Abstract
Metal-catalyzed reductive coupling has emerged as an alternative to the use of stoichiometric organometallic reagents in an increasingly diverse range of carbonyl and imine additions. In this monograph, the use of diene, allene and enyne pronucleophiles in intermolecular carbonyl and imine reductive couplings are surveyed along with related hydrogen auto-transfer processes.
Graphical Abstract

1. Introduction and Historical Perspective on Carbonyl Addition
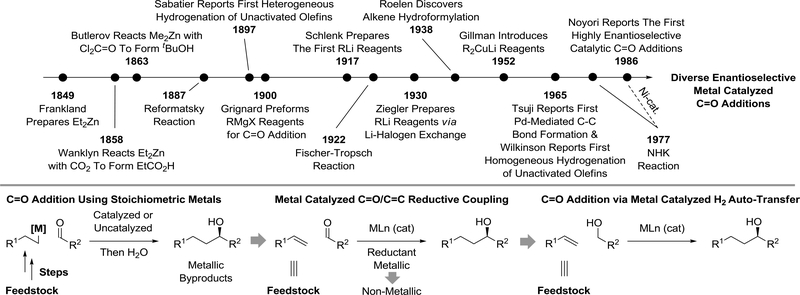
Following Frankland’s preparation of diethylzinc in 18491–3 were the first reports of the addition of premetalated C-nucleophiles to carbonyl compounds. For example, in 1858, Frankland’s protégé James Wanklyn described the addition of ethylsodium to carbon dioxide to form propionic acid assisted by sodium triethylzincate, NaZn(C2H5)3.4,5 Literature from this time period include references to the reaction of transient organometallics with carbonyl compounds, however, it was not until the systematic studies of Butlerov6,7 (and his “Kazan school” progeny)8,9 and Grignard10,11 that the addition of premetalated C-nucleophiles to carbonyl compounds took root as a major method for chemical synthesis. Subsequent milestones in organometallic and carbonyl addition chemistry include the generation of organolithium reagents12,13 and, therefrom, cuprates,14 the advent of palladium-mediated C-C coupling.15,16 Finally, in 1986 Noyori reported the first highly enantioselective catalytic method for carbonyl addition17 – a progenitor to the numerous methods now available for the catalytic enantioselective addition of non-stabilized carbanions and their equivalents to carbonyl compounds and imines (Figure 1).18–24
Figure 1.
Selected milestones in organometallic and carbonyl addition chemistry.
While carbonyl addition mediated by premetalated reagents continues to play an important role in chemical synthesis,25 the requisite organometallic reagents are hazardous, frequently require cryogenic conditions and generate stoichiometric quantities of metallic byproducts, which complicates large-volume applications. Metal-catalyzed reductive couplings of π-unsaturated reagents with carbonyl compounds provides a more ideal alternative to the use of stoichiometric organometallic reagents. Fischer-Tropsch type reactions (1922)26,27 and alkene hydroformylation (1938)28 may be considered the prototypical metal-catalyzed reductive C-C couplings. However, even with the advent of heterogeneous25 and homogenous hydrogenation,30 catalytic reductive couplings beyond carbon monoxide did not appear until much later and hydrogen-mediated reductive couplings were not systematically studied until the work of Krische.31–33 The discovery that nickel salts catalyze chromium(II)mediated couplings of organic halides to carbonyl compounds (the Nozaki-Hiyama-Kishi reaction)34–37 advanced the concept of metal-catalyzed reductive carbonyl addition, as well as the quest for more benign, less mass-intensive terminal reductants. Metal-catalyzed reductive coupling gradually emerged as a discrete field of inquiry.31–33,37–48
The arc of science traced from these early advances in organometallic chemistry to the current state-of-the-art in carbonyl addition chemistry defines a progression from (a) classical reactions of premetalated C-nucleophiles (with or without a metal catalyst)18–23 to (b) metal-catalyzed reductive couplings of π-unsaturated reactants (with metallic or non-metallic terminal reductants)38–48 and, finally, (c) carbonyl additions that proceed through alcohol-mediated hydrogen auto-transfer (Figure 1).24,43–48 The selective pressure of efficiency has guided this evolution: many reactions that traditionally exploit premetalated reagents can now be conducted catalytically in the absence of stoichiometric metals or byproducts with high levels of stereocontrol. Additionally, many transformations that have no counterpart in classical carbonyl or imine addition chemistry have been discovered.
In this review, intermolecular metal-catalyzed reductive couplings of 1,3-dienes, allenes or 1,3-enynes with carbonyl compounds and imines are surveyed. Discussion is restricted to processes that result in both C-H and C-C bond formation, ideally in (formal) additions of H2 across the π-unsaturated pronucleophile and C=X (X = O, NR) π-bond. Related reductive cyclizations,39 multi-component reductive couplings (alkylative/arylative,49–51 borylative52–58 or, more generally, bismetalative59–61) and reductive couplings to carbon dioxide62–67 are not covered and the reader is referred to the review literature and selected examples. Processes wherein the π-unsaturated pronucleophile is stoichiometrically reduced to a discrete nucleophilic species (for example, through hydrometalation) to which the carbonyl or imine partner is subsequently exposed, are not covered.68–70
2. Diene-C=X (X = O, NR) Reductive Coupling
2.1. Nickel
Following Mori and Sato’s seminal report in 1994 on the nickel-catalyzed reductive cyclization of dienyl aldehydes mediated by silane,71 related intermolecular diene-aldehyde reductive couplings were developed by Tamaru and Kimura in 1998 using triethylborane as terminal reductant.72 As illustrated in homoallylations of benzaldehyde, feedstock dienes such as isoprene and myrcene engage in C-C coupling with exceptional levels of regio- and 1,3-anti-diastereoselectivity at ambient temperature. The same year, an intermolecular silane-mediated variant of this process was reported by Mori and Sato using terminal dienes.73 While uniformly high levels of regioselectivity were accompanied by complete olefin (E:Z)-stereocontrol in additions to aryl aldehydes, related couplings of aliphatic aldehydes displayed incomplete levels of alkene stereoselectivity. Mori and Sato later showed that in reductive couplings of aldehydes with trialkylsilyl substituted dienes, Ph3P-modified nickel catalysts enable (E)selective allylation (not shown) whereas NHC-modified nickel catalysts enable (Z)-selective allylation.74 Further studies by Tamaru and Kimura revealed that diene-aldehyde reductive couplings mediated by diethylzinc are particularly effective for couplings of aliphatic aldehydes and ketones, although erosion of regioselectivity is observed in the latter case.75 Using the Ni(acac)2/Et3B catalyst system, lactols and aqueous glutardialdehyde undergo highly diastereoselective homoallylation illustrating compatibility with hydroxyl functional groups (Scheme 1).76 Diisobutylaluminum acetylacetonate is also a viable reductant in couplings of dienes bearing terminal aryl substituents, however, mixtures of linear and branched regioisomers are observed (not shown).77 Using the Ni(acac)2/Et3B catalyst system, cyclohexadiene-aldehyde reductive coupling is possible but roughly equimolar diastereomeric mixtures are observed (not shown).78
Scheme 1.
Nickel(0)-catalyzed diene-aldehyde reductive coupling
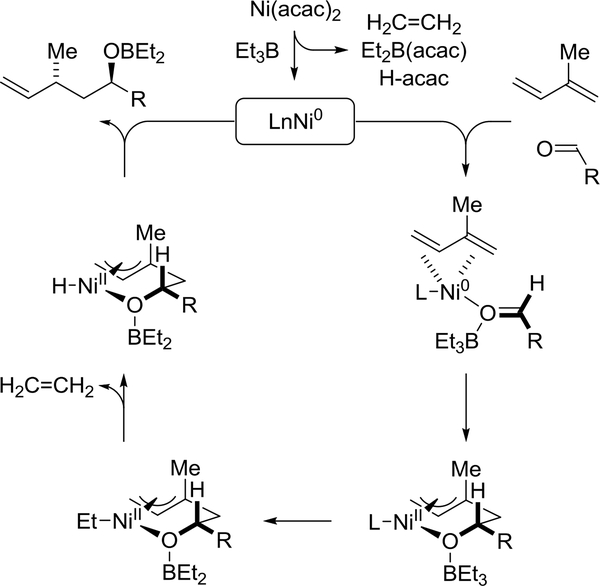
A general mechanism for Ni(acac)2/Et3B-catalyzed diene-aldehyde reductive coupling that accounts for the observed regio- and 1,3-anti-diastereoselectivity has been proposed (Scheme 2).79 Coordination of diene and aldehyde by nickel generates a nucleophilic π-complex, which upon triethylborane assisted oxidative coupling delivers the indicated π-allyl-oxanickelacycle. Computational studies on related nickel(0)-catalyzed alkyne-aldehyde reductive couplings mediated by triethylborane suggest the LUMOlowering effect evident upon the binding of triethylborane to the aldehyde accelerates oxidative coupling.80 As illustrated in elegant work by Ogoshi,81 reversible diene-aldehyde oxidative coupling is observed in stoichiometric reactions with nickel(0) to provide isolable π-allylalkoxynickel(II) complexes that have been characterized by single crystal X-ray diffraction analysis. Ethyl transfer to the nickel(II) center followed by β-hydride elimination provides a π-allylnickel hydride, which upon reductive elimination delivers the product of reductive coupling and returns nickel(II) to its zero-valent form.
Scheme 2.
Catalytic mechanism for nickel(0)-catalyzed diene-aldehyde reductive coupling
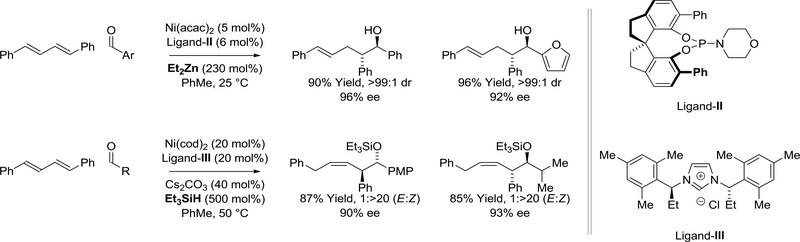
The development of general methods for enantioselective nickel(0)-catalyzed diene-aldehyde reductive coupling remains a largely unresolved challenge. In 2007, Zhou reported the anti-diastereo- and enantioselective reductive coupling of 1,4-diphenylbutadiene with aromatic aldehydes mediated by diethylzinc using a nickel catalyst modified by a monodentate spiro-phosphoramidite, ligand-II.82 The same year, Sato reported a silane-mediated diene-aldehyde reductive coupling using the C2-symmetric ligand-III.83 While this process delivers (Z)-homoallylic silyl ethers with excellent control of olefin geometry and diastereoselectivity, high levels of enantioselectivity were again only evident in reactions of 1,4-diphenylbutadiene (Scheme 3).
Scheme 3.
Enantioselective nickel(0)-catalyzed diene-aldehyde reductive couplings
The requirement of reductants that are pyrophoric (Et2Zn and Et3B) or those that are costly and mass-intensive (R3SiH) represents a major limitation associated with the methods described above. In a significant departure from prior art, Krische and Breit reported the reductive coupling of 2-substituted dienes wherein formaldehyde serves as both electrophile and reductant (Scheme 4).84 In these processes, the transient π-allylalkoxynickel(II) complex inserts formaldehyde and undergoes β-hydride elimination to deliver coupling products as the formate esters, which are cleaved in the course of isolation. Depending on the diene 2-substituent, a preference for C-C bond formation at either the C1 or C4 position is observed. For dienes incorporating alkyl or aryl groups at the 2-substituent, dieneformaldehyde oxidative coupling at C1 is kinetically preferred. For corresponding silicon- and tinsubstituted dienes, hyperconjugation between the C–Si or C–Sn σ-bond and the σ* orbital of the newly formed C-C bond renders oxidative coupling at C1 reversible, enabling oxidative coupling at C4 which is thermodynamically preferred. Reversibility in diene-carbonyl oxidative coupling and the kinetic vs thermodynamic preference for C1 vs C4 oxidative coupling, respectively, has been demonstrated in mechanistic studies by Ogoshi.81
Scheme 4.
Formaldehyde as electrophile and reductant in nickel(0)-catalyzed reductive couplings to 2substituted dienes
In 2004, Tamaru and Kimura reported the first nickel(0)-catalyzed reductive coupling of dienes and imines.85 This process conveniently generates the imine in situ from the aldehyde and p-anisidine with subsequent introduction of the nickel acetylacetonate, diene and diethylzinc. Coupling occurs predominantly at the diene C1 position to provide the products with good to complete levels of 1,3-syndiastereoselectivity. Using triethylborane as terminal reductant, the authors later extended this process to encompass imines derived from lactols.86 As reported by Singh in 2015, exposure of p-anisidine to aryl aldehydes bearing ortho-carboxy substituents enables tandem nickel(0)-catalyzed diene-imine reductive coupling-lactam formation to form isoindolinones and isoquinolinones (Scheme 5).87 For related 3component couplings of dienes, imines and organozinc reagents, the reader is referred to the review literature.50
Scheme 5.
Nickel(0)-catalyzed diene-imine reductive couplings
2.2. Ruthenium
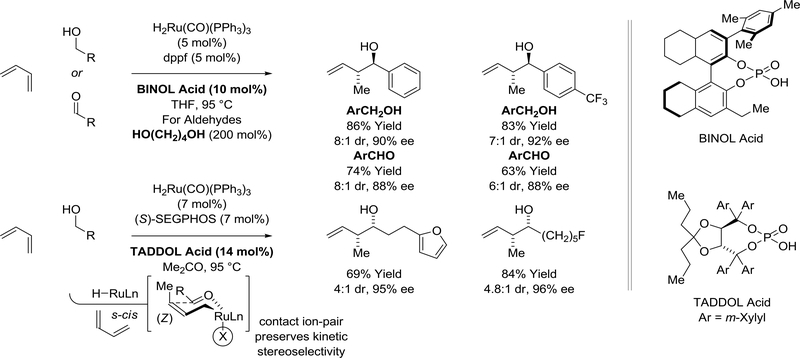
The first ruthenium(II)-catalyzed diene-carbonyl reductive coupling was reported in 2008 by Krische (Scheme 6).88 Using 2-propanol or formic acid as reductants, butadiene, isoprene or 2,3dimethylbutadiene react with aldehydes to form branched products of C-C coupling as single regioisomers. Unlike the nickel(0)-based catalysts systems, which operate through pathways involving diene-carbonyl oxidative coupling, the present ruthenium(II)-catalyzed processes involve diene hydrometalation to provide nucleophilic allylruthenium(II) intermediates. Related stoichiometric reactions of HClRu(CO)(PPh3)3 with 1,3-dienes to form well defined π-allylruthenium complexes have been documented.89 Carbonyl addition occurs by way of the primary σ-allyl haptomer through the indicated closed six-centered transition state. Under these conditions, primary alcohols transfer hydrogen to dienes to form aldehyde-allylruthenium pairs that combine to form homoallylic alcohols. The reaction of isoprene with d2-benzyl alcohol results in deuterium transfer to the allylic methyl (32% 2H) and allylic methine (14% 2H), corroborating reversible hydrometalation of the less substituted olefin with incomplete regiocontrol. The reaction products resist further dehydrogenation by coordination of the homoallylic olefin to the ruthenium center.90
Scheme 6.
Ruthenium(II)-catalyzed diene-aldehyde reductive couplings mediated by 2-propanol, formic acid or hydrogen auto-transfer
Relative and absolute stereocontrol is enforced using chiral ruthenium(II) complexes modified by (R)-DM-SEGPHOS in combination with 2-trialkylsilyl-butadienes (Scheme 7).91 Hydrometalation of 2trialkylsilyl-substituted dienes delivers crotylmetal species that exist predominantly as single geometrical isomers due to allylic strain.92,93 Stereospecific carbonyl addition provides the branched products of reductive coupling with high levels of syn-diastereo- and enantioselectivity. Carbonyl addition can be conducted from the aldehyde oxidation level using 2-propanol as terminal reductant or from the alcohol oxidation level via hydrogen auto-transfer. A significant extension in scope was made in 2017 by Brimble, who reports the crotylation of chiral alcohols to form polyketide stereotriads, which avoids the use of configurationally labile chiral α-stereogenic aldehydes.94
Scheme 7.
syn-Diastereo- and enantioselective ruthenium(II)-catalyzed reductive coupling of aldehydes with 2-trialkylsilyl substituted dienes mediated by 2-propanol or hydrogen auto-transfer
The ability to exploit butadiene itself, an abundant petrochemical feedstock, in regio- and stereocontrolled diene-carbonyl reductive coupling would represent a powerful alternative to stoichiometric reactions of chiral crotylmetal reagents.95 Using a ruthenium catalyst modified by a chiral phosphate counterion derived from H8-BINOL, direct anti-diastereo- and enantioselective hydrohydroxyalkylations of butadiene can be achieved from the alcohol or aldehyde oxidation level (Scheme 8).96 In the latter case, 1,4-butanediol is used as terminal reductant.97 Notably, the chiral counterion is the sole chiral inducing element. Corresponding syn-diastereo- and enantioselective butadiene-mediated carbonyl crotylations take advantage of match-mismatch effects between the indicated TADDOL-derived phosphate counterion and the chiral ligand, (S)-SEGPHOS.98 In both processes, butadiene hydroruthenation from the s-cis conformer delivers the (Z)-σ-crotylruthenium haptomer. For the more Lewis basic TADDOL-phosphate counterion, formation of a contact ion-pair with the ruthenium(II) center preserves kinetic (Z)-selectivity and stereospecific carbonyl addition provides the product of syn-crotylation. For the less Lewis basic BINOL-phosphate counterion, open coordination sites on ruthenium enable isomerization of the initially formed (Z)-σ-crotylruthenium haptomer to the thermodynamically preferred (E)-σ-crotylruthenium haptomer, which, in turn, provides the product of anti-crotylation. Computational studies suggest the transition state for aldehyde addition from the (Z)-σcrotylruthenium haptomer is facilitated by formation of a formyl hydrogen bond between the aldehyde C-H and phosphate oxo-moiety.99
Scheme 8.
Divergent diastereoselectivity in asymmetric ruthenium(II)-catalyzed butadiene-aldehyde reductive couplings
For 2-substituted dienes, the divergent behavior of neutral vs cationic ruthenium(II) catalysts manifests as regioisomeric carbonyl addition pathways. 2-Propanol-mediated reductive couplings of 2substituted dienes with paraformaldehyde illustrate this effect (Scheme 9).84,100–102 Whereas neutral ruthenium catalysts promote coupling at C3,83 ruthenium catalysts with greater cationic character promote coupling at C2.101 However, upon use of higher carbonyl partners, such as acetaldehyde (from ethanol), an erosion in C2-regioselectivity is observed.102 As corroborated by deuterium labelling studies (not shown), the collective data suggest the following mechanistic interpretation. Diene hydroruthenation at the less substituted olefin to form π-allyl A is kinetically preferred. For neutral ruthenium catalysts, this kinetic preference is preserved and C3 adducts are formed. In contrast, vacant coordination sites of cationic ruthenium complexes enable reversible diene hydroruthenation, allowing equilibration between π-allyl A and π-allyl B.103 A Curtin-Hammett scenario becomes operative. For small aldehydes (R1 = H), the transition state en route to C2-adducts is lower in energy. For larger aldehydes (R1 = Me), formation of a more congested quaternary carbon stereocenter elevates the energetic barrier to carbonyl addition, eroding C2-regioselectivity.
Scheme 9.
Divergent regioselectivity in ruthenium(II)-catalyzed diene-carbonyl reductive couplings
Ruthenium(0) complexes derived from Ru3(CO)12 and tricyclohexylphosphine catalyze the reductive coupling of dienes with α-ketoesters from the α-hydroxy ester oxidation level via hydrogen auto-transfer (Scheme 10).104 Butadiene, isoprene and myrcene deliver products of carbinol C-H (Z)-butenylation, prenylation and geranylation, respectively, as single regioisomers. A discrete mononuclear ruthenium(0) catalyst105 initiates the catalytic cycle by diene-carbonyl oxidative coupling.106,107 Transfer hydrogenolytic cleavage of the resulting oxaruthenacycle occurs through protonation at oxygen mediated by the α-hydroxy ester reactant followed by β-hydride elimination and C-H reductive elimination. This interpretation of the mechanism was corroborated by deuterium labelling studies. Upon use of deuterated rac-ethyl mandelate, the n-prenylated adduct incorporating deuterium exclusively at the cis-methyl group (2H 50%) was obtained. Under nearly identical conditions, related diene reductive couplings to isatins were achieved from the 3-hydroxy-2-oxindole oxidation level via hydrogen auto-transfer.108
Scheme 10.
Ruthenium(0)-catalyzed diene-ketone reductive coupling via hydrogen auto-transfer
Heteroaromatic ketones with vicinal dicarbonyl character undergo ruthenium(0)-catalyzed diene-carbonyl reductive coupling mediated by 2-propanol (Scheme 11).109 Alternatively, diene-carbonyl reductive coupling can be conducted from the secondary alcohol oxidation level via hydrogen autotransfer. The putative oxaruthenacycle intermediate was isolated and characterized by single crystal Xray diffraction. Remarkably, experiments involving diene exchange demonstrate reversible metallacycle formation.
Scheme 11.
Reversible oxidative coupling pathways in ruthenium(0)-catalyzed diene-ketone reductive coupling
As illustrated in formic acid-mediated reductive diene-dione [4+2] cycloadditions, πallylruthenium intermediates obtained upon diene-carbonyl oxidative coupling can be captured through intramolecular addition to the carbonyl moiety of a vicinal dione precursor (Scheme 12).110–113 Cycloaddition is possible from dione, ketol (not shown) or diol oxidation levels. Given the greater tractability and abundance of 1,2-diols, a focus was placed on diene-dione [4+2] cycloadditions via hydrogen auto-transfer. Acyclic dienes react with cyclic or acyclic diols to form [4+2] cycloadducts bearing bridgehead diols with complete syn-diastereoselectivity. Similarly, in reactions of cyclohexadiene, cyclic or acyclic diols provide syn-configured cycloadducts with complete levels of exoselectivity.112
Scheme 12.
Ruthenium(0)-catalyzed diene-dione reductive coupling resulting in [4+2] cycloaddition
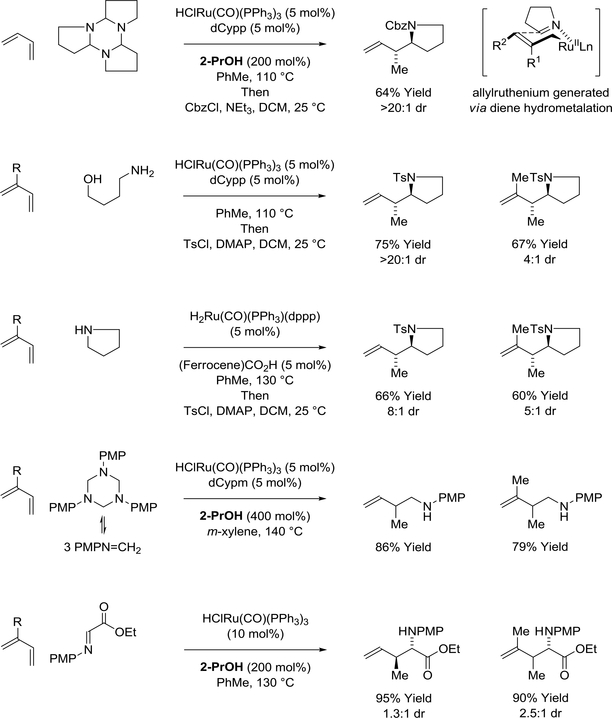
Using ruthenium(II) catalysts, 2-propanol-mediated reductive coupling of butadiene with the trimeric imine, dihydropyrrole, provides the branched product of addition in a completely regio- and anti-diastereoselective manner (Scheme 13).114 In related reactions of 4-aminobutanol, hydrogen transfer from the primary alcohol triggers cyclocondensation to form the imine, dihydropyrrole. The resulting allylruthenium-imine pair undergoes stereospecific addition through a closed transition structure to deliver the branched adducts with good to complete levels of anti-diastereoselectivity. Pyrrole itself can serve dually as reductant and imine proelectrophile to deliver identical products of addition. Other imines participate in ruthenium(II)-catalyzed diene reductive coupling mediated by 2propanol. For example, 1,3,5-tris(4-methoxyphenyl)-hexahydro-1,3,5-triazine, which undergoes thermal cycloreversion to generate formaldimines in situ, provides products of hydroaminomethylation as single regioisomers.115 Additionally, iminoacetates engage in regioselective 2-propanol-mediated reductive coupling with dienes, albeit with modest levels of anti-diastereoselectivity.116
Scheme 13.
Ruthenium(II)-catalyzed diene-imine reductive coupling mediated by 2-propanol or hydrogen auto-transfer
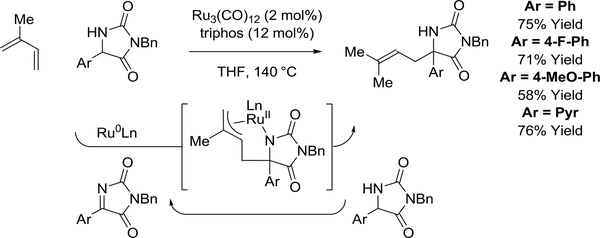
A single report of ruthenium(0)-catalyzed diene-imine reductive coupling via hydrogen autotransfer appears in the literature.117 Using a ruthenium(0) catalyst derived from Ru3(CO)12 and triphos [PhP(CH2CH2PPh2)2], isoprene reacts with aryl substituted hydantoins to give products of n-prenylation as single regioisomers (Scheme 14). In this process, hydantoin dehydrogenation is followed by dieneimine oxidative coupling to furnish a transient aza-ruthenacycle. Transfer hydrogenolysis of the azaruthenacycle mediated by the hydantoin releases the product and regenerates the requisite imine to close the catalytic cycle.
Scheme 14.
Ruthenium(0)-catalyzed diene-imine reductive coupling via hydrogen auto-transfer
2.3. Rhodium
Only two reports on rhodium catalyzed diene-carbonyl reductive coupling appear in the literature. In 2003, Krische described a reductive coupling of cyclohexadiene with α-ketoaldehydes mediated by hydrogen (Scheme 15).118 In 2009, Kimura described a triethylborane-mediated dienealdehyde reductive coupling, which displayed good levels of regio- and syn-diastereoselectivity.119
Scheme 15.
Rhodium(I)-catalyzed diene-carbonyl reductive couplings mediated by hydrogen and triethylborane
2.4. Iridium
Under the conditions of iridium catalyzed transfer hydrogenation, 2-propanol-mediated reductive coupling of 1,3-cyclohexadiene with aryl aldehydes provide products of carbonyl cyclohexenylation in good yield with complete levels of diastereocontrol (Scheme 16).120 Under nearly identical conditions, but in the absence of 2-propanol, 1,3-cyclohexadiene reductively couples to primary benzylic alcohols via hydrogen auto-transfer to furnish identical products with comparable levels of selectivity. In each case, small quantities of the regioisomeric γ,δ-unsaturated alcohols could be detected.
Scheme 16.
Iridium(I)-catalyzed cyclohexadiene-aldehyde reductive coupling mediated by 2-propanol or hydrogen auto-transfer
Cyclometalated π-allyliridium C,O-benzoates catalyze butadiene-aldehyde reductive coupling mediated by 1,4-butanediol97 to furnish products of carbonyl crotylation.121 Although enantioselective variants of this process were disclosed, diastereomeric mixtures were obtained (not shown). Under identical conditions, but in the absence of 1,4-butanediol, butadiene reductively couples to primary benzylic alcohols via hydrogen auto-transfer to furnish identical products with similar levels of selectivity.
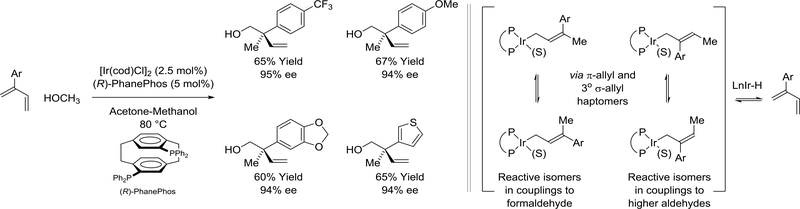
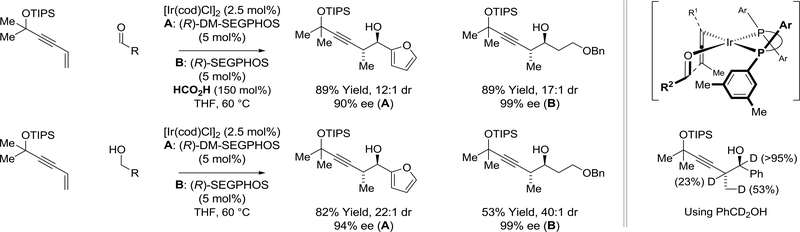
Using an iridium catalyst modified by (R)-PhanePhos, 2-substituted dienes engage in reductive couplings to formaldehyde via methanol-mediated hydrogen auto-transfer (Scheme 18).122 Notably, this process enables enantioselective formation of acyclic quaternary carbon stereocenters in the absence of stoichiometric byproducts.123 Whereas the indicated reactions of formaldehyde display complete C2-regioselectivity, reactions of higher aldehydes display complete C3-regioselectivity (not shown). The origins of regiodivergence were explored using deuterium labelling studies, which corroborate a CurtinHammett scenario wherein methanol dehydrogenation triggers rapid, reversible diene hydrometalation en route to regioisomeric allyliridium-carbonyl pairs. The energetic barrier to formaldehyde addition is lowest from the terminally disubstituted σ-allyliridium isomer. For higher aldehydes, the transition state energy for carbonyl addition at C2 increases due to greater steric congestion in formation of a quaternary carbon center. Hence, the 1,2-disubstituted allyliridium isomers become kinetically more reactive.
Scheme 18.
Iridium(I)-catalyzed reductive coupling of 2-substituted dienes with methanol via hydrogen auto-transfer
2.5. Titanium
A single study of titanium catalyzed diene-carbonyl reductive coupling was disclosed in 2005 by Moïse and Le Gendre (Scheme 19).124 Using substoichiometric quantities of titanocene dichloride in combination with poly(methylhydrosiloxane) (PMHS) as terminal reductant, diene-aldehyde reductive coupling occurred with complete regioselectivity and modest levels of anti-diastereoselectivity. Entry into the catalytic cycle occurs through the reaction of titanocene dichloride with n-BuLi in the presence of PMHS to form a titanium hydride. Diene hydrometalation generates a nucleophilic allyltitanium(IV) species, which engages in aldehyde addition. σ-Bond metathesis of the resulting titanium alkoxide with PMHS delivers the crotylation product and regenerates the titanium hydride to close the catalytic cycle.
Scheme 19.
Titanium catalyzed diene-aldehyde reductive coupling mediated by silane
2.6. Copper
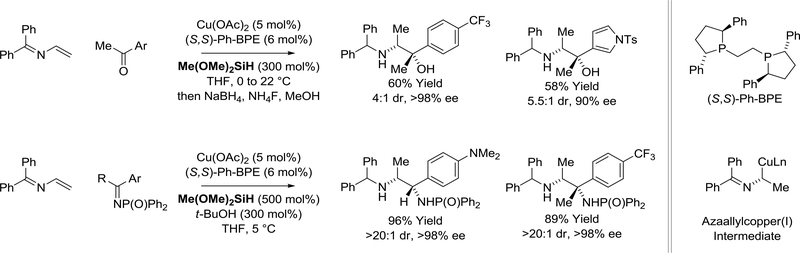
While the use of diene pronucleophiles in copper-catalyzed carbonyl reductive coupling is unknown, Malcolmson and co-workers recently demonstrated the viability of structurally related 2-azadienes (Scheme 20).125 Specifically, using a chiral copper catalyzed modified by (S,S)-Ph-BPE and silane as terminal reductant, 2-azadienes reacts with aryl ketones to generate 1,2-amino alcohols in a highly regio- and enantioselectives fashion. Entry into the catalytic cycle involves the conversion of Cu(OAc)2 to a copper(I) hydride. Hydrocupration of 2-azadiene delivers a nucleophilic azaallylcopper(I) species that undergoes ketone addition to form a copper(I) alkoxide, which upon σ-bond metathesis with the silane regenerates the copper hydride to close the catalytic cycle. Further imine reduction and cleavage of the silyl ether gives the benzhydryl-protected anti-1,2-amino alcohol products. More recently, the same authors successfully developed related 2-azadiene-imine reductive couplings to form differentially protected vicinal diamines with excellent control of diastereo- and enantioselectivity.126
Scheme 20.
Copper-catalyzed reductive coupling of 2-azadienes with aryl ketones and imines mediated by silane
3. Allene-C=X (X = O, NR) Reductive Coupling
3.1. Nickel
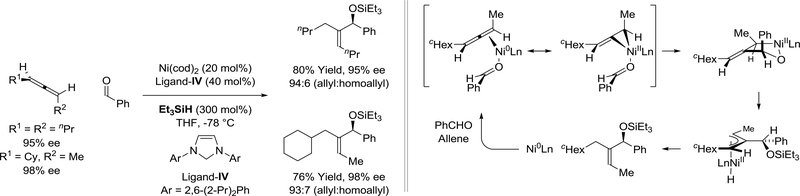
Nickel(0) complexes modified by carbene ligand-IV were reported by Jamison to catalyze the silane-mediated reductive coupling of chiral nonracemic 1,3-disubstituted allenes with aldehydes (Scheme 21).127–129 The reaction mechanism is initiated by stereospecific allene-aldehyde oxidative coupling to form an oxanickelacycle. σ-Bond metathesis with silane forms an 1,3-anti-π-allylnickel hydride, which upon regio- and stereoselective C-H reductive elimination delivers the product of carbonyl reductive coupling with excellent levels of axial-to-central chirality transfer and alkene (Z)stereoselectivity. In each case, small quantities of the isomeric homoallylic alcohols were formed.
Scheme 21.
Nickel(0)-catalyzed reductive coupling of chiral nonracemic allenes and aldehydes mediated by triethylsilane
3.2. Ruthenium
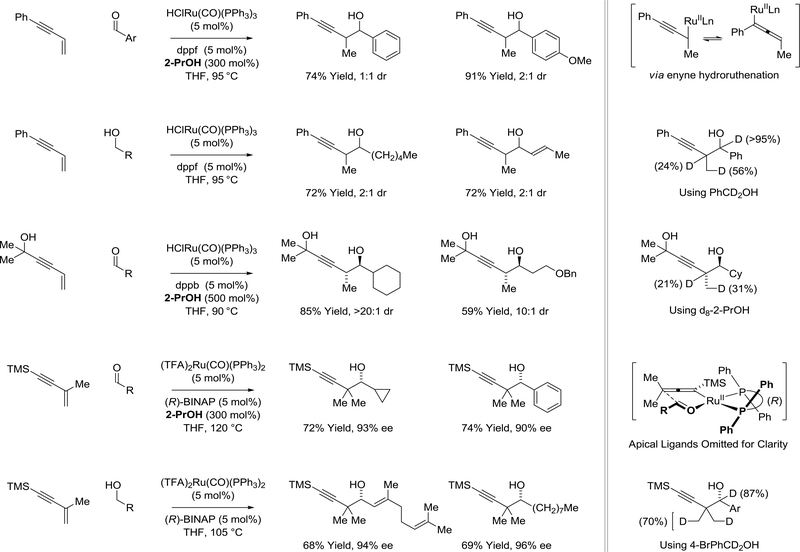
Phosphine-modified ruthenium(II) complexes bearing carbonyl ligands catalyze the 2-propanol-mediated reductive coupling of allenes to paraformaldehyde and higher aldehydes (Scheme 22).130 The mechanism involves allene hydrometalation to form a nucleophilic allylruthenium species that undergoes aldehyde addition through a closed 6-centered transition structure. In reactions of monosubstituted allenes, an appropriately defined allene substituent can enforce intervention of geometrically defined allylruthenium intermediates. For example, in 2-propanol-mediated reductive coupling of allenamides, stereospecific carbonyl addition occurs by way of the (E)-σ-allylruthenium haptomer to form vicinal anti-aminoalcohols as single diastereomers.131 Primary alcohols can serve dually as reductant and aldehyde pronucleophile in reductive couplings with allenamides under the conditions of hydrogen auto-transfer.132 Comparable levels of selectivity are observed from the aldehyde or alcohol oxidation level. In contrast, using 1,1-disubstituted allenes, high levels of antidiastereoselectivity are only evident in reactions conducted from the alcohol oxidation level.133 Furthermore, diastereoselectivities are highly concentration dependent and at lower concentrations higher diastereoselectivities are observed. A Curtin-Hammett scenario appears to be operative. As carbonyl addition is hindered sterically due to the formation of a quaternary carbon stereocenter, it is turn-over limiting. The transition state energy for carbonyl addition from the (E)-σ-allylruthenium haptomer, which leads to the anti-diastereomer, is lower than that from the corresponding (Z)-isomer, which provides the syn-diastereomer. At lower concentrations, equilibration of the transient (E)- and (Z)σ-allylruthenium isomers is fast with respective to carbonyl addition, allowing the (E)-isomer to be replenished.
Scheme 22.
Ruthenium(II)-catalyzed allene-aldehyde reductive coupling mediated by 2-propanol or hydrogen auto-transfer
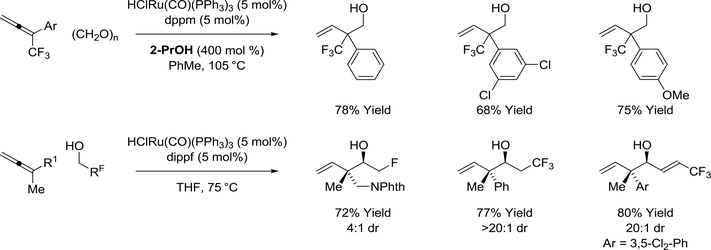
The formation of highly congested CF3-bearing quaternary carbon stereocenters is achieved upon ruthenium(II) catalyzed reductive coupling of CF3-allenes with paraformaldehyde mediated by 2propanol (Scheme 23).134 Formate esters, which are cleaved upon isolation, appear as minor reaction products, suggesting paraformaldehyde contributes to some extent as a terminal reductant. Under similar conditions, allene-aldehyde reductive coupling can be achieved using fluorinated alcohols as reductants and proelectrophiles.135 This capability is significant as the corresponding fluorinated aldehydes are highly intractable and, in many cases, are not commercially available. As dehydrogenation of fluorinated alcohols is significantly more endothermic than the corresponding aliphatic alcohols,136,137 reactions of monofluoro-, difluoro- and trifluoroethanol become increasingly inefficient.
Scheme 23.
Use of CF3-allenes and fluorinated alcohols in ruthenium(II)-catalyzed allene-aldehyde reductive coupling mediated by 2-propanol or hydrogen auto-transfer
The ability to exploit alkynes as latent allenes138 has expanded the scope of ruthenium catalyzed allene-aldehyde reductive coupling (Scheme 24).139–141 Using a cationic ruthenium catalyst generated upon the acid-base reaction of H2Ru(CO)(PPh3)3 and 2,4,6-(2-Pr)3PhSO3H, two discrete catalytic processes are enacted: (a) alkyne-to-allene isomerization and (b) allene-carbonyl reductive coupling via hydrogen auto-transfer. The cationic ruthenium(II) exists in equilibrium with a zero-valent ruthenium that promotes allene-aldehyde oxidative coupling. The resulting oxaruthenacycle ultimately provides the (Z)-homoallylic alcohol.139 The introduction of iodide and ligand-V (Josiphos SL-J009–1) enables suppression of oxidative coupling pathways. With these subtle changes, alkyne-to-allene isomerization pathways persist, however, the allene undergoes hydrometalation to form a nucleophilic allylruthenium species. Carbonyl addition by way of a closed transition structure provides the branched homoallylic alcohols with exceptional levels of anti-diastereo- and enantioselectivity.140 The ruthenium(II) complex derived from HClRu(CO)(PPh3)3 and dippf, bis(diisopropylphosphino)ferrocene, catalyzes conversion of acetylenic pyrroles to allenes, which participate in allene-aldehyde reductive coupling via hydrogen auto-transfer.141 The products, protected vicinal aminoalcohols, are generated with complete regio- and anti-diastereoselectivity.
Scheme 24.
Alkynes as latent allenes in ruthenium(0) and ruthenium(II) catalyzed allene-aldehyde reductive couplings via hydrogen auto-transfer
Only one study on ruthenium catalyzed allene-imine reductive coupling appears in the literature. Using a ruthenium(II) catalyzed modified by the chelating phosphine ligand 1,2bis(dicyclohexylphosphino)ethane (dCype), 1,1-disubstituted allenes and formaldimines engage in 2propanol-mediated reductive coupling to form homoallylic amines with complete branched regioselectivity (Scheme 25).142 The formaldimines are generated in situ through cycloreversion of PMPprotected hexahydro-1,3,5-triazines. This process represents a method for the hydroaminomethylation of π-unsaturated reactants beyond classical hydroformylation/reductive amination.
Scheme 25.
Ruthenium(II)-catalyzed allene-imine reductive coupling mediated by 2-propanol
3.3. Iridium
In 2007, Krische reported the first allene-aldehyde reductive coupling catalyzed by iridium (Scheme 26).143 Specifically, hydrogenation of dimethyl allene in the presence of activated aldehydes provided the products of carbonyl tert-prenylation with complete branched regioselectivity. Under an atmosphere of deuterium, deuterium is incorporated exclusively at the interior vinylic position (80% 2H). This result is consistent with a catalytic mechanism involving allene-aldehyde oxidative coupling, however, hydrometalative pathways involving allyliridium species cannot be excluded on the basis of this data alone. Shortly thereafter, related allene-aldehyde reductive couplings mediated by 2-propanol and hydrogen auto-transfer were demonstrated.144 Using d8-isopropanol as reductant, the indicated product of tert-prenylation incorporates deuterium predominantly at the internal vinylic position (85% 2H). A similar pattern of deuterium incorporation is observed in hydrogen auto-transfer reactions of d2benzyl alcohol, corroborating its dual role as reductant and carbonyl proelectrophile.
Scheme 26.
Iridium(I)-catalyzed allene-aldehyde reductive coupling mediated by hydrogen, 2propanol or hydrogen auto-transfer
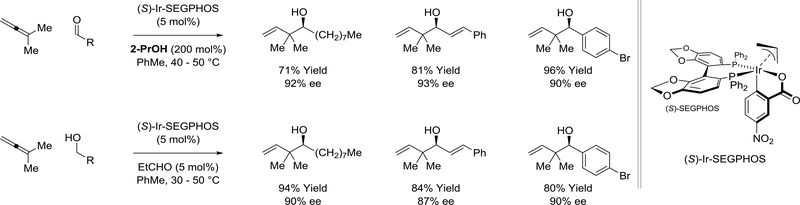
The cyclometalated π-allyliridium C,O-benzoate complex modified by (S)-SEGPHOS catalyzes the regio- and enantioselective reductive coupling of dimethylallene with aldehydes mediated by 2-propanol (Scheme 27).145,146 Aliphatic, α,β-unsaturated and aromatic aldehydes are converted to the products of carbonyl tert-prenylation with uniformly high levels of selectivity. Under the conditions of hydrogen auto-transfer, primary alcohols are converted to an identical set of products with similar levels of selectivity. This process represents a departure from the longstanding use of stoichiometric organometallic reagents in enantioselective carbonyl tert-prenylation.147
Scheme 27.
Enantioselective carbonyl tert-prenylation via iridium(I)-catalyzed dimethylallenealdehyde reductive coupling mediated by 2-propanol or hydrogen auto-transfer
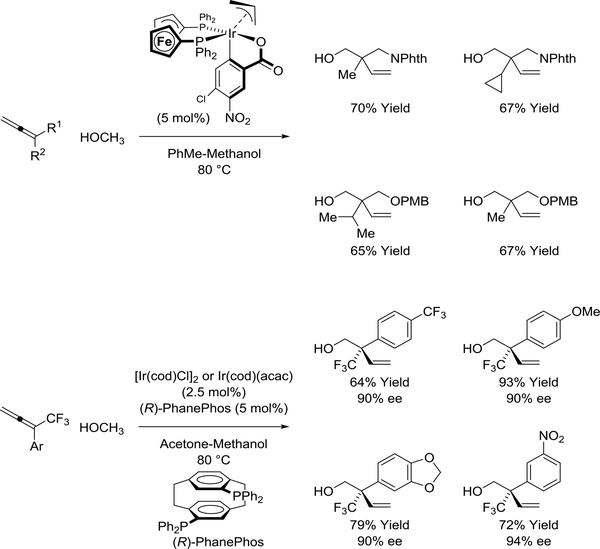
The cyclometalated π-allyliridium C,O-benzoate complex modified by DPPF catalyzes allene- formaldehyde reductive coupling via methanol-mediated hydrogen autotransfer (Scheme 28).148 This process enables direct, byproduct-free coupling of methanol, an abundant feedstock (35 million metric tons per year), to form highly congested quaternary carbon stereocenters. Using an iridium catalyst modified by (R)-PhanePhos, CF3-allenes react with methanol to form homoallylic alcohols with CF3-bearing quaternary carbon stereocenters with high levels of regio- and enantioselectivity.149 Such congested acyclic quaternary carbon stereocenters are exceptionally difficult to prepare in enantiomerically enriched form, with existing protocols for their construction largely restricted to conjugate additions to β,β-disubstituted CF3-enones and nitroolefins.123
Scheme 28.
Iridium(I)-catalyzed reductive coupling of 1,1disubstituted allenes via methanol-mediated hydrogen auto-transfer
3.4. Palladium
In 2000, a palladium(0) catalyzed allene-aldehyde reductive coupling mediated by tin(II) chloride was reported by Cheng (Scheme 29).150 Using dimethylallene, products of tert-prenylation were formed with complete regioselectivity. Additionally, monosubstituted allenes provided branched adducts with complete anti-diastereoselectivity. The authors propose the reaction proceeds through palladium catalyzed allene hydrostannylation to furnish an allylstannane that undergoes spontaneous aldehyde addition through a six-member chair-like transition state.
Scheme 29:
Palladium catalyzed allene-aldehyde reductive coupling mediated by stannous chloride
In 2015, a palladium(0)-catalyzed allene-anhydride reductive coupling mediated by silane was reported by Tsuiji and Fujihara (Scheme 30).151 The catalytic cycle is initiated by anhydride oxidation addition to form an acylpalladium(II) species, which upon allene carbopalladation forms a πallylpalladium intermediate. Silane-mediated hydride transfer to palladium followed by C-H reductive elimination delivers the reductive coupling product and returns palladium to its zero-valent form. The reaction products form as single regioisomers, albeit with incomplete control of alkene geometry. This method provides an alternative to the use of aldehydes as acyl donors in allene hydroacylation.152
Scheme 30.
Palladium(0)-catalyzed allene-anhydride reductive coupling mediated by silane
3.5. Copper
In 2018, Buchwald reported an enantioselective copper(I)-catalyzed allene-ketone reductive coupling mediated by silane (Scheme 31).153 Using a copper complex modified by ligand-VI, a wide range of methyl ketones were converted to the tertiary homoallylic alcohols with moderate to good levels of anti-diastereo- and enantioselectivity. The highest stereoselectivities were observed for allenes bearing branched alkyl substituents. The catalytic mechanism is postulated to involve allene hydrocupration to form a nucleophilic allylcopper(I) species. Ketone addition generates a copper(I) alkoxide, which upon σbond metathesis with silane delivers the homoallylic silyl ether (hydrolyzed upon isolation) and copper(I) hydride to close the catalytic cycle.
Scheme 31.
Copper(I)-catalyzed allene-ketone reductive coupling mediated by silane
In an earlier report (2016) by the same author, closely related conditions for copper-catalyzed allene-imine reductive coupling were described (Scheme 32).154 Remarkably, regiodivergent reductive coupling to form either branched or linear adducts was observed in response to the choice of nitrogen protecting group. The origins of this regiodivergence were probed using DFT calculations. Irreversible allene hydrocupration provides a primary σ-allylcopper intermediate. For the phosphinoyl imine, the phosphinoyl oxygen binds to the copper center in the transition state and allyl transfer provides the linear product. In contrast, for N-benzyl imines, the nitrogen atom coordinates the copper center causing imine addition to occur with allylic inversion to furnish the branched product.
Scheme 32.
Regiodivergent copper(I)-catalyzed allene-imine reductive coupling mediated by silane
4. Enyne-C=X (X = O, NR) Reductive Coupling
4.1. Nickel
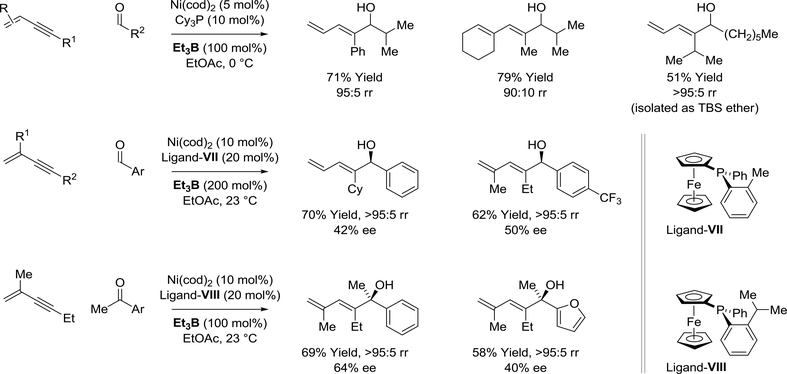
In 2004, Jamison reported an intermolecular nickel(0)-catalyzed enyne-aldehyde reductive coupling mediated by triethylborane (Scheme 33).155 High levels of regioselectivity in favor of C-C coupling at the acetylenic terminus of the enyne were accompanied by complete control of alkene geometry. Subsequent attempts to develop enantioselective variants of this process proved challenging. Using the monodentate P-chiral ferrocenyl phosphine ligand-VII, moderate levels of asymmetric induction were observed.156 Remarkably, under closely related reductive coupling conditions using the P-chiral ligand-VIII, unactivated ketones were competent electrophilic partners.157 Again, high levels of regioselectivity in favor of coupling at the acetylenic terminus were accompanied by complete control of alkene geometry and moderate enantioselectivities.
Scheme 33.
Nickel(0)-catalyzed enyne-aldehyde reductive coupling mediated by triethylborane
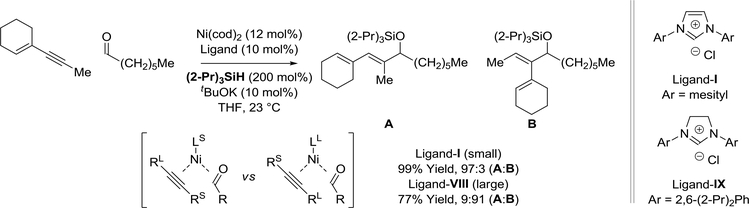
Computational studies by Houk158 and experimental studies by Montgomery,159 who observed a ligand-dependent inversion of regioselectivity, illuminate the origins of regioselectivity in enynealdehyde reductive coupling (Scheme 34). The collective data are consistent with the following interpretation. An oxidative coupling mechanism is operative in which an electronic bias for coupling at the acetylenic terminus is observed.160 For the large ligand-IX, this intrinsic bias is accentuated due to increased steric interactions between the ligand and the substituent at the acetylenic terminus. Hence, the substituent at the acetylenic terminus prefers to be placed distal to the metal center in the oxanickelacycle. Upon use of the smaller ligand-I, steric repulsion between the aldehyde and acetylenic substituents is greater than steric repulsion between the ligand and acetylenic substituent, which results in an inversion in regioselectivity.
Scheme 34.
Ligand-dependent inversion of regioselectivity in nickel(0)-catalyzed enyne-aldehyde reductive coupling mediated by silane
4.2. Rhodium
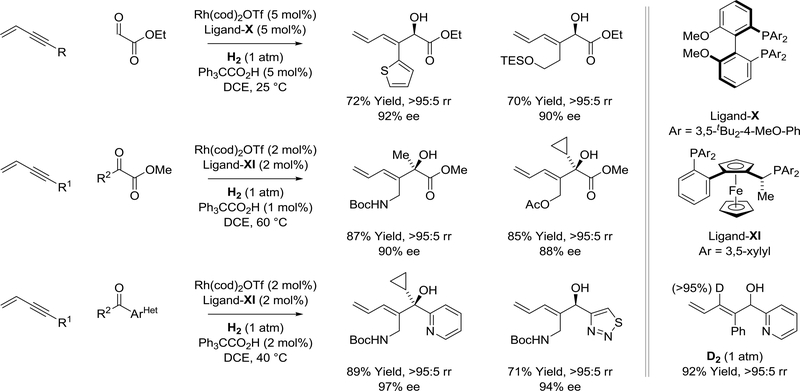
Highly enantioselective and byproduct-free enyne-carbonyl reductive coupling is achieved under the conditions of catalytic hydrogenation using chiral rhodium complexes modified by ligand-X or ligandXI (Scheme 35).161–163 Activated carbonyl compounds, such as glyoxalates161 and pyruvates,162 are required. The resulting dienyl alcohols form as single regioisomers with excellent levels of enantiomeric enrichment and, remarkably, conventional hydrogenation of the diene-containing products is not observed. This result can be explained as follows. The enyne reactant is a stronger π-acid than the diene product. Hence, once the carbonyl electrophile is fully consumed, excess enyne preferentially binds rhodium(I), retarding the rate of product hydrogenation. Beyond vicinal dicarbonyl electrophiles, certain heterocyclic aromatic aldehydes and ketones undergo reductive coupling with enyne pronucleophiles under hydrogenation conditions.163 Again, the resulting heteroaryl substituted secondary and tertiary dienyl alcohols are generated as single regioisomers in highly enantiomerically enriched form (Scheme 35).
Scheme 35.
Enantioselective rhodium(I)-catalyzed enyne-aldehyde and enyne-ketone reductive coupling mediated by hydrogen
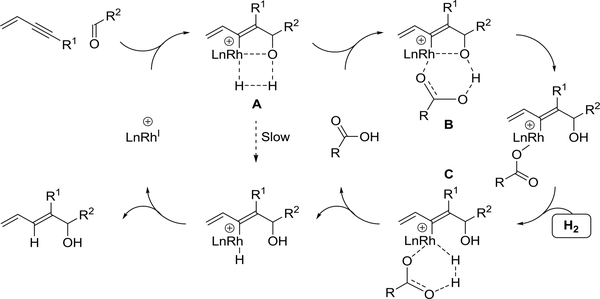
A mechanism for hydrogen-mediated enyne-carbonyl reductive coupling has been proposed and is supported by computational studies (Scheme 36).164,165 The catalytic cycle is initiated by alkyne-C=O oxidative coupling. Direct hydrogenolysis of the oxarhodacyclopentene by σ-bond metathesis with hydrogen through the 4-centered transition structure A is slow compared to metallacycle protonolysis by the carboxylic acid cocatalyst through the 6-centered transition structure B. The resulting rhodium carboxylate engages hydrogen in σ-bond metathesis through the 6-centered transition structure C to form an alkenylrhodium hydride, which upon C-H reductive elimination releases product and rhodium(I). Cationic rhodium complexes are essential, as these square planar non-contact ion pairs offer an additional coordination site, enabling simultaneous binding of enyne and carbonyl reactants. Additionally, unlike neutral rhodium complexes, the cationic complexes are slow to engage in hydrogen oxidative addition.166 These two factors act in concert to promote alkyne-C=O oxidative coupling pathways.
Scheme 36.
Catalytic mechanism for rhodium(I)-catalyzed enyne-carbonyl reductive coupling mediated by hydrogen
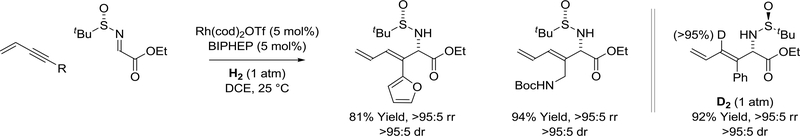
Rhodium catalyzed hydrogenation of 1,3-enynes in the presence of ethyl (N-tertbutanesulfinyl)iminoacetate results in reductive coupling to furnish diene-containing α-amino acid esters (Scheme 37).167 Coupling occurs in a completely regioselective manner at the acetylenic terminus of the enyne. Additionally, the nitrogen-bearing stereogenic center of the product is formed as a single diastereomer. Under an atmosphere of elemental deuterium, reductive coupling occurs with incorporation of a single deuterium atom at the former 2-position of the enyne, which is consistent with a mechanism involving enyne-imine oxidative coupling followed by hydrogenolytic cleavage of the resulting metallacycle.
Scheme 37.
Asymmetric rhodium(I)-catalyzed enyne-imine reductive coupling mediated by hydrogen
4.3. Ruthenium
The first enyne-mediated carbonyl propargylations were reported by Krische in 2008, who used a ruthenium(II) complex modified by dppf [bis(diphenylphosphino)ferrocene] to catalyze 2-propanolmediated enyne-aldehyde reductive coupling (Scheme 38).168 In these reactions, enyne hydrometalation delivers a nucleophilic allenylruthenium species that undergoes aldehyde addition. The stoichiometric reaction of HClRu(CO)(PPh3)3 with enynes to form σ-allenylruthenium complexes, which were characterized by single crystal X-ray diffraction, has been reported.169 Under these conditions, primary alcohols react with enynes to form products of carbonyl propargylation via hydrogen auto-transfer. The pattern of deuterium incorporation in isotopic labelling studies using α,α-d2-benzyl alcohol corroborate reversible and non-regioselective enyne hydroruthenation in advance of carbonyl addition. Deuterium is completely retained at the carbinol methine, suggesting the product is kinetically inert with respect to dehydrogenation due to internal alkyne coordination to ruthenium. It was later found that good to complete levels of anti-diastereoselectivity could be achieved in 2-propanol-mediated enyne-aldehyde reductive couplings using the indicated 2-propoxy-substituted enyne.170 Finally, (R)-BINAP-modified ruthenium complexes were shown to catalyze highly enantioselective 2-propanol-mediated enynealdehyde reductive couplings.171 Secondary homopropargyl alcohols bearing gem-dimethyl groups were obtained with uniformly high levels of enantioselectivity. Under these conditions, reductive coupling also could be achieved via hydrogen auto-transfer from aliphatic, allylic and benzylic alcohols. These processes represent an alternative to the use of preformed allenylmetal reagents in enantioselective carbonyl propargylation.172
Scheme 38.
Ruthenium(II)-catalyzed enyne-aldehyde reductive coupling mediated by 2-propanol and hydrogen auto-transfer
4.4. Iridium
The sole examples of iridium catalyzed enyne-carbonyl reductive coupling were reported by Krische in 2012 (Scheme 39).173 Using an iridium(I) catalyst in combination with commercially available SEGPHOS or DM-SEGPHOS ligands, aldehydes are subject to highly anti-diastereo- and enantioselective formic acid mediated propargylation. These transformations proceed via enyne hydrometalation to form an allenyl-iridium(I) species, which engages the aldehyde in addition through a closed six-centered transition state. Enyne-mediated propargylation from the alcohol oxidation level via hydrogen autotransfer displayed higher levels of anti-diastereo- and enantioselectivity. Deuterium labelling studies corroborate reversible, non-regioselective enyne hydrometalation in advance of carbonyl addition. Complete retention of deuterium at the carbonyl methine suggests the reaction products are inert with respect to dehydrogenation.
Scheme 39.
anti-Diastereoselective iridium(I)-catalyzed enyne-aldehyde reductive coupling mediated by formic acid or hydrogen auto-transfer
4.5. Copper
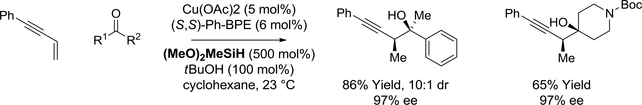
Recently, the first copper-catalyzed enyne-carbonyl reductive couplings were reported by Buchwald (Scheme 40).174 Using a copper complex modified by the chiral bidentate phosphine ligand (S,S)-Ph-BPE and (MeO)2MeSiH as terminal reductant, diverse ketones were converted to tertiary homopropagyl alcohols with good levels of syn-diastereo- and enantioselectivity. Aryl-methyl ketones underwent propargylation with highest levels of stereocontrol. Notably, the reaction could be run efficiently on 50 mmol scale with catalyst loadings as low as 0.2 mol%. A catalytic mechanism involving enyne hydrocupration to generate an allenylcopper(I) nucleophile is postulated. Ketone addition then generates a copper(I) alkoxide, which upon σ-bond metathesis regenerates the copper(I) hydride to close the catalytic cycle.
Scheme 40.
Diastereoselective copper(I)-catalyzed enyne-ketone reductive coupling mediated by silane
5. Applications in Natural Product Synthesis
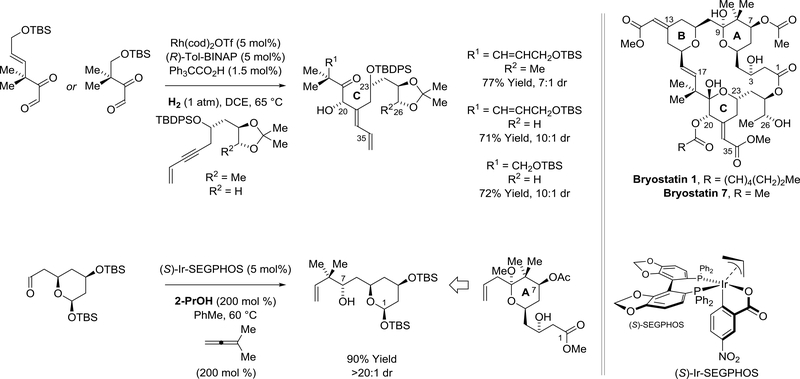
Although enantioselective carbonyl reductive couplings have only recently been developed, applications in natural product total synthesis have begun to emerge. Krische and coworkers prepared bryostatin 7175 and related seco-B-ring analogues (not shown)176,177 using enyne-carbonyl178 and allenecarbonyl179 reductive couplings (Scheme 41). To construct the bryostatin C-ring, a rhodium-catalyzed hydrogen-mediated enyne-α-ketoaldehyde reductive coupling forms the C20-C21 bond with control of the C20 carbinol stereochemistry and the C21 alkene geometry. The neopentyl carbinol motif of the bryostatin A-ring is generated through iridium-catalyzed reductive coupling of dimethyl allene with the indicated aldehyde using 2-propanol as terminal reductant. Using these hydrogenative and transferhydrogenative methods, the total synthesis of bryostatin 7 was achieved in fewer steps than any prior synthesis of a bryostatin family member.
Scheme 41.
Total synthesis of bryostatin 7 via asymmetric rhodium- and iridium-catalyzed hydrogenative and transfer-hydrogenative reductive couplings, respectively
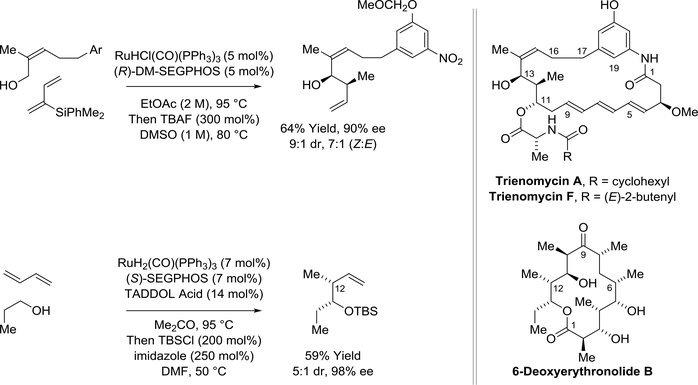
The triene-containing C17-benzene ansamycins, trienomycins A and F,180 and 6deoxyerythronolide B181 were prepared by Krische and coworkers via ruthenium-catalyzed syndiastereo- and enantioselective diene-carbonyl reductive coupling mediated by hydrogen auto-transfer (Scheme 42). For trienomycins A and F, a ruthenium catalyst modified by DM-SEGPHOS is used to react the indicated trisubstituted (Z)-allylic alcohol with a 2-trialkylsilyl substituted diene to form the product of carbonyl syn-crotylation. The ability to conduct carbonyl crotylation from the alcohol oxidative level avoids the use of the (Z)-enal, which is prone to geometrical isomerization. For the synthesis of deoxyerythronolide B, a ruthenium catalyst modified by SEGPHOS and a chiral phosphate counterion (previously indicated in Scheme 8) enabled direct use of butadiene itself as a crotyl donor.
Scheme 42.
Total syntheses of trienomycins A and F and 6-deoxyerythronolide B via rutheniumcatalyzed syn-diastereo- and enantioselective diene-carbonyl reductive coupling mediated by hydrogen auto-transfer
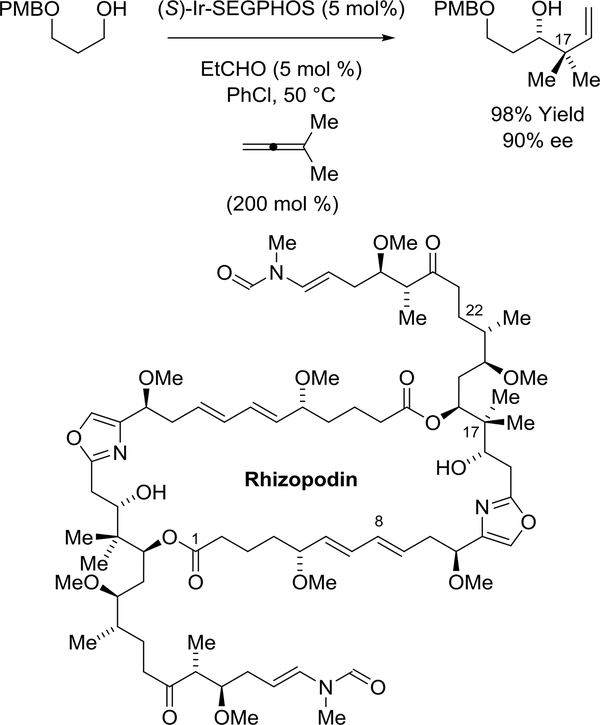
Menche and coworkers prepared the C8-C22 substructure of the actin-binding macrodiolide rhizopodin using an enantioselective iridium-catalyzed dimethyl allene-aldehyde reductive coupling under the conditions of alcohol-mediated hydrogen auto-transfer (Scheme 43).182,183 The reaction, which forms the C16-carbinol stereocenter, was conducted on multi-gram scale to furnish the product of tertprenylation in 98% yield. Chromatographic purification of the cyclometalated π-allyliridium catalyst (depicted in Scheme 41) led to an improvement in enantiomeric excess from 82% to 90% ee.183
Scheme 43.
Syntheses of the C8-C22 substructure of rhizopodin via enantioselective iridium-catalyzed allene-aldehyde reductive coupling via hydrogen auto-transfer
6. Conclusion and Outlook
Carbonyl and imine addition mediated by premetalated reagents has played a central role in chemical synthesis since the inception of Organic Chemistry as a field. However, the requisite organometallic reagents often pose issues of safety, selectivity, cost and waste. Metal catalyzed reductive coupling has emerged as an alternative to stoichiometric organometallic reagents in classical carbonyl and imine additions, but many important challenges remain. For example, many reductive couplings require pyrophoric (Et3B) or expensive/mass-intensive (R3SiH) terminal reductants. It would be preferable to exploit safe, inexpensive terminal reductants with low molecular weights, such as 2-propanol or H2. Perhaps most ideal are reductive couplings mediated by hydrogen auto-transfer, which altogether preclude the use of an exogenous terminal reductant (Scheme 44). Another major challenge involves the development of catalysts for the reductive coupling of ethylene and α-olefins,48 which are abundant feedstocks with production volumes exceeded only by alkanes. Late transition metals will likely be the key to unlock these unmet challenges, as their low oxaphilicity enables chemoselective reduction of π-unsaturated pronucleophiles in the presence of carbonyl and imine electrophiles. It is the authors’ hope that the collective efforts presented in this monograph will encourage future research toward catalytic carbonyl additions beyond stoichiometric metals.
Scheme 44.
Catalytic reductive coupling for carbonyl addition
Scheme 17.
Iridium(I)-catalyzed butadiene-aldehyde reductive coupling mediated by 1,4-butanediol or hydrogen auto-transfer
ACKNOWLEDGMENTS
The Robert A. Welch Foundation (F-0038) and the NIH-NIGMS (RO1-GM069445) are acknowledged for financial support.
Biography
Professor Michael J. Krische obtained a B.S. degree in Chemistry from the University of California at Berkeley (1989), where he performed research with Professor Henry Rapoport. After a year abroad as a Fulbright Fellow, he initiated doctoral studies at Stanford University with Professor Barry Trost as a Veatch Graduate Fellow. Following receipt of his Ph.D. degree (1996), he joined the laboratory of Professor Jean-Marie Lehn at the Université Louis Pasteur as an NIH Post-Doctoral Fellow. In 1999, he joined the faculty at the University of Texas at Austin. He was promoted directly to the rank of full professor (2004) and shortly thereafter appointed the Robert A. Welch Chair in Science (2007). Professor Krische has pioneered a new class of C-C bond formations that merge the characteristics of carbonyl addition and catalytic hydrogenation. Professor Krische’s research has garnered numerous awards, including the NSF-CAREER Award (2000), Cottrell Scholar Award (2002), Eli Lilly Granteeship for Untenured Faculty (2002), Frasch Award in Chemistry (2002), Dreyfus Teacher-Scholar Award (2003), Sloan Fellowship (2003), Johnson & Johnson Focused Giving Award (2005), Solvias Ligand Prize (2006), Presidential Green Chemistry Award (2007), ACS Corey Award (2007), Dowpharma Prize (2007), Novartis Lectureship (2008), Tetrahedron Young Investigator Award (2009), Humboldt Senior Research Award (2009–2011), Mukaiyama Award (2010), Glaxo-Smith-Kline Scholar Award (2011), ACS Cope Scholar Award (2012), and JSPS Fellow (2013), Eun Lee Lectureship, Korea (2015), Royal Society of Chemistry, Pedlar Award (2015), AAAS Fellow (2017).
Leyah A. Schwartz obtained a B.S. degree in chemistry from the University of Alabama at Birmingham in 2016, where she conducted undergraduate research in the laboratory of Professor Pengfei Wang. In Fall 2016, she entered the doctoral degree program at the University of Texas at Austin in the laboratory of Professor Michael J. Krische, where she is developing enantioselective alcohol-mediated iridiumcatalyzed reductive couplings of allene pronucleophiles.
Michael Holmes obtained a B.S. degree in Chemistry and Mathematics from the University of Canterbury in 2010 where he performed research in the laboratory of Professor Owen Curnow. He initiated doctoral studies under the supervision of Professor Robert Britton at Simon Fraser University in 2011, where he developed methods for the construction of tetrahydrofuranol containing natural products. Following receipt of his Ph.D. degree in 2016, he joined the laboratory of Professor Michael J. Krische at the University of Texas at Austin as an Eli Lilly postdoctoral fellow, where he is developing enantioselective alcohol-mediated iridium-catalyzed reductive couplings of allene pronucleophiles.
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).Frankland E Isolation of Organic Radicals. Ann 1849, 71, 171–213. [Google Scholar]
- (2).For a historical review, see: Seyferth D Zinc Alkyls, Edward Frankland, and the Beginnings of MainGroup Organometallic Chemistry. Organometallics 2001, 20, 2940–2955. [Google Scholar]
- (3).For a historical review, see: Thayer JS Historical Origins of Organometallic Chemistry. J. Chem. Educ. 1969, 46, 764–765. [Google Scholar]
- (4).Wanklyn JA Ueber die Bildung der Propionsäure aus Kohlensäure und eine Aethylverbindung. Ann. Chem. Pharm 1858, 107, 125–128. [Google Scholar]
- (5).For a historical review, see: Seyferth D Alkyl and Aryl Derivatives of the Alkali Metals: Useful Synthetic Reagents as Strong Bases and Potent Nucleophiles. 1. Conversion of Organic Halides to Organoalkali-Metal Compounds. Organometallics 2006, 25, 2–24. [Google Scholar]
- (6).Butlerov A, “Studien über die Einfachsten Verbindungen der Organischen Chemie,” Z. Chem. Pharm, 1863, 6, 484–497. [Google Scholar]
- (7).Butlerov AZ Sur l’Alcool Pseudobuylique Tertiare ou Alcool Méthylique triméthylé Bull. Soc. Chim. Fr 1864, 2, 106–116. [Google Scholar]
- (8).Lewis DE The Beginnings of the Synthetic Organic Chemistry: Zinc Alkyls and The Kazan’s School. Bull. Hist. Chem 2002, 27, 37–42. [Google Scholar]
- (9).Reformatsky S Neue Synthese Zweiatomiger Einbasischer Säuren aus den Ketonen. Ber. Dtsch. Chem. Ges 1887, 20, 1210–1211. [Google Scholar]
- (10).Grignard V Sur Quelques Nouvelles Combinaisons Organométalliques du Magnèsium et Leur Application à des Synthèses d’alcools et d’hydrocarbures. C. R. Acad. Sci 1900, 130, 1322–1325. [Google Scholar]
- (11).Also, see: Barbier P Synthèse du Diéthylhepténol. Compt. Rend 1899, 128, 110–111. [Google Scholar]
- (12).Schlenk W; Holtz J Über die Einfachsten Metallorganischen Alkaliverbindungen. Ber. Dtsch. Chem. Ges 1917, 50, 262–274. [Google Scholar]
- (13).Ziegler K; Colonius H Untersuchungen über Alkali‐Organische Verbindungen. V. Eine Bequeme Synthese Einfacher Lithiumalkyle. Liebigs Ann. Chem 1930, 479, 135–149. [Google Scholar]
- (14).Gilman H; Jones RG; Woods LA The Preparation of Methylcopper and some Observations on the Decomposition of Organocopper Compounds. J. Org. Chem 1952, 17, 1630–1634. [Google Scholar]
- (15).Tsuji J; Takahashi H Organic Syntheses by Means of Noble Metal Compounds. XII. Reaction of the Cyclooctadiene-Palladium Chloride Complex with Ethyl Malonate. J. Am. Chem. Soc 1965, 87, 32753276. [Google Scholar]
- (16).Tsuji J; Takahashi H; Morikawa M Organic Syntheses by Means of Noble Metal Compounds XVII. Reaction of π-Allylpalladium Chloride with Nucleophiles. Tetrahedron Lett. 1965, 6, 4387–4388. [Google Scholar]
- (17).Kitamura M; Suga S; Kawai K; Noyori R Catalytic Asymmetric Induction. Highly Enantioselective Addition of Dialkylzinc Reagents to Aldehydes. J. Am. Chem. Soc 1986, 108, 6071–6072. [DOI] [PubMed] [Google Scholar]
- (18).Noyori R; Kitamura M Enantioselective Addition of Organometallic Reagents to Carbonyl Compounds: Chirality Transfer, Multiplication and Amplification. Angew. Chem. Int. Ed 1991, 30, 49–69. [Google Scholar]
- (19).Soai K; Shibata T Alkylation of Carbonyl Groups In Comprehensive Asymmetric Catalysis I-III; Jacobsen EN, Pfaltz A, Yamamoto H, Eds.; Springer-Verlag Berlin Heidelberg: Germany, 1999; Vol. 2, pp 911–922. [Google Scholar]
- (20).Kobayashi S; Ishitani H Catalytic Enantioselective Addition to Imines. Chem. Rev 1999, 99, 10691094. [DOI] [PubMed] [Google Scholar]
- (21).Pu L; Yu H-B Catalytic Asymmetric Organozinc Additions to Carbonyl Compounds. Chem. Rev 2001, 101, 757–824. [DOI] [PubMed] [Google Scholar]
- (22).Trost BM; Weiss AH The Enantioselective Addition of Alkyne Nucleophiles to Carbonyl Groups. Adv. Synth. Catal 2009, 351, 963–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Garcia Celina; Martin Victor S. Asymmetric Addition to Ketones: Enantioselective Formation of Tertiary Alcohols. Curr. Org. Chem 2006, 10, 1849–1889. [Google Scholar]
- (24).Kim SW; Zhang W; Krische MJ Catalytic Enantioselective Carbonyl Allylation and Propargylation via Alcohol Mediated Hydrogen Transfer: Merging the Chemistry of Grignard and Sabatier Acc. Chem. Res 2017, 50, 2371–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Comprehensive Organic Synthesis, 2nd ed.; Knochel P; Molander GA, Eds.; Elsevier: Oxford, 2014; Vols. 1 and 2. [Google Scholar]
- (26).Fischer F; Tropsch H Process for the Synthesis of Alcohols and Other Oxygen-Compounds via Catalytic Reduction of Carbon Monoxide, DRP 411216, November 3, 1922.
- (27).Fischer F; Tropsch H Process for the Synthesis of Long Chain Hydrocarbons From Carbon Monoxide and Hydrogen in a Catalytic Method, DRP 484337, July 22, 1925.
- (28).Roelen O (to Chemische Verwertungsgesellschaft Oberhausen m.b.H.) German Patent DE 849548, 1938/1952; U.S. Patent 2327066, 1943; Chem. Abstr 1944, 38, 3631.
- (29.) Sabatier P; Senderens JB Action du Nickel sur l’Éthylène. Synthèse de l’Éthane. C. R. Hebd. Seances Acad. Sci. 1897, 124, 1358–1360. [Google Scholar]
- (30).Young JF; Osborn JA; Jardine FH; Wilkinson G Hydride Intermediates in Homogeneous Hydrogenation Reactions of Olefins and Acetylenes using Rhodium Catalysts. Chem. Commun 1965, 131–132. [Google Scholar]
- (31).Bower JF; Krische MJ Formation of C-C Bonds via Iridium Catalyzed Hydrogenation and Transfer Hydrogenation. Top. Organomet. Chem 2011, 34, 107–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Hassan A; Krische MJ Unlocking Hydrogenation for C-C Bond Formation: A Brief Overview of Enantioselective Methods. Org. Process Res. Devel 2011, 15, 1236–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Roane J; Holmes M; Krische MJ Reductive C–C Coupling via Hydrogenation and Transfer Hydrogenation: Departure from Stoichiometric Metals in Carbonyl Addition. Curr. Opin. Green Sus. Chem 2017, 7, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Okude Y; Hirano S; Hiyama T; Nozaki H Grignard-type Carbonyl Addition of Allyl Halides by Means of Chromous salt. A Chemospecific Synthesis of Homoallyl Alcohols. J. Am. Chem. Soc 1977, 99, 3179–3181. [Google Scholar]
- (35).Takai K; Tagashira M; Kuroda T; Oshima K; Utimoto K; Nozaki H Reactions of Alkenylchromium Reagents Prepared from Alkenyl Trifluoromethanesulfonates (Triflates) with Chromium(II) Chloride under Nickel Catalysis. J. Am. Chem. Soc 1986, 108, 6048–6050. [DOI] [PubMed] [Google Scholar]
- (36).Jin H; Uenishi J; Christ WJ; Kishi Y Catalytic Effect of Nickel(II) Chloride and Palladium(II) Acetate on Chromium(II)-Mediated Coupling Reaction of Iodo Olefins with Aldehydes. J. Am. Chem. Soc 1986, 108, 5644–5646. [Google Scholar]
- (37).Moragas T; Correa A; Martin R Metal-Catalyzed Reductive Coupling Reactions of Organic Halides with Carbonyl-Type Compounds. Chem. Eur. J 2014, 20, 8242–8258. [DOI] [PubMed] [Google Scholar]
- (38).Montgomery J Nickel-Catalyzed Reductive Cyclizations and Couplings. Angew. Chem. Int. Ed 2004, 43, 3890–3908. [DOI] [PubMed] [Google Scholar]
- (39).Krische MJ, Jang H-Y Metal Catalyzed Reductive Cyclization (C=C, C≡C, C=O Bonds) In Comprehensive Organometallic Chemistry III; Mingos M, Crabtree R, Eds. Elsevier: Oxford, 2006, Vol. 10, pp 493–536. [Google Scholar]
- (40).Metal Catalyzed Reductive C-C Bond Formation; Krische M, Eds.; Topics in Current Chemistry 279; Springer-Verlag Berlin Heidelberg: Germany, 2007. [Google Scholar]
- (41).Iida H; Krische MJ Catalytic Reductive Coupling of Alkenes and Alkynes to Carbonyl Compounds and Imines Mediated by Hydrogen. Top. Curr. Chem 2007, 279, 77–104. [Google Scholar]
- (42).Jeganmohan M; Cheng C-H Cobalt- and Nickel-Catalyzed Regio- and Stereoselective Reductive Coupling of Alkynes, Allenes, and Alkenes with Alkenes. Chem. Eur. J 2008, 14, 10876–10886. [DOI] [PubMed] [Google Scholar]
- (43).Shibahara F; Krische MJ Formation of C-C Bonds via Ruthenium Catalyzed Transfer Hydrogenation: Carbonyl Addition from the Alcohol or Aldehyde Oxidation Level. Chem. Lett 2008, 37, 1102–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Bower JF; Kim IS; Patman RL; Krische MJ Catalytic Carbonyl Addition through Transfer Hydrogenation: A Departure from Preformed Organometallic Reagents. Angew. Chem. Int. Ed 2009, 48, 34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Moran J; Krische MJ Formation of C-C Bonds via Ruthenium Catalyzed Transfer Hydrogenation. Pure Appl. Chem 2012, 84, 1729–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Ketcham JM; Shin I; Montgomery TP; Krische MJ Catalytic Enantioselective C-H Functionalization of Alcohols by Redox-Triggered Carbonyl Addition: Borrowing Hydrogen, Returning Carbon. Angew. Chem. Int. Ed 2014, 53, 9142–9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Perez F; Oda S; Geary LM; Krische MJ Ruthenium Catalyzed Transfer Hydrogenation for C-C Bond Formation: Hydrohydroxyalkylation and Hydroaminoalkylation via Reactant Redox Pairs. Top. Curr. Chem 2016, 374, 365–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Nguyen KD; Park BY; Luong T; Sato H; Garza VJ; Krische MJ Metal Catalyzed Reductive Coupling of Olefin-Derived Nucleophiles: Reinventing Carbonyl Addition. Science 2016, 354, 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Montgomery J Nickel-Catalyzed Cyclizations, Couplings, and Cycloadditions Involving Three Reactive Components. Acc. Chem. Res 2000, 33, 467–473. [DOI] [PubMed] [Google Scholar]
- (50).Kimura M; Tamaru Y Nickel-Catalyzed Reductive Coupling of Dienes and Carbonyl Compounds. Top. Curr. Chem 2007, 279, 173–207. [Google Scholar]
- (51).Patel SJ; Jamison TF Catalytic Three-Component Coupling of Alkynes, Imines, and Organoboron Reagent. Angew. Chem. Int. Ed 2003, 42, 1364–1367. [DOI] [PubMed] [Google Scholar]
- (52).Sieber JD; Morken JP Sequential Pd-Catalyzed Asymmetric Allene Diboration/αAminoallylation. J. Am. Chem. Soc 2006, 128, 74–75. [DOI] [PubMed] [Google Scholar]
- (53).Cho HY; Morken JP Diastereoselective Construction of Functionalized Homoallylic Alcohols by Ni-Catalyzed Diboron-Promoted Coupling of Dienes and Aldehydes. J. Am. Chem. Soc 2008, 130, 1614016141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Cho HY; Morken JP Ni-Catalyzed Borylative Diene−Aldehyde Coupling: The Remarkable Effect of P(SiMe3)3. J. Am. Chem. Soc 2010, 132, 7576–7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Cho HY; Yu Z; Morken JP Stereoselective Borylative Ketone-Diene Coupling. Org. Lett 2011, 13, 5267–5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Meng F; Haeffner F; Hoveyda AH Diastereo- and Enantioselective Reactions of Bis(pinacolato)diboron, 1,3-Enynes, and Aldehydes Catalyzed by an Easily Accessible Bisphosphine-Cu Complex. J. Am. Chem. Soc 2014, 136, 11304–11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Yeung K; Ruscoe RE; Rae J; Pulis AP; Procter DJ Enantioselective Generation of Adjacent Stereocenters in a Copper-Catalyzed Three-Component Coupling of Imines, Allenes, and Diboranes. Angew. Chem. Int. Ed 2016, 55, 11912–11916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Gan X-C; Zhang Q; Jia X-S; Yin L Asymmetric Construction of Fluoroalkyl Tertiary Alcohols through a Three-Component Reaction of (Bpin)2, 1,3-Enynes, and Fluoroalkyl Ketones Catalyzed by a Copper(I) Complex. Org. Lett 2018, 20, 1070. [DOI] [PubMed] [Google Scholar]
- (59).Suginome M; Nakamura H; Matsuda T; Ito Y Platinum-Catalyzed Silaborative Coupling of 1,3Dienes to Aldehydes: Regio- and Stereoselective Allylation with Dienes through Allylic Platinum Intermediates. J. Am. Chem. Soc 1998, 120, 4248–4249. [Google Scholar]
- (60).Saito N; Kobayashi A; Sato Y Nickel-Catalyzed Enantio- and Diastereoselective Three-Component Coupling of 1,3-Dienes, Aldehydes, and a Silylborane Leading to α-Chiral Allylsilanes. Angew. Chem. Int. Ed 2012, 51, 1228–1231. [DOI] [PubMed] [Google Scholar]
- (61).Cho HY; Morken JP Catalytic Bismetallative Multicomponent Coupling Reactions: Scope, Applications, and Mechanisms. Chem. Soc. Rev 2014, 43, 4368–4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Takaya J; Iwasawa N Hydrocarboxylation of Allenes with CO2 Catalyzed by Silyl Pincer-Type Palladium Complex. J. Am. Chem. Soc 2008, 130, 15254–15255. [DOI] [PubMed] [Google Scholar]
- (63).Takaya J; Sasano K; Iwasawa N Efficient One-to-One Coupling of Easily Available 1,3-Dienes with Carbon Dioxide. Org. Lett 2011, 13, 1698–1701. [DOI] [PubMed] [Google Scholar]
- (64).Zhu C; Takaya J; Iwasawa N Use of Formate Salts as a Hydride and a CO2 Source in PGePPalladium Complex-Catalyzed Hydrocarboxylation of Allenes. Org. Lett 2015, 17, 1814–1817. [DOI] [PubMed] [Google Scholar]
- (65).Tani Y; Kuga K; Fujihara T; Terao J; Tsuji Y Copper-Catalyzed C–C Bond-Forming Transformation of CO2 to Alcohol Oxidation Level: Selective Synthesis of Homoallylic Alcohols from Allenes, CO2, and Hydrosilanes. Chem. Commun 2015, 51, 13020–13023. [DOI] [PubMed] [Google Scholar]
- (66).Julia-Hernandez F; Gaydou M; Serrano E; van Gemmeren M; Martin R Ni- and Fe-Catalyzed Carboxylation of Unsaturated Hydrocarbons with CO2. Top. Curr. Chem 2016, 374, 1–38. [DOI] [PubMed] [Google Scholar]
- (67).Gui Y-Y; Hu N; Chen X-W; Liao L-L; Ju T; Ye J-H; Zhang Z; Li J; Yu D-G Highly Regio- and Enantioselective Copper-Catalyzed Reductive Hydroxymethylation of Styrenes and 1,3-Dienes with CO2. J. Am. Chem. Soc 2017, 139, 17011–17014. [DOI] [PubMed] [Google Scholar]
- (68).For example: Bareille L; Le Gendre P; Moïse C First Catalytic Allyltitanation Reactions. Chem. Commun 2005, 775–777. [DOI] [PubMed] [Google Scholar]
- (69).Hayashi N; Honda H; Yasuda M; Shibata I; Baba A Generation of Allylic Indium by Hydroindation of 1,3-Dienes and One-Pot Reaction with Carbonyl Compounds. Org. Lett 2006, 8, 45534556. [DOI] [PubMed] [Google Scholar]
- (70).Chang H-M; Cheng C-H Highly Regioselective and Stereoselective Allylation of Aldehydes via Palladium-Catalyzed in Situ Hydrostannylation of Allenes. Org. Lett 2000, 2, 3439–3442. [DOI] [PubMed] [Google Scholar]
- (71).Sato Y; Takimoto M; Hayashi K; Katsuhara T; Takagi K; Mori M Novel Stereoselective Cyclization via π-Allylnickel Complex Generation from 1,3-Diene and Hydride Nickel Complex. J. Am. Chem. Soc 1994, 116, 9771–9772. [Google Scholar]
- (72).Kimura M; Ezoe A; Shibata K; Tamaru Y Novel and Highly Regio- and Stereoselective NickelCatalyzed Homoallylation of Benzaldehyde with 1,3-Dienes. J. Am. Chem. Soc 1998, 120, 4033–4034. [Google Scholar]
- (73).Takimoto M; Hiraga Y; Sato Y; Mori M Nickel-Catalyzed Regio- and Stereoselective Synthesis of Homoallylic Alcohol Derivatives from Dienes and Aldehydes. Tetrahedron Lett 1998, 39, 4543–4546. [Google Scholar]
- (74).Sawaki R; Sato Y; Mori M Ligand-Controlled Highly Stereoselective Syntheses of E- and ZAllylsilanes from Dienes and Aldehydes Using Nickel Complex. Org. Lett 2004, 6, 1131–1133. [DOI] [PubMed] [Google Scholar]
- (75).Kimura M; Fujimatsu H; Ezoe A; Shibata K; Shimizu M; Matsumoto S; Tamaru Y NickelCatalyzed Homoallylation of Aldehydes and Ketones with 1,3-Dienes and Complementary Promotion by Diethylzinc or Triethylborane. Angew. Chem. Int. Ed 1999, 38, 397–400. [DOI] [PubMed] [Google Scholar]
- (76).Kimura M; Ezoe A; Tanaka S; Tamaru Y Nickel-Catalyzed Homoallylation of Aldehydes in the Presence of Water and Alcohols. Angew. Chem. Int. Ed. 2001, 40, 3600–3602. [DOI] [PubMed] [Google Scholar]
- (77).Sato Y; Sawaki R; Saito N; Mori M Nickel-Catalyzed Intermolecular Coupling of 1,3-Dienes and Aldehydes via Transmetalation of Nickelacycles with Diisobutylaluminum Acetylacetonate. J. Org. Chem 2002, 67, 656–662. [DOI] [PubMed] [Google Scholar]
- (78).Loh T-P; Song HY; Zhou Y Nickel-Catalyzed Homoallylation Reaction of Aldehydes with 1,3Dienes: Stereochemical and Mechanistic Studies. Org. Lett 2002, 4, 2715–2717. [DOI] [PubMed] [Google Scholar]
- (79).Kimura M; Ezoe A; Mori M; Tamaru Y Regio- and Stereoselective Nickel-Catalyzed Homoallylation of Aldehydes with 1,3-Dienes. J. Am. Chem. Soc 2006, 128, 8559–8568. [DOI] [PubMed] [Google Scholar]
- (80).McCarren PR; Liu P; Cheong PH-Y; Jamison TF; Houk KN Mechanism and Transition-State Structures for Nickel-Catalyzed Reductive Alkyne−Aldehyde Coupling Reactions. J. Am. Chem. Soc 2009, 131, 6654–6655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Ogoshi S; Tonomori K-I; Oka M.-a.; Kurosawa H Reversible Carbon-Carbon Bond Formation between 1,3-Dienes and Aldehyde or Ketone on Nickel(0). J. Am. Chem. Soc 2006, 128, 7077–7086. [DOI] [PubMed] [Google Scholar]
- (82).Yang Y; Zhu S-F; Duan H-F; Zhou C-Y; Wang L-X; Zhou Q-L Asymmetric Reductive Coupling of Dienes and Aldehydes Catalyzed by Nickel Complexes of Spiro Phosphoramidites: Highly Enantioselective Synthesis of Chiral Bishomoallylic Alcohols. J. Am. Chem. Soc 2007, 129, 2248–2249. [DOI] [PubMed] [Google Scholar]
- (83).Sato Y; Hinata Y; Seki R; Oonishi Y; Saito N Nickel-Catalyzed Enantio- and Diastereoselective Three-Component Coupling of 1,3-Dienes, Aldehydes, and Silanes Using Chiral N-Heterocyclic Carbenes as Ligands. Org. Lett 2007, 9, 5597–5599. [DOI] [PubMed] [Google Scholar]
- (84).Köpfer A; Sam B; Breit B; Krische MJ Regiodivergent Reductive Coupling of 2-Substituted Dienes to Formaldehyde Employing Ruthenium or Nickel Catalyst: Hydrohydroxymethylation via Transfer Hydrogenation. Chem. Sci 2013, 4, 1876–1880. [Google Scholar]
- (85).Kimura M; Miyachi A; Kojima K; Tanaka S; Tamaru Y Highly Stereo- and Regioselective NiCatalyzed Homoallylation of Aldimines with Conjugated Dienes Promoted by Diethylzinc. J. Am. Chem. Soc 2004, 126, 14360–14361. [DOI] [PubMed] [Google Scholar]
- (86).Mori T; Akioka Y; Onodera G; Kimura M Ni-Catalyzed Homoallylation of Polyhydroxy N,OAcetals with Conjugated Dienes Promoted by Triethylborane. Molecules 2014, 19, 9288–9306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Karmakar R; Suneja A; Bisai V; Singh VK Ni(II)-Catalyzed Highly Stereo- and Regioselective Syntheses of Isoindolinones and Isoquinolinones from in Situ Prepared Aldimines Triggered by Homoallylation/Lactamization Cascade. Org. Lett 2015, 17, 5650–5653. [DOI] [PubMed] [Google Scholar]
- (88).Shibahara F; Bower JF; Krische MJ Ruthenium Catalyzed C-C Bond Forming Transfer Hydrogenation: Carbonyl Allylation from the Alcohol or Aldehyde Oxidation Level Employing Acyclic 1,3Dienes as Surrogates to Preformed Allyl Metal Reagents. J. Am. Chem. Soc 2008, 130, 6338–6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Hiraki K; Ochi N; Sasada Y; Hayashida H; Fuchita Y; Yamanaka SJ Organoruthenium(II) Complexes Formed by Insertion Reactions of Some Vinyl Compounds and Conjugated Dienes into a Hydrido-Ruthenium Bond. Chem. Soc., Dalton Trans 1985, 873–877. [Google Scholar]
- (90).Shibahara F; Bower JF; Krische MJ Diene Hydroacylation from the Alcohol or Aldehyde Oxidation Level via Ruthenium-Catalyzed C-C Bond-Forming Transfer Hydrogenation: Synthesis of β,γUnsaturated Ketones. J. Am. Chem. Soc 2008, 130, 14120–14122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Zbieg JR; Moran J; Krische MJ Diastereo- and Enantioselective Ruthenium Catalyzed Hydrohydroxyalkylation of 2-Silyl-Butadienes: Carbonyl syn-Crotylation from the Alcohol Oxidation Level. J. Am. Chem. Soc 2011, 133, 10582–10586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Sato F; Kusakabe M; Kobayashi Y Highly Diastereofacial Selective Addition of Nucleophiles to 2Alkyl-3-Trimethylsilylalk-3-enyl Carbonyl Compounds. Stereoselective Preparation of β-Methylhomoallyl Alcohols and β-Hydroxy-α-Methyl Ketones. J. Chem. Soc. Chem. Commun 1984, 1130–1132. [Google Scholar]
- (93).Helm MD; Mayer P; Knochel P Preparation of Silyl Substituted Crotylzinc Reagents and Their Highly Diastereoselective Addition to Carbonyl Compounds. Chem. Commun 2008, 1916–1917. [DOI] [PubMed] [Google Scholar]
- (94).Pantin M; Hubert JG; Söhnel T; Brimble MA; Furkert DP Stereochemical Characterization of Polyketide Stereotriads Synthesized via Hydrogen-Mediated Asymmetric syn-Crotylation. J. Org. Chem 2017, 82, 11225–11229. [DOI] [PubMed] [Google Scholar]
- (95).Moran J; Krische MJ Enantioselective Carbonyl Allylation and Crotylation from The Alcohol Oxidation Level via C-C Bond Forming Transfer Hydrogenation In Asymmetric Synthesis II; Christmann M; Brase S; Eds. Wiley-VCH Verlag GmbH & Co. KGaA Weinheim, Germany, 2012, pp 187–196. [Google Scholar]
- (96).Zbieg JR; Yamaguchi E; McInturff EL; Krische MJ Enantioselective C-H Crotylation of Primary Alcohols via Hydrohydroxyalkylation of Butadiene. Science 2012, 336, 324–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (97).Maytum HC; Tavassoli B; Williams JMJ Reduction of Aldehydes and Ketones by Transfer Hydrogenation with 1,4-Butanediol. Org. Lett 2007, 9, 4387–4389. [DOI] [PubMed] [Google Scholar]
- (98).McInturff EL; Yamaguchi E; Krische MJ Chiral Anion Dependent Inversion of Diastereo- and Enantioselectivity in Carbonyl Crotylation via Ruthenium Catalyzed Butadiene Hydrohydroxyalkylation. J. Am. Chem. Soc 2012, 134, 20628–20631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (99).Grayson MN; Krische MJ; Houk KN Ruthenium-Catalyzed Asymmetric Hydrohydroxyalkylation of Butadiene: The Role of the Formyl Hydrogen Bond in Stereochemical Control. J. Am. Chem. Soc 2015, 137, 8838–8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (100).Paraformaldehyde and Methanol as C1-Feedstocks in Metal Catalyzed C-C Couplings of πUnsaturated Reactants: Beyond Hydroformylation,” Sam B; Breit B; Krische MJ Angew. Chem. Int. Ed 2015, 54, 3267. [DOI] [PubMed] [Google Scholar]
- (101).Smejkal T; Han H; Breit B; Krische MJ All Carbon Quaternary Centers via Ruthenium Catalyzed Hydroxymethylation of 2-Substituted Butadienes Mediated by Formaldehyde: Beyond Hydroformylation. J. Am. Chem. Soc 2009, 131, 10366–10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (102).Han H; Krische MJ Direct Ruthenium Catalyzed C-C Coupling of Ethanol: Diene HydroHydroxyethylation to Form All Carbon Quaternary Centers. Org. Lett 2010, 12, 2844–2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (103).Xue P; Bi S; Sung HHY; Williams ID; Lin Z; Jia G Isomerization of [Ru(η3allyl)Cl(CO)(PPh3)2]. Organometallics 2004, 23, 4735–4743. [Google Scholar]
- (104).Leung JC; Geary LM; Chen T-Y; Zbieg JR; Krische MJ Direct, Redox Neutral Prenylation and Geranylation of Secondary Carbinol C-H Bonds: C4 Regioselectivity in Ruthenium Catalyzed C-C Couplings of Dienes to α-Hydroxy Esters. J. Am. Chem. Soc 2012, 134, 15700–15703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (105).Sanchez-Delgado RA; Bradley JS; Wilkinson G Ru3(CO)12 Further Studies on the Homogeneous Hydroformylation of Alkenes by Use of Ruthenium Complex Catalysts J. Chem. Soc. Dalton Trans 1976, 399–404. [Google Scholar]
- (106).Chatani N; Tobisu M; Asaumi T; Fukumoto Y; Murai S Ruthenium Carbonyl-Catalyzed [2+2+1]-Cycloaddition of Ketones, Olefins, and Carbon Monoxide, Leading to Functionalized γButyrolactones. J. Am. Chem. Soc 1999, 121, 7160–7161. [Google Scholar]
- (107).Tobisu M; Chatani N; Asaumi T; Amako K; Ie Y; Fukumoto Y; Murai S Ru3(CO)12-Catalyzed Intermolecular Cyclocoupling of Ketones, Alkenes or Alkynes, and Carbon Monoxide. [2+2+1] Cycloaddition Strategy for the Synthesis of Functionalized γ-Butyrolactones. J. Am. Chem. Soc 2000, 122, 12663–12674. [Google Scholar]
- (108).Chen T-Y; Krische MJ Regioselective Ruthenium Catalyzed Hydrohydroxyalkylation of Dienes with 3-Hydroxy-2-Oxindoles: Prenylation, Geranylation and Beyond. Org. Lett 2013, 15, 2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (109).Park BY; Montgomery TP; Garza VJ; Krische MJ Ruthenium Catalyzed Hydrohydroxyalkylation of Isoprene Employing Heteroaromatic Secondary Alcohols: Isolation and Reversible Formation of the Putative Metallacycle Intermediate. J. Am. Chem. Soc 2013, 135, 1632016323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (110).Geary LM; Glasspoole BW; Kim MM; Krische MJ Successive C-C Coupling of Dienes to Vicinally Dioxygenated Hydrocarbons: Ruthenium Catalyzed [4+2] Cycloaddition across the Diol, Hydroxycarbonyl or Dione Oxidation Levels. J. Am. Chem. Soc 2013, 135, 3796–3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (111).Kasun ZA; Geary LM; Krische MJ Ring expansion of cyclic 1,2-diols to form medium sized rings via ruthenium catalyzed transfer hydrogenative [4+2] cycloaddition. Chem. Commun 2014, 50, 7545–7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (112).Sato H; Fukaya K; Poudel BS; Krische MJ Diols as Dienophiles: Bridged Carbocycles via Ruthenium(0) Catalyzed Transfer Hydrogenative Cycloadditions of Cyclohexadiene or Norbornadiene. Angew. Chem. Int. Ed 2017, 56, 14667–13471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (113).Sato H; Turnbull BWH; Fukaya K; Krische MJ Ruthenium(0) Catalyzed Cycloaddition of 1,2Diols, Ketols or Diones via Alcohol-Mediated Hydrogen Transfer. Angew. Chem. Int. Ed. 2018, 59, DOI: 10.1002/anie.201709916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (114).Chen T-Y; Tsutsumi R; Montgomery TP; Volchkov I; Krische MJ Ruthenium Catalyzed C-C Coupling of Amino Alcohols with Dienes via Transfer Hydrogenation: Redox-Triggered Imine Addition and Related Hydroaminoalkylations. J. Am. Chem. Soc 2015, 137, 1798–1801. [DOI] [PubMed] [Google Scholar]
- (115).Oda S; Franke J; Krische MJ Diene Hydroaminomethylation via Ruthenium-Catalyzed C-C Bond Forming Transfer Hydrogenation: Beyond Carbonylation. Chem. Sci 2016, 7, 136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (116).Zhu S; Lu X; Luo Y; Zhang W; Jiang H; Yan M; Zeng W Ruthenium(II)-Catalyzed Regioselective Reductive Coupling of α-Imino Esters with Dienes. Org. Lett 2013, 15, 1440–1443. [DOI] [PubMed] [Google Scholar]
- (117).Schmitt DC; Lee J; Dechert-Schmitt A-MR Yamaguchi E; Krische MJ Ruthenium Catalyzed Hydroaminoalkylation of Isoprene via Transfer Hydrogenation: Byproduct-free Prenylation of Hydantoins. Chem. Comm 2013, 6096–6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (118).Jang H-Y; Huddleston RR; Krische MJ A New Catalytic C-C Bond-Forming Hydrogenation: Reductive Coupling of Dienes and Glyoxals under Catalytic Hydrogenation Conditions. Angew. Chem. Int. Ed 2003, 42, 4074–4077. [DOI] [PubMed] [Google Scholar]
- (119).Kimura M; Nojiri D; Fukushima M; Oi S; Sonoda Y; Inoue Y Rh-Catalyzed Reductive Coupling Reaction of Aldehydes with Conjugated Dienes Promoted by Triethylborane. Org. Lett 2009, 11, 37943797. [DOI] [PubMed] [Google Scholar]
- (120).Bower JF; Patman RL; Krische MJ Iridium-Catalyzed C-C Coupling via Transfer Hydrogenation: Carbonyl Addition from the Alcohol or Aldehyde Oxidation Level Employing 1,3Cyclohexadiene. Org. Lett 2008, 10, 1033–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (121).Zbieg JR; Fukuzumi T; Krische MJ Iridium-Catalyzed Hydrohydroxyalkylation of Butadiene: Carbonyl Crotylation. Adv. Synth. Catal 2010, 352, 2416–2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (122).Nguyen KD; Herkommer D; Krische MJ Enantioselective Formation of All-Carbon Quaternary Centers via C-H Functionalization of Methanol: Iridium-Catalyzed Diene Hydrohydroxymethylation. J. Am. Chem. Soc 2016, 138, 14210–14213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (123).Feng J; Holmes M; Krische MJ Acyclic Quaternary Carbon Stereocenters via Enantioselective Transition Metal Catalysis. Chem. Rev 2017, 117, 12564–12580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (124).Bareille L; Le Gendre P; Moïse C; First catalytic allyltitanation reactions. Chem. Commun 2005, 775–777. [DOI] [PubMed] [Google Scholar]
- (125).Li K; Shao X; Tseng L; Malcolmson SJ 2-Azadienes as Reagents for Preparing Chiral Amines: Synthesis of 1,2-Amino Tertiary Alcohols by Cu-Catalyzed Enantioselective Reductive Couplings with Ketones. J. Am. Chem. Soc. 2018, 140, 598–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (126).Shao X; Li K; Malcolmson S Enantioselective Synthesis of anti-1,2-Diamines by Cu-Catalyzed Reductive Couplings of Azadienes with Aldimines and Ketimines. J. Am. Chem. Soc. 2018, 140, DOI: 10.1021/jacs.8b04750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (127).Ng S-S; Jamison TF Highly Enantioselective and Regioselective Nickel-Catalyzed Coupling of Allenes, Aldehydes, and Silanes. J. Am. Chem. Soc. 2006, 127, 7320–7321. [DOI] [PubMed] [Google Scholar]
- (128).Ng S-S; Jamison TF Nickel-Catalyzed Coupling of Terminal Allenes, Aldehydes, and Silanes. Tetrahedron 2005, 61, 11350–11359.s [DOI] [PubMed] [Google Scholar]
- (129).Ng S-S; Jamison TF Enantioselective and Regioselective Nickel-Catalyzed Multicomponent Coupling of Chiral Allenes, Aromatic Aldehydes, and Silanes. Tetrahedron 2005, 61, 11405–11417. [DOI] [PubMed] [Google Scholar]
- (130).Ngai M-Y; Skucas E; Krische MJ Ruthenium Catalyzed C-C Bond Formation via Transfer Hydrogenation: Branch-Selective Reductive Coupling of Allenes to Paraformaldehyde and Higher Aldehydes. Org. Lett 2008, 10, 2705–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (131).Skucas E; Zbieg JR; Krische MJ anti-Aminoallylation of Aldehydes via Ruthenium-Catalyzed Transfer Hydrogenative Coupling of Sulfonamido Allenes: 1,2-Aminoalcohols. J. Am. Chem. Soc 2009, 131, 5054–5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (132).Zbieg JR; McInturff EL; Krische MJ Allenamide Hydro-Hydroxyalkylation: 1,2-Aminoalcohols via Ruthenium Catalyzed Carbonyl anti-Aminoallylation. Org. Lett 2010, 12, 2514–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (133).Zbieg JR; McInturff EL; Leung JC; Krische MJ Amplification of anti-Diastereoselectivity via Curtin-Hammett Effects in Ruthenium Catalyzed Hydrohydroxyalkylation of 1,1-Disubstituted Allenes: Diastereoselective Formation of All-Carbon Quaternary Centers. J. Am. Chem. Soc. 2011, 133, 11411144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (134).Sam B; Montgomery TP; Krische MJ Ruthenium Catalyzed Reductive Coupling of Paraformaldehyde to Trifluoromethyl Allenes: CF3-Bearing All-Carbon Quaternary Centers. Org. Lett. 2013, 15, 3790–3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (135).Sam B; Luong T; Krische MJ Ruthenium-Catalyzed C-C Coupling of Fluorinated Alcohols with Allenes: Dehydrogenation at the Energetic Limit of β-Hydride Elimination. Angew. Chem. Int. Ed 2015, 54, 5465–5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (136).Gellman AJ; Dai Q Mechanism of β-Hydride Elimination in Adsorbed Alkoxides. J. Am. Chem. Soc. 1993, 115, 714–722. [Google Scholar]
- (137).Gellman AJ; Dai Q Fluorine Substituent Effects on Alkoxide Chemistry and Orientation on the Cu(100) Surface. J. Phys. Chem. 1993, 97, 10783–10789. [Google Scholar]
- (138).Haydl AM; Breit B; Liang T; Krische MJ Alkynes as Electrophilic or Nucleophilic Allylmetal Precursors in Transition Metal Catalysis. Angew. Chem. Int. Ed. 2017, 56, 11312–11325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (139).Park BY; Nguyen KD; Chaulagain MR; Komanduri V; Krische MJ Alkynes as Allylmetal Equivalents in Redox-Triggered C−C Couplings to Primary Alcohols: (Z)-Homoallylic Alcohols via Ruthenium-Catalyzed Propargyl C−H Oxidative Addition. J. Am. Chem. Soc 2014, 136, 11902–11905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (140).Liang T; Nguyen KD; Zhang W; Krische MJ Enantioselective Ruthenium Catalyzed Carbonyl Allylation via Alkyne-Alcohol C-C Bond Forming Transfer Hydrogenation: Allene Hydrometallation vs. Oxidative Coupling. J. Am. Chem. Soc. 2015, 137, 3161–3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (141).Zhang W Chen W; Xiao H; Krische MJ Carbonyl anti-(α-Amino)allylation via Ruthenium Catalyzed Hydrogen Auto-Transfer: Use of an Acetylenic Pyrrole as an Allylmetal Pronucleophile. Org. Lett 2017, 19, 4876–4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (142).Oda S; Sam B; Krische MJ Hydroaminomethylation Beyond Carbonylation: Allene-Imine Reductive Coupling by Ruthenium-Catalyzed Transfer Hydrogenation. Angew. Chem. Int. Ed 2015, 54, 8525–8528. [DOI] [PubMed] [Google Scholar]
- (143).Skucas E; Bower JF; Krische MJ Carbonyl Allylation in the Absence of Preformed Allyl Metal Reagents: Reverse Prenylation via Iridium-Catalyzed Hydrogenative Coupling of Dimethylallene. J. Am. Chem. Soc 2007, 129, 12678–12679. [DOI] [PubMed] [Google Scholar]
- (144).Bower JF; Skucas E; Patman RL; Krische MJ Catalytic C-C Coupling via Transfer Hydrogenation: Reverse Prenylation, Crotylation and Allylation from the Alcohol or Aldehyde Oxidation Level. J. Am. Chem. Soc 2007, 129, 15134–15135. [DOI] [PubMed] [Google Scholar]
- (145).Han SB; Kim IS; Han H; Krische MJ Enantioselective Carbonyl Reverse Prenylation from the Alcohol or Aldehyde Oxidation Level Employing 1,1-Dimethylallene as the Prenyl Donor. J. Am. Chem. Soc. 2009, 131, 6916–6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (146).(Addition/Correction) Han SB; Kim IS; Han H; Krische MJ Enantioselective Carbonyl Reverse Prenylation from the Alcohol or Aldehyde Oxidation Level Employing 1,1-Dimethylallene as the Prenyl Donor. J. Am. Chem. Soc 2010, 132, 12517–12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (147).Brown HC; Jadhav PK 3,3-Dimethylallyldiisopinocampheylborane: A Novel Reagent for Chiral Isoprenylation of Aldehydes. Synthesis of (+)- and (−)-Artemisia Alcohol in Exceptionally High Enantiomeric Purity. Tetrahedron Lett. 1984, 25, 1215–1218. [Google Scholar]
- (148).Moran J; Preetz A; Mesch RA; Krische MJ Iridium-Catalysed Direct C–C Coupling of Methanol and Allenes. Nature Chem. 2011, 3, 287–290. [DOI] [PubMed] [Google Scholar]
- (149).Holmes M; Nguyen KD; Schwartz LA; Luong T Krische, M. J. Enantioselective Formation of CF3‑Bearing All-Carbon Quaternary Stereocenters via C−H Functionalization of Methanol: Iridium Catalyzed Allene Hydrohydroxymethylation. J. Am. Chem. Soc 2017, 139, 8114–8117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (150).Chang H-M; Cheng C-H; Highly Regioselective and Stereoselective Allylation of Aldehydes via Palladium-Catalyzed in Situ Hydrostannylation of Allenes. Org. Lett. 2000, 22, 3439–3442. [DOI] [PubMed] [Google Scholar]
- (151).Fujihara T; Hosomi T; Cong C; Hosoki T; Terao J; Tsuji Y Palladium-Catalyzed Formal Hydroacylation of Allenes Employing Carboxylic Anhydrides and Hydrosilanes. Tetrahedron 2015, 71, 4570–4574. [Google Scholar]
- (152).Leung JC; Krische MJ Catalytic Intermolecular Hydroacylation of C-C π-Bonds in the Absence of Chelation Assistance. Chem. Sci 2012, 3, 2202–2209. [Google Scholar]
- (153).Tsai EY; Liu RY; Yang Y; Buchwald SL A Regio- and Enantioselective CuH-Catalyzed Ketone Allylation with Terminal Allenes. J. Am. Chem. Soc 2018, 1140, 2007–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (154).Liu RY; Yang Y; Buchwald SL Regiodivergent and Diastereoselective CuH-Catalyzed Allylation of Imines with Terminal Allenes. Angew. Chem. Int. Ed 2016, 55, 14077–14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (155).Miller KM; Luanphaisarnnont T; Molinaro C; Jamison TF Alkene-Directed, Nickel-Catalyzed Alkyne Coupling Reactions. J. Am. Chem. Soc 2004, 126, 4130–4131. [DOI] [PubMed] [Google Scholar]
- (156).Miller KM; Colby EA; Woodin KS; Jamison TF Asymmetric Catalytic Reductive Coupling of 1,3-Enynes and Aromatic Aldehydes. Adv. Synth. Catal 2005, 347, 1533–1536. [Google Scholar]
- (157).Miller KM; Jamison TF Highly Regioselective, Catalytic Asymmetric Reductive Coupling of 1,3Enynes and Ketones. Org. Lett 2005, 7, 3077–3080. [DOI] [PubMed] [Google Scholar]
- (158).Liu P; McCarren PR; Cheong PH-Y; Jamison TF; Houk KN Origins of Regioselectivity and Alkene-Directing Effects in Nickel-Catalyzed Reductive Couplings of Alkynes and Aldehydes. J. Am. Chem. Soc 2010, 132, 2050–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (159).Malik HA; Sormunen GJ; Montgomery J A General Strategy for Regiocontrol in NickelCatalyzed Reductive Couplings of Aldehydes and Alkynes. J. Am. Chem. Soc 2010, 132, 6304–6305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (160).Stockis A; Hoffman R Metallacyclopentanes and Bisolefin Complexes. J. Am. Chem. Soc 1980, 102, 2952–2962. [Google Scholar]
- (161).Hong Y-T; Cho C-W; Skucas E; Krische MJ Enantioselective Reductive Coupling of 1,3-Enynes to Glyoxalates Mediated by Hydrogen: Asymmetric Synthesis of β,γ-Unsaturated α-Hydroxy Esters. Org. Lett 2007, 9, 3745–3748. [DOI] [PubMed] [Google Scholar]
- (162).Kong J-R; Ngai M-Y; Krische MJ Highly Enantioselective Direct Reductive Coupling of Conjugated Alkynes and α-Ketoesters via Rhodium-Catalyzed Asymmetric Hydrogenation. J. Am. Chem. Soc 2006, 128, 718–719. [DOI] [PubMed] [Google Scholar]
- (163).Enantioselective Reductive Coupling of 1,3-Enynes to Heterocyclic Aromatic Aldehydes and Ketones via Rhodium-Catalyzed Asymmetric Hydrogenation: Mechanistic Insight into the Role of Brønsted Acid Additives. J. Am. Chem. Soc. 2006, 128, 16448–16449. [DOI] [PubMed] [Google Scholar]
- (164).Musashi Y; Sakaki S Theoretical Study of Rhodium(III)-Catalyzed Hydrogenation of Carbon Dioxide into Formic Acid. Significant Differences in Reactivity among Rhodium(III), Rhodium(I), and Ruthenium(II) Complexes. J. Am. Chem. Soc 2002, 124, 7588–7603. [DOI] [PubMed] [Google Scholar]
- (165).Liu P; Krische MJ; Houk KN Mechanism and Origins of Regio- and Enantioselectivities in Rh(I)Catalyzed Hydrogenative Couplings of 1,3-Diynes and Activated Carbonyl Partners: Intervention of a Cumulene Intermediate. Chem. Eur. J. 2011, 17, 4021–4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (166).Halpern J Mechanism and Stereoselectivity of Asymmetric Hydrogenation. Science 1982, 217, 401–407. [DOI] [PubMed] [Google Scholar]
- (167).Kong J-R; Cho C-W; Krische MJ Hydrogen-Mediated Reductive Coupling of Conjugated Alkynes with Ethyl (N-Sulfinyl)iminoacetates: Synthesis of Unnatural α-Amino Acids via RhodiumCatalyzed C-C Bond Forming Hydrogenation. J. Am. Chem. Soc. 2005, 127, 11269–11276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (168).Patman RL; Williams VM; Bower JF; Krische MJ Carbonyl Propargylation from the Alcohol or Aldehyde Oxidation Level Employing 1,3-Enynes as Surrogates to Preformed Allenylmetal Reagents: A Ruthenium Catalyzed C-C Bond Forming Transfer Hydrogenation. Angew. Chem. Int. Ed 2008, 47, 52205223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (169).Wakatsuki Y; Yamazaki H; Maruyama Y; Shimizu I Novel Regioselection in Insertion of a 1,4Disubstituted-1,3-Enyne into Ruthenium-Hydrogen Bonds. J. Chem. Soc., Chem. Commun. 1991, 261263. [Google Scholar]
- (170).Geary LM; Leung JC; Krische MJ Ruthenium Catalyzed Reductive Coupling of 1,3-Enynes and Aldehydes via Transfer Hydrogenation: anti-Diastereoselective Carbonyl Propargylation. Chem. Eur. J 2012, 18, 16823–16827. [DOI] [PubMed] [Google Scholar]
- (171).Nguyen KD; Herkommer D; Krische MJ Ruthenium-BINAP Catalyzed Alcohol C-H tertPrenylation via 1,3-Enyne Transfer Hydrogenation: Beyond Stoichiometric Carbanions in Enantioselective Carbonyl Propargylation. J. Am. Chem. Soc. 2016, 138, 5238–5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (172).Ding C-H; Hou X-L Catalytic Asymmetric Propargylation. Chem. Rev 2011, 111, 1914–1937. [DOI] [PubMed] [Google Scholar]
- (173).Geary LM; Woo SK; Leung JC; Krische MJ Diastereo- and Enantioselective Iridium Catalyzed Carbonyl Propargylation from the Alcohol or Aldehyde Oxidation Level: 1,3-Enynes as Allenylmetal Equivalents. Angew. Chem. Int. Ed 2012, 51, 2972–2976. [DOI] [PubMed] [Google Scholar]
- (174).Yang Y; Perry IB; Lu G; Liu P; Buchwald SL Copper-Catalyzed Asymmetric Addition of Olefin-Derived Nucleophiles to Ketones. Science 2016, 353, 144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (175).Lu Y; Woo SK; Krische MJ Total Synthesis of Bryostatin 7 via C-C Bond Forming Hydrogenation. J. Am. Chem. Soc. 2011, 133, 13876–13876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (176).Andrews Ian P.; Ketcham JM; Blumberg PM; Kedei N; Lewin NE; Krische MJ Synthesis of seco-B-Ring Bryostatin Analogue WN-1 via C-C Bond Forming Hydrogenation: Critical Contribution of the B-Ring in Determining Bryostatin- and PMA-Like Properties. J. Am. Chem. Soc 2014, 136, 13209–13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (177).Ketcham JM; Volchkov I; Chen T-Y; Blumberg PM; Kedei N; Lewin NE; Krische MJ Evaluation of Chromane-Based Bryostatin Analogues Prepared via Hydrogen-Mediated C-C Bond Formation: Potency Does Not Confer Bryostatin-Like Biology. J. Am. Chem. Soc 2016, 138, 13415–13423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (178).Enantioselective Reductive Coupling of Alkynes and α-Keto Aldehydes via Rhodium-Catalyzed Hydrogenation: An Approach to Bryostatin Substructures. Org. Lett 2006, 8, 891–894. [DOI] [PubMed] [Google Scholar]
- (179).Lu Y; Krische MJ Concise Synthesis of the Bryostatin A-Ring via Consecutive C−C Bond Forming Transfer Hydrogenations. Org. Lett 2009, 11, 3108–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (180).Del Valle DJ; Krische MJ Total Synthesis of (+)-Trienomycins A and F via C–C Bond-Forming Hydrogenation and Transfer Hydrogenation. J. Am. Chem. Soc. 2013, 135, 10986–10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (181).Gao X; Woo SK; Krische MJ Total Synthesis of 6-Deoxyerythronolide B via C–C Bond-Forming Transfer Hydrogenation. J. Am. Chem. Soc 2013, 135, 4223–4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (182).Kretschmer M; Menche D Stereocontrolled Synthesis of the C8–C22 Fragment of Rhizopodin. Org. Lett 2012, 14, 382–385. [DOI] [PubMed] [Google Scholar]
- (183).Kretschmer M; Dieckmann M; Li P; Rudolph S; Herkommer D; Troendlin J; Menche D Modular Total Synthesis of Rhizopodin: A Highly Potent G-Actin Dimerizing Macrolide. Chem. Eur. J 2013, 19, 15993–16018. [DOI] [PubMed] [Google Scholar]