Graphical abstract
Keywords: Functional magnetic resonance imaging (fMRI), Electrophysiology, Polyetheretherketone (PEEK), Hydroxyapatite (HA), Acrylic, Implant, Macaque, Osseointegration
Highlights
-
•
We created custom PEEK implants coated with hydroxylapatite to promote osseointegration.
-
•
Headposts and chambers were implanted with ceramic screws and the surgical incision was closed subcutaneously.
-
•
We prevented the animal from picking and scratching after surgery by using a head cap that protects the wound margin.
-
•
Implants integrated with the skull and remained robust after a year with no growth of granulation tissue.
-
•
MRI signal and contrast-to-noise ratio improved in NHPs implanted with our new methods.
Abstract
Background
Neuroscientists commonly use permanently implanted headposts to stabilize the head of nonhuman primates (NHPs) during electrophysiology and functional magnetic resonance imaging (fMRI). Here, we present improved methodology for MRI-compatible implants without the use of acrylic for head stabilization in NHPs.
New method
MRI is used to obtain a 3D-reconstruction of NHP skulls, which are used to create customized implants by modeling intersections with the bone. Implants are manufactured from PEEK using computer numerical control machining and coated with hydroxyapatite to promote osseointegration. Surgically, implants are attached to the skull with ceramic screws, while the skin flap is pulled over the implant and closed subcutaneously.
Results
Quality of blood oxygen level dependent (BOLD) fMRI signal is improved in animals implanted with our method as compared to traditional acrylic implants. Additionally, implants are well-integrated with the skull, remain robust for more than a year and without granulation tissue around the skin margin.
Comparison with existing method(s)
Previous improvements on NHP implants (Chen et al., 2017; McAndrew et al., 2012; Mulliken et al., 2015; Overton et al., 2017) lacked fMRI-compatibility, as they relied on titanium headposts and/or titanium screws. Thus, most fMRI studies in NHPs today still rely on the use of acrylic-based headposts for stabilization and the use of contrast-enhanced agents to improve MRI signal.
Conclusions
Our method preserves fMRI-compatibility and results in measurable improvement in BOLD signal without the use of contrast-enhanced agents. Furthermore, the long-term stability of our implants contributes positively to the wellbeing of NHPs in neuroscience research.
1. Introduction
In the past two decades, systems neuroscience has greatly benefited from the use of high-resolution functional magnetic resonance imaging (fMRI) in non-human primates (NHPs). By increasing spatial resolution, MR imaging allows studying cortical and subcortical networks with unprecedented functional and anatomical detail. However, with increasing spatial resolution and field strength, susceptibility artifacts also increase. Particularly in NHP fMRI, the presence of acrylic-based headposts and chambers results in magnetic field susceptibilities near the area of implantation. Here, we present a new refined method for MRI-compatible headpost and chamber implants and demonstrate improvements in T1 and T2-weighted (T2*) image quality by eliminating the use of acrylic for implantation.
Head stabilization in large animals such as macaque monkeys usually requires the use of permanently implanted headposts secured to an external reference frame. In cases where studies focus on electrophysiological recordings without the need for fMRI, headposts and recording chambers are commonly made out of titanium. Titanium implants are known to integrate very well with bone (Kumar and Narayan, 2014; Salvi et al., 2015) and are extensively used in human medicine for orthopedic surgery (Taddei et al., 2004). As designed for NHPs, titanium implants typically contain radially extending supporting legs that are bent during or prior to surgery to match the curvature of each individual animal skull. The process of bending titanium implants during surgery is time-consuming and a tight fit cannot always be achieved. Potential gaps left between the implant and the bone surface may result in poor osseointegration, which could increase the risk of infection and implant loss.
Recent technical developments have led to manufacturing customized titanium implants and chambers using 3D-printing technology (Chen et al., 2017) or utilizing computer numerical control (CNC) machining of chambers (McAndrew et al., 2012); both methods resulting in improved fit to the skull surface (Johnston et al., 2016). While the use of customized titanium implants might improve osseointegration, titanium disrupts the magnetic field of MRI scanners, causing image distortions and artifacts (Hargreaves et al., 2011). These effects are relatively minor in anatomical images (e.g. using FLASH or MPRAGE sequences); however, in functional MRI (e.g. spin echo or echo planar imaging) they are highly disruptive, rendering images unsuitable for proper analyses.
Therefore, neuroscientists who routinely use fMRI rely on implants that are made of durable plastic such as polyetheretherketone (PEEK). PEEK is a thermoplastic polymer with high resistance that is used in human medicine applications such as orthopedics with great success (Williams et al., 1987). However, compared to titanium, PEEK lacks osseointegrative properties (Ma and Tang, 2014). Thus, for firm stabilization of the implant assembly, researchers have mainly opted for the use of PEEK implants in combination with non-fMRI-compatible titanium screws (Lanz et al., 2013; McAndrew et al., 2012; Mulliken et al., 2015) or with acrylic-based bone cements. While acrylic-PEEK implants might offer strength and to a certain extent MRI compatibility (however see Fig. 7, Fig. 8), these implants fall behind in terms of biocompatibility and durability since the acrylic around the implant can introduce complications at the skin margin (Leggat et al., 2009).
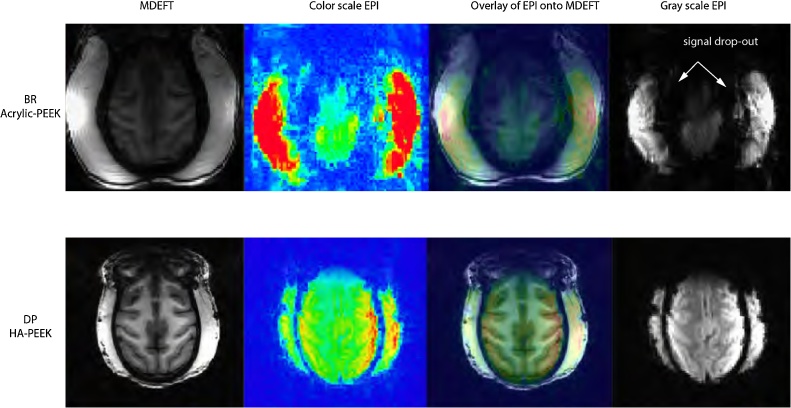
Fig. 7.
Comparison of anatomical and functional images between monkeys with HA-PEEK and Acrylic-PEEK implants.
Example anatomical and functional MRI images obtained from two monkeys (top, Acrylic-PEEK; bottom, HA-PEEK) showing the effects of the implants on MRI signal quality. For comparison, each image is shown at the same axial distance, at which the implant surrounds the most dorsal part of the brain. Acrylic-based implants may require the deflexion of the temporal muscles leading to an air-filled gap along the margin which causes abrupt changes in magnetic field susceptibility around the transition zones (e.g. bone/acrylic to brain tissue).
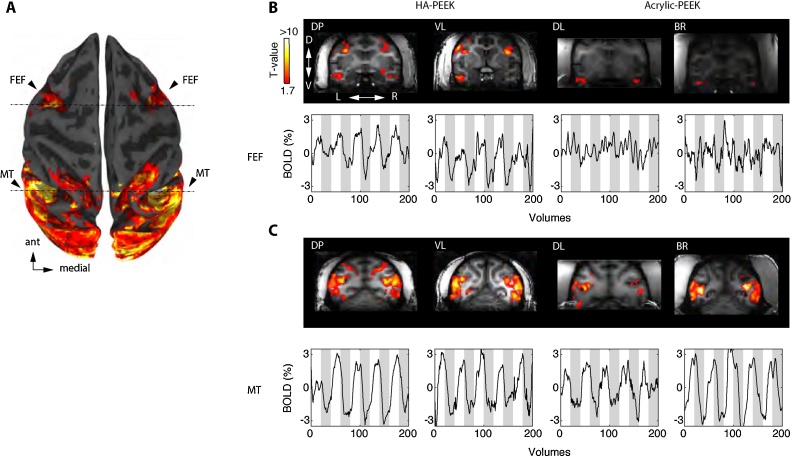
Fig. 8.
Functional activation of frontal eye fields in monkeys with HA-PEEK implants but not Acrylic-PEEK implants.
A. Brain surface reconstruction of monkey DP showing the overall visually evoked response during movie viewing. The activation pattern includes regions in the frontal eye fields (FEF) and the middle temporal area (MT). B. (Top) Coronal slices taken at the level corresponding to the anterior dashed line in A, in each individual monkey. Each animal was presented with periods of natural movies interleaved with periods of black screen and was allowed to freely look around and watch the movie. (Bottom) Example time courses for each monkey show the overall % BOLD modulation to 30-s movie segments (white background) and 30 s off-period (grey background). Notice the lack of activation and BOLD modulation in the FEF region for monkeys DL and BR with acrylic based headposts. C. Similar to B, except coronal slices are centered on area MT (posterior dashed line in A) and show % BOLD modulation plots. All data were obtained from a single run session (5 min of scanning) and illustrated using the same threshold level (q FDR = 0.05, p < 0.01, significance t-values > 1.9 and clipped at 10 for comparisons across animals). DP (T-value range 1.74–17.7; VL (T-value range 2.14–13.3; DL. (T-value range 2.52–13.5); BR (T-value range 2.50–10.8).
An important difference between implants used in medicine and those used in NHP research is that implants in humans are typically isolated from the exterior (by muscles and skin), while implants in NHPs are percutaneous: they include a structure outside the head that is used for head stabilization and/or neural recordings. The skin margin around the implant is formed of highly vascularized connective or granulation tissue. Normally, such granulation tissue forms as a part of the healing process; however, around an acrylic-based implant it actually prevents successful healing, often becomes irritated, requires frequent cleaning and still is highly susceptible to infection. Such infection is prone to spread which often results in a premature implant failure and negatively affects the animal’s welfare. Consequently, neuroscientists have become hesitant about the use of PEEK implants in NHPs (Lanz et al., 2013; McAndrew et al., 2012).
To address all the aforementioned issues with implants used for neuroimaging in NHPs, we developed an implant technique that offers MRI-compatibility, strength, and durability, and results in a healthy skin margin that does not require regular cleaning procedures. We used MRI data and pre-processing techniques to create a digital replica of the skull from each individual animal. These skull replicas were then used to design and manufacture customized skull-fitting headposts and recording chambers made of glass-filled PEEK and coated with hydroxyapatite (HA) at the base. These implants, which we refer to as hydroxyapatite-coated PEEK implants or HA-PEEK, were surgically implanted and secured onto the skull using ceramic screws and closed with a subcuticular suture; crucially, without the need for acrylic for stabilization.
2. Methods
2.1. Subjects
All data were obtained from eight rhesus monkeys (Macaca mulatta); four females (VL and DP: 6 years of age, weighing 7–9 kg; FL and AL: 4–5 years of age, weighing 6–9 kg) and four males (BR and DL: 11–15 years of age, weighing 14–17 kg; R and TL: 9–12 years of age, weighing 8–12 kg). The four females (VL, DP, FL, AL) were implanted using the proposed new method (HA-PEEK), while the four males (BR, DL, TL, and R) received traditional acrylic-based implants and serve as a control group (Acrylic-PEEK). Surgical procedures, postoperative care and implant cleaning methods for the Acrylic-PEEK animals were described earlier (Thiele et al., 2006). All surgical procedures were approved by the UK Home Office and comply with the Animal Scientific Procedures Act (1986) on the care and use of animals in research, including the European Directive on the protection of animals used in research (2010/63/EU).
2.2. Anesthesia during MRI
Pre-surgical scans under anesthesia were carried out for surgical planning and for 3D-reconstruction of the skull. For anesthesia, a combination of drugs was used. First, animals were sedated in their home cage with ketamine (10 mg/kg IM). Once immobile, animals were transferred to a preparatory room where a saphenous vein catheter was put in place and systemic analgesia administered (meloxicam 0.3 mg/kg IV). The animals were transferred to the MRI room, where general anesthesia was induced with propofol (2–4 mg/kg IV). The larynx was then desensitized (Intubease 20 mg/ml) and the trachea intubated with a cuffed oro-tracheal tube for maintenance anesthesia (sevoflurane, 2–3% in 100% oxygen). Lidocaine/prilocaine local anesthetic cream (EMLA 5%) was applied to the external acoustic meatus before placing the animal into a stereotaxic frame and ventilating it mechanically (Merlin, Vetronics Ltd, UK). Physiological parameters (end-tidal carbon dioxide, indirect blood pressure, blood oxygen saturation, pulse rate, respiratory rate, rectal temperature and gas analysis) were monitored and kept in desired ranges over the course of data acquisition. Scanning lasted approximately three hours from the time of initial sedation. Post-surgical anatomical scans were acquired in the awake state and were used to compare MRI signal quality between animals with different implant types (HA-PEEK vs. Acrylic-PEEK).
2.3. MRI data acquisition
MR images were acquired with a vertical 4.7 T magnet running ParaVision 5.1 (Bruker, BioSpin GmbH, Ettlingen, Germany) and equipped with a 4-channel phase array coil covering the whole head (https://www.wkscientific.com). For HA-PEEK monkeys, high-resolution pre-surgical anatomical images were acquired using a T1-weighted MDEFT pulse sequence with the following parameters: TR = 2000 ms, TI = 750 ms, TE = 3.95 ms, flip angle = 30°, slice thickness = 0.54 mm; matrix = 200 × 200 × 124 voxels, number of slices = 72, resolution = 0.525 × 0.525 × 0.54 mm3 voxel size. For monkeys DP and VL, four scans were acquired on each and combined in a grand average-MDEFT volume (see example in Fig. 1). For monkeys FL and AL, a volume coil was used to cover the whole head using an MDEFT pulse sequence with the following parameters: TR = 2000 ms, TE = 3.4 ms, flip angle = 30°, slice thickness = 0.63 mm; matrix = 154 × 154 × 102 voxels, number of slices = 100–102, resolution = 0.623 × 0.623 × 0.630 mm3 voxel size. For Acrylic-PEEK monkeys, all anatomical data was acquired during awake imaging.
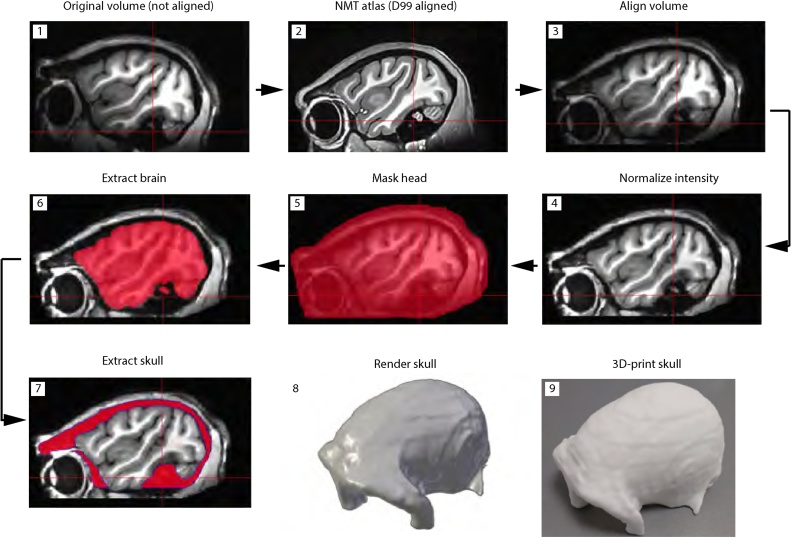
Fig. 1.
Workflow of anatomical MRI data pre-processing for implant design and surgical planning. Prior to the process of skull extraction, the T1 volume is aligned to the NMT template, which is further aligned with the D99 atlas space. Spatial intensity distributions are used to normalize the volume from surface coil inhomogeneities. The head and brain are segmented and subtracted from the normalized volume. Manual editing is done to remove non-skull voxels. The skull volume is rendered in STL format and 3D printed for surgical planning.
Before awake scanning, monkeys were trained to voluntarily approach the inside of a wooden box from which they learned to enter a cylindrical MRI-compatible chair. Inside the chair, the animals were trained to move their head out using positive reinforcement training techniques. Once their heads were outside of the chair a neck plate was locked in place to secure the animal inside the chair. The head was immobilized in the MRI chair using a head holding system and an implanted headpost.
For all animals, anatomical images were acquired with an MDEFT sequence with the following parameters: TR = 2000 ms, TI = 750 ms, TE = 3.95 ms, flip angle = 30°, slice thickness = 0.6 mm; matrix = 176 × 176 × 72 voxels, number of slices = 72, resolution = 0.6 × 0.6 × 0.6 mm3 voxel size. For functional scans, a continuous gradient-echo planar imaging (GE-EPI) sequence was used with the following parameters: TR = 1.5 s, TE = 18 ms, flip angle = 53°, FOV = 96 × 96 mm2, matrix = 96 × 96 voxels, number of slices = 18, slice thickness = 2 mm, resolution = 1.0 × 1.0 × 2 mm3, number of scans = 200. All experiments were performed with the same parameters and hardware settings across animal groups.
2.4. Skull reconstruction
A digital model of each individual animal’s skull was created from anatomical MRI data. AFNI/SUMA (Cox, 1996) software package was used for pre-processing and rendering (https://afni.nimh.nih.gov/download). The skull data were extracted using a custom-written script @Extract_skull while a second script @Create_skull rendered the result. The scripts for skull extraction are made available with this manuscript; individual functions and programs used by the scripts can be downloaded from the AFNI/SUMA website.
First, the T1 volume was aligned to the NMT (https://afni.nimh.nih.gov/NMT) macaque template (Seidlitz et al., 2018) using 3dAllineate function for affine transformation with 12 degrees of freedom. The NMT macaque template was previously aligned to the D99 atlas space using the same 3dAllineate function. Individual volumes were then normalized for coil non-uniformities and inhomogeneities in spatial intensity using 3dUniformize function. Next, the brain and muscle tissue were extracted using 3dSkullStrip, 3dAutomask and 3dcalc programs. The remaining low-T1 values corresponding to the cranial bone and to other low-signal non-cranial tissue were selected for manual editing using the AFNI mask GUI (see https://afni.nimh.nih.gov/Class_handouts for regions of interest). Finally, the edited skull volume was rendered in the STL format using IsoSurface program. The resulting STL file was used for implant design (https://www.rogue-research.com/veterinary/) and to 3D-print a skull replica (https://www.shapeways.com/) (Fig. 1), subsequently used for surgical planning. Since the NMT template was aligned to D99 template space and the D99 space is based on stereotaxic coordinates of the Kopf apparatus, MRI coordinates were used for surgical planning of the implants.
2.5. Implant design
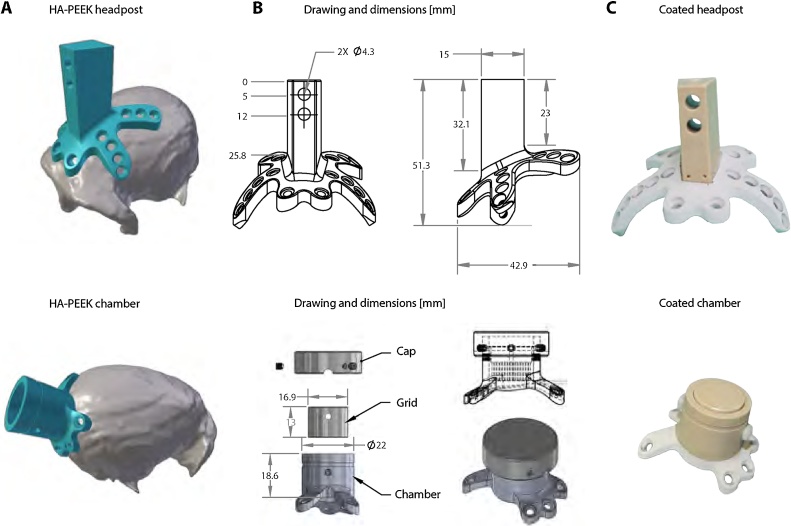
Implants were made using glass-filled PEEK which is 30–40% stronger than native PEEK. Custom-made HA-PEEK headposts were developed based on a trapezoid piece measuring 27 mm in height, 15 mm in length, 7 mm wide at the front and 11 mm wide at the back. The headpost piece was designed to rise perpendicular to the surface of the skull and to be firmly locked into a matching-shape head holder made of PEEK block with two screws at the center of the headpost (Fig. 2B). The top part of the trapezoid was replicated in a CAD file and the base was custom-designed for each animal by modeling intersections with the bone. The base included two anchor point frontally, two posterior transversal legs and two side legs. The screw holes on each leg were made to fit Thomas Recording ceramic screws type SA05 and SA06 (Thomas Recording, Giessen, Germany). Once completed, the CAD design was sent to a machine shop for manufacturing with a 5-axis CNC machine (Rogue Research, Montreal, Canada). After manufacturing, the piece was dispatched to Medicoat (http://www.medicoat.com) for HA coating.
Fig. 2.
Implant design based on the intersection with the skull surface. Implant designs for headpost (top) and chamber (bottom). A. STL files of HA-PEEK implants and rendered skull showing a tight surface intersection between the implants and the skull. B. Drawings and design features of headpost and chamber. The base of the headpost includes four legs for anchoring the implant around the circumference of the skull. The implant’s trapezoid-shaped part at the top is used to fix the animal’s head onto a head holder piece. The chamber includes a grid for electrophysiological recordings and a cap that is used to separate the craniotomy from the external environment. The holes on the legs of each of the implants were made to fit Thomas Recording ceramic screws. C. Images of the headpost and chamber before surgery showing hydroxyapatite (HA) coating.
PEEK chambers were designed with an inner diameter of 17 mm, an outer diameter of 22 mm and height of 18.6 mm (Fig. 2B). The legs supporting the chamber included three anchor points and two legs extending over the occipital ridge to avoid the potential risk of opening the mid-cranial suture. Two screw holes in the side wall of the chamber served to hold a grid for electrophysiological recordings. To prevent contamination of the interior of the chamber, a cap with stainless steel screws was used to seal the chamber.
2.6. Surgical procedures
Surgical placement of HA-PEEK implants followed the same anesthesia procedures for pre-medication and induction as described above for MRI, but with additional intravenous analgesia (alfentanil 0.4 μg/kg/min). Intravenous fluids (lactated Ringer’s solution 5 ml/kg/h), steroids (methylprednisolone 5.4 mg/kg/h) and antibiotic (cefotaxime 20 mg/kg/120 min) were also administered to help prevent dehydration, dura inflammation/cerebral swelling, and infection.
Neurosurgical techniques proceeded with a sterile Kopf pointer placed into a zero plate (Zeroing Bar Model 1750, David Kopf Instruments, CA, USA) for coordinate calibration of the anterior-posterior and mediolateral planes. The pointer was transferred into the stereotaxic frame (Kopf Model 1504, David Kopf Instruments, CA, USA) and lowered ventrally until it contacted the top of the head. The corresponding contact location was marked as the zero-reference coordinate. The pointer was then moved four centimeters anterior to the planned location for headpost implantation. The stereotaxic arm was then rotated 90° away from the animal to facilitate surgical procedures.
The headpost was then placed on top of the head to estimate and draw the trajectory of incision. A semi-circular incision was performed with a scalpel 3–5 cm posterior to the implant’s target location (Fig. 3A). Crucially, the skin flap was handled with great care, avoiding any trauma from forceps, keeping it moist, and ensuring it was not compressed in any way. The fascia was separated from the skin and both layers were kept moist with saline-soaked gauze. The temporal muscles were then separated from the skull using a bone scraper and the skull was scraped clean of remaining connective tissue (Fig. 3B).
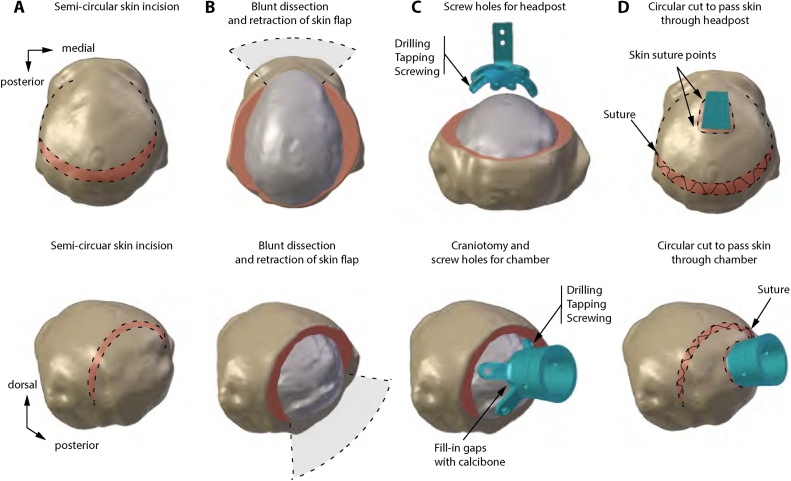
Fig. 3.
Surgical approach for anchoring MRI-compatible implants. Headpost and chamber implant surgery based on stereotaxic and MRI coordinates. A. The semi-circular skin incision shows the exposure of the fascia. B. Dissection of the fascia and exposure of the bone. The skin flap is retracted and maintained moist. Connective tissue is scraped from the skull and the temporal muscles retracted to facilitate placement of the implant. C. Drilling, tapping, and screwing allows locking the implant in place with ceramic screws. For chamber surgery, bone substitute material (Calcibon) serves to fill gaps between the chamber and the skull. D. The fascia is sutured, the skin is cut to allow passage of the implant through the skin. Finally, a subcutaneous suture is made to close the incision.
The stereotaxic arm was then repositioned to its original central position. The headpost piece was moved around the area to find the best fit on the skull. Once it was found, a manual drill was used to drill holes with a 2.5-mm diameter drillbit (Thomas Recording, Giessen, Germany). The drillbit included a depth stop to prevent accidental damage to the dura mater, while allowing space for a 2-mm thick implant leg and 2–4 mm of skull thickness. Drilling stopped once the skull was penetrated. After drilling, a 3.2-mm tap was used to thread the hole for the ceramic screws (SA05 and SA06). Surgeons took turns between screwing the left and right side of the implant to avoid biases in tightness of the implant. The process of drilling, tapping, and screwing was repeated until all holes were filled with screws (Fig. 3C). As a precaution and to counteract cerebral vasodilatation and brain swelling induced by sevoflurane, a mild hypocapnia (end-tidal carbon dioxide of 30–33 mmHg) was induced during the procedure.
Once implanted, the skin flap was unpacked from the moist gauze and the fascia realigned and sutured in place. The skin was extended over the headpost and a small (∼1 cm) incision was made to allow the post to pass through the skin. A continuous subcuticular suture was then used to close the wound (Fig. 3D) with an Aberdeen knot buried underneath the skin. The surgical procedure, from initial sedation to recovery, took approximately 6 h to complete.
For chamber surgeries, the same anesthesia procedures were used as previously described for the headpost implant. Chambers were designed to target the primary visual cortex of the right hemisphere of each animal. Once coordinates were marked, a semi-circular incision was made around the target implantation site and the skin was retracted along with the lateral muscles. The central position of the chamber was confirmed by setting the pointer to the specific coordinate location. A 15-mm wide craniotomy was then made with a trephine. Subsequently, the chamber was attached to the skull using ceramic screws (Fig. 3C). Once all ceramic screws were in place, gaps between the chamber and bone were filled with bone substitute paste Calcibone (Zimmer Biomet, U.S.A.). After the bone substitute paste dried up, the chamber was filled with sterile saline to confirm it was hermetic. In final steps, the fascia was sutured, the skin was cut and passed over the chamber, and a continuous subcuticular suture was made to close the wound (Fig. 3D).
After surgery, all animals were given buprenorphine (0.02 mg/kg, IM) and subsequently every eight hours as needed to alleviate pain and discomfort. In addition, animals also received meloxicam (0.2 mg/kg, IM) once daily for five days for pain relief. Cephalexin (15 mg/kg, IM) was also given twice daily for five days to prevent the risk of infection. Animals with post-operative complications of the incision due to picking and scratching received a protective cap (described further, see Fig. 6) and were treated with an antiseptic cream (Bepanthen) on the wound and skin margin once or twice per week until fully healed.
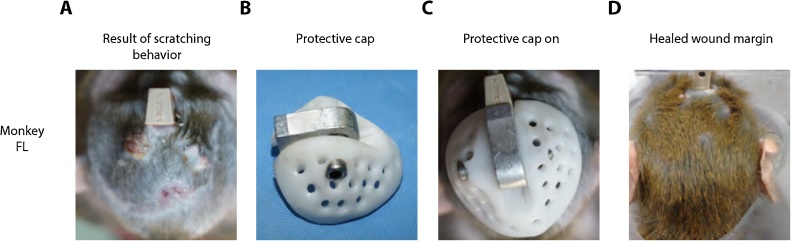
Fig. 6.
Protective cap worn after surgery to prevent wound picking.
A. Animal FL showed persistent scratching of the wound margin that prevented wound healing. B. Protective cap made out of fiber-glass material with small perforations that allow airflow around the skin margin. C. The cap protected the animal from self-inducing wounds around the implant and contributed to the overall healing of the wound margin D.
2.7. MRI data analyses
For all monkeys, a single session anatomical MRI volume (MDEFT T1 scan) was used for signal to noise ratio (SNR) measurements. The signal was measured in a spherical region of interest (ROI, n voxels = 401) placed over the frontal lobe, which included both grey and white matter voxels (signal). An equal size ROI was placed outside of the head (in background noise). The sample SNR was calculated by: SNR , where signal is the mean signal inside the brain and σ noise is the standard deviation of the background noise. SNR across the population was calculated by: SNR , where μ signal is the average signal across the population of monkeys and noise is the pooled variance in noise across the population of monkeys (n = 4). We compared the percent change in SNR: , where is the average SNR across the HA-PEEK group and is the average SNR across the Acrylic-PEEK group.
Headpost margins were measured with the digital measuring tool in MeshLab (http://www.meshlab.net). Margin size diameter was measured based on approximations of circumference from the margin. First, length measurements were taken from anterior to posterior (length) and medial to lateral (width) of the margin. The circumference was approximated by: . Further, we solve for the radius and the area under the circle to estimate the overall extend of the headpost implant (see Table 1).
Table 1.
Qualitative and quantitative measures of animals with HA-PEEK and Acrylic-PEEK implants.
| Monkey | Implant type | T1 mean signal | T1 SD signal | T1 Mean noise | T1 SD noise | Signal To Noise | T2* distortion | Margin Score index | Implant Length (cm) | Implant Width (cm) | Margin size (cm) | Implant size (cm2) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DP | HA | 5587.75 | 1390.56 | 322.49 | 91.06 | 61.36 | No | 0.16 | 2.2 | 1.5 | 5.91 | 2.78 |
| VL | HA | 7594.78 | 1227.87 | 454.43 | 131.36 | 57.82 | No | 0.16 | 2.2 | 2.0 | 6.60 | 3.47 |
| AL | HA | 5305.91 | 920.47 | 239.4 | 64.38 | 82.42 | No | 0.16 | 2.26 | 1.39 | 5.96 | 2.82 |
| FL | HA | 2076.51 | 2076.51 | 438.82 | 123.65 | 79.35 | No | 0.16 | 2.22 | 1.56 | 6.0 | 2.90 |
| DL | Acrylic | 3148.44 | 1267.91 | 414.38 | 120.64 | 26.10 | Yes | 0.83 | 6.0 | 4.2 | 13.76 | 21 |
| TL | Acrylic | 3391.46 | 982.28 | 642.53 | 245.88 | 13.79 | Yes | 0.5 | 6.3 | 6.3 | 17.52 | 24.4 |
| BR | Acrylic | 1857.516 | 502.21 | 280.18 | 86.61 | 21.45 | Yes | 0.83 | 6.1 | 5.0 | 17.19 | 23.53 |
| RC | Acrylic | 2767.736 | 727.58 | 378.81 | 109.22 | 25.34 | Yes | 1.0 | 7.8 | 5.6 | 21.33 | 36.20 |
For displaying functional MRI data, four anatomical scans were averaged, normalized, and segmented for grey and white matter. Whole-brain surfaces were rendered to obtain a semi-inflated white matter surface on which functional data were overlaid. Functional scans and analyses were conducted in four monkeys (DL and BR, both Acrylic-PEEK monkeys; DP and VL both HA-PEEK monkeys). Standard pre-processing techniques were used: slice-timing correction, spatial-smoothing (1.5 mm full width at half-maximum Gaussian kernel) and scaling of the time series at each voxel by the mean. Motion correction was also used to exclude volumes that contained motion shifts > 0.5 mm and/or rotations > 0.5° from regression analyses. Lastly, linear least-squares de-trending was used to remove non-specific variations (i.e. scanner drift).
After pre-processing, data were submitted to a general linear model with a single block-design regressor for the movie segments and the estimated motion regressors. For the movie stimulus, the regressor was generated by convolving a one-parameter gamma distribution estimate of the hemodynamic response function with the square-wave stimulus function. T-tests were performed by contrasting movie periods with baseline/blank periods effectively measuring contrast to noise ratio (CNR). Each movie segment lasted 30 s followed by a silent interval period of 30 s. Five movie segments were presented within a run for a total of 5 min per scan.
3. Results
After surgical implantation, the HA-coated PEEK headposts showed a very close fit to the skull in all monkeys (see an example for monkey VL in Fig. 4A). Similarly, the HA-PEEK chambers also showed a tight fit to the skull confirming the accuracy of the pre-surgical skull reconstruction and pre-surgical planning process (Fig. 4B). Each of these implants was finalized by closing the wound with a subcuticular suture buried inside the skin to prevent the animal from accessing it (Fig. 4).
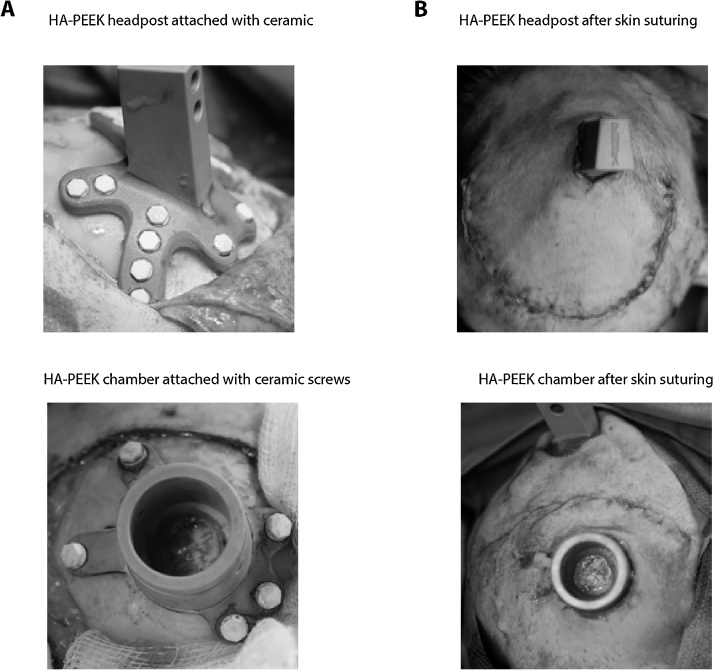
Fig. 4.
Close-fitting of implants during and after surgery.
A. Example image during surgery showing the tight fit of the HA-PEEK implants to the skull, and ceramic screw heads. B. Image after subcuticular suture to cover the skin over the implant. The chamber image (bottom) shows the central craniotomy with dura mater exposed.
After surgery, animals presented a slight swelling of the lateral muscles, which responded well to treatment with non-steroidal anti-inflammatory treatment. The incision healed well as a result of being away from the implant. Over the course of a month, the wound margin was entirely closed and healed (Fig. 5). Animal DP presented with a small (approximately 1 cm) lesion in the area as a result of scratching the surgical wound. In order to prevent wound picking and scratching, a protective cap was designed to cover the incision area (Fig. 6). Animal DP wore the cap for approximately one month allowing the wound to heal undisturbed. Animal DP was trained to willingly approach the chair and was calm while seated; as a result, we were able to treat her skin margin with Bepanthen cream every two days. By following this procedure for three weeks the wound margin healed completely. Animal FL also presented with scratching lesions and wore the cap for a period of two months after surgery due to additional complications. These included tightening of the skin around the top of the implant, which led to diminished blood supply and discoloration near the incision. The skin was treated with Bepanthen cream in the areas where wound picking and scratching caused skin damage including exposure of one of the ceramic screw heads (see Fig. 6). Skin treatment with Bepanthen was repeated for a period of two months two times a week the first month after surgery and once a week the second month after surgery. As a result of the treatment, the incision and skin surrounding the screws healed well (Fig. 6). Overall, in all four animals the skin healed very well around the incision in no more than two months after surgery. Subsequently, the skin margin around the headpost or chamber implants was monitored for signs of inflammation or infection.
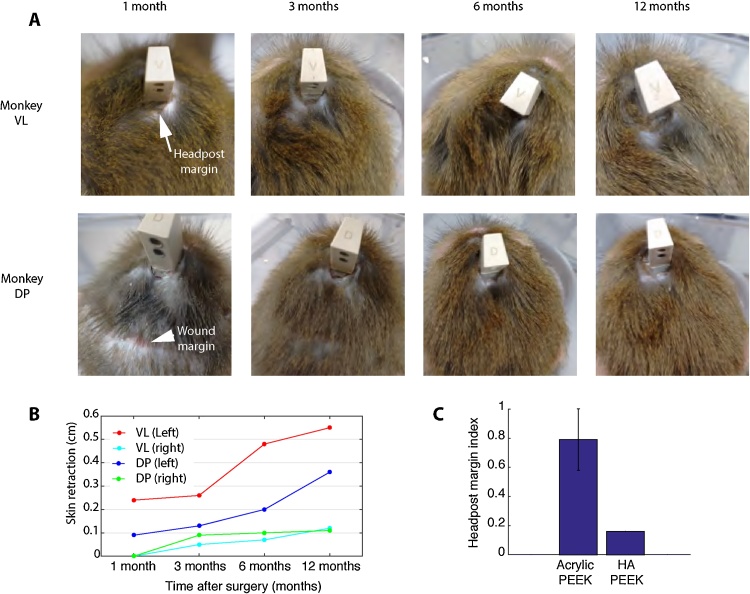
Fig. 5.
State of wound and implant margin over the course of one year.
A. Images of monkeys VL and DP showing the wound and headpost margins over the course of one year. Overall, the HA-PEEK headpost implants for both animals healed in less than 3-months. Monkey DP had a small scar tissue of approximately 1 cm in length as a result of scratching the incision (similarly to monkey FL, see Section 3 for details). However, over the course of the year, the incision remained stable and the implant margin remained free of granulation tissue or infection even without regular cleaning. B. After one year, we observed a small retraction of the skin, approximately of 0.5 cm in both animals.
3.1. HA-PEEK implants promote a healthy long-term skin margin
The health of animals with HA-PEEK headpost implants was monitored over the course of a year and their skin margins were compared to those animals with Acrylic-PEEK headposts implants. Acrylic is known to irritate the skin margin causing it to bleed and making it more prone to infection. The interface between acrylic and skin often develops granulation tissue that is highly vascularized, whereby the acrylic often prevents healing.
Comparisons between implant types were made with respect to the margin size and the overall health status of the margin (see Table 1 for a summary of all results) using a margin index. The skin condition was scored using the following criteria: dry skin – score 1, presence of granulation or connective tissue – score 2; presence of wet secretion, pus, or blood – score 3. The margins of all animals were examined visually and scored every three months and the scores were then summed across the monitoring period for an overall index representing the state of the margin. The margin index was scaled to the 0–1 range by dividing each individual score by the maximum score across animals (see Table 1). The margin indices from Acrylic-PEEK animals (mean, 0.79; ±SD, 0.20; N = 4) and HA-PEEK animals (mean, 0.16; ±SD, 0; N = 4) were significantly different among the groups (p = 0.0286, Mann-Whitney U test).
Additionally, the overall size of the headpost margin was measured for all animals in each group. Margin size measurements were taken from the surface reconstructions of the head and skin that resulted from the overall shape of the implant margin (see Section 2 and Table 1 for details). Overall the headpost margin size of HA-PEEK implant animals (mean, 6.11 cm; ±SD, 0.32; N = 4; range, 5.91–6.60 cm) were less than half the size of those with Acrylic-PEEK implants (mean, 17.45 cm; ±SD, 3.09; N = 4; range, 13.76–21.33 cm). Both, margin size and implant size measures (see Table 1) were found to be significantly different among the two groups (Mann-Whitney U test, p = 0.0286).
For HA-PEEK animals, the skin retraction which typically occurs around the headpost margin was also monitored over the course of a year. In two HA-PEEK animals, the skin retracted by about 0.5 cm on the left side of the implant (Fig. 5). For animals with HA-PEEK chambers, the skin margin presented a retraction of about 1 cm after three months with exposure of some of the screws. The area appeared also slightly swollen from time to time possibly due to occasional picking by the animal; however, the animal did not appear adversely affected. In general, the state of the chamber skin margin remained dry and healthy similarly to the headpost skin margin. For animals with Acrylic-PEEK, we did not evaluate the skin retraction from the time after surgery since the animals received their implants prior to the start of the present study. However, during the period of the study the margin size of Acrylic-PEEK animals remained the same and did not change over the course of a year.
3.2. Improvements in MRI signal and contrast to noise
T1 and T2* signal quality was compared between HA-PEEK animals and Acrylic-PEEK animals. At first glance, we noticed that the overall brightness of the brain image was decreased in monkeys with Acrylic-PEEK implants relative to HA-PEEK implants. Example images of monkey DP (HA-PEEK) and monkey BR (Acrylic-PEEK) illustrate this effect in T1 data (Fig. 7). In order to quantify the effect of implant type on MR signal quality, we measured and compared the signal-to-noise ratios (SNR) from T1 images between the groups (see Table 1 for individual SNR measures of each monkey). The population SNRs of HA-PEEK animals (signal average, 7075.14; population ±SD noise, 88.35; average SNR 80.03) were four times higher than those obtained from Acrylic-PEEK animals (signal average, 2791.28; population ±SD noise, 144.66; average SNR, 19.30). Comparisons across SNRs showed a significant difference (61.1% SNR reduction; p = 0.0286, Mann-Whitney U test) indicating that the Acrylic-PEEK implant affected the image quality in frontal regions.
In the echo-planar images or T2-weighted (T2*) data, the effects of the Acrylic-PEEK implants were even more pronounced with a significant amount of signal loss around areas of drastic spatial shifts in T2* signals (e.g. from muscle to bone or from acrylic to brain). As illustrated in Fig. 7 for monkey BR, the echo planar images were distorted and shrunk with a loss in T2* signal. Such signal losses and image distortions were present in all animals with Acrylic-PEEK implants, but not for those with HA-PEEK implants (Table 1). We examined the CNR by means of functional activation during free-viewing of natural-scene movies, which allowed to obtain reliable and consistent visual activations across all monkeys. All functional activations were corrected for false discovery rate (FDR) using a threshold at q FDR < 0.05 across all monkeys. The activation patterns involved a large-scale network consisting of areas involved in visual processing (Fig. 8A). These included low-level visual areas (V1–V4), motion-sensitive regions (MT and FST) and inferotemporal regions (TE, TEm, and TEO). Notably, a region that was exclusively activated in animals with HA-PEEK implants was the frontal eye fields (FEF). Selecting voxels from the FEF and area MT revealed blood oxygen level dependent (BOLD) signal modulation in both regions on HA-PEEK animals, but in Acrylic-PEEK animals we only observed modulation in area MT but not in FEF (Fig. 8B and C). Notably, area MT is far further from the anterior implant location than area FEF.
Based on the above measurements and comparisons between SNRs and CNRs across animal groups, we conclude that the Acrylic-PEEK implant interfered negatively with the MRI signal in frontal regions, particularly where the acrylic assembly is closer to the brain.
4. Discussion
MRI-based individual skull reconstructions enabled us to design customized PEEK implants, which were coated with hydroxyapatite at the base and were surgically implanted without the use of acrylic. Compared to Acrylic-PEEK implants, HA-PEEK implants improved the overall MRI signal magnitude and contrast to noise ratio in functional images. In addition, these HA-PEEK implants also promoted an overall healthy skin margin around the implant for more than a year contributing to the wellbeing of the involved non-human primates. In what follows, we discuss these improvements for MR imaging and skin margin development in the context of challenges of utilizing MRI-compatible materials and propose future improvements and alternatives to overcome these challenges.
4.1. Improvements for MR imaging
Both of the tested implant types (HA-PEEK and Acrylic-PEEK) allowed for measurements of BOLD-fMRI activation in awake monkeys. Movie stimuli elicited activity from a wide network of brain areas involved in processing visual information. These included early retinotopically organized visual cortical areas (V1–V4), motion-sensitive areas (MT/FST) and inferotemporal regions (TE, TEm, and TEO) implicated in face and object recognition among others (Fig. 8A). These findings are in accordance with previous fMRI studies in monkeys showing widespread visually driven activation (Logothetis et al., 1999). Activation of frontal regions was detectable in animals with HA-PEEK, but not in those with Acrylic-PEEK implants. These regions included the frontal eye fields (FEFs), a region involved in the control of saccadic eye movements and attention (Fig. 8B and C). The close proximity of FEFs to the muscle tissue and headpost acrylic assembly over the frontal lobe is the likely cause for the reduced BOLD activation in Acrylic-PEEK implanted animals. As evident from the anatomical images (Fig. 7), we found 61.1% SNR reduction in areas of close proximity to the implant in Acrylic-PEEK animals but not HA-PEEK animals where the SNR was at least four times as high (Table 1). The large volume of acrylic used to secure the implant to the skull, often applied in combination with displacement of the temporal muscles results in an air-filled gap along the margin causing abrupt changes in magnetic field susceptibility (see Fig. 7 for an example in monkey BR). Such differences in magnetic susceptibility generate changes in magnetic field gradients around the transition zones (e.g. bone/acrylic to brain tissue). The resulting spatially dependent frequency distribution of the raw MRI signal causes the corresponding signal dropout after image reconstruction, an effect similarly found for large cavities (e.g. in the orbitofrontal cortex and around the ear canals) (Goense et al., 2008). This effect is however absent in HA-PEEK monkeys explaining the overall increase in SNR as compared to Acrylic-PEEK animals. On T2* images, distortions became directly apparent in FEF (Fig. 7), precisely in the same voxels where low SNRs and lack of BOLD modulation were present (Fig. 8).
Previous monkey fMRI studies that relied on Acrylic-PEEK implants reported significant modulation of the FEF during visual stimulation (Ekstrom et al., 2008; Koyama et al., 2004; Russ and Leopold, 2015; Vanduffel et al., 2001). A number of methodological factors may account for this difference from our present results (e.g., field strength: 3 T vs. 4.7 T; the use contrast-enhanced agent MION vs. BOLD; seating position: sphinx vs. vertical; coil position, phase encoding direction, implant type and location on the head, etc.). An important factor is the distance of the coils to the brain, which in some cases has been minimized by implanting the coils directly into the skull (Janssens et al., 2012) resulting in greater SNRs as compared to the classic approach of placing coils outside of the head.
A limitation of our study is that the two implantation strategies are confounded by sex of the tested monkeys. Monkeys with Acrylic-PEEK implants were all males, while HA-PEEK monkeys were all females. The selection of female monkeys was driven by their smaller temporal muscles which allow placing surface coils closer to the brain resulting in an improved SNR. Moreover, smaller temporal muscles tend to generate fewer movement artifacts if jaw movements occur during an imaging run (Keliris et al., 2007). Consequently, it is possible that sex-related differences in the size of the temporal muscles contributed to better signal quality in the HA-PEEK monkeys. However, we argue that the pronounced lateral displacement of the temporal muscles due to the acrylic implant assembly and the resulting air-filled gap in Acrylic-PEEK animals was a significant factor affecting signal quality.
Studies using a 4.7 T vertical scanner have shown modulation of the FEF during naturalistic stimulation (Russ and Leopold, 2015) and when monkeys performed memory guided saccades (Kagan et al., 2010). However, the majority of these studies relied on contrast agents such as monocrystalline iron oxide nanoparticles (MION) to enhance CNR by a factor of 3–5 compared to the intrinsic BOLD signal (Leite et al., 2002). It is known that use of MION improve CNR and thus could “recover” functional activation from troublesome regions close to an acrylic-based implant. However, MION use comes at the cost of repeated intravenous injections and a risk of iron accumulation (Liu et al., 2013). An alternative potential solution for monkeys with acrylic implants, however limited, might be to add a ring of silicone around the skin margin to fill the air gap around the implant area, increase the BOLD signal, and thereby counteract the signal dropout. A more comprehensive and powerful solution would be to implement the use of the dynamic multi-coil technique (DYNAMITE) for magnetic field shimming of susceptibility-induced B0 distortions near the implant (Juchem et al., 2015).
In summary, our results show improvements in MR imaging quality measured by SNR and CNR in monkeys with HA-PEEK implants as compared to the classic acrylic-supported PEEK implants.
4.2. Improvement of percutaneous skin margin
Most implants used for fMRI or neural recordings in NHPs consist of a percutaneous headpost for head stabilization. Such implants, whether made of titanium or MRI-compatible PEEK, are surrounded by an open skin margin, which is exposed to the outside environment and thus susceptible to infections. This may lead to an increased chance for widespread implant infection and failure. In human medicine, PEEK implants are used successfully in spinal repairs (Ma and Tang, 2014; Williams et al., 1987); however, those implants are isolated from the exterior environment.
Here, we evaluated the overall state of both HA-PEEK and Acrylic-PEEK implants by visually monitoring the quality of the skin margins around the implant over a one-year period. In HA-PEEK monkeys, we observed that skin margins were dry, not infected nor inflamed, and lacked pink coloring; a sign of healthier skin margins (Fig. 5). On the other hand, the skin margin of Acrylic-PEEK animals consisted of granulation tissue, which was vascularized and more vulnerable to bacterial contamination. When formally scored, HA-PEEK implanted animals showed significantly better margin scores than animals with acrylic implants. Thus, our implant methodology contributed to the wellbeing of the NHPs.
In regard to acrylic implants, one fundamental issue with the use of acrylic is its porosity, which harbours microbiological contamination increasing the risk of infections particularly if the implant includes recording chambers. Thus, the aim of NHP implant refinement should be to minimise the use of acrylic. Moreover, typical Acrylic-PEEK implants consist of a large bulk of acrylic and, as a consequence, results in larger skin margins and a greater amount of tissue exposed for microbes to settle-in. Thus, another important advantage of our surgical approach with HA-PEEK implants is the reduction of the size of the skin margin. In HA-PEEK animals the skin margins around the headpost were significantly smaller (∼2 cm) compared to the skin margins in Acrylic-PEEK animals (>5 cm). The skin margins on two of the HA-PEEK animals retracted slightly (0.5 cm) after one year from surgery, and we believe they might retract even further in the future. However, the skin retraction did not adversely affect the quality of the implants and up to the time of writing (26 months after implantation) these skin margins remain healthy. In summary, HA-PEEK animals had smaller skin margins with a quality index significantly better than those with Acrylic-PEEK implants suggesting that our implant method can help to promote the wellbeing of the NHPs in neuroscience research.
4.3. Challenges and future potential improvements
4.3.1. Surgical and post-surgical procedures
Surgical procedures of the HA-PEEK implants were expedited by the precise fitting of the implant to the skull before surgery. In contrast, acrylic-based implants require building acrylic around the headpost in a layer-by-layer manner, a process that is time-consuming. In addition, the layers of acrylic require careful and skillful placement around the implant. Moreover, since the setting of acrylic is highly exothermic, it can cause damage to surrounding tissues, unless applied with adequate waiting times between layers.
Post-surgically, two animals in our HA-PEEK group initially presented with scratching and picking behavior around the skin incision. We prevented further picking and scratching by using a protective cap that was designed to cover the incision, preventing the animals from further self-injury. Both animals wore the head cap after surgery allowing the incision to heal without disturbance. Animal FL wore the cap for a period of two months after surgery as the animal faced additional complications besides wound picking and scratching. We followed the treatment procedure (as described above) and the incision, as well as the skin surrounding the screws, healed very well within a 2-month period after surgery (Fig. 6).
4.3.2. Skull reconstructions
Implants were designed from MRI-based skull reconstructions which were based on low-signal T1 values from bone structures. Since air-filled cavities and other anatomical structures might show similarly low-T1 values, selecting only bone structures for segmentation becomes a challenge. Manual segmentation was needed in order to create reliable skull reconstructions (see included dataset and scripts for skull extraction). An alternative solution to improve MRI-based skull reconstructions is to use black bone MRI (Eley et al., 2012), which relies on a lower flip angle that increases the contrast between bone and soft tissues at the expense of reduced contrast between individual soft tissues, such as grey and white matter. A more powerful solution is the use of computed tomography (CT), which offers excellent bone-to-soft tissue contrast thanks to the high attenuation of X-rays by calcified bone structures (Chen et al., 2017; Overton et al., 2017).
4.3.3. Implant design
Our implant design was based on radially extending legs that surrounded the frontal cranium (Fig. 2). Previous reports suggested that the legged design could result in skin retraction around the implant (Mulliken et al., 2015). However, similar to previous studies (Overton et al., 2017) we observed minimal skin retraction across all implanted animals within one year of assessment (Fig. 5). One potential advantage of the legless designs (Mulliken et al., 2015) is the ability to confine screws along the circumference of the chamber. Unfortunately, their reported design did not incorporate fMRI-compatibility as titanium screws were used to fix the implants to the cranium. To what extent legless carbon PEEK designs could provide the required long-term stability for headpost implants while preserving fMRI-compatibility remains, therefore, to be tested. With our legged design, we have achieved enough stability to avoid the use of any titanium material as to truly preserve fMRI-compatibility.
4.3.4. Hydroxyapatite coating
One crucial aspect of our implant design is the use of hydroxyapatite coating (HA). HA in the form of hydroxycarbonate apatite is the primary mineral component of bone tissue in mammals and is typically used for bone grafts, fillers and for coating of surgical implants in humans (Mucalo, 2015). While HA has been used extensively in human medicine, it has just begun to gain popularity in NHP neuroscience research mainly due to its osseointegration characteristics (Lanz et al., 2013; Overton et al., 2017). For our approach, we have used HA coated PEEK implants to promote osseointegration and potentially increase the strength of the PEEK material.
In regard to the implant strength, we indirectly evaluated the overall strength of implants by measuring movements during imaging. These measures were based on image displacements and deviations from normality during image acquisition, which ranged between 0.2–1.0 mm for both animal groups. Head fixations were robust and stable across all animals with or without acrylic and we believe that such robustness with the HA-PEEK implants was at least in part due to the osseointegrative properties of HA, but it might be also explained by the number of applied screws. Although at this point in our experimental timeline we cannot confirm osseointegration by assessing the crania post-mortem, we can confirm that these implants show durability and robustness similar to acrylic-PEEK and/or titanium implants.
4.3.5. Ceramic screws
Prior to surgery, skull reconstructions were used to evaluate the thickness of the cranium for screw fixations. The choice for ceramic screws is dictated by the need to avoid large MRI image distortions caused by titanium screws (Chen et al., 2017). A disadvantage of ceramic screws is that they tend to be brittle and can break, especially during implantation surgery (e.g. while torquing the screws). In order to accommodate ceramic screws for our implants, we designed implant legs with openings that incorporated the hexagonal head of the ceramic screw (see Section 2 and Fig. 4A). However, during surgery, it was not always possible to bury the screw entirely inside the leg due to slight angle imperfections between implant legs and cranial perforations. We believe that as a result, these screws with their sharp hexagonal heads might have caused discomfort and itchiness of the skin explaining the scratching and self-injury behavior observed in some of our NHPs. Thus, the use of screws with a smooth-surface head might be a better alternative, which could potentially prevent discomfort and tissue damage while in contact with the overlying skin.
4.3.6. ITAP design
Our results in four monkeys with HA-PEEK implants showed an improvement in the overall quality of the skin margins. However, in order to definitely improve the skin margin around an implant, a design must take into account the surrounding tissue. Recent developments in the treatment of human amputees have resulted in the so-called Intraosseous Transcutaneous Amputation Prosthesis (ITAP) design. The design is based on an osseointegrated prosthesis for limb replacement which is made with an additional perforated flange on the implant at the site where the skin interacts with the extruding implant. The ITAP design has been used in humans and animals and has resulted in significantly improved skin closure success and reduced infection rates (Pendegrass et al., 2006a, 2006b).
For NHP implants, developing the ITAP technique would require designing flanges customized to the target area or site of implantation. Such implant could be made with multiple angles and positions of the flange such that the flange would encompass the tissue around the head. However, implant designs with multiple angles that require small perforations for the flange could be challenging to manufacture using classic CNC machining. With advancements in 3D-printing technology, successful manufacturing of such designs might be possible. Importantly, in order to preserve MRI-compatibility, 3D-printing in PEEK material will be highly desirable (Overton et al., 2017). Currently, some companies offer 3D-printing in PEEK, but the technology and availability of PEEK 3D-printers are still in development as they require specialized high-temperature melting point spouts.
4.3.7. Alternative approaches
Studies in awake behaving monkeys require stabilization of the head during neural recording and/or functional imaging. For electrophysiology, customized titanium implants already offer a very good alternative to acrylic-based implants (Lanz et al., 2013; Overton et al., 2017). In terms of biocompatibility and osseointegration, titanium implants are probably a better choice than our HA-PEEK implants.
However, for our studies, the main goal is to conduct both functional MRI and electrophysiological measures and titanium material is not an option. Thus, we relied on robust material such as PEEK to preserve MRI-compatibility.
An alternative approach for head stabilization is the use of a head mask or head holding apparatus (Murnane and Howell, 2010; Slater et al., 2016; Srihasam et al., 2010) which avoids the use of invasive surgical procedures for headpost implantation. Such an approach is attractive from the perspective of animal welfare, however, it might require extensive training for animals to willingly cooperate for head restriction (Srihasam et al., 2010) and depending on the holding apparatus, residual instability could result in increased movement variance as compared to the headpost approach (Slater et al., 2016). Our methods, while still invasive by nature, improve image quality (without the use of contrast-enhanced agents), provide long-term rigidity and improve the welfare of animals by avoiding the use of acrylic for implantation.
5. Summary
We developed MRI based customized PEEK implants coated with hydroxyapatite which were surgically implanted onto NHP skulls without the use of acrylic for stabilization. These implants improved the overall MRI signal quality and contrast to noise ratio of functional images. Most importantly, these implants also promoted an overall healthy skin margin and were stable in the long term suggesting that our approach can contribute to the wellbeing of NHPs in neuroscience research.
Author contributions
Conceptualization, MOR and MCS; Design and Development, MOR, MCS, SF; Surgical Methods, MOR, MCS; Imaging Methods MOR and FB.; Investigation, MOR, MCS, MH; Formal Analysis, MOR; Writing – Original Draft, MOR; Writing – Review & Editing, MOR, MH, FB, SF, AT, KM, MCS, Funding Acquisition, AT, MCS; Supervision, MCS.
Acknowledgments
We would like to thank Drs. Henry Bertrand, Chris Blau, and Rocio Fernandez-Palacios-OConnor and other staff of the CBC for their help and effort during all the anesthesia procedures and training of the NHPs. We thank Prof. Nikos Logothetis and his team at MPI-BC Tübingen for implementing and teaching us on this implantation approach. We also thank Prof. Pawel Kusmierek for his helpful comments and revisions. Funded by NC3RsNC/P000940/1 and ERC OptoVision637638.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jneumeth.2018.09.013.
Contributor Information
Michael Ortiz-Rios, Email: Michael.Ortiz-Rios@newcastle.ac.uk.
Michael Christoph Schmid, Email: Michael.Schmid@newcastle.ac.uk.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Chen X., Possel J.K., Wacongne C., van Ham A.F., Klink P.C., Roelfsema P.R. 3D printing and modelling of customized implants and surgical guides for non-human primates. J. Neurosci. Methods. 2017;286:38–55. doi: 10.1016/j.jneumeth.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Ekstrom L.B., Roelfsema P.R., Arsenault J.T., Bonmassar G., Vanduffel W. Bottom-up dependent gating of frontal signals in early visual cortex. Science (80-.) 2008;321:414–417. doi: 10.1126/science.1153276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eley K.A., Mcintyre A.G., Watt-Smith S.R., Golding S.J. “Black bone” MRI: a partial flip angle technique for radiation reduction in craniofacial imaging. Br. J. Radiol. 2012;85:272–278. doi: 10.1259/bjr/95110289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goense J.B.M., Ku S.P., Merkle H., Tolias A.S., Logothetis N.K. fMRI of the temporal lobe of the awake monkey at 7 T. Neuroimage. 2008;39:1081–1093. doi: 10.1016/j.neuroimage.2007.09.038. [DOI] [PubMed] [Google Scholar]
- Hargreaves B.A., Worters P.W., Pauly K.B., Pauly J.M., Koch K.M., Gold G.E. Metal-induced artifacts in MRI. Am. J. Roentgenol. 2011;197:547–555. doi: 10.2214/AJR.11.7364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens T., Keil B., Farivar R., McNab J.A., Polimeni J.R., Gerits A., Arsenault J.T., Wald L.L., Vanduffel W. An implanted 8-channel array coil for high-resolution macaque MRI at 3T. Neuroimage. 2012;62:1529–1536. doi: 10.1016/j.neuroimage.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston J.M., Cohen Y.E., Shirley H., Tsunada J., Bennur S., Christison-Lagay K., Veeder C.L. Recent refinements to cranial implants for rhesus macaques (Macaca mulatta) Lab Anim. (NY) 2016;45:180–186. doi: 10.1038/laban.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juchem C., Umesh Rudrapatna S., Nixon T.W., de Graaf R.A. Dynamic multi-coil technique (DYNAMITE) shimming for echo-planar imaging of the human brain at 7 Tesla. Neuroimage. 2015;105:462–472. doi: 10.1016/j.neuroimage.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan I., Iyer A., Lindner A., Andersen R.A. Space representation for eye movements is more contralateral in monkeys than in humans. Proc. Natl. Acad. Sci. 2010;107:7933–7938. doi: 10.1073/pnas.1002825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keliris G.A., Shmuel A., Ku S.P., Pfeuffer J., Oeltermann A., Steudel T., Logothetis N.K. Robust controlled functional MRI in alert monkeys at high magnetic field: effects of jaw and body movements. Neuroimage. 2007;36:550–570. doi: 10.1016/j.neuroimage.2007.02.057. [DOI] [PubMed] [Google Scholar]
- Koyama M., Hasegawa I., Osada T., Adachi Y., Nakahara K., Miyashita Y. Functional magnetic resonance imaging of macaque monkeys performing visually guided saccade tasks: comparison of cortical eye fields with humans. Neuron. 2004;41:795–807. doi: 10.1016/s0896-6273(04)00047-9. [DOI] [PubMed] [Google Scholar]
- Kumar G., Narayan B. Classic Papers in Orthopaedics. 2014. Osseointegrated titanium implants: requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man; pp. 507–509. [DOI] [PubMed] [Google Scholar]
- Lanz F., Lanz X., Scherly A., Moret V., Gaillard A., Gruner P., Hoogewoud H.M., Belhaj-Saif A., Loquet G., Rouiller E.M. Refined methodology for implantation of a head fixation device and chronic recording chambers in non-human primates. J. Neurosci. Methods. 2013;219:262–270. doi: 10.1016/j.jneumeth.2013.07.015. [DOI] [PubMed] [Google Scholar]
- Leggat P.A., Smith D.R., Kedjarune U. Surgical applications of methyl methacrylate: a review of toxicity. Arch. Environ. Occup. Health. 2009;64:207–212. doi: 10.1080/19338240903241291. [DOI] [PubMed] [Google Scholar]
- Leite F.P., Tsao D., Vanduffel W., Fize D., Sasaki Y., Wald L.L., Dale A.M., Kwong K.K., Orban G.A., Rosen B.R., Tootell R.B.H., Mandeville J.B. Repeated fMRI using iron oxide contrast agent in awake, behaving macaques at 3 Tesla. Neuroimage. 2002;16:283–294. doi: 10.1006/nimg.2002.1110. [DOI] [PubMed] [Google Scholar]
- Liu G., Gao J., Ai H., Chen X. Applications and potential toxicity of magnetic iron oxide nanoparticles. Small. 2013;9:1533–1545. doi: 10.1002/smll.201201531. [DOI] [PubMed] [Google Scholar]
- Logothetis N.K., Guggenberger H., Peled S., Pauls J. Functional imaging of the monkey brain. Nat. Neurosci. 1999;2:555–562. doi: 10.1038/9210. [DOI] [PubMed] [Google Scholar]
- Ma R., Tang T. Current strategies to improve the bioactivity of PEEK. Int. J. Mol. Sci. 2014;15:5426–5445. doi: 10.3390/ijms15045426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAndrew R.M., Lingo VanGilder J.L., Naufel S.N., Helms Tillery S.I. Individualized recording chambers for non-human primate neurophysiology. J. Neurosci. Methods. 2012;207:86–90. doi: 10.1016/j.jneumeth.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucalo M.R. 2015. Hydroxyapatite (HAp) for Biomedical Applications; pp. 1–381. [Google Scholar]
- Mulliken G.H., Bichot N.P., Ghadooshahy A., Sharma J., Kornblith S., Philcock M., Desimone R. Custom-fit radiolucent cranial implants for neurophysiological recording and stimulation. J. Neurosci. Methods. 2015;241:146–154. doi: 10.1016/j.jneumeth.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murnane K.S., Howell L.L. Development of an apparatus and methodology for conducting functional magnetic resonance imaging (fMRI) with pharmacological stimuli in conscious rhesus monkeys. J. Neurosci. Methods. 2010;191:11–20. doi: 10.1016/j.jneumeth.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton J.A., Cooke D.F., Goldring A.B., Lucero S.A., Weatherford C., Recanzone G.H. Improved methods for acrylic-free implants in nonhuman primates for neuroscience research. J. Neurophysiol. 2017;118:3252–3270. doi: 10.1152/jn.00191.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendegrass C.J., Goodship A.E., Blunn G.W. Development of a soft tissue seal around bone-anchored transcutaneous amputation prostheses. Biomaterials. 2006;27:4183–4191. doi: 10.1016/j.biomaterials.2006.03.041. [DOI] [PubMed] [Google Scholar]
- Pendegrass C.J., Goodship A.E., Price J.S., Blunn G.W. Nature’s answer to breaching the skin barrier: an innovative development for amputees. J. Anat. 2006;209:59–67. doi: 10.1111/j.1469-7580.2006.00595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ B.E., Leopold D.A. Functional MRI mapping of dynamic visual features during natural viewing in the macaque. Neuroimage. 2015;109:84–94. doi: 10.1016/j.neuroimage.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi G.E., Bosshardt D.D., Lang N.P., Abrahamsson I., Berglundh T., Lindhe J., Ivanovski S., Donos N. Temporal sequence of hard and soft tissue healing around titanium dental implants. Periodontology 2000. 2015;68:135–152. doi: 10.1111/prd.12054. [DOI] [PubMed] [Google Scholar]
- Seidlitz J., Sponheim C., Glen D., Ye F.Q., Saleem K.S., Leopold D.A., Ungerleider L., Messinger A. A population MRI brain template and analysis tools for the macaque. Neuroimage. 2018;170:121–131. doi: 10.1016/j.neuroimage.2017.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater H., Milne A.E., Wilson B., Muers R.S., Balezeau F., Hunter D., Thiele A., Griffiths T.D., Petkov C.I. Individually customisable non-invasive head immobilisation system for non-human primates with an option for voluntary engagement. J. Neurosci. Methods. 2016;269:46–60. doi: 10.1016/j.jneumeth.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srihasam K., Sullivan K., Savage T., Livingstone M.S. Noninvasive functional MRI in alert monkeys. Neuroimage. 2010;51:267–273. doi: 10.1016/j.neuroimage.2010.01.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei E.B., Henriques V.A.R., Silva C.R.M., Cairo C.A.A. Production of new titanium alloy for orthopedic implants. Mater. Sci. Eng. C. 2004;24:683–687. [Google Scholar]
- Thiele A., Delicato L.S., Roberts M.J., Gieselmann M.A. A novel electrode-pipette design for simultaneous recording of extracellular spikes and iontophoretic drug application in awake behaving monkeys. J. Neurosci. Methods. 2006;158:207–211. doi: 10.1016/j.jneumeth.2006.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanduffel W., Fize D., Mandeville J.B., Nelissen K., Van Hecke P., Rosen B.R., Tootell R.B.H., Orban G.A. Visual motion processing investigated using contrast agent-enhanced fMRI in awake behaving monkeys. Neuron. 2001;32:565–577. doi: 10.1016/s0896-6273(01)00502-5. [DOI] [PubMed] [Google Scholar]
- Williams D.F., McNamara A., Turner R.M. Potential of polyetheretherketone (PEEK) and carbon-fibre-reinforced PEEK in medical applications. J. Mater. Sci. Lett. 1987;6:188–190. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.