SUMMARY
SETTING:
Pune, India.
OBJECTIVES:
To estimate the prevalence and risk factors of pre-diabetes mellitus (DM) and DM, and its associations with the clinical presentation of tuberculosis (TB).
DESIGN:
Screening for DM was conducted among adults (age ⩾ 18 years) with confirmed TB between December 2013 and January 2017. We used multinomial regression to evaluate the risk factors for pre-DM (glycated hemoglobin [HbA1c] ⩾ 5.7–6.5% or fasting glucose 100–125 mg/dl) and DM (HbA1c ⩾ 6.5% or fasting glucose ⩾ 126 mg/dl or random blood glucose > 200 mg/dl or self-reported DM history/treatment) and the association of dysglycemia with the severity of TB disease.
RESULTS:
Among 1793 participants screened, 890 (50%) had microbiologically confirmed TB. Of these, 33% had pre-DM and 18% had DM; 41% were newly diagnosed. The median HbA1c level among newly diagnosed DM was 7.0% vs. 10.3% among known DM (P < 0.001). DM (adjusted OR [aOR] 4.94, 95%CI 2.33–10.48) and each per cent increase in HbA1c (aOR 1.42, 95%CI 1.01–2.01) was associated with >1+smear grade or ⩽9 days to TB detection.
CONCLUSION:
Over half of newly diagnosed TB patients had DM or pre-DM. DM and increasing dysglycemia was associated with higher bacterial burden at TB diagnosis, potentially indicating a higher risk of TB transmission to close contacts.
Keywords: TB, pre-diabetes mellitus, diabetes mellitus, risk factors, clinical presentation, India
RÉSUMÉ
CONTEXTE :
Pune, Inde.
OBJECTIF :
Estimer la prévalence et les facteurs de risque de pré-diabète (DM) et de DM, et son association avec la présentation clinique de la tuberculose (TB)
SCHÉMA :
Une recherche de DM a été réalisée parmi des adultes (⩾ 18 ans) atteints de TB confirmée entre décembre 2013 et janvier 2017. Nous avons utilisé une régression multinomiale pour évaluer les facteurs de risque de pré-DM (HbA1c ⩾ 5,7 et jusqu’à 6,5% ou glycémie à jeun de 100–125 mg/dl) et DM (HbA1c ⩾ 6,5% ou glycémie a jeun ⩾ 126 mg/dl ou glycémie à toute heure > 200 mg/dl ou antécédents de DM/de traitement déclaré par les patients) et association de dysglycémie avec la gravité de la TB maladie.
RESULTATS :
Sur 1793 participants dépistés, 890 (50%) avaient une TB confirmée par microbiologie. Parmi eux, 33% avaient un pré-DM et 18%, un DM ; 41% ont été des diagnostics nouveaux. L’HbA1c médiane parmi les nouveaux cas de DM a été de 7,0% contre 10,3% parmi les DM connus (P < 0,001). Le DM (OR ajusté [ORa] 4,94 ; IC95% 2,33–10,48) et chaque augmentation de pourcentage de 1’HbA1c (ORa 1,42 ; IC95% 1,01–2,01) ont été associés avec une augmentation de >1+ du grade du frottis ou moins de 9 jours jusqu’à la détection de la TB.
CONCLUSION :
Plus de la moitié des nouveaux patients TB diagnostiqués avaient un DM ou un pré-DM. Le DM et une dysglycémie croissante ont été; associés avec une charge bactérienne plus élevée lors du diagnostic de TB, indiquant un risque potentiel plus élevé de transmission de la TB à des contacts proches.
RESUMEN
MARCO DE REFERENCIA:
La ciudad de Pune, en la India.
OBJETIVOS:
Calcular la prevalencia de pre-diabetes (DM) y DM y su asociación con el cuadro clínico inicial de la tuberculosis (TB).
MÉTODO:
Se llevó a cabo una detectión sistemática de la DM en los adultos (a partir de los 18 años) condiagnóistico confirmado de TB, de diciembre del 2013 a enero del 2017. Mediante un modelo de regresión polinómica se evaluaron los factores de riesgo de padecer pre-DM (HbA1c de 5,7% hasta 6,5% o glucemia en ayunas de 100 a 125 mg/dl) y DM (HbA1c ⩾6,5%, glucemia en ayunas ⩾126 mg/dl, una glucemia aleatoria > 200 mg/dl o un antecedente autorreferido de diagnóistico o tratamiento de la DM) y la asociación de la disglucemia con la gravedad de la enfermedad tuberculosa.
RESULTADOS:
De los 1793 participantes examinados, 890 presentaron TB con confirmación microbiológica (50%). De estos casos, el 33% tenía pre-DM y el 18% DM; en el 41% de los casos se trató de un diagnóstico nuevo. La mediana de la HbA1c en los casos recién diagnosticados fue 7,0% contra 10,3% en los pacientes con diagnóstico conocido (P < 0,001). Se asociaron con una gradatión de la baciloscopia superior a 1+o con ⩽9 días hasta la detectión de la TB, la DM (OR ajustado [aOR] 4,94; IC95% 2,33–10,48) y cada unidad de aumento de la HbA1c (aOR 1,42; IC95% 1,01–2,01).
CONCLUSIÓN:
Más de la mitad de los pacientes con diagnóstico reciente de TB presentó DM o pre-DM. La DM y un aumento de la disglucemia se asociaron con una mayor carga bacilar en el momento del diagnóstico de TB, lo cual puede indicar un mayor riesgo de transmisión de la enfermedad a los contactos cercanos.
THE INTERSECTION OF TUBERCULOSIS (TB) and diabetes mellitus (DM) has recently received global attention. DM increases the risk of TB by nearly three-fold, and persons with both TB and DM may have worse TB treatment outcomes.1–5 Globally, TB continues to be the leading cause of morbidity and mortality from any single infectious pathogen, with approximately 10.4 million incident cases and 1.8 million deaths in 2015.1 With diets rich in fat and refined carbohydrates, combined with decreasing daily physical activity, the burden of DM is reaching epidemic proportions, even in resource-limited settings.6,7 The TB epidemic in India is the largest in the world, with over 2.8 million cases occurring in 2015.1,8 India also has a staggering 69 million adults living with DM, the largest burden in the world.7 The convergence of these two enormous epidemics in India thus has major implications for global TB control.
Recent estimates suggest that DM may account for approximately 15% of all pulmonary TB (PTB) cases in high TB and DM burden countries.9 Data from India indicate a wide range of DM prevalence (6.5–33%) among TB cases; all of these estimates are much higher than for the general population.9–14 More recently, a report from South India showed that over 75% of TB cases had dysglycemia, with 54% meeting the criteria for DM.15 As the diet and genetics of the people and the epidemiology of dysglycemia are likely to vary in different parts of India, region-specific data are needed, particularly for Western India, where data are limited.
Previous research has shown that TB in people with DM may have a different clinical presentation than in those without DM.16 These differences include increased symptoms of TB, increased involvement of the lungs, and higher bacterial burdens. However, these findings have been inconsistently reported.16,17 We hypothesize that the clinical presentation of TB among people with DM will be more severe than in TB patients without DM. Furthermore, we hypothesize that glycemic status may alter the clinical severity of TB at disease presentation.
An understanding of the prevalence, risk factors and clinical presentation of TB according to pre-DM and DM status may be particularly informative for the early recognition of individuals at an increased risk for adverse TB treatment outcomes. Within a cohort study investigating the impact of DM on TB treatment outcomes, we present the prevalence and risk factors and the clinical presentation of TB patients with and without pre-DM and DM.
METHODS
Study design and study sites
Between 23 December 2013 and 4 January 2017, eligible individuals with suspected TB at the Revised National TB Control Programme (RNTCP) centers in Pune and Pimpri-Chinchwad Municipal Corporations (PCMCs) of Maharashtra, India, were approached by the study counsellors. They were referred to the clinical research site of Byramjee-Jeejeebhoy Medical College-Sassoon General Hospital (BJMC-SGH) in Pune. BJMC-SGH is a large, public-sector, tertiary-care teaching institution that serves approximately 7 million population in the surrounding urban, semi-urban and rural low-income populations. A second site, Dr D Y Patil Medical College, Pune, a private affiliated medical college with a large hospital catering to low- and lower-middle-income populations, initiated the study in July 2016, and obtained referrals from RNTCP centers in the PCMC region.
The Institutional Review Board of Johns Hopkins School of Medicine, Balitimore, MD, USA, and the ethics committees at BJMC-SGH and Dr D Y Patil Medical College, Pune, approved the project.
Study procedures
The eligibility criterion was adults aged ⩾18 years with suspicion of PTB. Those with a previous history of TB, current anti-tuberculosis treatment for at least 7 days and those diagnosed with rifampin-resistant TB were excluded.
Consenting participants were administered a questionnaire to collect information on demographics and medical history, including TB history and TB risk factors. All enrolled participants underwent anthropometric assessments and clinical evaluations. Chest radiography was performed on patients confirmed to have TB.
Laboratory investigations comprised a baseline fasting or random blood glucose test (Cobas c111; Roche Diagnostics, Rotkreuz, Switzerland), glycated hemoglobin (HbA1c) test (BioRad Laboratories, Hercules, CA, USA) and sputum evaluations. Spontaneously expectorated sputum was collected on two occasions—on the spot and early morning. All sputum specimens collected underwent direct smear for acid-fast bacilli (AFB) staining, Xpert® MTB/RIF (Cepheid, Sunnyvale, CA, USA) assays and culture (in Mycobacterial Growth Indicator Tube [MGIT] liquid culture and Löwenstein Jensen media). Mycobacterium tuberculosis growth on liquid culture was confirmed using p-nitrobenzoic acid, and cultures with confirmed growth of M. tuberculosis were subjected to phenotypic drug susceptibility testing.
Study definitions
Suspected TB was defined according to RNTCP criteria and included a cough for at least 2 weeks and any other symptoms suggestive of TB, such as fever, night sweats, loss of weight or loss of appetite. Confirmed TB was defined as positive AFB smear, Xpert results or M. tuberculosis complex in sputum culture. DM was defined as having either HbA1c ⩾6.5%, fasting blood glucose (FBG) level of ⩾126 mg/dl, random blood sugar > 200 mg/dl or DM diagnosis by self-reporting, or currently taking DM medication. Pre-DM was defined as HbA1c of ⩾5.7% and <6.5% or FBG of ⩾110 and <126 mg/dl. Body mass index (in kg/m2) was calculated using World Health Organization definitions for underweight (<18 kg/m2), normal (18–24.9 kg/m2), or overweight (⩾25 kg/m2).
Assessments of TB severity
Clinical presentation of TB among patients with pre-DM, DM and no DM was assessed using clinical, radiographic and laboratory findings collected at baseline. Using a published clinical score17 that gives one point to each major TB-related symptom—cough, fever, weight loss, night sweats, anorexia, hemoptysis and malaise—TB disease was classified as severe (⩾4) and non-severe TB (⩽3). Radiologic findings of cavitary lung lesions and/or the involvement of ⩾ 1 lung lobe, and microbiologic findings of >1+sputum AFB and/or shorter time to TB detection (TTD) in culture, were classified as severe TB disease. Shorter TTD by culture was defined as less than the median number of days by MGIT culture.
Statistical analysis
DM prevalence and binomial exact 95% confidence intervals (CIs) were calculated as the number of DM cases divided by the total number of microbiologically confirmed TB cases. Baseline categorical and continuous variables for sociodemographic, clinical characteristics and clinical presentation among confirmed PTB cases by DM were summarized using proportions and median values with interquartile range (IQR), respectively, and compared using either Fisher’s exact test or Wilcoxon rank-sum test; P < 0.05 was deemed statistically significant. Univariable and multivariable multinomial logistic regression was performed to assess the factors associated with pre-DM and DM among cases with confirmed TB in comparison with those without DM. All variables that had P < 0.1 in the univariable analysis and known risk factors for DM were used in the multivariable analysis to estimate adjusted odds ratios (aORs). In addition, univariable and multivariable logistic regressions were performed to determine the association of pre-DM and DM with TB disease severity. Data were analyzed using Stata v13.1 (StataCorp, College Station, TX, USA).
RESULTS
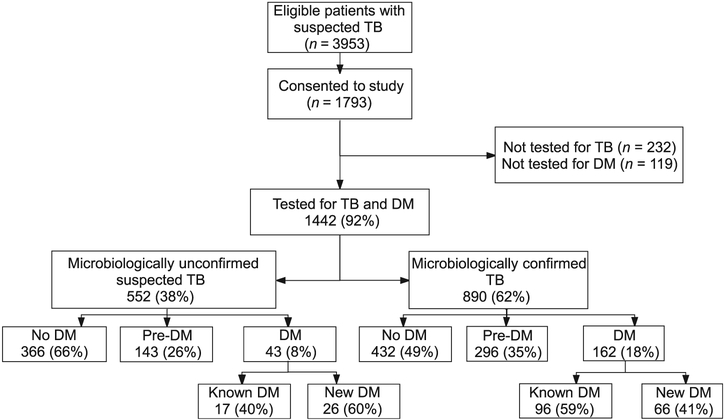
Prevalence of and factors associated with pre-diabetes mellitus and diabetes mellitus among pulmonary tuberculosis cases
Of 3953 eligible participants approached by the study team, 1793 (45%) consented to participate; 1442 (80%) underwent investigations for both TB and DM (Figure). Of these, 890 (62%) patients were diagnosed with microbiologically confirmed TB (Figure). The median age was 32 years (IQR 22–44); 589 (66%) were males (Table 1). The prevalence of pre-DM and DM was respectively 33% (95%CI 30–36) and 18% (95%CI 16–21). Of the 162 with DM, 96 (59%) were known to have DM and 66 (41%) were newly diagnosed. Prevalence of DM among TB patients aged <25 years was 2% (95%CI 0–8) compared with respectively 12% (95%CI 6–17) and 45% (95%CI 38–51) among TB patients aged 25–40 years and those aged >40 years. Overall, 42 (6%) TB patients had human immunodeficiency virus (HIV) infection, and 6 (6%) TB patients with DM had HIV. The median level of HbA1c for pre-DM was 6.0% (IQR 5.9–6.1) and for DM it was 9.1% (IQR 6.9ߝ11.6). The median level of HbA1c among cases with newly diagnosed DM was 7.0% (IQR 6.6–10.2) compared with 10.3% (IQR 8.6–12.1) among patients with known DM (P < 0.001).
Figure.
Flowchart of study enrollments and prevalence of pre-DM and DM among patients with suspected and confirmed TB in Pune, India. TB = tuberculosis; DM = diabetes mellitus.
Table 1.
Sociodemographic and clinical characteristics of TB patients by pre-DM and DM status, Pune, India
| Confirmed TB |
|||||
|---|---|---|---|---|---|
| Characteristics | Overall (n = 890) n (%) |
No DM (n = 432) n (%) |
Pre-DM (n = 296) n (%) |
DM (n = 162) n (%) |
P value |
| Sex | |||||
| Male | 589 (66) | 259 (60) | 205 (69) | 125 (77) | <0.001 |
| Female | 301 (34) | 173 (40) | 91 (31) | 37 (23) | |
| Age, years, median [IQR] | 32 [24–44] | 27 [23–35] | 30 [24–43] | 47 [40–55] | <0.001 |
| Age group, years | <0.001 | ||||
| <25 | 281 (32) | 180 (42) | 96 (32) | 5 (3) | |
| 25–40 | 347 (39) | 187 (43) | 120 (41) | 0 (25) | |
| >40 | 262 (29) | 65 (15) | 80 (27) | 4117 (72) | |
| Location | |||||
| Urban | 820 (92) | 403 (93) | 270 (91) | 147 (91) | 0.42 |
| Rural | 70 (8) | 29 (7) | 26 (9) | 15 (9) | |
| Smoked tobacco products | 890 | 432 | 296 | 162 | 0.30 |
| Non-smoker | 723 (81) | 356 (82) | 232 (78) | 135 (83) | |
| Smoker | 167 (19) | 76 (18) | 64 (22) | 27 (17) | |
| Alcohol | |||||
| No | 625 (70) | 298 (69) | 209 (71) | 118 (72) | 0.66 |
| Yes | 265 (30) | 134 (31) | 87 (29) | 44 (27) | |
| CAGE* | 0.84 | ||||
| <2 | 117 (44) | 59 (44) | 37 (43) | 21 (48) | |
| ⩾2 | 148 (56) | 75 (56) | 50 (57) | 23 (52) | |
| Household income, INR/month† | 0.27 | ||||
| <5000 | 208 (25) | 107 (27) | 70 (25) | 31 (20) | |
| >5000 | 625 (75) | 296 (73) | 205 (75) | 124 (80) | |
| Education | 0.003 | ||||
| More than primary | 644 (72) | 329 (76) | 215 (73) | 100 (62) | |
| None or primary | 246 (28) | 103 (24) | 81 (27) | 62 (38) | |
| Religion | 0.04 | ||||
| Non-Hindu | 214 (24) | 88 (20) | 82 (28) | 44 (27) | |
| Hindu | 676 (76) | 344 (80) | 214 (72) | 118 (73) | |
| Anemia‡ | 0.05 | ||||
| No | 59 (86) | 364 (84) | 247 (85) | 148 (92) | |
| Yes | 7123 (14) | 66 (15) | 44 (15) | 13 (8) | |
| HIV | 0.56 | ||||
| Negative | 529 (93) | 255 (93) | 173 (91) | 101 (94) | |
| Positive | 42 (7) | 19 (7) | 17 (9) | 6 (6) | |
| Body mass index§ | <0.001 | ||||
| Normal | 250 (28) | 102 (23) | 65 (22) | 83 (51) | |
| Underweight | 603 (68) | 326 (75) | 213 (72) | 64 (40) | |
| Overweight | 37 (4) | 4 (1) | 18 (6) | 15 (9) | |
CAGE score determines high alcohol dependence.
US$1 = 64 INR.
Defined as hemoglobin < 8 g/dl.
Underweight: <18.5 kg/m2; normal: 18.5–24.9 kg/m2; overweight: >25 kg/m2.
TB = tuberculosis; DM = diabetes mellitus; IQR = interquartile range; CAGE = 1) Have you ever felt you needed to cut down on your drinking? 2) Have people annoyed you by criticizing your drinking? 3) Have you ever felt guilty about drinking? 4) Have you ever felt you needed a drink first thing in the morning (eye-opener) to steady your nerves or to get rid of a hangover? INR = Indian rupee; HIV = human immunodeficiency virus.
In multivariate analyses, older age (aOR 1.97, 95%CI 1.17–3.30) and being overweight (aOR 4.49, 95%CI 1.36–14.83) were associated with pre-DM (Table 2). Furthermore, males (aOR 1.81, 95%CI 1.01–3.24) were more likely to be diagnosed with DM. Compared with TB patients aged <25 years, patients aged 25–40 years (aOR 10.05, 95%CI 2.99–33.77) and those aged >40 years (aOR 56.43, 95%CI 16.57–192.1) were more likely to have DM at TB diagnosis and being underweight was protective against DM (aOR 0.27, 95%CI 0.16–0.46).
Table 2.
Multinomial logistic regression analysis for risk factors for pre-DM and DM among confirmed TB patients, Pune, India
| Confirmed TB (n = 890) |
||
|---|---|---|
| Characteristics | Univariable analysis OR (95%CI) |
Multivariable analysis aOR (95%CI)* |
| Sex | ||
| Pre-DM | ||
| Male | 1.50 (1.10–2.06) | 1.44 (0.97–2.13) |
| Female | 1 | 1 |
| DM | ||
| Male | 2.26 (1.49–3.41) | 1.81 (1.01–3.24) |
| Female | 1 | 1 |
| Age group, years | ||
| Pre-DM | ||
| <25 | 1 | 1 |
| 25–40 | 1.20 (0.86–1.69) | 1.17 (0.78–1.75) |
| >40 | 2.31 (1.53–3.48) | 1.97 (1.17–3.30) |
| DM | ||
| <25 | 1 | 1 |
| 25–40 | 7.70 (2.97–19.95) | 10.05 (2.99–33.77) |
| >40 | 64.8 (25.34–165.7) | 56.43 (16.57–192.1) |
| Location | ||
| Pre-DM | ||
| Urban | 1 | — |
| Rural | 1.34 (0.77–2.32) | |
| DM | ||
| Urban | 1 | — |
| Rural | 1.42 (0.74–2.72) | |
| Smoking tobacco products | ||
| Pre-DM | ||
| Non-smokers | 1 | — |
| Smokers | 1.29 (0.89–1.87) | |
| DM | ||
| Non-smokers | 1 | — |
| Smokers | 0.94 (0.58–1.52) | |
| Alcohol | ||
| Pre-DM | ||
| No | 1 | — |
| Yes | 0.93 (0.67–1.28) | |
| DM | ||
| No | 1 | — |
| Yes | 0.83 (0.55–1.24) | |
| CAGE † | ||
| Pre-DM | ||
| CAGE <2 | 1 | — |
| CAGE ⩾2 | 1.06 (0.61–1.83) | |
| DM | ||
| CAGE <2 | 1 | — |
| CAGE ⩾2 | 0.86 (0.43–1.7) | |
| Religion | ||
| Pre-DM | ||
| Non-Hindu | 1 | 1 |
| Hindu | 0.67 (0.47–0.94) | 0.64 (0.42–0.97) |
| DM | ||
| Non-Hindu | 1 | 1 |
| Hindu | 0.69 (0.45–1.04) | 0.71 (0.40–1.28) |
| Anemia† | ||
| Pre-DM | ||
| No | 1 | 1 |
| Yes | 0.98 (0.65–1.49) | 1.31 (0.80–2.16) |
| DM | ||
| No | 1 | 1 |
| Yes | 0.48 (0.26–0.90) | 0.92 (0.41–2.07) |
| HIV status | ||
| Pre-DM | ||
| Negative | 1 | — |
| Positive | 1.32 (0.67– 2.61) | |
| DM | ||
| Negative | 1 | — |
| Positive | 0.79 (0.31– 2.05) | |
| Body mass index§ | ||
| Pre-DM | ||
| Normal | 1 | 1 |
| Underweight | 1.02 (0.72–1.46) | 1.02 (0.66–1.56) |
| Overweight/obese | 7.06 (2.29–21.79) | 4.49 (1.36–14.83) |
| DM | ||
| Normal | 1 | 1 |
| Underweight | 0.24 (0.16–0.36) | 0.27 (0.16–0.46) |
| Overweight/obese | 4.61 (1.47–14.41) | 1.98 (0.55–7.12) |
Adjusted for covariates with P < 0.1 in univariate analysis and covariates known to be associated with a risk of DM.
CAGE score determines high alcohol dependence.
Defined as hemoglobin < 8 g/dl.
Underweight: <18.5 kg/m2; normal: 18.5–24.9 kg/m2; overweight: >25 kg/m2.
TB = tuberculosis; DM = diabetes mellitus; OR = odds ratio; CI = confidence interval; aOR = adjusted OR; CAGE = 1) Have you ever felt you needed to cut down on your drinking? 2) Have people annoyed you by criticizing your drinking? 3) Have you ever felt guilty about drinking? 4) Have you ever felt you needed a drink first thing in the morning (eye-opener) to steady your nerves or to get rid of a hangover? HIV = human immunodeficiency virus.
Tuberculosis symptoms, radiologic features and microbiologic findings
The median duration of TB-related symptoms was 45 days for all patients (Table 3). Overall, 712 (84%) TB patients reported ⩾4 symptoms; of these, 280 (39%) had pre-DM and 140 (20%) had DM. In multivariable analysis, patients with DM were more than four-fold as likely to have ⩾4 symptoms (aOR 4.24, 95%CI 1.24–14.43) than those without DM (Table 4). Patients with pre-DM were nearly twice as likely to have ⩾4 symptoms (aOR 1.72, 95%CI 0.85–3.49); however, this association did not reach statistical significance.
Table 3.
Clinical presentation of TB by pre-DM and DM status, Pune, India
| Characteristics | No DM (n = 432) n (%) |
Pre-DM (n = 296) n (%) |
DM (n = 162) n (%) |
P value |
|---|---|---|---|---|
| Symptoms | ||||
| Duration of illness, days, median [IQR] | 45 [30–60] | 45 [30–90] | 45 [30–75] | 0.30 |
| Cough | 426 (99) | 296 (100) | 162 (100) | 0.05 |
| Duration, days, median [IQR] | 30 [15–60] | 30 [20–60] | 30 [20–60] | 0.25 |
| Fever | 370 (86) | 263 (89) | 145 (90) | 0.32 |
| Duration, days, median [IQR] | 20 [15–40]) | 30 [15–45] | 20 [12–45] | 0.14 |
| Night sweats | 250 (58) | 178 (60) | 95 (58) | 0.83 |
| Duration, days, median [IQR] | 15 [10–30] | 25 [15–45] | 21 [15–45] | 0.03 |
| Loss of appetite | 353 (82) | 250 (84) | 138 (85) | 0.50 |
| Duration, days, median [IQR] | 30 [15–60] | 30 [20–60] | 30 [15–60] | 0.42 |
| Weight loss | 343 (79) | 223 (75) | 127 (78) | 0.42 |
| Duration, days, median [IQR] | 30 [21–60] | 30 [30–60] | 45 [30–60] | 0.17 |
| Shortness of breath | 268 (62) | 192 (65) | 102 (63) | 0.74 |
| Duration, days, median [IQR] | 30 [15–60] | 30 [15–60] | 30 [15–60] | 0.75 |
| Physical examination | ||||
| Waist circumference, cm, median [IQR] | 69 [63–75] | 70 [65–76] | 78 [72–88] | <0.001 |
| Hip circumference, cm, median [IQR] | 82 [78–86] | 82 [78–86] | 86 [83–93] | <0.001 |
| Mid-upper arm circumference, cm, median [IQR] | 22 [20–23] | 21 [19–23] | 24 (21–25] | <0.001 |
| Body mass index, kg/m2, median [IQR] | 17 [15–18] | 17 [15–19] | 20 [17–23] | <0.001 |
| Hemoglobin, g/dl, median [IQR] | 11.8 [10.1–13.1] | 11.3 [10.3–12.6] | 12.3 [11.1–13.6] | 0.003 |
| Systolic blood pressure, mmHg, median [IQR] | 112 [110–114] | 112 [108–114] | 114 [110–120] | <0.001 |
| Diastolic blood pressure, mmHg, median [IQR] | 72 [70–76] | 72 [70–76] | 76 [70–80] | <0.001 |
| Chest radiography | ||||
| Normal | 0 | 2 (1) | 0 | 0.28 |
| Abnormal: not TB | 1 (0.6) | 1 (0.7) | 0 | |
| Abnormal: compatible with TB | 72 (40) | 45 (31) | 35 (40) | |
| Abnormal: highly suggestive of TB | 109 (60) | 99 (67) | 52 (60) | |
| Infiltrate | 117 (97) | 139 (96) | 87 (100) | 0.18 |
| Unilateral | 39 (21) | 25 (17) | 18 (21) | 0.22 |
| Bilateral | 139 (76) | 115 (78) | 69 (79) | |
| Unknown | 4 (2) | 7 (5) | 0 | |
| Number of lobes | ||||
| 1 | 28 (16) | 14 (10) | 12 (14) | 0.08 |
| 2 | 72 (41) | 64 (46) | 49 (56) | |
| >2 | 77 (44) | 62 (44) | 26 (30) | |
| Miliary infiltrates | 13 (7) | 6 (4) | 7 (8) | 0.37 |
| Cavitary | 84 (46) | 69 (47) | 39 (45) | 0.95 |
| Mycobacterial findings | ||||
| Direct smear result | ||||
| Negative | 135 (32) | 79 (27) | 34 (22) | 0.17 |
| Scanty | 66 (16) | 46 (16) | 25 (16) | |
| 1+ | 102 (24) | 77 (26) | 55 (35) | |
| 2+ | 67 (16) | 54 (19) | 30 (19) | |
| 3+ | 49 (12) | 35 (12) | 14 (9) | |
| Concentrated smear result | ||||
| Positive | 313 (75) | 227 (78) | 132 (84) | 0.06 |
| Time to MGIT result, days, median [IQR] | 8 [6–12] | 8 [5–11] | 8 [6–10] | 0.14 |
TB = tuberculosis; DM = diabetes mellitus; IQR = interquartile range; MGIT = Mycobacterial Growth Indicator Tube.
Table 4.
Diabetes as a risk factor for severe TB disease* using multinomial logistic regression analyses
| Univariable analysis |
Multivariable analysis† |
|||
|---|---|---|---|---|
| TB disease markers | RRR (95%CI) | P value | RRR | P value |
| TB symptoms | ||||
| Pre-DM | ||||
| <4 | Reference | — | Reference | — |
| ⩾4 | 1.49 (0.81–2.76) | 0.20 | 1.72 (0.85–3.49) | 0.14 |
| DM | ||||
| <4 | Reference | — | Reference | — |
| ⩾4 | 1.89 (0.82–4.36) | 0.13 | 4.24 (1.24–14.43) | 0.02 |
| Cavitary TB disease or ⩾1 lobe affected or miliary infiltrates | ||||
| Pre-DM | ||||
| Absent | Reference | — | Reference | — |
| Present | 1.34 (0.65–2.79) | 0.43 | 1.44 (0.64–3.20) | 0.38 |
| DM | ||||
| Absent | Reference | — | Reference | — |
| Present | 1.29 (0.55–3.04) | 0.56 | 1.99 (0.66–6.00) | 0.22 |
| Smear grade >1 + or TTD <9 days | ||||
| Pre-DM | ||||
| No | Reference | — | Reference | — |
| Yes | 1.24 (0.84–1.85) | 0.28 | 1.36 (0.89–2.08) | 0.15 |
| DM | ||||
| No | Reference | — | Reference | — |
| Yes | 3.12 (1.62–6.04) | 0.001 | 4.94 (2.33–10.48) | <0.001 |
Giving one point to each major TB-related symptom (cough, fever, weight loss, night sweats, anorexia, hemoptysis and malaise), TB disease was classified as severe (⩾4) and non-severe TB (<3). Radiologic findings of cavitary lung lesions and/or involvement of ⩾1 lung lobe, and microbiologic findings of >1+ sputum AFB and/or shorter TTD in culture was classified as severe TB disease. Shorter TTD by culture was defined as less than the median number of days required for MGIT culture.
Adjusted for age, sex, religion, education, body mass index and anemia.
TB=tuberculosis; RRR=relative risk reduction; CI=confidence interval; DM=diabetes mellitus; AFB=acid-fast bacilli; TTD=time to TB detection; MGIT=Mycobacterial Growth Indicator Tube.
On radiography, patients with pre-DM and DM had similar presentations according to pre-DM and DM status (P = 0.08) (Table 3). Cavitary TB disease, the involvement of more than one lung lobe, or miliary infiltrates suggestive of extensive lung in-volvement was seen in 374 (42%) TB patients; 134 (36%) had pre-DM and 79 (21%) had DM. In multivariable analysis, patients with DM and those with pre-DM were more likely to have extensive lung involvement, although neither association reached statistical significance (Table 4).
The median TTD was 8 days (IQR 6–11). Higher smear grade > 1+ or TTD < 9 days—suggestive of higher bacterial burden—was seen among 712 (84%) TB patients, 238 (84%) patients with pre-DM and 140 (93%) DM patients. In multivariate analysis, patients with DM had a nearly five-fold higher risk for higher smear grade and shorter TTD (aOR 4.94, 95%CI 2.33–10.48) (Table 4).
Among those with DM, with each unit increase in the HbA1c, the odds of having ⩾4 symptoms (aOR 1.15, 95%CI 0.73–1.81) was higher; however, this did not meet statistical significance. No association was seen with lung involvement and a unit increase in HbA1c level (aOR 1.04, 95%CI 0.77–1.41). Importantly, each unit increase in HbA1c level had 42% higher odds of higher smear grade and shorter TTD (aOR 1.42, 95%CI 1.01–2.01).
DISCUSSION
We found that over half of the newly diagnosed PTB patients in our study cohort had DM or pre-DM. It is not surprising to note that males and older TB patients had a higher risk for DM, whereas being underweight was protective against DM and pre-DM. These findings corroborated previously reported predictors of dysglycemia among patients with TB in India.12 We observed that the median HbA1c level of TB patients among newly diagnosed DM was considerably lower than that among known DM patients, and closer to the standard pre-DM glycemic range. Finally, TB patients with DM, specifically those with high HbA1c levels, had higher bacterial burdens at presentation.
The DM prevalence of 18% among TB cases in our study is representative of the study area.9–15 Specifically, the estimated prevalence of DM in the general population of Maharashtra state would be 6% based on our study results, assuming three-fold higher DM prevalence among TB cases,4 which is similar to reports for western India.18 Furthermore, the higher prevalence of DM reported by Kornfeld et al. (54%) is likely due to the higher median age (>45 years) of TB patients in their South Indian population.15 We also observed a 44% DM prevalence among TB patients aged >40 years.
It should be noted that lower HbA1c levels were observed among those newly diagnosed with DM compared with those with known DM.12,15 The median HbA1c level for newly diagnosed DM was marginally higher than the HbA1c-defined pre-DM range. While this finding has been observed in previous studies, the precise mechanism for this is unclear. While transient stress or inflammation-induced hyperglycemia is common in sepsis and chronic infections including TB,19–21 whether TB disease uncovers subclinical DM among those with epigenetic predisposition to DM has yet to be explored. Furthermore, whether new DM is transient with a similar pathophysiology to that of gestational DM (a transient DM that affects women with a genetic predisposition to DM)22 should also be considered and explored further in longitudinal studies.
Consistent with our study findings, previous research has shown that the number of symptoms may be higher among TB patients with DM.16,17,23 Furthermore, we found that patients with DM had over four-fold higher odds of higher mycobacterial burden, as demonstrated by higher smear grade and shorter TTD by culture. Moreover, each per cent increase in HbA1c level among those with DM was associated with higher mycobacterial burden, suggesting that uncontrolled glycemia could lead to severe TB disease at baseline, which has potential implications for managing DM in high TB burden countries. Our results also suggest that the risk of TB transmission to close contacts may be higher from TB patients with DM; however, this needs to be explored in community settings. Individuals with TB and DM have altered inflammation, T-helper type-1 immune responses, as well as defective neutrophil and macrophage function.24 In addition, TB-infected alveolar macrophages among DM patients may alter host cell recognition and delay innate immune responses.25–27 Whether these immune changes result in more severe TB disease among DM patients should be explored further.
Our study was conducted primarily at one state-run, public-sector facility and a second privately run facility in the last 6 months of the study. Selection bias may have occurred as we had to obtain participant consent for both TB and DM screening. However, the populations that were screened for DM in confirmed TB cases were from the national program, and were representative of India’s population. As our study was cross-sectional, we could not assess the temporality of the association between HbA1c level and disease severity. Despite these limitations, our results are generalizable to other TB populations in India, particularly those with a similar age range.11,13,14
The slower-than-expected decline in global TB incidence despite massive global TB control efforts could, in part, be attributed to the huge, unfolding epidemic of DM in resource-limited settings with high TB burdens.7,28 With the DM burden set to exceed 500 million people by 2030, and with India being the global frontrunner of this burden,7 a better understanding of TB and DM interactions is critical to inform optimal TB control efforts. Bidirectional screening for TB and DM has been recommended, but with very high TB and DM burdens, the resources required to screen all TB patients for DM and vice versa may limit the feasibility of these strategies.29,30 Our study adds to the growing body of literature supporting the view that screening for DM among TB cases can be tailored to specific regions and to specific subgroups within a target population. Furthermore, our results indicate that patients with DM may present with more severe TB disease. Future studies should investigate whether HbA1c levels among newly diagnosed DM patients revert to a normoglycemic range following successful anti-tuberculosis treatment and assess the role of host-directed therapies with metformin/statins and/or aggressive glycemic control in TB patients with DM to reduce disease severity and improve outcomes.
Acknowledgements
The authors thank the clinic and research staff of Byramjee-Jeejeebhoy Medical College (BJGMC) Sassoon General Hospital, Pune, and Dr D Y Patil Medical College, Pune, India, for their immense contributions.
This work was supported by the US National Institutes of Health (NIH; Bethesda, MD, USA), US National Institute of Allergy and Infectious Diseases (NIAID) R01AI097494 (JEG); NIH NIAID UM1AI069465 (VM, NG, AG); BJGMC Johns Hopkins University HIV TB Program funded by Fogarty International Center, NIH D43TW009574 (RL); NIH National Institute of Diabetes and Digestive and Kidney Diseases (NIDDKD) K24DK106414 and R01DK089174 (ES); and NIH Eunice Kennedy Shriver National Institute of Child Health (NICH) K99HD089753 (RS). Data in this manuscript were also collected as part of the Regional Prospective Observational Research for Tuberculosis (RePORT) India Consortium Government of India’s (GOI) Department of Biotechnology (DBT), the Indian Council of Medical Research (ICMR), the United States NIH, NIAID, Office of AIDS Research (OAR) and distributed by CRDF Global.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, DBT, the ICMR, or CRDF Global. Any mention of trade names, commercial projects, or organizations does not imply endorsement by any of the sponsoring organizations.
Conflicts of interest: none declared.
References
- 1.World Health Organization. Global tuberculosis report, 2016 WHO/HTM/TB/2016.13 Geneva, Switzerland: WHO, 2016. [Google Scholar]
- 2.Dooley KE, Chaisson RE. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis 2009; 9: 737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruslami R, Aarnoutse RE, Alisjahbana B, van der Ven AJ, van Crevel R. Implications of the global increase of diabetes for tuberculosis control and patient care. Trop Med Int Health 2010; 15: 1289–1299. [DOI] [PubMed] [Google Scholar]
- 4.Baker MA, Harries AD, Jeon CY, et al. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med 2011; 9: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jimenez-Corona ME, Cruz-Hervert LP, Garcia-Garcia L, et al. Association of diabetes and tuberculosis: impact on treatment and post-treatment outcomes. Thorax 2013; 68: 214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imamura F, Micha R, Khatibzadeh S, et al. Dietary quality among men and women in 187 countries in 1990 and 2010: a systematic assessment. Lancet Glob Health 2015; 3: e132–e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.International Diabetes Federation. Diabetes Atlas. 6th ed. Brussels, Belgium: IDF, 2014. [Google Scholar]
- 8.Central Tuberculosis Division, Government of India. Annual report, TB India 2016_Part 1. New Delhi, India: Government of India, 2016. http://tbcindia.nic.in/showfile.php?lid=3180. [Google Scholar]
- 9.India Tuberculosis-Diabetes Study Group. Screening of patients with tuberculosis for diabetes mellitus in India. Trop Med Int Health 2013; 18: 636–645. [DOI] [PubMed] [Google Scholar]
- 10.Balakrishnan S, Vijayan S, Nair S, et al. High diabetes prevalence among tuberculosis cases in Kerala, India. PLOS ONE 2012; 7: e46502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marak B, Kaur P, Rao SR, Selvaraju S. Non-communicable disease comorbidities and risk factors among tuberculosis patients, Meghalaya, India. Indian J Tuberc 2016; 63:123–125. [DOI] [PubMed] [Google Scholar]
- 12.Viswanathan V, Kumpatla S, Aravindalochanan V, et al. Prevalence of diabetes and pre-diabetes and associated risk factors among tuberculosis patients in India. PLOS ONE 2012; 7: e41367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dave P, Shah A, Chauhan M, et al. Screening patients with tuberculosis for diabetes mellitus in Gujarat, India. Public Health Action 2013; 3 (Suppl 1): S29–S33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nair S, Kumari AK, Subramonianpillai J, et al. High prevalence of undiagnosed diabetes among tuberculosis patients in peripheral health facilities in Kerala. Public Health Action 2013; 3 (Suppl 1): S38–S42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kornfeld H, West K, Kane K, et al. High prevalence and heterogeneity of diabetes in patients with TB in South India: a report from the effects of diabetes on tuberculosis severity (EDOTS) study. Chest 2016; 149: 1501–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skowroński M, Zozulińska-Ziółkiewicz D, Barinow-Wojewódzki A. Tuberculosis and diabetes mellitus—an underappreciated association. Arch Med Sci 2014; 10: 1019–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alisjahbana B, Sahiratmadja E, Nelwan EJ, et al. The effect of type 2 diabetes mellitus on the presentation and treatment response of pulmonary tuberculosis. Clin Infect Dis 2007; 45: 428–435. [DOI] [PubMed] [Google Scholar]
- 18.Anjana RM, Pradeepa R, Deepa M, et al. Prevalence of diabetes and prediabetes (impaired fasting glucose and/or impaired glucose tolerance) in urban and rural India: phase I results of the Indian Council of Medical Research-INdia DIABetes (ICMR-INDIAB) study. Diabetologia 2011; 54: 3022–3027. [DOI] [PubMed] [Google Scholar]
- 19.Rattanataweeboon P, Vilaichone W, Vannasaeng S. Stress hyperglycemia in patients with sepsis. J Med Assoc Thai 2009; 92 (Suppl 2): S88–S94. [PubMed] [Google Scholar]
- 20.Boillat-Blanco N, Ramaiya KL, Mganga M, et al. Transient hyperglycemia in patients with tuberculosis in Tanzania: implications for diabetes screening algorithms. J Infect Dis 2016; 213: 1163–1172. [DOI] [PubMed] [Google Scholar]
- 21.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest 2005; 115: 1111–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanthimathi S, Chidambaram M, Bodhini D, et al. Association of recently identified type 2 diabetes gene variants with gestational diabetes in Asian Indian population. Mol Genet Genomics 2017; 292: 585–591. [DOI] [PubMed] [Google Scholar]
- 23.Gil-Santana L, Almeida-Junior JL, Oliveira CA, et al. Diabetes Is associated with worse clinical presentation in tuberculosis patients from Brazil: a retrospective cohort study. PLOS ONE 2016; 11: e0146876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ronacher K, van Crevel R, Critchley J, et al. Defining a research agenda to address the converging epidemics of tuberculosis and diabetes. Part 2: Underlying biological mechanisms. Chest 2017; 152: 174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walsh MC, Camerlin AJ, Miles R, et al. The sensitivity of interferon-gamma release assays is not compromised in tuberculosis patients with diabetes. Int J Tuberc Lung Dis 2011; 15: 179–184. [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar NP, Sridhar R, Banurekha VV, et al. Type 2 diabetes mellitus coincident with pulmonary tuberculosis is associated with heightened systemic type 1, type 17, and other proinflammatory cytokines. Ann Am Thorac Soc 2013; 10: 441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar NP, Sridhar R, Nair D, Banurekha VV, Nutman TB, Babu S. Type 2 diabetes mellitus is associated with altered CD8(+) T and natural killer cell function in pulmonary tuberculosis. Immunology 2015; 144: 677–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harries AD, Kumar AM, Satyanarayana S, et al. Diabetes mellitus and tuberculosis: programmatic management issues. Int J Tuberc Lung Dis 2015; 19: 879–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kapur A, Harries AD, Lönnroth K, Wilson P, Sulistyowati LS. Diabetes and tuberculosis co-epidemic: the Bali Declaration. Lancet Diabetes Endocrinol 2016; 4: 8–10. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization & International Union Against Tuberculosis and Lung Disease. Collaborative framework for care and control of tuberculosis and diabetes. Geneva, Switzerland: WHO, 2011. [PubMed] [Google Scholar]