Abstract
Researchers have proposed that two processes featuring distinct types of inhibition support inhibitory control: a response threshold adjustment process involving the global inhibition of motor output and a conflict resolution process involving competitive inhibition among co-active response alternatives. To target the development of these processes, we measured the reaching behavior of 5–10-year-olds (Experiment 1) and adults (Experiment 2) as they performed an Eriksen flanker task. This method provided two key measures: initiation time (the time elapsed between stimulus onset and movement onset) and reach curvature (the degree to which a movement deviates from a direct path to the selected target). We suggest that initiation time reflects the response threshold adjustment process by indexing the degree of motoric stopping experienced before a movement is started, while reach curvature reflects the conflict resolution process by indexing the degree of co-activation between response alternatives over the course of a movement. Our results support this claim, revealing different patterns effects in initiation time and curvature, and divergent developmental trajectories between childhood and adulthood. These findings provide behavioral evidence for the dissociation between global and competitive inhibition, and offer new insight into the development of inhibitory control.
Keywords: cognitive development, executive function, flanker task, inhibitory control, reach tracking
In order to behave in a flexible, adaptive manner, children and adults must be able to suppress habitual or prepotent responses selectively. This capacity, known as inhibitory control, undergoes a protracted development, improving rapidly during early childhood and reaching its peak during late adolescence or adulthood (e.g., Carver, Livesey, & Charles, 2001; Davidson, Amso, Anderson, & Diamond, 2006; Diamond, 2002; Luna, 2009; Luna, Garver, Urban, Lazar, & Sweeney, 2004; Waszak, Li, & Hommel, 2010; Zelazo et al., 2013). Inhibitory control has been linked to a range of social and cognitive capacities during childhood, including theory of mind (Carlson, Moses, & Breton, 2002), emotion regulation (Carlson & Wang, 2007), and early math and reading ability (Blair & Razza, 2007). Individual differences in inhibitory control have also been linked to important outcomes in adulthood, including success in school and at work and levels of mental and physical health (see Diamond, 2013 for a review).
While the term inhibition is often used to refer to a unitary process or capacity, a growing body of research indicates that inhibitory control is supported by a number of dissociable processes that feature distinct types of inhibition (for a review, see Munakata et al., 2011). Given the important role that inhibitory control plays in supporting adaptive thought and behavior across the lifespan, a key challenge facing researchers is to identify how these dissociable processes function at different points in development. However, it is difficult to target these processes with traditional behavioral methods, as accuracy and response time provide limited insight into how different processes unfold leading up to a response (Song & Nakayama, 2009). To address this limitation, we use a technique known as reach tracking (e.g., Diedrich, Thelen, Smith, & Corbetta, 2000; Song & Nakayama, 2007) to target how two key processes underlying inhibitory control – a response threshold adjustment process and a conflict resolution process – are reflected in participants’ hand movements as they reach to touch a response target.
To outline our argument, we describe a prominent model of inhibitory control (Botvinick, Braver, Barch, Carter, & Cohen, 2001; Cohen, Dunbar, & McClelland, 1990; Cohen & Huston, 1994; Shenhav, Botvinick, & Cohen, 2013) to contextualize how the response threshold adjustment process and conflict resolution process function during performance of the Eriksen flanker task (Eriksen & Eriksen, 1974). We then review evidence indicating that these processes (a) feature different types of inhibition and (b) generate different patterns of trial sequence effects – effects in which qualities of a previous trail (e.g., trial n-1) influence performance on the current trial (trial n) (see e.g., Egner, 2007). Finally, following recent research using reach tracking with adults (Erb, Moher, Sobel & Song, 2016), we propose that two of the measures afforded by reach tracking – initiation time and curvature – can be used to target how each of these processes contribute to inhibitory control across the lifespan.
Inhibitory Control and the Flanker Task
The Eriksen flanker task (Eriksen & Eriksen, 1974) is one of the most widely used measures of inhibitory control, and was recently standardized for use in the NIH Toolbox (see Zelazo et al., 2013). In the task, participants identify a centrally presented target stimulus in the presence of distracting stimuli known as “flankers”. On congruent trials, the target and distractors cue the same response (e.g., ←←←←←) and the need for inhibitory control is minimal. On incongruent trials, the stimuli cue competing responses (e.g., ←←→←←) and inhibitory control is required to override the prepotent response generated by the distractors. A congruency effect is typically observed in the task, with higher error rates and response times on incongruent relative to congruent trials.
Contemporary models of inhibitory control propose that stimuli in the flanker task are processed along two different pathways: an automatic pathway that is sensitive to the overall stimulus array, and a control-demanding pathway that can be directed to focus on the centermost element in the array (e.g., Botvinick et al., 2001; Cohen et al., 1990; De Jong, Liang, & Lauber, 1994; Ridderinkhof, van der Molen, & Bashore, 1995; Shenhav et al., 2013). On incongruent trials, these pathways generate competing response activations, and the resulting conflict engages three key processes. First, a monitoring process linked to the dorsal anterior cingulate cortex (dACC) registers conflict between the competing response activations generated by the automatic and control-demanding pathways (Botvinick et al., 2001; Yeung, Botvinick, & Cohen, 2004). Second, a response threshold adjustment process temporarily inhibits motor output in response to signals of conflict from the dACC (Cavanagh et al., 2011; Frank, 2006; Munakata et al., 2011; Shenhav et al., 2013; Wiecki & Frank, 2013). This process is proposed to help balance speed-accuracy trade-offs by temporarily halting responding. This in turn allows additional time for a third conflict resolution process associated with the lateral prefrontal cortex (LPFC) to sway activation in favor of the appropriate response by providing top-down support to the control-demanding pathway (Shenhav et al., 2013).
Two distinct types of inhibition can be identified within this model of inhibitory control (Munakata et al., 2011). Global inhibition occurs in the response threshold adjustment process when signals of conflict from the dACC lead to the direct suppression of motor output. Competitive inhibition, on the other hand, occurs during the conflict resolution process, when increased activity along the control-demanding pathway suppresses activity in the competing automatic pathway through lateral inhibitory connections.
The response threshold adjustment process and conflict resolution process have also been linked to different patterns of trial sequence effects. For example, Sheth and colleagues (2012) used single-unit recordings to measure activity in the dACC while adult participants performed a Stroop-like interference task. They observed main effects of both current and previous trial congruency, resulting in the following pattern of effects in the magnitude of dACC activation: cC < iC < cI < iI (where lowercase letters denote previous trial congruency and uppercase letters denote current trial congruency). This pattern of trial sequence effects has since been suggested to reflect the response threshold adjustment process (Erb et al., 2016; Shenhav et al., 2013). On this view, response thresholds are adjusted on each trial, with incongruent trials increasing one’s response threshold from its previous position and congruent trials decreasing it (C < I). Trials preceded by an incongruent trial will therefore tend to feature higher response thresholds, while those preceded by a congruent trial will tend to feature lower thresholds (c < i).
While single-unit recordings of the dACC suggest that the response threshold adjustment process is sensitive to the congruency of both the current and previous trial (cC < iC < cI < iI), response times in the flanker task indicate that the conflict resolution process is influenced by a different pattern of trial sequence effects. Early research investigating trial sequence effects in the flanker task found that response times on incongruent trials were faster when the preceding trial was incongruent (iI trials) as opposed to congruent (cI trials) (e.g., Gratton, Coles, & Donchin, 1992). According to the conflict adaptation account of this finding, response times are faster on iI relative to cI trials because top-down resources are more likely to have been recently recruited on trials preceded by an incongruent trial (for a review, see Egner, 2007).
The conflict adaptation account of flanker task performance has been called into question, however, by research indicating that the response time difference between iI and cI trials is driven by a specific subset of responses; namely, those featuring a repeat of the previous trial’s response (Mayr, Awh, & Laurey, 2003; Nieuwenhuis et al., 2006). These studies found that response times were significantly longer on cI-r than iI-r trials (where “-r” indicates a response repeat) but not cI-c relative to iI-c trials (where “-c” indicates a response change). According to the feature integration account of these findings, elevated response times on cI-r trials reflect a stimulus-response (S-R) binding conflict that occurs when the S-R pair formed on the previous trial (e.g., stimulus = →→→→→, response = right) interferes with the formation of a new S-R pair on the current trial (e.g., stimulus =←←→←←, response = right) (Egner, 2007; Hommel, 2004; Nieuwenhuis et al., 2006).
On this view, activating one member of the S-R pair formed on the previous trial leads to the automatic activation of the other member. This generates S-R binding conflict on cI-r trials because participants must pair the response provided on the previous trial (e.g., right) with a different stimulus (e.g., ←←→←←) than that of the previous trial (e.g., →→→→→). Given that the appropriate S-R pair must be formed before top-down support can sway activation in favor of the control-demanding pathway, this S-R conflict can be understood to delay the conflict resolution process. Although iC-r trials also allow for S-R binding conflict, the conflict resolution process is not required to select the appropriate response on congruent trials. No such S-R binding conflict occurs on cC-r or iI-r trials, as these trials feature a repeat of both stimulus and response. Similarly, S-R binding does not occur on response change trials because these trials necessarily feature a stimulus change.
Measuring Different Processes of Inhibition
The results reviewed above suggest that two processes featuring distinct types of inhibition and different patterns of trial sequence effects support inhibitory control. To examine this hypothesis directly, Erb and colleagues (2016) presented adult participants with reach-tracking versions of the Stroop and flanker tasks. They proposed that two of the measures afforded by reach tracking – initiation time (the time elapsed between stimulus onset and movement onset) and reach curvature (the degree to which a movement deviates from a direct path to the selected target) – could be used to target the response threshold adjustment process and conflict resolution process, respectively.
Previous research using reach tracking suggests that the degree of curvature in a participant’s reach movement reflects how coactive different responses are over the course of a trial, and that participants routinely initiate a movement before the conflict resolution process has swayed activation in favor of a specific response (e.g., Freeman, Nakayama, & Ambady, 2013; Song & Nakayama, 2007). Erb et al. (2016) predicted that initiation time could be used to index the response threshold adjustment process, as higher response thresholds should lead to longer periods of motoric stopping and, consequently, longer initiation times. They also predicted that reach curvature could be used to target the conflict resolution process, with larger reach curvatures indicating that participants were more pulled toward the prepotent response before the conflict resolution process could sway activation in favor of the correct response.
The results of Erb et al. (2016) supported these predictions, with initiation times revealing the same pattern of trial sequence effects that was observed in single-unit recordings of the dACC (Sheth et al., 2012) and was later proposed to reflect the response threshold adjustment process (Shenhav et al., 2013): cC < iC < cI < iI. Reach curvatures revealed a main effect of current trial congruency, with larger curvatures on incongruent relative to congruent trials. An interaction between current and previous trial congruency was also observed, with significantly larger reach curvatures on the subset of incongruent trials that allowed for S-R binding conflict (cI trials) relative to those that did not (iI trials).1 This pattern of results is consistent with the claim that reach curvature can be used to index how the conflict resolution process unfolds over the course of a response. Thus, initiation time and curvature appear to capture distinct processes underlying inhibitory control in adults.
The Current Study
The current study builds on Erb et al. (2016) to address two key developmental questions. First, do the response threshold adjustment process and conflict resolution process make dissociable contributions to flanker task performance during childhood? If these processes function similarly in children as in adults, then children’s initiation times in the flanker task should reveal main effects of both current and previous trial congruency, resulting in the following pattern of effects: cC < iC < cI < iI. Children’s reach curvatures should be uniformly low on congruent trials, elevated on incongruent trials not featuring S-R binding conflict (cI-c, iI-c, and iI-r trials), and largest on incongruent trials featuring S-R binding conflict (cI-r trials).
Second, how do these processes contribute to the age-related changes in flanker task performance? While a number of studies have found that flanker task performance continues to improve into adulthood (Li, Hämmerer, Müller, Hommel, & Lindenberger, 2009; Waszak et al., 2010), it is unclear the extent to which the response threshold adjustment process and conflict resolution process contribute to these developmental gains. The available neurophysiology data indicate that key brain regions implicated in supporting these processes (the dACC and LPFC) undergo relatively prolonged development (Gogtay et al., 2004; Sowell et al., 2003; Velanova, Wheeler, & Luna, 2008), suggesting that both initiation time and reach curvature will reveal larger congruency effects in children than adults.
Numerous past studies have investigated inhibitory control using continuous behavioral and psychophysiological measures with both children (e.g., Checa, Castellanos, Abundis-Gutiérrez, & Rueda, 2014; Ridderinkhof & van der Molen, 1995; van de Laar, van den Wildenberg, van Boxtel, van der Molen, 2014) and adults (e.g., Eriksen, Coles, Morris, & O’Hara, 1985; Gratton et al., 1992; van Boxtel, van der Molen, Jennings, & Brunia, 2001). For example, Ridderinkhof and van der Molen (1995) had 5–12-year-olds and adults perform the flanker task by squeezing dynamometers with their left and right hands, which allowed the researchers to separate squeeze onset and squeeze closure. These measures revealed nearly identical gains in performance, with significant reductions in the size of the congruency effect occurring across childhood and no significant improvements occurring between 10–12-year-olds and adults. However, trial sequence effects were not analyzed in the study and, consequently, it is unclear whether squeeze onset and squeeze closure captured different underlying processes.
While trial sequence effects in the flanker task have been investigated in detail in adults (Gratton et al., 1992; Mayr et al., 2003; Nieuwenhuis et al., 2006; Schmidt & de Houwer, 2011; Ullsperger, Bylsma, & Botvinick, 2005; Verbruggen, Notebaert, Liefooghe, & Vandierendonck, 2006; Weissman, Jiang, & Egner, 2014), few studies have evaluated these effects in children, and data are particularly sparse for children under the age of 10 years (e.g., Cragg, 2016; Nieuwenhuis et al., 2006; Stins, Polderman, Boomsma, & de Geus, 2007; Takarae, Schmidt, Tassone, & Simon, 2009).2 Thus, a key goal of the present study is to evaluate the extent to which specific trial sequence effects contribute to developmental gains in flanker task performance. Given that incongruent trials featuring S-R binding conflict place greater – and possibly different – demands on inhibitory control relative to other trials, we expect that age-related gains in inhibitory control will be driven in large part by these trials.
Experiment 1
Five to 10-year-olds completed a child-friendly version of the flanker task by reaching to touch target locations on a digital display while wearing a small motion-tracking sensor on their index finger. If the response threshold adjustment process and conflict resolution process function similarly in children as in the adults featured in Erb et al. (2016), then children’s initiation times should reveal main effects of current and previous trial congruency, while children’s reach curvatures should be uniformly low on congruent trials, elevated on incongruent trials, and greatest on incongruent trials featuring S-R binding conflict (cI-r trials). Further, if these processes undergo significant development during middle childhood, then the congruency effects observed in initiation time and curvature should decrease with age.
Method
Participants
Sixty right-handed children (M = 91.4 months, SD = 19.6 months; 33 females) with normal reaching behavior and normal or corrected-to-normal vision participated in the study, with 12 children in each of five age groups (5-, 6-, 7-, 8- and 9–10-year-olds). The average age in months of each group was as follows: 65.0 (SD = 3.7), 79.0 (SD = 3.0), 90.1 (SD = 3.8), 101.6 (SD = 2.7), and 120.5 (SD = 6.6). Participants were recruited from a list of hospital births or through contact at a local children’s museum. All participants were tested in the laboratory on Brown University’s campus and received a small prize for their participation. The Institutional Review Board at Brown University approved the protocol.
Materials
The experiment was conducted using a rear-mounted projector to display the task on a Plexiglas screen that was arranged upright on a table approximately 48 cm in front of the participant. Participants initiated all movements from a Styrofoam starting block (2 × 2 × 2 cm) located 27 cm in front of the center of the screen. Reach movements and response selections were measured at a rate of approximately 160 Hz with an electromagnetic position and orientation recording system (Liberty, Polhemus). Hand position was measured with a small motion-tracking marker (2.26 cm long, 1.27 cm wide, and 1.14 high) weighing 0.13 ounces that was secured to the participant’s right index finger. The task was programmed in MATLAB (Mathworks).
Procedure
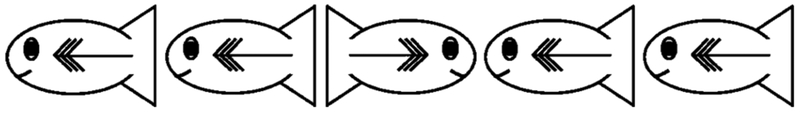
Participants were presented with a child-friendly version of the flanker task featuring yellow cartoon fish (adapted from Rueda et al., 2004; see Figure 1). Children identified which direction the fish in the center of the stimulus array was facing by touching one of two pieces of fish food (orange circles, 2 cm in diameter) located toward the top left or right of the screen. Each fish was 1.5 cm at its tallest and 3 cm long.
Figure 1.
Example of an incongruent stimulus array in the flanker task.
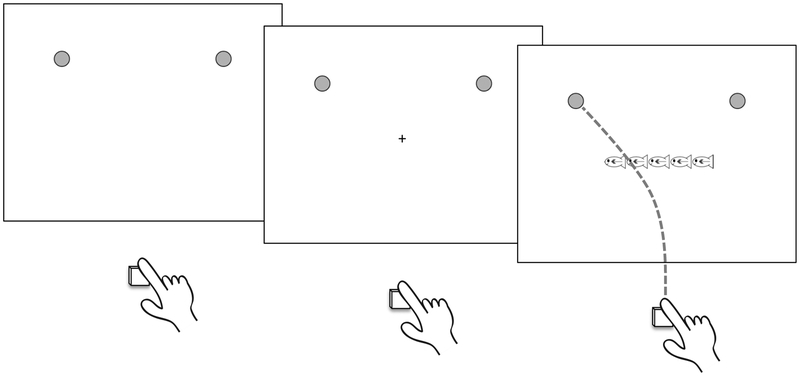
During each trial, a crosshair appeared 1 second before the stimulus array. The cue was located in the same location that the central target appeared, minimizing the demands placed on visual search (see Figure 2). A trial would not initiate until the child’s finger was resting on the Styrofoam starting block for 1 second. If the child’s hand moved from this location before stimulus onset, the task was paused and did not resume until the child returned their hand to the starting block for 1 second. Children had up to 10 seconds to respond following stimulus onset. A high tone sounded for correct responses provided in the allotted time (600 Hz for 200 milliseconds). A low tone sounded for incorrect responses or responses that exceeded the allotted time (300 Hz for 200 milliseconds).
Figure 2.
Illustration of a congruent trial in the flanker task presented in Experiment 1. Participants were instructed to respond by touching the target location cued by the centermost fish in the stimulus array.
Children first completed a nine-point calibration sequence followed by 16 baseline trials that required reaching to an image at the top left or right of the screen. Participants then received a practice block of 10 flanker trials before beginning the experiment. The experiment consisted of three blocks of trials. Each block featured 4 neutral trials in which the central target appeared without flankers, 16 congruent trials in which the central target and flankers cued the same response, and 16 incongruent trials in which the central target and flankers cued opposing responses (36 trials total). Half of the congruent and incongruent trials featured two flankers while the other half featured four (see Section 1 of the Supporting Information for results relating to this manipulation). Participants were reminded before each block to perform the task quickly. Three children (two 5-year-olds and one 7-year-old) declined to complete the third block of trials.
Data Processing
The processing procedures used in the current study were largely adapted from Moher and Song (2013). Three-dimensional resultant speed scalars were created for each trial using a differentiation procedure in MATLAB. These scalars were then submitted to a second order, low-pass Butterworth filter with a cutoff of 10 Hz. Movement onset was calculated as the first point on each trial after stimulus onset at which hand movement speed exceeded 25.4 cm/s. Each individual trial was visually inspected as in previous work (Song & Nakayama, 2006; 2007; 2008); for trials in which the default threshold clearly missed part of the movement or included substantial movement back to the starting point, thresholds were adjusted manually. Manual adjustments were most typically required when participants (a) rapidly pulled their finger away from the screen after having touched a target or (b) stopped entirely during their movement (e.g., after realizing that they had been moving toward the incorrect target). An average of 11.54% (SD = 11.13%) of trials were adjusted manually for each participant.
Trajectories for calculating curvature were measured in two-dimensional xy space by calculating a line from the start to the end point of the movement, and measuring the orthogonal deviation of the actual movement from that line at each sample. Curvature was defined as the maximum point of deviation in centimeters divided by the length of the line from the start to the end points of the movement in centimeters (following Desmurget, Jordan, Prablanc, & Jeannerod, 1997; Moher & Song, 2013).
Results
The first trial of each block was excluded from analysis. Average error rate was analyzed with a 2 (Current Trial Congruency: C or I) x 2 (Previous Trial Congruency: c or i) x 2 (Response Type: change or repeat) x 5 (Age Group: 5, 6, 7, 8, or 9–10 years of age) ANOVA. Average initiation time and curvature were calculated for all accurate trials that were not preceded by an error and were then analyzed via 2 × 2 × 2 × 5 ANOVAs of the form described above. Preliminary analyses revealed no difference between neutral and congruent trials and, consequently, neutral trials were included as congruent trials in the following analyses. In addition to initiation time and curvature, movement time (the time elapsed between stimulus onset and movement onset) and total time (the time elapsed between stimulus onset and response completion) were also measured. The results of these measures are presented in Section 1 of the Supporting Information.
Error Rate.
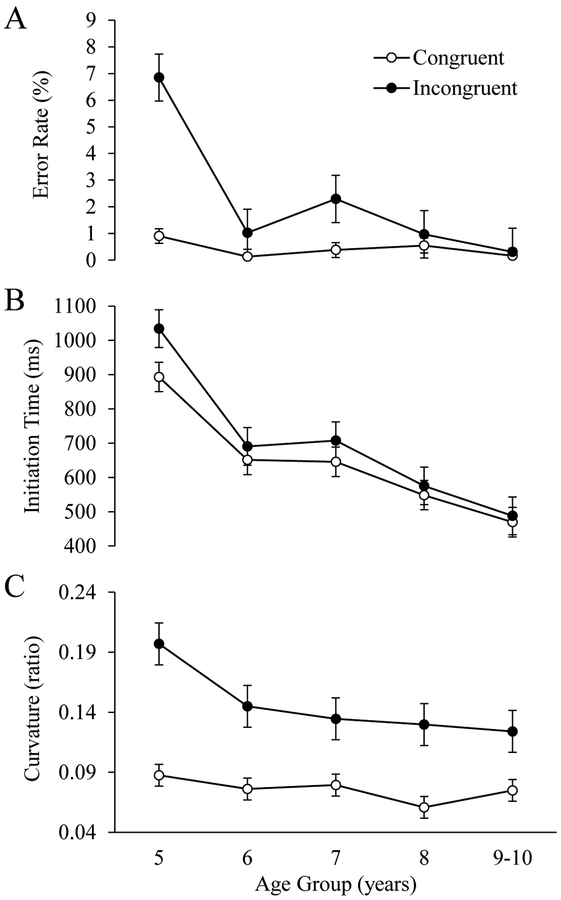
The average error rate for each trial type is shown in Figure 3. Given the clear floor effects on congruent trials and unequal variances among the trial types, we note that the following analyses should be interpreted with caution. Significant main effects of Current Trial Congruency, F(1, 55) = 20.96, p < .001, ηp2 = 0.28, and Age Group were observed, F(4, 55) = 9.57, p < .001, ηp2 = 0.41. Post-hoc analyses with Bonferroni corrections revealed significant differences in overall error rates between 5-year-olds and each of the other age groups, all p-values < .01 (see Figure 4A). A significant interaction between Current Trial Congruency and Age Group was also observed, F(4, 55) = 6.80, p < .001, ηp2 = 0.33. Post-hoc analyses with Bonferroni corrections revealed that the effect of Current Trial Congruency was significantly larger in 5-year-olds than in each of the other age groups, p-values < .05. None of the age groups differed significantly from one another.
Figure 3.
Children’s average error rate displayed as a function of current trial congruency (C, I), previous trial congruency (c, i), and response type (change, repeat). Error bars display standard errors.
Figure 4.
Children’s average error rate (A), initiation time (B), and curvature (C) as a function of current trial congruency and age group. Inaccurate trials are included in the averages (see text for details). Error bars display standard errors.
A significant interaction between Current and Previous Trial Congruency, F(1, 55) = 5.32, p = .025, ηp2 = 0.09, and a three-way interaction among Age Group, Current Trial Congruency, Previous Trial Congruency were also observed, F(4, 55) = 3.35, p < .016, ηp2 = 0.20. Follow-up tests revealed a significant interaction between Age Group and Current Trial Congruency on trials preceded by a congruent trial, F(4, 55) = 8.43, p < .001, ηp2 = 0.38, but not on trials preceded by an incongruent trial, F(4, 55) = 1.55, p = .20. On the subset of trials preceded by a congruent trial, post-hoc analyses with Bonferroni corrections revealed a significantly larger effect of Current Trial Congruency in 5-year-olds relative to each of the other age groups, p-values < .05. Thus, the difference in error rates between cI and cC trials decreased between 5 and 6 years of age. No other significant differences were observed among the age groups.
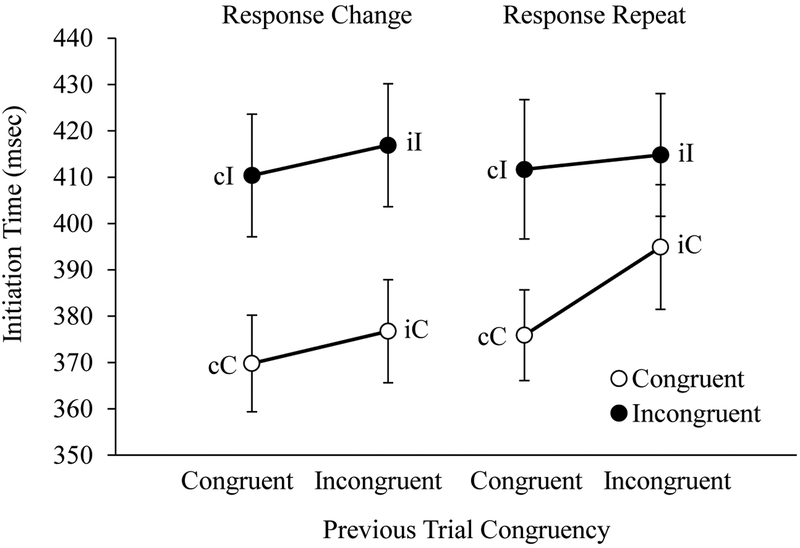
Initiation Time.
Average initiation time for each trial type is shown in Figure 5. As predicted, the ANOVA on initiation times revealed main effects of Current Trial Congruency, F(1, 55) = 16.69, p < .001, ηp2 = 0.23, and Previous Trial Congruency, F(1, 55) = 6.01, p = .017, ηp2 = 0.10. No interaction between Current and Previous Trial Congruency was observed, F(1, 55) = 0.17, p = .68. Thus, initiation times conformed to the same pattern of trial sequence effects observed in previous electrophysiology research by Sheth and colleagues (2012) and in previous reach tracking research with adults (Erb et al., 2016): cC < iC < cI < iI.
Figure 5.
Children’s average initiation time displayed as a function of current trial congruency (C, I), previous trial congruency (c, i), and response type (change or repeat). Error bars display standard errors.
A significant main effect of Age Group was observed, F(4, 55) = 14.59, p < .001, ηp2 = 0.51, indicating that overall initiation times decreased with age. Post-hoc analyses with Bonferroni corrections revealed significant differences in overall initiation times between 5-year-olds and each of the other age groups, all p-values < .01, a significant difference between 7- and 9–10-year-olds, p < .05, and a marginal difference between 6 and 9–10-year-olds, p = .06. Finally, a significant main effect of Response Type was also observed, F(1, 55) = 5.93, p < .018, ηp2 = 0.10. We suspect that this effect was driven by children’s anticipation of response change trials, as this observation is consistent with previous research investigating alternation behavior in children which indicates that older children (above 4.5 years of age) are biased to alternate between response options while younger children (around 3 years of age) are biased to perseverate on one option (Jeffrey & Cohen, 1965). This interpretation is also supported by research indicating that long response-to-stimulus intervals (RSIs) tend to encourage faster responding on response change than response repeat trials (e.g., Soetens, Boer, & Hueting, 1985). While a number of studies have not observed faster responding on response change than response repeat trials in children (for a review, see Smulders et al., 2005), these studies featured shorter RSIs than the current study.
The interaction between Age Group and Current Trial Congruency approached significance, F(4, 55) = 2.37, p = .064, ηp2 = 0.15, as did the interaction between Age Group and Previous Trial Congruency, F(4, 55) = 2.16, p = .085, ηp2 = 0.14. Given that 5-year-olds had higher error rates than the other age groups, it is plausible that these interaction effects were weakened by the disproportionate exclusion of trials from the youngest age group. When trials featuring errors were included in the analysis, the interaction between Age Group and Current Trial Congruency reached significance, F(4, 55) = 3.13, p = .022, ηp2 = 0.18 (see Figure 4B), while the interaction between Age Group and Previous Trial Congruency still remained at a marginal level, F(4, 55) = 2.27, p = .073, ηp2 = 0.14. Post-hoc analyses with Bonferroni corrections revealed no significant differences in the effect of Current Trial Congruency between any of the individual age groups, although the difference between 5-year-olds and 9–10-year-olds approached significance, p = .051.
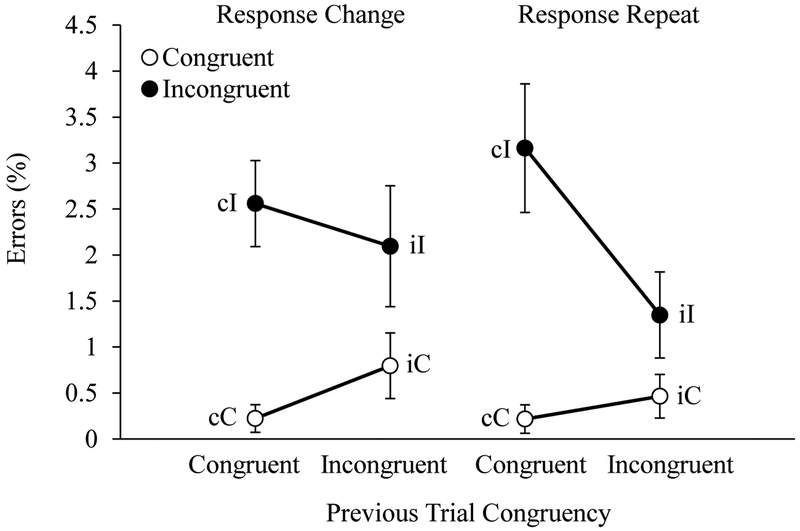
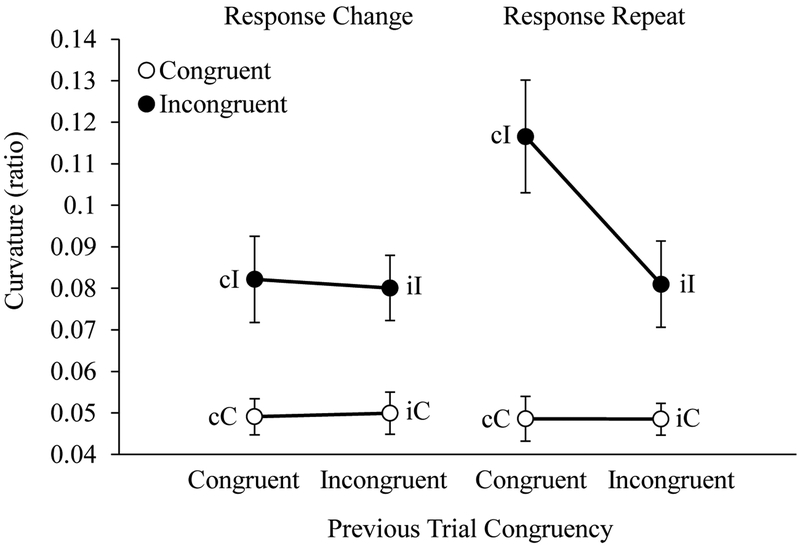
Curvature.
Average reach curvature for each trial type is shown in Figure 6. The ANOVA on reach curvatures revealed main effects of Current Trial Congruency, Previous Trial Congruency, and Response Type, all p-values < .001, all ηp2 values > .19. Further, all two-way interactions and the three-way interaction among these factors were significant, all p-values < .001, all ηp2-values > .20.
Figure 6.
Children’s average reach curvature displayed as a function of current trial congruency (C, I), previous trial congruency (c, i), and response type (change, repeat). Error bars display standard errors.
To account for these interactions, we first evaluated the effects of Previous Trial Congruency and Response Type on congruent and incongruent trials separately. No effect of Previous Trial Congruency, F(1, 59) = 1.21, p = .28, or Response Type, F(1, 59) = 0.83, p = .37, were observed on congruent trials. These findings are consistent with the prediction that reach curvatures on congruent trials would be uniformly low.
When the incongruent trials were analyzed alone, we observed significant main effects of Previous Trial Congruency, F(1, 59) = 18.35, p < .001, ηp2 = 0.25, and Response Type, F(1, 59) = 35.45, p < .001, ηp2 = 0.39. Additionally, there was a significant interaction between Previous Trial Congruency and Response Type, F(1, 59) = 24.94, p < .001, ηp2 = 0.31. Follow-up tests revealed a significant effect of Previous Trial Congruency on trials featuring a response repeat, F(1, 59) = 38.07, p < .001, ηp2 = 0.39, with larger reach curvatures on cI-r relative to iI-r trials. No effect of Previous Trial Congruency was observed on trials featuring a response change, F(1, 59) = 0.005, p = .94. Post-hoc analyses revealed no significant difference between iI-r trials and either the cI-c or iI-c trials, p-values = .14. These results support the claim that S-R binding conflict on cI-r trials delayed the conflict resolution process involving competitive inhibition.
The interaction between Age Group and Current Trial Congruency approached significance, F(4, 55) = 2.14, p = .089, ηp2 = 0.13. When trials featuring errors were included in the analysis, this interaction reached significance, F(4, 55) = 2.85, p = .032, ηp2 = 0.17 (see Figure 4C). Post-hoc analyses with Bonferroni corrections revealed a significantly larger effect of Current Trial Congruency in 5-year-olds relative to 7- and 9–10-year-olds, p-values < .05. No other significant differences were observed among the age groups.
Discussion
The results of Experiment 1 indicate that two of the measures afforded by reach tracking, initiation time and curvature, can be used to target dissociable processes underlying inhibitory control in 5- to 10-years-old, consistent with Erb et al.’s (2016) results with adults. Children’s initiation times revealed main effects of current and previous trial congruency, and matched the pattern of trial sequence effects proposed to reflect the dACC’s role in supporting the response threshold adjustment process: cC < iC < cI < iI (Shenhav et al., 2013; Sheth et al., 2012). These results suggest that conflict registered at the outset of a trial resulted in higher response thresholds with longer periods of motoric stopping.
Reach curvatures also revealed a main effect of current trial congruency, with larger curvatures on incongruent relative to congruent trials. While no effect of response type or previous trial congruency was observed on congruent trials, the subset of incongruent trials that featured S-R binding conflict (cI-r trials) generated significantly larger reach curvatures than the other incongruent trial types. These results are consistent with the claim that S-R binding conflict impedes the conflict resolution process on incongruent trials, which leads participants to be pulled toward the prepotent response for a longer period of time before top-down support can intervene.
It is important to note that because flanker number was manipulated in the task, a subset of iI-r trials featured a stimulus change (e.g., ←←→←← followed by ←→←). Thus, it is possible that S-R binding conflict could have occurred on certain iI-r trials, assuming that participants’ perceived the stimuli to be substantially different from one another. To test this possibility, we compared reach curvatures on iI-r trials featuring a stimulus change to those featuring a stimulus repeat. We observed no evidence of a difference between the two trial types, F(1, 59) = 0.13, p = .72. This finding, coupled with the observation that reach curvatures did not differ between iI-r trials and cI-c or iI-c trials, suggests that S-R binding conflict did not occur on iI-r trials.
The results of the current experiment revealed a number of age-related differences. Children’s overall error rates and initiation times decreased significantly with age. When incorrect responses were included in the analyses, initiation time and curvature revealed modest gains in inhibitory control (as indexed by the interaction between current trial congruency and age group), particularly between 5 and 6 years of age. Movement time and total time also revealed age-related gains in inhibitory control, again driven by differences between 5-year-olds and older age groups (see Section 1 of Supporting Information). These findings are consistent with the results of previous studies using similar child-friendly stimuli, which have found limited developmental gains in children’s response times between 6 to 10 years of age (e.g., Checa et al., 2014; Rueda et al., 2004).
Given that initiation time and reach curvature revealed similar interactions between age group and current trial congruency, our results indicate that the gains in inhibitory control made between 5 and 10 years of age reflect changes in the functioning of both the response threshold adjustment process and the conflict resolution process. However, it is unclear the extent to which each of these processes contribute to the developmental gains in inhibitory control observed between childhood and adulthood (Li et al., 2009; Waszak et al., 2010). We address this question in Experiment 2.
Experiment 2
In Experiment 2, we presented adult participants with a reach-tracking version of the flanker task analogous to that of Experiment 1. In light of previous reach tracking research with adults (Erb et al., 2016), we predicted that initiation time and reach curvature would reveal the same overall patterns of effects as those observed in Experiment 1. After testing this prediction, we investigate how the response threshold adjustment process and conflict resolution process change between childhood and adulthood by directly comparing 8–10-year-olds’ performance to that of adults.
Method
Participants
Twenty-four right-handed adults (M = 20.1 years, SD = 1.3 years; 14 females) with normal reaching behavior and normal or corrected-to-normal vision participated in the experiment. Participants received course credit for their participation. The Institutional Review Board at Brown University approved the protocol.
Materials
The same materials were used as in Experiment 1.
Procedure
Participants were presented with an array of dark grey arrows that pointed to the left or right and were instructed to indicate which direction the arrow in the center of the array pointed. Participants responded by reaching to touch one of two targets located toward the top left and right of a digital display while wearing a small tracking device on their index finger. The background of the display was white and the response targets were identical orange circles with a diameter of 2 cm. Half of the trials featured an array of three arrows (e.g.,←→←), while the other half featured an array of five arrows (e.g.,←←→←←) (see Section 2 of the Supporting Information for results relating to this manipulation). The three arrow arrays were 4.8 cm by 1.5 cm. The five arrow arrays were 8.2 cm by 1.5 cm.
The structure of the procedure was the same as Experiment 1, except that participants had 3 seconds to respond following stimulus onset instead of 10 seconds. Participants completed 16 baseline trials in which a solo target appeared at each of the target locations from the main task. These trials provided a baseline of participant’s reaching behavior and familiarized participants with the procedure. The experimental portion of the task was presented in five blocks of 48 trials. Each block of trials consisted of 24 congruent and 24 incongruent trials. The correct response was equally divided equally between the left and right targets. Before each block, participants were reminded to respond quickly while maintaining a high degree of accuracy. The first 10 trials of the first block were presented as practice trials and were excluded from further analysis.
Data Processing
Data were processed in the same manner as in Experiment 1. An average of 1.15% (SD = 3.62%) of trials were adjusted manually for each participant.
Results
The first trial of each block was excluded from analysis. Error rates were at floor (less than 1%) and were not analyzed further. Average initiation time and curvature were calculated for all accurate trials that were not preceded by an error and were then analyzed via a 2 (Current Trial Congruency: C or I) x 2 (Previous Trial Congruency: c or i) x 2 (Response Type: change or repeat) ANOVA. See Section 2 of Supporting Information for results from the movement time and total time measures.
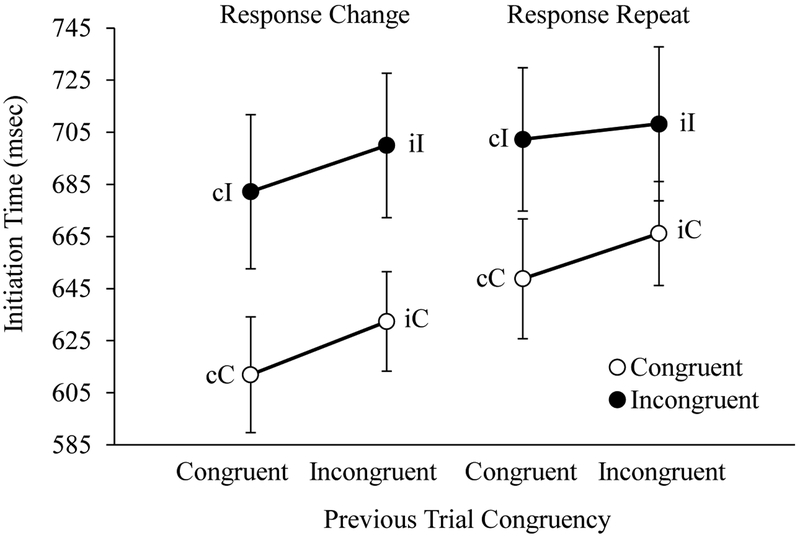
Initiation Time.
Average initiation time for each trial type is shown in Figure 7. As predicted, the ANOVA on initiation times revealed main effects of Current Trial Congruency, F(1, 23) = 60.36, p < .001, ηp2 = 0.72, and Previous Trial Congruency, F(1, 23) = 18.72, p < .001, ηp2 = 0.45. No interaction between Current and Previous Trial Congruency was observed, F(1, 23) = 1.63, p = .21. However, a significant interaction between Current Trial Congruency and Response Type was observed, F(1, 23) = 7.52, p = .012, ηp2 = 0.25. Follow-up tests revealed significantly higher initiation times on congruent trials featuring a response repeat relative to those featuring a response change, F(1, 23) = 9.48, p = .005, ηp2 = 0.29. No effect of Response Type was observed on incongruent trials, F(1, 23) = 0.03, p = .86. As in Experiment 1, these results support the claim that initiation time reflects the response threshold adjustment process involving the global inhibition of motor output.
Figure 7.
Adults’ average initiation time displayed as a function of current trial congruency (C, I), previous trial congruency (c, i), and response type (change or repeat) for adult participants. Error bars display standard errors.
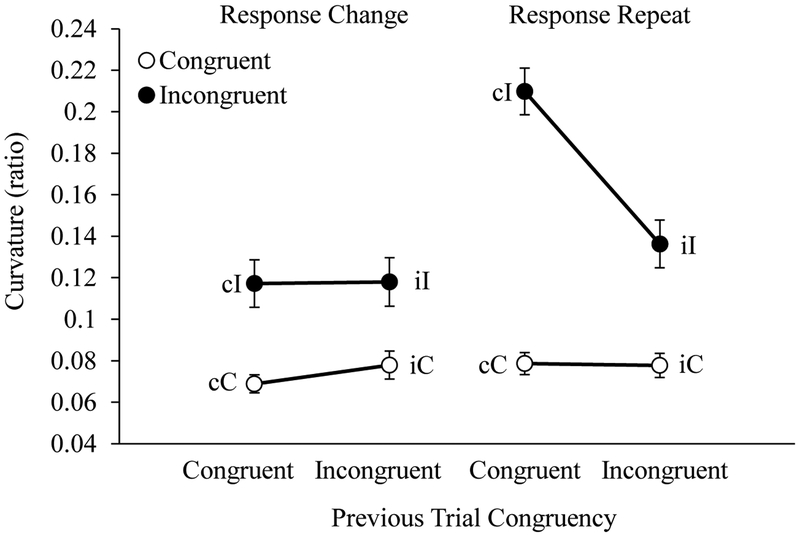
Curvature.
Average reach curvature for each trial type is shown in Figure 8. The ANOVA on reach curvatures revealed main effects of Current Trial Congruency, Previous Trial Congruency, and Response Type, all p-values < .01, all ηp2 values > .27. Further, all two-way interactions and the three-way interaction among these factors were significant, all p-values < .01, all ηp2-values > .32.
Figure 8.
Adults’ average reach curvature displayed as a function of current trial congruency (C, I), previous trial congruency (c, i), and response type (change or repeat) for adult participants. Error bars display standard errors.
To account for these interaction effects, we first evaluated the effects of Previous Trial Congruency and Response Type on congruent and incongruent trials separately. No effects of Previous Trial Congruency, F(1, 23) = 0.05, p = .83, or Response Type, F(1, 23) = 0.31, p = .83, were observed on congruent trials. These findings are consistent with the prediction that reach curvatures on congruent trials would be uniformly low. Significant main effects of Previous Trial Congruency, F(1, 23) = 14.98, p < .001, ηp2 = 0.39, and Response Type, F(1, 23) = 10.61, p = .003, ηp2 = 0.32, were observed on incongruent trials. Additionally, a significant interaction between Previous Trial Congruency and Response Type was observed, F(1, 23) = 21.02, p < .001, ηp2 = 0.48. Follow-up tests revealed a significant effect of Previous Trial Congruency on trials featuring a response repeat, F(1, 23) = 25.26, p < .001, ηp2 = 0.52, with larger reach curvatures on cI-r relative to iI-r trials. No effect of Previous Trial Congruency was observed on trials featuring a response change, F(1, 23) = 0.18, p = .67. These results support the claim that S-R binding conflict on cI-r trials delayed the conflict resolution process involving competitive inhibition.
Comparison of Child and Adult Data
Overall, adult performance presented the same patterns of trial sequence effects observed in children in Experiment 1 and in adults in Erb et al. (2016). Initiation times were significantly longer on incongruent trials and trials preceded by an incongruent trial. Adults’ reach curvatures were uniformly low on congruent trials, highest on cI-r trials, and intermediate on the remaining incongruent trial types (cI-c, iI-c, and iI-r). Again, we saw no difference between iI-r trials featuring a response change and those featuring a response repeat, F(1, 23) = 0.01, p = .93, indicating that S-R binding conflict did not occur on iI-r trials in the current experiment.
Next, we evaluated how inhibitory control changes between childhood and adulthood by directly comparing adult performance to that of the two oldest age groups from Experiment 1 with a series of 2 (Current Trial Congruency: C or I) x 2 (Previous Trial Congruency: c or i) x 2 (Response Type: change or repeat) x 2 (Age Group: 8–10-year-olds or adults) ANOVAs. As in our previous analyses, the first trial of each round was excluded from analysis and only accurate trials not proceeded by an error were analyzed. In order to equate for the number of trials presented in each age group, only the first 108 experimental trials collected with adults were included in the following analyses. This step was taken to guard against the possibility that age-related changes in performance could reflect practice or fatigue effects in the adult sample. To see a full comparison of child and adult performance, including all ages tested in Experiment 1 and all trials collected in Experiment 2, see Section 3 of the Supporting Information. Given our focus on developmental differences, we restrict our discussion to interactions with Age Group in the following analyses.
Initiation Time.
A significant interaction between Age Group and Response Type was observed, F(1, 46) = 4.37, p = .042, ηp2 = 0.09, with a larger difference between response repeat and response change trials in children (M = 25 ms, SD = 43 ms) than adults (M = 3 ms, SD = 21 ms). No significant interactions were observed with Age Group and Current Trial Congruency or Previous Trial Congruency, all p-values > .17.
Curvature.
Reach curvature revealed a significant two-way interaction between Age Group and Response Type, significant three-way interactions among Age Group, Current Trial Congruency, and Response Type, and Age Group, Previous Trial Congruency, and Response Type, and a significant four-way interaction among all the factors, all p-values < .05.
To account for these interaction effects, we split our analyses by Previous Trial Congruency. Follow-up tests revealed no interactions of Age Group with Current Trial Congruency or Response Type on trials preceded by an incongruent trials, p-values > .20. Trials preceded by a congruent trial revealed a significant interaction between Age Group and Response Type, F(1, 46) = 12.53, p < .001, ηp2= 0.21, and a significant three-way interaction among Age Group, Current Trial Congruency, and Response Type, F(1, 46) = 9.31, p = .004, ηp2= 0.17.
To further evaluate these interactions, we analyzed response change and response repeat trials separately. The interaction between Age Group and Current Trial Congruency was not significant on response change trials, F(1, 46) = 0.36, p = .55. However, it was significant on response repeat trials, F(1, 46) = 4.62, p = .037, ηp2= 0.09, with a larger difference between cI-r and cC-r trials in children (M = 0.115, SD = 0.090) than adults (M = 0.067, SD = 0.065).
Discussion
The comparison of child and adult performance revealed no significant age-related gains in inhibitory control in initiation time. Although the effect of response type on initiation time did decrease between 8–10-year-olds and adults, this improvement occurred across both congruent and incongruent trials. In contrast to initiation time, reach curvature did reveal significant age-related gains in inhibitory control. However, the observed gains were specific to a particular subset of trials; namely, those featuring S-R binding conflict. Reach curvatures on cI-r trials decreased significantly between childhood and adulthood, while the other trial types revealed no significant age-related improvements. These results indicate that the age-related gains in inhibitory control observed between 8–10 years of age and adulthood are driven by improvements in the conflict resolution process’ capacity to resolve S-R binding conflict.
In a separate experiment, we presented adult participants with the same child-friendly version of the task presented in Experiment 1. When we compared child and adult performance, we observed nearly identical results to those presented above (see Section 4 of Supporting Information). However, it was unclear whether the observed age-related changes in performance were driven by improved inhibitory control in adults or by differences in task difficulty (e.g., the child-friendly version may have been too easy for adults). In light of previous research indicating that the arrow version of the task is more difficult than the child-friendly version used in Experiment 1 (Rueda et al., 2004), we selected the arrow version of the task for Experiment 2 in an effort to equate task difficulty between the age groups. Taken together, the results presented above and the results presented in Sections 3 and 4 of the Supporting Information converge on the same conclusion: the age-related gains observed between middle childhood and adulthood in the flanker task are driven by changes in the functioning of the conflict resolution process on trials featuring S-R binding conflict.
General Discussion
The results of the current study indicate that two of the measures afforded by reach tracking, initiation time and reach curvature, can be used to target the functioning of the response threshold adjustment process and conflict resolution process in children and adults alike. Initiation times in Experiments 1 and 2 revealed main effects of current and previous trial congruency, consistent with the claim that conflict detected at the outset of a trial leads to higher response thresholds and, consequently, increased global inhibition of motor output. Reach curvatures across both experiments were uniformly low on congruent trials, elevated on incongruent trials without S-R binding conflict (cI-c, iI-c, and iI-r trials), and largest on incongruent trials with S-R binding conflict (cI-r trials). This pattern of effects is consistent with the claim that reach curvature reflects the relative co-activation of responses over the course of a movement and, therefore, can be used to index the conflict resolution process involving competitive inhibition (Erb et al., 2016).
The overall pattern of effects observed in initiation time and reach curvature were similar in children and adults, indicating that the response threshold adjustment process and conflict resolution process function in much the same manner in both age groups. The measures did, however, present a number of notable developmental differences. Both initiation time and reach curvature revealed relatively modest gains in inhibitory control during childhood (as evidenced by interactions between current trial congruency and age group), with pronounced improvements occurring between 5 and 6 years of age. These results suggest that both processes of interest follow similar developmental trajectories during childhood. However, only reach curvature revealed evidence of age-related gains in inhibitory control between childhood and adulthood, indicating that the protracted development of inhibitory control into adulthood is driven primarily by changes in the functioning of the conflict resolution process. These findings suggest that the response threshold adjustment process and conflict resolution process follow divergent developmental trajectories, though further research is needed to examine whether these results generalize to other tasks and testing conditions.
It is important to note that the inhibitory control advantage observed in adult reach curvatures was driven by a specific subset of trials; namely, incongruent trials featuring S-R binding conflict (cI-r trials). This finding raises a number of important questions concerning the nature of the gains in inhibitory control observed between childhood and adulthood. For example, were the developmental gains observed in the current study driven by cI-r trials because these trials were the most difficult and, therefore, the most likely to reveal age-related improvements? Or, were the developmental gains specific to cI-r trials because these trials placed unique – not just greater – demands on participants?
Research by Hommel, Kray, and Lindenberger (2011) provides some insight on this issue. They presented 9–10-year-olds, young adults (20–31 years of age), and older adults (64–76 years of age) with a task adopted from Hommel (1998) that systematically manipulated stimulus and response repetitions. Hommel and colleagues found that the effect of S-R binding conflict was more pronounced in the error rates of 9–10-year-olds than adults. In their discussion of this age-related change, the researchers referenced work by Colzato, van Wouwe, Lavender, and Hommel (2006) indicating that the ability to unbind and rebind event features (e.g., a particular stimulus and response) is linked to fluid intelligence, which improves during childhood and adolescence (Fry & Hale, 2000). Further, Hommel et al. note that the reconfiguring of event features has been proposed to involve neurophysiological processes linked to dopaminergic modulation, the functioning of which has also been found to relate to age and fluid intelligence (citing Bäckman, Nyberg, Lindenberger, Li, & Farde, 2006). Thus, the age-related gains in flanker task performance observed between 8–10-year-olds and adults in the current study may reflect changes specific to how efficiently event features can be reconfigured at different points in development, rather than improved inhibitory control (or conflict resolution) per se.
This account is consistent with recent work by Cragg (2016) in which 7-year-olds, 10-year-olds, and adults completed a modified version of the flanker task that did not allow stimulus or response repeats to occur. In contrast to previous developmental work (e.g., Li et al., 2009; Waszak et al., 2010), Cragg observed no age-related gains in inhibitory control on response conflict trials (akin to incongruent trials in the current study). The results of the current study suggest that the lack of age-related changes in Cragg (2016) may have resulted from the removal of associative priming effects such as S-R binding conflict. While further research is needed to evaluate the extent to which age-related gains in flanker task performance reflect improved inhibitory control or an improved capacity to reconfigure event features, our results present further evidence that age-related gains in these tasks are driven in part by a select subset of trials that feature greater – and possibly different – demands. Consequently, age-related gains in performance on these tasks may have been mischaracterized in the past.
Conflict Adaptation
An ongoing debate in the literature on inhibitory control concerns the extent to which performance on congruency tasks is influenced by conflict-driven modulations of top-down control. As noted in the Introduction, faster response times on iI relative to cI trials have been interpreted to reflect a conflict adaptation effect in which the recent recruitment of top-down support on one incongruent trial serves to facilitate performance on the next (e.g., Botvinick et al., 2001). While a number of studies have found that the difference between iI and cI trials is better explained by S-R binding conflict on cI-r trials than conflict adaptation on iI trials (Mayr et al., 2003; Nieuwenhuis et al., 2006), other studies featuring more than two responses and a larger stimulus set size have observed enhanced performance on iI relative to cI trials even after trials featuring S-R binding conflict were excluded from analysis (e.g., Kerns et al., 2004; Ullsperger et al., 2005; Verbruggen et al., 2006). However, recent research indicates that such attempts to control for S-R binding conflict can have the unintended consequence of introducing contingency learning effects in which participants learn to associate particular stimulus features with a specific response (for a discussion, see Schmidt, 2013). For example, when Schmidt and De Houwer (2011) controlled for both S-R binding conflict and contingency learning effects in the flanker task, they observed no performance differences between iI and cI trials, suggesting that conflict adaptation does not significantly contribute to flanker task performance.
In the current study, we observed no evidence of conflict adaptation in initiation time or reach curvature. Although reach curvatures were higher on cI relative to iI trials, this difference was specific to trials featuring a response repeat, indicating that the difference was driven by S-R binding conflict on cI-r trials and not conflict adaptation on iI trials. Children’s movement times in Experiment 1 were faster on iI relative to cI trials regardless of response type, suggesting a possible role for conflict adaptation (see Section 1 of Supporting Information). However, the difference between cC and iC trials was also marginally significant (p = .066), indicating that children’s movement times were generally faster on trials featuring a congruency repeat (cC and iI trials) than those featuring a congruency change (iC and cI trials). Interestingly, adults’ movement times did not show evidence of this general performance difference on congruency repeat and congruency change trials in Experiment 2 or the additional experiment reported in Section 4 of the Supporting Information. These findings suggest that children may experience a switch cost in the flanker task not encountered by adults, though further research is necessary to directly evaluate this possibility.
Methodological Considerations
Traditionally, inhibitory control in the Eriksen flanker task is assessed by evaluating the effect of current trial congruency on response times, accuracy, or a composite score of the two (e.g., Nieuwenhuis et al., 2006; Rueda et al., 2004; Waszak et al., 2010; Zelazo et al., 2013). While this approach has contributed greatly to our current understanding of inhibitory control and its development, the results of the present study highlight the promise of (a) collecting continuous behavioral measures and (b) evaluating the role of trial sequence effects when investigating inhibitory control in children. Reach tracking and related techniques such as mouse tracking have been used to study a wide range of topics in adults, including attention, categorization, numerical cognition, language, and decision making (for reviews, see Freeman, Dale, & Farmer, 2011 and Song & Nakayama, 2009). However, relatively little research has been conducted using these techniques with children (e.g., Anderson, Farmer, Goldstein, Schwade, & Spivey, 2011; Diedrich et al., 2000; Rommelse et al., 2007). Our results indicate that reach tracking is appropriate for use with children as young as five years of age, and presents a promising alternative to the behavioral methods traditionally used to assess children’s perception, cognition, and action.
The ability to isolate distinct patterns of effects underlying performance is particularly relevant for developmental research, as overall measures of performance such as response time may conceal age-related changes of interest. For example, total time revealed no age-related gains in inhibitory control between children 8–10 years of age and adults in current study (see Section 2 of Supporting Information), despite curvature and movement time revealing clear gains in performance. This is because total time is the product of both initiation time and movement time, which generated different patterns of trial sequence effects and followed different developmental trajectories in the current study. Thus, our findings indicate that reach tracking can be used to target developmental changes in performance that may be obscured by measures that reflect the outcome of a decision process but not its unfolding.
Conclusion
The current study presents evidence that reach tracking can be used to target how two key processes underlying inhibitory control function across the lifespan. Our findings indicate that a response threshold adjustment process involving the global inhibition of motor output and a conflict resolution process involving competitive inhibition among co-active responses follow different developmental trajectories between childhood and adulthood. The current study also presents a framework for future research to explore how each of these processes contributes to individual and group differences in inhibitory control. More broadly, our results contribute to a growing body of developmental research highlighting the importance of evaluating trial sequence effects in children’s cognitive performance (e.g., Cragg, 2012; Hommel et al., 2011; Kray, Karbach, & Blaye, 2012).
Supplementary Material
Research Highlights:
Two of the measures afforded by reach tracking, initiation time and curvature, revealed distinct patterns of trial sequence effects in the Eriksen flanker task in children 5 to 10 years of age and adults. We propose that the pattern of effects observed in initiation time reflects a response threshold adjustment process involving the global inhibition of motor output, while the pattern observed in reach curvature reflects a conflict resolution process involving competitive inhibition among co-active response alternatives.
Initiation time and reach curvature revealed similar gains in inhibitory control between 5 and 10 years of age, but only curvature revealed performance gains between 8–10-year-olds and adults.
The gains observed between childhood and adulthood were driven by a specific subset of trials, suggesting that age-related gains in inhibitory control have been mischaracterized in the past.
These results present a framework for future research to explore how the response threshold adjustment process and conflict resolution process contribute to individual and group differences in inhibitory control.
Acknowledgements:
This project is supported by NSF 1223777 to D.M.S and NIGMSNIH IDeA P20GM103645 to J.H.S.
Footnotes
Erb et al. (2016) used a three-response version of the flanker task that resulted in a larger number of response change than response repeat trials. Consequently, the researchers did not investigate the effect of response type (repeat vs. change).
Recently, Cragg (2016) has reported response time data indicating that children as young as 7 years of age present similar trial sequence effects to those observed in older children and adults (e.g., Nieuwenhuis et al., 2006), though the task did not assess the influence of response type (repeat vs. change) and, consequently, the role of S-R binding conflict was not evaluated. Similarly, work by Takarae and colleagues (2009) featured children younger than 10 (7–14 years), but the average age of their typically developing participants was greater than 10 years and the effect of response type was not reported.
References
- Anderson SE, Farmer TA, Goldstein M, Schwade J, & Spivey M (2011). Individual differences in measures of linguistic experience account for variability in the sentence processing skill of five-year-olds. Experience, variation, and generalization: Learning a first language (Trends in Language Acquisition Research), 7, 203–221. [Google Scholar]
- Bäckman L, Nyberg L, Lindenberger U, Li SC, & Farde L (2006). The correlative triad among aging, dopamine, and cognition: Current status and future prospects. Neuroscience & Biobehavioral Reviews, 30(6), 791–807. doi: 10.1016/j.neubiorev.2006.06.005 [DOI] [PubMed] [Google Scholar]
- Blair C, & Razza RP (2007). Relating effortful control, executive function, and false belief understanding to emerging math and literacy ability in kindergarten. Child Development, 78(2), 647–663. doi: 10.1111/j.1467-8624.2007.01019.x [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, & Cohen JD (2001). Conflict monitoring and cognitive control. Psychological Review, 108(3), 624–652. doi: 10.1037/0033-295X.108.3.624 [DOI] [PubMed] [Google Scholar]
- Carlson SM, Moses LJ, & Breton C (2002). How specific is the relation between executive function and theory of mind? Contributions of inhibitory control and working memory. Infant and Child Development, 11(2), 73–92. doi: 10.1002/icd.298 [DOI] [Google Scholar]
- Carlson SM, & Wang TS (2007). Inhibitory control and emotion regulation in preschool children. Cognitive Development, 22(4), 489–510. doi: 10.1016/j.cogdev.2007.08.002 [DOI] [Google Scholar]
- Carver AC, Livesey DJ, & Charles M (2001). Age related changes in inhibitory control as measured by stop signal task performance. International Journal of Neuroscience, 107(1–2), 43–61. doi: 10.3109/00207450109149756 [DOI] [PubMed] [Google Scholar]
- Cavanagh JF, Wiecki TV, Cohen MX, Figueroa CM, Samanta J, Sherman SJ, & Frank MJ (2011). Subthalamic nucleus stimulation reverses mediofrontal influence over decision threshold. Nature Neuroscience, 14(11), 1462–1467. doi: 10.1038/nn.2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checa P, Castellanos MC, Abundis-Gutiérrez A, & Rueda MR (2014). Development of neural mechanisms of conflict and error processing during childhood: Implications for self-regulation. Frontiers in Psychology, 5, 326. doi: 10.3389/fpsyg.2014.00326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Dunbar K, & McClelland JL (1990). On the control of automatic processes: A parallel distributed processing account of the Stroopeffect. Psychological Review, 97(3), 332–361. doi: 10.1037/0033-295X.97.3.332 [DOI] [PubMed] [Google Scholar]
- Cohen JD, & Huston TA (1994). Progress in the use of interactive models for understanding attention and performance In Umiltà C & Moscovitch M (Eds.), Attention and Performance XV (pp. 453–476). Cambridge, MA: MIT Press. [Google Scholar]
- Colzato LS, Van Wouwe NC, Lavender TJ, & Hommel B (2006). Intelligence and cognitive flexibility: Fluid intelligence correlates with feature “unbinding” across perception and action. Psychonomic Bulletin & Review, 13(6), 1043–1048. doi: 10.3758/BF03213923 [DOI] [PubMed] [Google Scholar]
- Cragg L (2016). The development of stimulus and response interference control in midchildhood. Developmental Psychology, 52(2), 242. doi: 10.1037/dev0000074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson MC, Amso D, Anderson LC, & Diamond A (2006). Development of cognitive control and executive functions from 4 to 13 years: Evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia, 44(11), 2037–2078. doi: 10.1016/j.neuropsychologia.2006.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong R, Liang CC, & Lauber E (1994). Conditional and unconditional automaticity: A dual-process model of effects of spatial stimulus-response correspondence. Journal of Experimental Psychology: Human Perception and Performance, 20(4), 731–750. doi: 10.1037/0096-1523.20.4.731 [DOI] [PubMed] [Google Scholar]
- Desmurget M, Jordan M, Prablanc C, & Jeannerod M (1997). Constrained and unconstrained movements involve different control strategies. Journal of Neurophysiology, 77(3), 1644–1650. [DOI] [PubMed] [Google Scholar]
- Diamond A (2002). Normal development of prefrontal cortex from birth to young adulthood: Cognitive functions, anatomy, and biochemistry In Stuss DT & Knight RT (Eds.), Principles of Frontal Lobe Function (466–503). London: Oxford University Press. [Google Scholar]
- Diamond A (2013). Executive functions. Annual Review of Psychology, 64, 135–168. doi: 10.1146/annurev-psych-113011-143750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrich FJ, Thelen E, Smith LB, & Corbetta D (2000). Motor memory is a factor in infant perseverative errors. Developmental Science, 3(4), 479–494. doi: 10.1111/1467-7687.00140 [DOI] [Google Scholar]
- Egner T (2007). Congruency sequence effects and cognitive control. Cognitive, Affective, & Behavioral Neuroscience, 7(4), 380–390. doi: 10.3758/CABN.7.4.380 [DOI] [PubMed] [Google Scholar]
- Erb CD, Moher J, Sobel DM, & Song JH (2016). Reach tracking reveals dissociable processes underlying cognitive control. Cognition, 152, 114–126. doi: 10.1016/j.cognition.2016.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen CW, Coles MG, Morris LR, & O’hara WP (1985). An electromyographic examination of response competition. Bulletin of the Psychonomic Society, 23(3), 165–168. doi: 10.3758/BF03329816 [DOI] [Google Scholar]
- Eriksen BA, & Eriksen CW (1974). Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & Psychophysics, 16(1), 143–149. doi: 10.3758/BF03203267 [DOI] [Google Scholar]
- Frank MJ (2006). Hold your horses: A dynamic computational role for the subthalamic nucleus in decision making. Neural Networks, 19(8), 1120–1136. doi: 10.1016/j.neunet.2006.03.006 [DOI] [PubMed] [Google Scholar]
- Freeman J, Dale R, & Farmer T (2011). Hand in motion reveals mind inmotion. Frontiers in Psychology, 2, 59. doi: 10.3389/fpsyg.2011.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JB, Nakayama K, & Ambady N (2013). Finger in flight reveals parallel categorization across multiple social dimensions. Social Cognition, 31(6), 792–805. doi: 10.1521/soco.2013.31.6.792 [DOI] [Google Scholar]
- Fry AF, & Hale S (2000). Relationships among processing speed, working memory, and fluid intelligence in children. Biological Psychology, 54(1), 1–34. doi: 10.1016/S0301-0511(00)00051-X [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, … & Rapoport JL (2004). Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America, 101(21), 8174–8179. doi: 10.1073/pnas.0402680101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MG, & Donchin E (1992). Optimizing the use of information: Strategic control of activation of responses. Journal of Experimental Psychology: General, 121(4), 480–506. doi: 10.1037/0096-3445.121.4.480 [DOI] [PubMed] [Google Scholar]
- Hommel B (1998). Event files: Evidence for automatic integration of stimulus-response episodes. Visual Cognition, 5(1–2), 183–216. doi: 10.1080/713756773 [DOI] [Google Scholar]
- Hommel B (2004). Event files: Feature binding in and across perception andaction. Trends in Cognitive Sciences, 8(11), 494–500. doi: 10.1016/j.tics.2004.08.007 [DOI] [PubMed] [Google Scholar]
- Hommel B, Kray J, & Lindenberger U (2011). Feature integration across the lifespan: Stickier stimulus–response bindings in children and older adults. Frontiers in Psychology, 2, 268. doi: 10.3389/fpsyg.2011.00268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey WE, & Cohen LB (1965). Response tendencies of children in a two-choice situation. Journal of Experimental Child Psychology, 2(3), 248–254. doi: 10.1016/0022-0965(65)90028-7 [DOI] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, Cho RY, Stenger VA, & Carter CS (2004). Anterior cingulate conflict monitoring and adjustments in control. Science, 303(5660), 1023–1026. doi: 10.1126/science.1089910 [DOI] [PubMed] [Google Scholar]
- Kray J, Karbach J, & Blaye A (2012). The influence of stimulus-set size on developmental changes in cognitive control and conflict adaptation. Acta psychologica, 140(2), 119–128. doi: 10.1016/j.actpsy.2012.03.005 [DOI] [PubMed] [Google Scholar]
- Li SC, Hämmerer D, Müller V, Hommel B, & Lindenberger U (2009). Lifespan development of stimulus-response conflict cost: Similarities and differences between maturation and senescence. Psychological Research, 73(6), 777–785. doi: 10.1007/s00426-008-0190-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B (2009). Developmental changes in cognitive control throughadolescence. Advances in Child Development and Behavior, 37, 233–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, & Sweeney JA (2004). Maturation of cognitive processes from late childhood to adulthood. ChildDevelopment, 75(5), 1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x [DOI] [PubMed] [Google Scholar]
- Mayr U, Awh E, & Laurey P (2003). Conflict adaptation effects in the absence of executive control. Nature Neuroscience, 6(5), 450–452. doi: 10.1038/nn1051 [DOI] [PubMed] [Google Scholar]
- Moher J, & Song JH (2013). Context-dependent sequential effects of target selection for action. Journal of Vision, 13(8), 10. doi: 10.1167/13.8.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munakata Y, Herd SA, Chatham CH, Depue BE, Banich MT, & O’Reilly RC (2011). A unified framework for inhibitory control. Trends in Cognitive Sciences, 15(10), 453–459. doi: 10.1016/j.tics.2011.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S, Stins JF, Posthuma D, Polderman TJ, Boomsma DI, & de Geus EJ (2006). Accounting for sequential trial effects in the flanker task: Conflict adaptation or associative priming? Memory & Cognition, 34(6), 1260–1272. doi: 10.3758/BF03193270 [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, & van der Molen MW (1995). A psychophysiological analysis of developmental differences in the ability to resist interference. Child Development, 66(4), 1040–1056. doi: 10.1111/j.1467-8624.1995.tb00921.x [DOI] [Google Scholar]
- Ridderinkhof KR, van der Molen MW, & Bashore TR (1995). Limits on the application of additive factors logic: Violations of stage robustness suggest a dual-process architecture to explain flanker effects on target processing. Acta Psychologica, 90(1), 29–48. doi: 10.1016/0001-6918(95)00031-O [DOI] [Google Scholar]
- Rommelse NN, Altink ME, Oosterlaan J, Buschgens CJ, Buitelaar J, De Sonneville LM, & Sergeant JA (2007). Motor control in children with ADHD and non-affected siblings: deficits most pronounced using the left hand. Journal of Child Psychology and Psychiatry, 48(11), 1071–1079. doi: 10.1111/j.1469-7610.2007.01781.x [DOI] [PubMed] [Google Scholar]
- Rueda MR, Fan J, McCandliss BD, Halparin JD, Gruber DB, Lercari LP, & Posner MI (2004). Development of attentional networks in childhood. Neuropsychologia, 42(8), 1029–1040. doi: 10.1016/j.neuropsychologia.2003.12.012 [DOI] [PubMed] [Google Scholar]
- Schmidt JR (2013). The Parallel Episodic Processing (PEP) model: dissociating contingency and conflict adaptation in the item-specific proportion congruent paradigm. Acta Psychologica, 142(1), 119–126. doi: 10.1016/j.actpsy.2012.11.004 [DOI] [PubMed] [Google Scholar]
- Schmidt JR, & De Houwer J (2011). Now you see it, now you don’t: Controlling for contingencies and stimulus repetitions eliminates the Gratton effect. Acta Psychologica, 138(1), 176–186. doi: 10.1016/j.actpsy.2011.06.002 [DOI] [PubMed] [Google Scholar]
- Shenhav A, Botvinick MM, & Cohen JD (2013). The expected value of control: An integrative theory of anterior cingulate cortex function. Neuron, 79(2), 217–240. doi: 10.1016/j.neuron.2013.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth SA, Mian MK, Patel SR, Asaad WF, Williams ZM, Dougherty DD, Bush G, & Eskandar EN (2012). Human dorsal anterior cingulate cortex neurons mediate ongoing behavioural adaptation. Nature, 488(7410), 218–221. doi: 10.1038/nature11239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smulders SF, Notebaert W, Meijer M, Crone EA, van der Molen MW, & Soetens E (2005). Sequential effects on speeded information processing: A developmental study. Journal of Experimental Child Psychology, 90(3), 208–234. doi: 10.1016/j.jecp.2004.10.003 [DOI] [PubMed] [Google Scholar]
- Soetens E, Boer LC, & Hueting JE (1985). Expectancy or automatic facilitation? Separating sequential effects in two-choice reaction time. Journal of Experimental Psychology: Human Perception and Performance, 11(5), 598. doi: 10.1037/0096-1523.11.5.598 [DOI] [Google Scholar]
- Song J-H, & Nakayama K (2006). Role of focal attention on latencies and trajectories of visually guided manual pointing. Journal of Vision, 6(9), 982–995. doi: 10.1167/6.9.11 [DOI] [PubMed] [Google Scholar]
- Song J-H, & Nakayama K (2007). Automatic adjustment of visuomotor readiness. Journal of Vision, 7(5), 2. doi: 10.1167/7.5.2 [DOI] [PubMed] [Google Scholar]
- Song J-H, & Nakayama K (2008). Target selection in visual search as revealed by movement trajectories. Vision Research, 48(7): 853–861. doi: 10.1016/j.visres.2007.12.015 [DOI] [PubMed] [Google Scholar]
- Song J-H, & Nakayama K (2009). Hidden cognitive states revealed in choice reaching tasks. Trends in Cognitive Sciences, 13(8), 360–366. doi: 10.1016/j.tics.2009.04.009 [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, & Toga AW (2003). Mapping cortical change across the human life span. Nature Neuroscience, 6(3), 309–315. doi: 10.1038/nn1008 [DOI] [PubMed] [Google Scholar]
- Stins JF, Polderman JT, Boomsma DI, & de Geus EJ (2007). Conditional accuracy in response interference tasks: Evidence from the Eriksen flanker task and the spatial conflict task. Advances in Cognitive Psychology, 3(3), 409–417. doi: 10.2478/v10053-008-0005-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takarae Y, Schmidt L, Tassone F, & Simon TJ (2009). Catechol-O-methyltransferase polymorphism modulates cognitive control in children with chromosome 22q11. 2 deletion syndrome. Cognitive, Affective, & Behavioral Neuroscience, 9(1), 83–90. doi: 10.3758/CABN.9.1.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullsperger M, Bylsma LM, & Botvinick MM (2005). The conflict adaptation effect: It’s not just priming. Cognitive, Affective, & BehavioralNeuroscience, 5(4), 467–472. doi: 10.3758/CABN.5.4.467 [DOI] [PubMed] [Google Scholar]
- van Boxtel GJ, van der Molen MW, Jennings JR, & Brunia CH (2001). A psychophysiological analysis of inhibitory motor control in the stop-signal paradigm. Biological Psychology, 58(3), 229–262. doi: 10.1016/S0301-0511(01)00117-X [DOI] [PubMed] [Google Scholar]
- van de Laar MC, van den Wildenberg WP, van Boxtel GJ, & van der Molen MW (2014). Development of response activation and inhibition in a selective stop-signal task. Biological Psychology, 102, 54–67. doi: 10.1016/j.biopsycho.2014.06.003 [DOI] [PubMed] [Google Scholar]
- Velanova K, Wheeler ME, & Luna B (2008). Maturational changes in anterior cingulate and frontoparietal recruitment support the development of error processing and inhibitory control. Cerebral Cortex, 18(11), 2505–2522. doi: 10.1093/cercor/bhn012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Notebaert W, Liefooghe B, & Vandierendonck A (2006). Stimulus-and response-conflict-induced cognitive control in the flanker task. Psychonomic Bulletin & Review, 13(2), 328–333. doi: 10.3758/BF03193852 [DOI] [PubMed] [Google Scholar]
- Waszak F, Li SC, & Hommel B (2010). The development of attentional networks: Cross-sectional findings from a life span sample. Developmental Psychology, 46(2), 337–349. doi: 10.1037/a0018541 [DOI] [PubMed] [Google Scholar]
- Weissman DH, Jiang J, & Egner T (2014). Determinants of congruency sequence effects without learning and memory confounds. Journal of Experimental Psychology: Human Perception and Performance, 40(5), 2022. doi: 10.1037/a0037454 [DOI] [PubMed] [Google Scholar]
- Wiecki TV, & Frank MJ (2013). A computational model of inhibitory control in frontal cortex and basal ganglia. Psychological Review, 120(2), 329–355. doi: 10.1037/a0031542 [DOI] [PubMed] [Google Scholar]
- Yeung N, Botvinick MM, & Cohen JD (2004). The neural basis of error detection: conflict monitoring and the error-related negativity. PsychologicalReview, 111(4), 931–959. doi: 10.1037/0033-295X.111.4.931 [DOI] [PubMed] [Google Scholar]
- Zelazo PD, Anderson JE, Richler J, Wallner-Allen K, Beaumont JL, & Weintraub S (2013). NIH toolbox cognition battery (CB): Measuring executive function and attention. Monographs of the Society for Research in Child Development, 78(4), 16–33. doi: 10.1111/mono.12032 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.