Abstract
The body’s biological stress response forms one of the foundations of adaptive behavior, including by promoting (and impairing) different forms of memory. This response transcends stressful experiences, underlying reactions to challenges and even reinforcers like addictive substances. Yet, drug-induced stress responses are rarely incorporated in models of addiction. Here we propose that drug-induced stress responses (particularly glucocorticoids) play a critical role in addictive behavior by modulating the formation of memories for substance use experiences. We review contributions of amygdala-, striatal-, and hippocampal-based memory systems to addiction, and reveal common effects of addictive drugs and acute stress on these different memories. We suggest that the contributions of drug-induced stress responses to memory may provide insights into mechanisms driving addictive behavior.
Keywords: stress, memory, addiction, hippocampus, striatum, amygdala
Memories for Drug Use
Drug seeking and drug use behaviors are powerfully informed by learning and memory. What was learned and what is remembered about prior experiences with these substances can have a profound impact on future responses to information or cues relating to these experiences. As illustrated by the following report by a patient with cocaine addiction, exposure to cues can lead to memory retrieval, triggering responses such as craving and relapse (reviewed in [1]):
It is 11:00am on a warm morning in July. You are walking down the street. All of a sudden, you run into Mary*. Your heart skips a beat. You think back to times you’ve gotten high together. You smile and feel a sense of excitement. You start to think about getting high.
*name changed
Such memory-driven effects, combined with evidence that drugs of abuse can act on neuroanatomical circuitry supporting memory, have fostered the conceptualization of addiction as a disorder of learning and memory (e.g., [2–7]).
Decades of research have demonstrated that different types of information are learned, or encoded, during everyday experiences, and that depending on the nature of this information, different processing mechanisms and neural networks, corresponding to different memory systems, are engaged ([8]; see Box 1 for examples related to drug use). These memory systems can function in parallel, each contributing to behavior [9], or they can compete for preferential expression [10]. Accordingly, theories describing the contributions of different memory systems to addiction describe both parallel and interactive effects (Table 1). These theories have been extremely influential, inspiring research programs and the development of addiction treatments (recent examples in [11, 12]). With recent evidence that memory for even a single experience can influence later decision-making [13, 14], understanding how memories for drug-related experiences are formed is a critical and timely question.
Box 1. Multiple Memories for Drug Use Experiences.
Figure I depicts a drug use experience. After work, rather than going home, Mr. X visits a local bar. He then spends a few hours at the bar and becomes intoxicated. As originally proposed by White (1996) [3], this experience contains many pieces of information that can be learned (or encoded) and later influence drug-seeking behavior:
Cue-response. Association between cues (like the street sign) and motor responses (turning left). Such associations are typically formed slowly over multiple repetitions, and depend on dorsolateral striatum. They are often contrasted with “goal-directed” (response-outcome) associations, which are sensitive to the outcome of making the response and involve medial prefrontal cortex and dorsomedial striatum [15].
Cue-affect. Association between neutral elements, or cues (like the bartender) and affective reaction (feeling pleasantly intoxicated). Affect can be positive (appetitive outcome) or negative (aversive outcome). Cue-affect association critically involves the amygdala [16, 17].
Cue-cue (context): Associations between multiple cues (door, decorations, stools), binding these into a context representation which depends on the hippocampus [18].
Table 1.
Proposed Roles for Multiple Memory Systems in Addiction
| Memory system | Proposed role in addiction | |||
|---|---|---|---|---|
| Parallel processes | cue - response | Promotes repetition of behaviors typically performed in presence of drug-related cues | ||
| cue - affect | Appetitive: promotes approach to drug-related cues (“incentive salience”) Aversive: promotes avoidance of withdrawal |
|||
| cue - cue | Promotes motivation/focus in drug- related contexts | |||
| Interactive systems | cue - response | > | response - outcome | Loss of control over drug-seeking behavior; responses increasingly triggered by drug-related cues |
| cue - response | > | cue - cue | Indirect facilitation of habitual behavior by impairing memory for competing, flexible associations | |
| cue - affect | → | cue - response | Appetitive: drug-related cue serves as conditioned reinforcer to promote response Aversive: drug-related cue signals withdrawal; promotes response to avoid |
|
We propose that these memories for drug-related experiences are critically modulated by the stress responses (particularly the release of glucocorticoids) that are triggered by using the drug. We first provide an overview of the glucocorticoid response as a modulator of memory and as a frequent consequence of consuming addictive substances. Next, we discuss common effects of addictive drugs and glucocorticoids on the amygdala-, striatal-, and hippocampal-based memory systems. We then highlight preliminary findings demonstrating that drug-induced glucocorticoids play a direct role in drug effects, particularly for learning and memory. Based on the emerging picture from this body of literature, we conclude by proposing novel directions for future research.
Stress as a Modulator of Memory
Across species, learning and memory can be profoundly influenced by exposure to acute (or short-term) stress. Acute stress can be decomposed into the stressor, or event, and the stress response, including a variety of different processes—cognitive, affective, behavioral, and physiological—that typically occur upon exposure to a stressor. Stressors are characterized as novel, unpredictable, and uncontrollable, resulting in a disruption of homeostasis [23]. Here we focus on the physiological component of the stress response, particularly the activation of the hypothalamic-pituitary-adrenal (HPA) axis. When the HPA axis is activated, the paraventricular nucleus of the hypothalamus releases corticotropin releasing factor (CRF), which leads the anterior pituitary to release adrenocorticotropin (ACTH), causing the adrenal cortex to release glucocorticoids (cortisol in humans, corticosterone in rodents). This hormonal response is part of an array of dynamic physiological changes, including the rapid release of catecholamines (including adrenaline and noradrenaline) from the adrenal medulla [24].
In addition to these peripheral effects, biological stress responses also influence central nervous system function. Glucocorticoids are lipophilic and readily cross the blood-brain barrier [23]. They directly influence the function (and, over time, the structure) of regions that are critical to forming and retrieving memories including the amygdala [25], striatum [26], and hippocampus (reviewed in [27]). Understanding the circumstances under which stress responses enhance and impair these memory systems is an active area of research. For example, interactions between glucocorticoids and adrenergic responses have been shown to be critical for the enhancing effects of acute stress on memory [28], and manipulating the adrenergic response can modulate the expression of different memory systems (e.g., [29]). In addition, acute stress can have opposite effects on memory if it occurs near encoding (frequently enhancing) or retrieval (frequently impairing memory) [30]. These findings raise questions about the role of adrenergic-glucocorticoid interactions in drug-related memories, and the influence of stress on the retrieval of drug-related information (see Outstanding Questions). In this Review we are focusing specifically on the role of glucocorticoids in the encoding of drug-related memories.
Addictive Drugs Induce Glucocorticoid Responses
In addition to stressors, addictive substances can acutely trigger an HPA axis response (reviewed in [31]; for CRF, see [32]). Although the mechanism varies by substance [31], there is strikingly consistent evidence for increases in glucocorticoids among non-dependent populations of rodents and humans (Table 2). Importantly, these findings do not imply that individuals “feel stressed” after taking these substances. That is, we are not arguing that because addictive drugs elicit glucocorticoid responses, they also trigger the negative affect that typically accompanies the stress response. Glucocorticoids may instead contribute to the reinforcing effects of addictive drugs, as supported by findings including preferential self-administration of corticosterone in rodents [33], and, in humans, euphoria after high doses of glucocorticoids [34]. Supporting the clinical relevance of glucocorticoids in addiction, targeting the glucocorticoid system has recently emerged as a potential approach in addiction therapeutics [35–37].
Table 2.
Glucocorticoid Responses to Addictive Substances Across Species
| Substance | Population | Effect on glucocorticoids |
|---|---|---|
| Cannabis | Rodent | Increase[38] |
| Human | Increase[39] | |
| Alcohol | Rodent | Increase[40] |
| Human | Increase[41] | |
| Stimulant | Rodent | Increase[42] |
| Human | Increase[43] | |
| Opiate | Rodent | Increase[44] |
| Human | Decrease[45] | |
| Nicotine | Rodent | Increase[46] |
| Human | Increase[47] |
Note: example references are provided (not an exhaustive list). Only studies on non-dependent populations are included.
Parallel Effects of Drugs and Glucocorticoids on Memory
As depicted in Box 1, different memories can be formed for drug use experiences, each of which has been shown to play a role in addiction (Table 1). We next review the effects of addictive drugs and acute stress on these memories. To facilitate comparisons between drugs and stress, we focus on studies in which acute stress or glucocorticoid manipulations occurred near the time that these memories were encoded (Box 2).
Box 2. Challenge: Comparing Intrinsic and Extrinsic Sources of Stress Responses.
The effects of the stress response on memory formation can be critically influenced by the relationship between the stressor and the information that is being learned. If the stress response is elicited by something intrinsic, or related to the learned information, memory is often enhanced; on the other hand, if the stress response comes from something extrinsic, or unrelated, memory is often impaired [48]. For example, rodents who learned a water maze showed better memory when the water was cold (triggering glucocorticoid release) than when water was warm [49], but showed worse memory if they were exposed to an unrelated stressor (restraint and tail shocks, also triggering glucocorticoid release) more than 30 minutes before learning [50]. This presents a challenge for relating the effects of stressor-induced (where the trigger of the stress response is typically extrinsic) to drug-induced (where the trigger is intrinsic) stress responses on memory.
We aim to address this challenge by focusing on acute stress studies in which the stressor occurred near the time of encoding. As the HPA axis response unfolds relatively slowly, with peak glucocorticoid release occurring on the order of minutes to tens of minutes post-stressor [23], there is an opportunity to compare intrinsic drug-induced and extrinsic stressor-induced sources of cortisol responses. That is, even if the drug itself produced a cortisol response, the spike in cortisol would not occur immediately upon drug administration. Thus, if an unrelated stressor occurred close to the time of encoding, it could produce a cortisol response with a similar time-course (and perhaps similar effects) to a drug-induced cortisol response. Indeed, it can be difficult to disentangle the effects of intrinsic vs. extrinsic stress responses from the delay between the stressor and encoding, as most studies to date measuring intrinsic stressors and memory also had a very short delay [51]. Convergence in time between stressor-induced glucocorticoid increases and encoding has been shown to be critical for the facilitating effects of acute stress on different types of memory [48, 52]. This has been particularly well-characterized in the hippocampus, as synaptic plasticity (a hallmark of learning) in hippocampal slices was enhanced if electrical stimulation occurred within minutes of corticosterone exposure, but not if there was an extended delay [53]. In humans, a recent meta-analysis found that increasing delays between stress exposure and the onset of encoding predicted worsening memory [51]. Together, these findings suggest that stress-induced and drug-related memory effects will be most comparable if acute stress occurs near the time of encoding.
Cue-affect associations
For many people, a straw is a neutral object. Yet for individuals who chronically use cocaine, repeated exposure to straws as part of drug use may imbue these formerly neutral objects with strong affective salience. This formation of associations between a cue and affect is supported by classical, or Pavlovian, conditioning. These associations may be aversive (e.g., cues are associated with withdrawal [22] or responses counteracting anticipated drug effects [54]) or appetitive (e.g., pleasant hedonic drug effects [4]). The amygdala is critically involved in associating a neutral cue (typically referred to as a conditioned stimulus, or CS) with an affective outcome (unconditioned stimulus, US). Memory is typically measured by the extent to which the CS can elicit a response (conditioned response, CR) indicating US anticipation. The lateral nucleus of the amygdala (LA) is particularly involved in aversive CS-US associations [16], with the basolateral amygdala (BLA) critical for linking the CS to the current appetitive US value [17].
The amygdala is also involved in associating neutral cues with drugs. For example, after CS-cocaine conditioning, presentation of the CS alone led a subset of BLA neurons to fire [55] and increased dopamine efflux in the amygdala [56]. Temporary inactivation of the BLA prior to CS-cocaine conditioning blocked later CS-cued reinstatement of drug-seeking behavior [57]. The amygdala also plays a key role in conditioned place preference (CPP; see Glossary), sometimes described as “conditioned cue preference” [3], in which rodents preferentially approach a previously drug-paired location. This behavior has been shown for natural reinforcers (e.g., [58, 59]) as well as multiple addictive substances [60]. Lesions of the LA (but not the dorsal striatum or fimbria/fornix) prior to conditioning blocked the development of food CPP [58], and lesions of the BLA prior to test abolished sucrose CPP [59]. Temporary inactivation of the amygdala prior to or immediately after conditioning blocked amphetamine CPP [61]. Conversely, successful acquisition of amphetamine CPP led to significantly elevated neuronal activity (c-Fos) in the BLA, which correlated with the magnitude of CPP expression [62]. Finally, functional neuroimaging studies in humans examining a variety of addictive substances have reported positive correlations between self-reported craving and cue-induced BOLD responses in the amygdala (reviewed in [63]).
For both appetitive and aversive conditioned associations, glucocorticoids facilitate the formation of memories as well as associated changes in the underlying neural circuitry. Both acute and chronic stress led to positive structural changes in the amygdala (particularly the BLA; [27, 64]). Acute glucocorticoid exposure facilitated immediate and sustained enhancement of BLA activity [65]. In the aversive domain, acute stress pre-training [66] and administration of corticosterone immediately post-training [67] each enhanced CRs (reviewed in [68]). In another aversive paradigm, conditioned taste aversion, administration of a glucocorticoid synthesis inhibitor (metyrapone) immediately post-training impaired later memory, whereas corticosterone (either into the BLA or systemically) enhanced memory [69]. In humans, acute stress pre-learning [70] and administration of hydrocortisone immediately post-learning [71] enhanced memory for CS-shock associations, as reflected by greater resistance to extinction (i.e., CRs persisted even when the CS no longer predicted shock). In addition, elevated cortisol levels after CS-shock conditioning positively correlated with later CRs [72].
In the appetitive domain, stress and glucocorticoids directly facilitated drug-related CPP. Although conditioning with corticosterone did not produce CPP [73], exposure to acute stress before conditioning enhanced cocaine CPP [74] and ethanol CPP [75]. Acute stress also enhanced morphine CPP in some strains of rats [76]. Similarly, autoshaping training led to increased corticosterone [77], particularly among rodents who made more autoshaping CRs [78] (with the caveat that the aversive method of sampling corticosterone may have influenced these results; see [77]). Few studies have examined acute stress effects on the acquisition of appetitive conditioning in humans, although there is evidence that individuals exposed to pre-learning stress “over-generalized” appetitive associations [79].
Interactions between the amygdala and other memory systems
One key function of cue-affect memory in addictive behavior is to influence the engagement of other forms of memory. For example, Wikler’s “two-factor theory” of relapse posits that environmental cues first become associated with feelings of withdrawal (through a process of amygdala-based cue-affect conditioning), which then promote cue-response (striatal-based) drug-seeking behavior to alleviate the conditioned aversive feelings [22]. Experimentally, drug-paired CSs can serve as “conditioned reinforcers” to promote acquisition of novel cue-response associations [80]. Presentation of an ethanol-paired CS in a Pavlovian-instrumental transfer (PIT) task was associated with enhanced cue-response behaviors for both ethanol and sucrose rewards [81] and, after extended cue-response training, the effects on cue-response behaviors for ethanol rewards became even stronger [82]. Similarly, a cocaine-paired CS promoted the performance of cue-response behaviors for a cocaine reward [83]. These effects can be very powerful: a cocaine-paired CS facilitated cue-response behavior for cocaine one year after a single CS-cocaine conditioning session [84]. Effects of glucocorticoids (post-training glucocorticoid receptor, GR, agonists) on PIT in rodents have been mixed [85, 86], although there is recent evidence that acute stress enhanced PIT in humans [87].
Beyond supporting memory for cue-affect associations, the amygdala plays a critical role in the effects of acute stress on memory by modulating the engagement of the striatum and hippocampus [28, 88]. For example, acute stress before training enhanced the use of striatal-based cue-response strategies in rodents and humans (see next section). This effect was blocked by temporary inactivation of the BLA in rodents [89], and was associated with greater amygdala BOLD signal and amygdala-striatal connectivity in humans [90]. BLA lesions also blocked both impairing [50] and enhancing [91] effects of stress on hippocampal memory. The BLA even arbitrated the stress-induced shift toward preferentially expressing striatal over hippocampal memory in both rodents [89] and humans [90] (for more discussion of this trade-off, see “Glucocorticoids and the balance between memory systems in addiction”). More research is needed to investigate whether the amygdala similarly modulates the acquisition of different drug-related associations (perhaps via drug-induced glucocorticoid release).
Cue-response associations
The repeated use of substances that characterizes addictive behavior is often colloquially referred to as a “bad habit”. Indeed, addictive behaviors have many features of automatized, habit-like processes: they can be performed with low effort (or awareness), are acquired slowly through practice, occur with increased speed and decreased variability [6], are described as “ritualistic” [7], and are progressively perceived as out of the user’s control and instead governed by triggering cues [19] (although there is debate about the relevance of these memories for human addiction; Box 3). The dorsolateral striatum (DLS) supports the formation of cue-response habits. In contrast, the dorsomedial striatum (DMS) and prelimbic cortex (medial prefrontal cortex [mPFC] in humans) are involved in goal-directed response-outcome associations [15, 92].
Box 3. Using Acute Stress Findings to Address Debates about Habit Memory in Addiction.
There is continued debate about the importance of habit memory for the behaviors observed in human addiction (e.g., [114]). Here we propose ways in which findings regarding the effects of stress responses could be used to address some of these issues.
Can “habits” develop in real-world settings of drug exposure? One challenge in examining the relevance of habit memory for human addiction concerns the rigid training routines used in the laboratory in many studies of habit formation (e.g., overtraining, interval reward schedules). As human drug users are fairly unlikely to experience such standardized routines [115], do they still form habits in the strict sense, and if so, how? It is possible that requirements for learning cue-response associations are relaxed for addictive drugs. For example, drug outcomes make rodents more likely to learn habitual cue-response associations (e.g., even without overtraining [94]; reviewed in [7]). This may be related to the drug-induced glucocorticoid response, as glucocorticoids also promote the formation of cue-response associations [101]. Further studies are needed to test whether drugs, and drug-induced glucocorticoid responses, can facilitate cue-response learning at the atypical exposure and reward schedules that reflect real-world drug use.
Does insensitivity to outcome devaluation measure “habit”? As discussed earlier, many experiments use persistent responding after an outcome has been devalued to infer that a response is habitual. It is possible however that this persistent behavior may instead indicate impairments in goal-directed learning [116]. Consistent with this alternative interpretation, acute stress has been shown to promote insensitivity to outcome devaluation [117], and there is evidence that it does so by impairing goal-directed (rather than enhancing habitual) learning. Both drugs [118] and acute stress responses [119] are known to impair PFC function. Acute stress was recently shown to particularly disrupt PFC processes needed for goal-directed learning [120] and to reduce (goal-directed) model-based learning [113]. These findings, together with evidence that individual differences in model-based learning predict insensitivity to devaluation [121], suggest that addictive drugs (perhaps through drug-induced glucocorticoid release) promote insensitivity to devaluation via impaired goal-directed learning.
Although most associations start as goal-directed (response-outcome), certain procedures, like overtraining and interval schedules of reward, make it more likely that habitual cue-response associations will be formed [15]. Critically, rodents are more likely to learn cue-response associations for addictive drug outcomes [7]. For example, whereas rodents trained for a food pellet outcome stopped responding when the food pellet was devalued, or paired with something aversive (evidence that they were goal-directed, or sensitive to outcome devaluation), rodents who were trained for an alcohol outcome were equally likely to respond regardless of whether the alcohol or a food pellet was devalued [93]. Similarly, rodents trained for an alcohol outcome were insensitive to outcome devaluation after 4 or 8 weeks of training, whereas rodents trained for a sucrose outcome were still sensitive to devaluation at 8 weeks [94]. In fact, rodents trained for an alcohol outcome were insensitive to devaluation after only 9 sessions [95]. Rodents trained to respond for a cocaine-sucrose solution also became insensitive to devaluation, whereas those trained to respond for lemon-sucrose remained sensitive to devaluation [96].
Recent evidence has demonstrated that glucocorticoids also facilitate DLS-dependent cue-response learning. Using outcome devaluation and contingency degradation procedures, recent studies have shown that chronic stress or chronic glucocorticoid exposure led rodents to form habitual cue-response associations for natural reinforcers [26, 97]. Humans exposed to acute stress before learning were also insensitive to outcome devaluation [98] (although this was likely not due to modulation of cue-response learning [99]). Using spatial navigation tasks in which striatal-based cue-response strategies could be used, both rodents [50, 100] and humans [90] exposed to stress before learning were more likely to learn cue-response associations, and rodents who received intra-DLS injections of glucocorticoids during learning were faster to shift toward using cue-response associations [101]. These latter findings suggest that stress (and glucocorticoids) may act to facilitate the formation of cue-response associations by directly enhancing striatal function.
Enhanced striatal-dependent responses and memory also characterize individuals with addiction. Patients showed higher dorsal striatal BOLD signal in response to drug cues, an effect that predicted worsening cannabis use-related problems among frequent cannabis users 3 years later [102], was associated with relapse among abstinent alcoholics [103], and, even among social drinkers, correlated with compulsive drinking behavior [104]. Suggestive of an interaction between glucocorticoid and drug cue-related circuitry, smokers exposed to acute stress before viewing drug cues had an enhanced cue-induced BOLD response in the dorsal striatum [105]. In the learning and memory domain, patients with cocaine use disorder were insensitive to devaluation in an appetitive task [106]. Patients with substance use disorder were better at performing learned cue-response behaviors and made more perseverative errors (i.e., repeated the old response) when learning a new response [107]. The neural mechanism supporting cue-response learning also differed for individuals with addiction: patients with alcohol use disorder (AUD) had greater BOLD signal in the DLS when learning cue-response associations, but attenuated mPFC signal for response-outcome associations, relative to controls [108]. Preclinically, young adults with a history of high cigarette, alcohol, and cannabis consumption were more likely to use a cue-response strategy in a spatial navigation task [109]. Using a sequential decision-making task designed to measure model-based/model-free strategies [110], relapsing alcoholics were shown to have impaired model-based learning (thought to reflect goal-directed behavior) and higher positive expectancies about alcohol [111].
Notably, exposure to acute stress led non-dependent individuals to show behavior similar to that of patients with addiction: acute stress enhanced cue-response learning (for positive outcomes [112]), increased use of cue-response strategies in spatial navigation [90], and impaired model-based learning (among individuals with low working memory capacity [113]).
In summary, drugs (and glucocorticoids) may promote the use of striatal memory through diverse pathways [5], including modulation by the amygdala [28], direct facilitation of striatal function [101], or impairment of competing (e.g., goal-directed) representations (Box 3). There is a need for more research to understand how striatal-dependent cue-response associations are facilitated among chronic drug users as well as naïve users responding for drug outcomes.
Cue-cue (context) associations
A major challenge in maintaining abstinence involves generalizing strategies learned in the clinic to contexts or environments in which drug use previously occurred. Unlike memory for associations between drug effects and discrete cues, memory for drug effects and contexts (or “stimulus configurations” [6]) includes associations between different cues. The hippocampus is critical for forming these associations and representing spatial information [18, 122].
The hippocampus also plays a critical role in drug-related context memories. For example, paradigms like context-induced reinstatement demonstrate that drug-seeking for several addictive substances, including cocaine, nicotine, alcohol, and heroin, is renewed when rodents are placed in an environment in which they had previously experienced the drug [123]. This behavior involves the hippocampus, as temporary inactivation of the dorsal hippocampus blocked reinstatement of cocaine seeking [124]. Context-induced reinstatement of cocaine and alcohol seeking elicited increased neuronal activity in the dorsal hippocampus [125] and CA3 subregion of the hippocampus, respectively [126]. In a similar protocol, ventral hippocampus inactivation before short-term context-cocaine conditioning blocked cocaine seeking reinstatement, and impaired ability to distinguish between cocaine-paired and saline-paired contexts [127]. More broadly, acute exposure to several addictive substances, including amphetamine [28], cocaine, and nicotine, enhanced hippocampal function and hippocampal-dependent processes like spatial memory [60]. Similar to the amygdala and dorsal striatum, functional neuroimaging studies have found that human drug users show increased hippocampal signal in response to drug cues [63].
The hippocampus is also highly sensitive to the effects of stress, and the mechanisms by which stress acts on this memory system have been well-characterized. The hippocampus has a high density of receptors that bind glucocorticoids [27], including both high-affinity mineralocorticoid (MR) and lower-affinity glucocorticoid (GR) receptors [53]. At moderate doses, glucocorticoid elevations near the time of encoding (see Box 2) promote hippocampal plasticity and formation of hippocampal-dependent memories [48]. As noted earlier, these enhancing effects may depend on an interaction between the glucocorticoid and adrenergic response, often making them specific to information that is emotionally arousing [28]. For example, exposure to acute stress around the time of training enhanced memory for aversive high arousal context associations in rodents [128], and enhanced memory in humans for cue-cue associations rated as highly arousing [129]. Suggestive of a common pathway between drugs and glucocorticoids, both acute stress and cocaine administration enhanced LTP in the ventral hippocampus [130]. As will be discussed in the next section, recent evidence supports an integral role for glucocorticoids in drug-related context memories.
Role of Drug-Induced Glucocorticoid Response in Acute Drug Effects
Beyond memories for drug-related experiences, the glucocorticoid response to addictive drugs plays a critical role in the effects of several substances. For example, blocking glucocorticoid synthesis before ethanol exposure dose-dependently blocked behavioral sensitization [131], and adrenalectomy before extended cocaine access slowed increasing self-administration and attenuated cocaine-induced reinstatement [132].
The drug-induced stress response may work in part by influencing the dopamine system. A recent review summarizing parallel effects of alcohol, nicotine, and acute stress on cellular processes highlighted shared downstream influence on dopaminergic pathways [133]. Behaviorally, selective inactivation of GR receptors on dopaminoceptive neurons (post-synaptic neurons containing dopamine receptors) led to reduced cocaine self-administration [134]. In addition, while nicotine exposure led to increased ethanol self-administration and attenuated dopamine response to ethanol, both effects were blocked by administering GR antagonists before nicotine exposure [46]. Although the mechanism(s) by which drug-induced stress responses contribute to drug effects require further investigation, these findings emphasize the importance of incorporating this process into models of addictive behaviors.
Drugs modulate learning and memory via glucocorticoids
Studies have begun to demonstrate a causal role for drug-related glucocorticoids in modulating drug-related learning and memory. For example, the amygdala is an important locus by which drug-induced stress responses modulate addictive behaviors. Administration of a CRF receptor antagonist into the amygdala (CeA) of ethanol-dependent rats led to decreased ethanol self-administration [135]. During abstinence (3 weeks), ethanol dependent rats had higher levels of GR mRNAs in the CeA (indicating greater receptor binding), and a GR receptor antagonist led to decreased ethanol self-administration [136]. More research is needed to determine whether these drug-induced glucocorticoid effects operate by influencing amygdala-dependent mnemonic processes (e.g., cue-affect memory, or amygdala-based modulation of other memory systems).
Learning cue-response associations to self-administer cocaine also critically involved glucocorticoids (reviewed in [137]). In a series of experiments, rats learned that a light (cue) indicated that lever pressing (response) would lead to intravenous cocaine injections (outcome). Although rats did not learn to respond to the cue at low cocaine doses, footshock (leading to elevated corticosterone) or corticosterone injections facilitated responding, with the number of cued responses made during learning positively correlating with corticosterone. Adrenalectomized rats did not learn to respond for any dose of cocaine, although corticosterone replacement somewhat rescued the behavior. These effects may be driven by GR receptors, as GR receptor blockade decreased cocaine self-administration in another protocol and impaired the ability to distinguish between “active” (responses delivered cocaine) and “inactive” cues [138].
Finally, learning and memory for drug-related cue-affect and cue-cue associations required glucocorticoids. Systemic blockade of glucocorticoid receptors during conditioning blocked ethanol CPP [139] and morphine CPP [140]. Inhibiting morphine-induced corticosterone synthesis during conditioning also blocked any enhancement of CPP due to prior stress exposure [141]. In addition, after conditioning (with nicotine [142] or cocaine [143, 144]), drug-paired contexts directly evoked glucocorticoid responses. Blocking these glucocorticoid responses with a GR antagonist prior to cocaine-paired context exposure dose-dependently attenuated reinstatement of cocaine-seeking [145].
Concluding Remarks
Different forms of memory have been shown to play key roles in addictive behavior across species. One mechanism that may promote the formation of memories for drug-related experiences is the drug-induced release of stress-related hormones, particularly glucocorticoids. We cite support for this hypothesis, including for parallel effects of drugs and acute stress on different memory systems, as well as preliminary evidence that blocking the drug-induced glucocorticoid response leads to widespread changes in drug effects as well as modulation of drug-related learning and memory. This integration of stress, memory, and addiction presents a compelling avenue for future research (see Outstanding Questions). We highlight two of these directions below.
Drug-induced glucocorticoid responses in a dysregulated stress system
The ubiquity of drug-induced glucocorticoid responses—and the fact that they are observed in non-dependent individuals—raises questions about how meaningful these responses are for the development of addictive behaviors. One simple model would be that individuals who go on to develop addictive behaviors also have larger drug-induced glucocorticoid responses. However, at fixed doses of drugs, dependent individuals who are currently using typically have a blunted drug-induced cortisol response compared to non-dependent individuals (e.g., cannabis: [146]; alcohol: [147]). It is worth noting that, as dependent individuals also self-administer higher doses, the ultimate level of drug-induced cortisol response may not differ or could even be higher as a function of level of drug use [148]. Further, depending on the type of memory, the relationship between cortisol and memory can be quadratic rather than linear (e.g., [23, 28, 129, 149]), making it unlikely that merely having higher cortisol responses would compel stronger drug-related memories.
In contrast to that simple model, the impact of glucocorticoid responses to addictive drugs should be understood in the context of broader dysregulation in HPA axis function. A large body of evidence demonstrates that individuals who chronically use addictive substances have atypical HPA axis responses. These alterations are not only the result of chronic substance exposure, as individuals at risk for developing addiction also have atypical HPA axis responses (e.g., stimulants: [118]; alcohol: [150]). This atypical response includes a shift in basal HPA axis function, leading to consistently elevated levels of cortisol [150] and blunted cortisol responses to acute stress (e.g., among individuals chronically using cannabis [151]; alcohol [152]; and nicotine [153]). Against this scaffold, the drug-induced cortisol response may play a stronger role in an addicted than a drug-naïve individual. As acute stress can enhance memory for related information [48], these drug-induced cortisol responses may particularly amplify memory for drug-related experiences. Stronger drug-related memories could then promote escalation of drug use [115] through several mechanisms, including enhancing the likelihood of choosing similar (drug-related) actions in the future (as recently shown in a non-drug domain; [14]). If cue-response memories are preferentially strengthened, this could increase performance of habitual drug-seeking or drug-use behaviors (see next section). More research is needed to characterize the influence of drug-induced cortisol, and glucocorticoids more broadly, on different forms of memory among individuals with dysregulated HPA axis function.
Glucocorticoids and the balance between memory systems in addiction
Early theories about memory systems in addiction posited that each type of memory could contribute to a dimension of addictive behavior (e.g., [3]; see Table 1). More recent theories instead suggest that an imbalance, or competition between memory systems (particularly hippocampal and striatal), plays a key role [5, 21]. Specifically, preferential use of striatal cue-response memory may drive habitual responding and eventually a loss of control over drug-seeking behavior [4, 5, 21]. This perspective has been informed by the stress literature, as acute stress elicits the preferential engagement of striatal over hippocampal memory (see [50] for an early demonstration in rodents; for a recent review, see [154]). This stress-induced shift from hippocampal to striatal memory has been shown to critically involve the amygdala [89, 90] and MR receptor [155]. Behaviorally, stress-induced impairments in hippocampal memory may be sufficient to cause this shift [156]. Yet, as we reviewed earlier, stress (and acute drug administration) can also enhance hippocampal function and promote context memory. How can these models be reconciled?
One possibility concerns the trajectory of addiction. Across a range of addictive substances, chronic drug exposure leads to pronounced impairments in hippocampal memory [60]. Thus, escalating use could lead to difficulties forming and updating hippocampal-based memories (e.g., for new learning new skills in treatment), and promote greater reliance on amygdala or striatal-based memories. More work is needed to explore the engagement of different memory systems throughout the progression of substance use.
It is also possible that the “context” memories which are so problematic in human addiction do not actually require hippocampal-based contextual representations. One theory of posttraumatic stress disorder posits that, rather than form contextual memories of a traumatic event that bind the different cues into a unified representation, patients instead form simple cue-affect (or cue-response) associations [157]. In the addiction domain, rodents could learn to prefer a drug-paired location in a typical CPP paradigm based only on local cue-drug associations rather than using hippocampal-based spatial discrimination [58]. This hypothesis is supported by the critical involvement of the amygdala in CPP (reviewed earlier). Similarly, both the BLA (particularly GR receptors within the BLA; [145]) and hippocampus contribute to context-induced reinstatement of drug seeking [123], suggesting a role for cue-affect associations. More research is needed to probe the nature of memories formed for drug-related contexts, and test whether there is indeed a shift in the engagement of different memory systems.
Disclaimer
R.S. serves on the scientific advisory board of Embera Neurotherapeutics, Inc.
Figure I.
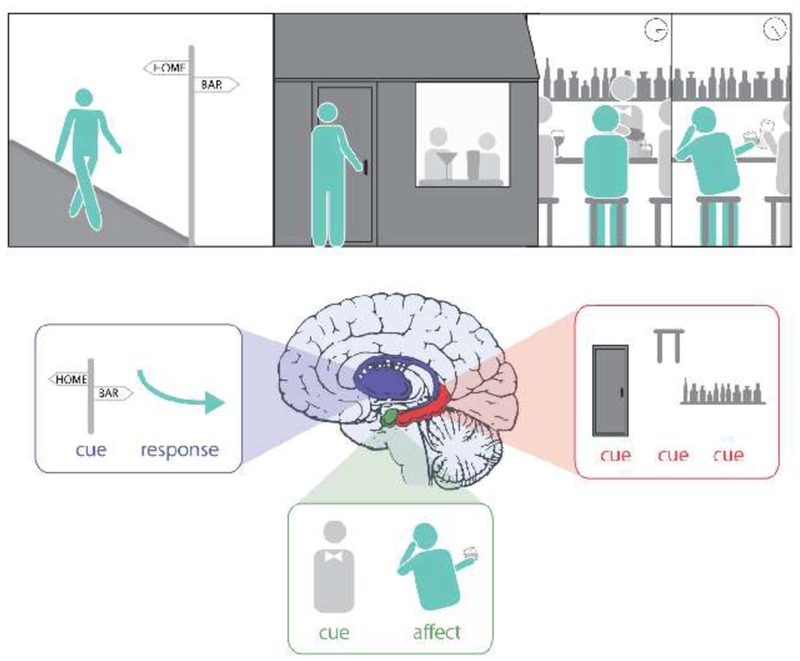
(for Box 1). Illustration of multiple memory systems in a drug-use episode. Top, an episode of substance use. Bottom, illustration of the different memories (associated with different memory systems) that can be formed during such an episode. Blue: dorsolateral striatum-dependent cue-response association; green: amygdala-dependent cue-affect association; red: hippocampus-dependent cue-cue (context) association. Note that other brain regions are also involved in the encoding and retrieval of these associations; here we focus on the critical “hubs” in these memory systems.
Highlights.
Memories for drug-use experiences, supported by different neural systems, contribute to the development and maintenance of addictive behaviors.
Addictive drugs and acute stress responses have common effects on these memory systems.
Addictive drugs can themselves elicit stress responses, including the release of glucocorticoids. Blocking glucocorticoid responses can change drug effects, including modulating drug-related learning and memory.
Acknowledgements
E.V.G. is supported by the Yale Neuroimaging Sciences Training Program (T32 DA022975); R.S. is supported by NIH grants including R01 AA20504, R01 AA013892, R01-DK099039 and 1UL1-TR001863–01.
Glossary
- Autoshaping
measure of appetitive Pavlovian conditioning, in which a rodent is presented with a lever before a response-independent delivery of food in another location. Although the rodent does not need to interact with the lever to receive the food, it acquires a set of motor responses (e.g., pressing and chewing) directed toward the lever
- Conditioned place preference
measure of cue-affect memory, in which a rodent experiences a reward (e.g., food or drug) in one compartment of a multi-compartment box. The amount of time spent in the rewarded compartment compared to other compartments is assessed
- Context-induced reinstatement
measure of context memory, in which rodents are trained to lever press to self-administer a drug in Context A, undergo extinction training (lever does not lead to drug delivery) in Context B, and are tested for reinstatement of lever press behavior after being reintroduced to Context A
- Conditioned taste aversion
measure of cue-affect memory in which a rodent experiences an aversive outcome (usually exposure to lithium chloride) after consuming a novel taste. Decrease in consumption of the novel taste after conditioning compared to acquisition is assessed
- Contingency degradation
measure of habit learning in which the link between a response and a rewarding outcome is diminished (e.g., reward is provided independent of the response). Persistence in performing the response after this procedure indicates that the learned association is habitual. Related to outcome devaluation
- Functional neuroimaging
Non-invasive technique often used to measure the blood oxygen level dependent (BOLD) response in humans. Greater BOLD signal in a region is interpreted as evidence of higher levels of underlying neuronal activity
- Model-based/model-free
problem-solving strategies, frequently measured in the context of a sequential decision-making task. Model-free learning relies on computationally efficient representations of previously rewarded actions, in which these actions are repeated and the reward value is not updated. This type of learning is thought to drive habitual behavior (but see [158] for limitations of this interpretation). Model-based learning is computationally much more sophisticated, involving representations of task contingencies to enable prospective planning, and is thought to reflect goal-directed behavior
- Pavlovian-instrumental transfer
measure of interaction between cue-affect and cue-response learning. Following cue-affect (Pavlovian) conditioning, cue-response (instrumental) behaviors are learned to receive the same outcome. If the cue-response behavior is performed more in the presence of the Pavlovian-conditioned cue, this indicates transfer
- Outcome devaluation
measure of habit learning in which the rewarding outcome is made less desirable through satiation or pairing the reward with something aversive. Persistence in performing the response that produces this outcome indicates that the learned association is habitual (but see Box 3). Related to contingency degradation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Outstanding Questions
Does the engagement of different memory systems, and the modulatory role of drug-induced stress responses, vary between addictive substances?
Are there sex differences in the contributions of different memory systems to addiction, or in how drug-induced stress responses modulate drug-related memories?
How do other components of the stress response (e.g., catecholamines) contribute to modulation of drug-related memories?
Would there be therapeutic benefits to blocking the drug-induced stress response? If so, at what stage in the development of addictive behaviors would these interventions be most helpful?
How does stress, itself a risk factor for relapse, play a role in the retrieval of drug-related memories?
How do escalating doses of addictive substances modulate the drug-induced stress response and the engagement of different memory systems?
References
- 1.Shaham Y et al. (2003) The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 168 (1–2), 3–20. [DOI] [PubMed] [Google Scholar]
- 2.Hyman SE et al. (2006) Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci 29, 565–98. [DOI] [PubMed] [Google Scholar]
- 3.White NM (1996) Addictive drugs as reinforcers: multiple partial actions on memory systems. Addiction 91 (7), 921–49; discussion 951–65. [PubMed] [Google Scholar]
- 4.Hogarth L et al. (2013) Associative learning mechanisms underpinning the transition from recreational drug use to addiction. Ann N Y Acad Sci 1282, 12–24. [DOI] [PubMed] [Google Scholar]
- 5.Goodman J and Packard MG (2016) Memory Systems and the Addicted Brain. Front Psychiatry 7, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tiffany ST (1990) A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychol Rev 97 (2), 147–68. [DOI] [PubMed] [Google Scholar]
- 7.Torregrossa MM and Taylor JR (2016) Neuroscience of learning and memory for addiction medicine: from habit formation to memory reconsolidation. Prog Brain Res 223, 91–113. [DOI] [PubMed] [Google Scholar]
- 8.Henke K (2010) A model for memory systems based on processing modes rather than consciousness. Nat Rev Neurosci 11 (7), 523–32. [DOI] [PubMed] [Google Scholar]
- 9.Goldfarb EV et al. (2016) Memory-Guided Attention: Independent Contributions of the Hippocampus and Striatum. Neuron 89 (2), 317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poldrack RA and Packard MG (2003) Competition among multiple memory systems: converging evidence from animal and human brain studies. Neuropsychologia 41 (3), 245–51. [DOI] [PubMed] [Google Scholar]
- 11.Marsden J et al. (2018) Memory-Focused Cognitive Therapy for Cocaine Use Disorder: Theory, Procedures and Preliminary Evidence From an External Pilot Randomised Controlled Trial. EBioMedicine 29, 177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mellentin AI et al. (2017) Cue exposure therapy for the treatment of alcohol use disorders: A meta-analytic review. Clin Psychol Rev 57, 195–207. [DOI] [PubMed] [Google Scholar]
- 13.Wimmer GE and Shohamy D (2012) Preference by association: how memory mechanisms in the hippocampus bias decisions. Science 338 (6104), 270–3. [DOI] [PubMed] [Google Scholar]
- 14.Bornstein AM et al. (2017) Reminders of past choices bias decisions for reward in humans. Nat Commun 8, 15958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yin HH and Knowlton BJ (2006) The role of the basal ganglia in habit formation. Nat Rev Neurosci 7 (6), 464–76. [DOI] [PubMed] [Google Scholar]
- 16.LeDoux JE (2000) Emotion circuits in the brain. Annu Rev Neurosci 23, 155–84. [DOI] [PubMed] [Google Scholar]
- 17.Baxter MG and Murray EA (2002) The amygdala and reward. Nat Rev Neurosci 3 (7), 563–73. [DOI] [PubMed] [Google Scholar]
- 18.Davachi L (2006) Item, context and relational episodic encoding in humans. Curr Opin Neurobiol 16 (6), 693–700. [DOI] [PubMed] [Google Scholar]
- 19.Everitt BJ and Robbins TW (2016) Drug Addiction: Updating Actions to Habits to Compulsions Ten Years On. Annu Rev Psychol 67, 23–50. [DOI] [PubMed] [Google Scholar]
- 20.McDonald RJ et al. (2004) Multiple memory systems: the power of interactions. Neurobiol Learn Mem 82 (3), 333–46. [DOI] [PubMed] [Google Scholar]
- 21.Schwabe L et al. (2011) Stress, habits, and drug addiction: a psychoneuroendocrinological perspective. Exp Clin Psychopharmacol 19 (1), 53–63. [DOI] [PubMed] [Google Scholar]
- 22.Wikler A (1965) Conditioning factors in opiate addiction and relapse In Narcotics (Wilner DI and Kassebaum GG eds), pp. 85–100, McGraw-Hill. [Google Scholar]
- 23.Lupien SJ et al. (2007) The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition. Brain Cogn 65 (3), 209–37. [DOI] [PubMed] [Google Scholar]
- 24.Joels M and Baram TZ (2009) The neuro-symphony of stress. Nat Rev Neurosci 10 (6), 459–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hermans EJ et al. (2014) Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends Neurosci 37 (6), 304–14. [DOI] [PubMed] [Google Scholar]
- 26.Dias-Ferreira E et al. (2009) Chronic stress causes frontostriatal reorganization and affects decision-making. Science 325 (5940), 621–5. [DOI] [PubMed] [Google Scholar]
- 27.McEwen BS et al. (2016) Stress Effects on Neuronal Structure: Hippocampus, Amygdala, and Prefrontal Cortex. Neuropsychopharmacology 41 (1), 3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roozendaal B and McGaugh JL (2011) Memory modulation. Behav Neurosci 125 (6), 797–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Packard MG and Wingard JC (2004) Amygdala and “emotional” modulation of the relative use of multiple memory systems. Neurobiol Learn Mem 82 (3), 243–52. [DOI] [PubMed] [Google Scholar]
- 30.Roozendaal B (2002) Stress and memory: opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiol Learn Mem 78 (3), 578–95. [DOI] [PubMed] [Google Scholar]
- 31.Armario A (2010) Activation of the hypothalamic-pituitary-adrenal axis by addictive drugs: different pathways, common outcome. Trends Pharmacol Sci 31 (7), 318–25. [DOI] [PubMed] [Google Scholar]
- 32.Zorrilla EP et al. (2014) Corticotropin releasing factor: a key role in the neurobiology of addiction. Front Neuroendocrinol 35 (2), 234–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piazza PV and Le Moal M (1997) Glucocorticoids as a biological substrate of reward: physiological and pathophysiological implications. Brain Res Brain Res Rev 25 (3), 359–72. [DOI] [PubMed] [Google Scholar]
- 34.McEwen BS (1987) Glucocorticoid-biogenic amine interactions in relation to mood and behavior. Biochem Pharmacol 36 (11), 1755–63. [DOI] [PubMed] [Google Scholar]
- 35.Donoghue K et al. (2016) Double-blind, 12 month follow-up, placebo-controlled trial of mifepristone on cognition in alcoholics: the MIFCOG trial protocol. BMC Psychiatry 16, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walter M et al. (2015) Effects of cortisol administration on craving in heroin addicts. Transl Psychiatry 5, e610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vendruscolo LF et al. (2015) Glucocorticoid receptor antagonism decreases alcohol seeking in alcohol-dependent individuals. J Clin Invest 125 (8), 3193–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kubena RK et al. (1971) Corticosterone elevation mediated centrally by delta 1-tetrahydrocannabinol in rats. Eur J Pharmacol 14 (1), 89–92. [DOI] [PubMed] [Google Scholar]
- 39.Cone EJ et al. (1986) Acute effects of smoking marijuana on hormones, subjective effects and performance in male human subjects. Pharmacol Biochem Behav 24 (6), 1749–54. [DOI] [PubMed] [Google Scholar]
- 40.Ellis FW (1966) Effect of ethanol on plasma corticosterone levels. J Pharmacol Exp Ther 153 (1), 121–7. [PubMed] [Google Scholar]
- 41.Jenkins JS and Connolly J (1968) Adrenocortical response to ethanol in man. Br Med J 2 (5608), 804–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moldow RL and Fischman AJ (1987) Cocaine induced secretion of ACTH, beta-endorphin, and corticosterone. Peptides 8 (5), 819–822. [DOI] [PubMed] [Google Scholar]
- 43.Heesch CM et al. (1995) Effects of cocaine on cortisol secretion in humans. Am J Med Sci 310 (2), 61–4. [DOI] [PubMed] [Google Scholar]
- 44.Nikodijevic O and Maickel RP (1967) Some effects of morphine on pituitary-adrenocortical function in the rat. Biochem Pharmacol 16 (11), 2137–42. [DOI] [PubMed] [Google Scholar]
- 45.Zis AP et al. (1984) Morphine inhibits cortisol and stimulates prolactin secretion in man. Psychoneuroendocrinology 9 (4), 423–7. [DOI] [PubMed] [Google Scholar]
- 46.Doyon WM et al. (2013) Nicotine decreases ethanol-induced dopamine signaling and increases self-administration via stress hormones. Neuron 79 (3), 530–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Newhouse PA et al. (1990) Neuroendocrine, physiologic, and behavioral responses following intravenous nicotine in nonsmoking healthy volunteers and in patients with Alzheimer’s disease. Psychoneuroendocrinology 15 (5–6), 471–84. [DOI] [PubMed] [Google Scholar]
- 48.Joels M et al. (2006) Learning under stress: how does it work? Trends Cogn Sci 10 (4), 152–8. [DOI] [PubMed] [Google Scholar]
- 49.Sandi C et al. (1997) Experience-dependent facilitating effect of corticosterone on spatial memory formation in the water maze. Eur J Neurosci 9 (4), 637–42. [DOI] [PubMed] [Google Scholar]
- 50.Kim JJ et al. (2001) Amygdala is critical for stress-induced modulation of hippocampal long-term potentiation and learning. J Neurosci 21 (14), 5222–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shields GS et al. (2017) The effects of acute stress on episodic memory: A meta-analysis and integrative review. Psychol Bull 143 (6), 636–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Diamond DM et al. (2007) The temporal dynamics model of emotional memory processing: a synthesis on the neurobiological basis of stress-induced amnesia, flashbulb and traumatic memories, and the Yerkes-Dodson law. Neural Plast 2007, 60803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Joels M et al. (2012) Unraveling the time domains of corticosteroid hormone influences on brain activity: rapid, slow, and chronic modes. Pharmacol Rev 64 (4), 901–38. [DOI] [PubMed] [Google Scholar]
- 54.Siegel S (2005) Drug Tolerance, Drug Addiction, and Drug Anticipation. Current Directions in Psychological Science 14 (6), 296–300. [Google Scholar]
- 55.Carelli RM et al. (2003) Basolateral amygdala neurons encode cocaine self-administration and cocaine-associated cues. J Neurosci 23 (23), 8204–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weiss F et al. (2000) Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc Natl Acad Sci U S A 97 (8), 4321–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.See RE et al. (2003) Muscarinic receptor antagonism in the basolateral amygdala blocks acquisition of cocaine-stimulus association in a model of relapse to cocaine-seeking behavior in rats. Neuroscience 117 (2), 477–83. [DOI] [PubMed] [Google Scholar]
- 58.White NM and McDonald RJ (1993) Acquisition of a spatial conditioned place preference is impaired by amygdala lesions and improved by fornix lesions. Behav Brain Res 55 (2), 269–81. [DOI] [PubMed] [Google Scholar]
- 59.Everitt BJ et al. (1991) The basolateral amygdala-ventral striatal system and conditioned place preference: further evidence of limbic-striatal interactions underlying reward-related processes. Neuroscience 42 (1), 1–18. [DOI] [PubMed] [Google Scholar]
- 60.Kutlu MG and Gould TJ (2016) Effects of drugs of abuse on hippocampal plasticity and hippocampus-dependent learning and memory: contributions to development and maintenance of addiction. Learn Mem 23 (10), 515–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hsu EH et al. (2002) The amygdala mediates memory consolidation for an amphetamine conditioned place preference. Behav Brain Res 129 (1–2), 93–100. [DOI] [PubMed] [Google Scholar]
- 62.Rademacher DJ et al. (2006) The neural substrates of amphetamine conditioned place preference: implications for the formation of conditioned stimulus-reward associations. Eur J Neurosci 24 (7), 2089–97. [DOI] [PubMed] [Google Scholar]
- 63.Jasinska AJ et al. (2014) Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neurosci Biobehav Rev 38, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sharp BM (2017) Basolateral amygdala and stress-induced hyperexcitability affect motivated behaviors and addiction. Transl Psychiatry 7 (8), e1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Karst H et al. (2010) Metaplasticity of amygdalar responses to the stress hormone corticosterone. Proc Natl Acad Sci U S A 107 (32), 14449–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shors TJ et al. (1992) Stress-induced facilitation of classical conditioning. Science 257 (5069), 537–9. [DOI] [PubMed] [Google Scholar]
- 67.Hui GK et al. (2004) Memory enhancement of classical fear conditioning by post-training injections of corticosterone in rats. Neurobiol Learn Mem 81 (1), 67–74. [DOI] [PubMed] [Google Scholar]
- 68.Raio CM and Phelps EA (2015) The influence of acute stress on the regulation of conditioned fear. Neurobiol Stress 1, 134–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miranda MI et al. (2008) Glucocorticoids enhance taste aversion memory via actions in the insular cortex and basolateral amygdala. Learn Mem 15 (7), 468–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Antov MI et al. (2013) Differential impact of the first and second wave of a stress response on subsequent fear conditioning in healthy men. Biol Psychol 94 (2), 456–68. [DOI] [PubMed] [Google Scholar]
- 71.Merz CJ et al. (2014) Cortisol modifies extinction learning of recently acquired fear in men. Soc Cogn Affect Neurosci 9 (9), 1426–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zorawski M et al. (2006) Effects of stress and sex on acquisition and consolidation of human fear conditioning. Learn Mem 13 (4), 441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dietz D et al. (2007) Corticosterone fails to produce conditioned place preference or conditioned place aversion in rats. Behav Brain Res 181 (2), 287–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McLaughlin JP et al. (2003) Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci 23 (13), 5674–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Matsuzawa S et al. (1998) Conditioned fear stress induces ethanol-associated place preference in rats. Eur J Pharmacol 341 (2–3), 127–30. [DOI] [PubMed] [Google Scholar]
- 76.Grakalic I et al. (2006) Effects of stress modulation on morphine-induced conditioned place preferences and plasma corticosterone levels in Fischer, Lewis, and Sprague-Dawley rat strains. Psychopharmacology (Berl) 189 (3), 277–86. [DOI] [PubMed] [Google Scholar]
- 77.Tomie A et al. (2002) Pavlovian autoshaping procedures increase plasma corticosterone levels in rats. Pharmacol Biochem Behav 72 (3), 507–13. [DOI] [PubMed] [Google Scholar]
- 78.Tomie A et al. (2000) Individual differences in pavlovian autoshaping of lever pressing in rats predict stress-induced corticosterone release and mesolimbic levels of monoamines. Pharmacol Biochem Behav 65 (3), 509–17. [DOI] [PubMed] [Google Scholar]
- 79.Kruse O et al. (2017) Altered reward learning and hippocampal connectivity following psychosocial stress. Neuroimage 171, 15–25. [DOI] [PubMed] [Google Scholar]
- 80.Di Ciano P and Everitt BJ (2004) Conditioned reinforcing properties of stimuli paired with self-administered cocaine, heroin or sucrose: implications for the persistence of addictive behaviour. Neuropharmacology 47 Suppl 1, 202–13. [DOI] [PubMed] [Google Scholar]
- 81.Corbit LH and Janak PH (2007) Ethanol-associated cues produce general pavlovian-instrumental transfer. Alcohol Clin Exp Res 31 (5), 766–74. [DOI] [PubMed] [Google Scholar]
- 82.Corbit LH and Janak PH (2016) Changes in the Influence of Alcohol-Paired Stimuli on Alcohol Seeking across Extended Training. Front Psychiatry 7, 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.LeBlanc KH et al. (2012) Pavlovian-to-instrumental transfer in cocaine seeking rats. Behav Neurosci 126 (5), 681–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ciccocioppo R et al. (2004) Stimuli associated with a single cocaine experience elicit long-lasting cocaine-seeking. Nat Neurosci 7 (5), 495–6. [DOI] [PubMed] [Google Scholar]
- 85.Pielock SM et al. (2013) Post-training glucocorticoid receptor activation during Pavlovian conditioning reduces Pavlovian-instrumental transfer in rats. Pharmacol Biochem Behav 104, 125–31. [DOI] [PubMed] [Google Scholar]
- 86.Zorawski M and Killcross S (2003) Glucocorticoid receptor agonist enhances pavlovian appetitive conditioning but disrupts outcome-specific associations. Behav Neurosci 117 (6), 1453–7. [DOI] [PubMed] [Google Scholar]
- 87.Pool E et al. (2015) Stress increases cue-triggered “wanting” for sweet reward in humans. J Exp Psychol Anim Learn Cogn 41 (2), 128–36. [DOI] [PubMed] [Google Scholar]
- 88.Packard MG and Goodman J (2012) Emotional arousal and multiple memory systems in the mammalian brain. Front Behav Neurosci 6, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Leong KC and Packard MG (2014) Exposure to predator odor influences the relative use of multiple memory systems: role of basolateral amygdala. Neurobiol Learn Mem 109, 56–61. [DOI] [PubMed] [Google Scholar]
- 90.Vogel S et al. (2017) Stress Induces a shift towards striatum-dependent stimulus-response learning via the mineralocorticoid receptor. Neuropsychopharmacology 42 (6), 1262–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Roozendaal B and McGaugh JL (1997) Basolateral amygdala lesions block the memory-enhancing effect of glucocorticoid administration in the dorsal hippocampus of rats. Eur J Neurosci 9 (1), 76–83. [DOI] [PubMed] [Google Scholar]
- 92.Devan BD et al. (2011) Parallel associative processing in the dorsal striatum: segregation of stimulus-response and cognitive control subregions. Neurobiol Learn Mem 96 (2), 95–120. [DOI] [PubMed] [Google Scholar]
- 93.Dickinson A et al. (2002) Alcohol seeking by rats: action or habit? Q J Exp Psychol B 55 (4), 331–48. [DOI] [PubMed] [Google Scholar]
- 94.Corbit LH et al. (2012) Habitual alcohol seeking: time course and the contribution of subregions of the dorsal striatum. Biol Psychiatry 72 (5), 389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mangieri RA et al. (2012) Ethanol seeking by Long Evans rats is not always a goal-directed behavior. PLoS One 7 (8), e42886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Miles FJ et al. (2003) Oral cocaine seeking by rats: action or habit? Behav Neurosci 117 (5), 927–38. [DOI] [PubMed] [Google Scholar]
- 97.Gourley SL et al. (2012) Action control is mediated by prefrontal BDNF and glucocorticoid receptor binding. Proc Natl Acad Sci U S A 109 (50), 20714–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schwabe L et al. (2011) Preventing the stress-induced shift from goal-directed to habit action with a beta-adrenergic antagonist. J Neurosci 31 (47), 17317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fournier M et al. (2017) Effects of psychosocial stress on the goal-directed and habit memory systems during learning and later execution. Psychoneuroendocrinology 77, 275–283. [DOI] [PubMed] [Google Scholar]
- 100.Schwabe L et al. (2010) Corticosteroids operate as a switch between memory systems. J Cogn Neurosci 22 (7), 1362–72. [DOI] [PubMed] [Google Scholar]
- 101.Siller-Perez C et al. (2017) Glucocorticoid administration into the dorsolateral but not dorsomedial striatum accelerates the shift from a spatial toward procedural memory. Neurobiol Learn Mem 141, 124–133. [DOI] [PubMed] [Google Scholar]
- 102.Vingerhoets WA et al. (2016) Cue-induced striatal activity in frequent cannabis users independently predicts cannabis problem severity three years later. J Psychopharmacol 30 (2), 152–8. [DOI] [PubMed] [Google Scholar]
- 103.Grusser SM et al. (2004) Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl) 175 (3), 296–302. [DOI] [PubMed] [Google Scholar]
- 104.Vollstadt-Klein S et al. (2010) Initial, habitual and compulsive alcohol use is characterized by a shift of cue processing from ventral to dorsal striatum. Addiction 105 (10), 1741–9. [DOI] [PubMed] [Google Scholar]
- 105.Dagher A et al. (2009) An acute psychosocial stress enhances the neural response to smoking cues. Brain Res 1293, 40–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ersche KD et al. (2016) Carrots and sticks fail to change behavior in cocaine addiction. Science 352 (6292), 1468–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McKim TH et al. (2016) Addiction History Associates with the Propensity to Form Habits. J Cogn Neurosci 28 (7), 1024–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sjoerds Z et al. (2013) Behavioral and neuroimaging evidence for overreliance on habit learning in alcohol-dependent patients. Transl Psychiatry 3, e337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bohbot VD et al. (2013) Caudate nucleus-dependent navigational strategies are associated with increased use of addictive drugs. Hippocampus 23 (11), 973–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Daw ND et al. (2011) Model-based influences on humans’ choices and striatal prediction errors. Neuron 69 (6), 1204–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sebold M et al. (2017) When Habits Are Dangerous: Alcohol Expectancies and Habitual Decision Making Predict Relapse in Alcohol Dependence. Biol Psychiatry 82 (11), 847–856. [DOI] [PubMed] [Google Scholar]
- 112.Lighthall NR et al. (2013) Stress modulates reinforcement learning in younger and older adults. Psychol Aging 28 (1), 35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Otto AR et al. (2013) Working-memory capacity protects model-based learning from stress. Proc Natl Acad Sci U S A 110 (52), 20941–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Robinson TE and Berridge KC (2008) Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci 363 (1507), 3137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Piazza PV and Deroche-Gamonet V (2013) A multistep general theory of transition to addiction. Psychopharmacology (Berl) 229 (3), 387–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Watson P and de Wit S (2018) Current limits of experimental research into habits and future directions. Curr Opin Behav Sci 20, 33–39. [Google Scholar]
- 117.Schwabe L and Wolf OT (2009) Stress prompts habit behavior in humans. J Neurosci 29 (22), 7191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sinha R (2001) How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 158 (4), 343–59. [DOI] [PubMed] [Google Scholar]
- 119.Arnsten AF (2009) Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci 10 (6), 410–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Devilbiss DM et al. (2017) Stress Degrades Prefrontal Cortex Neuronal Coding of Goal-Directed Behavior. Cereb Cortex 27 (5), 2970–2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gillan CM et al. (2015) Model-based learning protects against forming habits. Cogn Affect Behav Neurosci 15 (3), 523–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Moser EI et al. (2017) Spatial representation in the hippocampal formation: a history. Nat Neurosci 20 (11), 1448–1464. [DOI] [PubMed] [Google Scholar]
- 123.Crombag HS et al. (2008) Review. Context-induced relapse to drug seeking: a review. Philos Trans R Soc Lond B Biol Sci 363 (1507), 3233–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fuchs RA et al. (2005) The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology 30 (2), 296–309. [DOI] [PubMed] [Google Scholar]
- 125.McGlinchey EM and Aston-Jones G (2017) Dorsal Hippocampus Drives Context-Induced Cocaine Seeking via Inputs to Lateral Septum. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Marinelli PW et al. (2007) Effects of opioid receptor blockade on the renewal of alcohol seeking induced by context: relationship to c-fos mRNA expression. Eur J Neurosci 26 (10), 2815–23. [DOI] [PubMed] [Google Scholar]
- 127.Atkins AL et al. (2008) Hippocampal regulation of contextual cue-induced reinstatement of cocaine-seeking behavior. Pharmacol Biochem Behav 90 (3), 481–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sandi C and Pinelo-Nava MT (2007) Stress and memory: behavioral effects and neurobiological mechanisms. Neural Plast 2007, 78970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Goldfarb EV et al. (in press) Acute stress throughout the memory cycle: Diverging effects on associative and item memory. J Exp Psychol Gen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Keralapurath MM et al. (2014) Cocaine- or stress-induced metaplasticity of LTP in the dorsal and ventral hippocampus. Hippocampus 24 (5), 577–90. [DOI] [PubMed] [Google Scholar]
- 131.Pastor R et al. (2012) Role of corticotropin-releasing factor and corticosterone in behavioral sensitization to ethanol. J Pharmacol Exp Ther 341 (2), 455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mantsch JR et al. (2008) Surgical adrenalectomy with diurnal corticosterone replacement slows escalation and prevents the augmentation of cocaine-induced reinstatement in rats self-administering cocaine under long-access conditions. Neuropsychopharmacology 33 (4), 814–26. [DOI] [PubMed] [Google Scholar]
- 133.Ostroumov A and Dani JA (2018) Convergent Neuronal Plasticity and Metaplasticity Mechanisms of Stress, Nicotine, and Alcohol. Annu Rev Pharmacol Toxicol 58, 547–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ambroggi F et al. (2009) Stress and addiction: glucocorticoid receptor in dopaminoceptive neurons facilitates cocaine seeking. Nat Neurosci 12 (3), 247–9. [DOI] [PubMed] [Google Scholar]
- 135.Funk CK et al. (2006) Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci 26 (44), 11324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vendruscolo LF et al. (2012) Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci 32 (22), 7563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Goeders NE (2002) The HPA axis and cocaine reinforcement. Psychoneuroendocrinology 27 (1–2), 13–33. [DOI] [PubMed] [Google Scholar]
- 138.Fiancette JF et al. (2010) Mifepristone and spironolactone differently alter cocaine intravenous self-administration and cocaine-induced locomotion in C57BL/6J mice. Addict Biol 15 (1), 81–7. [DOI] [PubMed] [Google Scholar]
- 139.Rotter A et al. (2012) Glucocorticoid receptor antagonism blocks ethanol-induced place preference learning in mice and attenuates dopamine D2 receptor adaptation in the frontal cortex. Brain Res Bull 88 (5), 519–24. [DOI] [PubMed] [Google Scholar]
- 140.Fan YD et al. (2013) Blockage of glucocorticoid receptors during memory acquisition, retrieval and reconsolidation prevents the expression of morphine-induced conditioned place preferences in mice. Dongwuxue Yanjiu 34 (E1), E26–34. [DOI] [PubMed] [Google Scholar]
- 141.Der-Avakian A et al. (2005) Surgical and pharmacological suppression of glucocorticoids prevents the enhancement of morphine conditioned place preference by uncontrollable stress in rats. Psychopharmacology (Berl) 179 (2), 409–17. [DOI] [PubMed] [Google Scholar]
- 142.Davis KW et al. (2005) Plasma corticosterone in the rat in response to nicotine and saline injections in a context previously paired or unpaired with nicotine. Psychopharmacology (Berl) 180 (3), 466–72. [DOI] [PubMed] [Google Scholar]
- 143.DeVries AC et al. (1998) Conditioned release of corticosterone by contextual stimuli associated with cocaine is mediated by corticotropin-releasing factor. Brain Res 786 (1–2), 39–46. [DOI] [PubMed] [Google Scholar]
- 144.Stringfield SJ et al. (2017) Role of glucocorticoid receptor-mediated mechanisms in cocaine memory enhancement. Neuropharmacology 123, 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Stringfield SJ et al. (2016) Requisite role of basolateral amygdala glucocorticoid receptor stimulation in drug context-induced cocaine-seeking behavior. Int J Neuropsychopharmacol 19 (12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ranganathan M et al. (2009) The effects of cannabinoids on serum cortisol and prolactin in humans. Psychopharmacology (Berl) 203 (4), 737–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Merry J and Marks V (1969) Plasma-hydrocortisone response to ethanol in chronic alcoholics. Lancet 1 (7601), 921–3. [DOI] [PubMed] [Google Scholar]
- 148.Blaine SK et al. (in press) Craving, cortisol and behavioral alcohol motivation responses to stress and alcohol cue contexts and discrete cues in binge and non-binge drinkers. Addict Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.McCullough AM et al. (2015) Differential effects of stress-induced cortisol responses on recollection and familiarity-based recognition memory. Neurobiol Learn Mem 123, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Blaine SK and Sinha R (2017) Alcohol, stress, and glucocorticoids: From risk to dependence and relapse in alcohol use disorders. Neuropharmacology 122, 136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cuttler C et al. (2017) Blunted stress reactivity in chronic cannabis users. Psychopharmacology (Berl) 234 (15), 2299–2309. [DOI] [PubMed] [Google Scholar]
- 152.Sinha R et al. (2009) Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology 34 (5), 1198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kirschbaum C et al. (1993) Attenuated cortisol response to psychological stress but not to CRH or ergometry in young habitual smokers. Pharmacol Biochem Behav 44 (3), 527–31. [DOI] [PubMed] [Google Scholar]
- 154.Goldfarb EV and Phelps EA (2017) Stress and the trade-off between hippocampal and striatal memory. Curr Opin Behav Sci 14, 47–53. [Google Scholar]
- 155.Vogel S et al. (2016) Cognitive Adaptation under Stress: A Case for the Mineralocorticoid Receptor. Trends Cogn Sci 20 (3), 192–203. [DOI] [PubMed] [Google Scholar]
- 156.Goldfarb EV et al. (2017) Acute stress time-dependently modulates multiple memory systems. J Cogn Neurosci 29 (11), 1877–1894. [DOI] [PubMed] [Google Scholar]
- 157.Acheson DT et al. (2012) Hippocampal dysfunction effects on context memory: possible etiology for posttraumatic stress disorder. Neuropharmacology 62 (2), 674–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Dezfouli A and Balleine BW (2013) Actions, action sequences and habits: evidence that goal-directed and habitual action control are hierarchically organized. PLoS Comput Biol 9 (12), e1003364. [DOI] [PMC free article] [PubMed] [Google Scholar]


