Abstract
Circular RNAs (circRNAs) are a large class of transcripts in the mammalian genome. Although the translation of circRNAs was reported, additional coding circRNAs and the functions of their translated products remain elusive. Here, we demonstrate that an endogenous circRNA generated from a long noncoding RNA encodes regulatory peptides. Through ribosome nascent-chain complex-bound RNA sequencing (RNC-seq), we discover several peptides potentially encoded by circRNAs. We identify an 87-amino-acid peptide encoded by the circular form of the long intergenic non-protein-coding RNA p53-induced transcript (LINC-PINT) that suppresses glioblastoma cell proliferation in vitro and in vivo. This peptide directly interacts with polymerase associated factor complex (PAF1c) and inhibits the transcriptional elongation of multiple oncogenes. The expression of this peptide and its corresponding circRNA are decreased in glioblastoma compared with the levels in normal tissues. Our results establish the existence of peptides encoded by circRNAs and demonstrate their potential functions in glioblastoma tumorigenesis.
Functional peptides can be encoded by short open reading frames in non-coding RNA. Here, the authors identify a 87aa peptide encoded by the circular form of the long intergenic non-protein-coding RNA p53-induced transcript (LINC-PINT) that can reduce glioblastoma proliferation via interaction with PAF1 which sequentially inhibits the transcriptional elongation of some oncogenes.
Introduction
As determined by deep RNA sequencing and bioinformatics, circRNAs are widely expressed RNA transcripts found in different species1–6. CircRNAs are inherently resistant to exonuclease activity (resulting in higher stability than linear RNAs) and often show tissue- or developmental stage-specific expression2,7,8, implying that they possess important biological functions. To date, circRNAs have been shown to act as microRNA sponges9–11, to respond to and regulate neuronal synaptic function12, and to manipulate gene transcription in the nucleus13. A typical circRNA is generated through a back-splicing mechanism that covalently connects the 3′-end of a coding or non-coding exon to the 5′-end14–16. Until now, circRNAs have generally been considered non-coding RNAs (ncRNAs)3,9,12. However, recent evidence has demonstrated that functional peptides are encoded by short open reading frames (sORFs) in ncRNAs17–22. Additionally, certain synthetic circRNAs are translatable23,24, raising the question of whether natural circRNAs containing sORFs in mammalian cells are translated into proteins or peptides. Up-to-date reports showed that endogenous circRNAs, such as circZNF609, circMbl, circ-FBXW7, and circ-SHPRH, are translated in vivo, suggesting that additional coding circRNAs and their translated products have yet to be discovered25–29. Currently, three broad strategies have been employed to identify sORFs in ncRNAs19. These strategies include cross-species comparisons to identify conserved sequences, the examination of codon content or features to differentiate potential coding sORFs, and translational approaches to identify coding sORFs19. Even with these strategies, the identification of sORFs in circRNAs is difficult. First, although cross-species conserved sORFs have been identified in circRNAs, there are few computational tools, such as circRNADb, that are available to predict coding circRNAs30. Second, as most circRNAs are generated from protein-coding exons, the sORFs in circRNAs may overlap with ORFs in related mRNA sequences1,4. In such cases, it is difficult to distinguish the origin of a translated product. Third, the best tool for exploring sORFs, ribosome profiling, has not been broadly applied to circRNAs due to technical difficulties such as library construction3,31.
In this study, we show that human circRNAs generated from long ncRNAs that contain sORFs encode functional peptides. Through RNC-seq and bioinformatic analysis, we identify several previously uncharacterized peptides that are potentially encoded by circRNAs. Analysis of candidate peptides originating from previously designated ncRNAs confirms an 87-amino acid (aa) peptide, which we term PINT87aa, encoded by the circular form but not linear LINC-PINT, according to multiple translation-related lines of evidence. Functionally, PINT87aa, but not its corresponding circRNA, partially controls the cell proliferation and tumorigenesis of cancer cells. Upregulation of the expression of PINT87aa which is decreased in glioblastoma compared with their expression in normal tissues, induces tumor-suppressive effects in vitro and in vivo. Our study demonstrates that the biological and clinical implications of peptides translated from circRNAs are likely underestimated, and we provide some evidence that alternative splicing may produce functional peptides from previously designated non-coding genes.
Results
High-throughput sequencing of potential coding circRNAs
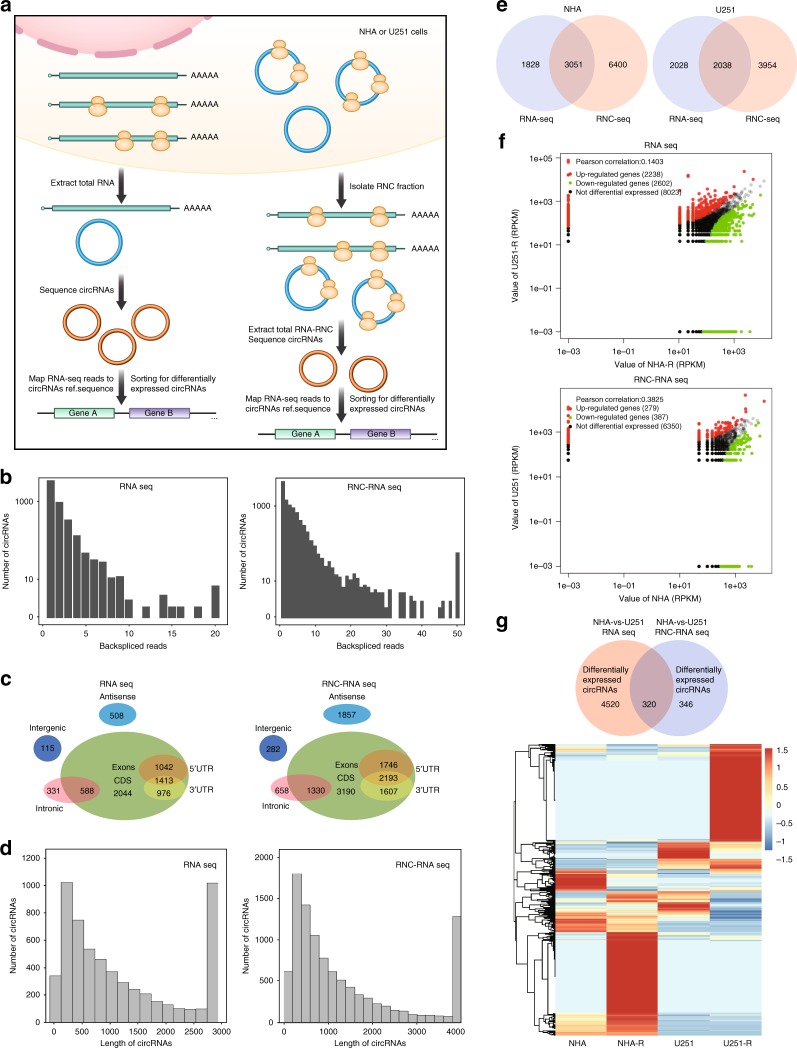
To generate an RNA-seq database of circRNAs (transcriptome sequencing) and RNC-RNAs (translatome sequencing), we used ribosomal RNA-depleted total RNA and RNC-RNAs from normal human astrocytes (NHA) and U251 glioblastoma cells (Fig. 1a). The brief procedure of RNC extraction is listed in the “Methods” section and is based on the article by Wang et al.32. Total RNA and RNC-RNAs were sequenced separately on an Illumina HiSeqTM 4000. The obtained reads were mapped to reference ribosomal RNA (Bowtie2, http://bowtie-bio.sourceforge.net/bowtie2/) and a reference genome (http://ccb.jhu.edu/software/tophat/)33,34, and 20mers from both ends of the unmapped reads were extracted and aligned to the reference genome to identify unique anchor positions within the splice site. Anchor reads that aligned in the reverse orientation indicated circRNA splicing was subjected to find_circ (https://omictools.com/find-circ-tool) to identify circRNAs2. The anchor alignments were then extended such that the complete read aligned, and the breakpoints were flanked by GU/AG splice sites. A candidate circRNA was called if it was supported by at least two unique back-spliced reads. As RNC-seq may have low identification rate, we designed to collect four times data in RNC-seq than that of RNA-seq. Through sequencing, a total of 15,189 circRNAs (7017 from RNA-seq and 12,863 from RNC-RNA seq) were identified, 4597 of which were matched in circBase35 (Fig. 1b and Supplementary Table 1). We next analyzed the characteristics of these circRNAs. The majority of the identified circRNAs were generated from exon coding sequences (CDS), but many circRNAs were derived from antisense or intronic regions (Fig. 1c). Most of the identified circRNAs were less than 1500 nucleotide (nt) in length (Fig. 1d), and the chromosome distribution was not unified between NHA and U251 circRNAs as previously reported36. Quantitatively, 4879 circRNAs were identified by using RNA-seq and 9451 circRNAs were identified by RNC-seq in NHA. 4066 circRNAs were identified by using RNA-seq and 5992 circRNAs were identified by using RNC-seq in U251 (Fig. 1e, RNC-seq has a larger sequencing depth compared with RNA-seq, see Supplementary Fig. 1a and Supplementary Table 2 and Supplementary Data 1). Among these candidates, 4840 and 666 differentially expressed circRNAs were identified in NHA and U251, respectively, from total RNA or RNC-RNAs with a false discovery rate (FDR) of ≤0.01 and a fold-change ≥2 (Fig. 1f). We cross-matched these differentially expressed candidates from total RNA and RNC-RNAs, and 320 overlapped circRNAs were further identified (Fig. 1g, upper). Gene Ontology (GO) enrichment analysis was performed for the host genes of these circRNAs (274 genes), and this gene set was enriched (FDR < 0.05) for GO molecular functions including protein binding and cellular component organization (Fig. 1g, lower and Supplementary Table 3). As mentioned previously, most circRNAs shared the same CDS with their host protein-coding genes. To exclude false-positive data from further investigation, we focused only on non-coding host genes among these 274 candidates. A total of ten non-coding host genes were found and in which five had coding-potential (Supplementary Table 4). After initial screening, we focused on LINC-PINT (ENSG00000231721) for further investigation. LINC-PINT is a tumor-suppressive long intergenic non-coding RNA (lincRNA) that is involved in Polycomb repressive complex 2 (PRC2)37. No evidence thus far has suggested that LINC-PINT is a coding RNA38,39.
Fig. 1.
Translatome sequencing and proteome profiling of potential coding circRNAs in normal and cancer cells. a Illustration of the screening protocol. Briefly, total RNAs or RNC-RNAs were isolated separately from NHA or U251 cells. Equal amounts of total RNA or RNC-RNA were reverse-transcribed and subjected to deep RNA sequencing. Identified differentially expressed circRNAs were annotated in the genome, and the host genes were cross-matched between NHA and U251. b RNA-seq read abundance distribution of identified circRNAs. Upper, total RNA seq; Lower, RNC-RNA seq. X-axis: the back-spliced read numbers of circRNAs detected by sequencing. Y-axis: the abundance of circRNAs classified by different read numbers. The majority of called circRNAs in the study were supported by more than 10 reads. c Venn plot showing the number of circRNAs derived from different genomic regions. Upper, total RNA seq; lower, RNC-RNA seq. d Length distribution of the identified circRNAs. Upper, total RNA seq; lower, RNC-RNA seq. X-axis: the length of circRNAs detected in this study. Y-axis: the abundance of circRNAs classified by different lengths. e The Venn plot of the numbers of called circRNAs in NHA and U251 by RNA-seq or RNC-seq. RNC-seq identified more circRNAs due to the higher sequencing depth (see Supplementary Figure 1). f Scatter plot of all differentially expressed circRNAs between NHA and U251 cells. Upper, total RNA-seq; lower, RNC-seq (x and y axes represent circRNA expression value, RPKM). g Upper, differentially expressed circRNAs between NHA and U251 cells in total RNA or RNC-RNA were cross-matched. A total of 320 differentially expressed circRNAs were identified, generated from 274 host genes. Lower, the host genes were subjected to GO enrichment analysis (The gene expression value in heatmap was normalized by Z score in each row.)
Identification of a circRNA formed by exon 2 of LINC-PINT
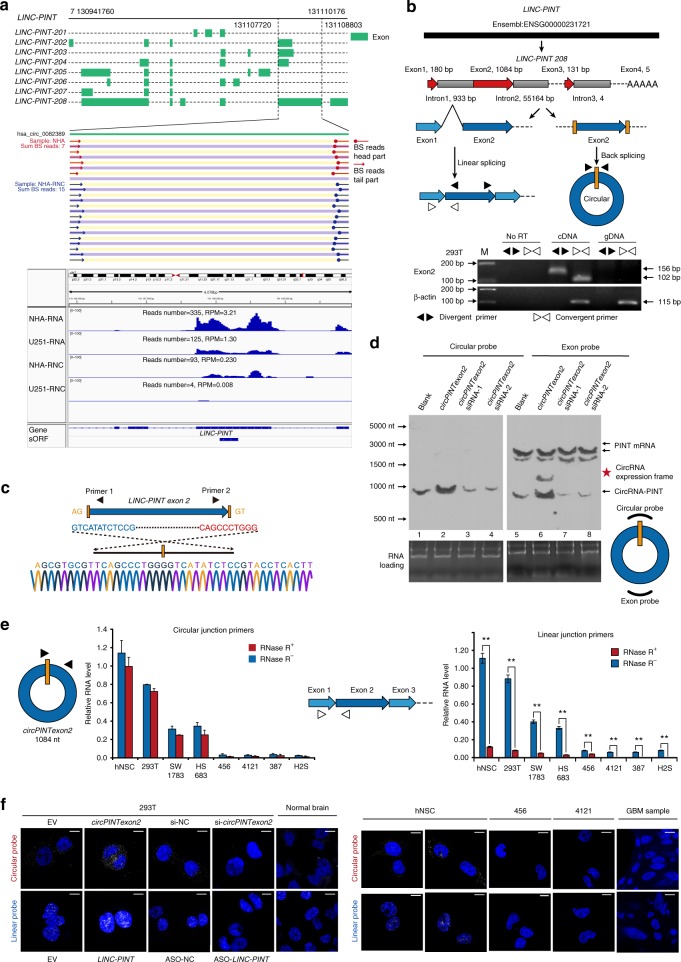
We first visualized the back-spliced reads of exon 2 of LINC-PINT in our sequencing data. As shown in Fig. 2a, upper panel, there were 15 back-spliced junction-specific reads in the RNC-RNA group compared with 7 reads in the total RNA group, implying that exon 2 of LINC-PINT was identified as a translatable circular RNA. In contrast, junction reads were not identified in either total RNAs or RNC-RNAs from U251. The IGV plot showed that reads number of exon 2 LINC-PINT were higher in NHA compared with U251, both in RNA-seq and RNC-seq. Notably, exon 1 and exon 3 reads were much lower than exon 2 reads in RNC-seq, implied that linear LINC-PINT is not translated (Fig. 2a, lower panel). The long exon 2 of LINC-PINT, which contains the 3′ AG receptor and 5′ GT donor sequences required for back-splicing, was identified as a circRNA in human CircBase (has circ-0082389, Fig. 2b, upper panel, and Supplementary Fig. 1b). Therefore, we first determined whether exon 2 of LINC-PINT formed an endogenous circRNA in human cells. Head-to-tail splicing was assayed by performing quantitative polymerase chain reaction (q-PCR) after reverse transcription with con/divergent primers specific for the linear or circular form of LINC-PINT (Fig. 2b, lower panel). The PCR products from divergent primers were analyzed via Sanger sequencing to reveal the junction of circular LINC-PINT exon 2 (Fig. 2c). To exclude the possibility that this back-splicing was attributable to genomic rearrangement or was a PCR artifact, we validated this circRNA through northern blotting with an exon probe or a circular probe, which recognize both the linear and circular forms or only the circular forms of LINC-PINT exon 2 (which we designated circPINTexon2), respectively. CircPINTexon2 was detected endogenously in 293T cells, while upregulated or decreased accordingly after synthetic circPINTexon2 plasmid overexpression or junction siRNA transfection (Fig. 2d, lanes 1–4, schematic diagram of the overexpression plasmid shown in Fig. 3f). Furthermore, exon probes detected both LINC-PINT and circPINTexon2, as shown in Fig. 2d, lanes 5–8. Q-PCR and fluorescence in situ hybridization (FISH) analyses with primers/probes specifically designed to detect the circular junction or linear junction further confirmed the existence of the head-to-tail spliced circular form of LINC-PINT exon 2 in human cell lines and tissues. Both linear LINC-PINT and circPINTexon2 were expressed in human neural stem cells (hNSC) and 293T cells, but their expression decreased in different glioma/brain tumor-initiating cells (BTIC) cell lines (Fig. 2e). LINC-PINT and circPINTexon2 presented different cellular localizations: linear LINC-PINT largely localized to the nucleus, whereas circPINTexon2 was mostly cytoplasmic (Fig. 2f and Supplementary Fig. 2).
Fig. 2.
Identification of exon 2 of LINC-PINT as a circRNA. a Upper, visualization of the forward reads within the exon 2 region in the LINC-PINT junction site of NHA cell in RNA-seq and RNC-seq. These junction reads are specific for circular form of LINC-PINT exon 2. Lower, IGV plot of all reads located on exon 2 of LINC-PINT in RNA-seq and RNC-seq. The IGV plot also included the reads on exon 1 and 3 of LINC-PINT. b Illustration of the annotated genomic region of LINC-PINT (Ensembl number: ENSG00000231721), the putative different mRNA splicing forms (linear splicing and head-to-tail splicing) and the validation strategy for LINC-PINT circular exon 2 (circPINTexon2). Divergent primers detected the circular form of circPINTexon2 in cDNA but not in gDNA. Convergent primers spanning exon 1 and exon 2 of LINC-PINT (variants LINC-PINT-208, shown in a) specifically detected the linear splicing form. β-actin was used as a linear RNA control. c Sanger sequencing was performed following PCR using the indicated divergent flanking primers to confirm the head-to-tail splicing of circPINTexon2 in 293T cells. d Northern blots of 293T total RNA with the exon probe and the junction-specific circular probe for circPINTexon2. Lanes 1–4 detected circPINTexon2 with circular probes. Lanes 5–8 detected circPINTexon2 and LINC-PINT with exon probes. CircPINTexon2-overexpression plasmid was shown in Fig. 3f. e Q-PCR followed by with junction-specific primers was used to detect the expression of circPINTexon2 in vitro. Primers specific for linear LINC-PINT were also used to detect LINC-PINT expression. RNase R treatment was used to validate circPINTexon2. Data are presented as mean ± s.e.m. from three independent experiments. **P < 0.01; ns, P > 0.05, determined by two-tailed Student’s t-tests. f FISH with junction-specific probes specific to circPINTexon2, whereas linear specific probes specific to linear LINC-PINT indicated their cellular localization in vitro. Normal brain tissues and GBM samples were stained with indicated probes. Overexpressed or knocked-down circPINTexon2 or LINC-PINT using corresponding plasmids or siRNA/ASOs in 293T cells to indicate the specificity of these probes. Scale bars, 10 μM. EV empty vector, si-NC random scrambled siRNA, circPINTexon2 circPINTexon2 overexpression vector, ASO LINC-PINT anti-sense oligos
Fig. 3.
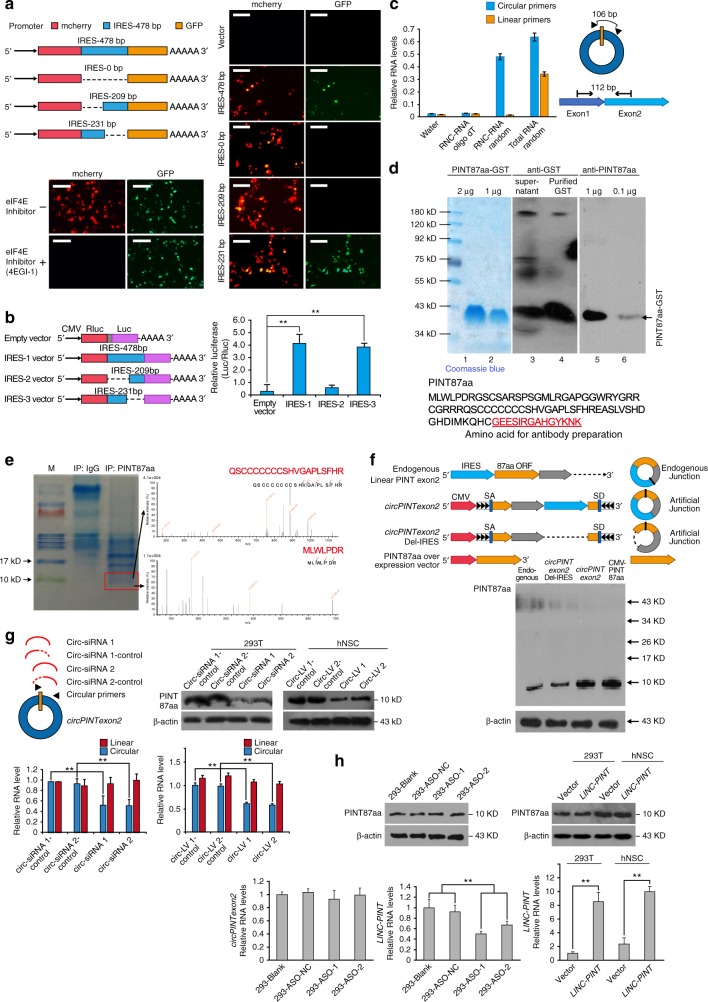
circPINTexon2 encodes an 87-aa peptide. a Full-length or truncated circPINTexon2 IRES (478, 231, and 209 bp) were cloned between mCherry and GFP as indicated to construct several reporter plasmids. These plasmids were transfected into 293T cells as indicated, with or without 4EGI-1 treatment. IF was performed to determine mCherry and GFP signals. Scale bars, 50 μM. b Rluc and Luc were tandemly cloned into the luciferase reporter plasmid, with or without the indicated truncated IRES between them. Luc/Rluc activities were measured in each transfected plasmid. c RNC-RNA or total RNA from 293T cells was extracted and reverse-transcribed using oligo-dT or random primers as indicated. Specific primers for circPINTexon2 or LINC-PINT were analyzed by using q-PCR. d Upper, antibody recognition test for the predicted 87-aa peptide. Lanes 1 and 2, Coomassie blue staining of the GST-PINT87aa fusion protein; lanes 3 and 4, Western blot performed with GST antibody; lanes 5 and 6, Western blot performed with PINT87aa antibody. Lower, the predicted 87-aa peptide sequence and antibody generation region was shown as indicated. e Endogenous immunoprecipitation using anti-PINT87aa antibodies in 293T cells. LC-MS/MS analysis following SDS-PAGE was performed to identified peptide sequences of PINT87aa. f Upper panel, endogenous circPINTexon2: endogenous formation of circPINTexon2; CircPINTexon2 vector: the artificial circPINTexon2 overexpression plasmid. Note the junction was moved inside the 87-aa ORF. CicrPINTexon2 Del-IRES vector: negative control, in which the IRES sequence was deleted from the artificial circPINTexon2 plasmid. 87-aa overexpression vector: positive control, in which the 87-aa ORF was cloned downstream of a linear CMV promoter. Lower panel, PINT87aa expression was tested in 293T cells after transfection of the plasmids indicated above. g circPINTexon2 and PIN87aa expression were determined using junction-specific siRNA or shRNA specific for circPINTexon2-transfected 293T or hNSC. h Left, LINC-PINT, circPINTexon2, and PIN87aa expression were determined in two ASOs specific for LINC-PINT-transfected 293T. Right, LINC-PINT and PINT87aa were determined in LINC-PINT stably transduced 293T and hNSC. b, c, g, h Data are presented as mean ± s.e.m. from three independent experiments. **P < 0.01; ns, P > 0.05, determined by two-tailed Student’s t-tests
CircPINTexon2 encodes a peptide
To test the hypothesis that circPINTexon2 is translatable (circPINTexon2 junction reads were identified in RNC-seq, and exon 2 reads on LINC-PINT were much higher than exon 1 and exon 3), we first analyzed all putative sORFs in LINC-PINT exon 2. We identified two sORFs, potentially encoding peptides 69 aa and 87 aa in length, in this exon that were highly conserved among multiple species (Supplementary Fig. 3). To explore whether these putative ORFs were active, we cloned full-length exon 2 of LINC-PINT into the PLCDH-ciR vector (with artificial side flanking sequences and SA/SD sequences), containing green fluorescent protein (GFP), without start or stop codons, fused immediately upstream of the stop codons of the two sORFs. Immunofluorescence (IF) results showed that only the second sORF, which encoded the 87-aa peptide, was expressed in 293T cells with the overexpression of the above vector (Supplementary Fig. 4). Although the 87-aa sORF was an active in-frame sORF in vivo compared with the 69-aa sORF, further investigations were needed to show that the 87-aa peptide was translated by endogenous circPINTexon2 but not linear LINC-PINT in vivo.
First, to demonstrate that circPINTexon2 contains a natural internal ribosome entry site (IRES), which is required for translation initiation in 5′-cap-independent coding RNAs40–42, we carried out a bioinformatics analysis (Supplementary Fig. 5) and an IRES activity test43, as shown in Fig. 3a. mCherry and GFP were cloned into a dual-cistronic reporter construct with or without the putative IRES (478 bp upstream of the 87-aa sORF) or with the indicated mutations between them (Fig. 3a, left). Under normal eIF4E conditions, both mCherry and GFP were detected with the putative IRES; in contrast, only mCherry was detected if the putative IRES was deleted. GFP was not detected when the sequence −478 to −231 upstream of the 87-aa sORF was deleted, indicating the IRES is located in this area. When eIF4E was inhibited (under treatment with 4EGI-1), the putative IRES induced the expression of GFP but not mCherry; thus, the natural IRES in circPINTexon2 induced ribosome entry and initiated translation. To quantitatively test the putative activity of circPINTexon2 IRES, we performed further experiments. Briefly, we used a tandem Rluc–Luc reporter plasmid in which the Rluc ORF was driven by a CMV promoter, and the Luc ORF was fused immediately after the RLuc stop codon, without any promoter between them. We cloned the IRES-478bp, IRES-209bp and IRES-231bp sequences between RLuc and Luc, and the luciferase activity of Luc relative to that of RLuc was measured for each plasmid. IRES-209bp presented the lowest luciferase activity (Luc/Rluc), close to that of the empty vector, indicating that IRES-209bp does not induce ribosome entry. IRES-478bp and IRES-231bp exhibited significantly higher Luc/Rluc activities than the empty vector, further supporting the notion that an active IRES is located upstream of the 87-aa sORF (Fig. 3b).
Next, we performed qPCR on the translating RNAs (RNC-RNAs) from 293T cells to further show that PIN87aa is encoded by circPINTexon2 but not linear LINC-PINT. RNC-RNAs were reverse-transcribed using oligo-dT (for mRNAs) or random primers (for all RNAs). Total RNAs were reverse-transcribed by random primers as a positive control. As shown in Fig. 3c, both circPINTexon2 and linear LINC-PINT-specific PCR products were amplified from cDNA reverse-transcribed from total RNAs with random primers, indicating that circPINTexon2 and linear LINC-PINT are both present in 293T cells. However, only circPINTexon2-specific PCR products were amplified from cDNA reverse-transcribed from RNC-RNAs with random primers, indicating that circPINTexon2 is bound to the ribosomal nascent chain complex and is undergoing translation. In contrast, linear LINC-PINT-specific PCR products were not detected in cDNA reverse-transcribed from RNC-RNAs with random primers or oligo-dT primers, indicating that linear LINC-PINT does not undergo translation. These data were consistent with our RNC-seq data described above (Fig. 2a, RNC-seq showed few reads on exon 1 and 3 of LINC-PINT, indicating that linear LINC-PINT was not translated).
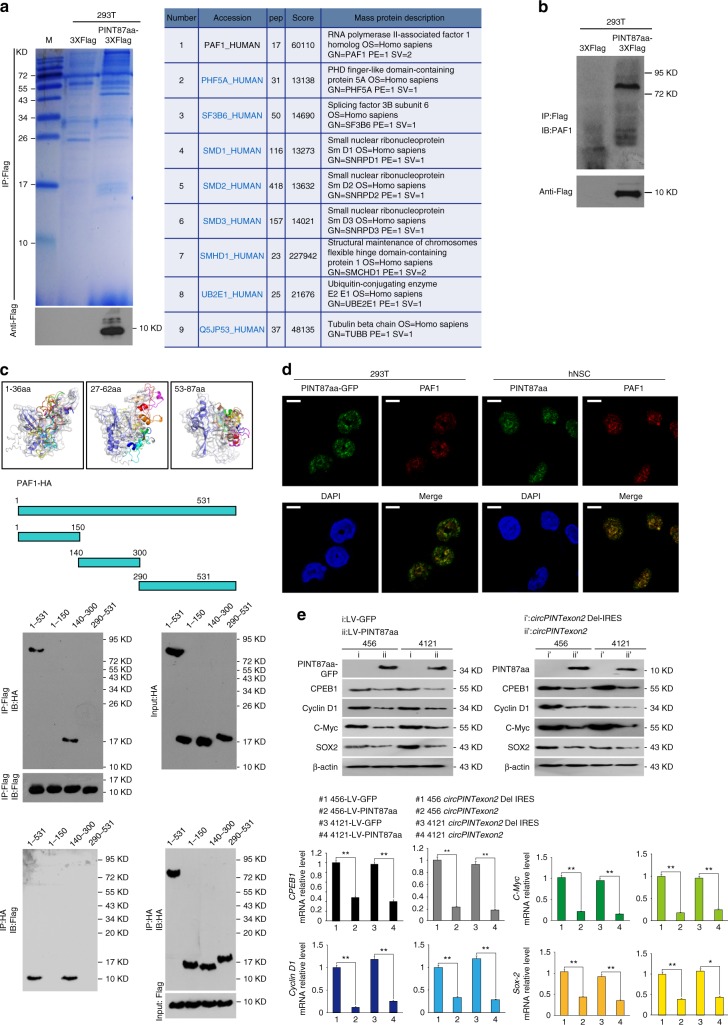
Based on the above information, we generated an antibody against the 87-aa peptide (antibody construction is described in the Supplementary Data 2). Immunoblotting showed that this antibody successfully recognized the predicted glutathione-S-transferase (GST)-fused protein (Fig. 3d). Endogenous immunoprecipitation using this antibody followed by LC-MS/MS in 293T cells further confirmed that the 10-kDa peptide sequences matched the predicted 87-aa sORF (Fig. 3e). Because the 87-aa sORF did not overlap the circPINTexon2 junction, we designed several synthetic plasmids to further test the coding ability of this circRNA (Fig. 3f, upper panel). In the circPINTexon2 vector, the circRNA junction was moved inside the 87-aa sORF, and circularization of this plasmid induced by side flanking sequences resulted in the formation of the same circRNA as the natural circPINTexon215. In contrast, the 87-aa sORF was not present in the circPINTexon2 vector’s linear reading frame. As shown in Figs. 2c and 3f, lower panel, northern blotting and western blotting indicated that transfection of this synthetic plasmid resulted in successful overexpression of circPINTexon2 and the 87-aa peptide. However, the transfection of control plasmids in which the IRES was deleted did not result in overexpression of the 87-aa peptide (Supplementary Fig. 6). Importantly, 87-aa peptide overexpression induced by this synthetic circRNA plasmid was as strong as that of the CMV-driven linear 87-aa sORF overexpression plasmid, demonstrating the high translation efficiency of circRNA44 (Fig. 3f, lower panel). We next used two siRNAs that specifically target the circular junction of circPINTexon2. Junction-specific siRNAs successfully reduced circPINTexon2 and 87-aa peptide levels without affecting linear LINC-PINT (Fig. 3g). In contrast, two antisense oligonucleotides (ASOs) specifically designed to target linear LINC-PINT did not decrease the expression of circPINTexon2 or the 87-aa peptide (Fig. 3h, left). Furthermore, stable overexpression of linear LINC-PINT in 293T/hNSC did not elevate PINT87aa expression (Fig. 3h, right). Collectively, these results demonstrated that the 87-aa peptide is produced by circPINTexon2 but not the linear form of LINC-PINT. We named this peptide PINT87aa.
Tumor-suppressive effects of PINT87aa in vitro
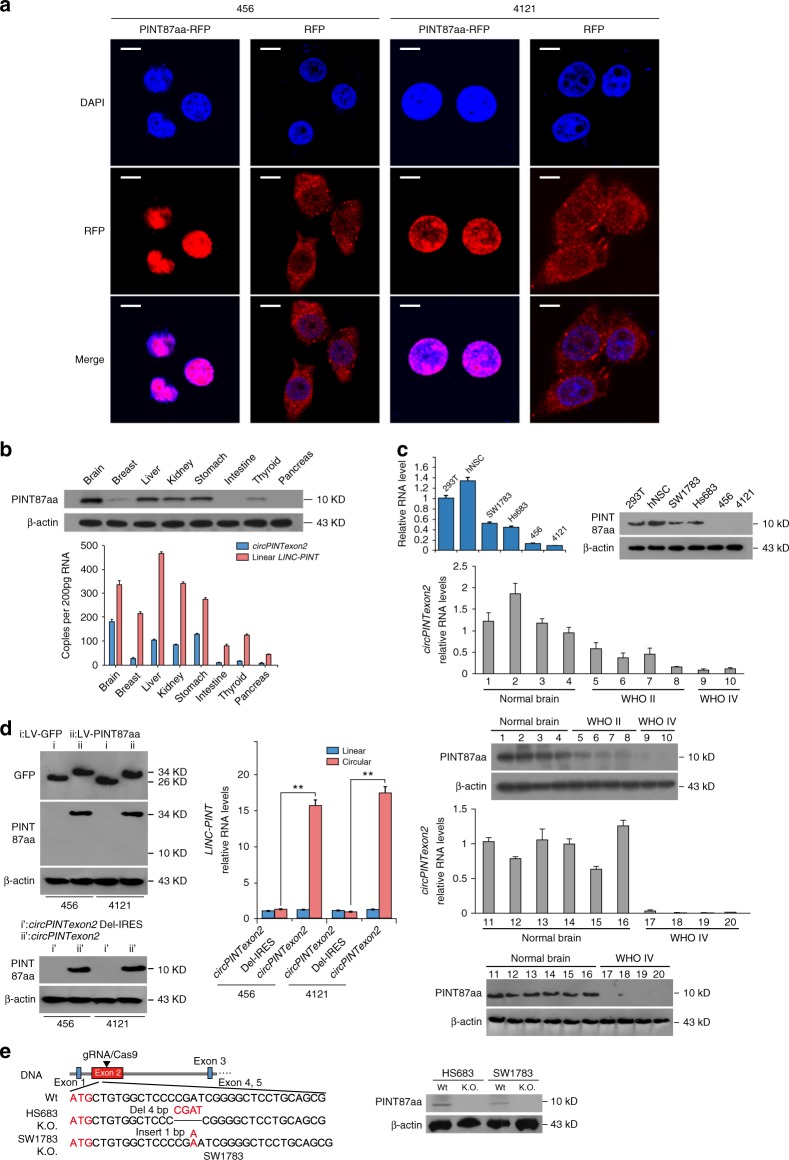
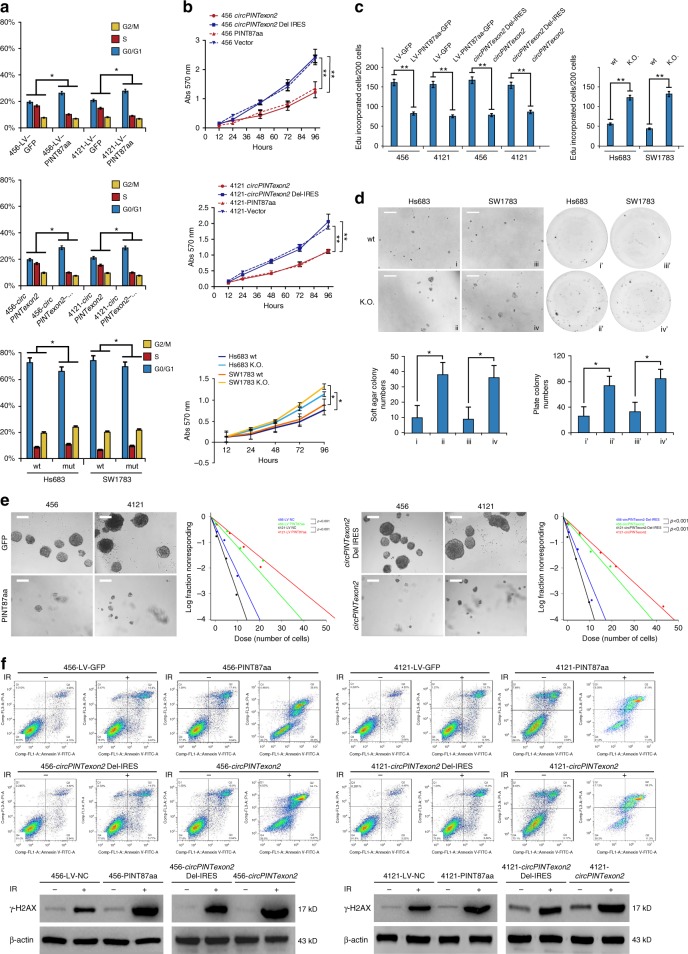
To investigate its possible biological functions, we first detected the cellular localization of PINT87aa. Using RFP fusion protein labeling, we found that this peptide was concentrated in nucleus, suggesting PINT87aa plays potential cellular regulatory roles (Fig. 4a). We next detected circPINTexon2 and PINT87aa baseline expression in several human tissues. CircPINTexon2 and PINT87aa were expressed in the brain, liver, kidney, and stomach but showed lower expression in the breast, intestine, thyroid, and pancreas tissues (Fig. 4b). Because PINT87aa were abundant in the human brain, we further detected its expression in several established glioma and BTIC cell lines. hNSC exhibited high PINT87aa expression, similar to 293T cells, whereas the anaplastic astrocytoma cell lines SW1783 and Hs683 exhibited the modest expression. The BTICs demonstrated the lowest levels of PINT87aa (Fig. 4c, left). The circPINTexon2 expression in these cell lines detected using junction-specific primers was consistent with PINT87aa levels (Fig. 4c, right). Decreased circPINTexon2 and PINT87aa expression also reflected WHO grades in clinical glioma samples (Fig. 4c, lower). To determine the biological roles of PINT87aa in tumor cells, we established 456 and 4121 cells that stably overexpressed the linear PINT87aa-GFP vector or the circular circPINTexon2 vector (Fig. 4d). Additionally, we generated CRISPR/Cas9-induced PINT87aa K.O. SW1783 and Hs683 cells (Fig. 4e and Supplementary Fig. 7). These cells were selected because 456 and 4121 exhibited very low PINT87aa expression, while SW1783 and Hs683 cells exhibited moderate PINT87aa expression. Compared with corresponding control cells, both 456 and 4121 cells overexpressing linear PINT87aa and circPINTexon2 exhibited G1 arrest and reduced cell proliferation without obvious cellular toxicity (Fig. 5a, b, left), whereas PINT87aa K.O. SW1783 and Hs683 cells showed increased cell cycle and cell proliferation rates (Fig. 5a, b, right). 456-PINT87aa and 4121-PINT87aa cells overexpressing both linear and circular vectors showed significant growth reduction (Fig. 5c, left), while PINT87aa K.O. SW1783 and Hs683 cells showed an enhanced cell proliferation and malignant phenotype in plate colony and soft agar (Fig. 5c, right; 5d). As expectation, 456-PINT87aa and 4121-PINT87aa cells overexpressing both linear and circular vectors showed less effective neuro-sphere formation (Fig. 5e). PINT87aa overexpression also enhanced the IR sensitivity in both 456 and 4121 cells (Fig. 5f). We also observed a slight inhibition of invasion ability in PINT87aa transduced 456 and 4121 cells, although this phenomenon may be induced by an accelerated cell proliferation (Supplementary Fig. 8a). In contrast, PINT87aa K.O. SW1783 and Hs683 cells showed radiation resistance compared with their parental cells (Supplementary Fig. 8b). Based on the above results, we showed that circPINTexon2 exerts its tumor-suppressive functions through PINT87aa instead of circPINTexon2.
Fig. 4.
Localization and expression of PINT87aa in cells and human tissues. a IF microscopy images of the cellular localization of the PINT87aa-RFP-fusion protein in 456 and 4121 BTIC cells showing stable overexpression. Scale bars, 20 μM. b The expression of circPINTexon2 and PINT87aa was detected in human brain, breast, liver, kidney, stomach, intestine, thyroid, and pancreas tissues. c Upper panel, the expression of circPINTexon2 and PINT87aa was detected in hNSC, 293T, Hs683, SW1783, 4121, 456, 387, and H2S cells. Lower panel, circPINTexon2 and PINT87aa levels were determined in normal brain and gliomas with different WHO grades. d Establishment of linear vector-transduced stable PINT87aa-GFP 456 and 4121 cells and artificial circPINTexon2-transduced stable PINT87aa 456 and 4121 cells. Data are presented as mean ± s.e.m. from three independent experiments. **P < 0.01; ns, P > 0.05, determined by two-tailed Student’s t-tests. e Establishment of PINT87aa K.O. SW1783 and Hs683 cells using CRISPR/Cas9 technology. Schematic illustration showing that the genomic regions of the LINC-PINT exon2 contained the 87-aa ORF. gRNAs designed to target the PINT87aa ORF are shown. Genomic K.O. effects were confirmed via Sanger sequencing, as shown in Supplementary Fig. 7. Western blotting revealed the effects of PINT87aa K.O. in SW1783 and Hs683 cells
Fig. 5.
Biological functions of PINT87aa. a Left, cell cycle was determined in PINT-87aa or circPINTexon2 stably overexpressed 456 and 4121 BTICs. Right, cell cycle analysis of PINT87aa K.O SW1783 and Hs683 cells. b MTT proliferation assay was examined in PINT87aa-GFP or circPINTexon2 stably overexpressed 456 and 4121 cells or PINT87aa K.O SW1783 and Hs683 cells. c Edu proliferation assay was examined in PINT87aa-GFP or circPINTexon2 stably overexpressed 456 and 4121 cells, PINT87aa K.O SW1783 and Hs683 cells and their control cells. d Soft-agar and plate colony assay was performed in PINT87aa K.O SW1783 and Hs683 cells and their control cells. Scale bar, 100 μM. e In vitro extreme limiting dilution assays (ELDAs) were performed to evaluate BTICs self-renewal capacity. Scale bar, 100 μM. Spheres were counted at 14 days. Cell density per well ranged from 1, 10, 25, 50, 100, 250, 500 to 1000. Each condition was tested in 10 independent wells. Neurosphere-forming capability was determined using the ELDA web-based tool (456, P < 0.001; 4121, P < 0.001, by ELDA analysis). f PINT-87aa or circPINTexon2 stably overexpressed 456 and 4121 BTICs and their control cells were subjected to 6 Gy radiation. DNA damage was determined by flow cytometry and γ-H2AX expression. a–d, f Data are presented as mean ± s.e.m. from three independent experiments. *P < 0.05; ns, P > 0.05, determined by two-tailed Student’s t-tests
PINT87aa regulates the RNA elongation of multiple oncogenes
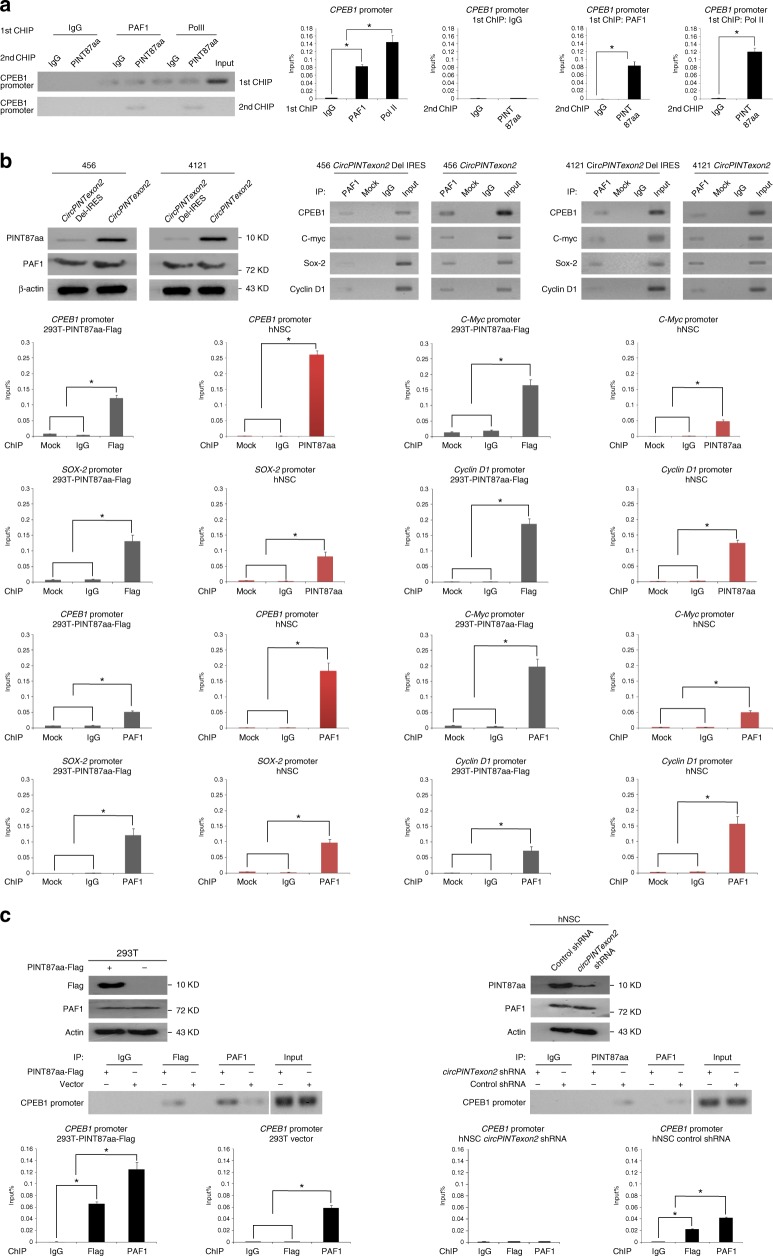
To further explore the potential molecular mechanisms underlying the tumor-suppressive functions of PINT87aa, we first checked some proto-oncogenes that are critical in glioma tumorigenesis in PINT87aa overexpressed BTICs. However, EGFR, MET, and PDGFR were not changed after PINT87aa or circPINTexon2 overexpression (Supplementary Fig. 8c). Next, we performed co-immunoprecipitation in PINT87aa-3XFlag-overexpressing 293T cells to identify its potential targeting molecules. As shown in Fig. 6a, left, 293T cells transfected with PINT87aa-3XFlag and corresponding control cells were subjected to immunoprecipitation using an anti-Flag antibody. The precipitates were subjected to LC-MS/MS to identify potential PINT87aa-interacting proteins. Among various candidates, PINT87aa was found potentially bound to the PAF1 protein complex (Fig. 6a, right). Immunoprecipitation further confirmed a mutual PINT87aa/PAF1 interaction in 293T cells (Fig. 6b). To investigate the potential direct interaction between PINT87aa and PAF1, PINT87aa conformations were modeled with PEP-FOLD and docked to PAF1 based on the ATTRACT2 force field through the PEP-SiteFinder pipeline45. Because PINT87aa was too long, it was split into three sections: 1–36 aa, 27–62 aa, and 53–87 aa. The top ten peptides in complex with PAF1 were visualized with different colors by PyMOL (The PyMOL Molecular Graphics System, Version 1.8 Schrödinger, LLC.), while protein–peptide interface residues (within 5 Å for each peptide) were labeled. As shown in Fig. 6c, upper left, PINT87aa most likely directly interacts with the middle region of PAF1. A direct binding assay using purified proteins further indicated that PINT87aa interacts with the 150–300-aa domain of PAF1 (Fig. 6d, lower panel), and the amino acid of R20, G21, P23, C32, R36, and S53 in PINT87aa are critical for PAF1 interaction (Supplementary Fig. 9). Furthermore, GFP-PINT87aa and PAF1 co-localized in the nucleus (Fig. 6e), suggesting PINT87aa may be involved in PAF1 target gene regulation. The PAF1 complex is involved in RNA II polymerase (Pol II) recruitment and regulating the transcriptional elongation of downstream genes46,47, and evidence has shown that PAF1 regulates potential oncogenes during human tumorigenesis, including that of gliomas48–50. We performed further experiments in PINT87aa-overexpressing 456 and 4121 cells to determine whether PINT87aa is involved in PAF1 target gene elongation. The mRNA of PAF1 downstream genes, including CPEB1, SOX-2, c-Myc, etc., was inhibited transcriptionally in PIN87aa-overexpressing 456 and 4121 cells compared with that in control cells (Fig. 6e). In PINT87aa K.O. Hs683 and SW1783 cells, the expression of these genes increased at both the mRNA and protein levels (Supplementary Fig. 10a). Further 2nd ChIP assay showed that PINT87aa and PAF1 were co-occupied in CPEB1 promoter (Fig. 7a), suggesting PINT87aa involved in PAF1/Pol II complex regulation. However, PINT87aa overexpression did not change PAF1 expression in 456 or 4121 BTICs. Instead, PINT87aa enhanced PAF1 and its target genes’ promoter interaction (Fig. 7b). PINT87aa overexpression or knocking down enhanced or decreased PAF1/CPEB1 promoter affinity, respectively (Fig. 7c), suggesting that PINT87aa could decide PAF1/CPEB1 promoter interaction. We assumed that PINT87aa may work as an anchor and keep PAF1 complex on target genes’ promoter, which sequentially pauses Pol II-induced mRNA elongation. Loss of PINT87aa or overexpression of PAF1, which was seen in many cancers, results in PAF1 losing its proper localization51. Freed PAF1 is sequentially involved in many other biological processes such as cell cycle regulation, histone modification, MAPK signaling transduction as well as cancer-stem-cell self-renewal51. LINC-PINT knockdown in SW1783 and Hs683 cells using specific ASOs increased cell viability but did not alter PAF1 downstream gene mRNA or protein levels (Supplementary Fig. 10b and c), indicating LINC-PINT and PINT87aa are involved in different signaling pathways. Interestingly, stable knockdown of PINT87aa in normal cells, such as NHA cells, also decreased cell vitality (Supplementary Fig. 11). PINT87aa appears to be required for normal cell survival, but its loss in cancer cells induces cell cycle acceleration and cell proliferation. LINC-PINT was reported to be regulated by p5337. We did not find PINT87aa altering p53 expression or affecting p53 downstream targets, in both p53 wild type or mutant cells (Supplementary Fig. 12a, b and c). In contrast, overexpression of p53 in A172 and 293T cells could upregulate PINT87aa (Supplementary Fig. 12d). We inferred that PINT87aa exerts biological functions independent of LINC-PINT although they are possibly both regulated by p53. Clearly, further investigations are still needed to clarify upstream signaling of PINT87aa.
Fig. 6.
PINT87aa directly interacts with the PAF1 complex and inhibits mRNA transcriptional elongation. a Left, immunoprecipitation was performed using an anti-Flag antibody in PINT87aa-3XFlag or empty vector transfected 293T cells. Western blotting using an anti-Flag antibody confirmed PINT87aa overexpression. The precipitates were subjected to LC-MS/MS to identify potential PINT87aa-interacting proteins. Right, PAF1 complex-related proteins were identified in PINT87aa precipitates. b Immunoprecipitation was performed in PINT87aa-3XFlag-transfected cells or control cells. Western blotting was performed using an anti-PAF1 antibody. c Upper, PINT87aa conformations were modeled with PEP-FOLD and docked to PAF1 based on the ATTRACT2 force field using the PEP-SiteFinder pipeline. PINT87aa was split into three segments: 1–36 aa, 27–62 aa, and 53–87 aa. The top ten peptides in complex with PAF1 were visualized with different colors using PyMOL (The PyMOL Molecular Graphics System, Version 1.8 Schrödinger, LLC.), while the protein–peptide interface residues (within 5 Å for each peptide) were labeled. Lower, the direct mutual interactions of PINT87aa with different domains of HA-tagged PAF1 were tested using purified proteins (Flag-tagged PINT87aa and HA-tagged PAF1). d IF was performed to determine PINT87aa-PAF1 colocalization in PINT87aa-GFP transfected 293T cells or hNSC. Scale bar, 20 μM. e The expression of PAF1 downstream genes was determined by performing Western blotting and q-PCR in PINT87aa- or circPINTexon2-overexpressed 456 and 4121 BTIC and their respective controls. Data are resented as mean ± s.e.m. from three independent experiments. **P < 0.01, ns, P > 0.05, determined by two-tailed Student’s t-tests
Fig. 7.
PINT87aa decides the localization of PAF1 complex on target genes promoter. a 2nd ChIP assay was used to determine the co-occupation of PAF1 complex/PINT87aa in CPEB1's promoter region by using RT-PCR or q-PCR. At least three independent experiments were performed. b ChIP assay was performed in circPINTexon2 stably overexpressed 456 and 4121 cells by using indicated antibodies. PCR products of indicated genes’ promoter were analyzed by using RT-PCR or q-PCR. c PINT87aa was overexpressed or knocked-down in 293T or hNSC cells by using indicated plasmid or shRNA, respectively. ChIP assay was performed by using indicated antibodies and PCR products of CPEB1 promoter region were analyzed by using RT-PCR or q-PCR. b, c At least three independent experiments were performed
The tumor-suppressive role of PINT87aa
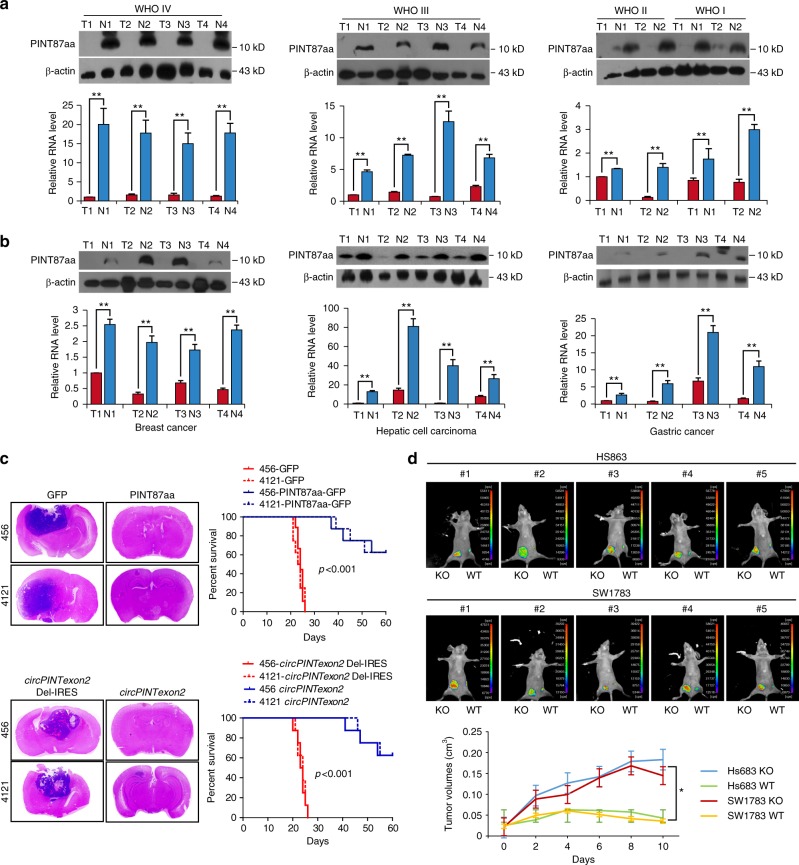
To understand the potential clinical implications of PINT87aa, we detected the expression of circPINTexon2 and PINT87aa in human glioma samples and paired adjacent normal tissues. CircPINTexon2 and PINT87aa were expressed in tumor-adjacent normal brain tissues, but their expression decreased in all brain tumor tissues, similar to LINC-PINT (Fig. 8a and Supplementary Fig. 13). Specifically, WHO grade IV glioblastomas exhibited the lowest expression levels of PINT87aa. In WHO grade I astrocytomas, PINT87aa was detectable, but levels were decreased compared with those in normal tissues. These data suggest that PINT87aa negatively impacts the clinical prognosis of glioma. We obtained similar results regarding circPINTexon2 and PINT87aa expression in other human malignancies, including breast cancer, hepatic cell carcinoma, and gastric cancer (Fig. 8b and Supplementary Fig. 13). Clearly, circPINTexon2 and PINT87aa downregulation is normal in human malignancies. In animal models, 456 and 4121 cells stably overexpressing PINT87aa either on linear or circular plasmids exhibited decreased in situ tumorigenic potential compared with control cells, as assessed by tumor growth and animal survival (Fig. 8c). Furthermore, Hs683 and SW1783 PINT87aa K.O. cells and control cells were subcutaneously injected into nude mice, and tumor growth was subsequently monitored with calipers and through in vivo fluorescence imaging (Fig. 8d). Compared with their parental cells, PINT87aa K.O. cells resulted in significantly increased xenograft tumor volumes, further supporting the anti-cancer effects of the PINT87aa peptide in vivo.
Fig. 8.
Clinical implications of circPINTexon2 and PINT87aa. a Expression of circPINTexon2 and PINT87aa in human glioma samples with different WHO grades and matched adjacent normal tissues. b Expression of circPINTexon2 and PINT87aa in breast cancer, hepatic cellular carcinoma, gastric cancer, and adjacent normal tissues. c Anti-cancer effects of PINT87aa in vivo; left, mice with brain xenograft tumors were sacrificed 60 days after implantation; right, Kaplan–Meier survival curves of mice implanted with 456 and 4121 cells stably overexpressing PINT87aa and their respective parental cells (N = 8 mice/treatment group, P < 0.001, two-tailed log-rank test). d Nude mice were subcutaneously injected with 5 × 106 Hs683 and SW1783 PINT87aa K.O. cells and their parental cells using Matrigel and were then monitored daily. Day 0 indicates the first day of injection. Tumor progression was monitored using a small animal imaging system or through caliper measurements. Error bars indicate standard error (N = 5 mice/treatment group, P < 0.01, two-tailed log-rank test)
Discussion
CircRNAs are a widespread RNA species in the human transcriptome4,52. In addition to acting as regulators of gene expression or development by adsorbing microRNAs, circRNAs were recently demonstrated to be critical in human malignancies53–55. However, only a few circRNAs contain perfect multiple microRNA trapping sites, raising the question of whether circRNAs exhibit functions beyond acting as microRNA sponges2,3. Recent evidence has confirmed the existence of functional peptides translated from sORFs in ncRNAs, including pri-microRNAs and lncRNAs21,22, suggesting that the coding potential of these ncRNAs has been largely underestimated. To explore the coding potential of circRNAs, we perform RNC-RNA deep sequencing and confirmed that an endogenous circRNA (circPINTexon2) encodes a tumor-suppressive peptide in human cells. Although the 87-aa sORF did not span the circPINTexon2 junction, multiple lines of evidence indicated that this peptide was not translated from its related linear RNA. Similarly, Pamudurti et al. and Yang et al. used ribosomal profiling to identify translatable circRNAs, showing that translatome high-throughput technologies are the most reliable methods to discover coding circRNAs25,27.
Although we identified approximately 320 potentially translatable circRNAs, we focused only on candidates whose host genes were previously designated as ncRNAs. This criterion may result in certain real coding-circRNAs being overlooked, but it reduces the false-negative rate to a maximum extent. Among the candidate coding-circRNAs, only few exhibit spanning junction sORFs. A spanning junction sORF is the distinctive feature of circRNA-encoded peptides. Most translatable circRNAs share similar sORFs with their related linear RNAs, although it is possible for their functions to differ. In addition, pre-mRNAs that generate long ncRNAs may also be back-spliced into translatable circRNAs, as is the case for LINC-PINT and circPINTexon2. Our discovery suggests that coding RNAs and ncRNAs are not very different. A recent publication described a coding circRNA database (circRNADb) in which 16,328 circRNAs were annotated as having putative ORFs, 7170 of which contained IRES elements. Furthermore, 46 circRNAs from 37 genes were reported to have corresponding proteins30. We match our screening results with circRNADb, but circPINTexon2 is not included because circRNADb excludes peptides less than 100 aa in length. Although this report did not provide experimental evidence supporting the coding ability of circRNAs, we believe that circRNADb provides an initial reference for screening translatable circRNAs based on our comparative results. Furthermore, our results show that circRNAs encoding peptides shorter than 100 aa should not be disregarded.
CircPINTexon2 was previously identified in several cancer cell lines with only 1–2 junction reads7. In a recently reported brain circRNA dataset, circPINTexon2 was not annotated8. The GC-rich junction region of circPINTexon2 or the sequencing depth may result in it being overlooked during high-throughput identification; nevertheless, absolute quantification PCR revealed that circPINTexon2 copy numbers were not necessarily very low in normal brains in our study and a most recent study supported that the translation efficiency of circRNAs may be higher than linear mRNA (most likely due to the higher stability of circRNAs)44.
Thus far, no coding circRNAs generated from long ncRNAs have been reported. Based on our results, circPINTexon2 exerts its biological functions independently in glioma, although it is generated from LINC-PINT gene. Glioma is a highly heterogenetic tumor56–58, which also gives rise to some limitations of our research. For instance, the PINT87aa expression was not evaluated in the molecular sub-types of GBM56; some of the experiments were performed not by using the patient-derived BTICs; and some molecular features such as chromosome 7 hypermethylation was not studied regarding to PINT87aa. Also, some critical genes variation such as c-Myc, sox-2 may also be induced by intra-tumoral heterogeneity instead of PINT87aa expression59. Nevertheless, our data supported that PINT87aa is a potential tumor-suppressive peptide and is lowly expressed in human cancers other than glioma. Substantial further works, including PINT87aa with GBM sub-types, PINT87aa upstream regulation or even single-cell level investigation, are needed for clarifying PINT87aa’s comprehensive tumor-suppressive roles.
Although no evidence showed that PINT87aa directly binds to DNA and act as a transcription factor, its interaction with PAF1 complex suggested its role during transcriptional elongation. To our knowledge, the binding partner of PAF1 complex decided its biological functions and cellular localization60–62. As a newly identified interacting protein, PINT87aa may serve its role in deciding PAF1 complex proper localization.
Our discovery of peptides encoded by circRNAs and their regulatory effects on human malignancies show that circRNAs may exert more biological functions than previously predicted. Additionally, our findings raise many questions. For example, how many translatable circRNAs are present in mammalian cells, and what are their general functions? With respect to this study, how does circPINTexon2 translocate into the cytoplasm, while LINC-PINT remains in the nucleus? Do circPINTexon2 and PINT87aa serve as specific tumor biomarkers for detection, intervention, and/or prognostication? Further investigations are clearly warranted to address these questions. Nevertheless, the present study shows that circRNA-encoded small peptides regulate human cancer behavior and may have important clinical implications and applications. Additionally, our results provide a fresh perspective regarding circRNAs and long ncRNAs, whose biological functions are largely yet to be revealed.
Methods
Human cancer and normal tissues
All human cancerous and adjacent normal tissues were collected from the 1st Affiliated Hospital of Sun Yat-sen University. The human materials were obtained with informed consent, and the study was approved by the Clinical Research Ethics Committee.
Cell culture and RNase-R treatments
All cells used in this study were tested for mycoplasma contamination. 293T, U251, A172, Hs683, and SW1783 cells were authenticated December 2015 by STR sequencing. 293T cell was purchased from ATCC (ATCC number: CRL-11268), and A172 cell was also from ATCC (ATCC number: CRL-1620). Specifically, A172 cells were not contained by U251MG as listed in ICLAC (http://iclac.org/databases/cross-contaminations), authenticated by STR sequencing. U251, Hs683, and SW1783 cells were kindly provided by Dr. Suyun Huang (MD Anderson). These cells were cultured in Dulbecco’s modified Eagle’s medium (Gibco, Carlsbad, CA) supplemented with 10% fetal bovine serum (Gibco, Carlsbad, CA) according to standard protocols. NHA were purchased from Lonza and cultured using an AGM Bullet Kit™ (Lonza, Walkersville, MD) as recommended by the manufacturer. BTICs including 456, 4121, 387, and H2S were kindly provided by Dr. Jeremy N. Rich (UCSD) and were cultured in Neurobasal medium (Gibco, Carlsbad, CA) with B27 (without vitamin A), basic fibroblast growth factor (20 ng ml−1, R&D, Indianapolis, IN) and epidermal growth factor (20 ng ml−1, R&D, Indianapolis, IN). hNSC, purchased from Gibco (Gibco, Carlsbad, CA, A15654), were cultured as the recommendation of the manufacturer (StemPro® NSC SFM (A10509-01)) supplemented with 2 mM GlutaMAX™-I Supplement (35050), 6 U/ml heparin (Sigma, St. Louis, MO, H3149), and 200 μM ascorbic acid (Sigma, St. Louis, MO, A8960). Only the early passages of hNSC were used (less than 3 passages). RNase-R (Epicentre Biotechnologies, Madison, WI) treatment (20 U/μl) was performed on total RNA (20 μg) at 37 °C for 15 min.
Ribosome-nascent chain complex (RNC) extraction
Cells were pre-treated with 100 μg/ml cycloheximide for 15 min, followed by pre-chilled phosphate buffered saline washes and the addition of 2 ml of cell lysis buffer [1% Triton X-100 in ribosome buffer (RB buffer): 20 mM HEPES-KOH (pH 7.4), 15 mM MgCl2, 200 mM KCl, 100 μg/ml cycloheximide, and 2 mM dithiothreitol]. After a 30-min ice bath, cell lysates were scraped and transferred to pre-chilled 1.5-ml tubes. Cell debris was removed by centrifuging at 16,200g for 10 min at 4 °C. Supernatants were transferred to the surface of 20 ml of sucrose buffer (30% sucrose in RB buffer). RNCs were pelleted after ultra-centrifugation at 185,000g for 5 h at 4 °C. RNC-RNA and total RNA were reverse-transcribed with a RevertAid H Minus First Strand cDNA Synthesis Kit (Thermo Fisher, Waltham, MA) using random hexamer primers (oligo-dTs primers for poly-A RNAs were used in Fig. 3c), following the manufacturer’s instructions. The cDNA was then subjected to PCR with specific primers using DreamTaq PCR Master Mix (Thermo Fisher, Waltham, MA) for 40 cycles (95 °C for 30 s and 68 °C for 60 s for each cycle).
Strand-specific RNA-seq library construction and sequencing
Total RNA was isolated using TRIzol (Life Technologies, Carlsbad, CA). After total RNA was extracted, rRNA was removed by using VAHTS Total RNA-seq (H/M/R) Library Prep Kit for Illumina (Vazyme Biotech Co., Ltd, Nanjing, China) to retain other types of RNA, including mRNAs and ncRNAs. The enriched mRNAs and ncRNAs were fragmented into short fragments in fragmentation buffer (MgCl2) integrated into VAHTS Total RNA-seq (H/M/R) Library Prep Kit for Illumina (Vazyme Biotech Co., Ltd, Nanjing, China), then the RNA fragments were reverse-transcribed into cDNA with random primers. Second-strand cDNA was synthesized with DNA polymerase I, RNase H, dNTPs (dUTP instead of dTTP), and buffer. Next, the cDNA fragments were purified with a QiaQuick PCR extraction kit, end-repaired, underwent poly(A) addition, and ligated to Illumina sequencing adapters. Then, UNG (uracil-N-glycosylase) was used to digest the second-strand cDNA. The digested products were size-selected via agarose gel electrophoresis, PCR-amplified, and sequenced using an Illumina HiSeqTM 4000 by Gene Denovo Biotechnology Co. (Guangzhou, China).
Bioinformatics analysis and identification of target circRNAs
The short reads alignment tool Bowtie2 was used to map reads to an rRNA database. The mapped rRNA reads were then removed. The remaining reads were further subjected to alignment and analysis. The rRNA removed reads were then mapped to a reference genome (Human genome hg38) using TopHat2 (version 2.0.3.12). Reads that mapped to genomes were discarded according to the mapping information recorded in bam files using in-house perl scripts, and unmapped reads were then collected for circRNAs identification. Next, 20mers from both ends of the unmapped reads were extracted using in-house perl scripts and aligned to the reference genome (bowtie2, version 2.3.0) to locate unique anchor positions within splice sites. Anchor reads that aligned in the reverse orientation (head-to-tail) indicated circRNA splicing and were then subjected to find_circ (version 1.2, https://github.com/marvin-jens/find_circ/) to identify circRNAs. The anchor alignments were then extended such that the complete aligned reads and the breakpoints were flanked by GU/AG splice sites. A candidate circRNA was called if it was supported by at least two unique back-spliced reads from at least one sample. Host genes of identified circRNAs were determined using in housed perls scripts according to gtf files from Ensembl database (http://www.ensembl.org/). CircRNAs were also blasted against circBase to determine if they have been reported. circRNAs that were not annotated were defined as newly discovered circRNAs. To quantify circRNAs, back-spliced junction reads were scaled to RPM (reads per million mapped reads) using the following formula:
In this formula, C is the number of back-spliced junction reads that uniquely align to a circRNA. N is the total number of back-spliced junction reads. Differential expression analysis was performed using edgeR in OmicShare, an online platform for data analysis (www.omicshare.com/tools). The default parameters of edgeR were used, and differentially expressed genes (DEGs) were selected based on log2 fold-changes ≥1 and q-values > 0.05. Because there were no replicates in this study, the biological coefficient of variation (BCV), which is the square-root of dispersions, was set to 0.01 following the suggestion of the edgeR official manual. GO enrichment analysis was performed for the host genes of differentially expressed circRNAs.
Northern blotting
Approximately 10–20 µg of total RNA and circular RNA was run in a 1.2% agarose gel containing formaldehyde. The RNA was then transferred to Amersham hybond-N1 membranes (GE Healthcare, Pittsburgh, PA). The membranes were hybridized with digoxin-labeled DNA oligonucleotides specific to LINC-PINT exon 2 in Church buffer (0.5 M NaPO4, 7% SDS, 1 mM EDTA, 1% BSA, pH 7.5) at 37 °C and washed in 2× SSC (300 mM NaCl, 30 mM Na-citrate, pH 7.0) with 0.1% SDS at room temperature. The membranes were finally exposed on phosphorimager screens and analyzed using Quantity One or Image Lab software (Bio-Rad, Foster City, CA).
RNA fluorescence in situ hybridization (FISH)
Oligonucleotide probes complementary to circular exon 2 of LINC-PINT and linear exon 2 of LINC-PINT were designed using the Clone Manager suite of analysis tools (Sci Ed Central, listed in Supplementary Table 5). Cells were seeded on glass coverslips in 12-well plates. The cells were washed in PBS, fixed in 4% paraformaldehyde for 15 min, and then permeabilized overnight in 70% ethanol. Next, the cells were washed twice in PBS containing 5 mM MgCl2 (PBSM) and rehydrated for 10 min in 50% formamide and 2× SSC. For FISH, cells were incubated at 37 °C in a solution containing 50% formamide, 2× SSC, 0.25 mg/ml Escherichia coli transfer RNA, 0.25 mg/ml salmon sperm DNA (Invitrogen, Carlsbad, CA), 2.5 mg/ml BSA (Roche, Indianapolis, IN), and fluorescently-labeled circular probes and linear probes at 125 nM (obtained from Generay Biotech Co, Ltd, Shanghai, China). After 12 h, the cells were washed twice for 20 min at 37 °C in 50% formamide and 2× SSC followed by 5-min washes for 4 times in PBS [with the penultimate wash containing 4,6-diamidino-2-phenylindole (DAPI)] and an additional brief wash in nuclease-free water. The cells were mounted in ProLong Gold (Invitrogen, Carlsbad, CA) and left overnight at room temperature.
Plasmids and transfection
GFP and mCherry sequences were amplified from the pEGFP-C1 and pLVX-mCherry-N1 vectors (Takara, Mountain View, CA), respectively. The wild-type and mutant IRES sequences of circular LINC-PINT were obtained through chemical gene synthesis. mCherry-IRES-GFP frames were obtained via overlap PCR and were then cloned into the psin-EF2 vector (Daen Gene Co, Ltd, Guangzhou, China) at the EcoRI and BamHI sites. GFP sequences without the ATG initiation codon and TAA stop codon were inserted into the C-terminal putative coding sequences of PINT87aa and circPINT69aa. Next, exon 2 of LINC-PINT, together with PINT87aa-GFP or circPINT69aa-GFP, was cloned into the psin-EF2 vector using the EcoRI and BamHI sites. For the artificial circRNA overexpression vectors, the junction of natural circPINTexon2 was moved inside the 87-aa ORF, and the side-flanking acceptor and donor sequences were added. The IRES sequence was deleted in the negative control plasmid, and the CMV-87aa-ORF linear overexpression vector was cloned as a positive control. The plasmids were transfected using Lipofectamine 3000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions.
Stable cell line generation
For stable PINT87aa overexpression, the artificial circPINTexon2 overexpression plasmid or the linear 87-aa-GFP ORF was cloned into the psin-EF2 vector using EcoRI and BamHI for lentiviral production. The cell lines were then infected, followed by selection with 2 μg/ml puromycin for 72 h. To generate PINT87aa stable knockdown cell lines, lentivirus-induced shRNA (GenePharma, Shanghai, China) was used according to the manufacturer’s instructions (shRNA sequences are listed in Supplementary Table 5).
Target DNA deletion using CRISPR/Cas9 technology
We designed CRISPR gRNAs to target the 87-aa ORF sequences of PINT87aa using the online software at http://crispr.mit.edu/. To clone the target sequences into the lentiCRISPRv2 (AddGene 52961) backbone, we synthesized oligos containing the same overhangs after BsmBI. The transfer plasmid lentiCRISPRv2-gRNA (1.2 µg) was co-transfected into 293T cells with the lentiviral packaging plasmids pVSVg (AddGene 8454, 0.5 µg) and psPAX2 (AddGene 12260, 1 µg). Sixty hours post-transfection, lentiviral infection of the SW1783 and HS683 cell lines was performed. After 48 h, cells were selected with 2 mg/ml puromycin for 1 week. Then, the cells were trypsinized and plated in 96-well plates at a seeding density of 1 cell/well. The cells were monitored for single colony formation and expanded upon confluency. A DNA extraction kit (QIAGEN, Germantown, MD) was used for DNA extraction. PCR was carried out to amplify the targeting region, and Sanger sequencing was performed to detect gene mutations.
Antibody generation and western blotting
A polyclonal antibody against the 87-aa peptide produced by circPINTexon2 was obtained by inoculating rabbits. The antibody was purified using affinity chromatography columns. After extraction with RIPA buffer and quantified with a BCA kit (Thermo, Waltham, MA, 23228), equal loading proteins of cell lysate or tissue lysate were added to each well of SDS PAGE. Followed by electrophoresising, transfer-membraning, and blocking with 5% non-fat milk in PBST for 1 h, then diluted primary antibodies were incubated at 4 °C overnight. After washing with PBST every 10 min for 3 times, diluted horseradish peroxidase (HRP)-conjugated secondary antibodies were incubated for 1 h at room temperature, then the signals were visualized. The 87-aa antibody was used at a 1:2000 dilution. The other primary antibodies used were anti-PAF1 (abcam; ab137519; 1:1000), anti-β-actin (Sigma; A5441; 1:5000), anti-GFP (abcam; ab1218; 1:3000), anti-flag (Sigma; F3165; 10 µg/ml; 1:1000), anti-HA (abcam; ab18181; 1:1000), anti-γ-H2AX (abcam; ab2893; 1:1000), anti-CPEB1 (abcam; ab155204; 1:5000), anti-Cyclin D1 (abcam; ab134175; 1:1000), anti-C-myc (abcam; ab51156; 1:1000), anti-SOX2 (abcam; ab97959; 1:1000), anti-H3 (abcam; ab1791; 1:1000), anti-EGFR (abcam; ab52894; 1:1000), anti-MET (abcam; ab51067; 1:1000), anti-PDGFR (abcam; ab32570; 1:5000), anti-P53 (abcam; ab26; 1:1000).
PCR
Reverse transcription for mRNA and circRNAs was performed using an MMLV-RT kit (Takara, Mountain View, CA) with random hexamers according to the manufacturer’s instructions. PCR was subsequently performed with a 1:10 dilution of reverse-transcribed cDNA. The PCR products were run in a 2% agarose gel. Real-time q-PCR (RT-q-PCR) was performed using an Applied Biosystems PCR System (ABI 7500). RT-qPCR SYBR Green Mix (Takara, Mountain View, CA) was employed, with forward and reverse primers at 50 nM in a 20-μl reaction system. Relative expression levels were calculated using the 2-ΔΔCT method. To determine the absolute quantity of RNA, the purified PCR product amplified from cDNA corresponding to the circPINTexon2 sequence was serially diluted to generate a standard curve. (Oligo sequences are listed in Supplementary Table 5.)
Immunoprecipitation (IP)
Cells were lysed in co-IP buffer [10 mM HEPES (pH 8.0), 300 mM NaCl, 0.1 mM EDTA, 20% glycerol, 0.2% NP-40, protease and phosphatase inhibitors]. The lysates were then centrifuged and cleared via incubation with 25 μl of protein A/G agarose for 1.5 h at 4 °C. The pre-cleared supernatant was subjected to IP using the indicated primary antibodies at 4 °C overnight. Then, the protein complexes were collected by incubating with 30 μl of protein A/G gel for 2 h at 4 °C. The collected protein complexes were separated via SDS-PAGE and analyzed by performing MS or blotting.
Neurosphere formation assay
Extreme limiting dilution analysis (ELDA) was performed to evaluate self-renewal capacity. BTICs were dissociated in TrypLE™ Select (Gibco, Carlsbad, CA) for 5 min at 37 °C. Cells were triturated into a single-cell solution. The solution was incubated with Hoechst (Thermo, Waltham, MA, 33342) for 30 min at 37 °C. Live cells were identified using a LIVE/DEAD staining kit (Thermo, Waltham, MA, L10119). Live cells were sorted into 96-well plates. Spheres were counted at 14 days. Cell density per well ranged from 1, 10, 25, 50, 100, 250, 500 to 1000. Each condition was tested in 10 independent wells. Volume of medium per well was 200 μl medium with growth factors spike-ins every 3–4 days. Neurosphere-forming capability was determined using the ELDA web-based tool (http://bioinf.wehi.edu.au/software/elda/).
Cell proliferation assays
Cells were seeded into 96-well plates. At the indicated time points, the cells were incubated with 100 μl of sterile MTT for 4 h at 37 °C, after which the medium was removed and replaced with 150 μl of DMSO. The absorbance was measured at 570 nm. All experiments were performed in triplicate.
EdU incorporation assay
8-Well chamber slides were coated with poly-L-lysine. Cells were then seeded at 40,000 cells per well. 10 μM EdU was added to each well. Cells were fixed after 24 h using 4% paraformaldehyde in PBS and stained using the Click-iT EdU kit as protocol (Invitrogen, Carlsbad, CA). Proliferation index was then determined by quantifying percentage of EdU labeled cells using confocal microscopy at 200× magnification. All experiments were performed in three biological replicates.
LC-MS analysis
Proteins were separated via SDS-PAGE, and gel bands were manually excised and digested with sequencing-grade trypsin (Promega, Madison, WI). The digested peptides were analyzed using a QExactive mass spectrometer (Thermo Fisher, Carlsbad, CA). Fragment spectra were analyzed using the National Center for Biotechnology Information nonredundant protein database with Mascot (Matrix Science).
Colony formation assays
Cells were plated in 6-well plates (1000 cells per plate), cultured for 10 days, fixed with 10% formaldehyde for 5 min, stained with 1.0% crystal violet for 30 s, and counted. All experiments were performed in three biological replicates.
ChIP and re-ChIP assays
For the ChIP assay, 2 × 106 cells were prepared with the ChIP assay kit (Cell Signaling Technology, Danvers, MA, 56383) according to the manufacturer’s instructions. The resulting precipitated DNA samples were analyzed by PCR. In the re-ChIP assay, 107 cells were used for the first-step ChIP. The DNA complexes were first immunoprecipitated using the indicated antibodies and then eluted by incubation for 30 min at 37 °C in 100 μl of 10 mM DTT. After centrifugation, the supernatant was diluted 50× with re-ChIP buffer and immunoprecipitated again using the indicated antibodies, as with the ChIP procedure. (Primer sequences are listed in Supplementary Table 5.) All experiments were performed in three biological replicates.
Cell cycle analyses
Cells were harvested via trypsinization, washed in ice-cold PBS, fixed in ice-cold 75% ethanol in PBS, centrifuged at 4 °C, and suspended in PBS. RNase A was then added at a final concentration of 4 mg/ml, followed by incubation at 37 °C for 30 min, after which 20 mg/ml propidium iodide (Beyotime, Shanghai, China) was added, and the samples were incubated for 20 min at room temperature. The cells were analyzed via flow cytometry. All experiments were performed in three biological replicates.
IF staining and confocal microscopy
Cells were grown on chamber slides precoated with poly-L-ornithine and fibronectin. The cells were fixed with 4% paraformaldehyde, permeabilized for 5 min with PBS containing 0.1% Triton X-100 (PBS-T), quenched with 50 mM NH4Cl in PBS-T, and blocked with 1% BSA in PBS-T. Immunostaining was performed with appropriate primary and secondary antibodies, and images were acquired using an Olympus FluoView FV1000 confocal microscope.
Animal care and ethics statement
Four-week-old female BALB/c-nu mice were purchased from the Laboratory Animal Center of Sun Yat-sen University. Mice were housed in a temperature-controlled (22 °C) and light-controlled pathogen-free animal facility with free access to food and water. All experimental protocols concerning the handling of mice were approved by the institutional animal care and use committee of Sun Yat-sen University.
Intracranial injection
We intracranially injected 2000 cells for each of the indicated BTIC types into nude mice. Eight mice were injected for each group. The mice were sacrificed after 60 days or when they showed clinical symptoms such as weight loss and orientation dysfunction. The brain of each mouse was harvested, fixed in 4% formaldehyde and embedded in paraffin. Tumor formation and phenotypes were determined through histologic analysis and assessed in hematoxylin and eosin-stained sections. Total survival curves were calculated.
Subcutaneous xenograft assay
Xenograft experiments were performed through the subcutaneous injection of 5 × 106 cancer cells into nude mice. Five mice were injected for each group. Day 0 indicates the first day of injection. Tumor progression was monitored by imaging with a Xenogen Spectrum small animal imaging system (Caliper, Hopkinton, MA) or via caliper measurements, as indicated.
Statistical analysis
Statistical tests were conducted using Prism (GraphPad) software unless otherwise indicated. Data are presented as mean ± s.e.m. from three independent experiments. For parametric data, unpaired, two-tailed Student’s t-tests were used. For non-parametric data, two-sided Mann–Whitney test was used. Data distribution was assumed to be normal, but this was not formally tested. The limiting dilution assay to test for neurosphere-forming capacity was analyzed with a Chi-squared test using the ELDA web-based tool (http://bioinf.wehi.edu.au/software/elda/). A level of P < 0.05 was used to designate significant differences. For all experiments, analyses were done in biological triplicate. No animals or data points were excluded from the analyses for any reason. Blinding and randomization were performed in all experiments. Statistical analyses for RNA-seq data are described above in the respective sections.
Electronic supplementary material
Acknowledgements
We are sincerely grateful to Dr. Jeremy N. Rich (UCSD) for kindly providing BTICs. This work was supported in part by the National Key Research and Development Program of China (2016YFA0503000 to N.Z.), the National Natural Science Outstanding Youth Foundation of China (81822033 to N.Z.), the National Natural Science Foundation of China (81370072, 81572477, 81772683 to N.Z. and 81802484 to K.H.), the Science and Technology Project of Guangdong Province (S2013050014535 and 2016A050502017 to N.Z.), the 863 Research Program (2014AA020504 to G.Z.)
Author contributions
N.Z. and S.H. conceived and designed the project. M.Z., K.Z., and X.X. planned and performed most of the experiments. N.Z., K.X., and S.H. wrote the manuscript. S.Y., P.W., N.H., and X.Y. helped with the experiments. H.L. designed and performed some of the experiments shown in Fig. 4. J.X. performed the gastric cancer experiments. F.X. performed the statistical analysis. J.L. provided experimental advice. G.Z. performed the RNC-RNA-related experiments. H.Z. performed the bioinformatic analysis. K.H. helped with the data organization and figure preparation. Y.Y. helped with the revision. S.H. and N.Z. supervised the study.
Data availability
The RNA-seq data obtained in this study have been deposited in the SRA database, with the accession code PRJNA401369. Other data that support the findings of this study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Maolei Zhang, Kun Zhao, Xiaoping Xu.
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-018-06862-2.
References
- 1.Jeck WR, et al. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19:141–157. doi: 10.1261/rna.035667.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeck WR, Sharpless NE. Detecting and characterizing circular RNAs. Nat. Biotechnol. 2014;32:453–461. doi: 10.1038/nbt.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo JU, Agarwal V, Guo H, Bartel DP. Expanded identification and characterization of mammalian circular RNAs. Genome Biol. 2014;15:409. doi: 10.1186/s13059-014-0409-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salzman J, Gawad C, Wang PL, Lacayo N, Brown PO. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS One. 2012;7:e30733. doi: 10.1371/journal.pone.0030733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kramer MC, et al. Combinatorial control of Drosophila circular RNA expression by intronic repeats, hnRNPs, and SR proteins. Genes Dev. 2015;29:2168–2182. doi: 10.1101/gad.270421.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ivanov A, et al. Analysis of intron sequences reveals hallmarks of circular RNA biogenesis in animals. Cell Rep. 2015;10:170–177. doi: 10.1016/j.celrep.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 7.Salzman J, Chen RE, Olsen MN, Wang PL, Brown PO. Cell-type specific features of circular RNA expression. PLoS Genet. 2013;9:e1003777. doi: 10.1371/journal.pgen.1003777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rybak-Wolf A, et al. Circular RNAs in the mammalian brain are highly abundant, conserved, and dynamically expressed. Mol. Cell. 2015;58:870–885. doi: 10.1016/j.molcel.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 9.Memczak S, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–338. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 10.Hansen TB, et al. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 11.Piwecka M, et al. Loss of a mammalian circular RNA locus causes miRNA deregulation and affects brain function. Science. 2017;357:eaam8526. doi: 10.1126/science.aam8526. [DOI] [PubMed] [Google Scholar]
- 12.You X, et al. Neural circular RNAs are derived from synaptic genes and regulated by development and plasticity. Nat. Neurosci. 2015;18:603–610. doi: 10.1038/nn.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Z, et al. Exon–intron circular RNAs regulate transcription in the nucleus. Nat. Struct. Mol. Biol. 2015;22:256–264. doi: 10.1038/nsmb.2959. [DOI] [PubMed] [Google Scholar]
- 14.Ashwal-Fluss R, et al. circRNA biogenesis competes with pre-mRNA splicing. Mol. Cell. 2014;56:55–66. doi: 10.1016/j.molcel.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 15.Zhang XO, et al. Complementary sequence-mediated exon circularization. Cell. 2014;159:134–147. doi: 10.1016/j.cell.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Starke S, et al. Exon circularization requires canonical splice signals. Cell Rep. 2015;10:103–111. doi: 10.1016/j.celrep.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Aspden JL, et al. Extensive translation of small open reading frames revealed by Poly-Ribo-Seq. eLife. 2014;3:e3528. doi: 10.7554/eLife.03528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galindo MI, Pueyo JI, Fouix S, Bishop SA, Couso JP. Peptides encoded by short ORFs control development and define a new eukaryotic gene family. PLoS Biol. 2007;5:e106. doi: 10.1371/journal.pbio.0050106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrews SJ, Rothnagel JA. Emerging evidence for functional peptides encoded by short open reading frames. Nat. Rev. Genet. 2014;15:193–204. doi: 10.1038/nrg3520. [DOI] [PubMed] [Google Scholar]
- 20.Ladoukakis E, Pereira V, Magny EG, Eyre-Walker A, Couso JP. Hundreds of putatively functional small open reading frames in Drosophila. Genome Biol. 2011;12:R118. doi: 10.1186/gb-2011-12-11-r118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lauressergues D, et al. Primary transcripts of microRNAs encode regulatory peptides. Nature. 2015;520:90–93. doi: 10.1038/nature14346. [DOI] [PubMed] [Google Scholar]
- 22.Magny EG, et al. Conserved regulation of cardiac calcium uptake by peptides encoded in small open reading frames. Science. 2013;341:1116–1120. doi: 10.1126/science.1238802. [DOI] [PubMed] [Google Scholar]
- 23.Chen CY, Sarnow P. Initiation of protein synthesis by the eukaryotic translational apparatus on circular RNAs. Science. 1995;268:415–417. doi: 10.1126/science.7536344. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Wang Z. Efficient backsplicing produces translatable circular mRNAs. RNA. 2015;21:172–179. doi: 10.1261/rna.048272.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pamudurti NR, et al. Translation of CircRNAs. Mol. Cell. 2017;66:9–21. doi: 10.1016/j.molcel.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Legnini I, et al. Circ-ZNF609 is a circular RNA that can be translated and functions in myogenesis. Mol. Cell. 2017;66:22–37. doi: 10.1016/j.molcel.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang Y, et al. Extensive translation of circular RNAs driven by N(6)-methyladenosine. Cell Res. 2017;27:626–641. doi: 10.1038/cr.2017.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang M, et al. A novel protein encoded by the circular form of the SHPRH gene suppresses glioma tumorigenesis. Oncogene. 2018;37:1805–1814. doi: 10.1038/s41388-017-0019-9. [DOI] [PubMed] [Google Scholar]
- 29.Yang, Y. et al. Novel role of FBXW7 circular RNA in repressing glioma tumorigenesis. J. Natl. Cancer Inst. 110 (2018). [DOI] [PMC free article] [PubMed]
- 30.Chen X, et al. circRNADb: a comprehensive database for human circular RNAs with protein-coding annotations. Sci. Rep. 2016;6:34985. doi: 10.1038/srep34985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ingolia NT, Ghaemmaghami S, Newman JR, Weissman JS. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science. 2009;324:218–223. doi: 10.1126/science.1168978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang T, et al. Translating mRNAs strongly correlate to proteins in a multivariate manner and their translation ratios are phenotype specific. Nucleic Acids Res. 2013;41:4743–4754. doi: 10.1093/nar/gkt178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim D, et al. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14:R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glazar P, Papavasileiou P, Rajewsky N. circBase: a database for circular RNAs. RNA. 2014;20:1666–1670. doi: 10.1261/rna.043687.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song X, et al. Circular RNA profile in gliomas revealed by identification tool UROBORUS. Nucleic Acids Res. 2016;44:e87. doi: 10.1093/nar/gkw075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marin-Bejar O, et al. Pint lincRNA connects the p53 pathway with epigenetic silencing by the Polycomb repressive complex 2. Genome Biol. 2013;14:R104. doi: 10.1186/gb-2013-14-9-r104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sauvageau M, et al. Multiple knockout mouse models reveal lincRNAs are required for life and brain development. eLife. 2013;2:e1749. doi: 10.7554/eLife.01749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Childs EJ, et al. Common variation at 2p13.3, 3q29, 7p13 and 17q25.1 associated with susceptibility to pancreatic cancer. Nat. Genet. 2015;47:911–916. doi: 10.1038/ng.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopez-Lastra M, Rivas A, Barria MI. Protein synthesis in eukaryotes: the growing biological relevance of cap-independent translation initiation. Biol. Res. 2005;38:121–146. doi: 10.4067/S0716-97602005000200003. [DOI] [PubMed] [Google Scholar]
- 41.Baranick BT, et al. Splicing mediates the activity of four putative cellular internal ribosome entry sites. Proc. Natl. Acad. Sci. U.S.A. 2008;105:4733–4738. doi: 10.1073/pnas.0710650105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mokrejs M, et al. IRESite: the database of experimentally verified IRES structures (www.iresite.org) Nucleic Acids Res. 2006;34:D125–D130. doi: 10.1093/nar/gkj081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Plank TD, Kieft JS. The structures of nonprotein-coding RNAs that drive internal ribosome entry site function. Wiley Interdiscip. Rev. RNA. 2012;3:195–212. doi: 10.1002/wrna.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wesselhoeft RA, Kowalski PS, Anderson DG. Engineering circular RNA for potent and stable translation in eukaryotic cells. Nat. Commun. 2018;9:2629. doi: 10.1038/s41467-018-05096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saladin A, et al. PEP-SiteFinder: a tool for the blind identification of peptide binding sites on protein surfaces. Nucleic Acids Res. 2014;42:W221–W226. doi: 10.1093/nar/gku404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen FX, et al. PAF1, a molecular regulator of promoter-proximal pausing by RNA polymerase II. Cell. 2015;162:1003–1015. doi: 10.1016/j.cell.2015.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strikoudis A, et al. Regulation of transcriptional elongation in pluripotency and cell differentiation by the PHD-finger protein Phf5a. Nat. Cell Biol. 2016;18:1127–1138. doi: 10.1038/ncb3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hubert CG, et al. Genome-wide RNAi screens in human brain tumor isolates reveal a novel viability requirement for PHF5A. Genes Dev. 2013;27:1032–1045. doi: 10.1101/gad.212548.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muntean AG, et al. The PAF complex synergizes with MLL fusion proteins at HOX loci to promote leukemogenesis. Cancer Cell. 2010;17:609–621. doi: 10.1016/j.ccr.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moniaux N, et al. The human homologue of the RNA polymerase II-associated factor 1 (hPaf1), localized on the 19q13 amplicon, is associated with tumorigenesis. Oncogene. 2006;25:3247–3257. doi: 10.1038/sj.onc.1209353. [DOI] [PubMed] [Google Scholar]
- 51.Karmakar S, et al. PD2/PAF1 at the crossroads of the cancer network. Cancer Res. 2018;78:313–319. doi: 10.1158/0008-5472.CAN-17-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lasda E, Parker R. Circular RNAs: diversity of form and function. RNA. 2014;20:1829–1842. doi: 10.1261/rna.047126.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guarnerio J, et al. Oncogenic role of fusion-circRNAs derived from cancer-associated chromosomal translocations. Cell. 2016;165:289–302. doi: 10.1016/j.cell.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 54.Du WW, et al. Foxo3 circular RNA retards cell cycle progression via forming ternary complexes with p21 and CDK2. Nucleic Acids Res. 2016;44:2846–2858. doi: 10.1093/nar/gkw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zheng Q, et al. Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat. Commun. 2016;7:11215. doi: 10.1038/ncomms11215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Verhaak RG, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Puchalski RB, et al. An anatomic transcriptional atlas of human glioblastoma. Science. 2018;360:660–663. doi: 10.1126/science.aaf2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ceccarelli M, et al. Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell. 2016;164:550–563. doi: 10.1016/j.cell.2015.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patel AP, et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344:1396–1401. doi: 10.1126/science.1254257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chaudhary K, Deb S, Moniaux N, Ponnusamy MP, Batra SK. Human RNA polymerase II-associated factor complex: dysregulation in cancer. Oncogene. 2007;26:7499–7507. doi: 10.1038/sj.onc.1210582. [DOI] [PubMed] [Google Scholar]
- 61.Sadeghi L, Prasad P, Ekwall K, Cohen A, Svensson JP. The Paf1 complex factors Leo1 and Paf1 promote local histone turnover to modulate chromatin states in fission yeast. EMBO Rep. 2015;16:1673–1687. doi: 10.15252/embr.201541214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dermody JL, Buratowski S. Leo1 subunit of the yeast paf1 complex binds RNA and contributes to complex recruitment. J. Biol. Chem. 2010;285:33671–33679. doi: 10.1074/jbc.M110.140764. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA-seq data obtained in this study have been deposited in the SRA database, with the accession code PRJNA401369. Other data that support the findings of this study are available from the corresponding author on reasonable request.