Abstract
The Global Vaccine Action Plan 2011–2020 (GVAP) aims to extend the full benefit of vaccination against vaccine-preventable diseases to all individuals. More than halfway through the Decade of Vaccines, countries classified as Middle-Income by the World Bank struggle to achieve several GVAP targets. Countries transitioning from Gavi, the Vaccine Alliance, represent a key sub-group of Middle Income Countries.
Through a review of available literature on the subject, this study documents the lack of comparative analyses on immunization system performance in countries transitioning from Gavi support. Despite increased emphasis on the importance of programmatic sustainability beyond financing through the Gavi 2016–2020 Strategy and availability of data, existing literature has predominantly documented challenges related to domestic financing of immunization.
This study complements a review of current literature with an analysis of country assessments conducted by immunization partners since 2011, in an effort to document programmatic challenges related to decision-making for immunization policy, delivery of services, and access to affordable and timely supply in Gavi transitioning countries.
In light of the findings, we suggest continued systematic compilation of country performance data beyond financing to inform policy-making, in particular for: (i) development of a more nuanced theory of change towards sustainable immunization programmes and (ii) measurement of progress and key areas for attention and investment.
Keywords: Gavi transition, Sustainability, Immunization, Middle-income countries
1. Introduction
Over the past decade, Middle Income Countries’ (MICs) access to vaccines has gained global attention. MICs struggle to achieve the targets set forward in the Global Vaccine Action Plan 2011–2020 (GVAP) and most either lack or will soon lose external financial and technical support [1], [2], [3].
As of July 2017, of 109 countries classified as MICs (GNI per capita between US$ 1006 and US$ 12,235) [4] by the World Bank, 42 receive financial and programmatic support through Gavi, the Vaccine Alliance [5]. Nevertheless, 26 of these countries have either transitioned or will soon transition from Gavi support [6]. As several countries with large populations below the poverty level have achieved middle-income status, MICs are now home to two-thirds of the world’s poorest people and account for two-thirds of deaths in children under the age of five [7].
According to the current Gavi transition and eligibility policies, a country enters a five year ‘transition phase’ when its average GNI per capita over three years equals or exceeds an eligibility threshold amount (US$1580 since 2015)2 [8]. Post-transition, countries do not receive further support from Gavi, although they may benefit from Gavi-comparable vaccine prices as well as limited alternative support from development partners [9].3 Gavi transitioning countries represent a key sub-group of MICs where the sustainability of immunization efforts is at stake.
Since 2011, immunization program assessments (transition assessments) have been conducted in these countries to identify challenges to transition. The assessments are led by national immunization programs with support from Gavi [10]. Following assessments, Gavi supports the country in developing a transition plan, consisting of short- to medium-term investments by the government and Gavi to address potential sustainability issues.
To date, results of these transition assessments and plans have not been systematically compiled and analysed for cross-country comparison, and existing literature on programmatic immunization challenges affecting transitioning countries is limited. This study aims to document and begin addressing this gap in order to inform policy-making for sustainable immunization programmes.
2. Methodology
2.1. Literature review
A systematic literature review was carried out in May 2016 to retrieve existing comparative analyses of programmatic performance for Gavi transitioning countries, in order to identify commonly reported challenges. Two sources of information were examined: (1) published peer-reviewed articles and (2) Gavi documents. The peer-reviewed literature search was restricted from January 2009 to the present, reflecting the time during which transition policies were designed.
For published literature, the following search strategy was employed in PubMed: (sustainab* OR challenges) AND (immunization OR vaccin*) AND (Gavi) AND (graduat* OR transition* OR middle income). Articles meeting the following criteria were included:
-
•
Discussion of Gavi transition or graduation policies and the resulting impact on transitioning countries;
-
•
Focus on one or more specific challenge areas (e.g. financing, supply chain, health workforce) in transitioning, lower-middle, and middle-income countries;
-
•
Focus on the progression or transition of lower-middle and/or middle-income countries in developing sustainable and independent immunization programmes;
-
•
Reference documents guiding the formation and progress of the graduation and transition programmes).
Articles in languages other than English, focussing on public health issues other than immunization, discussing immunization broadly without focussing on transition, and/or looking at a single vaccine-preventable disease were instead excluded.
Gavi documents were retrieved from the Gavi Programme and Policy Committee (PPC) webpage and WHO archives [11]. For the period of January 2009 to May 2016, the Committee minutes and Gavi Board meeting agendas were searched for the words “eligibility”, “transition”, and “graduation,” and matching background and supporting documents were retrieved from WHO archives. Relevant transition-related policies were additionally extracted from the library of policies on Gavi’s webpage (2009–2015) [12].
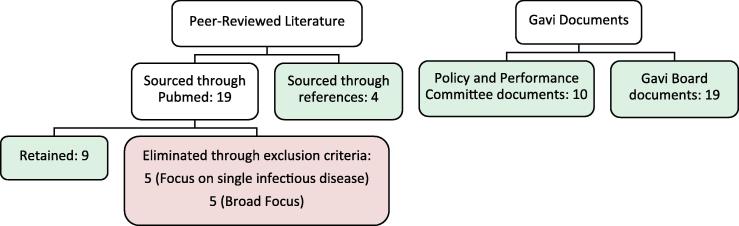
Fig. 1 illustrates the selection process and distribution of documents. Noting the estimated equivalence in quality, high variation in focus, and overall low number of peer-reviewed and Gavi documents retained for analysis, the articles were considered equally in the assessment. Findings were independently extracted from each document and compared, to generate a consensus on availability of information.
Fig. 1.
Literature review flow diagram.
2.2. Analysis of country assessments and plans
As a second step, all Gavi transition assessments (15), transition plans (13) available to WHO as of May 2016 were reviewed. Assessments and plans were retrieved from the Expanded Programme on Immunization Team (EPI) in the WHO Immunization, Vaccines, and Biologicals Department (IVB). Table 1 provides a list of transitioning countries assessed by this study.
Table 1.
Gavi transitioning countries with available transition assessments.a
| Country (WHO) | Region | Gavi status (2017) | Transition plan |
|---|---|---|---|
| 1. Angola | Africa | Accelerated Transition phase | 2015–2017 |
| 2. Armenia | Europe | Accelerated transition phase | 2014–2015 |
| 3. Azerbaijan | Europe | Accelerated transition phase | 2014–2015 |
| 4. Bhutan | South-East Asia | Fully self-financing phase | 2015 |
| 5. Bolivia | Americas | Accelerated transition phase | 2015–2017 |
| 6. Congo Republic | Africa | Accelerated transition phase | 2015–2017 |
| 7. Georgia | Europe | Accelerated transition phase | 2015–2017 |
| 8. Ghana | Africa | Accelerated transition phase | Not Available |
| 9. Guyana | Americas | Fully self-financing phase | 2015–2017 |
| 10. Honduras | Americas | Fully self-financing phase | 2014–2015 |
| 11. Moldova | Europe | Fully self-financing phase | 2014–2016 |
| 12. Mongolia | Western Pacific | Fully self-financing phase | 2012–2014 |
| 13. Papua New Guinea | Western Pacific | Accelerated transition phase | Not Available |
| 14. Sri Lanka | South-East Asia | Fully self-financing phase | 2015 |
| 15. Uzbekistan | Europe | Accelerated transition phase | 2014–2015 |
As of January 2016.
Rather than follow a strictly defined template, assessments are generated from a high-level guide that is flexibly adapted to each country’s circumstances. Information gaps were thus anticipated and addressed with additional sources of information (Table 2).
Table 2.
Additional sources of information.
| Additional source of information | Description | Source |
|---|---|---|
| Expanded Program on Immunization (EPI) Reviews | Comprehensive, periodic evaluations of country EPI programs by immunization partners | WHO (IVB) |
| Gavi Annual Program Reviews (APR) and Joint Appraisals | Annual in-country multi-stakeholder review of the implementation progress and performance of Gavi grant support to a country [28] | Gavi and WHO (IVB) |
| Comprehensive Multi-Year Plans (cMYPs) | Multi-year strategic plans developed by countries to assess costs of an immunization programme to meet GVAP goals and mobilize finances [29] | WHO (IVB) and country inquiries) |
| Effective Vaccine Management (EVM) Assessments | Country-generated assessments to monitor and assess supply chain management [30] | WHO (IVB) and country inquiries |
| World Development Indicators of the World Bank | A collection of national, regional, and global development indicators with official WB data [4] | World Bank: Online World Databank |
| WHO Immunization Repository | An online database to manage and review information on new vaccine introductions, immunization services, and accelerated disease control in countries | Internal WHO Immunization Repository |
| Gavi Country Information | A compilation of data on Gavi-eligible and transitioning countries including basic indicators, a history of Gavi support, financial information, and coverage data [31] | Online Gavi Country Hub |
Transition assessments, transition plans, and supplemental sources of information were analyzed to understand country progress towards immunization related targets across four programmatic areas: (i) decision-making, (ii) political commitment and financial sustainability, (iii) demand for and equitable delivery of vaccines, and (iv) access to timely and affordable supply. These areas represent key pillars of the Immunization Partners’ Shared Strategy for Sustainable Access to vaccines in MICs, referred to as the MIC Strategy, endorsed by the WHO Strategic Advisory Group of Experts in Immunization (SAGE) in April 2015 [3]. While the MIC Strategy Framework was not developed specifically to analyse the sustainability of immunization programmes in Gavi transitioning countries, the framework was jointly developed by international partners and middle-income countries (including several supported by Gavi), to address concerns of MICs that are financing immunization efforts with national resources. The Framework thus provides a reasonable indication of sustainability challenges for this analysis.
Within this framework, specific indicators and targets were used to measure performance and challenges in study countries. In the absence of universally accepted standards, the indicators and targets used are either commonly accepted (e.g. GVAP targets) or derived from median values/reported challenges in sample countries. Basic statistical analysis was performed to determine the share of Gavi countries meeting, or failing to meet, targets. Thematic content analysis of qualitative information available in transition assessments and plans was further completed following the MIC Strategy Framework’s programmatic areas. Finally, the initiatives identified in the plans were matched to needs identified in the transition assessments.
2.3. Limitations
Several methodological limitations are noteworthy. The study surveys 15 of 26 Gavi transitioning countries, since transition assessments were not completed or available at the time of analysis for the remaining 11 countries (India, Lao PDR, Solomon Islands, Nigeria, Nicaragua, Cuba, Indonesia, Kiribati, Timor-Leste, Vietnam, Ukraine). Noting that several large countries are excluded, the data may overlook challenges impacting a significant share of the transitioning country population.
Moreover, there is currently a lack of consensus within Gavi on the path to sustainable immunization programs and thus on related criteria and indicators. While the MIC Strategy and GVAP provide a useful framework for analysis, the indicators and targets used in this analysis were not specifically developed to study Gavi transitioning countries and important considerations may be overlooked.
Finally, the lack of standardized templates and processes coupled with changing assessment teams impacts the comparability of assessment data.
These limitations could be addressed as data becomes increasingly available, Gavi develops a more targeted theory of change, and related monitoring/evaluation frameworks and targeted tools are developed to assess progress towards sustainability of immunization in transitioning countries.
3. Results
3.1. Literature review
The literature search produced a total of 52 documents, of which 42 were retained for analysis (see Table S1 for a summary of results). Gavi documents focus exclusively on transitioning countries, while peer-reviewed articles include analyses of Gavi-ineligible MICs. However, both Gavi publications and peer-reviewed literature focus heavily on appraising financial challenges for countries [3], [9], [13], [14], [15], [16], [17].
When reviewing the broader performance of immunization programmes in Gavi transitioning countries, most papers use the third dose of Diphtheria, Tetanus, and Pertussis (DTP3) immunization coverage as a proxy for overall programme impact without reviewing specific programmatic challenges. There are two exceptions: a Gavi document [14] and a peer-reviewed article [16] that review the transition experience of six countries (Angola, Bhutan, Republic of Congo, Georgia, Moldova, and Mongolia). Nonetheless, while these two articles offer a useful view on country procurement practices, national regulatory authorities and country capacity for immunization planning and advocacy, they provide examples of issues rather than a comprehensive review of countries’ challenges. One article elaborating on the rationale for recent Gavi transition policy updates also provides a high-level discussion of programmatic challenges [13]. However, aside from a list of established National Immunization Technical Advisory Bodies (NITAGs), the article does not provide a comprehensive analysis beyond financing challenges.
Gavi documents supporting access to Gavi-like prices provide data on the number of countries with potential payment and procurement inefficiencies and stress vaccine pricing as key factors affecting countries’ ability to sustainably finance programmes [9], [15].
A more systematic, although limited, analysis of immunization programmes is provided in an article addressing challenges experienced by the broader middle-income country group [18]. Here, Gavi transitioning countries do not receive special attention and it is thus not possible to identify unique constraints.
Finally, two papers specifically focused on national decision-making processes provide a review of available information from country case studies (15 countries) [19] or country interviews (95 countries) [20] to understand factors affecting decision-making on new vaccine adoption in low- and middle-income countries. These papers conclude that the local burden of disease data, vaccine prices and the cost implications of adopting a new vaccine are of particular importance in new vaccine adoption decisions in lower-middle-income countries (LMICs) and that the underlying driver for vaccine adoption decisions in Gavi-eligible countries was the desire to seize windows of opportunity for Gavi funding [19], [20].
3.2. Analysis of country assessments and plans
Table 3, Table 4, Table 5 provide results of the analysis. The online Appendix provides further detail.
Table 3.
Summary of results.
 |
ii % of countries under study (15).
iii % of countries with available data.
iv WHO JRF asks countries to identify reasons for hesitancy to accept vaccines according to the national schedule. A country identifying 1+ reasons for hesitancy is classified as having a vaccine hesitancy issue. (See above-mentioned references for further information.)
Table 4.
Issues identified by area in transition assessments.
| Area | Sub-area | % of Countries with 1+ Issue | Issues identified |
|---|---|---|---|
| Decision-Making (NITAG) | – | 40% |
|
| Political Commitment and Financial Sustainability | – | 67% |
|
| Demand for and Equitable Delivery of Vaccines | Data Management | 60% |
|
| Communication | 80% |
|
|
| Human Resources for Health | 67% |
|
|
| Supply Chain | 80% |
|
|
| Access to Timely and Affordable Supply | Procurement | 73% |
|
| NRA | 53% |
|
|
Table 5.
Alignment between transition assessment issues and transition plan activities, by programmatic area.
 |
The table provides an overview of the alignment between transition assessments and transition plans for each country. Each column represents a country (n = 13), and each row indicates an issue. Green: an issue was identified in a transition assessment and was addressed in the transition plan. Yellow: an issue was not explicitly identified in the assessment but was nonetheless addressed. Red: an issue was identified in the transition assessment but not addressed in the plan. Grey: no issue identified or addressed.
3.2.1. Decision-making processes
The analysis first examined decision-making processes determining a country’s capacity to undertake timely and evidence-based immunization policy and programmatic choices. Effective immunization decision-making is particularly important for countries that fully fund immunization programs and thus require strong evidence to secure sufficient domestic financial resources while relying less on international recommendations for immunization policies [20]. NITAGs are formal multidisciplinary bodies of national experts that aim to provide independent, evidence-based guidance to national policymakers on immunization-related decisions [21]. WHO recommends the establishment of NITAGs to enable governments to develop objective policies independent of external influence and to improve evidence-based decision-making on immunization [21]. Although most transitioning countries have progressed toward establishing NITAGs in line with GVAP targets, 60% of the countries under study lack a functional NITAG, particularly African countries [21]. Forty percent of transition assessments note issues in this area and this issue was not addressed by transition plans for only 3 countries. Transition plans generally present two types of support activities in this area: (a) establishing a NITAG and (b) enhancing access and use of disease surveillance data to support evidence-based decisions.
3.2.2. Political commitment and financial sustainability
With a loss of financial support from Gavi, transitioning countries must primarily rely on domestic resources to fund immunization services. Thirteen of the fifteen countries under study have a budget line for immunization as per GVAP recommendations and 62% finance a large portion of both routine immunization and related vaccines with government sources. Nonetheless, significant variation exists, with highest coverage in the American region countries for instance. Nearly half of sample countries have defaulted at least once on Gavi co-financing requirements, particularly countries in the African region, and 67% of transition assessments report shortfalls in financing for immunization.
Transition plans largely recognize these well-understood weaknesses, recommending the construction of legal frameworks to ensure the political priority of immunization as well as the development of resource mobilization strategies linked to updated financial projections, cost and economic analyses, and fiscal space assessments.
3.2.3. Demand for and equitable delivery of vaccines
The analysis of demand for and equitable delivery of vaccines indicates that only 25% of countries under study have not reported major data quality issues and that two thirds of countries lack human resources to adequately provide quality health services, including immunization. Only 38% of countries have a communication plan for immunization activities, with the highest investment in communication noted in the African region. Vaccine management is problematic in 60% of countries and, on average, countries have experienced more than one stock-out per year between 2010 and 2015, with the most concerning results noted in the European region. Hesitancy among vaccine users is also flagged by most study countries (92%) and appears to be an important area of concern.
Transition assessments have identified challenges with data management, communication, human resources for health and supply chain in 60, 80, 67 and 80% of countries, respectively. To address these concerns, transition plans incorporate the following initiatives: training in data management and use (including support to implement electronic data systems at the community level), activities to develop national communication strategies and targeted media (i.e. posters, radio and television clips), communication training programs for key health officials, and capacity-building activities to train human resources on cold chain logistics and basic immunization practices. Overall, 19 of 45 issues identified across 11 countries are not explicitly addressed by transition plans.
3.2.4. Access to timely and affordable supply
According to data from the Gavi transition assessments and from the WHO Regulatory Systems Strengthening team, nearly half of the study countries utilize a mixed-procurement method to ensure timely access to affordable supply, procuring Gavi-funded vaccines and occasionally other routine vaccines through the UNICEF Supply Division (SD).
Transition assessments have evaluated whether countries procuring partly or fully through the United Nations (UN) can continue to do so following the termination of Gavi support. Only 4 out of 14 countries that procure through a UN organization will face barriers in continuing to use UN procurement due to national legislation requiring local procurement of vaccines.
Transition assessments have also evaluated the capacity of self-procuring or prospective self-procuring countries. The assessments report critical self-procurement capacity issues in 7 out of 9 applicable countries. Collectively, across all procurement methods, 73% of countries under study have identified at least one red flag in their current procurement practices. Beyond procurement, most transition assessments also noted barriers to accessing affordable vaccine pricing.
Transition plans are largely individualized in addressing procurement challenges and include activities such as training on procurement methods, improving registration procedures, mid-level management training, improving the government understanding of UNICEF procurement processes, and enabling timely procurement of traditional and new vaccines.
To conclude, the analysis reviewed the strength of National Regulatory Authorities (NRAs), which are essential for assuring the safety, efficacy and quality of vaccines used in national immunization programmes [22]. WHO has developed standard criteria for evaluating NRAs across six key regulatory functions: marketing authorization and licensing; post-marketing surveillance; NRA lot release, laboratory access; regulatory inspections; and regulatory oversight of clinical trials [22]. A review of existing NRA assessments concluded that a majority of the study countries do not have a functional regulatory system. Transition assessments identified challenges in 53% of countries distributed across all NRA functions in all regions. Transition plans address country-specific NRA shortfalls, with a particular focus on strengthening market authorization and licensing as well as pharmacovigilance, particularly AEFI surveillance. Activities to improve NRA lot release and laboratory access functions are not specifically noted in transition plans. Nonetheless, the majority of plans calls for the development of blueprints for NRA strengthening external to the transition plan. These activities may thus be described in such NRA-specific documents.
4. Discussion
Through a systematic literature review, this study confirms limited comparative analyses on immunization system performance in countries transitioning from Gavi support. Peer-reviewed and Gavi literature focus primarily on financial challenges. However, while the ability to sustainably fund immunization programmes is an area of justified concern, immunization partners have increasingly emphasized the importance of ensuring the programmatic sustainability of immunization through Gavi’s 2016–2020 Strategy [23]. Closely monitoring countries’ performance beyond financing is thus becoming key to informed policy-making, particularly for: (i) the development of a more nuanced theory of change towards sustainable immunization programmes; (ii) measurement of progress and understanding of key areas for attention and for investment; and (iii) stimulation of further discussion around eligibility criteria for external support beyond GNI.
Several existing concerns on the programmatic sustainability of immunization programmes are confirmed by a review of existing transition assessments and plans and commonly accepted immunization indicators.
4.1. Decision-making processes
The data indicates shortcomings in the use of evidence-based decision-making in transitioning countries, raising an important concern as countries lose Gavi support and further rely on autonomous decision-making for immunization policies and new vaccine introductions. Transition plans attempt to address this issue and Gavi is currently investing over 30% of its transition resources on strengthening “information, data and systems for decision-making” [24]. Despite this investment, the limited time remaining for several countries to complete their Gavi transition presents a considerable risk. Establishing systems that generate reliable data and institutionalizing fact-based decision-making requires time.
4.2. Political commitment and financial sustainability
Our analysis confirms previous conclusions on immunization financing. Country performance is difficult to assess without clear targets, yet transitioning countries encounter identifiable challenges. In particular, a review of in-country assessments reveals a lack of skills and processes to develop sound financing and resource mobilization strategies. Under its Strategic Focus Area of Sustainability, Gavi has committed to investments in financial planning and resource mobilization. Yet, at present, only 3% of transition investments are dedicated to supporting countries with inadequate financial planning or management and budgeting capacity [23]. Moreover, a potential shortfall of the Gavi transition process is that it tends to focus exclusively on immunization and may thus be unable to identify system-wide constraints to sustainability nor integrated and sector-wide approaches to overcome them.
Future challenges in the area of sustainable financing include: i) matching the concurrent withdrawal of other donors (e.g. the Global Fund) with national-level efforts to ensure adequate fiscal space and financing for the entire health sector; and ii) ensuring the sustainability of alternative means of support [25], [26]. In fact, despite considerable investments by Gavi for developing and applying tools to assess immunization financing challenges as well as for costing immunization programmes, options to help countries mobilize national resources for immunization are underdeveloped. Positive experiences from the European and Latin American regions may prove useful in guiding policy.
4.3. Demand for and equitable delivery of vaccines
The review of vaccine hesitancy, communication for immunization, availability of skilled human resources, and effective vaccine management reveals critical issues across all assessments. Transition plans invest important resources to address weaknesses in these areas, but not consistently (36% of identified challenges don’t seem to be addressed). Furthermore, available time and resources may be limited and a complete interruption of external support may endanger progress. Options to continue providing limited support to countries should be explored, perhaps through stronger integration of the immunization sector with the broader health system and through longer term financial mechanisms, such as concessional loans. A concern that emerges less clearly from our review of transition assessments, but which may warrant dedicated attention moving forward, is the consistent discussion of equity issues and solutions in all countries.
4.4. Access to timely and affordable supply
An appraisal of challenges affecting access to affordable vaccine supplies provides informative results. In the area of procurement, concerns have been expressed regarding the ability of countries to continue procuring through UN agencies following the termination of Gavi support. Our analysis suggests that this problem may be limited to a few countries. In these cases, with advocacy to modify regulatory procedures, immunization partners must support countries in allowing for regulatory exceptions in the short- to medium-term.
Of greater concern is a generalized need to strengthen in-country practices for both UN and self-procurement. Considering the limited tools and support provided by the international immunization community for procurement, coupled with limited clarity on the role of different development agencies, this is a significant finding. Through transition plans, Gavi is only initiating investment in this area. Additionally, these investments may be too small to address long-term issues linked to weak pharmaceutical and procurement laws requiring several years and strong political commitment to change.
Finally, vaccine affordability has received significant attention in transition assessment discussions, with countries flagging important concerns. Yet, as a result of recent manufacturers’ commitments to affordable prices for Gavi transitioning countries, a relatively smooth transition should be possible, supported by a recent analysis [27]. It may be important to ensure available pricing commitments are promptly communicated to countries to ease anxiety and inform policy making.
While notable differences in performance by geographical region did not emerge from our results, nor are conclusions possible given the small available sample of countries, future comparative analyses could review a larger volume of data to better target investments. So far, results indicate some geographical heterogeneity in terms of the main areas of need, confirming the importance of tailored approaches for each country during the Gavi transition process. However, within each programme area studied, countries experience comparable challenges requiring similar types of support. These results encourage the current timid interest by development partners to invest in peer platforms to boost learning and leverage country knowledge and past experience—a sustainable approach to external support following Gavi transition.
In collaboration and consultation with countries, industry, and civil society organizations, global immunization partners developed a Middle Income Strategy endorsed by WHO SAGE in April 2015 to address several challenges encountered by MICs never been eligible for Gavi support. With some variability, challenges experienced by Gavi transitioning countries are similar [3]. While the MIC Strategy currently remains largely unfunded, the transition out of Gavi of several highly populated countries may improve awareness and interest in this mandate.
5. Conclusion
Middle Income Countries (MICs) losing support from Gavi, the Vaccine Alliance, require special attention to achieve Global Vaccine Action Plan (GVAP) targets. This study examined available literature and programmatic data for 15 transitioning countries documenting and beginning to address a gap in systematic analysis of programmatic performance and challenges. The analysis suggests that the transition period between a Gavi-supported programme and a self-sufficient immunization programme represents a key opportunity for targeted investments to address shortfalls, but also that a complete interruption of support following exit may jeopardize sustainability. Transitioning countries are struggling across four programmatic areas: decision-making; political commitment and financial sustainability; equitable delivery of vaccines; and access to timely and affordable supply. Continued monitoring of countries’ performance beyond financing is thus key to informed policy-making on sustainability of immunization efforts spurred by external donor support. Development of a shared theory of change towards sustainable immunization programmes and a related monitoring and evaluation framework would allow clearer measurement of progress, gaps, and understanding of key areas for future investment.
Acknowledgments
Acknowledgements
The authors would like to thank Juliette Puret for early data collection and input; Khaem Alireza, Sarah Alkenbrack, Logan Brenzel, Thomas Cherian, Adam Cohen, Santiago Cornejo, Laure Dumolard, Ulla Griffiths, Xiao Xian Huang, Patrick Lydon, Thomas O’Connel, Minal Patel, Claudio Politi, and Kamel Senouci, for helpful discussions and suggestions; and Carmen Au for copy-editing.
Conflict of interest
All authors declare no conflicts of interest.
Footnotes
Of note, this threshold is above the World Bank’s middle income threshold and all Gavi transitioning countries are thus MICs by definition.
Since its inception in 2000, Gavi has had incredible success in increasing and streamlining donor funding for immunization for target countries. While global entities, such as WHO and UNICEF, have broader mandates encompassing all member states, they have struggled to continue resourcing for country support activities beyond the Gavi eligible countries.
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.vaccine.2018.06.012.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.World Health Organization. Global Vaccine Action Plan, 2011-2020; 2013.
- 2.Strategic Advisory Group of Experts on Immunization. Assessment Report of the Global Vaccine Action Plan; 2015.
- 3.Cernuschi T, Ghandi G. A Shared Middle Income Countries Strategy; 2015.
- 4.World Development Indicators. World Bank 2016. https://data.worldbank.org/data-catalog/world-development-indicators [accessed June 1, 2017].
- 5.Gavi. Facts and Figures 2017. http://www.gavi.org/about/mission/facts-and-figures/ [accessed June 1, 2017].
- 6.Gavi Secretariat. Gavi Transition Process 2017. http://www.gavi.org/support/sustainability/transition-process/ [accessed June 1, 2017].
- 7.World Health Organization. Number of Deaths (Thousands), Data by WB Income Group 2016. http://apps.who.int/gho/data/view.main.CM1300NWB?lang=en [accessed June 1, 2017].
- 8.Secretariat Gavi. Eligibility and Transition. Policy. 2015 [Google Scholar]
- 9.Gavi Secretariat. 07- Gavi Support for Access to Appropriate Pricing for Gavi Graduated Countries, 10-11 June 2015; 2015.
- 10.Rwabukwisi F.C., Bawah A.A., Gimbel S., Phillips J.F., Mutale W., Drobac P. Health system strengthening: A qualitative evaluation of implementation experience and lessons learned across five African countries. BMC Health Serv Res. 2017;17 doi: 10.1186/s12913-017-2662-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gavi. Gavi Programme and Policy Committee 2017. http://www.gavi.org/about/governance/gavi-board/committees/programme-policy-committee/ [accessed June 1, 2017].
- 12.Gavi. Gavi Documents 2017. http://www.gavi.org/library/gavi-documents/ [accessed June 1, 2017].
- 13.Kallenberg J., Mok W., Newman R., Nguyen A., Ryckman T., Saxenian H. Gavi’s transition policy: Moving from development assistance to domestic financing of immunization programs. Health Aff. 2016;35:250–258. doi: 10.1377/hlthaff.2015.1079. [DOI] [PubMed] [Google Scholar]
- 14.Gavi Secretariat. 06- Graduation – Financial and Programmatic Sustainability for Graduating Countries, March 2013; 2013.
- 15.Gavi Secretariat. 07- Gavi Support for Access to Appropriate Pricing for Gavi Graduates and Other Lower Middle Income Countries, 5-6 May 2014; 2014.
- 16.Saxenian H., Hecht R., Kaddar M., Schmitt S., Ryckman T., Cornejo S. Overcoming challenges to sustainable immunization financing: Early experiences from GAVI graduating countries. Health Policy Plan. 2015;30:197–205. doi: 10.1093/heapol/czu003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saxenian H., Cornejo S., Thorien K., Hecht R., Schwalbe N. An analysis of how the GAVI alliance and low- and middle-income countries can share costs of new vaccines. Health Aff. 2011;30:1122–1133. doi: 10.1377/hlthaff.2011.0332. [DOI] [PubMed] [Google Scholar]
- 18.Kaddar M., Schmitt S., Makinen M., Milstien J. Global support for new vaccine implementation in middle-income countries. Vaccine. 2013;31 doi: 10.1016/j.vaccine.2012.11.085. [DOI] [PubMed] [Google Scholar]
- 19.Makinen M., Kaddar M., Molldrem V., Wilson L. New vaccine adoption in lower-middle-income countries. Health Policy Plan. 2012;27 doi: 10.1093/heapol/czs036. [DOI] [PubMed] [Google Scholar]
- 20.Burchett H.E.D., Mounier-Jack S., Griffiths U.K., Biellik R., Ongolo-Zogo P., Chavez E. New vaccine adoption: Qualitative study of national decision-making processes in seven low- and middle-income countries. Health Policy Plan. 2012;27 doi: 10.1093/heapol/czs035. [DOI] [PubMed] [Google Scholar]
- 21.Adjagba A., Senouci K., Biellik R., Batmunkh N., Faye P.C., Durupt A. Supporting countries in establishing and strengthening NITAGs: Lessons learned from 5 years of the SIVAC initiative. Vaccine. 2015;33:588–595. doi: 10.1016/j.vaccine.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. National Regulatory Authorities 2016. http://www.who.int/immunization_standards/national_regulatory_authorities/role/en/ [accessed July 1, 2016].
- 23.Gavi Secretariat. Gavi Alliance Strategy 2016 – 2020; 2014.
- 24.Gavi Secretariat. 03a - Update on Country Programmes, Annex C; 2016.
- 25.World Health Organization. Health Financing 2017. http://www.who.int/health_financing/en/ [accessed June 1, 2017].
- 26.World Health Organization. Developing a National Health Financing Strategy: A Reference Guide 2017. http://www.who.int/health_financing/tools/developing-health-financing-strategy/en/ [accessed June 1, 2017].
- 27.World Health Organization. Fact Sheet: Vaccine Pricing for Gavi Transitioning Countries. Geneva; 2016.
- 28.Gavi Secretariat. Joint Appraisals. Gavi Alliance; 2016.
- 29.Gavi Secretariat. Immunization Planning and Financing. World Heal Organ Immunization, Vaccines, Biol 2016. http://www.who.int/immunization/programmes_systems/financing/en/ [accessed June 1, 2017].
- 30.Effective Vaccine Management (EVM) Initiative. World Heal Organ Immunization, Vaccines, Biol 2016.
- 31.Country Hub. Gavi 2016. http://www.gavi.org/country/.
- 62.Global Equity Initiative Harvard University. Human Resources for Health: Overcoming the Crisis n.d. http://www.who.int/hrh/documents/JLi_hrh_report.pdf [accessed June 1, 2017].
Further reading
- 32.Gavi Secretariat. 01a- country eligibility policy revision, October 2009; 2009.
- 33.Gavi Secretariat. 01b- Graduation policies for gavi eligibility, October 2009; 2009.
- 34.Secretariat Gavi. Gavi Alliance Country Eligibility. Policy. 2009 [Google Scholar]
- 35.Gavi Secretariat. GAVI Alliance Graduation Policy n.d.; 2009.
- 36.Gavi Secretariat. 06b- GAVI Eligibility Graduation Procedures, 17-18 November 2009; 2009.
- 37.Secretariat Gavi. 06a- Gavi alliance eligibility. Policy. November 2009;17–18:2009. [Google Scholar]
- 38.Gavi Secretariat. 07- Co-financing policy revision: principles and objectives, 16-17 June 2010; 2010.
- 39.Gavi Secretariat. 01- Co-financing Policy Revision, 21-22 October 2010; 2010.
- 40.Secretariat Gavi. Revised Co-Financing. Policy. 2010 [Google Scholar]
- 41.Gavi Secretariat. 11d- Co-Financing Policy Revision, 1 December 2010; 2010.
- 42.Secretariat Gavi. 10- Dr. Gonzalez’ Proposal on Co-Financing. September 2011;28–30:2011. [Google Scholar]
- 43.Secretariat Gavi. B- Review of Eligibility Threshold. November 2011;16–17:2011. [Google Scholar]
- 44.Gavi Secretariat. 13- Gavi engagement with graduating countries, 9-10 October 2013; 2014.
- 45.Gavi Secretariat. 07- Gavi alliance strategy 2016-2020, 21-22 November 2013; 2013.
- 46.Gavi Secretariat. 08- Gavi’s Approach to Graduation, 5-6 May 2014; 2014.
- 47.Norwegian Institute of Public Health. Evaluation of the GAVI Alliance Co-financing Policy n.d.:1–148.
- 48.Gavi Secretariat. 06b- review of co-financing policy, 7–8 October 2014; 2014.
- 49.Gavi Secretariat. 06a- Review of Eligibility and Graduation Policies, 7-8 October 2014; 2014.
- 50.Gavi Secretariat. Gavi alliance board retreat: analysis and discussion on eligibility, graduation, and co-financing, 24–25 March 2015; 2015.
- 51.Gavi Secretariat. Co-Financing Policy, Gavi Alliance; 2015.
- 52.Gavi Secretariat. 04- Report to the Programme and Policy Committee, 4-6 May 2015; 2015.
- 53.Gavi Secretariat. 05- Strengthening Country Transitions Out of Gavi Support, 10-11 June 2015; 2015. doi:10.1016/0735-1097(96)00175-1.
- 54.Secretariat Gavi. 06- Review of Gavi’s Co-financing. Policy. June 2015;10–11:2015. [Google Scholar]
- 55.Gavi Secretariat. 09- Implementation of the 2016-2020 Strategy: Strategic Focus Area on Sustainability, 12-13 May 2016; 2016.
- 56.Berkley S. Improving access to vaccines through tiered pricing. Lancet. 2014;383:2265–2267. doi: 10.1016/S0140-6736(13)62424-1. [DOI] [PubMed] [Google Scholar]
- 57.Valdés W., Janusz C.B., Molina Aguilera I.B., Mendoza L., Díaz I.Y., Resch S. Tracking financial flows for immunization in Honduras. Vaccine. 2015;33:A85–A92. doi: 10.1016/j.vaccine.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 58.Schütte C., Chansa C., Marinda E., Guthrie T.A., Banda S., Nombewu Z. Cost analysis of routine immunisation in Zambia. Vaccine. 2015;33:A47–A52. doi: 10.1016/j.vaccine.2014.12.040. [DOI] [PubMed] [Google Scholar]
- 59.Ngabo F., Levin A., Wang S., Gatera M., Rugambwa C., Kayonga C. A cost comparison of introducing and delivering pneumococcal, rotavirus and human papillomavirus vaccines in Rwanda. Vaccine. 2015;33:7357–7363. doi: 10.1016/j.vaccine.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shen A.K., Weiss J.M., Andrus J.K., Pecenka C., Atherly D., Taylor K. Country ownership and gavi transition: Comprehensive approaches to supporting new vaccine introduction. Health Aff. 2016;35:272–276. doi: 10.1377/hlthaff.2015.1418. [DOI] [PubMed] [Google Scholar]
- 61.Feikin DR, Flannery B, Hamel MJ, Stack M HP. Vaccines for Children in Low- and Middle-Income Countries. Heal Dis Control Priorities, 3rd ed. (vol. 2), The International Bank for Reconstruction and Development/The World Bank; n.d. [chapter 10]. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.