Abstract
CD4+ T cells are essential for sustaining CD8+ T cell responses during a chronic infection. The adoptive transfer of virus-specific CD4+ T cells has been shown to efficiently rescue exhausted CD8+ T cells. However, the question of whether endogenous virus-specific CD4+ T cell responses can be enhanced by certain vaccination strategies and subsequently reinvigorate exhausted CD8+ T cells remains unexplored. In this study, we developed a CD4+ T cell epitope-based heterologous prime-boost immunization strategy and examined the efficacy of this strategy using a mouse model of chronic lymphocytic choriomeningitis virus (LCMV) infection. We primed chronically LCMV-infected mice with a Listeria monocytogenes vector that expressed the LCMV glycoprotein-specific I-Ab-restricted CD4+ T cell epitope GP61–80 (LM-GP61) and subsequently boosted the primed mice with an influenza virus A (PR8 strain) vector that expressed the same CD4+ T cell epitope (IAV-GP61). This heterologous prime-boost vaccination strategy elicited strong anti-viral CD4+ T cell responses, which further improved both the quantity and quality of the virus-specific CD8+ T cells and led to better control of the viral loads. The combination of this strategy and the blockade of the programmed cell death-1 (PD-1) inhibitory pathway further enhanced the anti-viral CD8+ T cell responses and viral clearance. Thus, a heterologous prime-boost immunization that selectively induces virus-specific CD4+ T cell responses in conjunction with blockade of the inhibitory pathway may represent a promising therapeutic approach to treating patients with chronic viral infections.
Keywords: CD4+ T cell epitope, CD8+ T cell exhaustion, chronic viral infection, prime-boost
Introduction
During an acute viral infection, virus-specific effector CD8+ T cells greatly expand, secrete copious amounts of inflammatory cytokines and exert potent cytolytic activities on virus-infected cells. Altogether, these responses lead to the efficient clearance of the infection. After the acute elimination of the viral infection, the majority of the effector CD8+ T cells die by apoptosis, although a small fraction of these effector cells survive and progressively differentiate into long-lived memory cells that are capable of conferring immediate protection on re-encountering the same virus.1,2 However, in chronic viral infections, such as HIV, HCV and mouse lymphocytic choriomeningitis virus Clone 13 strain (LCMV-Cl13) infection, the long-term persistence of the viral antigens drives the differentiation of functionally exhausted virus-specific CD8+ T cells. These cells are characterized by impaired cytokine secretion, compromised proliferation potential and a diminished cytolytic activity.3,4,5,6 Moreover, exhausted CD8+ T cells exhibit a prolonged upregulation of the expression of an array of inhibitory receptors including programmed cell death-1 (PD-1), lymphocyte activation gene 3 (Lag3) and T cell immunoglobulin domain and mucin domain 3 (Tim-3).7,8,9 These inhibitory molecules mediate signaling pathways that profoundly influence the functional state of the exhausted CD8+ T cells in chronic viral infections.7,10,11,12,13
Although exhausted CD8+ T cells often lose the potential to differentiate into memory T cells, they are not terminally differentiated cells.14,15 This point is supported by the evidence that targeting of the PD-L1/PD-1 inhibitory pathway using antibody blockade can partially reprogram the exhausted CD8+ T cells and effectively restore their function.16,17,18,19 Moreover, exhausted CD8+ T cells are not functionally inert and still maintain a certain level of ability to limit viral replication during chronic infection.14,15,20,21,22,23,24 The non-terminal differentiation state and partially retained effector function of exhausted CD8+ T cells lay the foundation for therapeutic vaccines to target and reinvigorate the exhausted CD8+ T cells, which could potentially lead to efficient virus control. In support of this concept, the combination of PD-1 antibody blockade and VSV-based vectors that express the LCMV-GP33 epitope can efficiently boost exhausted GP33-specific CD8+ T cells and improve virus control in vaccinated animals that are chronically infected with LCMV-Cl13.25
CD4+ T cells provide essential assistance in the maintenance of both the effector function and the population of exhausted CD8+ T cells.26 Transient depletion of CD4+ T cells before LCMV-Cl13 infection markedly attenuates the number and function of exhausted LCMV-specific CD8+ T cells.4,27,28 Similarly, the gradual loss of CD4+ T cells in chronic HIV infection promotes the exhaustion of HIV-specific CD8+ T cells.29 In addition, the adoptive transfer of virus-specific CD4+ T cells into mice chronically infected with LCMV-Cl13 has been shown to greatly enhance the number and effector function of exhausted CD8+ T cells and increase the control of viral replication.30 Paradoxically, the immunization of naive animals with a vaccine regimen that selectively induces CD4+ T cell responses to LCMV glycoprotein GP66-specific followed by chronic infection with LCMV-Cl13 results in lethal immunopathology.31 However, it is not known whether the induction of virus-specific CD4+ T cell responses by the vaccination of mice that are already chronically infected would contribute to virus control or result in lethal immunopathology.
The preparation and subsequent adoptive transfer of a large number of virus-specific CD4+ T cells for the treatment of patients with chronic viral infections is obviously challenging and economically burdensome in clinical settings. Therefore, the development of a vaccination strategy that effectively induces endogenous viral-specific CD4+ T cell responses in vivo would be optimal. In this study, we examined the efficacy and safety of a heterologous prime-boost immunization strategy designed to elicit virus-specific CD4+ T cell responses in mice chronically infected with LCMV-Cl13.
Materials and methods
Mice, bacteria and immunization
The C57BL/6J mice were purchased from The Jackson Laboratories. The lymphocytic choriomeningitis virus (LCMV) clone 13 (Cl13) strains were obtained from Dr Rafi Ahmed, Emory University. The mice were infected intravenously (i.v.) with LCMV-Cl13 (2 × 106 PFU) at 6–10 weeks of age, and both sexes were included without randomization or blinding. The LCMV-Cl13-infected mice were immunized with 1 × 106 PFU of Listeria monocytogenes that expressed a CD4+ T cell epitope derived from LCMV GP61–80 (LM-GP61) 32 and with 0.5 LD50 (50% of the lethal dose) of influenza A virus (PR8 strain) that expressed the epitope GP61–80 (IAV-GP61) or the CD8+ T cell epitope GP33–41 (IAV-GP33).33 The immunizations with LM-GP61 and the IAV-GP61/GP33 was injected intraperitoneally (i.p.).
The Listeria strain was grown with agitation at 37 °C in Brain-Heart Infusion broth containing streptomycin (100 μg/ml), and the Listeria cells and culture supernatants were harvested at A600 nm=1.5.
No statistical methods were used to predetermine the sample size. The number of mice necessary to reach statistical significance for each experiment was based on previous experience. The immunized mice were housed in accordance with the institutional biosafety regulations of the Third Military Medical University. All mice were used in accordance with the guidelines of the Institutional Animal Care and Use Committees of the Third Military Medical University.
Flow cytometry and antibodies
Major histocompatibility complex (MHC) class I peptide tetramers of the H-2Db complex with LCMV GP33–41 and GP276–286 were obtained from Dr Rafi Ahmed (Emory University). MHC class II (I-Ab) tetramers specific for the LCMV epitope of glycoprotein amino acids 66–77 were provided by the tetramer core facility of the US National Institutes of Health (Emory). The antibodies used for flow cytometry are listed in Supplementary Table 1. Surface staining was performed using PBS containing 2% BSA or FBS (wt/vol). For the analysis of intracellular cytokine production, the splenocytes were first stimulated with the indicated peptides (0.2 μg/ml) and brefeldin A for 5 h at 37 °C. Following surface staining, intracellular cytokine staining and Ki-67 staining were performed using a Cytofix/Cytoperm Fixation/Permeabilization Kit (554714, BD Biosciences, San Jose, CA, USA) according to the manufacturer’s instructions. To detect degranulation, the splenocytes were stimulated for 5 h in the presence of the indicated peptide (0.2 μg/ml) and brefeldin A, as well as anti-CD107a and anti-CD107b antibodies (BD Biosciences). The MHC class II tetramers were stained by incubating the tetramers with cells for 1 h at 37 °C. CXCR5 staining was performed as previously described.23 Foxp3 staining was performed after the surface staining using a Foxp3 Staining Buffer Set (eBioscience, San Diego, CA, USA) according to the manufacturer’s instructions. The samples were collected using a FACSCanto (BD Bioscience) flow cytometer and analyzed using FlowJo (Treestar, Ashland, OR, USA).
Virus titration
The LCMV viral loads in the tissue samples were quantified using qRT-PCR as previously described.34 For quantification of LCMV-Cl13 viral loads, the weight of harvested tissues from infected mice was measured. After homogenization, the total RNA was then extracted from the tissue homogenate using TIANAMP Virus RNA Kit (TIANGEN, China) and was subjected to reverse transcription using the RevertAid Minus First Strand cDNA Synthesis Kit (Thermo, USA) following the manufacturer’s instructions, while LCMV-specific glycoprotein primer (GP-R: 5'-GCAACTGCTGTGTTCCCGAAAC-3') was used for cDNA synthesis. The qPCR assay with LCMV glycoprotein-specific primer pairs (GP-R, 5'-GCAACTGCTGTGTTCCCGAAAC-3', and GP-F 5'-CATTCACCTGGACTTTGTCAGACTC-3') was then used to evaluated the viral loads in the tissue samples. The Cq (quantification cycle) values from RNA samples of 10-fold serial diluted LCMV-Armstrong virus that has already been titrated by plaque assay was used to set up the standard curve. The pfu of LCMV-Cl13 in tissues was calculated with the following: lg(pfu)=slope*Cq+y-intercept; pfu/g=pfu calculated from above/tissue weight.
ELISA
The LCMV-specific serum antibody titers were determined using ELISA as previously described35 with HRP-conjugated goat anti-mouse IgG secondary antibodies (Southern Biotech, Birmingham, AL, USA).
In vivo antibody blockade
For the PD-L1 blockade, 200 μg of rat anti-mouse PD-L1 antibody (10F.9G2; BioXcell, West Lebanon, NH, USA) was administered (i.p.) every 3 days for a total of three times. To deplete the CD4+ T cells, the mice were administered 500 μg of anti-mouse CD4 antibody (GK1.5; BioXcell) i.p. on day −1 and day 1 after LCMV-Cl13 infection. To deplete the CD8+ T cells, the mice were injected i.p. with 500 μg of anti-mouse CD8 antibody (YTS 169.4; BioXcell).
Statistical analysis
The statistical analysis was conducted using Prism 6.0 (GraphPad, La Jolla, CA, USA). For virus titration, a Mann-Whitney U-test was used to calculate the P-values where noted. For all other analyses, a two-tailed unpaired Student’s t-test with a 95% confidence interval was used to calculate the P-values.
Results
Heterologous prime-boost vaccination using the CD4+ T cell epitope-based therapeutic vaccine efficiently reduces the viral load in mice chronically infected with LCMV-Cl13
To examine the efficacy of the CD4+ T cell epitope-based vaccination, we first primed the LCMV-Cl13-infected mice on day 21 with the L. monocytogenes vector that expressed the LCMV glycoprotein-specific I-Ab-restricted CD4+ T cell epitope GP61–80 (LM-GP61). We subsequently boosted the primed mice 7 days later with an influenza virus A (PR8 strain) vector that expressed the same CD4+ T cell epitope (IAV-GP61, Figure 1a). Control mice that had been infected with LCMV-Cl13 were treated with i.p. injections of PBS at the times of priming and boost.
Figure 1.
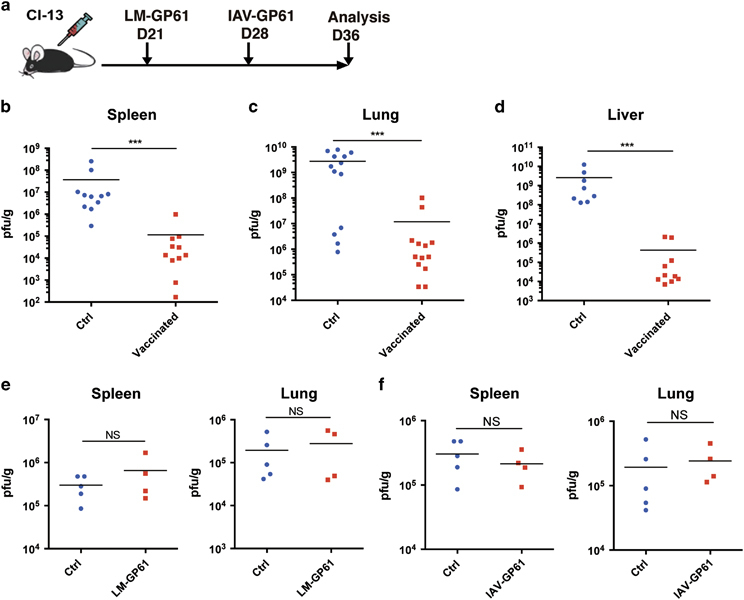
CD4+ T cell epitope-based heterologous prime-boost vaccination efficiently reduced the viral load in mice with chronic LCMV-Cl13 infections. (a) The heterologous prime-boost vaccination strategy using a CD4 T cell epitope-based therapeutic vaccine. (b–d) Viral titers in the indicated tissues of the control (Ctrl) and vaccinated mice on day 8 post boost (n=10–13 mice per group). (e, f) Viral titers in the indicated tissues of the chronically infected control mice and the chronically infected mice that were vaccinated with LM-GP61 or IAV-GP61 alone (Ctrl, n=5, LM-GP61/IAV-GP61, n=4). The data are representative of three independent experiments and were analyzed using Mann–Whitney U-tests (b–f). The error bars (b–f) denote the s.e.m. ***P<0.001. LCMV, lymphocytic choriomeningitis virus; NS, not significant.
On day 8 post boost, we measured the viral load in non-lymphoid (lung and liver) and lymphoid (spleen) tissues of the vaccinated and control mice. Strikingly, we observed a 100- to 1000-fold reduction in the viral load in all of the examined tissues from the vaccinated mice compared with those from the control mice (Figures 1b–d). The reduction in viral load was presumed to be attributable to the integrated prime and boost vaccination based on the fact that immunization of LCMV-Cl13-infected mice with either LM-GP61 or IAV-GP61 alone did not significantly diminish the viral burden in the vaccinated mice compared with the control mice (Figures 1e and f). Notably, the efficient control of viral replication using this vaccination strategy did not seem either to cause the death of the immunized animals or result in overt immunopathology, as indicated by the 100% survival of the immunized mice and the similar levels of alanine aminotransferase and aspartate transaminase in the vaccinated and control mice (Supplementary Figures 1a and b). Furthermore, the body weights of the vaccinated infected mice were modestly increased compared with the non-vaccinated infected mice (Supplementary Figure 1c).
Heterologous prime-boost immunization with vectors expressing an LCMV-specific CD4+ T cell epitope reinvigorates the exhausted CD8+ T cells in mice with chronic LCMV-Cl13 infections
Even when they are functionally exhausted, virus-specific CD8+ T cells have a pivotal role in the control of viral replication during chronic infection.14,15,20,21,22,23,24 The efficient reduction in viral load prompted us to examine whether our immunization strategy restored the CD8+ T cell responses during chronic LCMV-Cl13 infection. Notably, we observed a dramatic increase in both the frequency and number of virus-activated total CD44hiCD8+ T cells in the spleens of the vaccinated LCMV-Cl13-infected mice compared with the non-vaccinated LCMV-Cl13-infected mice (Figure 2a). Consistently, we also noted that although the frequencies of LCMV glycoprotein (GP33 and GP276)-specific CD8+ T cells in the spleens of the vaccinated mice were similar to those in the control mice (Supplementary Figures 2a and b), the numbers of GP33 and GP276-specific CD8+ T cells were significantly higher in the vaccinated mice compared with the control mice (Figure 2b).
Figure 2.
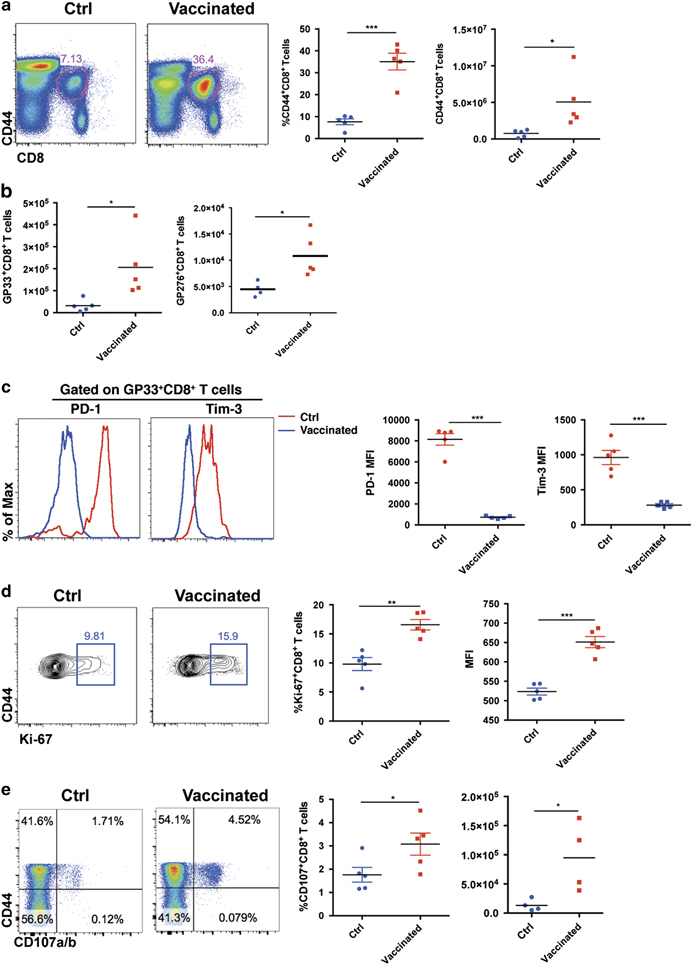
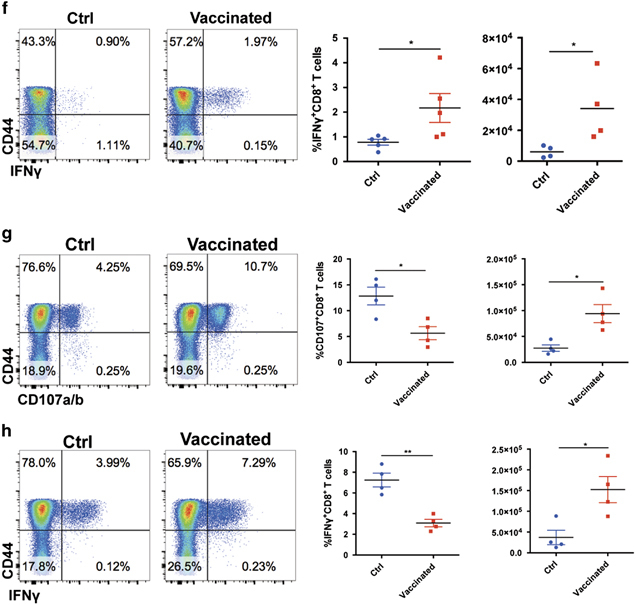
CD4+ T cell epitope-based heterologous prime-boost vaccination recruited exhausted CD8+ T cells during chronic viral infection. On day 8 post boost: (a) The frequencies and numbers of virus-activated CD44hiCD8+ T cells in the spleens of the control and vaccinated mice (n=5). (b) The numbers of LCMV-GP33- and LCMV-GP276-specific CD8+ T cells in the spleens of the control and vaccinated mice (n=5). (c) PD-1 and Tim-3 expression in the virus-specific CD8+ T cells of the control and vaccinated mice (n=5). MFI, mean fluorescence intensity. (d) The expression of Ki-67 in the CD44hiCD8+ T cells of the control and vaccinated mice (n=5). (e–h) Upon stimulation with LCMV-specific peptides, the frequency and number of surface CD107a/b+- and IFN-γ-producing CD8+ T cells in the spleens of the control and vaccinated mice (e, f: stimulated by GP33–41, n=5; g, h; stimulated by GP276–286, n=4). The data are representative of three independent experiments and were analyzed using two-tailed unpaired t-tests (b–f). The error bars (b–f) denote the s.e.m. *P<0.05; **P<0.01; ***P<0.001.
The expression of inhibitory molecules including PD-1 and Tim-3 is a hallmark of exhausted CD8+ T cells. These molecules render these exhausted cells less proliferative and less functional.7,8,9 The greater number of virus-specific CD8+ T cells and the lower viral load in the vaccinated LCMV-Cl13-infected mice compared with the control LCMV-Cl13-infected mice suggested that vaccination could have reinvigorated the exhausted CD8+ T cells. To test this point, we analyzed the expression of PD-1 and Tim-3 in the cells of the vaccinated and control LCMV-Cl13-infected mice. Our results demonstrated that the virus-specific CD8+ T cells in the vaccinated mice exhibited much lower expression levels of both PD-1 and Tim-3 compared with the control mice (Figure 2c). In agreement with these results, the virus-specific CD8+ T cells in the vaccinated mice expressed higher levels of Ki-67 than those of the control mice, which suggested that the proliferation of these cells was enhanced after vaccination (Figure 2d). More importantly, we observed a remarkable increase in the IFN-γ secretion and surface expression of CD107a/b by the virus-specific CD8+ T cells upon LCMV-specific peptide stimulation in the vaccinated mice compared with the control mice (Figures 2e and f). In addition to the GP33 epitope-specific CD8+ T cell responses, we found that the prime-boost vaccination also led to an increase in the IFN-γ production and surface expression of CD107a/b by virus-specific CD8+ T cells upon GP276 peptide stimulation (Figure 2g).
Recent reports have shown that a CXCR5+CD8+ T cell subset within the exhausted CD8+ T cell pool has a critical role in controlling the viral replication in a chronic viral infection.23,36,37 We did not observe significant changes in the percentage of CXCR5+ CD8+ T cells in the vaccinated mice, but the number of CXCR5+ CD8+ T cells increased primarily due to the increased number of total virus-specific CD8+ T cells in the vaccinated mice (Supplementary Figure 2c). To determine whether the CD8+ T cell response was essential for the control of viral replication in the prime-boost vaccination, we depleted the CD8+ T cells at the time of priming (Supplementary Figures 2d and e). As expected, we observed no improvement in the control of viral replication in the vaccinated mice under these conditions (Supplementary Figure 2f). These results suggested that a single CD4+ epitope-based prime-boost vaccination strategy is capable of inducing broad anti-virus CD8+ T cell responses. Taken together, our results demonstrate that the CD4+ T cell epitope-based heterologous prime-boost strategy greatly improves both the quantity and quality of exhausted virus-specific CD8+ T cells, which then efficiently control chronic infections.
Vaccine-induced CD4+ T cell responses are essential for the control of chronic viral infections
The assistance provided by CD4+ T cells is essential to sustain, and sufficient to improve, the functionality of virus-specific exhausted CD8+ T cells during chronic infection.26,27,28,29 In this study, we examined whether our vaccination strategy was able to induce potent virus-specific CD4+ T cell responses. On day 8 post boost, we observed a marked increase in the frequency and number of total virus-activated CD44hiCD4+ T cells and I-Ab restricted LCMV-GP66 epitope-specific CD4+ T cells in the spleens of the vaccinated mice relative to the control mice (Figures 3a–c). Furthermore, the prime-boost vaccination of LCMV-Cl13-infected mice also greatly improved the functionality of the LCMV-specific CD4+ T cells, as indicated by the increased frequency of IFN-γ +CD4+ T cells, TNFα+CD4+ T cells and IL-2+CD4+ T cells as well as the production of IFN-γ, TNFα and IL-2 by antigen-specific CD4+ T cells in the vaccinated mice compared with the control mice (Figures 3d and e). In addition, the boosted CD4+ T cells demonstrated an increased polyfunctionality compared with the non-boosted control CD4+ T cells (Figure 3f). However, we observed virus-specific CD8+ T cell responses and viral loads that were similar in both the vaccinated and control mice after the prime-boost vaccination when we depleted the CD4+ T cells one day before priming (Figures 3g–i); the depletion efficiency of CD4+ T cell is shown in Supplementary Figure 3b. In addition, a subdominant H-2Kb-restricted GP70–77 CD8+ T cell epitope lies within the GP61–80 CD4+ T cell epitope.38 The dependence of the CD4+ T cell responses also indicated that this subdominant CD8+ T cell epitope was unlikely to be involved in the control of viral load. Moreover, the virus-specific CD4+ T cell response was strongly enhanced only when the LM-GP61 prime and IAV-GP61 boost immunization were combined. Single immunization with LM-GP61 or IAV-GP61 did not significantly improve the virus-specific CD4+ T cell response (Supplementary Figures 3c–f). These data collectively demonstrate that the CD4+ T cell epitope-based prime-boost strategy efficiently induces virus-specific CD4+ T cell responses that are essential for reinvigorating virus-specific exhausted CD8+ T cells in chronically infected mice.
Figure 3.
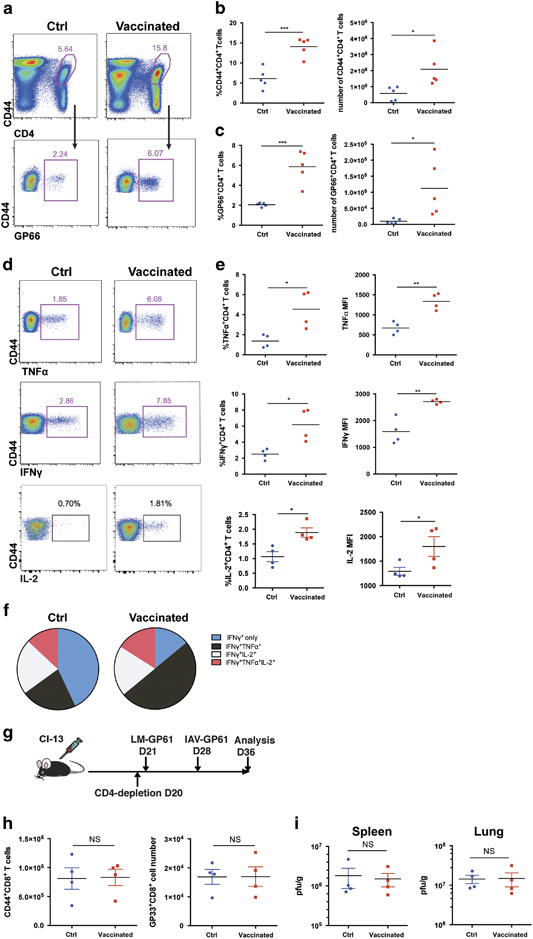
CD4+ T cell epitope-based heterologous prime-boost vaccination induced potent virus-specific CD4+ T cell responses that were essential for the control of viral replication. On day 8 post boost: (a–c) The frequency and number of the total virus-activated CD44hiCD4+ T cells and LCMV-GP66 epitope-specific CD4+ T cells in the spleens of the control and vaccinated mice (n=5). (d, e) The cytokine production by the LCMV-specific CD4+ T cells in the spleens of the control and vaccinated mice (n=4) upon stimulation with an LCMV-specific peptide (GP61–77). (f) Polyfunctionality of the CD4+ T cells in the spleens of the control and vaccinated mice. The colors represent the cytokines coproduced per cell. (g) CD4+ T cells were depleted in the control and vaccinated mice on the day before the LM-GP66 priming. (h) The numbers of virus-activated CD44hiCD8+ T cells and LCMV-GP33-specific CD8+ T cells in the spleens of the CD4+ T cell-depleted control mice and vaccinated mice (n=4). (i) Viral titers in the indicated tissues of the CD4+ T cell-depleted control and vaccinated mice (n=4). The data are representative of three independent experiments and were analyzed using two-tailed unpaired t-tests (b, c, e, h) and Mann–Whitney U-tests (i). The error bars denote the s.e.m. *P<0.05; **P<0.01; ***P<0.001. LCMV, lymphocytic choriomeningitis virus; MFI, mean fluorescence intensity; NS, not significant.
Heterologous prime-boost immunization with vectors expressing an LCMV-specific CD4+ T cell epitope boosts the humoral responses of mice with chronic LCMV-Cl13 infections
In addition to the cellular immune responses, IgG-mediated humoral responses are also involved in the control of viral replication during chronic infection.39 Follicular helper CD4+ T (TFH) cells have a critical role in the initiation and maintenance of the germinal center (GC) B cell response that subsequently gives rise to antigen-specific memory B cells and antibody-secreting plasma cells.40,41 On day 8 post boost, we observed an accumulation of more virus-specific Foxp3−CD44hiCXCR5hiCD4+ TFH cells in the spleens of the vaccinated mice than in those of the control mice (Figure 4a). Consistent with this result, the vaccinated mice exhibited a higher frequency and number of PNAhiFAShiCD19+ GC B cells in their spleens than the control mice (Figure 4b). Consequently, we observed a remarkable increase in LCMV-specific IgG titers in the sera of the vaccinated mice compared with the control mice (Figure 4c).
Figure 4.
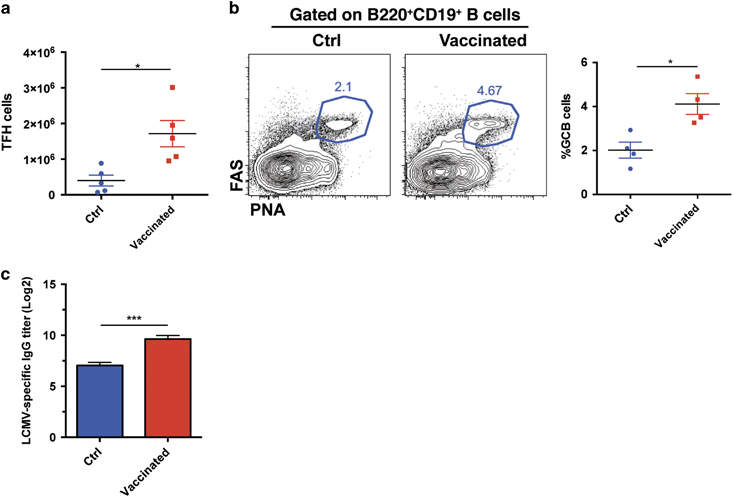
The CD4+ T cell epitope-based heterologous prime-boost vaccination boosted the humoral responses in chronically infected mice. On day 8 post boost: (a) The number of virus-specific TFH cells in the spleens of the control and vaccinated mice (n=5). (b) The frequency and number of GC B cells in the spleens of the control and vaccinated mice (n=4). (c) Titration of LCMV-specific IgG in the sera of the control and vaccinated mice (n=4). The data are representative of three independent experiments and were analyzed using two-tailed unpaired t-tests. The error bars denote the s.e.m. *P<0.05; ***P<0.001.
In addition to modulating the B cell responses, our vaccination strategy seemed to reduce the PD-L1 expression in antigen-presenting cells including macrophages and dendritic cells (DCs) and to slightly reduce the frequency of regulatory T cells in the spleens of the vaccinated mice compared with the control mice (Supplementary Figures 4 and 5).
Inclusion of the LCMV-specific CD8+ T cell epitope has minimal effect on the efficacy of the CD4+ T cell epitope-based prime-boost vaccination in chronically infected mice
Next, we sought to determine whether the inclusion of an LCMV-specific CD8+ T cell epitope would further enhance the efficacy of the CD4+ T cell epitope-based prime-boost vaccination in chronically infected mice (Figure 5a). Interestingly, the addition of an IAV vector that expressed the H-2-Db-restricted, LCMV-specific GP33 epitope (IAV-GP33) to the LM-GP61 and IAV-GP61 prime-boost vaccination failed to increase the numbers of either the total CD44hiCD8+ T cells or the LCMV-GP33 epitope-specific CD8+ T cells compared with the LM-GP61/IAV-GP61 prime-boost immunization (Figures 5b and c). Furthermore, the addition of the IAV-GP33 immunization to the LM-GP61/IAV-GP61 immunization also seemed to have minimal effect on the functionality of the virus-specific CD8+ T cells based on the similar numbers of CD107a/b- and IFN-γ positive CD8+ T cells upon LCMV-specific peptide stimulation in the LM-GP61/IAV-GP61/IAV-GP33 immunization compared with the LM-GP61/IAV-GP61 immunization (Figures 5d and e). Consistent with these results, these two immunization groups exhibited comparable viral titers in the spleens and livers (Figures 5f and g). These results therefore demonstrated that pre-existing virus-specific CD8+ T cells in chronically infected mice stimulated with a CD4+ T cell epitope-based prime-boost strategy are sufficient to control the chronic viral infection.
Figure 5.
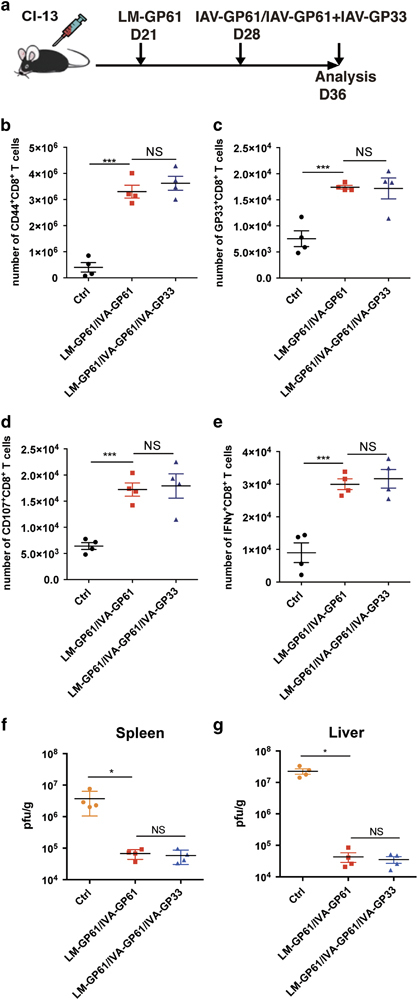
Inclusion of the LCMV-specific CD8+ T cell epitope had a minimal effect on the efficacy of the CD4+ T cell epitope-based heterologous prime-boost vaccination. (a) The setup of the experiment. At the time of the boost immunization, the vaccinated mice were injected with IVA-GP61 alone or with IVA-GP61 and IVA-GP33. On day 8 post boost: (b–e) The number of virus-activated CD44hiCD8+ T cells (b), LCMV-GP33-specific CD8+ T cells (c), surface CD107a/b+CD8+ T cells (d) and IFNγ-producing CD8+ T cells (e) in the spleens of the control, IVA-GP66-boosted and IVA-GP61+IVA-GP33-boosted mice (n=4). (f, g) Viral titers in the spleens (f) and livers (g) of the control, IVA-GP61-boosted and IVA-GP61+IVA-GP33-boosted mice (n=4). The data are representative of three independent experiments and were analyzed using two-tailed unpaired t-tests (b–e) and Mann–Whitney U-tests (f, g). The error bars denote the s.e.m. *P<0.05; ***P<0.001. LCMV, lymphocytic choriomeningitis virus; NS, not significant.
PD-L1 blockade further improves the efficacy of the CD4+ T cell epitope-based prime-boost vaccination in chronically infected mice
Blockade of the PD-1-PD-L1 signaling axis has been proven to reinvigorate exhausted CD8+ T cells in chronic viral infections.16,17,18,19 We next sought to examine whether PD-L1 antibody blockade would further enhance the efficacy of the CD4+ T cell epitope-based prime-boost immunization (Figure 6a). Consistent with previous reports, PD-L1 blockade efficiently reduced the viral load in mice with chronic LCMV-Cl13 infections (Figures 6b–d). Moreover, when combined with the LM-GP61/IAV-GP61 prime-boost immunization, the PD-L1 antibody further reduced the viral load compared with either the single PD-L1 antibody blockade or the LM-GP61/IAV-GP61 prime-boost vaccination (Figures 6b–d). Therefore, these results indicated that a combination of immune checkpoint blockade and CD4+ T cell epitope-based prime-boost immunization may represent a novel strategy for treating chronic viral infections.
Figure 6.
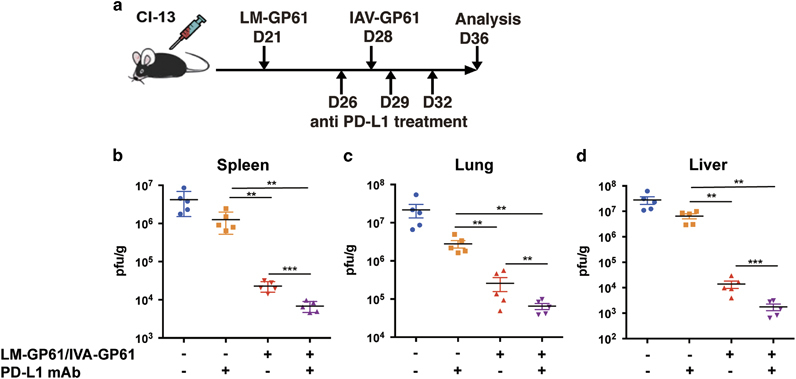
PD-L1 blockade improved the efficacy of the CD4+ T cell epitope-based heterologous prime-boost vaccination. (a) The setup of the experiment. (b–d) Viral titers in the spleens (b), lungs (c) and livers (d) of the mice that received the various treatments. The data are representative of three independent experiments and were analyzed using Mann–Whitney U-tests (b–d). The error bars denote the s.e.m. **P<0.01.
Discussion
CD4+ T cells have a critical role in regulating the effector function and population size of exhausted CD8+ T cells during chronic viral infections.4,26,27,28 The transient depletion of CD4+ T cells during the initial priming phase of the CD8+ T cell response drives the differentiation of more exhausted phenotypes of virus-specific CD8+ T cells and leads to the rapid turnover of the exhausted CD8+ T cell pool and high viral loads in animals.27 Supplementing the CD4+ T cells by adoptive transfer of virus-specific CD4+ T cells efficiently improves the functionality and quantity of the exhausted CD8+ T cells and greatly reduces the viral loads.30 However, this strategy is difficult to achieve in a clinical setting because of the immense complexity and cost of acquiring sufficient autologous, virus-specific CD4+ T cells for adoptive transfer. In this study, we demonstrated that a prime-boost immunization with Listeria and influenza vectors that expressed a single virus-specific CD4+ T cell epitope efficiently induced a potent endogenous CD4+ T cell response, which further expanded the number and rescued the effector function of the exhausted CD8+ T cells. Importantly, this immunization protocol resulted in a dramatic reduction in the viral loads in the immunized animals without causing overt immunopathology. Thus, compared with the adoptive transfer of CD4+ T cells, heterologous prime-boost immunization of chronically infected hosts to enhance the virus-specific CD4+ T cell responses may represent a simple, safe and cost-effective strategy to reinvigorate the exhausted CD8+ T cells for the treatment of chronic viral diseases.
The prime-boost immunization with Listeria and influenza vectors that expressed LCMV GP61-specific CD4+ T cell epitopes also increased the humoral immune responses, dampened the regulatory T cell responses and decreased the expression of PD-L1 in the antigen-presenting cells of animals with LCMV-Cl13 infections. It is plausible that these effects synergistically enhanced the CD8+ T cell responses. The increase in follicular helper CD4+ T cells may lead to an increase in the secretion of IL-21, a cytokine that is essential to sustain the virus-specific CD8+ T cell responses during chronic infections.42,43 However, the diminished number of regulatory CD4+ T cells may favor the expansion of the exhausted CD8+ T cells.44 Moreover, a downregulation of the expression of PD-L1 in APCs could potentially attenuate the negative effects of the PD-1/PD-L1 pathway on exhausted CD8+ T cells.19,25 Nonetheless, the exact mechanism by which the heterologous prime-boost strategy elicited virus-specific CD4+ T cell responses that were specific to a single epitope and profoundly improved the quantity and functional quality of the exhausted CD8+ T cells awaits further investigation.
Immunization of naive mice with a Listeria vector that expressed the LCMV glycoprotein-specific I-Ab restricted CD4+ T cell epitope GP61–80 and subsequent challenge with LCMV-Cl13 resulted in generalized inflammation and multi-organ system failure.31 Rapid and potent stimulation of a large number of pre-existing memory Th1 cells by uncontrolled viral replication may lead to this phenotype. Furthermore, the rapid increase in the virus-specific CD4+ T cell responses appeared to dampen both the virus-specific CD8+ T cell and B cell responses,31 potentially through the effects of the innate cell-secreted type I interferons that are stimulated by strong Th1 responses.45,46,47,48 In contrast, our heterologous prime-boost therapeutic strategy elicited an increase in the virus-specific CD4+ T cell response that was not as aberrant as that observed when a primarily immunized host received challenges with persistent viruses. These increased CD4+ T cells further provided potent help to both the exhausted CD8+ T cells and the virus-specific B cells as well as innate cells, which probably synergistically improved the control of viral replication without overt immunopathology. The tight control of the increased CD4+ T cell responses following the prime-boost strategy may be largely attributed to the fact that this strategy was designed to therapeutically treat hosts that were already chronically infected, in which the virus-specific CD4+ T cells were exhausted 49 and were therefore limited with respect to the undergoing excessive proliferation after immunization.
Notably, the inclusion of an influenza virus vector that expressed a CD8+ T cell epitope during the boost phase of our immunization strategy did not seem to further enhance the number or effector function of the virus-specific CD8+ T cells or reduce the viral load. This phenotype may indicate that the pre-existing pool of exhausted CD8+ T cells in the chronically infected animals following the heterologous prime-boost immunization with vectors expressing virus-specific CD4+ T epitopes was sufficient to effectively control the viral loads. Therapeutic vaccination with VSV that expressed the LCMV GP33-specific CD8+ T cell epitope plus anti-PD-L1 blockade could effectively control the viral load in LCMV-Cl13 infected animals.25 Therefore, it was of great interest to investigate the efficacy of combining a CD4+ T cell epitope-based heterologous prime-boost therapeutic vaccination and a CD8+ T cell epitope boost with blockade of the PD-1/PD-L1 pathway. However, it remains to be determined whether the inclusion of a vector expressing a CD8+ T cell epitope at both the prime and boost stages could be more efficient in controlling viral loads.
The combination of blockade of the PD-L1/PD-1 pathway and the LM-GP61/IAV-GP61 prime-boost immunization was shown to reduce viral loads more effectively than the prime-boost immunization alone. Recent studies have demonstrated that a unique exhausted CD8+ T cell subset that expresses the chemokine receptor CXCR5 is the main subset that responds to a monoclonal antibody-mediated blockade of the PD-1/PD-L1 pathway.23,36 It will, therefore, be interesting to investigate whether the combination of PD-L1/PD-1 blockade and the LM-GP61/IAV-GP61 prime-boost immunization exerts its effects mainly on CXCR5+CD8+ T cells to improve the control of viral loads. In addition, IL-2 has been shown to synergize with the PD-1/PD-L1 blockade to promote the response of exhausted CD8+ T cells and substantially reduce the viral loads.50 Therefore, it might be worthwhile to investigate the efficacy of a combination of PD-L1/PD-1 blockade, IL-2 administration and heterologous prime-boost immunization with vectors that express virus-specific CD4+ T cell epitopes.
In conclusion, we have developed a simple, safe and effective immunization strategy that induced strong endogenous virus-specific CD4+ T cell responses in chronically infected hosts. This strategy subsequently rescued the exhausted virus-specific CD8+ T cells to more efficiently control the viral loads. PD-1 blockade further improved this rescue. Therefore, a combination of the blockade of inhibitory pathways and a heterologous prime-boost immunization that enhances the virus-specific CD4+ T cell responses may represent a novel approach to treating patients with chronic viral diseases.
Electronic supplementary material
Acknowledgements
The work was supported by National Basic Research Program of China (973 program, 2013CB531500, to LY) and the National Natural Science Foundation of China (81471624 to LY).
Conflict of interest
The authors declare a conflict of interest. A patent associated with a CD4+ T cell epitope-based therapeutic vaccine has been filed (L Ye and R He).
Electronic supplementary material
Supplementary Information for this article can be found on the Cellular & Molecular Immunology website 10.1038/cmi.2017.3
References
- 1.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams MA, Holmes BJ, Sun JC, Bevan MJ. Developing and maintaining protective CD8+ memory T cells. Immunol Rev. 2006;211:146–153. doi: 10.1111/j.0105-2896.2006.00389.x. [DOI] [PubMed] [Google Scholar]
- 3.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 4.Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, et al. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zuniga EI, Macal M, Lewis GM, Harker JA. Innate and adaptive immune regulation during chronic viral infections. Annu Rev Virol. 2015;2:573–597. doi: 10.1146/annurev-virology-100114-055226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crawford A, Wherry EJ. The diversity of costimulatory and inhibitory receptor pathways and the regulation of antiviral T cell responses. Curr Opin Immunol. 2009;21:179–186. doi: 10.1016/j.coi.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin HT, Anderson AC, Tan WG, West EE, Ha SJ, Araki K, et al. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci USA. 2010;107:14733–14738. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blackburn SD, Crawford A, Shin H, Polley A, Freeman GJ, Wherry EJ. Tissue-specific differences in PD-1 and PD-L1 expression during chronic viral infection: implications for CD8 T-cell exhaustion. J Virol. 2010;84:2078–2089. doi: 10.1128/JVI.01579-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blackburn SD, Shin H, Freeman GJ, Wherry EJ. Selective expansion of a subset of exhausted CD8 T cells by alphaPD-L1 blockade. Proc Natl Acad Sci USA. 2008;105:15016–15021. doi: 10.1073/pnas.0801497105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Workman CJ, Cauley LS, Kim IJ, Blackman MA, Woodland DL, Vignali DA. Lymphocyte activation gene-3 (CD223) regulates the size of the expanding T cell population following antigen activation in vivo. J Immunol. 2004;172:5450–5455. doi: 10.4049/jimmunol.172.9.5450. [DOI] [PubMed] [Google Scholar]
- 14.Speiser DE, Utzschneider DT, Oberle SG, Munz C, Romero P, Zehn D. T cell differentiation in chronic infection and cancer: functional adaptation or exhaustion? Nat Rev Immunol. 2014;14:768–774. doi: 10.1038/nri3740. [DOI] [PubMed] [Google Scholar]
- 15.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15:486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freeman GJ, Wherry EJ, Ahmed R, Sharpe AH. Reinvigorating exhausted HIV-specific T cells via PD-1-PD-1 ligand blockade. J Exp Med. 2006;203:2223–2227. doi: 10.1084/jem.20061800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458:206–210. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuller MJ, Callendret B, Zhu B, Freeman GJ, Hasselschwert DL, Satterfield W, et al. Immunotherapy of chronic hepatitis C virus infection with antibodies against programmed cell death-1 (PD-1) Proc Natl Acad Sci USA. 2013;110:15001–15006. doi: 10.1073/pnas.1312772110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 20.Deng K, Pertea M, Rongvaux A, Wang L, Durand CM, Ghiaur G, et al. Broad CTL response is required to clear latent HIV-1 due to dominance of escape mutations. Nature. 2015;517:381–385. doi: 10.1038/nature14053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones RB, Walker BD. HIV-specific CD8(+) T cells and HIV eradication. J Clin Invest. 2016;126:455–463. doi: 10.1172/JCI80566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zehn D, Utzschneider DT, Thimme R. Immune-surveillance through exhausted effector T-cells. Curr Opin Virol. 2016;16:49–54. doi: 10.1016/j.coviro.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 23.He R, Hou S, Liu C, Zhang A, Bai Q, Han M, et al. Follicular CXCR5-expressing CD8+ T cells curtail chronic viral infection. Nature. 2016;537:412–428. doi: 10.1038/nature19317. [DOI] [PubMed] [Google Scholar]
- 24.Johnson S, Bergthaler A, Graw F, Flatz L, Bonilla WV, Siegrist CA, et al. Protective efficacy of individual CD8+ T cell specificities in chronic viral infection. J Immunol. 2015;194:1755–1762. doi: 10.4049/jimmunol.1401771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ha SJ, Mueller SN, Wherry EJ, Barber DL, Aubert RD, Sharpe AH, et al. Enhancing therapeutic vaccination by blocking PD-1-mediated inhibitory signals during chronic infection. J Exp Med. 2008;205:543–555. doi: 10.1084/jem.20071949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomsen AR, Johansen J, Marker O, Christensen JP. Exhaustion of CTL memory and recrudescence of viremia in lymphocytic choriomeningitis virus-infected MHC class II-deficient mice and B cell-deficient mice. J Immunol. 1996;157:3074–3080. [PubMed] [Google Scholar]
- 27.Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Battegay M, Moskophidis D, Rahemtulla A, Hengartner H, Mak TW, Zinkernagel RM. Enhanced establishment of a virus carrier state in adult CD4+ T-cell-deficient mice. J Virol. 1994;68:4700–4704. doi: 10.1128/jvi.68.7.4700-4704.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalams SA, Buchbinder SP, Rosenberg ES, Billingsley JM, Colbert DS, Jones NG, et al. Association between virus-specific cytotoxic T-lymphocyte and helper responses in human immunodeficiency virus type 1 infection. J Virol. 1999;73:6715–6720. doi: 10.1128/jvi.73.8.6715-6720.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aubert RD, Kamphorst AO, Sarkar S, Vezys V, Ha SJ, Barber DL, et al. Antigen-specific CD4 T-cell help rescues exhausted CD8 T cells during chronic viral infection. Proc Natl Acad Sci USA. 2011;108:21182–21187. doi: 10.1073/pnas.1118450109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Penaloza-MacMaster P, Barber DL, Wherry EJ, Provine NM, Teigler JE, Parenteau L, et al. Vaccine-elicited CD4 T cells induce immunopathology after chronic LCMV infection. Science. 2015;347:278–282. doi: 10.1126/science.aaa2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen H, Slifka MK, Matloubian M, Jensen ER, Ahmed R, Miller JF. Recombinant Listeria monocytogenes as a live vaccine vehicle for the induction of protective anti-viral cell-mediated immunity. Proc Natl Acad Sci USA. 1995;92:3987–3991. doi: 10.1073/pnas.92.9.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mueller SN, Langley WA, Li G, Garcia-Sastre A, Webby RJ, Ahmed R. Qualitatively different memory CD8+ T cells are generated after lymphocytic choriomeningitis virus and influenza virus infections. J Immunol. 2010;185:2182–2190. doi: 10.4049/jimmunol.1001142. [DOI] [PubMed] [Google Scholar]
- 34.McCausland MM, Crotty S. Quantitative PCR technique for detecting lymphocytic choriomeningitis virus in vivo. J Virol Methods. 2008;147:167–176. doi: 10.1016/j.jviromet.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rasheed MA, Latner DR, Aubert RD, Gourley T, Spolski R, Davis CW, et al. Interleukin-21 is a critical cytokine for the generation of virus-specific long-lived plasma cells. J Virol. 2013;87:7737–7746. doi: 10.1128/JVI.00063-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Im SJ, Hashimoto M, Gerner MY, Lee J, Kissick HT, Burger MC, et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. 2016;537:417–421. doi: 10.1038/nature19330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leong YA, Chen Y, Ong HS, Wu D, Man K, Deleage C, et al. CXCR5+ follicular cytotoxic T cells control viral infection in B cell follicles. Nat Immunol. 2016;17:1187–1196. doi: 10.1038/ni.3543. [DOI] [PubMed] [Google Scholar]
- 38.Homann D, Lewicki H, Brooks D, Eberlein J, Mallet-Designe V, Teyton L, et al. Mapping and restriction of a dominant viral CD4+ T cell core epitope by both MHC class I and MHC class II. Virology. 2007;363:113–123. doi: 10.1016/j.virol.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harker JA, Lewis GM, Mack L, Zuniga EI. Late interleukin-6 escalates T follicular helper cell responses and controls a chronic viral infection. Science. 2011;334:825–829. doi: 10.1126/science.1208421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 41.Xu L, Cao Y, Xie Z, Huang Q, Bai Q, Yang X, et al. The transcription factor TCF-1 initiates the differentiation of T(FH) cells during acute viral infection. Nat Immunol. 2015;16:991–999. doi: 10.1038/ni.3229. [DOI] [PubMed] [Google Scholar]
- 42.Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009;324:1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson LD, Jameson SC. Immunology. A chronic need for IL-21. Science. 2009;324:1525–1526. doi: 10.1126/science.1176487. [DOI] [PubMed] [Google Scholar]
- 44.Penaloza-MacMaster P, Kamphorst AO, Wieland A, Araki K, Iyer SS, West EE, et al. Interplay between regulatory T cells and PD-1 in modulating T cell exhaustion and viral control during chronic LCMV infection. J Exp Med. 2014;211:1905–1918. doi: 10.1084/jem.20132577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilson EB, Yamada DH, Elsaesser H, Herskovitz J, Deng J, Cheng G, et al. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science. 2013;340:202–207. doi: 10.1126/science.1235208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moseman EA, Wu T, de la Torre JC, Schwartzberg PL, McGavern DB. Type I interferon suppresses virus-specific B cell responses by modulating CD8+ T cell differentiation. Sci Immunol 2016; 1. [DOI] [PMC free article] [PubMed]
- 47.Fallet B, Narr K, Ertuna YI, Remy M, Sommerstein R, Cornille K et al. Interferon-driven deletion of antiviral B cells at the onset of chronic infection. Sci Immunol 2016; 1. [DOI] [PMC free article] [PubMed]
- 48.Sammicheli S, Kuka M, Di Lucia P, de Oya NJ, De Giovanni M, Fioravanti J et al. Inflammatory monocytes hinder antiviral B cell responses. Sci Immunol 2016; 1. [DOI] [PMC free article] [PubMed]
- 49.Crawford A, Angelosanto JM, Kao C, Doering TA, Odorizzi PM, Barnett BE, et al. Molecular and transcriptional basis of CD4(+) T cell dysfunction during chronic infection. Immunity. 2014;40:289–302. doi: 10.1016/j.immuni.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.West EE, Jin HT, Rasheed AU, Penaloza-Macmaster P, Ha SJ, Tan WG, et al. PD-L1 blockade synergizes with IL-2 therapy in reinvigorating exhausted T cells. J Clin Invest. 2013;123:2604–2615. doi: 10.1172/JCI67008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


