Abstract
Immunohistochemistry (IHC) is a robust scientific tool whereby cellular components are visualized within a tissue, and this method has been and continues to be a mainstay for many reproductive biologists. IHC is highly informative if performed and interpreted correctly, but studies have shown that the general use and reporting of appropriate controls in IHC experiments is low. This omission of the scientific method can result in data that lack rigor and reproducibility. In this editorial, we highlight key concepts in IHC controls and describe an opportunity for our field to partner with the Histochemical Society to adopt their IHC guidelines broadly as researchers, authors, ad hoc reviewers, editorial board members, and editors-in-chief. Such cross-professional society interactions will ensure that we produce the highest quality data as new technologies emerge that still rely upon the foundations of classic histological and immunohistochemical principles.
Keywords: histology, immunohistochemistry, controls, formalin fixed paraffin embedded, reproductive tissues
Immunohistochemistry is a critical tool in the reproductive sciences, and we suggest that our field adopts the guidelines set forth by the Histochemical Society to maximize the rigor and reproducibility of our data.
Introduction
Immunohistochemistry (IHC) is the concept of selectively imaging proteins (which act as antigens) in the context of a tissue by exploiting the basic immunology concept of antigen–antibody interactions. During this process, an antibody binds to a specific antigen, which is then visualized by direct or indirect methods as a chromogenic or fluorescent signal. IHC is an essential technique in reproductive biology that enables the analysis of complex cellular and tissue architecture (such as in the male and female gonads and reproductive tracts), where structure and interaction between cell types informs both physiology and pathology. A challenge of IHC is that the identity or the presence or absence of a molecule in a tissue sample can never be proven. However, a preponderance of evidence can and should be generated for each experiment that supports the specificity of the antigen–antibody interaction [1]. Like any other research method, the validity of IHC results relies heavily on experimental design—including the use of rigorous controls. Without these appropriate controls, inaccurate conclusions can be drawn from false-positives and false-negatives. Unfortunately, there is a general lack of performance or reporting of controls in IHC experiments across the scientific literature, and this omission has the potential to lead to unverified and irreproducible findings [1].
The problem
The plethora of antibodies that are commercially available is a major factor in the inability of investigators to reproduce IHC data because not all antibodies are created equal. There are greater than 300 companies selling over 2 million antibodies, which in 2011 represented a market worth $1.6 billion [2]. However, of these antibodies, only about 250 000–500 000 are actually unique “core” antibodies. This is due to larger companies purchasing the same antibody from smaller companies, relabeling them, and then reselling them. Thus, researchers may mistakenly be under the impression that they are purchasing different antibodies from different suppliers, when, in fact, they may simply be the same [2]. Associated with this large market and mass production is batch-to-batch variation, which can produce significantly different results [2]. Various antibodies may also exhibit cross-reactivity, recognizing multiple epitopes beyond the ones that they are designed to detect. Moreover, there is a wide range of antibodies and labels used to localize antigens for various techniques, and researchers may inadvertently use antibodies for the incorrect applications. These issues surrounding antibodies have resulted in them being considered a major problem with reproducibility in biomedical research [2, 3].
However, antibodies are only one part of the equation that is driving the reproducibility crisis. Just as significant as the antibody problem is the long lost scientific method which Webster's New World College Dictionary defines as “a method of research in which a hypothesis is tested by means of a carefully documented control experiment that can be repeated by another researcher.” Although the scientific method has existed for hundreds of years and seems so commonsensical, it is often overlooked in daily practice. When designing and performing experiments, the researcher must ask “is the data reproducible?” If the data is reproducible, then the next question should be “is it valid?” Reproducible but invalid data can be generated with a poor-quality antibody if proper controls are omitted. The waning of the scientific method could be due to a number of factors such as poor training, lack of emphasis on including technical information in presentations or publications, or lenient journal requirements for reporting experimental design. Paying more attention to experimental design and the validation of reagents, including antibodies, is an opportunity to improve reproducibility [4].
The solution
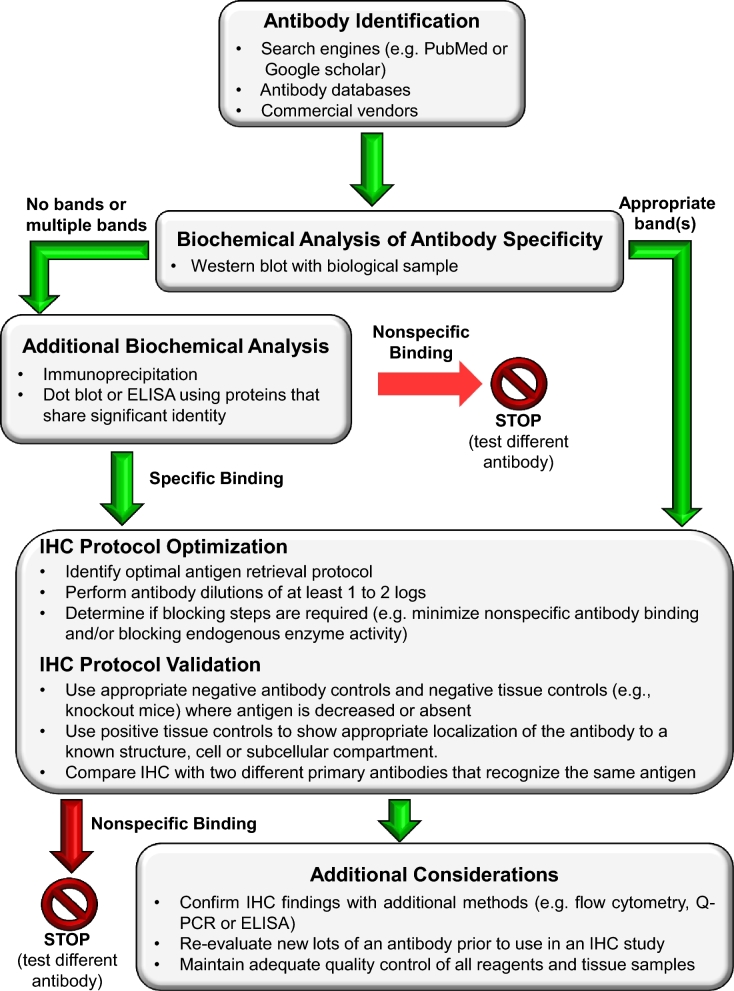
To improve the validity of IHC data, the Histochemical Society has published guidelines for the experimental design of IHC research, and here we summarize the key concepts and provide a decision aid that can be used when initiating a new IHC study [1, 5, 6] (Figure 1). Once potential antibodies have been identified, their specificity must be validated. Antibody specificity refers to the ability of a particular antibody to bind to a specific epitope on an antigen that was used for immunization. In IHC, specific staining describes that in which the reaction product used to detect the primary antibody (i.e., chromogenic or fluorescent) is localized only to regions in tissue where the target antigen is expressed [5]. Nonspecific staining can occur when an interaction besides the specific antibody-antigen occurs. Of note, nonspecific staining can have very precise cellular localizations. For this very reason, it is imperative to include rigorous controls to show that the antibodies used for IHC are binding specifically to the target antigen within a tissue and to avoid being misled by potential nonspecific staining.
Figure 1.
A decision aid outlining key steps in the validation of antibodies and optimization of protocols for immunohistochemistry (adapted from [7]).
The western blot is the most commonly used method to evaluate the biochemical specificity of the antibody–antigen interaction [5]. Complex biological samples from the same tissue or cells in which the antibody will be used for IHC should be used to demonstrate that an antibody recognizes a specific antigen rather than purified recombinant proteins. If an antibody has biochemical specificity, it should recognize a single band at the appropriate molecular weight. If multiple bands are observed, potential isoforms or post-translational modifications should be considered. If no bands are observed, it is possible that the antibody detects a conformational epitope rather than a linear epitope in the primary structure of the protein which is accessible by western blots where the proteins have been denatured. In this case, native gels or other assays can be used to confirm biochemical specificity.
However, biochemical specificity does not prove the identity of what has been bound by the antibody within the context of a tissue, and thus additional positive and negative controls for antibodies and tissues are necessary (Table 1) [1, 5, 6]. In general, a negative control is a treatment, condition, or cohort where no effect is expected, whereas a positive control is one where a known response is expected. In IHC, negative antibody controls are used to demonstrate that the reaction visualized is due to the specific interaction of the epitope of the target molecule and paratope of the antibody/affinity reagent. This is important because, in addition to the molecule targeted by the antibody, signal can indicate detection of structurally related molecules or nonspecific binding of primary/secondary antibodies to other tissue components. A negative control antibody replaces the primary antibody in an IHC protocol, and it is useful for determining specificity because it shares all the same characteristics of the primary antibody (i.e., nonspecific binding characteristics) except for its ability to detect the specific epitope on the antigen of interest. For polyclonal antibodies, preimmune serum from the animal prior to immunization with the antigen is optimal. However, sera from the same species used to raise the antibody can also be used. For monoclonal antibodies, isotype-specific immunoglobulins used at the same protein concentration as the primary antibody are used. The omission of primary antibody alone is not a suitable negative control for nonspecific binding in tissues because it only provides information as to whether the secondary antibody binds nonspecifically or whether there is background due to the detection system. As a positive control for an antibody, IHC is typically performed with two independent antibodies made against different epitopes of the antigen. Similar staining patterns with two antibodies are considered a strong positive control of specificity.
Table 1.
Summary of key IHC controls.
| Antibody controls | ||
|---|---|---|
| • Provides evidence that the primary antibody is specifically binding to its epitope within a tissue | ||
| • Accounts for variability inherent in biological assays | ||
| Control type | Example | |
| Negative | Preimmune serum | |
| Negative | Commercial sera from the same species where antibody was produced | |
| Negative | Isotype control | |
| Positive | Independent antibody raised against the same antigen | |
| Tissue controls | ||
| • Confirms the validity of the staining pattern within a tissue | ||
| • Accounts for variability inherent in biological assays | ||
| Control type | Example | |
| Negative | Section of tissue that is known not to express the target antigen | |
| Negative | Samples that are genetically engineered or modified so that they do not express the target antigen | |
| Positive | Section of tissue where the target antigen is known to be expressed | |
| Positive | Section of tissue that contains anatomical structures where the target antigen is known to localize (structure, cell, subcellular localization) | |
| Additional controls | ||
| Control Type | Example | Function |
| Endogenous tissue background | Section of unstained tissue | Detects endogenous signals that could be confused for positive staining |
| No primary antibody | Section of tissue stained with everything except the primary antibody | Provides evidence that the staining is produced from detection with the primary antibody and not by the detection system or specimen |
| Absorption | Use of a primary antibody that has been preabsorbed with an excess of antigen | Used to validate antibody specificity (*use in combination with other specificity controls) |
Tissue controls confirm the validity of a staining pattern. Negative controls include use of tissues that do not express the target antigen or are modified to lack it. On the other hand, positive controls include tissues known to express the target antigen. The ideal positive control is a positive anatomical control, which is a specimen containing the targeted molecule in its known location (cell type or subcellular compartment). In this type of control, the presence of antigen in the specimen is known and is not the target of an experimental analysis. Typically, positive and negative controls are run in parallel to experimental samples. Common pitfalls to avoid in IHC are the incorrect use of antibody preabsorption as a negative control, the use of secondary antibodies only as a negative control, or the use of cells lines that overexpress a target protein as a positive control.
In addition to performing rigorous controls, IHC protocols must also be optimized for each antibody and tissue type (Figure 1). Factors that can be modified empirically include antigen retrieval conditions, antibody dilutions, and blocking buffers. A comprehensive discussion of these steps is covered in [5, 6].
A call to action
Scientific advances are hampered when investigators fail to use proper controls in any technique including IHC. Therefore, we encourage the reproductive science and medicine community to heed a call to action. As researchers and authors we have the opportunity to partner with our colleagues in the Histochemical Society, who are experts in IHC, and adopt their guidelines. As authors, ad hoc reviewers, journal editorial board members, and editors-in-chief, we can implement and adhere to more stringent standards. We recommend to journals that publish IHC data to require explicit reporting of appropriate controls (positive, negative, and specificity) as well as detailed experimental paradigms (comprehensive information on antigen retrieval, blocking and wash buffers, antibody identity and concentrations, detection methods, and timing). The need to adhere to such guidelines has been echoed by many [5–7]. Although it has previously been reported that ∼89% of journals containing IHC data do not have specific guidelines on controls or even mention controls [1], there are a few, such as Biology of Reproduction (BOR), that provide clear author guidelines regarding IHC controls (Table 2). BOR also specifies details that should be reported with respect to the use of antibodies. While these author guidelines are an important first step, ad hoc reviewers and editorial boards must be required specifically to critically evaluate these criteria.
Table 2.
Summary of IHC-relevant guidelines in BOR.
| Data Type | Specifications/guidelines |
|---|---|
| Immunohistochemistry/Immunofluorescence | Appropriate, representative controls need to be reported (either in the figures or in supplemental data) |
| Antibodies | • Supplementary antibody table |
| • Materials and methods | |
| - Positive and negative controls | |
| - Validation information | |
| - Lot number | |
| - Associated references | |
| - RRID | |
| • Antibody registry [10] | |
| - If antibody is not registered, then author needs to register and obtain an RRID by the revision stage of manuscript submission | |
| BOR endorsed reference | Saper C.B. An open letter to our readers on the use of antibodies. J Comp Neurol 2005. 493(4):477–488. |
Beyond the publishing sphere, increased access to training courses such as the Immunohistochemisty and Microscopy offered by the Marine Biological Laboratory will improve authors’ understanding of what appropriate IHC controls entail. Additionally, creation and dissemination of formalized decision aids for effective validation of IHC would enable rigor and reproducibility of IHC results coupled to the creation of global antibody registries or databases (Figure 1) [6]. In fact, the Resource Identification portal was developed to support the Resource Identification Initiative to help researchers identify validated reagents with a persistent and unique Research Resource Identifier (RRID) to improve reproducibility [8].
Conclusions
In summary, cross discipline and professional society interactions between reproductive biology and the Histochemical Society will ensure the generation of the highest quality data that keeps pace with technical advances in areas such as automated IHC, quantitative microscopy, and digital pathology that can be applied effectively and accurately to reproductive cells and tissues across species. As we move closer toward personalized medicine, more standardized controls for tools such as IHC are needed to improve quality assurance required for clinical specificity and sensitivity [9]. Therefore, it is our responsibility as scientists to hold each other to these critical standards in IHC experimentation.
Acknowledgments
The authors would like to acknowledge all the faculty of the 2016 Immunohistochemistry and Microscopy course held at the Marine Biological Laboratory, Woods Hole for increasing our awareness of these critical concepts.
Footnotes
Grant Support: This work was supported by the Center for Reproductive Health After Disease (P50HD076188) from the National Institutes of Health National Center for Translational Research in Reproduction and Infertility (NCTRI), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01HD093726 to FED), and the Master of Science in Reproductive Science and Medicine Program at Northwestern.
References
- 1. Hewitt SM, Baskin DG, Frevert CW, Stahl WL, Rosa-Molinar E. Controls for immunohistochemistry: the Histochemical Society's standards of practice for validation of immunohistochemical assays. J Histochem Cytochem 2014; 62:693–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baker M. Reproducibility crisis: blame it on the antibodies. Nature 2015; 521:274–276. [DOI] [PubMed] [Google Scholar]
- 3. Baker M. Antibody anarchy: a call to order. Nature 2015; 527:545–551. [DOI] [PubMed] [Google Scholar]
- 4. Freedman LP, Venugopalan G, Wisman R. Reproducibility2020: progress and priorities. F1000Res 2017; 6:604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Frevert CW, Johnson B, Stahl WL. Immunohistochemistry: antibody specificity. In: McManus LM, Mitchell RN (eds.), Pathobiology of Human Disease. San Diego: Academic Press; 2014:3807–3816. [Google Scholar]
- 6. Goodwin PJB, Frevert C. Microscopy, immuno-histochemistry, digital imaging, and quantitative microscopy. In: Treuting PM, Dintzis SM, Montine KS (eds.), Comparative Anatomy and Histology. Elsevier/Academic Press; 2017: 53–66. [Google Scholar]
- 7. Elliott K, McQuaid S, Salto-Tellez M, Maxwell P. Immunohistochemistry should undergo robust validation equivalent to that of molecular diagnostics. J Clin Pathol 2015; 68:766–770. [DOI] [PubMed] [Google Scholar]
- 8. Bandrowski A, Brush M, Grethe JS, Haendel MA, Kennedy DN, Hill S, Hof PR, Martone ME, Pols M, Tan SC, Washington N, Zudilova-Seinstra E, Vasilevsky N. The Resource Identification Initiative: A cultural shift in publishing. The Journal of Comparative Neurology 2016; 524(1):8–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Torlakovic EE, Nielsen S, Vyberg M, Taylor CR. Getting controls under control: the time is now for immunohistochemistry. J Clin Pathol 2015; 68:879–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Antibody Registry, 3.0 ed 2018. http://antibodyregistry.org/ [Google Scholar]