Abstract
Ascites fluid is present in over 30% of diagnosed ovarian cancer (OC) cases and is produced when tumor cells metastasize into the peritoneal cavity. Because of increased interest in the immune status of ovarian cancer patients who present with metastatic ascites and the potential role that glycosylation plays with functions of the different immunoglobulins such as complement-dependent and antibody-dependent cytotoxicity largely through the activity of Fc receptors.
Immunoglobulins IgA and IgG were affinity isolated from OC ascites fluids, N-glycans were released and analyzed while site specific glycan analyses of glycopeptides was performed using tandem MS/MS with nano-LC Chip/QTOF MS. Results revealed the heterogeneity of N-glycans present in each glycosylation site of IgA1, IgA2 and IgG. Interestingly, fucosylated glycans were present in certain sites while other sites contained no fucosylated glycans. Highly sialylated N-glycans were present in other IgA sites suggesting different functional roles for each specific site.
Keywords: glycoproteomics, IgG/IgA, ovarian cancer ascites, mass spectrometry
Introduction
Ovarian cancer is a leading cause of cancer mortality among women in the United States [1] largely due to late diagnosis and metastasis of tumors. Ascites is fluid accumulating in the abdominal/peritoneal cavity and ascites reportedly occurs in over 30% of women diagnosed with ovarian cancer [2, 3]. Tumor cells metastasize from the primary ovarian tumor into the abdominal space where they find a rich environment that enables them to proliferate and thrive in spite of immune surveillance that the tumor cells are somehow able to evade. Some of the most abundant proteins in ovarian cancer ascites are immunoglobulins IgA, IgG and IgM [4]. All isotypes of immunoglobulins (Igs) are present in external secretions such as ascites with amounts and distributions different from those found in plasma [5].
Aberrant immunoglobulins reactive against tumor antigens were identified in the circulation of women with ovarian cancer [6], and immune complexes of IgG and IgA are present in OC ascites fluid [7]. Glycosylation of immunoglobulins is important for their stability, recognition and immune function. In humans, IgG has one N-linked glycosylation site in its heavy chain at N297, whereas IgA has two isoforms, IgA1 and IgA2 with two N-linked sites in IgA1 at N144 and N340 and five N-glycosylation sites: N47, N92, N131, N205 and N327 in IgA2 [8].
We have applied analytical glycoproteomic methods using mass spectrometry to analyze global and site specific glycosylation of IgG and IgA isolated from ascites fluid obtained from patients with advanced stage ovarian cancer. Since glycosylation can have a significant impact on immunoglobulin structure and function, identifying the abundance and types of N-glycans attached to immunoglobulins in malignant OC ascites will help us to better understand the local immune response to combat growth of tumor cells and help prevent metastasis.
Material and Methods
Metastatic ovarian cancer ascites were obtained from the Cancer Center Biorepository (UCDCC #183) with patient consent utilizing a UC Davis institutionally approved IRB protocol. Ascites specimens were de-identified and contained no patient identifiers before distribution to the lab for analysis. Ascites fluid samples were centrifuged at 300-450 × g (1200-1400 rpm, Beckman GPR centrifuge) for 10 min to pellet cells. Supernatants (fluid) were aliquoted and frozen for further molecular analyses (−80°C).
Isolation of IgA and IgG from ascites fluid
Twenty μl of ascites fluid was used for binding of IgG to Protein G agarose (GE Healthcare) or for binding of IgA to polyclonal anti-IgA antibodies bound to agarose (Sigma Aldrich) using spin columns (BioRad, Hercules, CA). After washing, bound IgG or IgA were eluted with 100 mM formic acid and lyophilized. Binding reactions (IgG or IgA) were performed in triplicate: one aliquot was used for total N-glycan analysis, a second aliquot used for proteomic analysis, and a third used for pronase digestion followed by site specific glycopeptide analysis. PNGase F was used to release the N-glycans for total N-glycan analysis as previously described [9, 10]. A retrosynthetic theoretical glycan library [11] was used for glycan identification with a 15 ppm mass error allowance. Five µg of each sample was analyzed by 10% SDS-PAGE (BioRad, Hercules, CA) and Coomassie blue staining.
Trypsin digestion and protein identification of the isolated IgA and IgG fractions
Trypsin digestion was performed as previously described [12]. After trypsin digestion and clean-up (ZipTip, Omix C18 100 ml tips, Agilent, Santa Clara CA), peptides were lyophilized. Lyophilized peptides were reconstituted and subjected to nano-LC Chip/TOF MS (Agilent 6520) as previously described [13]. MS and MS/MS spectra were acquired in a data dependent manner (positive ion mode). Data were converted to Mascot Generic files (MGF) using Masshunter Qualitative analysis (version B 03.01, Agilent Technologies). Protein identification was performed using X!Tandem (www.thegpm.org) using the human Swissprot database. An MS tolerance of 10 ppm and a MS/MS tolerance of 100 ppm was used. Trypsin was enzyme and up to two missed cleavage sites were allowed. Carbamidomethylation (complete), oxidation of methionine and deamidation of asparagine and glutamine (potential modifications) were modifications used.
Pronase Digestion and Glycopeptide Cleanup
Pronase E was covalently coupled to CNBr activated Sepharose beads as described previously [14, 15]. Isolated IgA or IgG were added to pronase-beads and incubated (37°C, 24 h). Glycopeptide digests were desalted and enriched using graphitized carbon cartridges as described previously [16]. Glycopeptides were eluted with 20% ACN) (v/v) and 0.05% trifluoroacetic acid (TFA) in 40% ACN, combined and lyophilized. The fraction was reconstituted in 10–20 μL water and 2 μL injected into the LC/MS.
Analysis of glycopeptides from pronase digestion
Glycopeptide solutions were analyzed using an HPLC-Chip/Q-TOF system with a 96 well plate auto sampler, HPLC-Chip Cube (Agilent Technologies, Santa Clara, CA), and Agilent 6520 Q-TOF MS detector as previously described [17]. MS and MS/MS spectra were acquired in positive ion mode with an acquisition time of 1587 ms per spectrum and acquisition rate of 0.63 spectra per second. MS data was acquired over a mass range of 400_3000 m/z, MS/MS data was acquired over 50_3000 m/z mass range. Mass calibration was enabled using reference masses of m/z 622.029, 922.010, 1221.991, 1521.972, 1821.952, 2121.933, 2421.914, and 2721.895 (ESI-TOF Tuning Mix G1969_85000, Agilent Technologies, Santa Clara, CA). Data analysis was performed on Agilent Mass Hunter (Agilent Technologies Inc.). The mass list of the glycopeptide precursor ions from the MS/MS analysis was analyzed with our in-house software GP finder for rapid glycopeptide assignment, which is an improvement on the previously developed GlycoX [18]. All glycopeptide assignments were made within a specified tolerance level (20 ppm). Each glycopeptide identified was further verified by tandem mass spectrometry (MS/MS) for detailed structural information.
Results
IgA and IgG isolation from OC ascites fluids
Immunoglobulins IgA and IgG were isolated from OC ascites fluids obtained from different individuals (Ascites 1 and Ascites 412) using Protein G Sepharose for IgG and anti-human IgA (α-chain specific) agarose for IgA. The presence of IgA and IgG in these samples was confirmed by SDS PAGE followed by protein staining (Figure 1) showing the purification of the heavy (55 kDa) and light (25 kDa) chains of IgAs from each ascites fluid, and the heavy (53 kDa) and light chains (25 kDa) of IgGs isolated from each ascites fluid.
Figure 1.
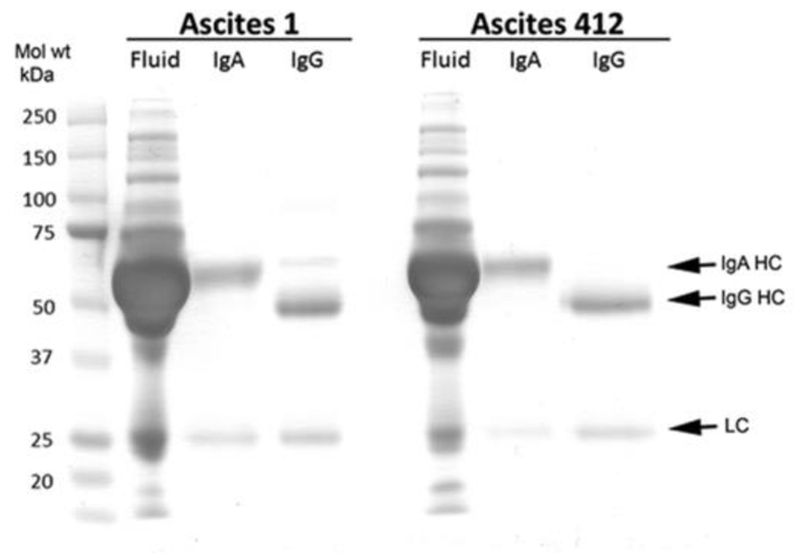
10% SDS PAGE of isolated IgG and IgA from Ascites 1 (asc1) and Ascites 412 (asc412) stained for protein. HC = heavy chain; LC = light chain. Mol. wt markers (kDa) are on left side of gel. Fluid is the original ascites fluid
N-glycan analysis of IgA and IgA isolated from two OC ascites fluids
N-linked glycans were enzymatically released (see methods) from purified IgA and IgG isolated from the two ascites samples (asc1 and asc412). Total N-glycan analysis identified 41 different N-glycans present in IgA and 34 different N-glycans in IgG from the four samples (Supplemental Tables S1 and S2). N-glycan compositions are shown according to the following order: H represents hexose, N represents HexNac, F represents fucose and S represents sialic acid, followed by the number of each type of glycan (Supplemental Tables S1 and S2). The top 15 most abundant N-glycans present in the affinity purified IgA samples from asc1 and asc412 are shown in Figure 2A by order or highest abundance. They were mostly fucosylated, sialylated biantennary and bisected N-glycans, H5N5F1S2, H5N4F1S2, H5N5F1S1, H5N4S1, H3N5, H5N4F1S1, H4N5F1, H3N5F1, H4N5S1, H5N4S2, H5N5S1, H4N5, H5N5F1, H4N5F1S1, and H5N2, which. The combined percentage of the top 15 N-glycans for asc1 is 69.37% and 68.95 for asc412 showing similar results for N-glycans from the two ascites samples with some differences. Since mass spectrometry analysis is unable to distinguish the different types of hexose (mannose, glucose or galactose), structures presented are based on glycan biology and experience with glycan structural analysis by MS, the glycan structures, which have been validated [19].
Figure 2.
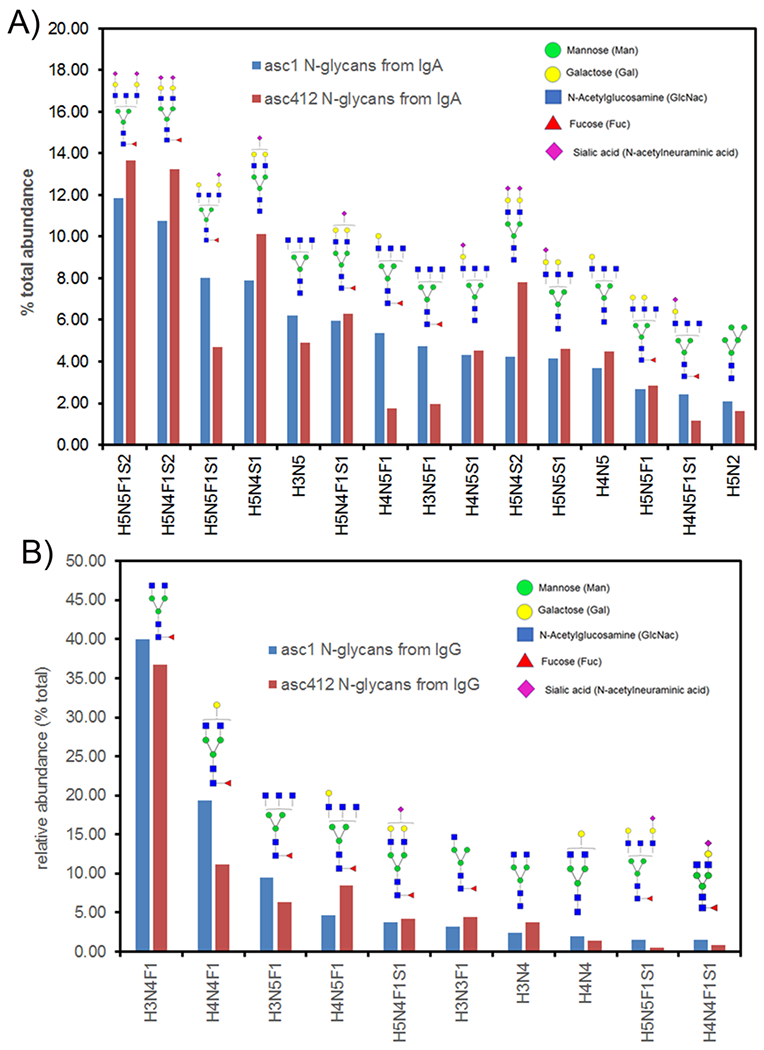
N-glycans identified in IgA and IgG from OC ascites fluid. A) Top 15 N-glycans from IgA isolated from asc1 and asc412 fluids, organized by abundance; B) Top 10 most abundance N-glycans in IgG isolated from asc1 and asc412 fluids. Blue bars represent N-glycans from asc1; red bars represent those from asc412. Annotated N-glycan structures are shown with mannose (green circle); galactose (yellow circle); GlcNac (N-acetyl glucosamine)(blue square); fucose (red triangle) and sialic acid (S)(purple diamond).
Comparison of N-glycans isolated from IgG isolated from the two ascites samples (Supplemental Table S2) showed that there were fewer and less heterogeneous N-glycans in IgG compared with IgA, likely due to the presence of a single N-glycan sites in the IgG heavy chain versus more sites in IgA1 and IgA2. The top 10 N-glycans in IgGs identified in asc1 and asc412 samples (Figure 2B)were H3N4F1, H4N4F1, H3N5F1, H4N5F1, H5N4F1S1, H3N3F1, H3N4, H4N4, H5N5F1S, and H4N4F1S1. The top two N-glycans in IgG were H3N4F1 and H4N4F1, which comprised 59.40% and 47.90% of the total N-glycans for asc1 and asc412 respectively showing the predominance of these glycans in ascites IgG and showing little variability in IgG N-glycans from OC ascites. H3N4F1, H4N4F1, H4N5F1, and H5N4F1 are all core fucosylated glycans commonly identified on IgG [20, 21].
Specific sites of glycosylation and heterogeneity of glycans attached to IgA and IgG sites were identified in ascites fluid samples
Analysis of site specific glycosylation of glycopeptides was performed by pronase digestion [14, 15] followed by tandem MS/MS using nanoLC Chip/QTOF MS [17]. This method digests the protein with pronase, a mixture of proteinases. The protein is completely digested except for the area of the protein where glycans are attached since they protect the region of the protein from protease digestion. Lyophilized glycopeptides after SPE with graphitized carbon affinity chromatography were reconstituted and analyzed for site specific glycosylation by nano-LC Chip/qTOF MS.
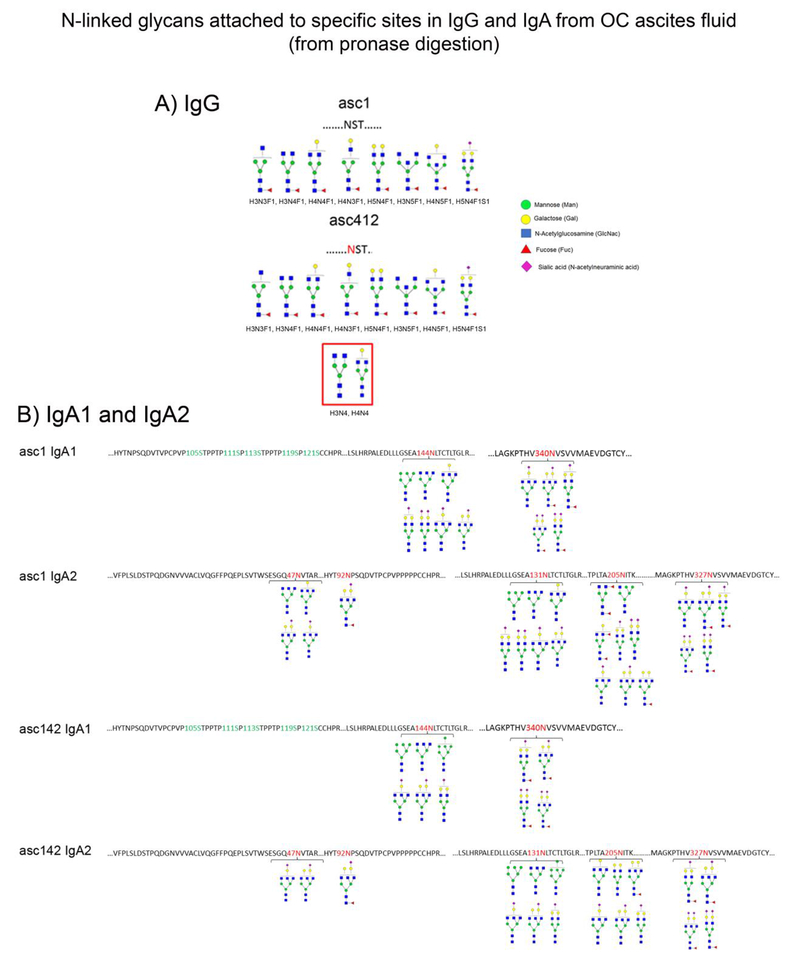
Results from glycomic site-specific analysis of ascites IgG by tandem MS/MS are shown in Figure 3A (shown as glycan structures). IgG1 and IgG2 each have one N-linked glycosylation site, located in the constant region of the heavy gamma chain [22]. Analysis of IgG isolated from Ascites 1 (asc1) showed 8 different glycans attached to single site N297: H3N3F1, H3N4F1, H4N4F1, H4N3F1, H5N4F1, H3N5F1, H4N5F1, H5N4F1S1, all core fucosylated with 6 complex, 2 bisected and one sialylated N-glycan present (Figure 3A, top). N-glycans attached to IgG site N297 in asc412 (Figure 3A, bottom) had the same eight N-glycans, but also contained two non-fucosylated glycans, H3N4, H4N4 (red box).
Figure 3.
N-glycans from site specific analyses of IgG and IgA isolated from asc1 and asc412 ascites fluids. Peptides were digested by pronase followed by site specific glycan analysis using nLC Chip/qTOF MS. A) N-glycans identified in site N297 in IgG heavy chain from asc1 and asc412. Red box identifies non-fucosylated biantennary N-glycans in asc412; B) N-glycans identified in specific N-glycan sites in IgA1 and IgA2 isolated from OC asc1 and asc412. N-glycan sites are indicated in the sequence by red numbers for N144, N340 in IgA1 and N47, N92, N131, N205 and N327 in IgA2 for each ascites fluid. Structures of N-glycans identified by tandem MS/MS and based on composition are indicated below each glycan site in IgG, IgA1 and IgA2 from asc1 and asc412.
IgA glycan site identification in ascites fluid
IgA has two isoforms, IgA1 and IgA2. IgA1 has two N-glycosylation sites: N144 and N340 and IgA2 has five N-glycosylation sites: N47, N92, N131, N205 and N327. Different groups of N-glycans were identified attached to each site (Figure 3B). Interestingly the glycans attached to N144 in both asc1 and asc412 did not contain any core fucoses, whereas the second site in IgA1, N340 contained mostly N-glycans with core fucose. Both sites contained sialylated glycans with the second site at N340 highly sialylated with multiple sialic acids attached to bi and tri antennary glycans. There was heterogeneity in N-glycans identified as attached to IgA1 site N144, which included a high mannose N-glycan, Man6 (H6N2) identified attached as attached to IgA1 at N144 in both ascites fluids. Glycans H5N4 and H5N5S1 were found to be attached to IgA1 N144 from asc1, but not to the same site in asc412. Another high mannose Man7 (H7N2) was found attached to N144 in IgA1 from asc412 and not to the same site in asc1. For the second N-linked site in IgA1, N340, four of the five N-glycans identified attached to this site in asc1 (H5N4F1S1, H5N5F1S1, H5N5F1S2, H5N4F1S2) were also identified in asc412. There was an additional N-glycan, H4N5F0S1 attached to N340 in asc1.
In IgA2, where there were five N-linked sites identified (N47, N92, N131, N205, N327) (Figure 3B) with greater heterogeneity observed. Interestingly, IgA2 does not contain the hinge region, which makes it less flexible than IgA1, which has the hinge region. There were two sites, N47 and N97 nearthe N-terminus (Figure 3B). The first site, N47 contained N-glycans without core fucose, similar to the N144 site in IgA1. Asc1 contained two other glycans, H3N5, H4N5 that were not identified in asc412 N144. One of these two glycans, H3N5 was also detected in IgA1, in the N144 site showing similarity between these sites in the different IgA isoforms. The nearby site at N92 was determined to contain only one N-glycan H5N5F1S1, which was present in asc1 and asc412, showing conservation and specificity of this fucosylated and sialylated N-glycan for this particular site. The two N-glycans sites identified in the N-terminus of IgA2 were not present in IgA1, but the first site, N47 does show a particularly strong similarity to the types of glycans identified in the later N-glycan sites, N144 in IgA1 and N131 in IgA2, possibly showing evolutionary genetic duplication of this site. N-linked site N131 in IgA2 contained similar N-glycans, all without any core fucose and about 50% sialylated, with some double sialylated in asc1. The next site, N205 showed the most heterogeneity between the two ascites fluids with glycans partly fucosylated and with an antennary fucose in asc1. Multiple sialic acids also identified in the same site, although the fucose and sialic acid were not always on the same glycan, but on different glycans, which could change their charge and recognition by other molecules. There were two glycopeptides with the same amino acid sequence in IgA1 and IgA2. One sequence LSLHRPALEDLLLGSEANLTCTLTGLR (underlined N indicates site of N-glycan attachment) that were located at N144 in IgA1 and N131 in IgA2 and a second glycopeptide sequence GKPTHVNVSVVMAEVDGTCY was located at N340 in IgA1 and N327 in IgA2 (Figure 3B). Because the amino acid sequence is the same for the glycopeptide it was impossible for us to know which IgA (IgA1 or IgA2) the N-glycans were attached to, so the N-glycans reported might have been attached to either one or the other site or to both, which is why the glycans listed at these sites are the same for each ascites fluid (asc1 and asc412). Furthermore, the N-glycans attached to the sites in asc1 were slightly different for asc412. Identified at the asc1 N144/N131 site in asc1 IgA1 and IgA2 were N-glycans H6N2, H3N5, H4N5, H5N4S1, H5N4S2, H4N5S1, H5N5S1 (Figure 3B, top two panels), whereas in N-glycans attached to these sites in asc142 were identified as H6N2, H3N5, H7N2, H4N5S1, H5N5, H5N4S1 (Figure 3B, bottom two panels).
Examples of site specific N-glycan analysis and different N-glycans attached to the same glycan site analyzed by tandem MS/MS analysis from pronase digested peptides
Examples of the tandem MS/MS analysis of specific N-glycan sites in IgA1 and IgA2 isolated from the two ascites fluids (asc1 and asc412) are shown in Figure 4A-E. Figure 4A shows tandem CID MS/MS fragment results from a bisected glycan H3N5 N-glycan attached to either or both N144 in IgA1 or N131 in IgA2 from both ascites fluids since these sites share the same amino acid sequence (Figure 3B). A second N-glycan (H5N4S1), which was also identified attached to the same amino acid sequence (N144 in IgA1 and N131 IgA2) in both ascites fluids, is shown in Figure 4B thus showing how tandem MS/MS identified two different N-glycans attached to the same amino acid sequence. Unfortunately, we cannot tell if these glycans are attached to IgA1 or to IgA2 because the sequences are identical for this glycopeptide so more peptide sequence would be needed to distinguish N-glycans attached to this site in each IgA isoforms (IgA1 or IgA2).
Figure 4.
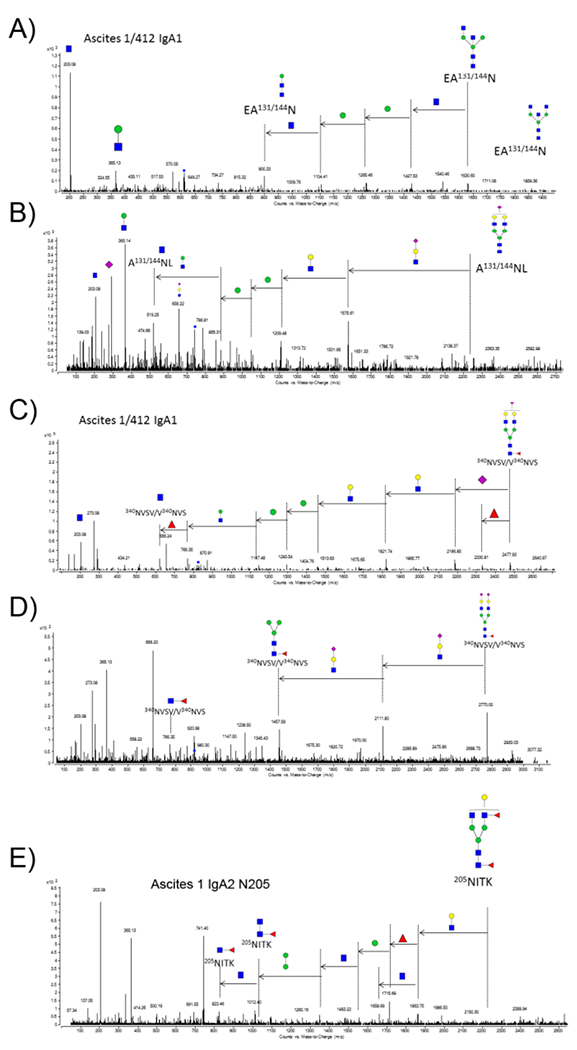
Tandem MS/MS analysis of N-glycans produced by pronase digestion. A) MS/MS analysis of N-glycan H3N5F0S0 attached to the same IgA1 site (N131/144) in both ascites fluids (asc1 and asc412); B) MS/MS analysis of a different N-glycan H5N4F0S1 attached to the same site; C) MS/MS analysis of N-glycan H5N4F1S1 attached to N340 in IgA1 in both ascites fluids; D) MS/MS of N-glycan H5N4F1S2 attached to N340 in IgA1 in both ascites fluids; E) MS/MS of N-glycan H4N4F2S0 attached to glycan site N205 in asc1 IgA2, but not detected in asc412 IgA2.
The next tandem MS/MS figure (Figure 4C) shows the presence of N-glycan H5N4F1S1 attached at another N-glycosylation site in IgA1 (N340), which shared the same amino acid sequence in IgA2 at N327. A second N-glycan H5N4F1S2 is shown by tandem MS/MS attached to the same peptide sequence (Figure 4D). Both glycans are biantennary with a core fucose attached. CID fragmentation identifies one as having one sialic acid (Figure 4C) and the other having two sialic acids (Figure 4D). The peptide sequence is the same for both glycopeptides, with the difference being the presence of the second sialic acid on the larger glycopeptides (2770 m/z for the larger glycopeptide (Figure 4D) versus 2477.93 m/z for the smaller glycopeptide with the single sialic acid attached (Figure 4C). In this way, the N-glycans attached to each site are separately identified.
A tandem MS analysis of one of the N-glycopeptides produced by pronase digestion shows the tandem MS/MS analysis of H4N4F2 (Figure 4E), a glycan that was attached to IgA2 site N205 in the asc1 fluid (Figure 3B). This particular glycan contained the sialyl Lewis X (SLeX) structure with a core and antennary fucose and was identified in asc1, but not identified in the same site in asc412.
Proteomic analysis of isolated IgA and IgG fractions
Proteomic analysis of the IgA and IgG isolated from ascites was performed and verified the presence and subtypes of IgG and IgA(Supplemental Table S3). The affinity purified IgG sample isolated from asc1 contained IgG1, IgG2 and IgG3 gamma chain C region. Ig gamma-1 chain C region was the most predominant protein identified in this sample. IgG light chains kappa and lambda were identified, and IgA1 was also identified in this sample. Affinity purified IgG fraction from asc142 mainly contained unique peptides from the Ig gamma C-chain regions from IgG1, IgG4 and IgG2 and kappa and lambda light chain regions.
Proteomic analysis of IgA isolated by immunoprecipitation from asc1 identified mainly Ig alpha-1 chain C region, Ig kappa chain C region, Ig mu chain C region, with unique peptides also identified from serum albumin, Ig gamma-3 chain C region, Ig mu heavy chain disease protein, Ig lambda-2 chain C regions, and Ig gamma-1 chain C region and interestingly, polymeric immunoglobulin receptor (PIgR). The IgA samples isolated from asc412 contained IgA2, also contained IgG1 along with other identified glycosylated proteins, haptoglobin, clusterin, Apo-J, fibrinogen beta chain and alpha-1-antitrypsin. This proteomic analysis of the affinity purified IgG and IgA from the ovarian cancer ascites fluid samples show that these samples were enriched for IgA and IgG, but that other glycosylated proteins were also present. These additional glycosylated proteins could have contributed to the overall total N-glycans profiled for each fraction, which is why it is so important to analyze glycosylation of specific glycopeptides to identify each glycosylation site and glycan heterogeneity at each site.
Discussion
Immunoglobulins IgG and IgA were isolated from ascites fluid samples obtained from different patients using affinity methods and further analyzed for specific protein sites and glycan heterogeneity at each site, thus showing our ability to identify protein sources of N-glycosylation in OC ascites fluid and assess the heterogeneity of N-glycans attached to each site. Furthermore, since IgG and IgA are among the most abundant glycosylated proteins in ascites fluid [23], N-glycans attached to these proteins are likely a source of the most abundant N-glycans being measured in total N-glycan analysis of OC ascites fluid as previously reported [24] . These results demonstrate our ability to identify N-glycans attached to some of the major sources of N-glycosylation (IgG and IgA) in OC ascites.
Glycosylation of human IgA1 is about 6% to 7% of the IgA molecular mass and 8% to 10% of the mass of human IgA2 myeloma proteins [25], largely due to the greater number (5 sites) in IgA2 and in IgA1. We observed that consistency in the type of N-glycans attached to specific sites in IgA1 and IgA2. By specific site analysis (Figure 3B), more fucosylated sites were detected in asc1 IgA1 site N340 and IgA2 sites N205 and N327 and also in asc412 IgA1 sites N47, N340 and N327 in IgA2, whereas in both ascites samples non-fucosylated and high mannose N-glycans were more present in IgA1 site N144 and IgA2 sites N47 and N1131. Sialylated N-glycans were identified throughout the glycosylation sites in IgA1 and IgA2 in both ascites samples with all N-glycans highly sialylated and fucosylated in site N327. This consistency of non-fucosylated glycans attached to N144 and fucosylated glycans attached to N340 in both asc1 and asc142 may have functional consequences for IgA1. Consistently, only one glycan, H5N5F1S1 was attached to N92 in both ascites IgA2. Endo et al. reported a small amount of high mannose type glycans in IgA [26]. We observed high mannose (Man6 and Man7) in N144 of IgA1 and N131 of IgA1 from both OC ascites sample. Glycosylation of IgA1 and IgA2 is likely to be important for IgA function such as mediating phagocytosis and other activities of neutrophils, monocytes, macrophages or dendritic cells and can downregulate the release of inflammatory cytokines [27]. Glycosylation of IgA in OC ascites may affect how it forms immune complexes without inciting a strong inflammatory response and serve to provide a protective environment for tumor cells to evade immune surveillance.
Heterogeneity due to glycosylation of IgG and IgA
There are reportedly 36 possible N-glycan structures for IgG showing the possible heterogeneity in this protein [28]. Only biantennary or bisected N-glycans are attached to IgG, which are largely core fucosylated with some sialylation [28]. N-glycans in IgG often contain core fucosylation [29] which may also restrict the type of glycosylation. It has been reported that approximately 96% of all neutral IgG glycans contain core fucose [20]. However, if core fucosylation is missing an enhancement of ADCC can occur [29]. Interestingly in our site-specific analysis of IgG isolated from ascites fluids, two N-glycans H3N4F0S0 and H4N3F0S0 in IgG from ascites412 were identified that did not contain a core fucose (Figure 3A), so it is possible that IgG containing these non-fucosylated glycans might have higher ADCC activity in OC ascites fluid.
IgA1 has 2 glycosylation sites, but also has multiple O-linked sites in its hinge region (not analyzed in this study) that may also be important for IgA1 stability since this region is labile to protease activity. These O-linked sites will add to the heterogeneity of the different IgA1 isoforms that likely translates into different functional consequences. In contrast, IgA2 has 5 N-linked glycosylation sites, but no hinge region with O-linked glycans. Already, we can see that certain N-glycans sites in IgA1 and IgA2 show a preference for non-fucosylated or fucosylated glycans. There is a high degree of sialic acids in IgA1 and IgA2, which would give this molecule a mostly negative charge, which would also impact structure, recognition by other molecules and cells.
Types of glycosylation
Sialyl Lewis X and Lewis X antigens have been implicated as cancer antigen epitopes [30, 31]. Both glycan epitopes were specifically identified in one site (N205) on asc 1 IgA2 using our site-specific MS/MS pronase digestion method (Figure 3B) whereas we were unable to detect this type of glycosylation in our total glycan profile showing why it is important to perform site specific glycan analysis on individual proteins in ascites fluid. These specific glycan epitopes on Ig alpha-2 chain C region have been reported to be elevated in breast cancer plasma [30]. SLeX and LeX N-glycans were recently reported on IgA, IgM, IgG and other plasma proteins [19]. Of biological interest is the important interaction between SLeX and E-selectin in cancer and metastasis [32], especially since there is increased SLeX containing glycans in tumor antigens [33] that may promote metastasis. Selectins are molecules that contain a type C lectin domain at their N-termini which recognizes sialylated, fucosylated carbohydrate ligands of the sialyl Lewis X (sLex) type [34]. How SLeX and LeX on IgA might interact with E-selectins in ascites fluid or on endothelial cells and either inhibit or promote metastasis remains to be determined.
Conclusion
How the immune system adapts to the presence and growth of tumor cells in ascites fluid remains to be completed elucidated, but more analytical analysis of the glycosylation of immunoglobulins IgG and IgA can help us to better understand immune surveillance and evasion of OC tumor cells and possible metastasis of these cells to other organ sites. Through careful analysis of glycopeptides obtained from purified IgG and IgA isolated from two different ovarian cancer ascites fluids we performed total glycan profiling and N-glycan analysis of specific glycosylation sites in IgG and IgA from OC ascites. Since glycosylation is highly important for function of immunoglobulins, N-glycan profiling of specific glycans sites in IgG and IgA can possibly help us to better understand how certain glycan structures might contribute to the stability and function of these immunoglobulins in metastatic ascites fluid and perhaps how glycosylation of these molecules could impact the growth and survival of ovarian cancer tumor cells.
Supplementary Material
Acknowledgements
We acknowledge the technical assistance from Camille Rodriguez who assisted with the affinity IgA and IgG purification from the ascites fluid. Funding for the project was provided by the Ovarian Cancer Research Foundation (OCRF)(Miyamoto) and by privately donated funds to the UC Davis Cancer Center (Leiserowitz) and NIH R01GM049077 (Lebrilla). Patient samples were acquired with the assistance of the UC Davis Cancer Center Biorepository (Dr. Regina Gandour Edwards, Director).
Abbreviations
- asc
ascites
- F
fucose
- H
hexose
- IgA
immunoglobulin A
- IgG
immunoglobulin G
- Igs
immunoglobulins
- Man
mannose
- N
hexNac
- OC
ovarian cancer
- PIgR
polymeric immunoglobulin receptor
- S
sialic acid
- SLeX
sialyl Lewis X
Footnotes
Conflict of Interest Statement
All authors have no financial or commercial conflicts of interest.
References
- [1].Siegel R, Naishadham D, Jemal A, Cancer statistics, 2013. CA Cancer J Clin 2013, 63, 11–30. [DOI] [PubMed] [Google Scholar]
- [2].Ahmed N, Stenvers KL, Getting to know ovarian cancer ascites: opportunities for targeted therapy-based translational research. Front Oncol 2013, 3, 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Puiffe ML, Le Page C, Filali-Mouhim A, Zietarska M, et al. , Characterization of ovarian cancer ascites on cell invasion, proliferation, spheroid formation, and gene expression in an in vitro model of epithelial ovarian cancer. Neoplasia 2007, 9, 820–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Elschenbroich S, Ignatchenko V, Clarke B, Kalloger SE, et al. , In-depth proteomics of ovarian cancer ascites: combining shotgun proteomics and selected reaction monitoring mass spectrometry. J Proteome Res 2011, 10, 2286–2299. [DOI] [PubMed] [Google Scholar]
- [5].Woof JM, van Egmond M, Kerr MA, in: Mestecky J, Lamm ME, Strober W, Bienenstock J, et al. (Eds.), Mucosal Immunology, Elsevier, London: 2005, pp. 251–265. [Google Scholar]
- [6].Gercel-Taylor C, Bazzett LB, Taylor DD, Presence of aberrant tumor-reactive immunoglobulins in the circulation of patients with ovarian cancer. Gynecol Oncol 2001, 81, 71–76. [DOI] [PubMed] [Google Scholar]
- [7].Silburn PA, Khoo SK, Daunter B, Hill R , et al. , Types of immune complexes in the ascitic fluid of women with carcinoma of the ovary. Int Arch Allergy Appl Immunol 1983, 71, 219–223. [DOI] [PubMed] [Google Scholar]
- [8].Mestecky J, Moro I, Kerr MA, Woof JM, in: Mestecky J, Lamm ME, Strober W, Bienenstock J , et al. (Eds.), Mucosal Immunology, Elsevier, London: 2005, pp. 153–181. [Google Scholar]
- [9].Chu CS, Ninonuevo MR, Clowers BH, Perkins PD, et al. , Profile of native N-linked glycan structures from human serum using high performance liquid chromatography on a microfluidic chip and time-of-flight mass spectrometry. Proteomics 2009, 9, 1939–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ruhaak LR, Taylor SL, Miyamoto S, Kelly K, et al. , Chip-based nLC-TOF-MS is a highly stable technology for large-scale high-throughput analyses. Anal Bioanal Chem 2013, 405, 4953–4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kronewitter SR, De Leoz ML, Strum JS, An HJ, et al. , The glycolyzer: automated glycan annotation software for high performance mass spectrometry and its application to ovarian cancer glycan biomarker discovery. Proteomics 2012, 12, 2523–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Miyamoto S, Ruhaak LR, Stroble C, Salemi MR, et al. , Glycoproteomic Analysis of Malignant Ovarian Cancer Ascites Fluid Identifies Unusual Glycopeptides. J Proteome Res 2016, 15, 3358–3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wu S, Grimm R, German JB, Lebrilla CB, Annotation and structural analysis of sialylated human milk oligosaccharides. J Proteome Res 2011, 10, 856–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Dodds ED, Seipert RR, Clowers BH, German JB, Lebrilla CB, Analytical performance of immobilized pronase for glycopeptide footprinting and implications for surpassing reductionist glycoproteomics. J Proteome Res 2009, 8, 502–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Clowers BH, Dodds ED, Seipert RR, Lebrilla CB, Site determination of protein glycosylation based on digestion with immobilized nonspecific proteases and Fourier transform ion cyclotron resonance mass spectrometry. J Proteome Res 2007, 6, 4032–4040. [DOI] [PubMed] [Google Scholar]
- [16].An HJ, Peavy TR, Hedrick JL, Lebrilla CB, Determination of N-glycosylation sites and site heterogeneity in glycoproteins. Anal Chem 2003, 75, 5628–5637. [DOI] [PubMed] [Google Scholar]
- [17].Nwosu CC, Seipert RR, Strum JS, Hua SS, et al. , Simultaneous and extensive site-specific N- and O-glycosylation analysis in protein mixtures. J Proteome Res 2011, 10, 2612–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kronewitter SR, An HJ, de Leoz ML, Lebrilla CB, et al. , The development of retrosynthetic glycan libraries to profile and classify the human serum N-linked glycome. Proteomics 2009, 9, 2986–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Aldredge D, An HJ, Tang N, Waddell K, Lebrilla CB, Annotation of a serum N-glycan library for rapid identification of structures. J Proteome Res 2012, 11, 1958–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pucic M, Knezevic A, Vidic J, Adamczyk B, et al. , High throughput isolation and glycosylation analysis of IgG-variability and heritability of the IgG glycome in three isolated human populations. Mol Cell Proteomics 2011, 10, M111 010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hong Q, Lebrilla CB, Miyamoto S, Ruhaak LR, Absolute Quantitation of Immunoglobulin G and Its Glycoforms Using Multiple Reaction Monitoring. Anal Chem 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wuhrer M, Stam JC, van de Geijn FE, Koeleman CA, et al. , Glycosylation profiling of immunoglobulin G (IgG) subclasses from human serum. Proteomics 2007, 7, 4070–4081. [DOI] [PubMed] [Google Scholar]
- [23].Alexandrakis MG, Moschandrea J, Kyriakou DS, Alexandraki R, Kouroumalis E, Use of a variety of biological parameters in distinguishing cirrhotic from malignant ascites. Int J Biol Markers 2001, 16, 45–49. [PubMed] [Google Scholar]
- [24].Miyamoto S, Ruhaak LR, Stroble C, Salemi MR, et al. , Glycoproteomic Analysis of Malignant Ovarian Cancer Ascites Fluid Identifies Unusual Glycopeptides. J Proteome Res 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Tomana M, Niedermeier W, Mestecky J, Skvaril F, The differences in carbohydrate composition between the subclasses of IgA immunoglobulins. Immunochemistry 1976, 13, 325–328. [DOI] [PubMed] [Google Scholar]
- [26].Endo T, Mestecky J, Kulhavy R, Kobata A, Carbohydrate heterogeneity of human myeloma proteins of the IgA1 and IgA2 subclasses. Mol Immunol 1994, 31, 1415–1422. [DOI] [PubMed] [Google Scholar]
- [27].Mestecky J, Mucosal immunology, Elsevier Academic Press, Amsterdam ; Boston: 2005. [Google Scholar]
- [28].Guile GR, Rudd PM, Wing DR, Prime SB, Dwek RA, A rapid high-resolution high-performance liquid chromatographic method for separating glycan mixtures and analyzing oligosaccharide profiles. Anal Biochem 1996, 240, 210–226. [DOI] [PubMed] [Google Scholar]
- [29].Huhn C, Selman MH, Ruhaak LR, Deelder AM, Wuhrer M, IgG glycosylation analysis. Proteomics 2009, 9, 882–913. [DOI] [PubMed] [Google Scholar]
- [30].Cho W, Jung K, Regnier FE, Sialylated Lewis x antigen bearing glycoproteins in human plasma. J Proteome Res 2010, 9, 5960–5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hakomori S, Tumor-associated carbohydrate antigens defining tumor malignancy: basis for development of anti-cancer vaccines. Adv Exp Med Biol 2001, 491, 369–402. [DOI] [PubMed] [Google Scholar]
- [32].Julien S, Ivetic A, Grigoriadis A, QiZe D, et al. , Selectin ligand sialyl-Lewis x antigen drives metastasis of hormone-dependent breast cancers. Cancer Res 2011, 71, 7683–7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Laidler P, Litynska A, Tumor cell N-glycans in metastasis. Acta Biochim Pol 1997, 44, 343–357. [PubMed] [Google Scholar]
- [34].Higai K, Shibukawa K, Muto S, Matsumoto K, Targeted proteo-glycomics analysis of Sialyl Lewis X antigen expressing glycoproteins secreted by human hepatoma cell line. Anal Sci 2003, 19, 85–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.