Abstract
Early chronic stress has enduring implications for physical and mental health outcomes. Hair cortisol concentration (HCC) has emerged as a marker of cumulative cortisol exposure, yet HCC in infants is not well understood. We examined how infant HCC relates to widely used basal salivary cortisol measures, maternal HCC, and environmental context in 111 infants assessed at 6 and 12 months of age. Maternal HCC at 6 and 12 months was correlated with infant HCC at 12 months. At 12 months, infant HCC was positively associated with waking salivary cortisol concentration (SCC), evening SCC, and area under the curve (AUC), but was independent of diurnal slope. Breastfeeding was associated with lower HCC, whereas increased sleep disruption was related to flatter slope. Reduced nighttime sleep duration was related both to higher HCC and to flatter slope. A person-focused analysis indicated that the combination of high HCC and flattened slope was associated with more environmental risks, highlighting the importance of investigating the interplay between HCC and diurnal cortisol slope. Results support the validity of HCC as a marker of cumulative cortisol exposure in infancy, while emphasizing the value of including multiple cortisol measures assessing distinct aspects of Hypothalamic-Pituitary-Adrenal (HPA) function.
Keywords: Infancy, Hair Cortisol, Sleep, Breastfeeding, Socioeconomic Status, Diurnal Cortisol
Introduction
Early life stress has long-term consequences, making children more vulnerable to physical and mental health problems in adulthood (Anda, Butchart, Felitti, & Brown, 2010). The HPA axis, of which the end product is cortisol, is immature at birth and sensitive to early experiences (Gunnar & Talge, 2008). Caregivers regulate infant physiological stress and serve as a social buffer, which in turn shapes the infant’s stress regulation capacities for the future (Gunnar & Donzella, 2002). Infants who experience early life stress are at risk for abnormal HPA activity. Chronic activation of the stress response creates wear and tear on the body that, according to the allostatic load model, leads to multi-systemic physiological dysregulation that increases the individual’s risk for physical and mental health problems (McEwen, 2006). A better understanding of the mechanisms that shape HPA axis function in infancy is crucial to inform prevention and intervention programs to support optimal development of biological stress systems and promote long-term health.
Thus far, salivary cortisol has been used most extensively to investigate early physiological stress during this critical maturational period. Because salivary cortisol only assesses stress at the moment of saliva collection, it is often used as a biomarker of an infant’s acute stress or diurnal rhythm. Hair cortisol, on the other hand, indexes cumulative cortisol exposure (i.e., both basal circulating cortisol levels and physiological reaction to stress over time). As such, it may be more indicative of allostatic load. Cortisol is deposited in the hair shaft as it grows, such that, taking into account the rate of growth, a hair sample can provide a timeline of long-term cortisol exposure (Meyer & Novak, 2012; Russell, Koren, Reider, & Van Uum, 2012). In recent years, its relative ease in collection compared to salivary cortisol has led to rapid adoption of hair cortisol measures in studies of various animal species and adult humans, and it has potential as a developmental marker of HPA function as well. We must first understand the role of HCC in infancy before deploying it in long-term studies that investigate lifelong health implications of early physiological stress or allostatic load. Few studies, however, have used hair cortisol in infants and young children, so its developmental significance and association with salivary cortisol are relatively unknown. Given that hair and basal salivary cortisol represent different aspects of the same rapidly developing physiological system, they may demonstrate differential associations with the child’s environment and experiences. Discerning where the two methods converge and diverge is critical in understanding the infant’s immature HPA axis and how early life stress is best measured and conceptualized. Yet, to our knowledge, there are no published studies of infant salivary and hair cortisol studied in conjunction.
Hair and Salivary Cortisol
The few studies of older children and adults that have explored the overlap between hair and basal salivary cortisol find inconsistent support for an association between HCC and salivary cortisol AUC from waking to bedtime (an estimate of cumulative exposure across daytime waking hours). In pregnant women HCC was related to AUC, but only when looking at both over the same time frame (D’Anna-Hernandez, Ross, Natvig, & Laudenslager, 2011). Morning SCC has been correlated with HCC in young adults (Xie et al., 2011). Only two studies have investigated the overlap between salivary cortisol and HCC in children. Girls’ HCC at 7 years of age was correlated with cortisol reactivity to challenge at 3 years of age (Oullette et al., 2015). In a different sample of girls, HCC was related to salivary cortisol AUC calculated from the time of waking to 60 minutes later and also to morning SCC, but not to diurnal slope (Vanaelst et al., 2012). Taken together, these few studies suggest a possible association between HCC and salivary cortisol. Given that HCCs in infancy are higher and more variable than those in older children and adults (e.g., Karlén et al., 2013), the relation between HCC and basal salivary cortisol measures may differ across development. Research is needed to investigate the associations between different measures of basal salivary cortisol and HCC in infancy, when the HPA axis is rapidly developing.
Maternal and Child Cortisol
The relation between maternal and child basal salivary cortisol is well established (e.g., Bright, Granger, & Frick, 2012; Letourneau, Watson, Duffett-Leger, Hegadoren, & Tryphonopoulos, 2011; Stenius et al., 2008). Less is known about how a mother’s HCC relates to her child’s HCC. Pregnant women’s HCC levels in the second and third trimester were related to their infants’ HCC at one year of age (Karlén et al., 2013), yet concurrent associations between maternal and infant HCC were not found at 9 and 12 months of age (Liu, Snidman, Leonard, Meyer, & Tronick, 2016). Later in childhood, HCC has been positively correlated in mothers and their 7-year-old daughters (sons were not included in the study; Ouellette et al., 2015). More research is needed to better understand the association between mother and offspring HCC levels, particularly in infancy and early childhood.
Familial Correlates of Hair and Salivary Cortisol
Despite the established importance of caregivers in social buffering of the infant’s HPA axis, only a few studies have examined familial correlates of infant or childhood HCC. In infancy and childhood, higher HCC has been related to greater maternal parenting stress, depressive symptoms, psychological distress (Palmer et al., 2013) and increased stressful life events (Vanaelst et al., 2013), though other studies have demonstrated no relation between HCC and maternal perceived stress or depressive scores in infants and children (Liu et al., 2016; Ouellette et al., 2015). In infants, the association of SES with HCC has not been comprehensively examined, though living in an apartment versus a house has been related to higher HCC (Karlen et al., 2013; Palmer et al., 2013), whereas maternal education was independent of infant HCC in several studies (Karlen et al., 2013; Palmer et al., 2013; Liu et al., 2016). In older children, lower maternal education and lower neighborhood SES have been related to higher HCC (Vaghri et al., 2013; Vliegenthart et al., 2016). Taken together, the literature suggests the potential relevance of familial factors in child cumulative cortisol exposure, though the particular factors and nature of effect are inconsistent from study to study and may vary across developmental periods as well as study population.
Familial factors, including SES, maternal depression, and parenting, have been examined more extensively in relation to salivary cortisol in infancy and early childhood. Lower SES has been related to higher basal salivary cortisol in infants and children (e.g., Clearfield, Carter-Rodriguez, Merali, & Shober, 2014; Lupien, King, Meaney, & McEwen, 2000). Increased maternal depression has been related to higher child basal SCC in some cases (Ashman, Dawson, Panagiotides, Yamada, & Wilkinson, 2002; Brennan et al., 2008; Lupien et al., 2000) but not in others (Laurent et al., 2013). In infants and young children, maternal sensitivity has been related to steeper diurnal slope (Ben-Dat Fisher et al., 2007; Letourneau et al., 2011), lower AUC (Philbrook et al., 2014; Letourneau et al., 2011) and lower basal SCC (Blaire et al., 2011; Philbrook et al., 2014). Negative parenting has been associated with higher SCC (Blaire et al., 2011; Taylor et al., 2012). Importantly, however, the conceptualization of maternal sensitivity varies across studies, and in many studies multiple indices of maternal sensitivity were unrelated to basal SCC (e.g., Philbrook et al., 2014; Letourneau et al., 2011). Despite inconsistencies in measurement and results, basal salivary cortisol research suggests a general pattern of a) greater maternal sensitivity relating to smaller AUC, lower basal SCC and steeper slopes, and b) maternal depression as a possible predictor of child flattened slope and elevated basal SCC.
Breastfeeding, Sleep, and Cortisol
Although we know that infants are exposed to maternal cortisol in breast milk (Hamosh, 2001), the relation between breastfeeding and infant cortisol is still not well understood. Breastfeeding has been related to higher basal SCCs, particularly in boys (Cao et al., 2009; Forns et al., 2014), and maternal-infant synchrony in evening SCCs was found for breastfed babies only (Neelon et al., 2015). Yet the association of breastfeeding with infant HCC and salivary cortisol is understudied, most likely because of sample-size constraints in separating out breastfed versus formula-fed infants.
In adults, sleep disruption is related to a higher cortisol awakening response (CAR), greater AUC and a flatter diurnal slope (Bostock & Steptoe, 2013). The few studies that have examined sleep and basal salivary cortisol in infancy support the sensitivity of the infant HPA axis to sleep. In infants, taking more daytime naps was associated with higher waking SCC and CAR, and later sleep onset was positively related to CAR (Stalder et al., 2013). In 2–4-year-olds, forced sleep restriction resulted in lower waking SCC the next morning, and day-time naps resulted in a CAR (Gribbin, Watamura, Cairns, Harsh, & LeBourgeois, 2012). Thus, even after a short time asleep, waking triggers a cortisol response in infants. It is possible that nighttime wakings may result in cortisol awakening responses that could affect overall cortisol output, and thus, infant HCC.
Current Study
The purpose of the current study was to gain a more comprehensive understanding of how different aspects of the HPA axis are related to each other and to environmental influences during infancy, a time when the HPA axis is rapidly developing. We assessed maternal HCC and maternal psychosocial factors when infants were 6 months old, and at 12 months we measured infant HCC and salivary cortisol, maternal HCC, breastfeeding, sleep, and SES. This is, to our knowledge, the first study to examine the overlap between hair and salivary cortisol in infancy, an interplay not well understood even in later childhood and adulthood. To better understand the mother’s role in her infant’s cumulative cortisol exposure, we examined mother-infant HCC synchrony both concurrently and across time. To advance our understanding of how maternal psychosocial factors are related to infant HPA functioning, we explored how maternal depression and maternal parenting stress predicted both infant hair and salivary cortisol at 12 months, rather than one or the other. This study builds on the few existing studies investigating how breastfeeding and sleep are related to salivary cortisol in infancy, and is the first to examine how they relate to infant HCC. By investigating the methodological and developmental significance of HCC and the interplay between hair and salivary cortisol, we aim to pave the way for further research on allostatic load and the long-term implications of distinct aspects of infant HPA function for later physiological stress regulation.
Methods
Participants
One hundred twenty-five infants were enrolled in the study, but 14 infants did not provide useable hair cortisol values and were excluded from analysis. Of these 14 infants, three had mothers who declined infant hair collection, 10 infants were on medication, and one infant was breastfed while the mother was on a medication that could affect cortisol values. This resulted in a final sample of 111 dyads (M=12.18 mos.). Of these 111 infants, 90 had participated in an assessment at six months as well (M=6.67 mos.). All infants were singletons who had no known hearing, visual, neurological, or developmental disorders, and whose mothers were fluent in English. Mothers were mostly college-educated (87.2%). See Table 1 for additional demographic information. Participants were recruited from a department-maintained database of families interested in participating in research; from publically available state birth records; and from online advertising.
Table 1.
Demographic Characteristics of Participating Families (12 month visit)
| Maternal age (years) | |
| M(SD) | 33.42 (4.29) |
| Infant age (months) | |
| Home Visit M (SD) | 6.67 (.44) |
| Lab Visit M (SD) | 12.18 (.72) |
| Maternal ethnicity | |
| Caucasian | 73.4% |
| Asian | 11.0% |
| Black | 6.4% |
| Hispanic | 3.7% |
| Native American | .9% |
| Multiracial/Other | 4.6% |
| Infant ethnicity | |
| Caucasian | 65.5% |
| Asian | 4.5% |
| Black | 4.5% |
| Hispanic | 1.8% |
| Native American | 0% |
| Multiracial/Other | 23.6% |
| Income-to-needs ratio | |
| M(SD) | 5.59 (3.62) |
| Maternal education (% with at least a 4 year college degree) |
87.2% |
| Paternal education (% with at least a 4 year college degree) |
77.4% |
| Maternal occupational prestige (1-5 scale) |
3.89 (1.04) |
| M(SD) | |
| Paternal occupational prestige (1-5 scale) |
3.77 (1.16) |
| M(SD) |
General Procedure
This study was approved by the university institutional review board and parents gave informed consent prior to participation. Parents were compensated for their time and infants received a small gift. A subset of infants participated in a home visit conducted when the infant was 6 months old, which lasted about one hour. The visit included maternal questionnaires, collection of a maternal hair sample, a mother-infant free play interaction, and infant behavioral assessments.
When infants were approximately 12 months old families were invited to participate in a laboratory visit that lasted about 90 minutes. The visit included maternal questionnaires and collection of both maternal and infant hair samples, as well as behavioral and electrophysiological assessments not included in the current analyses. Mothers were trained on home saliva collection, and research staff picked up the infant saliva samples upon completion of collection.
Hair cortisol.
Hair cortisol collection procedures followed our validated methods (Meyer, Novak, Hamel, & Rosenberg, 2014). Maternal hair samples were collected at the 6-month home visit, and maternal and infant hair samples were collected at the 12-month lab visit. A small amount (15–30mg) of hair was collected representing the 3 cm closest to the scalp. Because washing, hair straightening or dyeing, and styling products may affect hair cortisol concentration (HCC), mothers were asked about their and their infants’ hair history including the frequency that the hair got wet. Human scalp hair grows at approximately 1 cm per month (LeBeau et al., 2011), so the 3 cm sample indexed cortisol output over the past 3 months. Hair samples were stored in plastic tubes, and were frozen at −20° C until cortisol analysis. Hair samples were washed twice with isopropanol and dried and ground to powder. Cortisol was extracted into methanol and the sample was reconstituted in assay buffer. Reconstituted extracts were analyzed for cortisol using a sensitive and selective enzyme immunoassay. Assay readout was converted to pg cortisol per mg of dry hair weight. Intra- and inter-assay CVs were less than 3% and 5% respectively. Raw HCC values were log transformed because the data were not normally distributed. Values were not winsorized. Most mothers in this sample provided useable HCC values at the 6-month visit (95.5%) and 12-month visit (92.8%). Of those without useable HCC at 6 months, one mother did not consent to collection and three mothers were on medications that could affect cortisol values. At 12 months, four mothers did not consent to collection and four were on medication that could affect cortisol values. Most infants had useable HCC at 12 months (88.8%).
At 6 and 12 months, mothers’ HCC was unrelated to frequency of washing (6 mos: Spearman’s rho =.011, p =.922; 12 mos: Spearman’s rho =.−.057, p =.572) or to color treatment of hair (6 mos: t(76)=.333, p=.740; 12 mos: t(101)=1.177, p=.242 ). Frequency of washing was also unrelated to the infants’ HCC at 12 months (Spearman’s rho=−.084, p=.389). Therefore, it was not necessary to correct for variation in hair care habits.
Salivary cortisol.
Infant 12-month salivary cortisol was collected in the home. Mothers were instructed to collect samples from their infants immediately upon the infant’s waking and at the infant’s bedtime (just before the last feeding, and at least an hour since the infant last napped) on three days when they spent most of the day together and when the infant was not sick. This diurnal sampling was repeated across 3 days. Mothers were instructed to collect the samples when it had been at least an hour since the infant was last fed. Compliance with these instructions was assessed via home diaries.
An infant-safe synthetic swab (Salimetrics, State College, PA) was placed in the infant’s mouth for 60 seconds. Mothers were instructed to keep samples frozen until the 3 days of sampling were completed, after which samples were collected by research staff. Salivary samples were frozen at −20° C until they were sent to Trier Laboratories in Germany for assay. Salivary cortisol concentrations were determined employing a competitive solid phase time-resolved fluorescence immunoassay with flouromeric end point detection (DELFIA; Dressendörfer, Kirschbaum, Rohde, Stahl, & Strasburger, 1992).
Two full days of sampling was the required minimum for inclusion in analyses, and 70 infants (63.1%) met this criterion. Specifically, 47 infants had usable samples for all three days and 23 infants had usable samples for two days. Saliva samples were not returned for 27 infants, nine infants provided fewer than two days of usable samples, two infants’ salivary cortisol values were excluded because the mother was breastfeeding and taking oral steroids or the infant was taking medication that could affect cortisol levels, and one infant was excluded due to a collection error. Infants with sufficient salivary samples did not significantly differ on any demographic variables than infants without sufficient salivary samples.
Four variables were calculated to assess diurnal cortisol for the infant, standardized, and averaged across the days of sampling: waking SCC, bedtime SCC, slope across the day, and area under the curve with respect to ground (AUCg). Prior to averaging across days, statistically extreme values for these 70 infants that were not explained by illness or medications were winsorized to three standard deviations from the mean. Nine infants required winsorizing of wake SCC, bed SCC, slope, or AUC on at least one of the 3 days of sampling, for a total of 25 values requiring winsorizing (wake=2; bed=5; slope=10; AUC=8). Values were log transformed as necessary to meet the assumptions of normality. Values across all sampling days were then averaged to form an aggregate for each of the four variables, and each demonstrated adequate internal consistency (wake α=.604; bed α=.672; slope α=.587; AUCg α=.602). Slope assessed regulation of cortisol levels across the day, as a healthy diurnal rhythm is characterized by a morning spike upon waking followed by a decline throughout the day (Fries, Dettenborn, & Kirschbaum, 2009). Slopes were computed using the rise-over-run formula: change across the day in SCC divided by the time elapsed from morning to bedtime sample. AUCg was calculated as an estimate of cumulative cortisol exposure using the trapezoid formula (Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, 2003). Given that AUC indexes cumulative cortisol exposure across the day and that slope is indexing change across the day, it is possible the two can diverge. For example, flat slope could either reflect levels that stay high across the day or low across the day, resulting in very different AUC values. Thus, it is important to include both when looking at the overlap with HCC.
Although emergent at this age, there is some suggestion that infants show a cortisol awakening response (Stalder et al., 2013). Thus, average time since waking when the morning sample was taken (M=16.38 mins., SD=15.92 mins.) was used to control for variability in the time of sampling relative to the cortisol awakening response (CAR), which peaks approximately 30 minutes after waking. Infant waking SCC, slope and AUCg were each regressed on the square of the time since waking when the morning sample was taken, and standardized residuals were saved. These standardized residuals, which represented standardized salivary cortisol values after correcting for time of sampling, were used as the measures of waking SCC, slope, and AUCg in all subsequent salivary cortisol analyses.
Mother-infant free-play interaction.
The mother-infant interaction was assessed in the home during the 6-month visit using a 6-minute exploratory play paradigm (Feldman & Eidelman, 2003; Feldman, 2007). Mothers were provided with six age-appropriate standardized toys and told to play with their infants as they normally would at home. The video camera was positioned facing the mother and infant in such a way that the toys, mother’s and infant’s faces and bodies were in view.
Interactions were micro-coded, frame-by-frame, with Noldus 11.0 (The Vaggenigen, Netherlands), using the methods of Feldman and colleagues (Atzil, Hendler, & Feldman, 2011; Feldman, Gordon, & Zagoory-Sharon, 2011). Mother and infant gaze, affect, vocalizations and interaction with toys were coded every 30th of a second. Interrater reliability was calculated for one minute of each interaction video and yielded a range of kappas from 84-.97. Maternal Positive Affect indexed the proportion of time the mother demonstrated positive affect (e.g., smiling, laughter). Motherese indexed the proportion of time the mother spent using infant-directed speech. Maternal Intrusiveness indexed the proportion of time that the mother spent taking a toy away from the infant. Maternal Negative Affect indexed the proportion of time that the mother displayed any negative affect.
Maternal parenting stress.
Maternal parenting stress was assessed at the 6-month visit using the Parenting Stress Index (PSI; Abidin, 1995), 4th Edition short form, a 36-item parent report rated on a 5-point Likert scale. Mothers with less than a 10 on the Defensive Responding scale are considered defensive responders, and their PSI scale scores were not included in subsequent analyses. The Total Stress scale was used (possible range=36–180). The PSI is validated for use with parents of children aged 1 month to 12 years (Abidin, 1995).
Maternal depression.
Maternal depression was measured at the 6-month visit with the Center for Epidemiologic Studies-Depression Scale (CES-D; Radloff, 1977), a 20-item self-report questionnaire. The CES-D has internal, concurrent, and predictive validity and has been used widely with mothers of infants (Bureau, Easterbrooks, & Lyons-Ruth, 2009). Possible scores range from 0 to 60, with a clinical cutoff of 16.
Infant sleep.
At the 12-month visit, mothers completed the Brief Infant Sleep Questionnaire (BISQ; Sadeh, 2004). Infant nighttime sleep duration was defined as the time in hours that the infant typically spends asleep between 7 pm and 7 am. For the purpose of this study, nighttime sleep duration was dichotomized as either at least 10 hours per night or less than 10 hours per night. Room sharing was defined as either sleeping in the parent’s bed or the parent’s room and number of night wakings was dichotomized as either 2 or more wakings per night, or less than 2 night wakings. Because room sharing and number of night wakings were significantly related to one another (χ2(103)=27.158, p<.001) and conceptually similar, they were standardized and averaged to create a sleep disruption composite (Cronbach’s alpha=.679). Although those sleeping less than 10 hours per night also had more sleep disruption (t=2.44, p=.017), nighttime sleep duration had poor internal consistency with the sleep disruption aggregate (Cronbach’s alpha=.078), so sleep disruption and nighttime sleep duration were kept separate.
Breastfeeding.
At the 12-month visit, mothers reported on breastfeeding history, and infants were classified based on whether or not the infant had been breastfed in the past three months. This time window was chosen based on the period indexed by the hair cortisol sample.
Socioeconomic status.
Mothers reported maternal and paternal occupation; the highest educational level attained by mother and father; household composition; and household income. Occupational prestige was coded using the Job Zone coding scheme from the Occupational Information Network (O*NET, http://www.onetonline.org/help/online/zones), which ranks U. S. Census-based occupational categories on a 1–5 scale based on the education, experience, and training required. Income and household composition were used to compute income-to-needs ratio based on the 2013 federal poverty level. Parent occupational prestige, parent education, and income-to-needs ratio were standardized and averaged to yield an SES composite.
Analysis Plan
First, SES was correlated with all other variables to determine whether it needed to be controlled for in subsequent analyses. SES was included as a covariate in subsequent analyses when it was related to both the cortisol dependent variable and to at least one of the independent variables in the analysis. Pearson correlations then examined concurrent relations of infant HCC to infant waking SCC, bedtime SCC, AUCg and slope across the day at 12 months of age, as well as predictive and concurrent relations between maternal HCC (at 6 and 12 months) and infant HCC (at 12 months) to better understand HCC as a marker of infant cumulative cortisol exposure. To assess maternal psychosocial correlates of infant cortisol, Pearson correlations were conducted on the longitudinal sample relating maternal parenting stress, mother-infant interaction variables, and maternal depressive symptoms at 6 months to infant HCC and salivary cortisol at 12 months. Next, t-tests or correlations were conducted to examine the association of infant HCC and salivary cortisol with breastfeeding, nighttime sleep duration, and sleep disruption at 12 months of age. Stepwise regressions determined which of these uniquely contributed to variance in infant HCC and salivary cortisol.
Results
Preliminary Analyses
Table 2 presents means and standard deviations for the raw infant and maternal cortisol measures. Descriptive statistics for behavioral and environmental variables are indicated in Table 3. Maternal intrusiveness and maternal negative affect hardly ever occurred in our sample, and thus were not included in subsequent analyses.
Table 2.
Raw Hair and Salivary Cortisol Values
| M(SD) | |
|---|---|
| sample range | |
|
Infant | |
| Wake SCC (µg/Dl) – 12mo | .40 (.24) |
| N=70 | .04 – 1.64 |
| Bed SCC (µg/Dl) – 12 mo | .13 (.26) |
| N=70 | .01 – 2.05 |
| AUCg (µg/Dl/hour) – 12 mo | 3.26 (3.12) |
| N=70 | .34 – 22.79 |
| Diurnal slope (µg/Dl/hour) – 12 mo | .020 (.032) |
| N=70 | −.18 – .07 |
| HCC (Pg/Mg) – 12 mo | 86.26 (183.63) |
| N=111 | 3.60 – 1530.80 |
|
Mother | |
| HCC (Pg/Mg) – 6 mo | 38.60 (87.14) |
| N=86 | .30 – 641.20 |
| HCC (Pg/Mg) – 12 mo | 27.33 (30.86) |
| N=103 | 3.80 – 189.70 |
Table 3.
Descriptive Statistics of Environmental Measures
| Maternal Factors (N=89) | M(SD) |
| sample range | |
| CESD total score | 9.87 (7.01) |
| 0-33 | |
| Maternal Positive Affect | 14.3% (14.5%) |
| 1-79% | |
| Maternal Motherese | 22.5% (18.6%) |
| 0-56% | |
| Maternal Negative Affect | 0% (0%) |
| 0-3% | |
| Maternal Intrusiveness | 1% (1%) |
| 0-4% | |
| Maternal Total Parenting Stress Score (PSI) | 71.56 (11.44) |
| 46.00-105.97 | |
| Breastfeeding and Sleep (N=106) | Frequency or M(SD) |
| Duration of sleep >/= 10 hours/night | |
| (%) | 81.7% |
| Sleep Disruption | .01(.88) |
| M(SD) | −.75 – 1.35 |
| Breastfed in last 3 months | |
| (%) | 72.8% |
Note. Descriptive statistics for maternal psychosocial measures are from the longitudinal sample at 6 months. Maternal Total Parenting Stress Score (PSI) is the mean and SD excluding defensive responders. Breastfeeding and sleep descriptive statistics are from the full 12-month sample. Sleep disruption is a standardized aggregate score.
Infants from higher SES families had significantly steeper cortisol slopes (r(70)=.291, p<.05), and SES was significantly higher for breastfed infants (t(102)=−3.697, Cohen’s d=.774, p<.001) and for infants sleeping 10 hours or more a night (t(103)=−2.658, Cohen’s d=.605, p<.01). No other variables were related to SES. SES, therefore, was included as a covariate in analyses relating breastfeeding and nighttime sleep duration to slope, because it was associated with both the independent and dependent variables. Other than the significant association between sleep disruption and nighttime sleep duration reported in the methods, no other environmental correlates were related to one another.
Infant Hair and Salivary Cortisol at 12 Months
As seen in Table 4a, higher infant HCC was associated with greater infant waking SCC, bedtime SCC, and AUCg, but not with diurnal slope. This suggests that infant cumulative cortisol exposure (i.e., HCC) and daily rhythms (i.e., slope) are tapping different biological stress constructs. To explore whether these distinct aspects of HPA function were differentially related to breastfeeding, sleep, and maternal factors, both infant HCC and diurnal slope were used as outcome variables in further analyses.
Table 4a.
Correlations between infant hair and salivary cortisol at 12 months
Note.
p<.05;
p<.01;
p<.001
Mother and Infant Hair Cortisol
Higher maternal HCC was strongly related to higher infant HCC both within and across age (Table 4b).
Table 4b.
Associations between infant hair cortisol and mother hair cortisol
Note.
p<.05;
p<.01;
p<.001
Relations Between Maternal Factors at 6 Months and Infant Cortisol at 12 Months
HCC.
Infant HCC was not related to maternal parenting stress, maternal depressive symptoms, or motherese and maternal positive affect during the mother-infant interaction.
Diurnal slope.
Infant slope was also not related to any of the maternal factors at 6 months.
Concurrent Associations of Breastfeeding and Sleep with Infant Cortisol at 12 Months
HCC.
Breastfed infants had lower levels of HCC at 12 months (t(103)=2.305, Cohen’s d=−0.532, p=.023). Infants who slept 10 hours or more each night at 12 months had lower HCC than infants who slept less than 10 hours (t(104)=2.545, Cohen’s d=−0.638, p=.012). Sleep disruption was not related to infant HCC at 12 months. Infant HCC was regressed on breastfeeding and nighttime sleep duration using stepwise regression, and the overall model was significant (F=5.087, p=.026; Table 5). After accounting for the variance in infant HCC explained by nighttime sleep duration, breastfeeding status did not explain additional variance in infant HCC at 12 months. After correction for false discovery rate, all associations remained significant. This method controls for the expected proportion of falsely rejecting the null hypothesis, and is a more powerful method than the more traditional false positive rate approach (Benjamini & Hochberg, 1995).
Table 5.
Stepwise Regression Models Predicting Infant Cortisol from Breastfeeding and Sleep
|
Infant Hair Cortisola | |||||
| Variable | B | SE | β | p | Model Adjusted R2 |
| 12 mo. sleep duration | −.620 | .271 | −.230 | .026 | .043 |
|
Infant Slopeb | |||||
| Variable | B | SE | β | p | Model Adjusted R2 |
| 12 mo. sleep duration | 1.210 | .286 | .468 | .000 | .207 |
Note.
sleep duration and breastfeeding were both entered into the stepwise regression; breastfeeding did not remain in the model.
sleep duration, sleep disruption, and SES were entered into a stepwise regression; SES and sleep disruption did not remain in the model.
Diurnal slope.
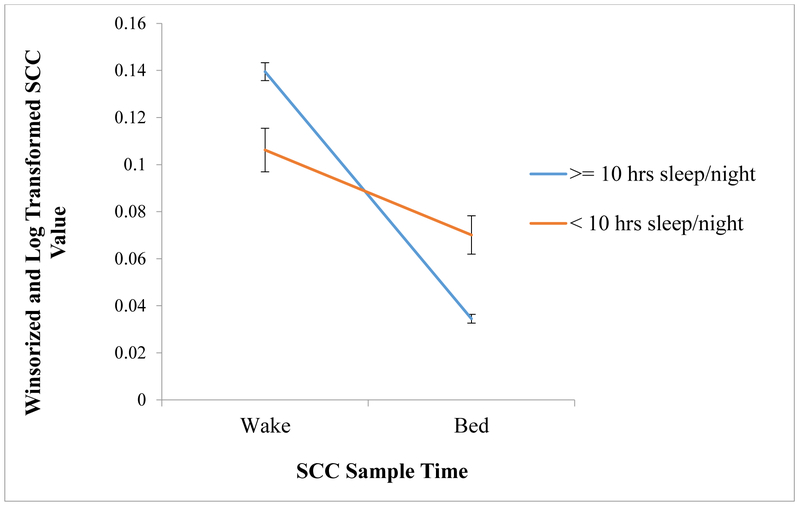
Sleep duration and sleep disruption were related to infant slope at 12 months. Specifically, infants with 10 hours or more nighttime sleep had steeper slopes across the day (t(66)=−4.239, Cohen’s d=1.619, p<.001) than those with less than 10 hours of nighttime sleep (see Figure 1). Infants with lower sleep disruption had steeper slopes (r=−.295, p=.016). Breastfeeding was not related to diurnal slope (t(64)=−.601, Cohen’s d=.179 p=.550). Again, these associations remained significant after correction for false discovery rate. To determine which factors uniquely contribute to variance in infant diurnal slope at 12 months, nighttime sleep duration, sleep disruption, and SES were entered into a stepwise regression. As seen in Table 5, only nighttime sleep duration remained in the model, accounting for about 21% of the variance in infant diurnal slope (F=17.966, p<.0001). Sleep disruption and SES did not uniquely explain variation in infant slope above and beyond that explained by nighttime sleep duration.
Figure 1.
Slope across the day by sleep duration classification
Note. Y-axis indicates SCC value after winsorizing and log transformation. Standardized SCC values were not used in order to make it easier to interpret the slope across the day. X-axis indicates the SCC sample time, showing the decline in basal SCC across the day.
Post hoc Analyses: Interaction Between Infant HCC and Diurnal Slope
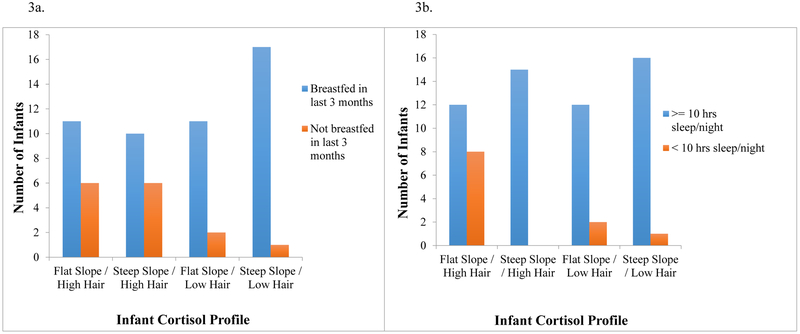
To investigate whether the combination of the infant’s HCC and diurnal cortisol slope provides additional information beyond looking at each separately, we created a person-focused variable that represented four distinct combinations of HCC and slope. We decided to examine the interaction between diurnal cortisol slope and HCC for several reasons. First, there is little known about the emergence of the circadian cortisol rhythm in infancy. The four studies that have looked at this find a great deal of individual variation in when this emerges in infancy (Antonini, Jorge, & Moreira, 2000; de Weerth, Zijl, & Buitelaar, 2003; Price, Close, & Fielding, 1983; Santiago, Jorge, & Moreira, 1996). Because there is no consensus in the field as to when the circadian cortisol rhythm arises, slope by itself is hard to interpret at this stage in development. High HCC combined with flat slope suggest to us the possibility of frequent cortisol responses to stressors across the day which contribute to overall elevated levels and a flattened slope. On the other hand, an infant could have a flat slope because of slower development of the circadian cortisol rhythm, but elevated HCC would not be expected in this case. Thus, looking at slope and HCC together may shed more light on our understanding of the overall HPA functioning in early development. Secondly, from a more clinical perspective, flatter slopes and elevated HCC are both considered “at risk” (e.g., Bernard, Butzin-Dozier, Rittenhouse, & Dozier, 2010; Palmer et al., 2013), yet the two have never been investigated together to see if the combination of flat slope and high HCC adds further information. Median splits were performed on both infant HCC and slope, dichotomizing infant HCC into “low” and “high” and infant slope into “flat” and “steep.” The four groups were then created as follows: flat slope/low HCC (14 infants, 20%), steep slope/low HCC (19 infants, 27.1%), flat slope/high HCC (21 infants, 30%), and steep slope/high HCC (16 infants, 22.9%). One-way ANOVAs and chi square difference tests examined differences between these infant profiles on all environmental factors that were significantly related to either cortisol measure alone (i.e., SES, breastfeeding, nighttime sleep duration, and sleep disruption).
There was a significant difference between infant cortisol profiles on SES as determined by a one-way ANOVA (F(3,66)=5.405, η2p=.082, p=.002; Figure 2a). A Bonferroni post-hoc test revealed that SES was lower for infants with flat slope/high HCC compared both to infants with flat slope/low HCC (Mean difference=−.808, p=.022) and to those with steep slope/low HCC (Mean difference=−.875, p=.004). There was a significant difference between infant cortisol groups on sleep disruption (F(3,65)=4.370, η2p=.175, p=.007; Figure 2b), with a Bonferroni post-hoc test showing that sleep disruption was higher for infants with flat slope/high HCC compared to infants with a steep slope/high HCC (Mean difference=.883, p=.019), with no statistical differences between the other groups.
Figure 2.
Socioeconomic status (a) and sleep disruption (b) by infant cortisol profile.
Note. The Y-axis indicates mean standardized SES score (2a) and mean standardized sleep disruption score (2b) for each infant cortisol profile. The X-axis represents all four infant cortisol profiles. Standard errors are indicated by the error bars.
Chi square difference tests were performed to determine whether the infant cortisol profiles differed on sleep duration or breastfeeding outcomes. There were no significant differences between the four cortisol profiles on breastfeeding (χ2(3, 64)=6.772, p=.080). Indeed, as can be seen in Figure 3a, there is clearly a main effect of HCC on breastfeeding, but no interaction with slope. The infant cortisol profiles did differ on sleep duration (χ2(3, 66)=12.321, p=.006). Figure 3b suggests that it is the combination of flat slope and high HCC, rather than either flat slope or elevated HCC on their own, that is associated with less than 10 hours of nighttime sleep.
Figure 3.
Breastfeeding status (a) and sleep duration status (b) by infant cortisol profile.
Note. The Y-axis indicates number of infants the X-axis represents all four infant cortisol profiles.
Discussion
This study investigated the association of HCC and salivary cortisol in 12-month-old infants, environmental correlates of infant HCC and diurnal cortisol slope, and the interplay between these two distinct aspects of the infant’s HPA axis in predicting environmental risks. Maternal HCC was related to infant HCC both concurrently and across time. At 12 months, there was significant overlap between infant basal salivary cortisol and HCC measures, such that infants with higher HCC had higher AUCg, waking SCC, and bedtime SCC. Infants’ diurnal slope was not related to HCC, suggesting that HCC and diurnal slope are measuring distinct aspects of HPA function: cumulative exposure vs. regulation across the day, respectively. Neither slope nor HCC at 12 months was related to maternal psychosocial factors at 6 months. Both were sensitive to sleep, such that sleep problems were related to higher HCC and flatter slope, but only HCC was related to breastfeeding. Person-focused analyses looking at infant HCC and diurnal slope together suggests that the interplay between the two is particularly informative. The combination of high HCC and flat slope was associated with more risk factors than any other infant cortisol profile. Taken together, these results suggest the validity of using HCC to assess infant cumulative cortisol exposure, and the importance of measuring both HCC and diurnal rhythm. Infant HPA function is complex and multiple measures of infant cortisol are required for a comprehensive understanding of its environmental correlates and long-term implications.
The present results are the first to demonstrate an association of HCC with AUC in infancy, which is consistent with the literature in children and adults (D’Anna-Hernandez, Ross, Natvig, & Laudenslager, 2011; Ouellette et al., 2015). Not surprisingly, correlations between the two were moderate. Both are measuring cumulative exposure, but AUC is calculated only from waking hours across the day, and averaged across only a few days. HCC, on the other hand, captures cumulative exposure across day and night and over a period of several months. As previously seen in children, HCC was unrelated to diurnal slope (Vanaelst et al., 2012), demonstrating their measurement of different constructs of the physiological stress system (cumulative exposure and diurnal rhythm, respectively). Infant HCC was related to waking and bedtime SCC, contrary to research that found no association between HCC and waking levels in older children (Vanaelst et al. 2012). This highlights the importance of the infant’s basal SCC at the start and end of the day in their cumulative cortisol exposure, and raises the possibility that HCC and SCC are differentially related in infancy and childhood. The expected association between hair cortisol and AUC provides support for the validity of using hair cortisol to assess cumulative cortisol exposure in infancy.
Mothers with higher HCC at 6 and 12 months had infants with higher HCC at 12 months. This is contradictory to the only other study exploring postnatal maternal-infant synchrony of HCC that found no relation at 9 months or 12 months of age (Liu et al., 2016), but is consistent with patterns of mother-child synchrony in basal SCC (e.g., Bright et al., 2012; Letourneau et al., 2011; Stenius et al., 2008), and in HCC in older children (Ouellette et al., 2015). Although it is not clear why contradictory findings were found in infancy, HPA regulation is emerging during this period and may result in variable associations across studies that use different populations, collection techniques and ages. There are several possible reasons as to why synchrony between the mother and infant exists. First, the importance of mothers in the regulation of their children’s HPA axis, such that mothers who are more stressed may in turn be less able to help their child regulate their own stress, is likely to contribute to this synchrony (Gunnar & Talge, 2008). Indeed, research demonstrating a stronger relationship between maternal and child HCC when there is poor parenting and high maternal HCC provides support for this mechanism (Ouellette et al., 2015). Second, mothers and their infants may be exposed to similar environmental stressors, thus resulting in more similar cumulative cortisol exposure (Stenius et al., 2008). Third, it is possible that mother and child have related HCC because HCC is genetically influenced, and the mother and child share some overlapping genes. Several twin studies indicating heritability of basal salivary cortisol (Bartels et al., 2003; Ouellet-Morin et al., 2008; Ouellet-Morin et al., 2009), as well as the demonstrated heritability of hair cortisol in monkeys (Fairbanks et al., 2011), suggest that this is likely partially responsible. No research to date, however, has explored the heritability of HCC in humans. Lastly, it is also possible that postnatal mother-infant synchrony may be a result of prenatal maternal HCC and fetal programming of the HPA axis. Mothers’ prenatal HCC has been shown to predict infant HCC at 1 and 3 years of age (Karlén et al., 2013), yet that study did not measure mothers’ postnatal HCC to disentangle these associations. Similarly, our results cannot address this question as we did not collect mothers’ prenatal HCC. More research is needed to separate prenatal and postnatal effects of maternal HCC on infant stress physiology. Regardless of the mechanism, which is likely to be some combination of all four, our results provide support for the importance of the mother in the infant’s cumulative cortisol exposure, not just acute cortisol levels, and further support the validity of HCC as an index of infant cumulative cortisol exposure.
Sleep, characterized as nighttime sleep duration and sleep disruption, was differentially related to HCC and cortisol diurnal slope. Slope was related to both duration and disruption, whereas HCC was only associated with nighttime sleep duration. Infants sleeping less than 10 hours a night had higher HCC and flatter slopes (i.e., poorer regulation of diurnal rhythm). Increased sleep disruption was also related to flatter slopes. Although lower SES infants were more likely to sleep less than 10 hours per night, the association of sleep with HCC and diurnal rhythm was not attributable to SES. Given that our data indicate an opposite pattern from research on infant cortisol reactivity that shows room sharing with the parent may serve as a protective factor against high salivary cortisol reactivity (Beijers, Riksen-Walraven, & de Weerth, 2013; Tollenaar, Beijers, Jansen, Riksen-Walraven, & de Weerth, 2012), sleep may be differentially affecting different aspects of infant HPA function. Additionally, what was conceptualized as a positive protective buffer may actually be representative of a more overall flattened, or blunted, physiological stress system.
While these results suggest that targeting infant sleep may be a possible avenue for intervention, it is important to note that the association between the two does not tell us about directions of effect. For example, having high cortisol at bedtime could delay or disrupt sleep, and having higher bedtime SCC is consistent with both higher HCC and flattened slope. Thus, it is possible that these infants may be more resistant to sleep interventions. Although limited, research on the emergence of circadian rhythm demonstrates an overall pattern of the parallel emergence of sleep and diurnal cortisol rhythms, or the sleep cycle preceding the diurnal cortisol rhythm (Antonini et al., 2000; de Weerth et al., 2003; Price et al., 1983; Santiago et al., 1996) However, great individual variation exists in the timing of both sleep and circadian rhythms, as well as the order of the two, and there is currently no longitudinal work examining the long-term developmental significance of the timing of diurnal cortisol rhythm. More research is needed to better understand the causal relation between infant cortisol and sleep.
Breastfeeding infants had lower HCC, but did not differ in diurnal cortisol slope. Although maternal cortisol is transmitted to infants through breastmilk (Hamosh, 2001), the skin-to-skin contact and co-regulation associated with the experience of breastfeeding may at the same time have suppressive effects on infant cortisol production, with a net effect of cumulatively lower HCC. Diurnal slope did not relate to breastfeeding status, but mothers were instructed to take the waking sample prior to feeding and wait one hour after feeding before collecting the evening sample. Thus, any transient effects on infant cortisol levels might not be reflected in the saliva samples. Notably, the lower HCC of breastfed infants in our sample is opposite of the two studies demonstrating higher basal SCC in breastfed infants (Cao et al., 2009; Forns et al., 2014). Although low SES mothers are less likely to breastfeed, the association between breastfeeding and HCC in our sample was not explained by SES. While breastfeeding and non-breastfeeding infants may differ on other family or maternal characteristics, these factors are unlikely to explain the lower HCC in breastfed infants, given that none of the maternal factors in our study were related to HCC. More research is needed to better understand the multi-faceted relationship between breastfeeding and cortisol exposure, both chronic and acute.
Both infant HCC and slope were unrelated to all maternal factors explored in this study. Contrary to Azak et al. (2013), mothers with more depressive symptoms did not have infants with flatter slopes. This inconsistency regarding the association between maternal depression and maternal perceived stress with child cortisol levels is, however, not uncommon in the literature (e.g., Palmer et al., 2013; Ouellette et al., 2015). Thus, the relation between maternal depression and other psychosocial factors with child cortisol is still not well understood and warrants further investigation across development. Unlike previous research demonstrating no difference in diurnal rhythm for lower SES infants compared to higher SES infants (Clearfield, Carter-Rodriguez, Merali, & Shober, 2014), we found that infants from lower SES families had flatter diurnal slopes.
In our study, HCC and diurnal slope were unrelated and differentially sensitive to breastfeeding and certain aspects of sleep, demonstrating that they may tap distinct aspects of HPA functioning – cumulative exposure (HCC) versus regulation across the day (diurnal slope). Furthermore, research has demonstrated that environmental risks such as maternal depression, living with birth parents when under Child Protective Services, and regular foster care, are related to flattened slope (e.g., Azak et al., 2013; Bernard et al., 2010; Fisher, Stoolmiller, Gunnar, & Burraston, 2007) and that maternal and familial risk factors are also associated with elevated HCC (Karlen et al., 2013; Palmer et al., 2013; Vaghri et al., 2013), but nothing is known about the interplay between the two. Yet, looking at the interplay between two distinct aspects of the HPA axis can provide a more comprehensive understanding of the infant’s developing HPA system. Because there is a large amount of variation in when circadian cortisol rhythm emerges, slope on its own is hard to interpret during infancy. An infant with frequent responses to stressors across the day could, as a result, have both flattened slope (because levels stay high across the day) and elevated HCC. On the other hand, an infant may have flat slope because their circadian cortisol rhythm has not yet emerged, in which case high HCC would not be expected. Given both our clinical and physiological reasoning, we anticipated that infants showing a combination of high HCC and flattened slope might be those experiencing the most environmental risk. Indeed, our results indicated just that. Infants were classified as 1) steep slope/high HCC 2) steep slope/low HCC 3) flat slope/high HCC and 4) flat slope/low HCC. Infants with flat slope/high HCC, which we considered a dysregulated cortisol profile, were lower SES than infants with flat slope/low hair or steep slope/low HCC. These same flat slope/high HCC infants also had greater sleep disruption than infants with steep slope/high HCC. Furthermore, infants with flat slope/high HCC appear to be more likely to sleep less than 10 hours/night than the other infant cortisol profiles. Thus, considering diurnal slope or HCC alone does not tell the whole story; it is only by examining the interplay between the two that a cortisol profile of infants with flat slope/high HCC emerged as particularly associated with environmental risk. This suggests that any one cortisol measure may not be linearly related to risk. Instead, by looking at several aspects of HPA function in concert we can identify more complex patterns of dysregulation. Importantly, the cortisol profile that is the most associated with risk may change across development. For example, later in childhood and adulthood the combination of low HCC and flat slope, representing maladaptive downregulation of the HPA system, may be particularly “at risk.” Future work should investigate how the combination of HCC and diurnal rhythm interact with environmental risk and developmental outcomes at different points in development.
Limitations
The mother-infant interaction in the current study yielded minimal maternal negativity. Potentially, this lack of maternal negativity reflected the developmental stage of the infants. Maternal negativity may increase as infants get older when frustrations and conflict are more likely to arise in the mother-child interaction. Assessing mother-child interactions across various ages in relation to cortisol measures would help address this question. It is also possible that the task itself, rather than the infant’s age, was not optimal to elicit variability in maternal behavior. The free-play situation is relatively non-stressful and may not elicit maternal insensitivity as well as a more stressful situation (e.g., when the infant is upset, or during a structured task). In fact, one study found that infant cortisol was related to maternal sensitivity only when assessed in a nighttime changing routine, not a daytime free-play interaction (Philbrook et al., 2014). A second limitation of the current research is the relatively low-risk sample. A great majority of mothers were college educated and middle class with relatively high income-to-needs ratios. Yet even in our low-risk sample, we found that SES was significantly related to infant diurnal slope, emphasizing the sensitivity of the infant HPA axis to even modest variations in socioeconomic context. Third, because infant HCC is an emerging literature, it has not yet been determined if the rate of hair growth in infants is the same as that observed in adults (1 cm/month), so we cannot be certain if 3 cm of hair indexed precisely 3 months of cortisol. Lastly, we relied on parent report for waking and sampling times for salivary cortisol collection, which can introduce error to “time since waking” measures because parents may not know exactly when their infant wakes up in the morning, nor accurately record when the infant’s saliva sample was extracted.
Conclusions
We find support for HCC as a valid marker of cumulative cortisol exposure in infancy. Furthermore, our results clearly indicate that infant HCC and diurnal slope are both sensitive to the certain aspects of the environment, but in distinct ways. Investigating the interplay of these different aspects of the HPA axis suggested that dysregulated HPA functioning is nuanced even as early as infancy, and likely dependent on the interaction of various parts of the HPA axis (e.g., high HCC and flattened slope). This highlights the importance of using a more comprehensive approach to our understanding of HPA functioning. Further research is needed to continue investigating the interplay of HCC and salivary cortisol in infancy, as well as across development. Although we provide support for the sensitivity of infant HCC and diurnal slope to the environment, and the importance of the interplay between infant HCC and diurnal slope, nothing is known about the long-term implications of certain cortisol profiles or characteristics. More longitudinal research is needed to better understand various physiological and behavioral outcomes associated with early cortisol dysregulation in infancy. Longitudinal work can also begin to tease apart the direction of effect between cortisol and certain risk factors (e.g., sleep). HPA functioning is nuanced and complex as early as infancy, and our methodological approach should reflect that complexity.
Research Highlights.
We demonstrated maternal-infant synchrony in hair cortisol concentration (HCC) both concurrently and across time.
Infant HCC related to salivary cortisol area under the curve (AUC) but was independent of diurnal slope.
Breastfeeding and nighttime sleep duration were both associated with lower HCC in infants. Person-focused analyses indicated that the combination of high HCC and flat diurnal slope was particularly related to environmental risks.
Acknowledgements
The authors thank Ryan Johnson and Katie Kao for assisting with data collection and Kendra Rosenberg for assaying the hair samples. This research is supported by a National Institute of Child Health and Human Development 1R03HD082550 grant to Dr. Tarullo. We are grateful to the families who participated.
References
- Abidin R, 1995. Parenting Stress Index (PSI), third ed. Psychological Assessment Resources, Lutz, Florida. [Google Scholar]
- Anda RF, Butchart A, Felitti VJ, & Brown DW (2010). Building a framework for global surveillance of the public health implications of adverse childhood experiences. American Journal of Preventative Medicine, 39(1), 93–98. [DOI] [PubMed] [Google Scholar]
- Antonini SR, Jorge SM, & Moreira AC (2000). The emergence of salivary cortisol circadian rhythm and its relationship to sleep activity in preterm infants. Clinical endocrinology, 52(4), 423–426. [PubMed] [Google Scholar]
- Ashman SB, Dawson G, Panagiotides H, Yamada E, & Wilkinson CW (2002). Stress hormone levels of children of depressed mothers. Development and Psychopathology,14(02), 333–349. [DOI] [PubMed] [Google Scholar]
- Atzil S, Hendler T, & Feldman R (2011). Specifying the neurobiological basis of human attachment: brain, hormones, and behavior in synchronous and intrusive mothers. Neuropsychopharmacology, 36(13), 2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azak S, Murison R, Wentzel‐Larsen T, Smith L, & Gunnar MR (2013). Maternal depression and infant daytime cortisol. Developmental Psychobiology, 55(4), 334–351. [DOI] [PubMed] [Google Scholar]
- Bartels M, de Geus EJ, Kirschbaum C, Sluyter F, & Boomsma DI (2003). Heritability of daytime cortisol levels in children. Behavior Genetics,33(4), 421–433. [DOI] [PubMed] [Google Scholar]
- Beijers R, Riksen-Walraven JM, & de Weerth C (2013). Cortisol regulation in 12-month-old human infants: Associations with the infants’ early history of breastfeeding and co-sleeping. Stress: The International Journal on the Biology of Stress, 16(3), 267–277. 10.3109/10253890.2012.742057 [DOI] [PubMed] [Google Scholar]
- Ben‐Dat Fisher D, Serbin LA, Stack DM, Ruttle PL, Ledingham JE, & Schwartzman AE (2007). Intergenerational predictors of diurnal cortisol secretion in early childhood. Infant and Child Development, 16(2), 151–170. [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological), 289–300. [Google Scholar]
- Bernard K, Butzin-Dozier Z, Rittenhouse J, & Dozier M (2010). Cortisol production patterns in young children living with birth parents vs children placed in foster care following involvement of Child Protective Services. Archives of pediatrics & adolescent medicine, 164(5), 438–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Granger DA, Willoughby M, Mills‐Koonce R, Cox M, Greenberg MT, Kivlighan KT, & Fortunato CK (2011). Salivary cortisol mediates effects of poverty and parenting on executive functions in early childhood. Child Development, 82(6), 1970–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock S, & Steptoe A (2013). Influences of early shift work on the diurnal cortisol rhythm, mood and sleep: within-subject variation in male airline pilots. Psychoneuroendocrinology, 38(4), 533–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PA, Pargas R, Walker EF, Green P, Jeffrey Newport D, & Stowe Z (2008). Maternal depression and infant cortisol: influences of timing, comorbidity and treatment. Journal of Child Psychology and Psychiatry, 49(10), 1099–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright MA, Granger DA, & Frick JE (2012). Do infants show a cortisol awakening response?. Developmental Psychobiology, 54(7), 736–743. [DOI] [PubMed] [Google Scholar]
- Bureau JF, Easterbrooks M, & Lyons-Ruth K (2009). Maternal depressive symptoms in infancy: Unique contribution to children’s depressive symptoms in childhood and adolescence?. Development and Psychopathology, 21(02), 519–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell BM, & Bradley RH (1984). Home observation for measurement of the environment. Little Rock: University of Arkansas at little Rock. [Google Scholar]
- Cao Y, Rao SD, Phillips TM, Umbach DM, Bernbaum JC, Archer JI, & Rogan WJ (2009). Are breast-fed infants more resilient? Feeding method and cortisol in infants. The Journal of Pediatrics, 154(3), 452–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clearfield MW, Carter-Rodriguez A, Merali A-R, & Shober R (2014). The effects of SES on infant and maternal diurnal salivary cortisol output. Infant Behavior and Development, 37(3), 298–304. 10.1016/j.infbeh.2014.04.008 [DOI] [PubMed] [Google Scholar]
- D’Anna-Hernandez KL, Ross RG, Natvig CL, & Laudenslager ML (2011). Hair cortisol levels as a retrospective marker of hypothalamic-pituitary axis activity throughout pregnancy: comparison to salivary cortisol. Physiology & Behavior, 104(2), 348–353. 10.1016/j.physbeh.2011.02.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Weerth C, Zijl RH, & Buitelaar JK (2003). Development of cortisol circadian rhythm in infancy. Early human development, 73(1), 39–52. [DOI] [PubMed] [Google Scholar]
- Dressendörfer RA; Kirschbaum C; Rohde W; Stahl F & Strasburger CJ (1992). Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. Journal of Steroid Biochemistry and Molecular Biology, 43(7): 683–692. [DOI] [PubMed] [Google Scholar]
- Fairbanks LA, Jorgensen MJ, Bailey JN, Breidenthal SE, Grzywa R, & Laudenslager ML (2011). Heritability and genetic correlation of hair cortisol in vervet monkeys in low and higher stress environments. Psychoneuroendocrinology, 36(8), 1201–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R, & Eidelman AI (2003). Direct and indirect effects of breast milk on the neurobehavioral and cognitive development of premature infants. Developmental Psychobiology, 43(2), 109–119. [DOI] [PubMed] [Google Scholar]
- Feldman R (2007). Parent–infant synchrony and the construction of shared timing; physiological precursors, developmental outcomes, and risk conditions. Journal of Child Psychology and Psychiatry, 48(3‐4), 329–354. [DOI] [PubMed] [Google Scholar]
- Feldman R, Gordon I, & Zagoory‐Sharon O (2011). Maternal and paternal plasma, salivary, and urinary oxytocin and parent–infant synchrony: considering stress and affiliation components of human bonding. Developmental Science, 14(4), 752–761. [DOI] [PubMed] [Google Scholar]
- Fisher PA, Stoolmiller M, Gunnar MR, & Burraston BO (2007). Effects of a therapeutic intervention for foster preschoolers on diurnal cortisol activity. Psychoneuroendocrinology, 32(8), 892–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forns J, Vegas O, Julvez J, Garcia‐Esteban R, Rivera M, Lertxundi N, Guxens M, Fano E, Ferrer M, Grellier J, Ibarluzea J, & Sunyer J (2014). Association between child cortisol levels in saliva and neuropsychological development during the second year of life, Stress and Health, 30(2), 142–148. [DOI] [PubMed] [Google Scholar]
- Fries E, Dettenborn L, & Kirschbaum C (2009). The cortisol awakening response (CAR): facts and future directions. International Journal of Psychophysiology, 72(1), 67–73. [DOI] [PubMed] [Google Scholar]
- Grey KR, Davis EP, Sandman CA, & Glynn LM (2013). Human milk cortisol is associated with infant temperament. Psychoneuroendocrinology, 38(7), 1178–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribbin CE, Watamura SE, Cairns A, Harsh JR, & LeBourgeois MK (2012). The cortisol awakening response (CAR) in 2‐ to 4‐year‐old children: Effects of acute nighttime sleep restriction, wake time, and daytime napping. Developmental Psychobiology, 54(4), 412–422. 10.1002/dev.20599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, & Donzella B (2002). Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology, 27(1), 199–220. [DOI] [PubMed] [Google Scholar]
- Gunnar M, & Talge NM (2008). Neuroendocrine measures in developmental research, in: Schmidt LA, Segalowitz SJ. (Eds.), Developmental Psychophysiology: Theory, Systems, and Methods. Cambridge University Press, New York, pp. 343–364. [Google Scholar]
- Hamosh M (2001). Bioactive factors in human milk. Pediatric Clinics of North America, 48(1), 69–86. [DOI] [PubMed] [Google Scholar]
- Karlén J, Frostell A, Theodorsson E, Faresjö T, & Ludvigsson J (2013). Maternal influence on child HPA axis: a prospective study of cortisol levels in hair. Pediatrics, 132(5), e1333–e1340. [DOI] [PubMed] [Google Scholar]
- Laurent HK, Leve LD, Neiderhiser JM, Natsuaki MN, Shaw DS, Fisher PA, Marceau K, Harold GT, & Reiss D (2013). Effects of parental depressive symptoms on child adjustment moderated by hypothalamic pituitary adrenal activity: within-and-between-family risk. Child Development, 84(2), 528–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBeau MA, Montgomery MA, & Brewer JD (2011). The role of variations in growth rate and sample collection on interpreting results of segmental analyses of hair. Forensic Science International, 210(1), 110–116. [DOI] [PubMed] [Google Scholar]
- Letourneau N, Watson B, Duffett-Leger L, Hegadoren K, & Tryphonopoulos P (2011). Cortisol patterns of depressed mothers and their infants are related to maternal–infant interactive behaviours. Journal of Reproductive and Infant Psychology, 29(5), 439–459. [Google Scholar]
- Liu CH, Snidman N, Leonard A, Meyer J, & Tronick E (2016). Intra‐individual stability and developmental change in hair cortisol among postpartum mothers and infants: Implications for understanding chronic stress. Developmental Psychobiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, King S, Meaney MJ, & McEwen BS (2000). Child’s stress hormone levels correlate with mother’s socioeconomic status and depressive state. Society of Biological Psychiatry, 48(10), 976–980. [DOI] [PubMed] [Google Scholar]
- McEwen B, 2006. Protective and damaging effects of stress mediators: Central role of the brain. Dialogues in Clinical Neuroscience, 8(4), 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JS, Novak MA, 2012. Minireview: Hair cortisol: a novel biomarker of hypothalamic-pituitary-adrenocortical activity. Endocrinology, 153(9), 4120–4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J, Novak M, Hamel A, & Rosenberg K (2014). Extraction and analysis of cortisol from human and monkey hair. Journal of Visualized Experiments, (83), e50882, http://doi: 10.3791/50882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neelon SEB, Stroo M, Mayhew M, Maselko J, & Hoyo C (2015). Correlation between maternal and infant cortisol varies by breastfeeding status. Infant Behavior and Development, 40, 252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellet-Morin I, Boivin M, Dionne G, Lupien SJ, Arseneault L, Barr RG, Perusse D, & Tremblay RE (2008): Variations in heritability of cortisol reactivity to stress as a function of early familial adversity among 19-month-old twins. Archives of General Psychiatry, 65(2), 211–218. [DOI] [PubMed] [Google Scholar]
- Ouellet-Morin I, Dionne G, Pérusse D, Lupien SJ, Arseneault L, Barr RG, Tremblay RE, & Boivin M (2009). Daytime cortisol secretion in 6-month-old twins: genetic and environmental contributions as a function of early familial adversity. Biological Psychiatry, 65(5), 409–416. [DOI] [PubMed] [Google Scholar]
- Ouellette SJ, Russell E, Kryski KR, Sheikh HI, Singh SM, Koren G, & Hayden EP (2015). Hair cortisol concentrations in higher‐and lower‐stress mother–daughter dyads: A pilot study of associations and moderators. Developmental Psychobiology, 57(5), 519–534. [DOI] [PubMed] [Google Scholar]
- Palmer FB, Anand KJ, Graff JC, Murphy LE, Qu Y, Völgyi E, Rovnaghi CR, Moore A, Tran QT, & Tylavsky FA (2013). Early adversity, socioemotional development, and stress in urban 1-year-old children. The Journal of Pediatrics, 163(6), 1733–1739. [DOI] [PubMed] [Google Scholar]
- Philbrook LE, Hozella AC, Kim BR, Jian N, Shimizu M, & Teti DM (2014). Maternal emotional availability at bedtime and infant cortisol at 1 and 3 months. Early Human Development, 90(10), 595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DA, Close GC, & Fielding BA (1983). Age of appearance of circadian rhythm in salivary cortisol values in infancy. Archives of Disease in Childhood, 58(6), 454–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, & Hellhammer DH (2003). Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology, 28(7), 916–931. [DOI] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D scale a self-report depression scale for research in the general population. Applied Psychological Measurement,1(3), 385–401. [Google Scholar]
- Russell E, Koren G, Rieder M, Van Uum S, 2012. Hair cortisol as a biological marker of chronic stress: current status, future directions and unanswered questions. Psychoneuroendocrinology 37(5), 589–601. [DOI] [PubMed] [Google Scholar]
- Sadeh A (2004). A brief screening questionnaire for infant sleep problems: validation and findings for an Internet sample. Pediatrics, 113(6), e570–e577. [DOI] [PubMed] [Google Scholar]
- Santiago LB, Jorge SM, & Moreira AC (1996). Longitudinal evaluation of the development of salivary cortisol circadian rhythm in infancy. Clinical endocrinology, 44(2), 157–161. [DOI] [PubMed] [Google Scholar]
- Stalder T, Bäumler D, Miller R, Alexander N, Kliegel M, & Kirschbaum C (2013). The cortisol awakening response in infants: Ontogeny and associations with development-related variables. Psychoneuroendocrinology, 38(4), 552–559. [DOI] [PubMed] [Google Scholar]
- Stenius F, Theorell T, Lilja G, Scheynius A, Alm J, & Lindblad F (2008). Comparisons between salivary cortisol levels in six-months-olds and their parents. Psychoneuroendocrinology, 33(3), 352–359. 10.1016/j.psyneuen.2007.12.001 [DOI] [PubMed] [Google Scholar]
- Taylor ZE, Spinrad TL, VanSchyndel SK, Eisenberg N, Huynh J, Sulik MJ, & Granger DA (2013). Sociodemographic risk, parenting, and effortful control: Relations to salivary alpha‐amylase and cortisol in early childhood. Developmental Psychobiology, 55(8), 869–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollenaar MS, Beijers R, Jansen J, Riksen-Walraven JMA, & de Weerth C (2012). Solitary sleeping in young infants is associated with heightened cortisol reactivity to a bathing session but not to a vaccination. Psychoneuroendocrinology, 37(2), 167–177. 10.1016/j.psyneuen.2011.03.017 [DOI] [PubMed] [Google Scholar]
- Vaghri Z, Guhn M, Weinberg J, Grunau RE, Yu W, & Hertzman C (2013). Hair cortisol reflects socio-economic factors and hair zinc in preschoolers. Psychoneuroendocrinology, 38(3), 331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanaelst B, Huybrechts I, Bammann K, Michels N, de Vriendt T, Vyncke K, Sioen I, Iacoviello L, Günther K, Molnar D, Lissner L, Rivet N, Raul JS, & De Henauw S (2012). Intercorrelations between serum, salivary, and hair cortisol and child-reported estimates of stress in elementary school girls. Psychophysiology, 49(8), 1072–1081. 10.1111/j.1469-8986.2012.01396.x [DOI] [PubMed] [Google Scholar]
- Vanaelst B, Michels N, De Vriendt T, Huybrechts I, Vyncke K, Sioen I, Bammann K, Rivet N, Raul JS, Molnar D, & De Henauw S (2013). Cortisone in hair of elementary school girls and its relationship with childhood stress. European Journal of Pediatrics, 172(6), 843–846. 10.1007/s00431-013-1955-1 [DOI] [PubMed] [Google Scholar]
- Vliegenthart J, Noppe G, van Rossum EFC, Koper JW, Raat H, van den Akker ELT (2015). Socioeconomic status in children is associated with hair cortisol levels as a biological measure of chronic stress. Psychoneuroendocrinology. [DOI] [PubMed] [Google Scholar]
- Xie Q, Gao W, Li J, Qiao T, Jin J, Deng H, & Lu Z (2011). Correlation of cortisol in 1-cm hair segment with salivary cortisol in human: hair cortisol as an endogenous biomarker. Clinical Chemistry and Laboratory Medicine, 49(12), 2013–2019. [DOI] [PubMed] [Google Scholar]