Abstract
Cardiovascular diseases are often associated with impaired lipid metabolism. Animal models are useful for deciphering the physiological mechanisms underlying these pathologies. However, lipid metabolism is contrasted between species limiting the transposition of findings from animals to human. Hence, we aimed to compare extended lipid profiles of several animal species to bring new insights in animal model selections. Human lipid phenotype was compared with those of 10 animal species. Standard plasma lipids and lipoprotein profiles were obtained by usual methods and lipidomic analysis was conducted by liquid chromatography-high-resolution mass spectrometry (LC-HRMS). As anticipated, we found contrasted lipid profiles between species. Some of them exhibited similar plasma lipids to human (non-human primate, rat, hamster, pig), but only usual lipid profiles of pigs were superimposable with human. LC-HRMS analyses allowed the identification of 106 other molecular species of lipids, common to all samples and belonging to major lipid families. Multivariate analyses clearly showed that hamster and, in a lower extent mouse, exhibited close lipid fingerprints to that of human. Besides, several lipid candidates that were previously reported to study cardiovascular diseases ranged similarly in human and hamster. Hence, hamster appeared to be the best option to study physiological disturbances related to cardiovascular diseases.
Introduction
Atherothrombotic cardiovascular diseases (ACVD) are the leading cause of death worldwide and are widely associated with lipid disturbances1–3. Animal models are essential to understand the physiological mechanisms of ACVD in humans. However, lipoprotein metabolism and vascular physiology are often contrasted between species limiting the transposition of findings from animals to human4,5. Such differences should be considered for animal model selection and for data understanding within a realistic extrapolation framework for a better assessment of disturbances involved in humans6.
Most of lipid comparisons between species are primarily focused on plasma lipoprotein profiles and major lipid components such as total cholesterol (TC) and triglycerides (TG)7. However, other circulating lipids within or out lipoproteins could also potentially be involved in the process of atherosclerotic lesions. In that respect, glycerophospholipids and sphingolipids have been shown to be significantly altered during the atherosclerotic progression in apoE−/− mice8, and other studies showed that elevated concentrations of lysophosphatidylcholines (LPC) and sphingomyelins (SM) were also correlated with atherosclerosis development9,10. These findings have been recently supported by human prospective ACVD outcome studies11.
Here we explored an extended lipid profile of nine animal species including non-human primate, dog, cat, pig, horse, bovine, hamster, mouse and rat along with human. Some of these species were already described as potential models for human ACVD12. We aimed to investigate both global (non-targeted) and specific (targeted) lipid profiles in those species to create global lipid fingerprints and then assess the similarity and differences compared with humans.
Results
Lipoprotein profiles
Plasma lipids were measured for the different animal species and are displayed in Table 1. In contrast to human, FPLC profiles showed most of species carried cholesterol primarily within HDL particles (Fig. 1). For NHP, mouse, dog, cat, horse and cow, LDL cholesterol was markedly lower than HDL cholesterol (Fig. 1). In contrast, pig displayed a lipoprotein profile comparable to human characterized by elevated LDL cholesterol. Of note, hamster and rat exhibited similar lipoprotein profiles characterized by a strong overlap between both LDL and HDL particles (Fig. 1). Finally, triglyceride-rich lipoproteins (VLDL) were mainly detected in human and non-herbivorous species.
Table 1.
Plasma lipid concentrations in human and animal plasma.
| Species | Strain | n | TC | TG | CE | PL |
|---|---|---|---|---|---|---|
| Human | Caucasian | 6 | 201 ± 34 | 62 ± 13 | 164 ± 28 | 231 ± 19 |
| NHP | Cynomolgus | 6 | 126 ± 8 | 56 ± 10 | 106 ± 7 | 212 ± 33 |
| Mouse | C57BL/6 | 6 | 135 ± 7 | 111 ± 27 | 114 ± 6 | 261 ± 17 |
| Rat | Wistar | 6 | 133 ± 8 | 64 ± 13 | 111 ± 8 | 223 ± 15 |
| Hamster | Golden Syrian | 6 | 172 ± 33 | 87 ± 25 | 134 ± 28 | 263 ± 30 |
| Pig | West white pork | 6 | 133 ± 15 | 43 ± 8 | 116 ± 14 | 129 ± 13 |
| Bovine | Holstein | 6 | 99 ± 34 | 11 ± 4 | 84 ± 31 | 89 ± 22 |
| Horse | Lusitanian | 6 | 136 ± 18 | 29 ± 16 | 114 ± 16 | 186 ± 26 |
| Dog | Beagle | 6 | 320 ± 61 | 39 ± 6 | 266 ± 45 | 344 ± 35 |
| Cat | European | 6 | 248 ± 43 | 31 ± 9 | 209 ± 38 | 231 ± 18 |
Values are mean ± standard deviation (3 males, 3 females) and are expressed in mg/dL.
TC, total cholesterol; TG, triglycerides; CL, cholesterol esters; PL, phospholipids.
Figure 1.
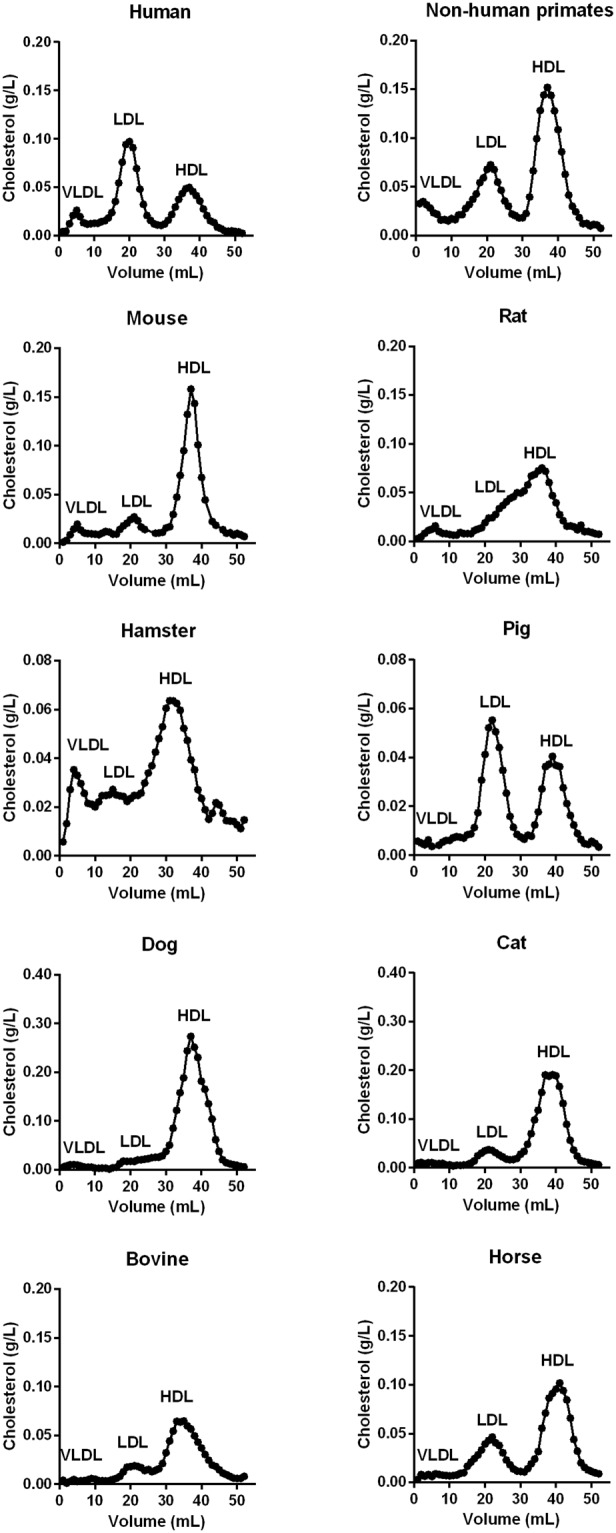
Lipoprotein profiles of studied species. The mean lipoprotein profiles (n = 6) were obtained by fast performance liquid chromatography (FPLC).
Non-targeted lipidomics
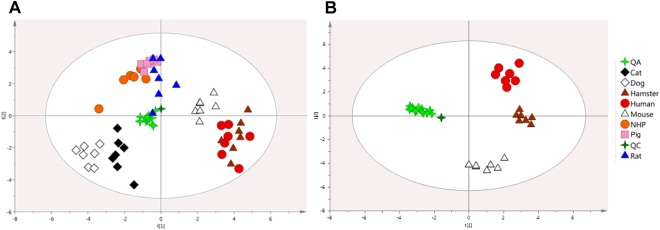
A dataset of 4,899 variables common to all species was obtained from raw data. An unsupervised PCA model was applied to get an overview of variance between lipid profiles of species (Fig. 2). The analytical QA samples were well-clustered on the PCA model and plotted together with the biological QC sample attesting the quality of the analysis. In addition, the individual biological QCspecies samples were plotted within each corresponding species plasma samples. This established PCA model explained 54% of the total variability and highlighted clustering between some animal species. Bovine and equine species (the herbivorous group), were characterized by a relatively low level of TG in line with biochemical measurements. This could likely explain their clustering and discrimination from the other species (Fig. 2). In sharp contrast, humans were plotted together with hamsters and were both well discriminated from the other species. Of note, no clear difference was observed between genders for each species. This underlined that sex differences were likely masked by those of species.
Figure 2.
Comparison of non-targeted plasma lipid fingerprints of studied species. Principal component analysis (PCA) model based on 4,899 features extracted from the lipid fingerprints of human and non-human plasma samples (n = 10 × 6). R2X = 0.540; Q2 = 0.362. PC contributions: PC1 = 0.156, PC2 = 0.101.
Targeted lipidomics
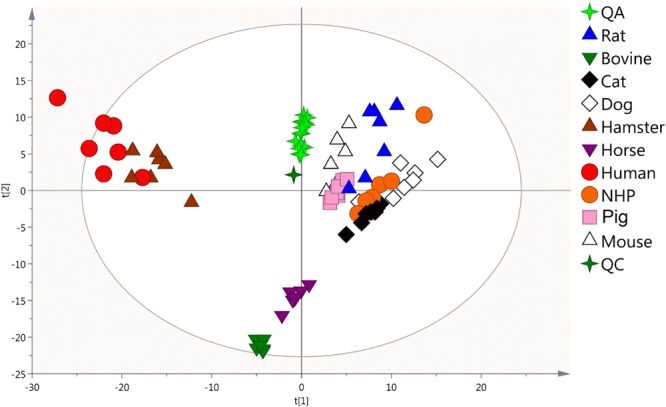
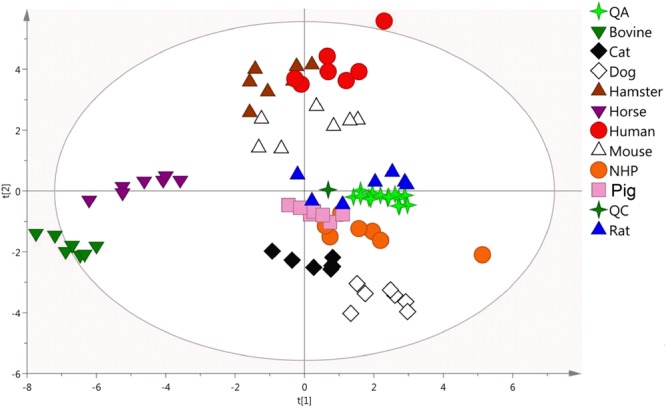
By querying a lipid library of 260 molecular species (Table 2), 106 were clearly and accurately identified from the 4,899 variables including CE, ceramides (Cer), SM, LPC, lysophosphatidylethanolamines (LPE), phosphatidylcholines (PC), phosphatidylethanolamines (PE), phosphatidylinositols (PI), diglycerides (DG), and TG (Supplemental Table S2). These lipids were subsequently used to build a new PCA model, exhibiting an acceptable clustering of QA and QC samples and explaining 75% of the total variability (Fig. 3). Similarly to the previous non-targeted PCA model, this one based on the 106 identified lipids showed a clustering of the different samples and individual QCspecies for each species. Based on abundances of these 106 lipid markers, the intra-species gender difference was irrelevant in comparison to the inter-species lipid profile variance. In addition, bovine and equine plasma samples were again plotted together and clearly discriminated from the other study samples. Targeting a better understanding of the possible clustering between other species, an additional PCA model was established taking out bovine and equine species. This third PCA model (Fig. 4A) showed three separated groups of animals: 1) dog and cat; 2) pig, rat and NHP; 3) mouse, hamster and human. This targeted model also exhibited a clustering of mice along with hamsters and humans. Again, no difference was observed between genders for each species.
Table 2.
Details of the in-house database used for lipid identification in positive ionization mode.
| Classification | Number of lipids | Major adduct | RT range (min) |
|---|---|---|---|
| Fatty acyls | |||
| Fatty esters | 12 | [M + H]+ | 0.51–4.12 |
| Glycerolipids | |||
| TG | 77 | [M + NH4]+, [M + Na]+ | 16.36–22.03 |
| DG | 6 | [M + NH4]+, [M + Na]+ | 13.35–15.69 |
| Glycerophospholipids | |||
| LPE | 5 | [M + H]+ | 1.76–2.38 |
| PE | 16 | [M + H]+ | 11.43–13.72 |
| LPC | 16 | [M + H]+ | 1.45–3.85 |
| PC | 53 | [M + H]+ | 8.93–15.83 |
| PI | 4 | [M + NH4]+ | 6.91–12.56 |
| Sphingolipids | |||
| SM | 30 | [M + H]+ | 9.25–16.85 |
| Cer | 14 | [M + H-H2O]+, [M+H]+ | 13.24–17.64 |
| Sterol lipids | |||
| CE | 19 | [M+NH4]+, [M + Na]+ | 18.54–20.17 |
| Free sterols | 8 | M + H-H2O]+, [M + H]+ | 6.51–12.32 |
| TOTAL | 260 | ||
RT, retention time; n/a, not applicable; TG, triglyceride; DG, diglyceride; LPE, lysophosphatidylethanolamine; PE, phosphatidylethanolamine; LPC, lysophosphatidylcholine; PC, phosphatidylcholine; PI, phosphatidylinositol; SM, sphingomyelin; Cer, ceramide; CE, cholesteryl ester.
Figure 3.
Comparison of targeted plasma lipid fingerprints of studied species. Principal component analysis (PCA) model based on 106 identified lipids from the lipid fingerprints of human and non-human plasma samples (n = 10 × 6). R2X = 0.744; Q2 = 0.577. PC contributions: PC1 = 0.242, PC2 = 0.153.
Figure 4.
Comparison of targeted plasma lipid fingerprints of humans, mice and hamsters. (A) Principal component analysis (PCA) model based on 106 identified lipids from the lipid fingerprints of human and non-herbivorous animal species plasma samples (n = 8 × 6), R2X = 0.753; Q2 = 0.498. PC contributions: PC1 = 0.223, PC2 = 0.136. (B) PCA model based on 106 identified lipids from the lipid fingerprints of human, mouse and hamster plasma samples (n = 3 × 6), R2X = 0.776; Q2 = 0.698. PC contributions: PC1 = 0.302, PC2 = 0.169.
Comparison of mouse and hamster with human
A fourth PCA model was built with only human, mouse and hamster (Fig. 4B) and this last one underlined again similarities between human and hamster. Univariate analyses were therefore carried out on global lipid class abundances between those species (Table 3). Further evaluations of the lipid profile similarity between humans and each of animal species were investigated. For each animal species, the relative abundances of the 106 lipids identified were compared to those obtained in human (Fig. 5). As anticipated, strong correlations were found between human and hamster (r = 0.816, p < 0.0001) or mouse (r = 0.810, p < 0.0001). For other species, r correlation coefficients were significant (p values range 0.001–0.05) with lower r coefficients (range 0.467–0.690).
Table 3.
Mean fold changes (n = 6) of plasma lipid classes between human and hamster or mouse.
| Lipid class | Number of species | Fold change (vs human) | |
|---|---|---|---|
| Hamster | Mouse | ||
| Ceramides | 6 | 0.78 | 0.50* |
| Sphingomyelins | 5 | 0.40** | 0.12** |
| Phosphatidylcholines | 38 | 1.23 | 1.19 |
| Phosphatidylethanolamines | 7 | 0.95 | 1.02 |
| Phosphatidylinositols | 2 | 1.29 | 3.04** |
| Cholesteryl esters | 5 | 0.68 | 3.03** |
| Diglycerides | 3 | 0.32** | 0.37** |
| Triglycerides | 40 | 0.79 | 1.16 |
Values were compared using a Mann-Whitney comparison test (*p < 0.05, **p < 0.01).
Figure 5.
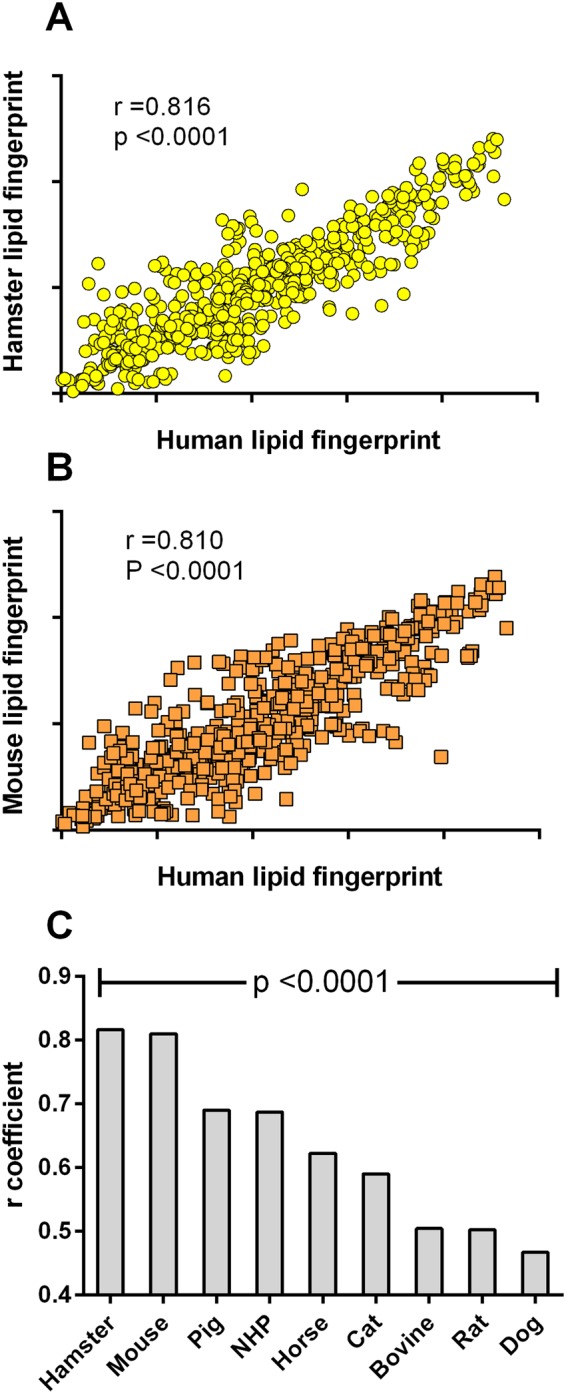
Correlation of lipid marker abundances between human and animals. (A) Pearson correlation obtained with the mean relative abundances (n = 6) of identified lipids (n = 106) (A) between hamster and human and (B) between mouse and human. (C) Pearson correlation coefficients (p < 0.0001) obtained with the relative abundances of identified lipids between human and each animal species (n = 106).
Discussion
Animal models are widely used to investigate and understand the physiopathology of diseases and to facilitate the development of treatments. Lipidomics helps to select relevant lipid biomarkers to target lipid disturbances and related ACVD. From a wide range of animal lipidomic phenotypes, we showed that hamster and, in a lower extent mouse, displayed similar lipid fingerprints compared with human beside traditional plasma lipids such as total cholesterol or triglycerides. This finding confirms these two species are convenient models to investigate some lipid disorders and related events.
We confirmed that human and pig lipoprotein profiles were similar and characterized by elevated LDL cholesterol compared to HDL cholesterol levels13. This is likely related to the physiological and anatomical similarities between both species14. With regard to rats and hamsters, similar lipoprotein profile analysis showed comparable distribution of cholesterol within HDL and LDL. Lower LDL cholesterol concentrations, compared with HDL cholesterol, were detected in these two species as in NHP, cat, mouse, dog, horse and cow in agreement with previous reports15–18. Elevated HDL cholesterol levels in mouse, dog, horse and cow could be closely related to a very low or not detected Cholesteryl Ester Transfer Protein (CETP) activity, which plays a critical role in lipid metabolism, especially by mediating cholesterol ester transfer from HDL to apolipoprotein B-containing lipoproteins (i.e. VLDL and LDL)15,19.
Lipid evaluation in ACVD risk is primarily focused on TC, TG, LDL and HDL cholesterol. However, detailed analyses of lipidome could improve risk prediction by targeting specific molecular species possibly involved in atherosclerosis or related diseases. In that respect, sphingomyelins were shown to be positively and independently related to the presence of coronary artery disease20 and lowering plasma ceramides was associated with decreased plasma cholesterol and inhibition of atherogenesis in apoE*3 Leiden and LDLR−/− mice21. Epidemiological studies also revealed that plasma CE, DG, PE, LPE, LPC and PI species could be associated with cardiovascular diseases (CVD)22,23. More recently, PC metabolites were strongly associated with multiple CVD risk factors in teenagers24. In diabetic patients, a lipid signature including sphingolipids, phospholipids, sterols and glycerolipids, was associated with cardiovascular events and cardiovascular death25. Some similar lipid species were increased in apoE−/− mouse plasma exhibiting atherosclerotic lesions26. These findings suggested that these lipid species could be powerful biomarkers for cardiovascular risk evaluation.
In that context, we performed a non-targeted analysis to give a global overview of similarities and differences between species. Our data unraveled a strong clustering between human and hamster which were well discriminated from the other species. From the main lipid classes found in plasma27, we identified 106 common molecular lipid species useful to better discriminate species. By targeting only these compounds, we showed that humans, mice and hamsters shared similar lipid profiles. This was reinforced by univariate analyses which revealed strong correlations between human and hamster or mouse unlike the other species such as dog and rat.
To date, only one study has investigated plasma lipid profile comparisons between healthy humans and animal species (rat, mouse and rabbit)28. From the 206 common identified lipids consisting of glycerophospholipids (LPC, LPE, PC, PE, PI), sphingolipids (SM, Cer, sulfatides), glycerolipids (DG) and sterol lipids (CE), abundances of 163, 166, and 151 metabolites were significantly different in mice, rats, and rabbits, respectively, when compared with humans28. In line with our data (Supplemental Table S2), the authors also showed lower sphingolipid abundances as well as elevated LPC and longer unsaturated PC levels in rodents in comparison to humans. Compared with human, our data showed that LPC were overall higher in animals (except for pig and horse), while PE and PI ranged similarly. We also noticed that PC carrying shorter or more saturated fatty acids (<C34, <3 double bonds) were significantly lower in animals. In contrast, PC carrying longer polyunsaturated acids (>C34, >3 double bonds) tended to be more abundant in animals than in humans. Noteworthy, our data clearly showed that hamster and human displayed similar PC profiles unlike other species (18 out of 33 PCs). Besides, it has been previously reported that PC (35:4) and PC (36:1) could be powerful markers involved in CVD25. PC (35:4) abundances were found similar between human and hamster, mouse, NHP, cat or bovine, while PC (36:1) abundances were close to those measured for human, mice and NHP but 2-fold higher in hamster. Except for dog, sphingolipid levels were significantly lower in animal species than in humans. However, human and animal species shared similar patterns for SM (d18:1/20:1) which was proposed as a precursor marker for CVD29, and no differences were shown between hamster and human regarding major ceramide species found in human plasma30. Triglycerides carrying shorter or less unsaturated fatty acids (<C54, <5 double bonds) were significantly lower in animal species than in humans except for hamsters. In contrast, TG carrying higher and polyunsaturated fatty acids (>C54, >5 double bonds) were more abundant in animal species than in humans. However, our results highlighted that hamsters and mice displayed a TG profile closer to that of humans than others species (36 out of 44 TGs). No significant difference was found between hamsters or mice and humans regarding to TG (54:2) which was identified as most consistently linked to CVD23. Human and animal species significantly displayed different levels of CEs likely due to the inter-species modulations of CETP. Again, no significant differences were observed between hamsters and humans regarding to CE (16:1) which has been recently associated with CVD25.
A limitation of the study is the small number of samples per species (6 individuals). However, we aimed to extend the analysis to a maximum of animal species with an appropriate group size to obtain meaningful multivariate analyses. Another hurdle could be the selection of 3 males and 3 females per animal species as lipid phenotypes are well-known to be impacted by gender. We chose this option to underline inter-species differences in lipid fingerprints independently of gender. In that respect, our data showed that sex differences were minimal compared to those of species. Nevertheless, this suggests that further analyses are warranted in this field.
In summary, this is the first study that explored molecular lipid species in plasma from humans and nine animal species besides usual plasma lipids. We showed that hamster and human lipid profiles were comparable beside cholesterol and TG. We also identified 89 and 102 common molecular species between human and mouse or hamster, respectively. In contrast to mice, hamster and human also shared 13 common lipids belonging to Cer, PI and CE classes, which can be used to identify the metabolic variation associated with early atherosclerosis and to select biomarkers for ACVD11. Of note, it has also been shown that hamsters are able to develop a diet-induced atherogenesis with a close similarity to the human lipoprotein profile and foam cell formation28–35, while transgenic mice are required to investigate such physiological aspects (mainly apoE or LRL receptor knockout mice)12. Finally, hamsters also share with humans an exclusively hepatic and intestinal production of apolipoprotein B100 and B48, respectively35, as well as a CETP activity. Reflecting the human setting, the hamster appeared to be the most relevant model to monitor a time-dependent development of atherosclerosis and to identify plasma lipids involved in underlying mechanisms.
Methods
Plasma samples and study design
Plasma EDTA samples were obtained from 10 species (Table 1) namely human, non-human primate (NHP), dog, cat, pig, horse, bovine, hamster, mouse and rat. Samples were collected from healthy and overnight fasted males (n = 3) and females (n = 3) at ONIRIS (Veterinary school of Nantes, France). Plasma collections were conducted in agreement with the animal welfare guidelines and approval of ONIRIS Ethics Committee. Human plasma samples were collected at the Nantes hospital. All of the participants provided written informed consent, and the study was approved by the local human Ethics committee (Nantes Hospital, France). All of the methods were performed in accordance with the relevant guidelines and regulations. All samples were stored at −80 °C prior to analysis that was performed in the month following collection.
Lipoprotein profiling and biochemical analysis
Plasma lipoprotein fractions, including very low-density lipoprotein (VLDL), low-density lipoprotein (LDL) and high-density lipoprotein (HDL), were separated by Fast Protein Liquid Chromatography (FPLC, Amersham Pharmacia Biotech Inc., Orsay, France) as described previously36. TC, TG, cholesteryl esters (CE) and phospholipids (PL) were assayed in plasma and FPLC fractions using enzymatic test kits from Boehringer Mannheim GmbH (Mannheim, Germany).
Non-targeted lipidomic analysis
All solvents were purchased from Biosolve (Valkenswaard, Netherlands). The non-targeted analysis was performed on the full set of plasma samples (n = 6 individuals × 10 species). One quality control sample (QCspecies) per species was prepared by pooling 5 µL from each individual sample of the same species. Then, a quality control sample (QC) was formed by pooling 5 µL aliquots from each QCspecies sample, and subsequently split in two 25 µL aliquots. Lipids were extracted from plasma and QC samples (25 µL) as reported previously37. In brief, 225 µL of ice-cold methanol were initially added to defrost plasma samples. Following 10 seconds vortex, 750 µL of ice-cold methyl-ter-butyl ether (MTBE) containing exogenous internal standards [10 µmol/L; Cer (d18:1/17:0), SM (d18:1/17:0), CE (19:0), d31-LPC (16:0), d62-PC (32:0), d62-PE (32:0), PI (31:1), and d5-TG (50:0); Avanti Polar Lipids, Inc., Alabaster, AL, USA] were added and the mixture was vortexed for 10 seconds. Finally, 188 µL of water were added and vortexed for 10 seconds. The final mixture was centrifuged (10,000 g; 10 minutes, 5 °C) and 600 µL of supernatant were transferred to LC-MS vial to be evaporated to dryness under a nitrogen stream (room temperature). Dried samples were reconstituted with 110 µL mixture of acetonitrile/isopropanol/water (65/30/5, v/v/v). A quality assurance sample (QA) was prepared by pooling 10 µL from each vial. This QA sample was injected throughout the analytical batch for correction and normalization purposes38. Ultra-performance liquid chromatography-high-resolution mass spectrometry (UPLC-HRMS) analyses were performed on a SynaptTM G2 HRMS Q-TOF mass spectrometer equipped with an electrospray ionization (ESI) interface operating in the positive ionization mode, and an Acquity H-Class® UPLCTM device (Waters Corporation, Milford, MA, USA). Samples were randomized and injected (5 µL) altogether with QA extracts onto a reversed-phased LC column. Lipids were eluted as detailed in the Supplemental Table S1. The total number of samples was: 6 individuals by 10 species, 1 QCspecies by 10 species, 1 QC and 1 QA repeatedly injected throughout the batch. The full-HRMS mode was applied for lipid detection (mass-to-charge ratio (m/z) range 200–1,200) at a mass resolution of 25,000 full-widths at half maximum (continuum mode). The ionization settings were as follows: capillary voltage, +2 kV; cone voltage, 30 V; desolvation gas (N2) flow rate, 900 L/h; desolvation gas/source temperatures, 550/120 °C. Leucine enkephalin solution at 2 µg/mL (50% acetonitrile) was infused at a constant flow rate of 10 µL/min in the lockspray channel, allowing for correction of the measured m/z throughout the batch (theoretical m/z 556.2771). Data acquisition was achieved using MassLynxTM software version 4.1 (Waters Corporation).
Data processing
Raw files initially processed with MassLynx software were converted to mzXmL format with MSConvert version 3.0.3347 (Vanderbilt University, Nashville, TN, USA). The converted raw data were processed by XCMS software version 1.38.0 running under R version 3.2.0. The Matchedfilter algorithm was used with following parameters: step = 0.03, fwhm = 10, snthresh = 10 and mzdiff = 0.01. Furthermore, “Obiwarp” method was selected for peaks alignment and “group density” function was used for grouping peaks (bw = 9, mzwid = 0.008, minsamp = 5 and max = 30). The function “Fill Peaks” was discarded. Consequently, all non- integrated peaks by XCMS software were affected with Non Attributed (NA) annotation in the generated dataset table. The features presenting “NA” annotations or zeros were processed in a manner to replace the “NA” annotations or the zeros of each feature with randomly generated values ranging between −30% and +30% of the lowest detected signal of each corresponding feature, avoiding hence artificial zero abundance values. A filtration step was automatically performed aiming at eliminating the different isotopomers (isotopic pattern) (M + 1, M + 2 and M + 3) and possible adducts (sodium, potassium, ammonium acetate, sodium ammonium acetate and acetonitrile) of a same compound. Such a filtration was performed using a home-made informatics tool taking into account the two following criteria: a maximum mass difference of 3 mDa and a maximum retention time variation of ±2 seconds with the compound ion adduct. Finally, features exhibiting a CV > 30% in the QA batches were discarded for further data analysis step. Relative abundance of variables (putative lipid markers) was obtained from peak areas normalized to exogenous internal standards. Lipid markers were then extracted from the detected variables using an in-house database containing 260 reference lipid standards, their exact mass measured, their elemental compositions with a mass error of ±5 ppm, their retention times (±30 seconds), and their fragmentation patterns in tandem mass spectrometry (Table 2).
Statistical analysis
Targeting a comprehensive comparison of our sample profiles originating from the different human and animal species, unsupervised “Principal Component Analysis” (PCA) models were used with dedicated software (SIMCA-P+, version 13.0, Umetrics, Umea, Sweden). A logarithmic transformation and Pareto scaling were applied to generate unsupervised PCA giving a general overview of the main discriminations and assessing the analytical robustness through the QA samples clustering. Models validity was appraised using permutation tests and CV-ANOVA. Univariate statistical analyses were performed with GraphPad Prism software (version 6.0, GraphPad Software Inc., La Jolla, CA, USA). Correlations were carried out using the parametric Pearson test and groups were compared using the Mann-Whitney test. Results were considered significant at p < 0.05.
Electronic supplementary material
Acknowledgements
We are grateful to the Biogenouest CORSAIRE core facility for their financial support. The authors thank the Therassay core facility for the provision of FPLC AKTA© and the staff of the Clinical Investigation Center of the University Hospital in Nantes, especially Eliane Hivernaud for her help with patients and blood collection.
Author Contributions
Z.K., M.K. and M.C. conceived and designed research; Z.K., M.M., A.A., S.B.C., F.F., E.D. and M.D. performed experiments and laboratory analyses; Z.K., J.P., M.M. and M.C. analyzed data; Z.K., J.P., M.M., M.K. and M.C. edited and revised manuscript; all authors approved final version of manuscript.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zied Kaabia and Julie Poirier contributed equally.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-34329-3.
References
- 1.GBD 2013 Mortality and Causes of Death Collaborators Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barquera S, et al. Global Overview of the Epidemiology of Atherosclerotic Cardiovascular Disease. Arch Med Res. 2015;46:328–338. doi: 10.1016/j.arcmed.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Graham I, Cooney MT, Bradley D, Dudina A, Reiner Z. Dyslipidemias in the prevention of cardiovascular disease: risks and causality. Curr Cardiol Rep. 2012;14:709–720. doi: 10.1007/s11886-012-0313-7. [DOI] [PubMed] [Google Scholar]
- 4.Jové M, Pamplona R, Prat J, Arola L, Portero-Otín M. Atherosclerosis prevention by nutritional factors: a meta-analysis in small animal models. Nutr Metab Cardiovasc Dis. 2013;23:84–93. doi: 10.1016/j.numecd.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Vilahur G, Padro T, Badimon L. Atherosclerosis and thrombosis: insights from large animal models. J. Biomed Biotechnol. 2011;2011:907575. doi: 10.1155/2011/907575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daugherty A, et al. Recommendation on Design, Execution, and Reporting of Animal Atherosclerosis Studies: A Scientific Statement From the American Heart Association. Arterioscler Thromb Vasc Biol. 2017;37:e131–e157. doi: 10.1161/ATV.0000000000000062. [DOI] [PubMed] [Google Scholar]
- 7.Kolovou G, Kolovou V, Mavrogeni S. Lipidomics in vascular health: current perspectives. Vasc Health Risk Manag. 2015;11:333–342. doi: 10.2147/VHRM.S54874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dang VT, Huang A, Zhong LH, Shi Y, Werstuck GH. Comprehensive Plasma Metabolomic Analyses of Atherosclerotic Progression Reveal Alterations in Glycerophospholipid and Sphingolipid Metabolism in Apolipoprotein E-deficient Mice. Sci Rep. 2016;6:35037. doi: 10.1038/srep35037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsumoto T, Kobayashi T, Kamata K. Role of lysophosphatidylcholine (LPC) in atherosclerosis. Curr Med Chem. 2007;14:3209–3220. doi: 10.2174/092986707782793899. [DOI] [PubMed] [Google Scholar]
- 10.Bismuth J, Lin P, Yao Q, Chen C. Ceramide: a common pathway for atherosclerosis? Atherosclerosis. 2008;196:497–504. doi: 10.1016/j.atherosclerosis.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pechlaner R, Kiechl S, Mayr M. Potential and Caveats of Lipidomics for Cardiovascular Disease. Circulation. 2016;134:1651–1654. doi: 10.1161/CIRCULATIONAHA.116.025092. [DOI] [PubMed] [Google Scholar]
- 12.Russell JC, Proctor SD. Small animal models of cardiovascular disease: tools for the study of the roles of metabolic syndrome, dyslipidemia, and atherosclerosis. Cardiovasc Pathol. 2006;15:318–330. doi: 10.1016/j.carpath.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Bergen WG, Mersmann HJ. Comparative aspects of lipid metabolism: impact on contemporary research and use of animal models. J. Nutr. 2005;135:2499–2502. doi: 10.1093/jn/135.11.2499. [DOI] [PubMed] [Google Scholar]
- 14.Bergen WG, Brandebourg TD. Regulation of lipid deposition in farm animals: Parallels between agriculture and human physiology. Exp Biol Med (Maywood). 2016;241:1272–1280. doi: 10.1177/1535370216654996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsutsumi K, Hagi A, Inoue Y. The relationship between plasma high density lipoprotein cholesterol levels and cholesteryl ester transfer protein activity in six species of healthy experimental animals. Biol Pharm Bull. 2001;24:579–581. doi: 10.1248/bpb.24.579. [DOI] [PubMed] [Google Scholar]
- 16.Vitić J, Stevanović J. Comparative studies of the serum lipoproteins and lipids in some domestic, laboratory and wild animals. Comp Biochem Physiol B. 1993;106:223–229. doi: 10.1016/0305-0491(93)90030-9. [DOI] [PubMed] [Google Scholar]
- 17.Ramaswamy M, Wallace TL, Cossum PA, Wasan KM. Species differences in the proportion of plasma lipoprotein lipid carried by high-density lipoproteins influence the distribution of free and liposomal nystatin in human, dog, and rat plasma. Antimicrob Agents Chemother. 1999;43:1424–1428. doi: 10.1128/AAC.43.6.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dillard A, Matthan NR, Lichtenstein AH. Use of hamster as a model to study diet-induced atherosclerosis. Nutr Metab (Lond). 2010;7:89. doi: 10.1186/1743-7075-7-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ha YC, Barter PJ. Differences in plasma cholesteryl ester transfer activity in sixteen vertebrate species. Comp Biochem Physiol B. 1982;71:265–269. doi: 10.1016/0305-0491(82)90252-8. [DOI] [PubMed] [Google Scholar]
- 20.Jiang XC, et al. Plasma sphingomyelin level as a risk factor for coronary artery disease. Arterioscler Thromb Vasc Biol. 2000;20:2614–2618. doi: 10.1161/01.ATV.20.12.2614. [DOI] [PubMed] [Google Scholar]
- 21.Bietrix F, et al. Inhibition of glycosphingolipid synthesis induces a profound reduction of plasma cholesterol and inhibits atherosclerosis development in APOE*3 Leiden and low-density lipoprotein receptor−/− mice. Arterioscler Thromb Vasc Biol. 2010;30:931–937. doi: 10.1161/ATVBAHA.109.201673. [DOI] [PubMed] [Google Scholar]
- 22.Meikle PJ, et al. Plasma lipidomic analysis of stable and unstable coronary artery disease. Arterioscler Thromb Vasc Biol. 2011;31:2723–2732. doi: 10.1161/ATVBAHA.111.234096. [DOI] [PubMed] [Google Scholar]
- 23.Stegemann C, et al. Lipidomics profiling and risk of cardiovascular disease in the prospective population-based Bruneck study. Circulation. 2014;129:1821–1831. doi: 10.1161/CIRCULATIONAHA.113.002500. [DOI] [PubMed] [Google Scholar]
- 24.Syme C, et al. Glycerophosphocholine Metabolites and Cardiovascular Disease Risk Factors in Adolescents: A Cohort Study. Circulation. 2016;134:1629–1636. doi: 10.1161/CIRCULATIONAHA.116.022993. [DOI] [PubMed] [Google Scholar]
- 25.Alshehry ZH, et al. Plasma Lipidomic Profiles Improve on Traditional Risk Factors for the Prediction of Cardiovascular Events in Type 2 Diabetes Mellitus. Circulation. 2016;134:1637–1650. doi: 10.1161/CIRCULATIONAHA.116.023233. [DOI] [PubMed] [Google Scholar]
- 26.Chen Y, et al. Atherosclerotic dyslipidemia revealed by plasma lipidomics on ApoE−/− mice fed a high-fat diet. Atherosclerosis. 2017;262:78–86. doi: 10.1016/j.atherosclerosis.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 27.Quehenberger O, et al. Lipidomics reveals a remarkable diversity of lipids in human plasma. J. Lipid Res. 2010;51:3299–3305. doi: 10.1194/jlr.M009449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishikawa M, et al. Comparison of circulating lipid profiles between fasting humans and three animal species used in preclinical studies: mice, rats and rabbits. Lipids Health Dis. 2015;14:104. doi: 10.1186/s12944-015-0104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernandez C, et al. Plasma lipid composition and risk of developing cardiovascular disease. PLoS One. 2013;8:e71846. doi: 10.1371/journal.pone.0071846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Croyal M, et al. Fenofibrate decreases plasma ceramide in type 2 diabetes patients: A novel marker of CVD? Diabetes Metab. 2018;44:143–9. doi: 10.1016/j.diabet.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 31.Nistor A, Bulla A, Filip DA, Radu A. The hyperlipidemic hamster as a model of experimental atherosclerosis. Atherosclerosis. 1987;68:159–73. doi: 10.1016/0021-9150(87)90106-7. [DOI] [PubMed] [Google Scholar]
- 32.Kowala MC, Nunnari JJ, Durham SK, Nicolosi RJ. Doxazosin and cholestyramine similarly decrease fatty streak formation in the aortic arch of hyperlipidemic hamsters. Atherosclerosis. 1991;91:35–49. doi: 10.1016/0021-9150(91)90185-6. [DOI] [PubMed] [Google Scholar]
- 33.Sima A, Stancu C, Constantinescu E, Ologeanu L, Simionescu M. The hyperlipemic hamster - a model for testing the anti-atherogenic effect of amlodipine. J. Cell Mol Med. 2001;5:153–162. doi: 10.1111/j.1582-4934.2001.tb00148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhathena J, et al. Diet-induced metabolic hamster model of nonalcoholic fatty liver disease. Diabetes Metab Syndr Obes. 2011;4:195–203. doi: 10.2147/DMSO.S18435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dalbøge LS, et al. A Hamster Model of Diet-Induced Obesity for Preclinical Evaluation of Anti-Obesity, Anti-Diabetic and Lipid Modulating Agents. PLoS One. 2015;10:e0135634. doi: 10.1371/journal.pone.0135634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chétiveaux M, et al. The differential apoA-I enrichment of prebeta1 and alphaHDL is detectable by gel filtration separation. J. Lipid Res. 2002;43:1986–1993. doi: 10.1194/jlr.D200024-JLR200. [DOI] [PubMed] [Google Scholar]
- 37.Matyash V, Liebisch G, Kurzchalia TV, Shevchenko A, Schwudke D. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J. Lipid Res. 2008;49:1137–1146. doi: 10.1194/jlr.D700041-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dunn WB, et al. Human Serum Metabolome (HUSERMET) Consortium. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat Protoc. 2011;6:1060–1083. doi: 10.1038/nprot.2011.335. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.