Abstract
Interactions between metal nanoparticles and support materials can strongly influence the performance of catalysts. In particular, reducible oxidic supports can form suboxides that can decorate metal nanoparticles and enhance catalytic performance or block active sites. Therefore, tuning this metal-support interaction is essential for catalyst design. Here, we investigate reduction-oxidation-reduction (ROR) treatments as a method to affect metal-support interactions and related catalytic performance. Controlled oxidation of pre-reduced cobalt on reducible (TiO2 and Nb2O5) and irreducible (α-Al2O3) supports leads to the formation of hollow cobalt oxide particles. The second reduction results in a twofold increase in cobalt surface area only on reducible oxides and proportionally enhances the cobalt-based catalytic activity during Fischer-Tropsch synthesis at industrially relevant conditions. Such activities are usually only obtained by noble metal promotion of cobalt catalysts. ROR proves an effective approach to tune the interaction between metallic nanoparticles and reducible oxidic supports, leading to improved catalytic performance.
Tuning metal-support interaction can strongly influence the performance of a catalyst, and is thus essential for catalyst design. Here, the authors investigate reduction-oxidation-reduction treatments as a method to affect metal-support interactions of cobalt-based catalysts in Fischer-Tropsch synthesis.
Introduction
Metal particles catalyze major chemical processes, including the synthesis of bulk chemicals and transportation fuels1–4. The catalytic reactions that are involved occur mainly at the metal surface. Therefore, the surface-to-volume ratio of these particles is typically maximized through nanosizing, leading to adequate exposure of the active sites and efficient utilization of the metal5,6. Supports play a critical role in stabilizing the reactive nanoparticles but also in generating new interphase phenomena, such as electron transfer or shape rearrangement7–9. These phenomena can have ambivalent consequences for the catalytic performance such as promoting activity and selectivity or blocking active sites, which decreases the catalyst efficiency.
A renowned example of support effects occurs when reducible metal oxide supports (TiO2, Nb2O5, CeO2, etc.) are partially reduced by hydrogen spillover from the metal nanoparticle10, i.e., the so-called strong metal–support interaction (SMSI)11,12. As a result, suboxide species are generated from the support and these may migrate onto the metal nanoparticles surface13–16. In the case of cobalt catalysts for the Fischer–Tropsch (FT) synthesis, i.e., the conversion of synthesis gas (H2/CO) into ultra clean fuels and chemicals, the effect of SMSI is twofold. On one hand, the cobalt catalysts benefit from the mildly Lewis acidic character of the suboxide species, as it increases the intrinsic activity (turnover frequency, TOF) and selectivity towards C5+ products17,18. The increased TOF is believed to originate from the interface between the metal and suboxide where the CO-dissociation rate is enhanced19–21. On the other hand, the catalytic activity can be lowered by a dense overlayer of suboxide species that block the access to the active sites14. The interplay between promotion of the catalytic performance and blockage of active sites is not clear and control over the metal-support interactions may thus provide an opportunity to increase the number of accessible, promoted active sites.
The SMSI state can be reversed by oxidative treatments. However, this reversibility has been mainly studied for noble metals, while reports of base metals are scarce12,22–24. Soled et al.25 studied the impact of SMSI on H2-chemisorption for cobalt nanoparticles supported on titania. The H2-uptake was initially suppressed because of site blockage but increased significantly after a reduction–oxidation–reduction (ROR) treatment. This brought H2-chemisorption and TEM and XRD results into closer agreement, but the effect of ROR on the structure of the cobalt particles as well as its implications for catalysis remained unclear. Besides reversal of the SMSI state, ROR has been applied to regenerate deactivated catalysts through redispersion of the active metal26–29. The work on catalyst regeneration focused, however, on cobalt catalysts supported on irreducible oxides, such as Al2O3 and SiO2, in which SMSI effects do not play a role.
In this work, we investigate the possibility to tune the metal-support interactions of cobalt supported on reducible metal oxides via reduction-oxidation-reduction treatments with the aim to enhance the catalytic activity in the Fischer–Tropsch synthesis. To this end, Co/TiO2 and Co/Nb2O5 are synthesized and subjected to ROR treatments. Co on irreducible α-Al2O3 is prepared for comparison. After the first reduction, oxidation above 100 °C leads to the formation of hollow cobalt oxide particles. Upon re-reduction, the H2-uptake doubles for the reducible metal oxides due to optimization of the SMSI state and not by cobalt redispersion, while the H2-uptake decreases for α-Al2O3. The modified metal-support interaction on reducible supports drastically enhances the catalytic activity under industrially relevant FT conditions, effectively doubling the number of available active sites without changing their TOF. Modulation of the metal–support interaction via ROR is thus a promising strategy to boost the catalytic performance and to greatly improve the efficiency of metal catalysts supported on reducible oxides.
Results
Reduction
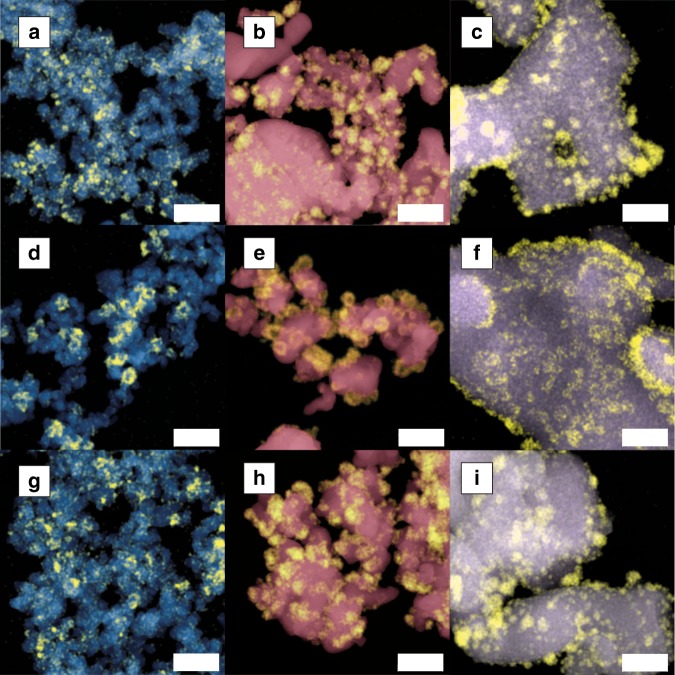
Cobalt particles supported on reducible oxides TiO2 and Nb2O5, and on irreducible oxide α-Al2O3 were prepared by incipient wetness impregnation followed by drying and thermal treatment. The nominal cobalt loading was 6 wt% and experimentally determined loadings are given in Supplementary Table 1. H2-chemisorption analysis was performed on the pristine samples, which were reduced at 350 °C prior to the measurement (referred to as R-samples). After H2-chemisorption, the samples were passivated by exposure to air at room temperature and subsequently analyzed using scanning transmission electron microscopy-energy-dispersive X-ray spectroscopy (STEM-EDX, Fig. 1a–c). The cobalt particles were rather uniformly distributed over TiO2 and Nb2O5 while the spatial distribution was more heterogeneous on α-Al2O3.
Fig. 1.
Electron microscopy images of the samples upon reduction-oxidation-reduction treatments. STEM-EDX mapping of Co/TiO2, Co/Nb2O5, and Co/α-Al2O3 at various stages of the ROR treatment. Co is depicted in yellow, Ti in blue, Nb in red, and Al in violet. a–c Samples after reduction at 350 °C and passivation (R-samples). d–f Samples after reduction at 350 °C and oxidation at 200 °C (RO200-samples). g–i Samples after reduction at 350 °C, oxidation at 200 °C, and reduction at 220 °C (RO200R-samples) and passivation. The scale bars corresponds to 100 nm. Additional electron microscopy results for these samples can be found in Supplementary Figures 1–3
H2-chemisorption and STEM-EDX results for the R-samples are given in Table 1. The theoretical H2-uptake was calculated for cobalt particles using the surface-average particle diameter derived from STEM-EDX images. This theoretical value was compared to the experimental uptake measured by H2-chemisorption. In the case of R-TiO2 and R-Nb2O5, a substantial discrepancy in H2-uptake was observed between both methodologies. This is explained by partial coverage of the metallic cobalt surface by suboxide species leading to suppressed uptake in the H2-chemisorption measurement. It is important to note that the total amount of suboxide species is probably low, because no overlayers could be detected with STEM-EDX nor with in situ TEM of Co/Nb2O5 under reducing conditions in a previous study30. No large discrepancy between the theoretical and experimental H2-uptake of R-Al2O3 was observed, because suboxides were not formed.
Table 1.
The results of cobalt particle size analyses using STEM-EDX and H2-chemisorption for the pristine samples after reduction at 350 °C (R-samples) and the samples after reduction at 350 °C, oxidation at 200 °C, and reduction at 220 °C (RO200R-samples)
| Sample | Particle diameter D[3,2]±standard deviation (nm)a | Theoretical H2-uptake (µmolH2∙gCo−1)a | Experimental H2-uptake (µmolH2∙gCo−1)b |
|---|---|---|---|
| R-samples | |||
| R-TiO2 | 11 ± 3.4 | 787 | 341 |
| R-Nb2O5 | 19 ± 10 | 437 | 265 |
| R-Al2O3 | 13 ± 6.5 | 666 | 631 |
| RO200R-samples | |||
| RO200R-TiO2 | 10 ± 3.8 | 865 | 783 |
| RO200R-Nb2O5 | 18 ± 7.9 | 470 | 657 |
| RO200R-Al2O3 | 21 ± 11 | 412 | 499 |
aDerived from STEM-EDX
bDerived from H2-chemisorption
Reduction–oxidation
After the first reduction treatment at 350 °C, the R-samples were subjected to an oxidative treatment at temperatures varying between 30 and 400 °C (referred to as RO30- to RO400-samples). The temperature-programmed reduction (TPR) profiles of pristine Co3O4/Nb2O5 and RO30-Nb2O5 to RO400-Nb2O5 are shown in Fig. 2a. The pristine sample displayed two-step, consecutive reduction from Co3O4 to CoO (280–300 °C), and from CoO to Co (300–400 °C). Samples that had been reduced and oxidized at temperatures up to 100 °C showed only one sharp reduction peak at 230 °C corresponding to the reduction of CoO. Therefore, the second reduction of these samples can be performed at substantially lower temperature than the first one. Based on the amount of consumed H2, we found that some metallic cobalt was still present on those samples, probably as a metallic core covered by a CoO layer. Further increasing the oxidation temperature to 150 °C led to broadening of the narrow peak (RO150-Nb2O5) and eventually resulted in a two-step reduction profile, associated with the formation of Co3O4 during oxidation (RO200-Nb2O5). Upon oxidation at 400 °C, the reduction profile resembled that of the pristine Co3O4/Nb2O5 sample.
Fig. 2.
The effect of the oxidation temperature on the structure of Co/Nb2O5. a Temperature-programmed reduction profiles, b schematic illustration, and c STEM-EDX images of Co/Nb2O5 after reduction and oxidation at various temperatures (30–400 °C). Co is depicted in yellow, Nb in red. The scale bar corresponds to 100 nm
The RO-samples were characterized by STEM-EDX mapping. Samples oxidized at low temperatures appeared unchanged (Fig. 2b, c). However, at oxidation temperatures of 200 °C, hollow cobalt oxide particles were observed (Fig. 1d–f). The formation of these hollow particles was ascribed to the Kirkendall effect31–33. During oxidation, an initial oxide layer forms around the metallic particle and at higher temperature, the smaller Co2+ or Co3+ cations diffuse outwards through the oxide layer faster than the larger O2- anions diffuse inwards, forming shells of cobalt oxide leaving behind empty cores. At an oxidation temperature of 400 °C, the hollow cobalt oxide particles got distorted and agglomerated.
Reduction–oxidation–reduction
The samples after an ROR cycle were characterized by H2-chemisorption (Fig. 3a). The second reduction of the ROR treatment was performed at 220 °C, whereas the R-samples had been reduced at 350 °C. For the reducible oxides, the H2-uptake significantly increased at oxidation temperatures above 100 °C. In the case of TiO2, it changed from 341 µmolH2 gCo−1 for R-TiO2 to 783 µmolH2 gCo−1 for RO200R-TiO2. Similarly, for Nb2O5 it increased from 265 µmolH2 gCo−1 for R-Nb2O5 to 657 µmolH2 gCo−1 for RO200R-Nb2O5. On α-Al2O3 however, a substantial decrease of H2-uptake was observed after ROR treatments. The degree of reduction (DOR) was determined by TPR directly after the last reduction for all R- and RO200R-samples and for RO400R-Nb2O5 (Supplementary Figure 4 and Supplementary Table 2). The DOR for the R-samples was 97–98% and decreased slightly to 84–95% after ROR. Because these changes were minor, the H2-chemisorption results were not corrected for the DOR.
Fig. 3.
The effect of ROR treatments on the exposed cobalt surface area and particle size. a Hydrogen uptake for Co/TiO2 (blue squares), Co/Nb2O5 (red circles), and Co/α-Al2O3 (violet triangles) after various reduction–oxidation–reduction treatments, as determined by H2-chemisorption. The results of the pristine samples after reduction at 350 °C (open symbols) are included at 0 on the x-axis. b–d Particle size distributions of cobalt supported on TiO2, Nb2O5, and α-Al2O3, respectively, after reduction at 350 °C (open bars) and after reduction at 350 °C followed by oxidation at 200 °C and reduction at 220 °C (solid filled bars), as determined by STEM-EDX. e Schematic illustration of the effect of the ROR treatment on cobalt supported on reducible oxides
The particle sizes of the RO200R-samples were investigated using STEM-EDX (Fig. 1g–i). The spatial distribution of the particles over the supports was not markedly different from that in the R-samples (Fig. 1a–c). Furthermore, the average size of the cobalt particles was similar to that of the R-samples, despite the significantly different values obtained by H2-chemisorption (Fig. 3b–d, Table 1). In fact, the theoretical H2-uptake now approached the values of the experimental H2-uptake.
On TiO2, the increase in H2-uptake with a similar average particle size implied that ROR did not induce substantial redispersion of cobalt. Rather, the ROR treatment resulted in a higher fraction of accessible metallic cobalt surface through a decrease of the surface coverage by suboxide species (Fig. 3e). On Nb2O5, however, the situation is more complex, because the experimental H2-uptake after ROR was higher than the theoretical one. The additional H2-uptake could be rationalized in two ways. First, redispersion of few large cobalt particles could have formed small particles, which would be undetectable by STEM-EDX. Second, hydrogen could have spilled over to Nb2O5, in line with the fact that Nb2O5 has been proven to facilitate hydrogen storage34,35. On α-Al2O3, the same Kirkendall behavior occurred at oxidation temperatures above 100 °C (Fig. 1f), but here the H2-uptake decreased on all ROR-samples due to particle growth.
ROR has been reported to regenerate deactivated catalysts through redispersion of the active metal via the Kirkendall effect26–29. However, the work on catalyst regeneration focused on cobalt catalysts supported on irreducible oxides, such as Al2O3 and SiO2, in which SMSI effects do not play a role. Furthermore, we ascribe the absence of net redispersion in our samples to different initial cobalt particle sizes. The particles in our R-TiO2 (11 nm) and R-Al2O3 (13 nm) were substantially smaller than those reported in literature (22 nm)29. According to Sadasivan et al.36, only cobalt particles above a certain critical size give rise to redispersion upon ROR treatments.
The trends observed with H2-chemisorption for the reducible oxides can be understood based on the previously discussed results. The Kirkendall effect was most likely key in tuning the SMSI during ROR, because at oxidation temperatures up to 100 °C, where this effect was not observed, the increase in H2-uptake was minor. The H2-uptake only increased significantly when the Kirkendall effect took place. On the other side of the optimum, at an oxidation temperature of 400 °C, the high temperature induced particle growth from 10 up to 15 nm for RO400R-TiO2, as determined using STEM-EDX (Supplementary Figure 5). This reduced the metallic surface area and counteracted the positive effect of the ROR treatment on the SMSI.
Further indications for the optimization of SMSI effects through ROR were found when the second reduction was performed at a higher temperature (Fig. 4). The H2-uptake decreased for the RO200R-TiO2 and RO200R-Nb2O5 samples with increasing reduction temperature. At 350 °C, the H2-uptake values returned to the values of the initial R-samples that were reduced once. Apparently, the mild temperature of the second reduction was essential to keep the concentration or mobility of the suboxide species low, while it was sufficiently high to reduce cobalt oxide to metallic cobalt. Additionally, the H2-uptakes of the R-samples at reduction temperatures of 220 and 350 °C were comparable. The substantial increase in H2-uptake for the RO200R-samples was thus not merely caused by reduction at lower temperature. In contrast, the H2-uptake of the Co/α-Al2O3 catalysts increased slightly at higher reduction temperatures.
Fig. 4.
The influence of the temperature of the second reduction on the exposed cobalt surface area. a Hydrogen uptake as a function of the second reduction temperature. Dashed bars correspond to the pristine samples after reduction at 220 or 350 °C and solid filled bars correspond to the samples after reduction at 350 °C followed by oxidation at 200 °C and reduction at 220 or 350 °C. b Schematic illustration of the effect of the reduction temperature on catalysts supported on reducible oxides
Fischer-Tropsch
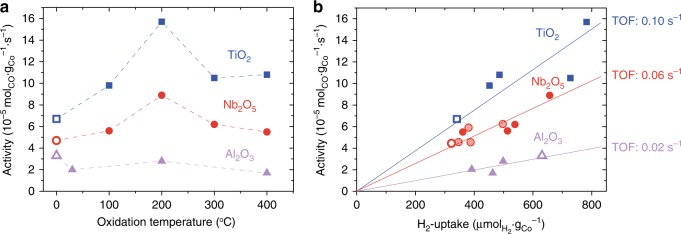
The catalytic activity of Co/TiO2 and Co/Nb2O5 in FT after ROR followed the same trend as H2-chemisorption, i.e., increased activity with the optimum at an oxidation temperature of 200 °C (Fig. 5a). Notably, the activity doubled on TiO2 and Nb2O5 as a result of the RO200R treatment and the enhancement was stable over at least 100 h time-on-stream (Supplementary Figure 6). As expected, the activity of Co/α-Al2O3 was not enhanced, indicating that the positive effect of the ROR treatment was specific for catalysts supported on reducible oxides. The C5+-selectivity for all catalysts was 85–89% and did not change significantly as a result of the ROR treatments (Supplementary Table 3).
Fig. 5.
The effect of the ROR treatment on the catalytic activity in the Fischer–Tropsch synthesis. FT was performed at 20 bar, 220 °C, H2/CO = 2 V/V, GHSV = 1000–13000 h−1, time-on-stream = 90–100 h, CO conv. = 15–34% (TiO2), 17–25% (Nb2O5), and 8–12% (Al2O3). a Cobalt-weight-based catalytic activity of Co/TiO2 (blue squares), Co/Nb2O5 (red circles), and Co/Al2O3 (violet triangles) after various reduction–oxidation–reduction treatments. The activity of the pristine samples after reduction at 350 °C (R-samples, open symbols) is included at 0 on the x-axis. b Cobalt-weight-based activity as a function of hydrogen uptake for the R-samples (open symbols) and the samples after reduction at 350 °C followed by oxidation between 30 and 400 °C and reduction at either 220 °C (filled symbols) or at 250 °C (dashed symbols); values for the turnover frequencies (TOF) have been calculated from the slopes of the lines
The observed cobalt-weight-based activities of RO200R-TiO2 (16 × 10−5 molCO gCo−1 s−1) and RO200R-Nb2O5 (8.9 × 10−5 molCO gCo−1 s−1) are exceptionally high. Compared to literature12,30,37–41, activities in this range are normally only achieved by promoting a cobalt-based catalyst with noble metals (Supplementary Figure 7). Tuning of the metal–support interactions by ROR or other pretreatments might thus provide an attractive alternative to promotion with expensive and scarce noble metals.
The ROR treatments increased the catalytic activity of Co/TiO2 and Co/Nb2O5 with respect to the R-samples. The increase in activity was proportional to the H2-uptake (Fig. 5b), indicating that the surface-specific activity or turnover frequency (TOF, based on activity and H2-chemisorption data; see SI for details) was constant and more surface sites with identical characteristics became available. The opposite was observed with irreducible α-Al2O3, where the activity decreased after ROR and less surface sites were available albeit at constant TOF. Clearly, the TOF was support-dependent and increased in the order α-Al2O3 < Nb2O5 < TiO2.
The high TOF values for the reducible oxides show that Co surface sites were promoted by TiOx or NbOx in the R- and ROR-samples. In addition, the TOF was independent of ROR, meaning that ROR did not change the nature of the promoted active sites. Therefore, the enhanced cobalt-weight-based activity of Co/TiO2 and Co/Nb2O5 implied that the number of promoted active sites increased as a result of ROR. Furthermore, the effect of the second reduction temperature on the activity is included as well in Fig. 5b for Co/Nb2O5. Reduction at 250 °C gave only a moderate increase in H2-chemisorption and a corresponding increase in activity, while reduction at 220 °C doubled the H2-uptake and activity.
Although the TOF of cobalt catalysts has been reported to be support independent37,42, recent publications have shown that the promoting effect of the support can be substantial17,18,21,30. This promoting effect on the TOF has been related to the Lewis acidity of the metal oxide: an increase in acidity leads to an increase in TOF17,18. The Lewis acid character of oxidic materials originates from exposed metal centers43, meaning that the structural arrangement and oxidation state of the ions will determine their acid strength44. Jeong et al.45 have recently reported an acidity scale based on the intramolecular charge transfer energy between organic molecules and several metal oxides (Supplementary Table 4). The values reported for the supports used in our work do predict higher TOF for TiO2 and Nb2O5 compared to Al2O3, but the comparison between TiO2 and Nb2O5 requires further analysis. As mentioned before, Lewis acidity depends on the oxidation state of the cation, thus values for lower oxidation states in suboxides (e.g., Ti3+, Nb4+, or Nb3+) gain importance for reducible metal oxides as shown in the work of Boffa et al.21,46. Because the oxidation state and structural arrangement of the relevant suboxides are not completely established, the formal Lewis acidity remains uncertain.
In summary, ROR treatments were investigated as a tool to tune metal-support interactions in supported cobalt catalysts to improve their performance in the FT synthesis. Reduction of cobalt oxide supported on reducible (TiO2 and Nb2O5) and irreducible (α-Al2O3) oxides followed by controlled oxidation led to hollow cobalt oxide particles via the Kirkendall effect. A second reduction resulted in a twofold increase in H2-uptake for the TiO2- and Nb2O5-supported samples and a decrease for the α-Al2O3-supported sample. This effect was attributed to modulation of the metal–support interactions rather than redispersion of the cobalt phase. The modified metal–support interactions greatly increased the catalytic activity in the FT synthesis, as the activity doubled on TiO2 and Nb2O5 and decreased slightly on α-Al2O3. This increased activity was caused by more accessible cobalt surface sites as revealed by the constant turnover frequency, while preserving the full promotional effect of the TiOx or NbOx species. The more efficient utilization of the cobalt and support interphase led to exceptionally active catalysts. Tailoring of metal-support interactions via ROR treatments is thus an effective method to improve the activity of metal catalysts supported on reducible oxides.
Methods
Synthesis
The samples were synthesized by incipient wetness impregnation using aqueous cobalt nitrate solutions. The precursor solutions were prepared by dissolving appropriate amounts of cobalt nitrate hexahydrate (99+%, Acros Organics) in Milli-Q water to obtain 6 wt% cobalt loading on each catalyst. The supports, TiO2 (P25, Evonik, SABET: 50 m2 g−1, PV: 0.30 cm3 g−1), Nb2O5 (pseudo-hexagonal TT-phase, CBMM, SABET: 9 m2 g−1, PV: 0.06 cm3 g−1), and α-Al2O3 (BASF, SABET: 7 m2 g−1, PV: 0.02 cm3 g−1), were sieved to a grain size of 75–150 µm. Nb2O5 was obtained from amorphous niobium oxide hydrate (HY-340, AD/4465, CBMM) by calcination in a muffle oven at 600 °C for 4 h.
For a typical impregnation, 2 g of support was pre-dried at 80 °C for 1 h under vacuum. Just before the impregnation, the vacuum was released and a pore filling amount of precursor solution was added dropwise to the support under magnetic and manual stirring. In the case of Co/TiO2, 2 g of sample was dried and heat treated in fluidized bed mode in a U-shaped glass reactor following a previously reported procedure28. The sample was first dried at 80 °C for 2 h and heat treated directly afterwards at 250 °C for 2 h. Both steps were performed in 1 L min−1 N2 upward flow with a heating ramp of 2 °C min−1. Co/Nb2O5 and Co/α-Al2O3 catalysts were dried for 12 h at 60 °C overnight in stagnant air and subsequently calcined in a fixed bed reactor under N2 flow (1 L min−1) at 350 °C for 2 h with a heating ramp of 3 °C min−1. Multiple batches of 2 g were mixed to obtain a substantial amount of sample. The obtained samples were pressed and sieved to a grain size of 75–150 µm.
Ex situ RO treatments were performed in U-shaped glass reactors. To this end, batches of 500 mg were first reduced at 350 °C for 2 h (2 °C min−1) in 120 mL min−1 of 25 vol% H2 in N2 and subsequently cooled to 100 °C. At this point, the reactor was flushed with N2 and cooled down further to 30 °C. The oxidation step followed directly after the reduction and was performed in 95 mL min−1 of 5 vol% O2 in N2 at various temperatures up to 400 °C for 2 h with a 2 °C min−1 heating ramp.
Characterization
STEM combined with high-angle annular dark-field (STEM-HAADF) and EDX analyses were performed on an FEI Talos F200X. This microscope was equipped with a high-brightness field emission gun (X-FEG) and a Super-X G2 EDX detector and operated at 200 kV. The EM samples were prepared by suspending the samples in 2-propanol (>99.9%, Sigma-Aldrich) using sonication and dropcasting the suspension on a carbon-coated Cu grid (200 mesh). The cobalt particle size was determined using the iTEM software and at least 200 particles were analyzed. The reduced and passivated samples were corrected for a 3 nm CoO layer and the particle surface averaged diameters (D[3,2]) were calculated.
H2-chemisorption was measured on a Micromeritics ASAP 2020 C using ~200 mg of sample. The catalysts were reduced in pure H2 prior to the measurement. The pristine samples were reduced for 2 h (1 °C min−1) at 350 °C, while the RO-samples were reduced at 220 °C. After reduction, the samples were evacuated, cooled to 150 °C and analyzed at that temperature. The metallic surface area and average particle diameter were calculated assuming a cobalt cross-sectional area of 0.0662 nm2, an H/Co stoichiometry of 1 and spherical cobalt particles26.
Inductively coupled plasma-optical emission spectroscopy was performed on a SPECTRO ARCOS. The samples were prepared by extraction of cobalt using aqua regia.
TPR profiles were measured using a Micromeritics Autochem 2920, equipped with a TCD detector. Typically, 100 mg sample was reduced up to 1000 °C with 5 °C min−1 in a flow of 5 vol% H2 in Ar. To determine the degree of reduction, the samples were first reduced in the TPR setup in a flow of pure H2 for 2 h at 350 or 220 °C depending of the stage in the ROR treatment and then, the TPR profile was recorded from 50 to 800 °C in a flow of 5 vol% H2 in Ar. The cobalt that had not been reduced was quantified from the areas of the peaks between 250 and 700 °C.
Fischer-Tropsch
The catalytic performance was measured using an Avantium Flowrence 16 parallel reactor setup. Stainless steel reactors (ID = 2 mm) were loaded with 75–100 mg of catalyst (75–150 µm), and diluted with 100 mg SiC (212–425 µm). Prior to reaction, the catalysts were reduced in situ for 8 h (1 °C min−1) in 25 vol% H2 in He at 1 bar. The pristine samples were reduced at 350 °C, while the RO-samples were reduced at 220 °C. After reduction, the temperature was lowered to 180 °C (3 °C min−1) and the pressure was increased to 20 bar under H2 flow. Finally, synthesis gas with H2/CO = 2 V/V (5 vol% He as internal standard) was introduced and the temperature was raised to 220 °C with 1 °C min−1. The product stream was analyzed online with an Agilent 7890A GC. The hydrocarbon products were analyzed on an Agilent J&W PoraBOND Q column connected to an FID. The permanent gasses were separated on a ShinCarbon ST column and quantified using a TCD. Definitions of the selectivity and activity, expressed as CO conversion, cobalt time yield and TOF, are included as Supplementary Methods.
Electronic supplementary material
Acknowledgments
Companhia Brasileira de Metalurgia e Mineração (CBMM), Shell Global Solutions, and the Netherlands Association for Scientific Research (NWO) are thanked for financial support. K.P.d.J. acknowledges the European Research Council, EU FP7 ERC Advanced Grant no. 338846. Robson Monteiro (CBMM), Rogério Ribas (CBMM), and Peter Munnik (Shell Global Solutions) are acknowledged for useful discussions. Wouter Lamme, Jessi van der Hoeven, Nynke Krans and Mark Meijerink (STEM-EDX), and Helen de Waard (ICP-OES) are acknowledged for the measurements indicated.
Author contributions
C.H.M. and T.W.v.D. contributed equally to the synthesis, characterization, evaluation of the catalytic performance and writing of the manuscript; K.P.d.J. contributed to experimental design, data analysis, and manuscript writing.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Carlos Hernández Mejía, Tom W. van Deelen.
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-018-06903-w.
References
- 1.Rayment T, Schlögl R, Thomas JM, Ertl G. Structure of the ammonia synthesis catalyst. Nature. 1985;315:311–313. doi: 10.1038/315311a0. [DOI] [Google Scholar]
- 2.Torres Galvis HM, et al. Supported iron nanoparticles as catalysts for sustainable production of lower olefins. Science. 2012;335:835–838. doi: 10.1126/science.1215614. [DOI] [PubMed] [Google Scholar]
- 3.Zečević J, Vanbutsele G, de Jong KP, Martens JA. Nanoscale intimacy in bifunctional catalysts for selective conversion of hydrocarbons. Nature. 2015;528:245–248. doi: 10.1038/nature16173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behrens M, et al. The active site of methanol synthesis over Cu/ZnO/Al2O3 industrial catalysts. Science. 2012;336:893–898. doi: 10.1126/science.1219831. [DOI] [PubMed] [Google Scholar]
- 5.Campbell CT, Parker SC, Starr DE. The effect of size-dependent nanoparticle energetics on catalyst sintering. Science. 2002;298:811–814. doi: 10.1126/science.1075094. [DOI] [PubMed] [Google Scholar]
- 6.Munnik P, de Jongh PE, de Jong KP. Recent developments in the synthesis of supported catalysts. Chem. Rev. 2015;115:6687–6718. doi: 10.1021/cr500486u. [DOI] [PubMed] [Google Scholar]
- 7.Lykhach Y, et al. Counting electrons on supported nanoparticles. Nat. Mater. 2016;15:284–288. doi: 10.1038/nmat4500. [DOI] [PubMed] [Google Scholar]
- 8.Divins NJ, Angurell I, Escudero C, Pérez-Dieste V, Llorca J. Influence of the support on surface rearrangements of bimetallic nanoparticles in real catalysts. Science. 2014;346:620–623. doi: 10.1126/science.1258106. [DOI] [PubMed] [Google Scholar]
- 9.Kitano M, et al. Ammonia synthesis using a stable electride as an electron donor and reversible hydrogen store. Nat. Chem. 2012;4:934–940. doi: 10.1038/nchem.1476. [DOI] [PubMed] [Google Scholar]
- 10.Karim W, et al. Catalyst support effects on hydrogen spillover. Nature. 2017;541:68–71. doi: 10.1038/nature20782. [DOI] [PubMed] [Google Scholar]
- 11.Tauster SJ, Fung SC, Garten RL. Strong metal–support interactions. Group 8 noble metals supported on TiO2. J. Am. Chem. Soc. 1978;100:170–175. doi: 10.1021/ja00469a029. [DOI] [Google Scholar]
- 12.Tauster SJ, Fung SC, Baker RT, Horsley JA. Strong interactions in supported-metal catalysts. Science. 1981;211:1121–1125. doi: 10.1126/science.211.4487.1121. [DOI] [PubMed] [Google Scholar]
- 13.Matsubu JC, et al. Adsorbate-mediated strong metal–support interactions in oxide-supported Rh catalysts. Nat. Chem. 2017;9:120–127. doi: 10.1038/nchem.2607. [DOI] [PubMed] [Google Scholar]
- 14.Zhang S, et al. Dynamical observation and detailed description of catalysts under strong metal−support interaction. Nano Lett. 2016;16:4528–4534. doi: 10.1021/acs.nanolett.6b01769. [DOI] [PubMed] [Google Scholar]
- 15.de la Peña O’Shea VA, Álvarez Galván MC, Platero Prats AE, Campos-Martin JM, Fierro JLG. Direct evidence of the SMSI decoration effect: the case of Co/TiO2 catalyst. Chem. Commun. 2011;47:7131–7133. doi: 10.1039/c1cc10318k. [DOI] [PubMed] [Google Scholar]
- 16.Xu M, et al. TiO2–x-modified ni nanocatalyst with tunable metal–support interaction for water–gas shift reaction. ACS Catal. 2017;7:7600–7609. doi: 10.1021/acscatal.7b01951. [DOI] [Google Scholar]
- 17.Johnson GR, Bell AT. Effects of Lewis acidity of metal oxide promoters on the activity and selectivity of Co-based Fischer–Tropsch synthesis catalysts. J. Catal. 2016;338:250–264. doi: 10.1016/j.jcat.2016.03.022. [DOI] [Google Scholar]
- 18.Prieto G, et al. Cobalt-catalyzed Fischer-Tropsch synthesis: chemical nature of the oxide support as a performance descriptor. ACS Catal. 2015;5:3323–3335. doi: 10.1021/acscatal.5b00057. [DOI] [Google Scholar]
- 19.Maitlis, P. M. & Zanotti, V. The role of electrophilic species in the Fischer–Tropsch reaction. Chem. Commun. 0, 1619–1634 (2009). [DOI] [PubMed]
- 20.Vannice MA. Hydrogenation of CO and carbonyl functional groups. Catal. Today. 1992;12:255–267. doi: 10.1016/0920-5861(92)85044-M. [DOI] [Google Scholar]
- 21.Boffa AB, Lin C, Bell AT, Somorjai GA. Lewis acidity as an explanation for oxide promotion of metals: implications of its importance and limits for catalytic reactions. Catal. Lett. 1994;27:243–249. doi: 10.1007/BF00813909. [DOI] [Google Scholar]
- 22.Foger K. Reactions of n-butane and neopentane on titania-supported iridium catalysts. J. Catal. 1982;418:406–418. doi: 10.1016/0021-9517(82)90323-2. [DOI] [Google Scholar]
- 23.Braunschweig EJ, Logan AD, Datye AK, Smith DJ. Reversibility of strong metal–support interactions on Rh/TiO2. J. Catal. 1989;118:227–237. doi: 10.1016/0021-9517(89)90313-8. [DOI] [Google Scholar]
- 24.Pan CJ, et al. Tuning/exploiting strong metal–support interaction (SMSI) in heterogeneous catalysis. J. Taiwan Inst. Chem. Eng. 2017;74:154–186. doi: 10.1016/j.jtice.2017.02.012. [DOI] [Google Scholar]
- 25.Soled SL, et al. Control of metal dispersion and structure by changes in the solid-state chemistry of supported cobalt Fischer–Tropsch catalysts. Top. Catal. 2003;26:101–109. doi: 10.1023/B:TOCA.0000012990.83630.f9. [DOI] [Google Scholar]
- 26.Weststrate CJ, et al. Cobalt Fischer–Tropsch catalyst regeneration: the crucial role of the kirkendall effect for cobalt redispersion. Top. Catal. 2011;54:811–816. doi: 10.1007/s11244-011-9698-6. [DOI] [Google Scholar]
- 27.Saib AM, et al. Fundamental understanding of deactivation and regeneration of cobalt Fischer–Tropsch synthesis catalysts. Catal. Today. 2010;154:271–282. doi: 10.1016/j.cattod.2010.02.008. [DOI] [Google Scholar]
- 28.Saib AM, et al. Fundamental science of cobalt catalyst oxidation and reduction applied to the development of a commercial Fischer−Tropsch regeneration process. Ind. Eng. Chem. Res. 2014;53:1816–1824. doi: 10.1021/ie4027346. [DOI] [Google Scholar]
- 29.Hauman MM, et al. Re-dispersion of cobalt on a model Fischer–Tropsch catalyst during reduction–oxidation–reduction cycles. ChemCatChem. 2012;4:1411–1419. doi: 10.1002/cctc.201200034. [DOI] [Google Scholar]
- 30.den Otter JH, Yoshida H, Ledesma C, Chen D, de Jong KP. On the superior activity and selectivity of PtCo/Nb2O5 Fischer–Tropsch catalysts. J. Catal. 2016;340:270–275. doi: 10.1016/j.jcat.2016.05.025. [DOI] [Google Scholar]
- 31.Yin Y, et al. Formation of Hollow Nanocrystals Through the Nanoscale Kirkendall effect. Science. 2004;304:711–714. doi: 10.1126/science.1096566. [DOI] [PubMed] [Google Scholar]
- 32.Chernavskii PA, Pankina GV, Zaikovskii VL, Peskov NV, Afanasiev P. Formation of hollow spheres upon oxidation of supported cobalt nanoparticles. J. Phys. Chem. C. 2008;112:9573–9578. doi: 10.1021/jp077007o. [DOI] [Google Scholar]
- 33.González-Carballo JM, Sadasivan S, Landon P, Tooze RP. Synthesis of cobalt nanodumbbells and their thermal stability under H2, H2/CO and O2 atmospheres. Mater. Charact. 2016;118:519–526. doi: 10.1016/j.matchar.2016.06.031. [DOI] [Google Scholar]
- 34.Barkhordarian G, Klassen T, Bormann R. Fast hydrogen sorption kinetics of nanocrystalline Mg using Nb2O5 as catalyst. Scr. Mater. 2003;49:213–217. doi: 10.1016/S1359-6462(03)00259-8. [DOI] [Google Scholar]
- 35.Dolci F, Di Chio M, Baricco M, Giamello E. Niobium pentoxide as promoter in the mixed MgH2/Nb2O5 system for hydrogen storage: a multitechnique investigation of the H2 uptake. J. Mater. Sci. 2007;42:7180–7185. doi: 10.1007/s10853-007-1567-0. [DOI] [Google Scholar]
- 36.Sadasivan S, Bellabarba RM, Tooze RP. Size dependent reduction–oxidation–reduction behaviour of cobalt oxide nanocrystals. Nanoscale. 2013;5:11139–11146. doi: 10.1039/c3nr02877a. [DOI] [PubMed] [Google Scholar]
- 37.Iglesia E. Design, synthesis, and use of cobalt-based Fischer–Tropsch synthesis catalysts. Appl. Catal. A Gen. 1997;161:59–78. doi: 10.1016/S0926-860X(97)00186-5. [DOI] [Google Scholar]
- 38.Sun X, et al. Manufacture of highly loaded silica-supported cobalt Fischer–Tropsch catalysts from a metal organic framework. Nat. Commun. 2017;8:1680. doi: 10.1038/s41467-017-01910-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nabaho D, Niemantsverdriet JW, Claeys M, Van Steen E. Hydrogen spillover in the Fischer–Tropsch synthesis: an analysis of gold as a promoter for cobalt–alumina catalysts. Catal. Today. 2016;275:27–34. doi: 10.1016/j.cattod.2015.12.019. [DOI] [Google Scholar]
- 40.Eschemann TO, Oenema J, de Jong KP. Effects of noble metal promotion for Co/TiO2 Fischer–Tropsch catalysts. Catal. Today. 2016;261:60–66. doi: 10.1016/j.cattod.2015.06.016. [DOI] [Google Scholar]
- 41.Lögdberg S, et al. Effect of water on the space–time yield of different supported cobalt catalysts during Fische–Tropsch synthesis. Appl. Catal. A Gen. 2011;393:109–121. doi: 10.1016/j.apcata.2010.11.030. [DOI] [Google Scholar]
- 42.Iglesia E, Soled SL, Fiato RA. Fischer–Tropsch synthesis on cobalt and ruthenium. Metal dispersion and support effects on reaction rate and selectivity. J. Catal. 1992;137:212–224. doi: 10.1016/0021-9517(92)90150-G. [DOI] [Google Scholar]
- 43.Corma A. Inorganic solid acids and their use in acid-catalyzed hydrocarbon reactions. Chem. Rev. 1995;95:559–614. doi: 10.1021/cr00035a006. [DOI] [Google Scholar]
- 44.Kreissl HT, et al. Structural studies of bulk to nanosize niobium oxides with correlation to their acidity. J. Am. Chem. Soc. 2017;139:12670–12680. doi: 10.1021/jacs.7b06856. [DOI] [PubMed] [Google Scholar]
- 45.Jeong NC, Lee JS, Tae EL, Lee YJ, Yoon KB. Acidity scale for metal oxides and Sanderson’s electronegativities of lanthanide elements. Angew. Chem. Int. Ed. 2008;47:10128–10132. doi: 10.1002/anie.200803837. [DOI] [PubMed] [Google Scholar]
- 46.Boffa AB, Lin C, Bell AT, Somorjai GA. Promotion of CO and CO2 hydrogenation over Rh by metal oxides: the influence of oxide lewis acidity and reducibility. J. Catal. 1994;149:149–158. doi: 10.1006/jcat.1994.1280. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.