Cellular and Molecular Immunology advance online publication, 5 March 2018; 10.1038/cmi.2018.170
Regulatory T (Treg) cells comprise diverse subsets of immunosuppressive cells that play a pivotal role in regulating immune homeostasis and preventing autoimmunity. In cancers, Treg cells can suppress antitumor immune responses and support the establishment of an immunosuppressive tumor micro-environment, thus promoting immune evasion and cancer progression.1 Treg cells are increased or activated in the tumor microenvironment, which is associated with a poor clinical outcome.2 Although CD4+ Treg cells have been extensively studied, the lack of universal markers to distinguish CD8+ Treg cells from conventional CD8+ T cells means that the function of CD8+ Treg cells in cancer has not been fully characterized. Now, an increasing body of research has revealed that CD8+ Treg cells (CD8+ CD25+ Foxp3+, CD25+CD122+Foxp3+ and CD8+CD28−)3–5 accumulate in the tumor microenvironment and suppress antitumor immunity (Fig. 1). However, the influence of CD8+ Treg cells on tumor progression in ovarian cancer (OC) is less clear. Moreover, there are a limited number of studies describing the molecular signatures involved in the induction of CD8+ Treg cells.
Fig. 1.
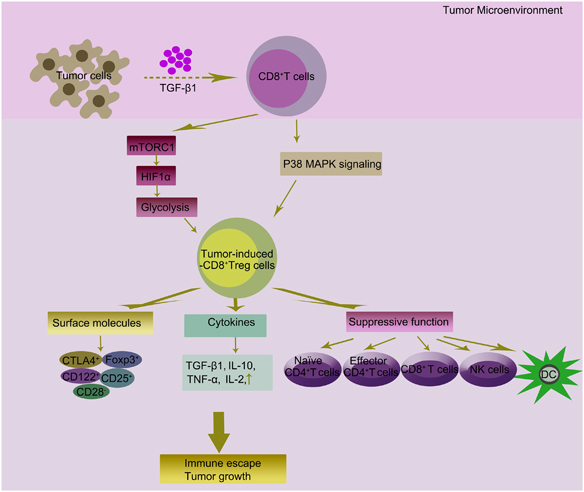
Tumor-induced CD8+ Treg cells and their potential role in tumor microenvironment. TGF-β1 can be released by cancer cells in the tumor microenvironment. The suppressive activity of Treg cells mainly mediated through tumor-derived exosome TGF-β1. We previously reported that TGF-β1 secreted by OC cells could generate CD8+ Treg cells by promotion of the p38 MAPK signaling pathway. Tumor microenvironment influences T-cell immune responses by altering cellular metabolism. mTORC1 regulates glucose metabolism in CD8+ Treg cells’ differentiation through regulating the expression of HIF1α. Treg cells use different strategies to inhibit target cells within the tumor mass. Among the surface molecules expressed by CD8+ Treg cells, CD25, CD122, CD28, CTLA-4 and Foxp3 have a well-demonstrated role in promoting tumor progression. Treg cells secrete several immune-modulatory cytokines (TGF-β, IL-10, TNF-α and IL-2). Tumor-induced-CD8+ Treg cells could also directly modulate activation and function of immune cells. CD8+ Treg cells may play a role in promoting imbalance of the immune system in tumor growth. These immune-tolerance mechanisms may also be exploited by cancer cells to achieve immune escape, which becomes more pronounced with tumor formation, progression and metastasis. CTLA-4, cytotoxic T-lymphocyte associated protein 4; HIF1α, hypoxia-inducible factor 1α; IL, interleukin; MAPK, mitogen-activated protein kinase; mTORC1, mammalian target of rapamycin complex 1; OC, ovarian cancer; TGF-β, transforming growth factor β;TNF-α, tumor necrosis factor α;Treg, regulatory T
We recently identified the expression of Treg markers in CD8+ T cells isolated from peripheral blood and fresh tumor tissues of OC patients. We detected a higher percentage of CD8+ Treg cells in OC patients compared with benign ovarian tumor patients and healthy controls.6 The immune-suppressive T-cell markers CD25, cytotoxic T-lymphocyte associated protein 4 (CTLA-4) and Foxp3 were upregulated, whereas expression of the immune activation marker CD28 was downregulated in these cells. We also showed that levels of Foxp3 in CD8+ T cells were positively associated with tumor stage in OC patients, suggesting that CD8+Foxp3+ Treg cells contribute to the progression of OC. This highlights the role of CD8+Foxp3+ Treg cells as predictors of clinical outcome in OC patients. Then we determined whether the tumor microenvironment had the ability to convert CD8+ effector T cells into suppressor cells. We observed phenotypic similarities between CD8+ Tcells in transwell co-culture system and CD8+ T cells obtained from OC tumor tissues, including upregulated Foxp3 and CTLA-4 and downregulated CD28 expression levels.6 The in vitro-induced CD8+ Treg cells also secreted higher concentrations of transforming growth factor β1 (TGF-β1), interferon (IFN)-γ, tumor necrosis factor (TNF)-α,and interleukin (IL)−2. The Foxp3+CTLA-4+ and TGF-β1+ phenotype indicated that the induced CD8+ Treg cells were functionally activated, which was confirmed by their ability to suppress CD4+ T-cell proliferation partially by TGF-β1and IFN-γ. These data imply that CD8+ Treg cells accumulated in OC tissue in vivo and inducible in vitro may dampen antitumor immunity and contribute to tumor immune evasion.
TGF-β1 also has a crucial role in the development of Treg cells,7 and CD4+ CD25− T cells deficient in the TGF-β signaling pathway cannot be converted into Foxp3+ iTreg cells.8 We showed that OC SKOV3 cells induced CD8+ Treg cells by secreting TGF-β1and that OC patients expressed high levels of TGF-β1, correspondingly, increased TGFβ1 secretion was demonstrated in supernatant from the co-culture system of CD8+ T cells and SKOV3. Additionally, TGF-β1 levels were positively correlated with the percentage of CD8+ Treg cells in OC.9 Consistent with previous reports,10 we also confirmed that high expression TGF-β1 at least partially contributed to the suppressive function of in vitroinduced CD8+ Treg cells. Compared with CD8+ T cells cultured alone, CD8+ T cells co-cultured with SKOV3 cells exhibited marked activation of p38 mitogen-activated protein kinase (MAPK), which could be inhibited by a TGF-β1-neutralizing antibody. Moreover, the TGF-β1-triggered conversion to CD8+ Treg cells in the tumor microenvironment CD8+ Treg cells in vitro was dose-dependently blocked by the p38-specific inhibitor SB203580, indicating that the induction of CD8+ Treg cells depended in part on TGF-β1-activated p38 MAPK signaling. These findings indicate that TGF-β1 is a potential target in the treatment of OC.
We further investigated the molecular signatures that contributed to the induction of CD8+ Treg cells by comparing the expressed gene profiles when CD8+ T cells were cultured with or without SKOV3 cells.11 DNA microarray data showed that 73% of previously reported CD8+ Treg cell molecular markers were significantly upregulated during co-culture with SKOV3 cells. Among them, ITGAX, also known as CD11c, was particularly noteworthy. This observation is consistent with previous reports that CD11chighCD8+ Treg cells possess potent cytotoxicity to target cells via the Fas/Fas ligand pathway and that this subset of CD8+ Treg cells can inhibit the CD4+ T-cell-mediated immune response by killing the activated CD4+ Tcells.12 Interestingly, we found that the expression of glycolysis genes in CD8+ Tcells was decreased during co-culture with SKOV3 cells, while their expression was negatively correlated with Foxp3 expression in CD8+ Treg cells. This indicated that the glycolysis pathway may contribute to the differentiation and generation of CD8+ Treg cells. Further efforts areneededtofully reveal theunderlying mechanism of CD8+ Treg cells’ differentiation and activation in an ovarian microenvironment.
Taken together, these findings suggest that CD8+ Treg cells accumulate in human OC tissues and display an activated phenotype with suppressive functions. Although CD8+ Treg cells represent only a small fraction of CD8+ T cells in vivo, targeting CD8+ Treg cells may potentiate the antitumor immunity and rebalance the OC microenvironment. We are attempting to confirm the clinical relevance of this hypothesis.
Acknowledgements
We are grateful to the technical support from National Key Clinical Department of Laboratory Medicine of Jiangsu Province Hospital. This study was supported by Natural Science Foundation of China (No.81772779), Jiangsu Province’sKey Provincial Talents Program (No. ZDRCA2016003) and Key Laboratory for Medicine of Jiangsu Province of China (No. ZDXKB2016005), a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
S. Zhang and M. Wu contributed equally to this work.
References
- 1.Wang H, Franco F, Ho PC. Metabolic regulation of tregs in cancer: opportunities for immunotherapy. Trends Cancer. 2017;3:583–592. doi: 10.1016/j.trecan.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017;27:109–118. doi: 10.1038/cr.2016.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiniwa Y, et al. CD8+ Foxp3+ regulatory T cells mediate immunosuppression in prostate cancer. Clin. Cancer Res. 2007;13:6947–6958. doi: 10.1158/1078-0432.CCR-07-0842. [DOI] [PubMed] [Google Scholar]
- 4.Chen C, et al. Changes of CD4+ CD25+ FOXP3+ and CD8+ CD28 − regulatory T cells in non-small cell lung cancer patients undergoing surgery. Int. Immunopharmacol. 2014;18:255–261. doi: 10.1016/j.intimp.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Chaput N, et al. Identification of CD8+ CD25+ Foxp3+ suppressive T cells in colorectal cancer tissue. Gut. 2009;58:520–529. doi: 10.1136/gut.2008.158824. [DOI] [PubMed] [Google Scholar]
- 6.Zhang S, et al. Analysis of CD8+ Treg cells in patients with ovarian cancer: a possible mechanism for immune impairment. Cell. Mol. Immunol. 2015;12:580–591. doi: 10.1038/cmi.2015.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Csencsits K, Wood SC, Lu G, Bishop DK. Transforming growth factor-beta1 gene transfer is associated with the development of regulatory cells. Am. J. Transplant. 2005;5:2378–2384. doi: 10.1111/j.1600-6143.2005.01042.x. [DOI] [PubMed] [Google Scholar]
- 8.WangQ YuT, et al. Sorafenib reduces hepatic infiltrated regulatory T cells in hepatocellular carcinoma patients by suppressing TGF-beta signal. JSurg. Oncol. 2013;107:422–427. doi: 10.1002/jso.23227. [DOI] [PubMed] [Google Scholar]
- 9.Wu M, et al. TGF-beta1 contributes to CD8+ Treg induction through p38 MAPK signaling in ovarian cancer microenvironment. Oncotarget. 2016;7:44534–44544. doi: 10.18632/oncotarget.10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palomares O, et al. Regulatory T cells and immune regulation of allergic diseases: roles of IL-10 and TGF-beta. Genes Immun. 2014;15:511–520. doi: 10.1038/gene.2014.45. [DOI] [PubMed] [Google Scholar]
- 11.Wu M, et al. Gene expression profiling of CD8+ T cells induced by ovarian cancer cells suggests a possible mechanism for CD8+ Treg cell production. Cell. Prolif. 2016;49:669–677. doi: 10.1111/cpr.12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Z, et al. CD11c(high)CD8+ regulatory T cell feedback inhibits CD4 T cell immune response via Fas ligand-Fas pathway. J. Immunol. 2013;190:6145–6154. doi: 10.4049/jimmunol.1300060. [DOI] [PubMed] [Google Scholar]


