SUMMARY
SETTING:
A large, impoverished squatters’ settlement (favela), Rio de Janeiro, Brazil.
OBJECTIVE:
To assess the community impact of active case finding for tuberculosis (TB) compared to an enhanced case-finding strategy.
DESIGN:
A pair-matched, cluster-randomized trial comparing household symptom screening and spot sputum collection (Arm 1) vs. distribution of an educational pamphlet (Arm 2) was performed in a large Brazilian favela. We compared TB case-notification rates, time from symptom onset to treatment start and treatment completion proportions between arms. Fourteen neighborhoods (estimated population 58 587) were pair-matched by prior TB case rates and randomly allocated to one of two interventions. TB was diagnosed using acid-fast bacilli smears. New TB cases were interviewed and clinic records were reviewed.
RESULTS:
A total of 193 TB cases were identified in the 14 study neighborhoods (incidence proportion 329 per 100 000 population). The case identification rate in Arm 1 was 934/100 000 person-years (py) vs. 604/100 000 py in Arm 2 (RR 1.55, 95%CI 1.10–1.99). No significant differences were found in time from cough onset to treatment start or proportion completing treatment.
CONCLUSIONS:
A door-to-door case-finding campaign was more effective (while ongoing) at detecting prevalent cases and influencing people to come for care than leafleting, but no differences were seen in time to treatment start or treatment completion.
Keywords: tuberculosis, active case detection, cluster randomization, Brazil
RÉSUMÉ
CONTEXTE:
Un grand bidonville appauvri (favela) à Rio de Janeiro, Brésil.
OBJECTIF:
Evaluer l’impact sur la collectivité d’un dépistage actif des cas de tuberculose (TB) par comparaison avec une stratégie de dépistage renforcé.
SCHÉMA:
On a mené dans une grande favela brésilienne un essai apparié et randomisé par grappes comparant le dépistage des symptômes au domicile avec collecte de crachats sur place (Bras 1) versus la distribution d’une brochure de formation (Bras 2). Nous avons comparé les taux de déclaration des cas de TB, la durée séparant le début des symptômes du début du traitement et les proportions d’achèvement du traitement entre les deux bras. On a apparié par paires 14 quartiers (population estimée 58 587 personnes) en fonction des taux antérieurs des cas de TB et on les a attribués au hasard à l’une des deux interventions. Le diagnostic de TB a été obtenu par les frottis à la recherche de bacilles acidor ésistants. Les nouveaux cas de TB ont été interviewés et on a passé en revue les dossiers cliniques.
RÉSULTATS:
Dans les 14 quartiers de l’étude, on a identifié 193 cas de TB (proportion d’incidence 329/100 000). Le taux d’identification des cas dans le Bras 1 a été de 934/100 000 années/personne (ap) vs. 604/100 000 ap dans le Bras 2 (RR 1,55 ; IC95% 1,10–1,99). On n’a pas trouvé de différences significatives en matière de durée séparant le début de la toux et le début du traitement ou de proportion d’achèvement du traitement.
CONCLUSION:
Une campagne de dépistage des cas de porte à porte a été plus efficiente que la distribution de brochures pour la détection des cas prévalents et pour influencer les gens à se présenter pour les soins. On n’a trouvé aucune différence en matière de durée avant le début du traitement ou d’achèvement du traitement.
RESUMEN
MARCODEREFERENCIA:
Un extenso asentamiento ilegal pobre (favela) en Río de Janeiro, Brasil.
OBJETIVO:
Evaluar los efectos de una intervención activa de búsqueda de casos de tuberculosis (TB), en comparación con un reforzamiento de la estrategia de búsqueda de casos.
MÉTODOS:
Se llevó a cabo un estudio aleatorizado por conglomerados emparejados, en el cual se comparó la detección sistemática domiciliaria de síntomas y la recogida de muestras de esputo (grupo de estudio 1) con la distribución de un folleto educativo (grupo de estudio 2) en una extensa favela en el Brasil. Se compararon las tasas de notificación de casos, el lapso entre la aparición de síntomas y el comienzo del tratamiento y la compleción del tratamiento entre ambos grupos de estudio. Se aparearon 14 vecindarios (con una población calculada de 58 587 habitantes), en función de las tasas previas de casos de TB y se asignó a cada uno en forma aleatoria una de las dos intervenciones. El diagnóstico de TB se estableció mediante la baciloscopia del esputo. Se entrevistó a los casos nuevos de TB y se analizaron los expedientes clínicos.
RESULTADOS:
Se detectaron 193 casos de TB en los 14 vecindarios estudiados (incidencia 329 por 100 000 habitantes). La tasa de detección en el grupo 1 fue 934/100 000 años-persona (ap), comparada con 604/100 000 ap en el grupo 2 (riesgo relativo 1,55; IC95% 1,10–1,99). No se encontraron diferencias significativas en el lapso entre la aparición de la tos y el comienzo del tratamiento ni en la proporción de tratamientos completos.
CONCLUSIÓN:
Una campaña puerta a puerta de detección de casos fue más eficaz en detectar los casos prevalentes y en estimular a las personas a buscar atención que la sola distribución de folletos, pero no se observaron diferencias en el tiempo transcurrido hasta el comienzo del tratamiento ni en la proporción de tratamientos completos.
CASE DETECTION under the World Health Organization (WHO) DOTS strategy is passive, relying on patients to self-identify. The detection of smear-positive tuberculosis (TB) still lags behind stated goals (70%), with only 63% of acid-fast bacilli (AFB) smear-positive cases detected in 2008.1 Effective strategies to increase case detection are badly needed.2 Active and enhanced case finding (ACF and ECF) are strategies in which the onus is on the health care system to identify TB cases,3 seeking them out in the community (ACF) or raising awareness of and reducing barriers to diagnosis (ECF).4,5 Mathematical models suggest that effective ACF campaigns could contribute to global reduction in cases and deaths under the DOTS strategy,6–9 but data assessing the community impact of ACF are sparse.
We undertook a cluster-randomized trial to deter mine the impact of an intensive community-wide ACF campaign on case notification rates, time from symptom onset to treatment initiation and treatment outcomes compared with a less intensive ECF intervention conducted simultaneously in an area with high TB incidence and ready access to local health services.
STUDY POPULATION AND METHODS
Setting
The study was conducted in a large favela (squatter’s settlement) in Rio de Janeiro City, from August 2005 to March 2006. The favela is a densely populated, impoverished and drug- and violence-ridden area of the city, with population estimates of approximately 62 000 in a 1.4 km2 area,10 and an estimated TB incidence of 565 per 100 000 population.11 Two basic health posts, including a community-based TB program with cure rates of around 90%, serve the favela.11
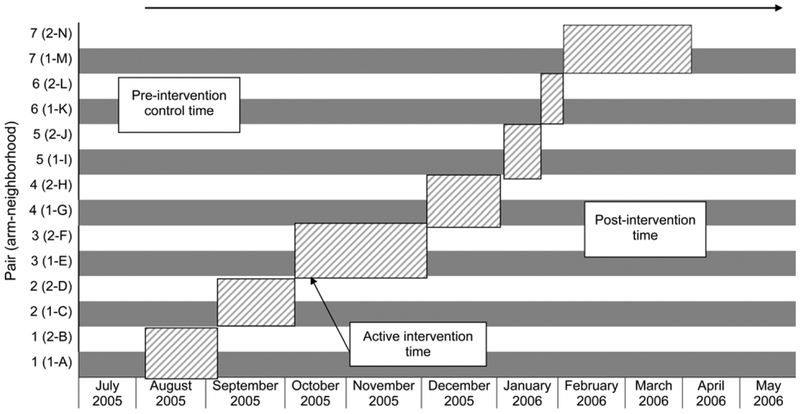
The Municipal Health Department of Rio de Janeiro has divided the favela into 15 neighborhoods, which were used as our units of intervention. Fourteen neighborhoods were pair-matched based on 2004 estimated TB notification rates, leaving one pilot area. One of each pair was randomly allocated to receive door-to-door ACF (Arm 1), while the other was allocated to receive home delivery of an informational pamphlet (Arm 2). The interventions took place between 10 August 2005 and 22 March 2006, using a unidirectional cross-over format in which matched pairs of neighborhoods received the intervention serially over time (Figure 1). Interventions were completed in each pair before commencing in the next pair.
Figure 1.
Study timeline. Diagonal hatch = active intervention time; grey = door-to-door neighborhoods (Arm 1); white = pamphlet neighborhoods (Arm 2); top arrow = total study time (10 August–22 May).
Intervention
The study consisted of two phases: a community-wide, door-to-door intervention, followed by structured interviews with patients diagnosed with active TB during the study period. The total study period included the complete intervention time plus 60 days after the end of the last intervention (283 days).
Phase 1
In Arm 1 neighborhoods, all households with at least one member aged ⩾18 years received a 7-question TB symptom survey after providing verbal consent. Children aged <18 years were interviewed by proxy. Those reporting cough for ⩾3 weeks were asked to provide a sputum specimen. A second sputum collection pot was left with the household and collected the following day.
In Arm 2 neighborhoods, an informational pamphlet from a national televised TB awareness campaign describing symptoms of TB, the free TB services available and encouraging attendance at the local health clinic by those with symptoms, was left under the door at all homes. Literacy rates in the favela were postulated at 74% at last census.12 Images from the televised campaigns may also have been recognizable without literacy.
The community health agent program (Favela Programa de Agentes Comunitários de Saúde [PACS]) of the Municipal Health Department provided the 50 community health agents (CHAs) from the favela and two supervising nurses who were involved in the project.
TB diagnosis was based on positive AFB smear (Ziehl-Neelsen) and clinical assessment. Mycobacterial culture was not routinely available. Pulmonary TB was defined, as standard of care at the clinic, as a chest X-ray suggestive of TB and at least one positive sputum smear. As this was a community intervention targeted at identifying and treating symptomatic individuals, all presenting cases not already on TB treatment were included. All AFB smear-positive individuals were given an appointment at the public health clinic (Clinic A) serving the area. Diagnosis and treatment for TB is provided free of charge in Brazil, and all patients were offered directly observed therapy (DOT).
Phase 2
Phase 2 consisted of interviewing newly diagnosed TB patients at Clinic A to obtain information on demographics, type, number and duration of symptoms, and reason for seeking treatment. Treatment initiation date and outcomes were abstracted from clinic log books.
The study protocol and consent forms were approved by both the Johns Hopkins University School of Medicine Institutional Review Board and the Comitê de Ética of the Health Secretariat of Rio de Janeiro City.
Outcome measures
The primary outcome was TB case notification rates in the two treatment arms. Secondary outcomes included duration of pre-treatment symptoms and proportions of treatment completion between study arms. Two time periods of analysis were established for the primary outcome: 1) the number of days each neighborhood pair experienced active intervention (‘active intervention’) and 2) the number of days during active intervention plus 60 days follow-up for each pair to assess duration of effect.4 Treatment outcomes for TB patients were recorded in March 2007.
Randomization
Using data from the Rio de Janeiro public health surveillance system (Sistema de Informações de Agravos de Notificação [SINAN]), the favela’s 14 neighborhoods were matched into seven pairs with similar 2004 case notification rates using a constrained randomization scheme with a relative difference of 5% between marginal rates.13 One of these permutations was selected at random using MS Excel’s RAND command (MicroSoft, Redmond, WA, USA).
Sample size
For a pair-matched study with the community as the unit of analysis, seven pairs of communities and an estimated coefficient of variation of 0.36, we had 80% power to detect a two-fold increase in case detection in the ACF arm from an estimated rate in pilot of 0.018.14 The coefficient of variation measured for the duration of our study was 0.44.
Analysis
Paired t-tests were used to evaluate the mean paired difference between case notification rates in communities assigned to each intervention.15 Rate ratios were calculated16 and 95% confidence intervals (CIs) were based on bootstrap standard deviations from resampling the matched pairs.17 Data were analyzed using Stata® 8 (Stata Corp, College Station, TX, USA) and Microsoft Excel® (MicroSoft). For the comparison of time from symptom onset to treatment, cluster-level analyses were adjusted by covariates.16 A linear-r egression model including all likely covariates except the intervention was fit to the individual data. The paired t-test was performed on neighborhood-aggregated residuals. Potential covariates were determined as those with P ⩽ 0.1 in univariate linear regression. Wilcoxon matched pairs signed rank test was used to test median time differences from symptom onset to treatment. Treatment outcomes were identified according to WHO definitions18 as successful (cured, completed therapy) or unsuccessful (abandoned, died, transferred, failed therapy), and analyzed using the paired t-test.
RESULTS
Overall, 193 cases were identified in an estimated population of 58 587 during the 283 days of the study, for an incidence proportion of 329/100 000. Case notification rates by neighborhood are presented in Table 1.
Table 1.
Tuberculosis case detection rates by neighborhood
| Neighbor- hood (pair) |
Estimated Residents n |
Intervention Days n |
Person- years |
Intervention days plus 60 days |
Person-years for intervention days plus 60 days |
Cases During Intervention n |
Cases during Intervention plus 60 days n |
Case detection rate, intervention time % |
Case detection rate, intervention days plus 60 % |
|---|---|---|---|---|---|---|---|---|---|
| A(1) | 4264 | 27 | 315 | 87 | 1016 | 7 | 10 | 0.022 | 0.010 |
| B(1) | 7287 | 27 | 539 | 87 | 1737 | 7 | 15 | 0.013 | 0.009 |
| C(2) | 5428 | 24 | 357 | 84 | 1248 | 3 | 6 | 0.008 | 0.005 |
| D(2) | 7730 | 24 | 508 | 84 | 1779 | 4 | 10 | 0.008 | 0.006 |
| E(3) | 5054 | 45 | 623 | 105 | 1454 | 3 | 3 | 0.005 | 0.002 |
| F(3) | 6110 | 45 | 753 | 105 | 1758 | 1 | 3 | 0.001 | 0.002 |
| G(4) | 2330 | 20 | 128 | 80 | 511 | 2 | 6 | 0.016 | 0.012 |
| H(4) | 5045 | 20 | 276 | 80 | 1106 | 2 | 3 | 0.007 | 0.003 |
| I (5) | 1223 | 12 | 40 | 72 | 241 | 0 | 0 | 0 | 0 |
| J(5) | 2074 | 12 | 68 | 72 | 409 | 0 | 0 | 0 | 0 |
| K(6) | 2275 | 14 | 87 | 74 | 461 | 0 | 1 | 0 | 0.002 |
| L(6) | 3464 | 14 | 133 | 74 | 702 | 0 | 1 | 0 | 0.001 |
| M(7) | 3603 | 49 | 484 | 109 | 1076 | 4 | 7 | 0.008 | 0.006 |
| N(7) | 2774 | 49 | 372 | 109 | 829 | 2 | 9 | 0.005 | 0.011 |
Door-to-door (Arm 1)
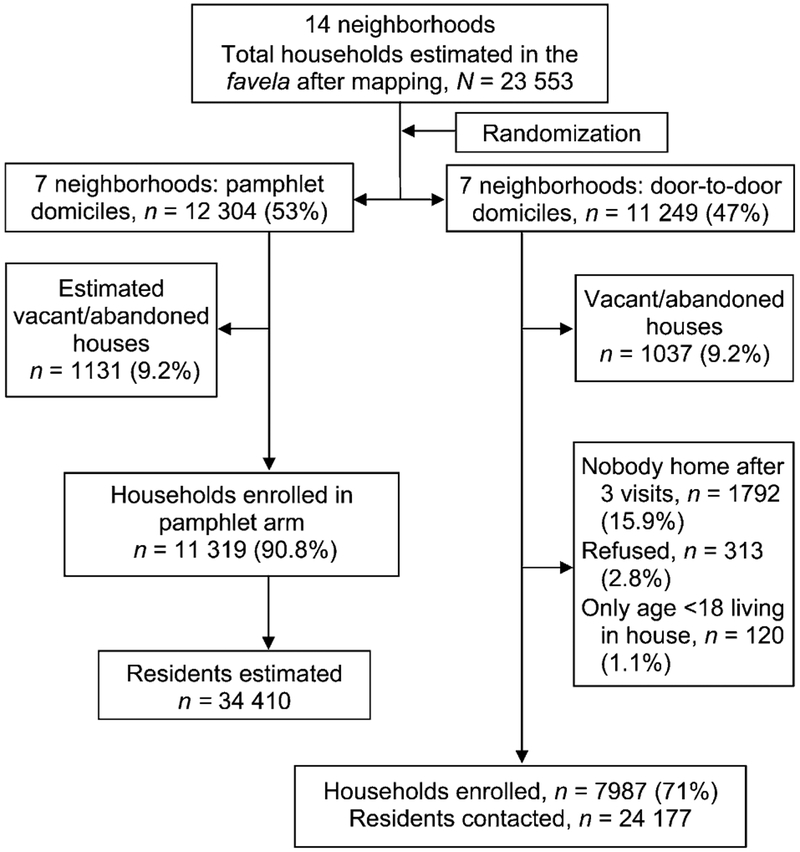
Of an estimated 11 249 households with 24 177 residents in the seven communities assigned to Arm 1 (Figure 2), 10 992 households and 23 865 residents (98.7%) were screened by CHAs. Data were collected for 12 067 (51%) females and 11 774 (49%) males interviewed in Arm 1. The mean age was 27 years (range <1–93, median 25, interquartile range 13–38). A total of 430 (1.8%) were identified as having respiratory symptoms and investigated for TB; among those with respiratory symptoms detected in the door-to-door investigation, 12 (2.8%) were identified as AFB sputum-positive cases (detection rate 50/100 000).
Figure 2.
Study flow diagram.
During the entire 283-day study period, Clinic A identified 92 TB cases from Arm 1 neighborhoods. During the period of active intervention, 19 cases were identified from Arm 1 neighborhoods, a case-notification rate of 934 per 100 000 person-years (py). For the intervention time plus 60 days, 32 cases were identified from Arm 1 neighborhoods (516/100 000 py; Tables 1 and 2).
Table 2.
Cluster and patient data by study arm
| Arm 1 door-to-door n (%) or mean ± SD |
Arm 2 Pamphlet n (%) or mean ± SD |
|
|---|---|---|
| Total (N = 193) | 92 | 101 |
| Total houses identified during study | 11249 | 12 304 |
| Total residents identified | 24177 | 34410 |
| Cluster size | 3454 ± 1568 | 4927 ± 2226 |
| Distance from bus line to clinic, km* | 0.18 ± 0.09 | 0.25 ± 0.26 |
| Cases identified per cluster | 13 ±7 | 14 ± 12 |
| Altitude, m* | 111.90 ± 15.98 | 92.22 ± 20.45 |
| Age, years | 31 ± 13.7 | 32 ± 15.4 |
| Sex | ||
| Male | 51 (55) | 65 (64) |
| Female | 41 (45) | 36 (36) |
Based on a 3-point estimate using Google Earth™ (Google, Mountain View, CA, USA).
SD = standard deviation.
Pamphlet (Arm 2)
Of an estimated 12 304 households in Arm 2, 11 319 households with an estimated 34 410 residents received the pamphlets (Table 2, Figure 2). During the 283-day study period, Clinic A identified 101 TB cases from the neighborhoods in Arm 2. During the active interven tion, 16 cases were identified from Arm 2 neighborhoods, a case-notification rate of 604/100 000 py. For the intervention time plus 60 days, 41 cases were identified from Arm 2 neighborhoods (493/100 000 py).
Outcome 1: Comparison of case-notification rates
During the active intervention, case-notification rates were significantly higher in Arm 1 than Arm 2 (rate ratio 1.55, 95%CI 1.10–1.99). However, case-notification rates were not significantly different during the extended time period (active intervention time plus 60 days (rate ratio 1.05, 95%CI 0.56–1.54; Table 3).
Table 3.
Rate ratios and 95%CIs for tuberculosis case detection
| Comparison | Arm 1 (door-to-door Symptom screen) |
Arm 2 (pamphlet delivery) |
Rate ratio (95%CI)* |
|---|---|---|---|
| Intervention time | 9.34/1000 py | 6.04/1000 py | 1.55(1.10–1.99) |
| Intervention + 60 days | 5.16/1000 py | 4.93/1000 py | 1.05(0.56–1.54) |
Outcome 2: Comparison of times to diagnosis
Of 193 persons, 103 (53%) with active TB were interviewed in the second phase. The study arms were not significantly different in terms of demographics, although Arm 1 cases were more likely to smoke (Table 4). Seventy-nine cases were identified post-intervention and the remaining 24 cases were diagnosed before the intervention in their neighborhoods. Of those identified post-intervention, all 79 reported one or more of the following: cough, fever, shortness of breath, night sweats or weight loss, with 73/79 (92.4%) reporting at least cough. No significant difference in time to treatment between the two study arms was found when controlling for paired neighborhoods (Table 5).
Table 4.
Demographic data on interviewed cases, by study arm
| Arm 1 door to door (n = 53) n (%) |
Arm 2 Pamphlet (n = 50) n (%) |
Robust P value |
|
|---|---|---|---|
| Sex | |||
| Male | 28 (53) | 33 (66) | 0.14 |
| Female | 25 (47) | 17(34) | |
| Age, years | |||
| ⩾18 | 50 (94) | 49 (98) | 0.23 |
| <18 | 3(6) | 1(2) | |
| Ethnicity (n = 102) | |||
| White | 35 (66) | 32 (65) | |
| Black | 15(28) | 12(25) | 0.49 |
| Mixed race | 3(6) | 5(10) | |
| White | 35 (66) | 32 (67) | 0.93 |
| Non-White | 18(34) | 17(33) | |
| Marital status | |||
| Married/living in partnership | 20 (38) | 24 (48) | |
| Single/separated/divorced/widowed | 33 (62) | 26 (52) | 0.21 |
| Smoking status | |||
| Current smoker | 18(34) | 7(14) | 0.03 |
| Not current smoker | 35 (66) | 43 (86) | |
| Ever smoked | 41 (77) | 31 (63) | 0.22 |
| Never smoked | 12(23) | 18(37) | |
| Rooms in house (n = 102)* | |||
| 1 | 16(30) | 7(15) | |
| 2 | 19(36) | 23 (46) | 0.02 |
| 3 | 16(30) | 14(29) | |
| 4 | 2(4) | 1(2) | |
| >4 | 0 | 4(8) | |
| Crowded (n = 102)† | |||
| No | 38 (72) | 42 (86) | 0.06 |
| Yes | 15 (28) | 7(14) | |
| Minimum salaries (n = 100)‡ | |||
| <1 | 6(12) | 9(19) | |
| 1–<2 | 25 (49) | 18(35) | 0.54 |
| 2–<4 | 16(31) | 15(31) | |
| 4–<6 | 4(8) | 5(10) | |
| 6–<8 | 0 | 2(4) | |
| Very low income‡ | |||
| >2 minimum salaries | 20 (39) | 22 (45) | 0.55 |
| ⩽2 minimum salaries | 31 (61) | 27 (55) | |
| Educational level | |||
| No formal education | 3(6) | 8(16) | 0.65 |
| 1–5 years | 29(55) | 14(28) | |
| ⩾6 years | 21 (39) | 28 (56) |
Excluding kitchen or bathroom. One case was homeless, therefore missing.
Difference between number of people in house and number of rooms in house is ⩾3.
A ‘minimum salary’ is $350 Brazilian reals, which equated to US$161 on 7 August 2006.
Table 5.
Times to treatment start from symptom onset by study arm, cases identified post-intervention only (n = 79)
| Days Mean (range) |
95%CI | Median |
P value for medians |
|
|---|---|---|---|---|
| Cough time (n = 73) | ||||
| Pamphlet | 53 (7–224) | 38.1–68.1 | 56 | |
| Door-to-door | 57(10–336) | 32.8–81.9 | 32 | 0.83* |
| Any symptom time Pamphlet | ||||
| Door-to-door | 74 (15–336) | 48.9–98.5 | 56 | |
| Found at home vs. not at home | 65 (7–336) | 42.6–87.3 | 56 | 0.75* |
| Cough time only (n = 73) | ||||
| At home | 93 (28–336) | 7.6–178.4 | 56 | |
| Other door-to-door | 53 (7–336) | 30.5–74.8 | 30 | 0.23† |
| All other | 54 (7–336) | 41.9–66.1 | 52.5 | |
| Any symptom time | ||||
| At home | 95 (28–336) | 22.7–167.0 | 56 | |
| Other door-to-door | 66 (7–336) | 44.8–87.8 | 56 | 0.59† |
| All other | 75 (7–336) | 59.2–90.8 | 56 |
Wilcoxon signed rank test.
Median test.
CI = confidence interval.
Twelve cases were found at their homes by our door-to-door symptom screen, although one had already presented for care and was therefore reassigned as self-identified. Nine were interviewed. Mean and median times from cough onset to treatment start were substantially higher in cases found at home than in those self-reporting to the clinic, but were not statistically significantly different (Table 5). No demographic differences were found between cases found at home and those reporting to the clinic, but those found at home were more likely to be current or ever smokers (100% vs. 72%, P = 0.04).
Outcome 3: Completion of therapy
Of 193 participants, 182 had a final outcome listed by March 2007: 88 in Arm 1 and 94 in Arm 2. Overall, 156 patients were treated successfully (86%). Treatment completion rates were not significantly different between the two study arms: 84% (n = 74) of those in Arm 1 vs. 87% (n = 82) in Arm 2.
DISCUSSION
Our study found that ACF using CHAs for a door-to-door symptom screen was successful at identifying cases of TB, and resulted in significantly higher case notification rates than pamphlets alone while ongoing. Treatment completion rates were high, regardless of detection method. Future studies on the cost-effectiveness of the two methods would help policy makers determine whether to make the investment in active case finding.
In our study, ACF worked significantly better than ECF at detecting cases while ongoing, but this effect was not enduring. It is possible that having a CHA come to the door is not something that people remember later if they develop symptoms. However, studies from several disciplines show increased effectiveness of personal interaction over educational literature alone,19–21 and our interviewed patients indicated that CHA visits were an important factor in the decision to seek care. Another interpretation is that informational interventions take longer to motivate people to seek care, but also work fairly well. Increasing awareness of TB is likely to increase case detection, and both arms of this study were subject to TB promotion.
Using door-to-door screening, we found 12 smear-positive TB cases with cough, only one of whom (8%) had already sought care for his illness. All subsequently began anti-tuberculosis treatment. Cases found in Arm 1 were more likely than cases from Arm 2 to be current smokers. This finding is likely due to the method in which suspect cases were identified in Arm 1, which was to ask about cough. As smokers tend to have longer diagnostic delays and worse treatment outcomes,22 ACF may help reduce transmission among coughing smokers and increase their chance of treatment success.
Cases detected at the door reported fewer symptoms. This supports findings by other authors23–26 that TB patients identified through ACF were less ill than those who self-identified. However, unlike Cassels et al.’s23 and Santha et al.’s24 findings, cases found in our ACF arm were equally likely to complete therapy as those who self-identified, probably due to the favela’s highly successful DOT program. This underscores the importance of using ACF in areas with documented strong DOTS programs, thus providing the best opportunity for successful treatment of detected cases.
We did not detect differences in time to diagnosis between the two study arms. Shargie et al. found that in rural Ethiopia, ECF significantly reduced the time to diagnosis but had no impact on overall case finding and case notification rates when compared to the control arm.27
Our study had several unique strengths. To our knowledge, this is the first randomized trial of two types of case finding. By using an interviewer to whom administrative designation of ‘neighborhoods’ was unknown, we were able to avoid possible selection and interviewer biases. We demonstrate very low refusal rates and high therapy completion rates. This study was conducted in a highly challenging setting, with outbreaks of violence periodically interrupting the intervention. That we were able to detect a difference in these circumstances speaks highly of the practicability of door-to-door case screening and the conscientious dedication of the staff.
One limitation of our study is that small numbers of large clusters limit statistical power. We addressed this challenge by using methods specifically designed for these studies, and matched neighborhoods on a very important predictor of future TB incidence—past TB incidence. Despite this limitation, we were able to detect a difference in our study arms.
To evaluate ACF in the context of usual practice, we used AFB positivity as the primary diagnostic tool. While AFB smear-negative cases in the door-to-door arm of the study could have been missed, and may contribute to transmission,28 our results would have been biased toward the null.
For Phase 2, our protocol prioritized smear-positive, pulmonary, incident adult cases of TB, and only 53% of identified cases were interviewed because of unexpected workload constraints of the interviewing nurse. Although no significant differences in age, sex or study arm were identified between those interviewed and those not interviewed (data not shown), the relatively small number of cases interviewed may have limited our power to detect differences between study arms in time from symptom onset to diagnosis.
We believe the findings for this study are applicable to other areas of Latin America and certainly to other favelas. That ACF and DOT have been successfully implemented in the largest favela in Latin America may encourage future application. Longer term follow-up will provide data for the crucial question of whether increasing case detection under an effective DOT program results in reduced incidence over time.
CONCLUSION
Comprehensive door-to-door symptom screening was more immediately effective than door-to-door distribution of informational pamphlets at detecting cases, and identified cases with a high likelihood of transmission yet a low likelihood of prompt care seeking. No differences were seen in time to treatment start or treatment completion.
Acknowledgements
The authors thank the Favela PACS program. Partial funding was provided by United States Agency for International Development/Brasil H454–991–2416 and National Institutes of Health grants K01 AI066994; K24 AI 001637; U19-AI45432.
References
- 1.World Health Organization. Global tuberculosis control: surveillance, planning, financing: WHO report 2007 WHO/HTM/TB/2007.376. Geneva, Switzerland: WHO, 2007. [Google Scholar]
- 2.World Health Organization. An expanded DOTS framework for effective tuberculosis control. Geneva, Switzerland: WHO, 2002. [PubMed] [Google Scholar]
- 3.Golub JE, Mohan CI, Comstock GW, Chaisson RE. Active case finding of tuberculosis: historical perspective and future prospects. Int J Tuberc Lung Dis 2005; 9: 1183–1203. [PMC free article] [PubMed] [Google Scholar]
- 4.Jaramillo E The impact of media-based health education on tuberculosis diagnosis in Cali, Columbia. Health Policy Plan 2001; 16: 68–73. [DOI] [PubMed] [Google Scholar]
- 5.Becx-Bleumink M, Wibowo H, Apriani W, Vrakking H. High tuberculosis notification and treatment success rates through community participation in central Sulawesi, Republic of Indonesia. Int J Tuberc Lung Dis 2001; 5: 920–925. [PubMed] [Google Scholar]
- 6.Dowdy DW, Chaisson RE, Moulton LH, Dorman SE. The potential impact of enhanced diagnostic techniques for tuberculosis driven by HIV: a mathematical model. AIDS 2006; 20: 751–762. [DOI] [PubMed] [Google Scholar]
- 7.Dye C, Watt CJ, Bleed DM, Williams BG. What is the limit to case detection under the DOTS strategy for tuberculosis control? Tuberculosis (Edinb) 2003; 83: 35–43. [DOI] [PubMed] [Google Scholar]
- 8.Murray CJL, Salomon JA. Modeling the impact of global tuberculosis control strategies. Proc Natl Acad Sci USA 1998; 95: 13881–13886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray CJL, Salomon JA. Expanding the WHO tuberculosis control strategy: rethinking the role of active case finding. Int J Tuberc Lung Dis 1998; 2 (Suppl 1): S9–S15. [PubMed] [Google Scholar]
- 10.Prefeitura da Cidade do Rio de Janeiro. Projeção da população, segundo as Regiões Administrativas—2001/2020 (Tabela N° 697); Rio de Janeiro, RJ, Brazil: Janeiro PdCdRd, 2000. [Google Scholar]
- 11.Cavalcante SC, Soares ECC, Pacheco A, Chaisson RE, Durovni B, Team DE. Community DOT for tuberculosis in a Brazilian favela: comparison with a clinic model. Int J Tuberc Lung Dis 2007; 11: 544–549. [PubMed] [Google Scholar]
- 12.Cavallieri F, Sigard MF. O analfabetismo na cidade do Rio de Janeiro 1991–2000. Rio de Janeiro, RJ, Brazil: Prefeitura da Ciu dade do Rio de Janiero, 2002. [Google Scholar]
- 13.Moulton LH. Covariate-based constrained randomization of group-randomized trials. Clin Trials 2004; 1: 297–305. [DOI] [PubMed] [Google Scholar]
- 14.Hayes RJ, Bennett S. Simple sample size calculation for cluster-randomized trials. Int J Epidemiol 1999; 28: 319–326. [DOI] [PubMed] [Google Scholar]
- 15.Brookmeyer R, Chen YQ. Person-time analysis of paired community intervention trials when the number of communities is small. Stat Med 1998; 17: 2121–2132. [DOI] [PubMed] [Google Scholar]
- 16.Bennett S, Parpia T, Hayes R, Cousens S. Methods for the analysis of incidence rates in cluster randomized trials. Int J Epidemiol 2002; 31: 839–846. [DOI] [PubMed] [Google Scholar]
- 17.Efron B, Tibshirani R. An introduction to the bootstrap. London, UK: Chapman and Hall, 1993. [Google Scholar]
- 18.World Health Organization. Treatment of tuberculosis: guidelines for national programmes WHO/CDS/TB/2003.313. G eneva, Switzerland: WHO, 2003. [Google Scholar]
- 19.Taylor VM, Hislop TG, Jackson JC, et al. A randomized controlled trial of interventions to promote cervical cancer screening among Chinese women in North America. J Natl Cancer Inst 2002; 94: 670–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramsaroop S, Reid M, Adelman R. Completing an advance directive in the primary care setting; what do we need for success? J Am Geriatr Soc 2007; 55: 277–283. [DOI] [PubMed] [Google Scholar]
- 21.Huang M, Liu C, Chi Y, Thomas K, Huang C. Effects of educational intervention on changing parental practices for recurrent febrile convulsions in Taiwan. Epilepsia 2002; 43: 81–86. [DOI] [PubMed] [Google Scholar]
- 22.Yang JY, Hsueh PR, Jan IS, et al. The effect of smoking on tuberculosis: different patterns and poorer outcomes. Int J Tuberc Lung Dis 2005; 11: 143–149. [PubMed] [Google Scholar]
- 23.Cassels A, Heineman E, LeClerc S, Gurung PK, Rahut CB. Tuberculosis case finding in Eastern Nepal. Tubercle 1982; 63: 175–185. [DOI] [PubMed] [Google Scholar]
- 24.Santha T, Renu G, Freiden TR, et al. Are community surveys to detect tuberculosis in high prevalence areas useful? Results of a comparative study from Tiruvallur District, South India. Int J Tuberc Lung Dis 2003; 7: 258–265. [PubMed] [Google Scholar]
- 25.Jackson-Sillah D, Hill P, Fox A, Brookes R, Donkor S, Lugos M. Screening for tuberculosis among 2381 household contacts of sputum-smear-positive cases in The Gambia. Trans R Soc Trop Med Hyg 2007; 101: 594–601. [DOI] [PubMed] [Google Scholar]
- 26.den Boon S, Verver S, Lombard CJ, et al. Comparison of symptoms and treatment outcomes between actively and passively detected tuberculosis cases: the additional value of active case finding. Epidemiol Infect 2008; 136: 1342–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shargie EB, Morkve O, Lindtjorn B. Tuberculosis case-finding through a village outreach programme in a rural setting in southern Ethiopia: community randomised trial. Bull World Health Organ 2006; 84: 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Behr MA, Warren SA, Salamon H, et al. Transmission of Mycobacterium tuberculosis from patients smear-negative for acid-fast bacilli. Lancet 1999; 353: 444–449. [DOI] [PubMed] [Google Scholar]