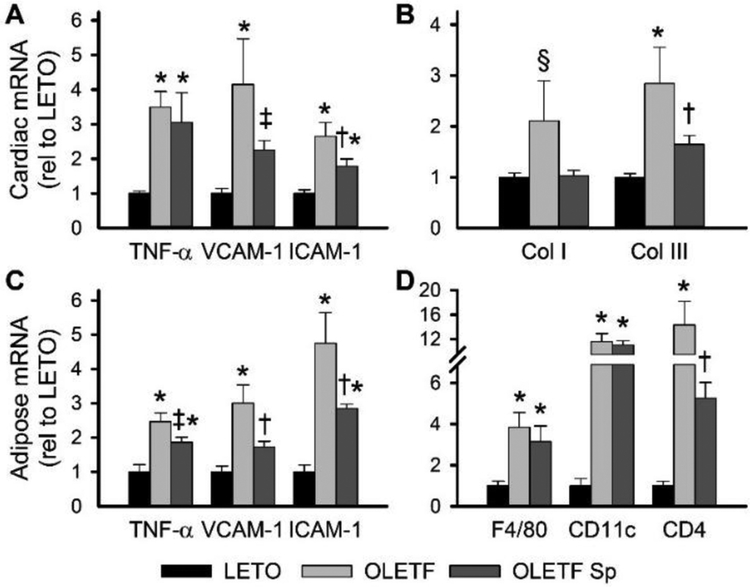
Phenotypically, in addition to increased body weight and adipose expansion, 32 week old OLETF rats exhibited elevated fasting plasma glucose, cholesterol, and triglycerides but similar systolic blood pressure compared to age-matched LETO rats (Table 1). Treatment of OLETF rats for 3 weeks with Sp resulted in no change in body or tissue (heart and adipose) weights, fasting plasma measures (glucose, insulin, cholesterol, and triglycerides), serum potassium, or systolic blood pressure compared to placebo-treated OLETF rats (Table 1). Plasma aldosterone was reduced in OLETF rats compared to LETO rats, as previously described [30], and was increased by Sp treatment (Table 1). OLETF rats exhibited increased cardiac expression of pro-inflammatory (TNF-α, VCAM-1, ICAM-1) and fibrosis-related (increased collagen III and trend for increased collagen I) genes compared to LETO rats (Figure 2A and2B). Similarly, in retroperitoneal adipose tissue, OLETF rats exhibited increased expression of pro-inflammatory (TNF-α, VCAM-1, ICAM-1) and immune cell marker (F4/80, CD11c, CD4) genes compared to LETO rats, indicative of adipose immune cell infiltration (Figure 2C and2D). Treatment of OLETF rats with Sp attenuated cardiac pro-inflammatory (VCAM-1, ICAM-1) and fibrosis-related (collagen III) gene expression in concert with reduced adipose pro-inflammatory (TNF-α, VCAM-1, ICAM-1) and lymphocyte (CD4) gene expression (Figure 2).
Figure 2.
Cardiac and adipose pro-inflammatory and fibrotic markers are elevated in Otsuka Long-Evans Tokushima Fatty (OLETF) rats and attenuated by mineralocorticoid receptor antagonism. Cardiac gene expression of pro-inflammatory (A; tumor necrosis factor-α [TNF-α], vascular cell adhesion molecular-1 [VCAM-1], and intracellular adhesion molecule-1 [ICAM-1]) and fibrosis (B, collagen I [Col I] and collagen III [Col III]) genes in hearts from Long-Evans Tokushima Otsuka (LETO), OLETF, and OLETF rats treated with spironolactone (Sp). Adipose gene expression of pro-inflammatory (C, TNF-α, VCAM-1, ICAM-1) and leukocyte (D, F4/80 and CD11c for macrophages; CD4 for T cells) genes in retroperitoneal adipose from LETO, OLETF, and OLETF Sp rats. Values are mean±SE; n=5–8; *p<0.05 versus LETO, †p<0.05 versus OLETF, ‡p=0.08 versus OLETF, §p=0.07 versus LETO.
3.3. MR inhibition normalized coronary endothelium-dependent vasodilation but not coronary constriction in OLETF rats.
As demonstrated in Figure 1, compared to LETO rats, 32 week old OLETF rats exhibit impaired endothelium-dependent vasodilation to acetylcholine, no change in endothelium-independent vasodilation to SNP (data not shown), and enhanced ET-1-induced vasoconstriction. In addition, at this age, OLETF rats exhibit insulin-induced coronary vasoconstriction as opposed to insulin-induced vasodilation in LETO rats (Figure 3). Lastly, 32 week old OLETF rats exhibit increased coronary vasoconstriction to the thromboxane A2 mimetic U46619 but not the L-type Ca2+ channel opener BayK-8644 (Figure 3). Treatment of OLETF rats with Sp for 3 weeks largely normalized impairments in acetylcholine- and insulin-induced vasodilation but did not reduce the enhanced vasoconstriction induced by ET-1 or U46619 despite modestly attenuating vasoconstriction to BayK-8644 (Figure 3).
Figure 3.
Impaired coronary arteriolar vasodilation, but not enhanced vasoconstriction, in OLETF rats is treated by mineralocorticoid receptor antagonism. A, Coronary vasodilation to acetylcholine and insulin in arterioles from 32 week old Long-Evans Tokushima Otsuka (LETO) control, untreated Otsuka Long-Evans Tokushima Fatty (OLETF) rats, and OLETF treated with spironolactone (OLETF Sp). B, Coronary vasoconstriction to endothelin-1, thromboxane A2 mimetic U46619, and the L-type Ca2+ channel opener BayK-8644. Values are mean±SE; n=5–12; *p<0.05 versus LETO, †p<0.05 versus OLETF.
3.4. MR inhibition induces pronounced differential vascular gene expression in OLETF rats.
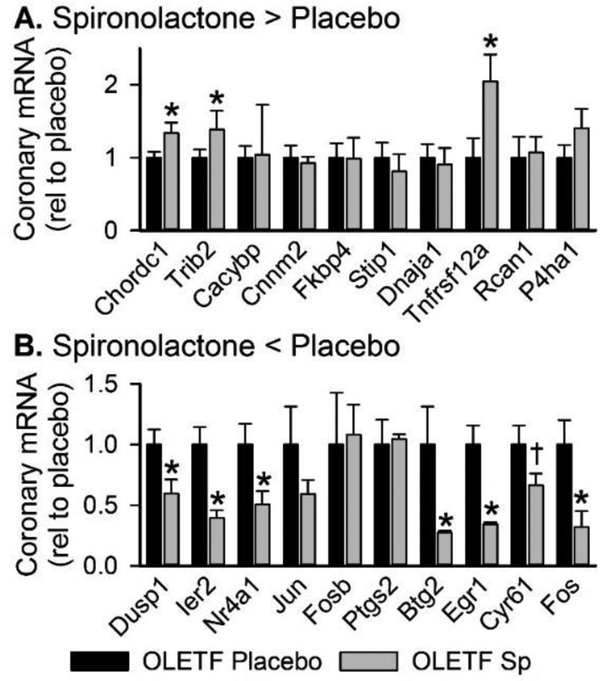
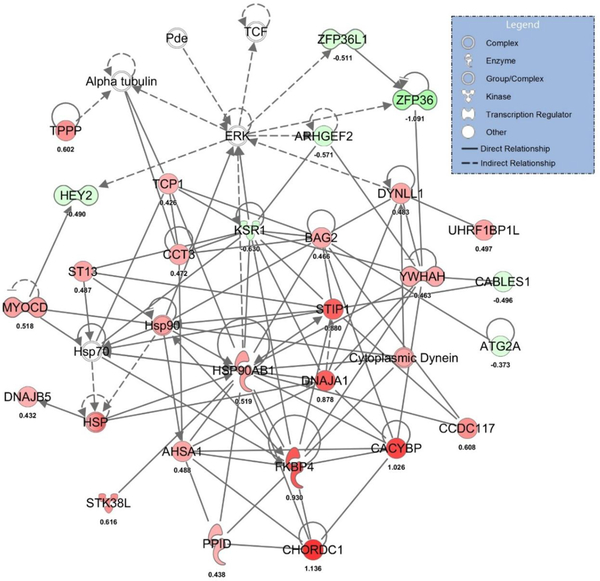
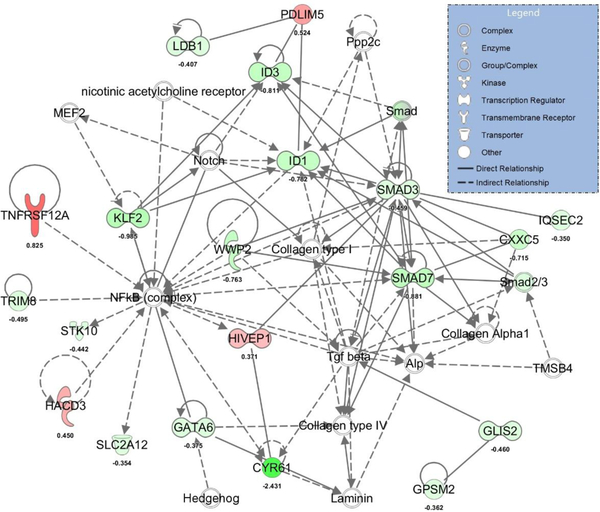
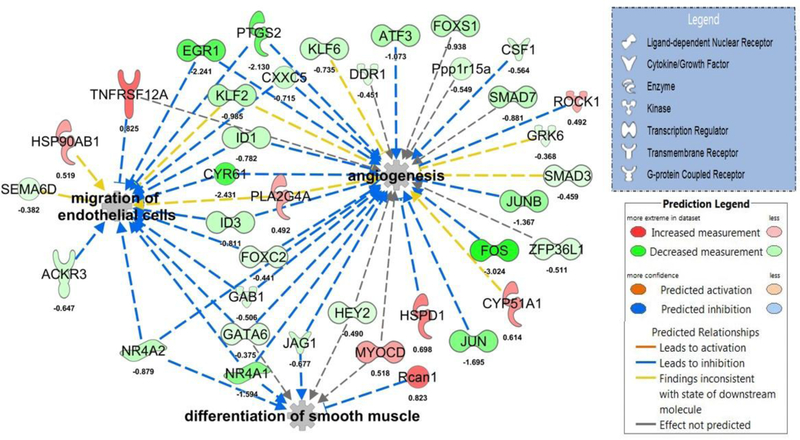
RNA-Seq analysis identified 157 differentially expressed transcripts (70 up- and 87 down-regulated) in whole aortas from Sp-treated compared to placebo-treated OLETF rats (Supplemental Table 4). The top up- and down-regulated mRNAs are presented in Table 2. Examination of coronary expression of these differentially expressed aortic genes in the coronary artery are presented in Figure 4 and these data reveal strong agreement between Sp-induced changes in aortic and coronary artery gene expression for many genes. Using all differentially expressed genes, IPA identified ‘NRF2-mediated oxidative stress response’, ‘glucocorticoid receptor signaling’, and ‘IL-2 signaling’ as the top regulated canonical pathways (Table 2). The top regulated gene networks identified by IPA in Sp versus placebo-treated rats included ‘drug metabolism, endocrine system development and function, lipid metabolism’ (network 1, IPA score 55, Figure 5) and ‘gene expression, cardiovascular system development and function, cellular movement’ (network 2, IPA score 37, Figure 6). Examination of potential upstream regulators mediating the measured changes in gene expression by Sp in IPA predicted inhibition of PDGF-BB, FOXO1, TNF, VEGF-A, NFκB, and IFN-γ signaling as well as activation of DHT, HDAC, NKX2–3, and NFE2L2 (i.e., NRF2) signaling (Supplemental Table 5). Lastly, based on all differentially expressed genes, assessment of the potential impact of Sp on biological functions by IPA revealed predicted inhibition of angiogenesis (IPA z-score −2.508), migration of endothelial cells (IPA z-score −2.172), and differentiation of smooth muscle (IPA z-score −2.00) by MR antagonism in OLETF rats (Figure 7).
Table 2.
Top up- and down-regulated genes and canonical pathways between spironolactone and placebo-treated OLETF treated rats
| Entrez ID | Symbol | Gene Name | FDR | Log Fold |
|---|---|---|---|---|
| Spironolactone-treated > Placebo-treated | ||||
| 26973 | Chordc1 | Cysteine And Histidine Rich Domain Containing 1 | 1.00E-07 | 1.14 |
| 28951 | Trib2 | Tribbles Pseudokinase 2 | 1.87E-07 | 1.07 |
| 27101 | Cacybp | Calcyclin Binding Protein | 2.40E-04 | 1.03 |
| 54805 | Cnnm2 | Cyclin And CBS Domain Divalent Metal Cation Transport Mediator 2 | 6.19E-05 | 0.94 |
| 2288 | Fkbp4 | FK506 Binding Protein 4 | 7.28E-06 | 0.93 |
| 10963 | Stip1 | Stress Induced Phosphoprotein 1 | 5.53E-05 | 0.88 |
| 3301 | Dnaja1 | DnaJ Heat Shock Protein Family (Hsp40) Member A1 | 2.31E-02 | 0.88 |
| 51330 | Tnfrsf12a | Tumor Necrosis Factor Receptor Superfamily, Member 12A | 5.36E-04 | 0.83 |
| 1827 | Rcan1 | Regulator Of Calcineurin 1 | 1.80E-03 | 0.82 |
| 5033 | P4ha1 | Prolyl 4-Hydroxylase Subunit Alpha 1 | 1.28E-04 | 0.82 |
| Spironolactone-treated < Placebo-treated | ||||
| 1843 | Dusp1 | Dual Specificity Phosphatase 1 | 2.02E-13 | −1.46 |
| 9592 | Ier2 | Immediate Early Response 2 | 2.90E-08 | −1.55 |
| 3164 | Nr4a1 | Nuclear Receptor Subfamily 4 Group A Member 1 | 3.92E-09 | −1.59 |
| 3725 | Jun | Jun Proto-Oncogene, AP-1 Transcription Factor Subunit | 3.62E-12 | −1.70 |
| 2354 | Fosb | FosB Proto-Oncogene, AP-1 Transcription Factor Subunit | 1.08E-05 | −1.74 |
| 5743 | Ptgs2 | Prostaglandin-Endoperoxide Synthase 2 | 1.87E-06 | −2.13 |
| 7832 | Btg2 | BTG Anti-Proliferation Factor 2 | 1.32E-26 | −2.17 |
| 1958 | Egr1 | Early Growth Response 1 | 3.08E-35 | −2.24 |
| 3491 | Cyr61 | Cysteine Rich Angiogenic Inducer 61 | 1.39E-19 | −2.43 |
| 2353 | Fos | Fos Proto-Oncogene, AP-1 Transcription Factor Subunit | 1.09E-21 | −3.02 |
| Top regulated canonical pathways | p value | Ratio | ||
| NRF2-mediated oxidative stress response | 1.22E-08 | 12/193 | ||
| Glucocorticoid receptor signaling | 9.45E-07 | 12/288 | ||
| IL-2 signaling | 5.81E-06 | 6/64 | ||
| Thrombopoetin signaling | 6.36E-06 | 6/65 | ||
| IGF-1 signaling | 9.96E-06 | 7/106 | ||
| ERK/MAPK signaling | 1.18E-05 | 9/199 | ||
| Estrogen-dependent breast cancer signaling | 1.70E-05 | 6/77 | ||
| Erythropoetin signaling | 2.28E-05 | 6/81 | ||
Figure 4.
Gene expression of differentially expressed aortic transcripts in coronary artery by PCR. The 10 top (A) up- and (B) down-regulated aortic transcripts determined by RNA-Seq were assessed in coronary arteries from placebo- and spironolactone (Sp)-treated Otsuka Long-Evans Tokushima Fatty (OLETF) rats by RT-PCR. Values are mean±SE; n=4–5; *p<0.05 versus OLETF Placebo, †p=0.06 versus OLETF Placebo.
Figure 5.
The top regulated gene network in Sp versus placebo-treated OLETF rats (IPA score 61). Up-regulated hubs indicated in red and down-regulated hubs indicated in green with brighter coloring indicating greater differences between Sp and placebo-treated rats. For convenience, log fold change differences of individual genes are provided below each gene.
Figure 6.
The second most highly regulated gene network in Sp versus placebo-treated OLETF rats (IPA score 37). Up-regulated hubs indicated in red and down-regulated hubs indicated in green with brighter coloring indicating greater differences between Sp and placebo-treated rats. For convenience, log fold change differences of individual genes are provided below each gene.
Figure 7.
Predicted impact of mineralocorticoid receptor antagonism on biological functions. Predictions based on IPA assessment of differentially expressed aortic transcripts in spironolactone- versus placebo-treated Otsuka Long-Evans Tokushima Fatty (OLETF) rats. The biological functions of angiogenesis (IPA z-score −2.508), migration of endothelial cells (IPA z-score −2.172), and differentiation of smooth muscle (IPA z-score −2.00) are predicted to be inhibited by spironolactone in this model. For convenience, log fold change differences of individual genes are provided below each gene.
4. Discussion
Emerging evidence suggests a principle role for inappropriate MR signaling underlying the development of coronary microvascular dysfunction in the setting of obesity and type 2 diabetes [5, 19, 20]. Accordingly, the present results demonstrate that systemic MR antagonism with Sp attenuates (i.e., treats) established coronary microvascular dysfunction and reduces pro-inflammatory/fibrotic gene expression in visceral adipose and cardiac tissues independent of blood pressure in a model recapitulating later stages of human type 2 diabetes. To begin to identify potential mechanisms underlying the vascular benefit of MR antagonism in diabetes, the vascular transcriptome was sequenced revealing a number of differentially regulated mRNA transcripts and networks. To our knowledge, this is the first investigation of the vascular transcriptomic signature underlying the therapeutic impact of MR antagonism in diabetes, thus providing a unique snapshot of potential MR-dependent mechanisms of diabetes-related vascular dysfunction.
The natural history of obesity and diabetes in the OLETF rat closely resembles that in humans vis-à-vis the late (i.e., adult) onset of hyperglycemia and overt diabetes as well as the development of coronary microvascular dysfunction [22–24]. Indeed, impaired CFR has been reported in this model as early as 15 weeks of age concomitant with coronary perivascular fibrosis [24]. Our findings extend these data by demonstrating impaired endothelium-dependent vasodilation at 5–8 weeks of age preceding the reported change in CFR and the progressive development of enhanced coronary vasoconstrictor responses beginning at 20 weeks of age. Although the temporal relationship between impaired endothelium-dependent vasodilation and reduced CFR is unclear in human patients, both are independent predictors of future cardiovascular morbidity and mortality in the absence of overt coronary atherosclerosis [3, 31, 32]. It has previously been established that coronary arteriolar dilation to ACh in rats is NO-dependent [33] and that NO inhibition and removal of the endothelium enhance ET-1-mediated coronary constriction [34, 35]. Thus, the impairment of ACh-induced vasodilation and the progressive increase in ET-1-mediated constriction in coronary arterioles of the OLETF rat may arise from reduced NO bioavailability as we previously reported in skeletal muscle feed arteries in this model [36]. Increased ET-1-induced constriction in this model may also involve sensitization of coronary ET-1 receptors as was previously reported in obese dogs [37] consistent with our previous report of reduced ET-1 type A receptors in the OLETF rat [36]. Nonetheless, these findings indicate that endothelial vasodilator dysfunction may be the earliest coronary defect in obesity leading to additional impairments of coronary function and impaired myocardial blood flow regulation.
It has recently been proposed that the coronary dysfunction associated with obesity and diabetes, as well as subsequent impairment of cardiac diastolic dysfunction, arises principally from a systemic pro-inflammatory state [1]. Our data are consistent with this paradigm demonstrating increased pro-inflammatory and pro-fibrotic gene expression in both visceral adipose and cardiac tissues of the OLETF rat. Importantly, and in line with this proposition, MR antagonism with Sp reduced both adipose and cardiac pro-inflammatory/pro-fibrotic gene expression in concert with restored coronary endothelial function with no change in blood pressure. These data are consistent with our recent report that MR antagonism reversed coronary dysfunction as well as adipose and cardiac inflammation in the obese Zucker rat model of insulin resistance [5]. Together with available evidence in patients with type 2 diabetes [19, 20], these studies demonstrate a clear impact of MR antagonism to treat obesity/diabetes-associated coronary microvascular endothelial dysfunction. Surprisingly, however, MR antagonism did not attenuate diabetes-associated increases of coronary vasoconstrictor responses despite a modest reduction of vasoconstriction to L-type Ca2+ channel activation. The latter finding is consistent with the recent report that vascular L-type Ca2+ channels are upregulated by smooth muscle MR activation in aging [8, 9]. Together, these data suggest that mechanisms other than MR activation may be principle contributors to enhanced coronary constriction despite the improvement of endothelial function following MR antagonism in the obese, diabetic OLETF rat. Importantly, it should be noted that MR antagonism in both the obese Zucker [5] and OLETF rat models is beneficial despite reduced plasma aldosterone levels indicating a limitation of plasma aldosterone as an index of pathologic MR activation.
In order to delineate potential mechanisms underlying the benefit of MR antagonism on vascular function in the setting of obesity and diabetes, we employed RNA Seq coupled with IPA analysis of differentially expressed genes. In total, RNA Seq revealed 157 differentially expressed genes in aortas from OLETF rats following MR antagonism. MR-dependent regulation of vascular cell gene expression has previously been evaluated via short-term treatment of isolated vessels or cultured vascular cells with the MR agonist aldosterone [13, 14, 16, 38, 39]. Conversely, our study examined the impact of chronic MR antagonism with Sp in vivo on the vascular transcriptome in the setting of established obesity and diabetes. In this way, to our knowledge, this is the first study of its kind focused on delineating potential transcriptomic mechanisms underlying the vascular benefit of MR antagonism in obesity/diabetes. It should be noted that only four genes (Egr-1, Nedd9, Tsc22d3, Zfp281) identified in this study overlap with genes differentially expressed by acute MR activation in healthy cells/vessels ex vivo [14, 16, 38]. Together, these results support a growing recognition of significant context dependency of MR-dependent gene regulation [40] warranting further examination in disease states associated with inappropriate renin-angiotensin-aldosterone system activation.
Vascular transcriptomic analysis in Sp- versus placebo-treated OLETF rats revealed several differentially regulated transcripts and pathways which likely contribute to the Sp-mediated improvement of coronary microvascular vasodilation, a primary functional outcome of this study. First, Sp treatment resulted in upregulation of aortic Hsp90ab1 mRNA as well as upregulation of aortic Ahsa1 and Ahsa2, highlighted in the top regulated gene network determined by IPA. Hsp90 is a molecular chaperone and key mediator of agonist-mediated endothelial NO synthase (eNOS) activation and NO production [41]. Further, Ahsa1 and Ahsa2 (aka AHA1) are Hsp90 co-chaperones directly involved in agonist-induced Hsp90 phosphorylation, eNOS activation, and NO production [42]. These findings implicate improved dynamic regulation of eNOS-mediated NO production as a potential mechanism underlying enhanced coronary endothelium-dependent vasodilation following MR blockade in diabetes. Second, consistent with reduced cardiac and adipose pro-inflammatory/pro-fibrotic gene expression by PCR, RNA Seq revealed transcriptomic changes indicative of reduced vascular inflammation and oxidative stress following MR blockade. Specifically, several of the top genes downregulated by MR blockade are subunits necessary for pro-inflammatory AP-1 signaling (Jun, Fosb, Fos) and upstream regulator analysis by IPA was enriched for predicted inhibition of endogenous pro-inflammatory/pro-fibrotic mediators by Sp (PDGF-BB, TNF, NFκB, etc). In addition, the top canonical pathway in IPA impacted by MR blockade was ‘NRF2-mediated oxidative stress response’. NRF2 signaling is a critical positive regulator of antioxidant defenses, the activation of which attenuates vascular oxidative stress [43, 44]. Genes of this canonical pathway (Dnaja1, Dnajb5, Ephx1, Fos, Gab1, Hacd3, Jun, Junb, Jund, Pik3c3, Rras2, Stip1) are altered by Sp in a manner suggesting activation of this pathway consistent with NRF2 (NFE2L2) being identified by IPA as a predicted upstream regulator activated by Sp. These data suggest a broad impact of MR antagonism on vascular redox state in diabetes involving not only reduction of well described pro-oxidant actions of MR activation [6] but also a less well appreciated enhancement of antioxidant defenses in the vasculature warranting further exploration. Taken together, these data support the paradigm that vascular inflammation/endothelial activation underlies coronary (and ultimately cardiac) dysfunction in obesity/diabetes and that the benefit of MR antagonism involves reduced vascular inflammation and improved regulation of NO bioavailability.
Examination of potential biological functions impacted by MR antagonism via IPA lends additional support to reduced vascular inflammation and endothelial activation with Sp in diabetes. Specifically, IPA predicted that MR antagonism will inhibit migration of endothelial cells, smooth muscle cell differentiation, and angiogenesis in line with identification of VEGF-A as a Sp-inhibited upstream regulator. Endothelial activation and oxidative stress are key steps in the initiation of endothelial migration, particularly in the context of angiogenesis. Interestingly, available evidence for the role of MR signaling in angiogenesis is inconsistent. For instance, in models of hindlimb ischemia via femoral artery occlusion, MR antagonism improved angiogenesis [45] while infusion of the MR agonist aldosterone has been reported to enhance [46] or attenuate [47] angiogenesis via MR-dependent mechanisms. Thus, further studies regarding the role of vascular MR signaling in peripheral angiogenesis are warranted, particularly in the setting of obesity and diabetes where this has not been examined.
Beyond the changes in gene expression consistent with our functional data, RNA-Seq revealed other novel Sp-induced changes in the diabetic vascular transcriptome. Notably, MR antagonism resulted in downregulation of the α1D adrenergic receptor (Adra1d) and atypical chemokine receptor 3 (Ackr3) gene expression in OLETF rats. Recent evidence indicates that these receptors, along with chemokine (C-X-C motif) receptor (CXCR) 4, engage in a dynamic process of hetero-oligomeric complex formation in vascular smooth muscle [48, 49]. The makeup of these complexes modulates α1D-dependent responses (i.e., vasoconstriction) and this previously unreported downregulation of Adra1d itself as well as the modulatory Ackr3 by MR antagonism is worthy of further examination as sensitization of coronary α1 adrenergic receptors and increased α1-dependent coronary vasoconstriction have been reported in prediabetic dogs [50]. Our transcriptomic analysis also revealed pronounced downregulation of prostaglandin-endoperoxide synthase 2 (Ptgs2; i.e., cyclooxygenase 2) as well as upregulation of acyl-CoA synthetase long-chain family member 4 (Acsl4). These directional changes would be expected to reduce vascular production of the vasoconstrictor prostaglandin E2 (PGE2) by directly reducing Ptgs2 that produces PGE2 [51] as well as reducing membrane phospholipid substrate for Ptgs2 via increased Acsl4 [52]. These MR-associated alterations may provide novel mechanistic insight into the previously reported impairment of endothelium-dependent vasodilation in a cyclooxygenase-dependent manner in aldosterone-infused mice [17].
In summary, results of the present study identify a variety of novel vascular transcripts and gene networks associated with the benefit of MR antagonism to reverse coronary microvascular vasodilator dysfunction in diabetes. Importantly, this benefit occurred independent of changes in blood pressure or serum potassium. These results are clinically relevant given the growing appreciation of the role of coronary microvascular dysfunction in the etiology of cardiac disease [53] as well as the powerful association of coronary microvascular dysfunction and cardiac mortality in diabetic patients [3]. The efforts of the present study therefore provide novel insight into and the first report of potential vascular-specific targets linked to MR activation in diabetes worthy of further examination. Ultimately, enhanced understanding of vascular MR signaling in diabetes holds promise to address the critical need for improved coronary flow regulation in diabetes in order to prevent cardiac diastolic dysfunction, the primary cardiac defect in heart failure with preserved ejection fraction.
Supplementary Material
5. Acknowledgements
We gratefully acknowledge critical evaluation of this manuscript by Dr. Iris Jaffe and the assistance of Dr. M. Harold Laughlin, Nathan Rehmer, Mona Garro-Kacher, Pam Thorne, Grace Meers, and Kristyn Sanders.
6. Funding
This work was supported by the Department of Veterans Affairs BLR&D [CDA-2 IK2 BX002030 to SBB, VA Merit Grant I01 BX003271–01 to RSR], the National Institutes of Health [R01 HL136386 to SBB], the University of Missouri Department of Medicine Research Council [to SBB], and the use of facilities and resources at the Harry S Truman Memorial Veterans Hospital in Columbia, MO.
Abbreviations:
- ACh
acetylcholine
- BP
blood pressure
- CD4
cluster of differentiation 4
- CD11c
cluster of differentiation 11c
- Col I
collagen I
- Col III
collagen III
- CFR
coronary flow reserve
- ET-1
endothelin-1
- F4/80
EGF-like module-containing mucin-like hormone receptor-like 1
- ICAM-1
intercellular adhesion molecular-1
- LETO
Long-Evans Tokushima Otsuka
- MR
mineralocorticoid receptor
- OLETF
Otsuka Long-Evans Tokushima Fatty
- PSS
physiological salt solution
- SNP
sodium nitroprusside
- Sp
spironolactone
- TNF-α
tumor necrosis factor-α
- U46619
thromboxane A2 mimetic
- VCAM-1
vascular cell adhesion molecule-1
Footnotes
Chemical compounds studied in this article: Spironolactone (PubChem CID: 5833), Acetylcholine (PubChem CID: 187), Insulin (PubChem CID: 118984375), Sodium Nitroprusside (PubChem CID: 11963622), Endothelin-1 (PubChem CID: 16212950), U-46619 (PubChem CID: 5311493), Bay-K 8644 (PubChem CID: 2303)
Declarations of Interest: none
6. References
- [1].Paulus WJ, Tschöpe C, A Novel Paradigm for Heart Failure With Preserved Ejection Fraction: Comorbidities Drive Myocardial Dysfunction and Remodeling Through Coronary Microvascular Endothelial Inflammation, J. Am. Coll. Cardiol 62(4) (2013) 263–271. [DOI] [PubMed] [Google Scholar]
- [2].Taqueti VR, Everett BM, Murthy VL, Gaber M, Foster CR, Hainer J, Blankstein R, Dorbala S, Di Carli MF, Interaction of Impaired Coronary Flow Reserve and Cardiomyocyte Injury on Adverse Cardiovascular Outcomes in Patients Without Overt Coronary Artery Disease, Circulation 131(6) (2015) 528–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Murthy VL, Naya M, Foster CR, Gaber M, Hainer J, Klein J, Dorbala S, Blankstein R, Di Carli MF, Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus, Circulation 126(15) (2012) 1858–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Suhrs HE, Michelsen MM, Prescott E, Treatment strategies in coronary microvascular dysfunction: a systematic review of interventional studies, Microcirculation (2017) doi: 10.1111/micc.12430. [DOI] [PubMed] [Google Scholar]
- [5].Bender SB, DeMarco VG, Padilla J, Jenkins NT, Habibi J, Garro M, Pulakat L, Aroor AR, Jaffe IZ, Sowers JR, Mineralocorticoid Receptor Antagonism Treats Obesity-Associated Cardiac Diastolic Dysfunction, Hypertension 65(5) (2015) 1082–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].McCurley A, Jaffe IZ, Mineralocorticoid receptors in vascular function and disease, Mol. Cell. Endocrinol 350(2) (2012) 256–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].DuPont JJ, Hill MA, Bender SB, Jaisser F, Jaffe IZ, Aldosterone and Vascular Mineralocorticoid Receptors: Regulators of Ion Channels Beyond the Kidney, Hypertension 63(4) (2014) 632–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].DuPont JJ, McCurley A, Davel AP, McCarthy J, Bender SB, Hong K, Yang Y, Yoo J-K, Aronovitz M, Baur WE, Christou DD, Hill MA, Jaffe IZ, Vascular mineralocorticoid receptor regulates microRNA-155 to promote vasoconstriction and rising blood pressure with aging, JCI Insight 1(14) (2016) e88942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].McCurley A, Pires PW, Bender SB, Aronovitz M, Zhao MJ, Metzger D, Chambon P, Hill MA, Dorrance AM, Mendelsohn ME, Jaffe IZ, Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors, Nat. Med 18(9) (2012) 1429–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Barrett Mueller K, Bender SB, Hong K, Yang Y, Aronovitz M, Jaisser F, Hill MA, Jaffe IZ, Endothelial Mineralocorticoid Receptors Differentially Contribute to Coronary and Mesenteric Vascular Function Without Modulating Blood Pressure, Hypertension 66(5) (2015) 988–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sherajee SJ, Fujita Y, Rafiq K, Nakano D, Mori H, Masaki T, Hara T, Kohno M, Nishiyama A, Hitomi H, Aldosterone induces vascular insulin resistance by increasing insulin-like growth factor-1 receptor and hybrid receptor, Arterioscler. Thromb. Vasc. Biol 32(2) (2012) 257–63. [DOI] [PubMed] [Google Scholar]
- [12].Caprio M, Newfell BG, la Sala A, Baur W, Fabbri A, Rosano G, Mendelsohn ME, Jaffe IZ, Functional mineralocorticoid receptors in human vascular endothelial cells regulate intercellular adhesion molecule-1 expression and promote leukocyte adhesion, Circ. Res 102(11) (2008) 1359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jaffe IZ, Mendelsohn ME, Angiotensin II and aldosterone regulate gene transcription via functional mineralocortocoid receptors in human coronary artery smooth muscle cells, Circ. Res 96(6) (2005) 643–50. [DOI] [PubMed] [Google Scholar]
- [14].Newfell BG, Iyer LK, Mohammad NN, McGraw AP, Ehsan A, Rosano G, Huang PL, Mendelsohn ME, Jaffe IZ, Aldosterone regulates vascular gene transcription via oxidative stress-dependent and -independent pathways, Arterioscler. Thromb. Vasc. Biol 31(8) (2011) 1871–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jaffe IZ, Newfell BG, Aronovitz M, Mohammad NN, McGraw AP, Perreault RE, Carmeliet P, Ehsan A, Mendelsohn ME, Placental growth factor mediates aldosterone-dependent vascular injury in mice, J. Clin. Invest 120(11) (2010) 3891–3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sekizawa N, Yoshimoto T, Hayakawa E, Suzuki N, Sugiyama T, Hirata Y, Transcriptome analysis of aldosterone-regulated genes in human vascular endothelial cell lines stably expressing mineralocorticoid receptor, Mol. Cell. Endocrinol 341(1–2) (2011) 78–88. [DOI] [PubMed] [Google Scholar]
- [17].Schafer N, Lohmann C, Winnik S, van Tits LJ, Miranda MX, Vergopoulos A, Ruschitzka F, Nussberger J, Berger S, Luscher TF, Verrey F, Matter CM, Endothelial mineralocorticoid receptor activation mediates endothelial dysfunction in diet-induced obesity, Eur. Heart J 34(45) (2013) 3515–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tune JD, Goodwill AG, Sassoon DJ, Mather KJ, Cardiovascular consequences of metabolic syndrome, Trans. Res 183 (2017) 57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Joffe HV, Kwong RY, Gerhard-Herman MD, Rice C, Feldman K, Adler GK, Beneficial effects of eplerenone versus hydrochlorothiazide on coronary circulatory function in patients with diabetes mellitus, J. Clin. Endocrinol. Metab 92(7) (2007) 2552–8. [DOI] [PubMed] [Google Scholar]
- [20].Garg R, Rao AD, Baimas-George M, Hurwitz S, Foster C, Shah RV, Jerosch-Herold M, Kwong RY, Di Carli MF, Adler GK, Mineralocorticoid receptor blockade improves coronary microvascular function in individuals with type 2 diabetes, Diabetes 64(1) (2015) 236–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T, Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima Fatty (OLETF) strain, Diabetes 41(11) (1992) 1422–1428. [DOI] [PubMed] [Google Scholar]
- [22].Rector RS, Thyfault JP, Uptergrove GM, Morris EM, Naples SP, Borengasser SJ, Mikus CR, Laye MJ, Laughlin MH, Booth FW, Ibdah JA, Mitochondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non-alcoholic fatty liver disease in an obese rodent model, J. Hepatol 52(5) (2010) 727–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kawano K, Hirashima T, Mori S, Natori T, OLETF (Otsuka Long-Evans Tokushima Fatty) rat: a new NIDDM rat strain, Diabetes Res. Clin. Pract 24, Supplement(0) (1994) S317–S320. [DOI] [PubMed] [Google Scholar]
- [24].Yu Y, Ohmori K, Kondo I, Yao L, Noma T, Tsuji T, Mizushige K, Kohno M, Correlation of functional and structural alterations of the coronary arterioles during development of type II diabetes mellitus in rats, Cardiovasc. Res 56(2) (2002) 303–11. [DOI] [PubMed] [Google Scholar]
- [25].Bostick B, Habibi J, DeMarco VG, Jia G, Domeier TL, Lambert MD, Aroor AR, Nistala R, Bender SB, Garro M, Hayden MR, Ma L, Manrique C, Sowers JR, Mineralocorticoid receptor blockade prevents Western diet-induced diastolic dysfunction in female mice, Am. J. Physiol. Heart Circ. Physiol 308(9) (2015) H1126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].de Gasparo M, Joss U, Ramjoue HP, Whitebread SE, Haenni H, Schenkel L, Kraehenbuehl C, Biollaz M, Grob J, Schmidlin J, et al. , Three new epoxy-spirolactone derivatives: characterization in vivo and in vitro, J. Pharmacol. Exp. Ther 240(2) (1987) 650–6. [PubMed] [Google Scholar]
- [27].Bender SB, Tune JD, Borbouse L, Long X, Sturek M, Laughlin MH, Altered mechanism of adenosine-induced coronary arteriolar dilation in early-stage metabolic syndrome, Exp. Biol. Med. (Maywood) 234(6) (2009) 683–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Benjamini Y, Hochberg Y, Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing, Journal of the Royal Statistical Society. Series B (Methodological) 57(1) (1995) 289–300. [Google Scholar]
- [29].Laye MJ, Rector RS, Warner SO, Naples SP, Perretta AL, Uptergrove GM, Laughlin MH, Thyfault JP, Booth FW, Ibdah JA, Changes in visceral adipose tissue mitochondrial content with type 2 diabetes and daily voluntary wheel running in OLETF rats, J. Physiol 587(Pt 14) (2009) 3729–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Noguchi S, Ohno Y, Aoki N, Adrenocortical insufficiency in Otsuka Long-Evans Tokushima Fatty rats, a type 2 diabetes mellitus model, Metabolism. 56(10) (2007) 1326–1333. [DOI] [PubMed] [Google Scholar]
- [31].Murthy VL, Naya M, Taqueti VR, Foster CR, Gaber M, Hainer J, Dorbala S, Blankstein R, Rimoldi O, Camici PG, Di Carli MF, Effects of Sex on Coronary Microvascular Dysfunction and Cardiac Outcomes, Circulation 129(24) (2014) 2518–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, Nour KR, Quyyumi AA, Prognostic value of coronary vascular endothelial dysfunction, Circulation 106(6) (2002) 653–8. [DOI] [PubMed] [Google Scholar]
- [33].LeBlanc AJ, Shipley RD, Kang LS, Muller-Delp JM, Age impairs Flk-1 signaling and NO-mediated vasodilation in coronary arterioles, Am. J. Physiol. Heart Circ. Physiol 295(6) (2008) H2280–H2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bender SB, Klabunde RE, Altered role of smooth muscle endothelin receptors in coronary endothelin-1 and alpha1-adrenoceptor-mediated vasoconstriction in Type 2 diabetes, Am. J. Physiol. Heart Circ. Physiol 293(4) (2007) H2281–8. [DOI] [PubMed] [Google Scholar]
- [35].Shipley RD, Muller-Delp JM, Aging decreases vasoconstrictor responses of coronary resistance arterioles through endothelium-dependent mechanisms, Cardiovasc. Res 66(2) (2005) 374–383. [DOI] [PubMed] [Google Scholar]
- [36].Bender SB, Newcomer SC, Harold Laughlin M, Differential vulnerability of skeletal muscle feed arteries to dysfunction in insulin resistance: impact of fiber type and daily activity, Am. J. Physiol. Heart Circ. Physiol 300(4) (2011) H1434–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Knudson JD, Rogers PA, Dincer UD, Bratz IN, Araiza AG, Dick GM, Tune JD, Coronary vasomotor reactivity to endothelin-1 in the prediabetic metabolic syndrome, Microcirculation 13(3) (2006) 209–18. [DOI] [PubMed] [Google Scholar]
- [38].Ducros E, Berthaut A, Mirshahi SS, Faussat AM, Soria J, Agarwal MK, Mirshahi M, Aldosterone modifies hemostasis via upregulation of the protein-C receptor in human vascular endothelium, Biochem. Biophys. Res. Commun 373(2) (2008) 192–196. [DOI] [PubMed] [Google Scholar]
- [39].Nakamura Y, Suzuki S, Suzuki T, Ono K, Miura I, Satoh F, Moriya T, Saito H, Yamada S, Ito S, Sasano H, MDM2: A Novel Mineralocorticoid-Responsive Gene Involved in Aldosterone-Induced Human Vascular Structural Remodeling, Am. J. Pathol 169(2) (2006) 362–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].DuPont JJ, Jaffe IZ, 30 YEARS OF THE MINERALOCORTICOID RECEPTOR: The role of the mineralocorticoid receptor in the vasculature, J. Endocrinol 234(1) (2017) T67–T82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Michel T, Vanhoutte PM, Cellular signaling and NO production, Pflügers Archiv 459(6) (2010) 807–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Desjardins F, Delisle C, Gratton J-P, Modulation of the Cochaperone AHA1 Regulates Heat-Shock Protein 90 and Endothelial NO Synthase Activation by Vascular Endothelial Growth Factor, Arterioscler. Thromb. Vasc. Biol 32(10) (2012) 2484–2492. [DOI] [PubMed] [Google Scholar]
- [43].Levonen A-L, Inkala M, Heikura T, Jauhiainen S, Jyrkkänen H-K, Kansanen E, Määttä K, Romppanen E, Turunen P, Rutanen J, Ylä-Herttuala S, Nrf2 Gene Transfer Induces Antioxidant Enzymes and Suppresses Smooth Muscle Cell Growth In Vitro and Reduces Oxidative Stress in Rabbit Aorta In Vivo, Arterioscler. Thromb. Vasc. Biol 27(4) (2007) 741–747. [DOI] [PubMed] [Google Scholar]
- [44].Ashino T, Yamamoto M, Yoshida T, Numazawa S, Redox-Sensitive Transcription Factor Nrf2 Regulates Vascular Smooth Muscle Cell Migration and Neointimal Hyperplasia, Arterioscler. Thromb. Vasc. Biol 33(4) (2013) 760–768. [DOI] [PubMed] [Google Scholar]
- [45].Kobayashi N, Fukushima H, Takeshima H, Koguchi W, Mamada Y, Hirata H, Machida Y, Suzuki N, Yokotsuka F, Tabei K, Kobayashi E, Fukuda N, Ishimitsu T, Effect of Eplerenone on Endothelial Progenitor Cells and Oxidative Stress in Ischemic Hindlimb, Am. J. Hypertens 23(9) (2010) 1007–1013. [DOI] [PubMed] [Google Scholar]
- [46].Michel F, Ambroisine M-L, Duriez M, Delcayre C, Levy BI, Silvestre J-S, Aldosterone Enhances Ischemia-Induced Neovascularization Through Angiotensin II–Dependent Pathway, Circulation 109(16) (2004) 1933–1937. [DOI] [PubMed] [Google Scholar]
- [47].Fujii M, Inoki I, Saga M, Morikawa N, K.-i. Arakawa, S. Inaba, K. Yoshioka, T. Konoshita, I. Miyamori, Aldosterone inhibits endothelial morphogenesis and angiogenesis through the downregulation of vascular endothelial growth factor receptor-2 expression subsequent to peroxisome proliferator-activated receptor gamma, J. Steroid Biochem Mol. Biol 129(3–5) (2012) 145–152. [DOI] [PubMed] [Google Scholar]
- [48].Albee LJ, Eby JM, Tripathi A, LaPorte HM, Gao X, Volkman BF, Gaponenko V, Majetschak M, α1‐Adrenergic Receptors Function Within Hetero‐Oligomeric Complexes With Atypical Chemokine Receptor 3 and Chemokine (C‐X‐C motif) Receptor 4 in Vascular Smooth Muscle Cells, J. Am. Heart Assoc 6(8) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bach HH, Wong YM, Tripathi A, Nevins AM, Gamelli RL, Volkman BF, Byron KL, Majetschak M, Chemokine (C-X-C Motif) Receptor 4 and Atypical Chemokine Receptor 3 Regulate Vascular α(1)-Adrenergic Receptor Function, Mol. Med 20(1) (2014) 435–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Dincer UD, Araiza AG, Knudson JD, Molina PE, Tune JD, Sensitization of coronary alpha-adrenoceptor vasoconstriction in the prediabetic metabolic syndrome, Microcirculation 13(7) (2006) 587–95. [DOI] [PubMed] [Google Scholar]
- [51].Soler M, Camacho M, Escudero J-R, Iñiguez MA, Vila L, Human Vascular Smooth Muscle Cells but Not Endothelial Cells Express Prostaglandin E Synthase, Circ. Res 87(6) (2000) 504–507. [DOI] [PubMed] [Google Scholar]
- [52].Golej DL, Askari B, Kramer F, Barnhart S, Vivekanandan-Giri A, Pennathur S, Bornfeldt KE, Long-chain acyl-CoA synthetase 4 modulates prostaglandin E2 release from human arterial smooth muscle cells, J. Lipid Res 52(4) (2011) 782–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Pries AR, Reglin B, Coronary microcirculatory pathophysiology: can we afford it to remain a black box?, Eur. Heart J 38(7) (2017) 478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.